Optimization of Demineralization and Pyrolysis Performance of Eucalyptus Hydrothermal Pretreatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Demineralization
2.3. Sample Preparation by Microwave-Assisted Digestion
2.4. ICP-OES Measurements
2.5. Process Optimization
2.6. Component Analysis
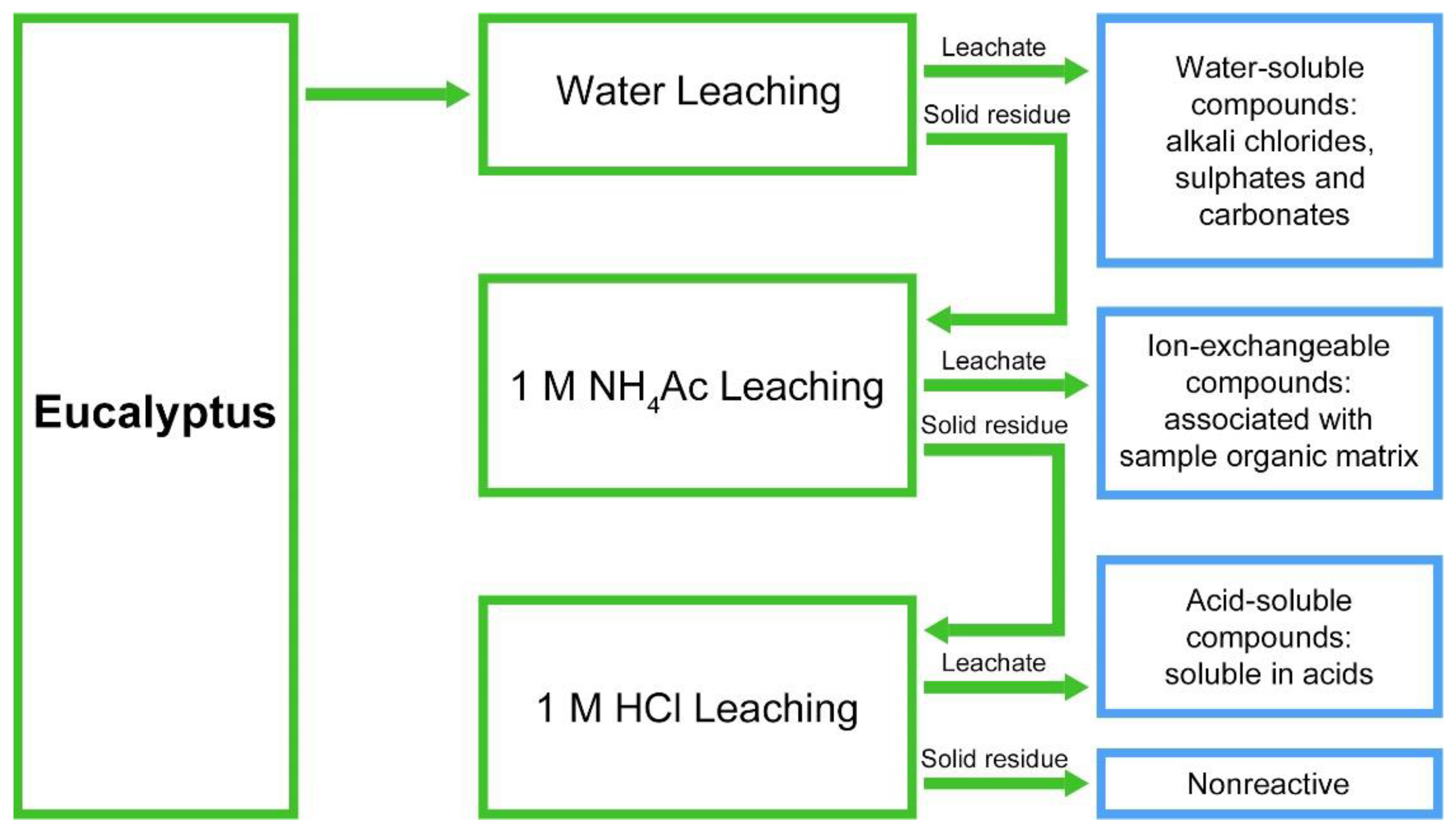
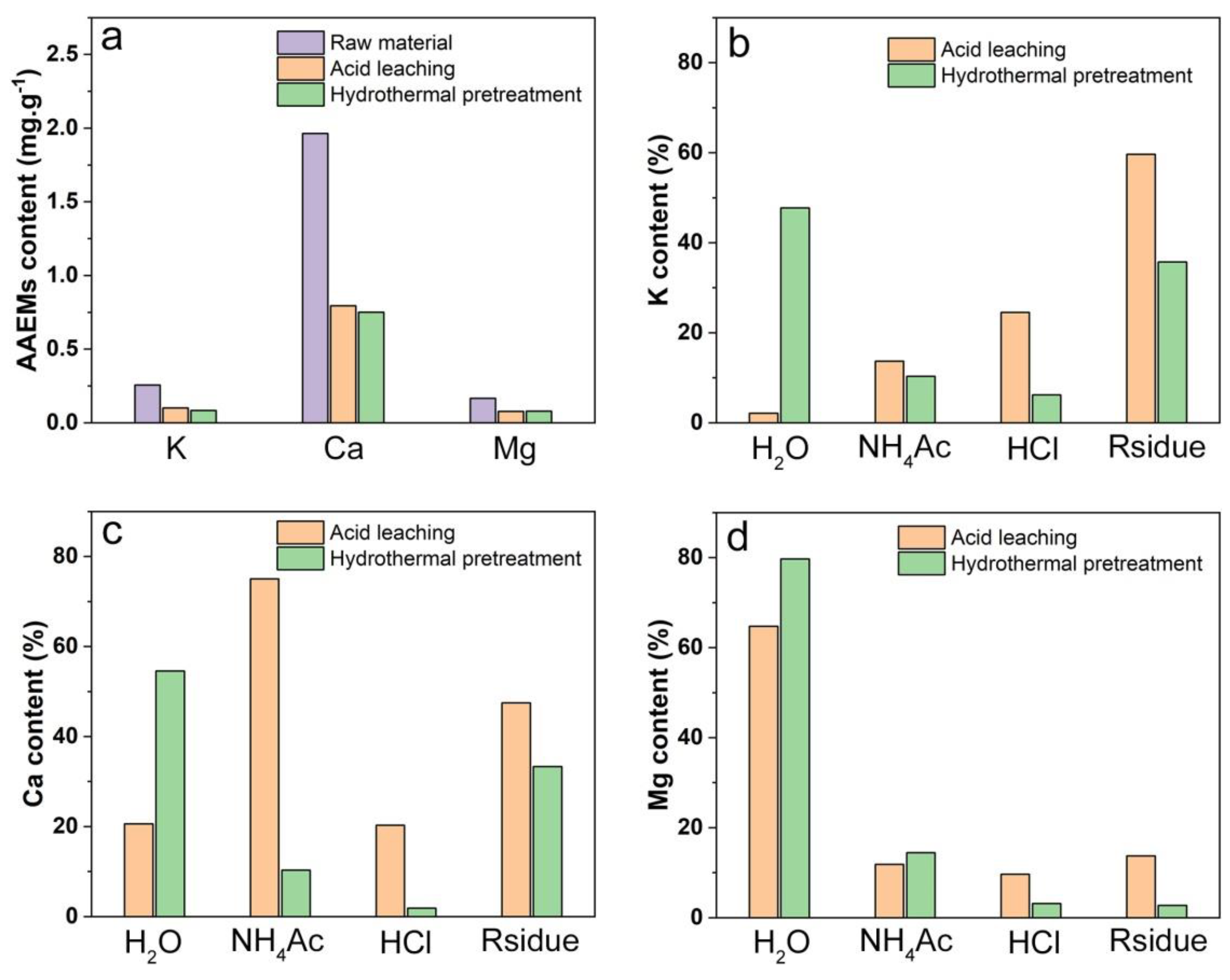
2.7. Chemical Fractionation
2.8. Rapid Pyrolysis of Eucalyptus
2.9. Pyrolysis Performance Characterization
3. Results and Discussion
3.1. Response Surface Design and Results
3.2. Interaction between Reaction Factors
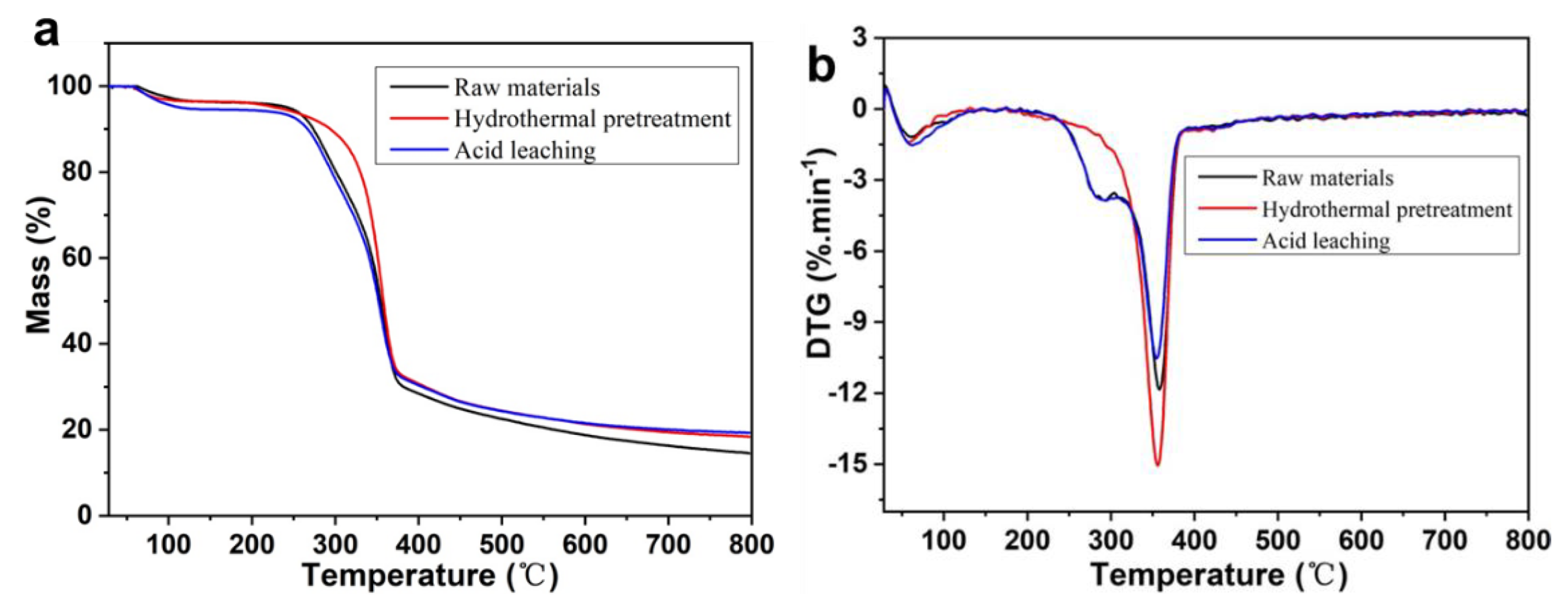
3.3. Thermal Stability Analysis
3.4. Pyrolysis Performance Analysis
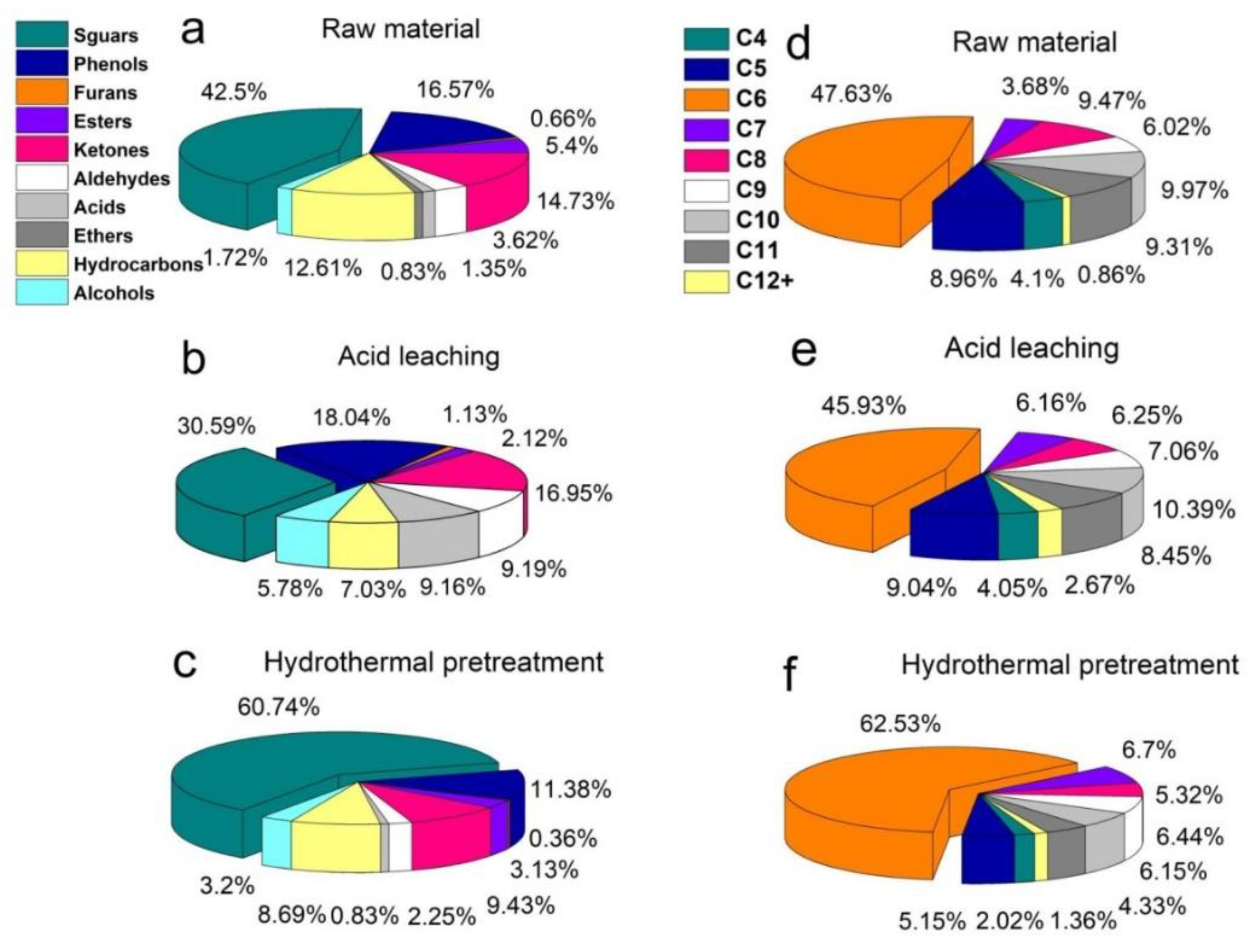
3.5. Chemical Composition and Distribution of Bio-Oil
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Johnsson, F.; Kjärstad, J.; Rootzén, J. The threat to climate change mitigation posed by the abundance of fossil fuels. Clim. Policy 2019, 19, 258–274. [Google Scholar] [CrossRef] [Green Version]
- Zeng, H.; Liu, B.; Li, J.; Li, M.; Peng, M.; Qin, C.; Liang, C.; Huang, C.; Li, X.; Yao, S. Efficient separation of bagasse lignin by freeze–thaw-assisted p-toluenesulfonic acid pretreatment. Bioresour. Technol. 2022, 351, 126951. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Zhu, J.; Hou, Y.; Qin, C.; Chen, W.; Nong, Y.; Liao, Z.; Liang, C.; Bian, H.; Yao, S. Effect of temperature on simultaneous separation and extraction of hemicellulose using p-toluenesulfonic acid treatment at atmospheric pressure. Bioresour. Technol. 2022, 348, 126793. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Li, Y.; Cao, L.; Zhu, J.; Deng, B.; Hou, Y.; Liang, C.; Huang, C.; Qin, C.; Yao, S. High efficiency and clean separation of eucalyptus components by glycolic acid pretreatment. Bioresour. Technol. 2021, 341, 125757. [Google Scholar] [CrossRef] [PubMed]
- Bian, H.; Chen, L.; Dong, M.; Fu, Y.; Wang, R.; Zhou, X.; Wang, X.; Xu, J.; Dai, H. Cleaner production of lignocellulosic nanofibrils: Potential of mixed enzymatic treatment. J. Clean. Prod. 2020, 270, 122506. [Google Scholar] [CrossRef]
- Nguyen, T.-V.; Clausen, L.R. Techno-economic analysis of polygeneration systems based on catalytic hydropyrolysis for the production of bio-oil and fuels. Energy Convers. Manag. 2019, 184, 539–558. [Google Scholar] [CrossRef]
- Patel, M.; Kumar, A. Production of renewable diesel through the hydroprocessing of lignocellulosic biomass-derived bio-oil: A review. Renew. Sustain. Energy Rev. 2016, 58, 1293–1307. [Google Scholar] [CrossRef]
- Xiong, Z.; Wang, Y.; Syed-Hassan, S.S.A.; Hu, X.; Han, H.; Su, S.; Xu, K.; Jiang, L.; Guo, J.; Berthold, E.E.S.; et al. Effects of heating rate on the evolution of bio-oil during its pyrolysis. Energy Convers. Manag. 2018, 163, 420–427. [Google Scholar] [CrossRef]
- Dai, L.; Wang, Y.; Liu, Y.; Ruan, R.; He, C.; Yu, Z.; Jiang, L.; Zeng, Z.; Tian, X. Integrated process of lignocellulosic biomass torrefaction and pyrolysis for upgrading bio-oil production: A state-of-the-art review. Renew. Sustain. Energy Rev. 2019, 107, 20–36. [Google Scholar] [CrossRef]
- Liang, J.; Shan, G.; Sun, Y. Catalytic fast pyrolysis of lignocellulosic biomass: Critical role of zeolite catalysts. Renew. Sustain. Energy Rev. 2021, 139, 110707. [Google Scholar] [CrossRef]
- Zhang, L.; Li, S.; Li, K.; Zhu, X. Two-step pyrolysis of corncob for value-added chemicals and high quality bio-oil: Effects of pyrolysis temperature and residence time. Energy Convers. Manag. 2018, 166, 260–267. [Google Scholar] [CrossRef]
- Deng, L.; Ye, J.; Jin, X.; Che, D. Transformation and release of potassium during fixed-bed pyrolysis of biomass. J. Energy Inst. 2018, 91, 630–637. [Google Scholar] [CrossRef]
- Persson, H.; Kantarelis, E.; Evangelopoulos, P.; Yang, W. Wood-derived acid leaching of biomass for enhanced production of sugars and sugar derivatives during pyrolysis: Influence of acidity and treatment time. J. Anal. Appl. Pyrolysis 2017, 127, 329–334. [Google Scholar] [CrossRef]
- Chen, D.; Mei, J.; Li, H.; Li, Y.; Lu, M.; Ma, T.; Ma, Z. Combined pretreatment with torrefaction and washing using torrefaction liquid products to yield upgraded biomass and pyrolysis products. Bioresour. Technol. 2017, 228, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Asadieraghi, M.; Wan Daud, W.M.A. Characterization of lignocellulosic biomass thermal degradation and physiochemical structure: Effects of demineralization by diverse acid solutions. Energy Convers. Manag. 2014, 82, 71–82. [Google Scholar] [CrossRef]
- Dai, L.; Wang, Y.; Liu, Y.; Ruan, R.; He, C.; Duan, D.; Zhao, Y.; Yu, Z.; Jiang, L.; Wu, Q. Bridging the relationship between hydrothermal pretreatment and co-pyrolysis: Effect of hydrothermal pretreatment on aromatic production. Energy Convers. Manag. 2019, 180, 36–43. [Google Scholar] [CrossRef]
- Yao, Z.; Ma, X.; Xiao, Z. The effect of two pretreatment levels on the pyrolysis characteristics of water hyacinth. Renew. Energy 2020, 151, 514–527. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, H.; Yang, H.; Zhang, Y. The effect of acid washing pretreatment on bio-oil production in fast pyrolysis of rice husk. Cellulose 2019, 26, 8465–8474. [Google Scholar] [CrossRef]
- Dong, Q.; Zhang, S.; Zhang, L.; Ding, K.; Xiong, Y. Effects of four types of dilute acid washing on moso bamboo pyrolysis using Py–GC/MS. Bioresour. Technol. 2015, 185, 62–69. [Google Scholar] [CrossRef]
- Mosqueda, A.; Wei, J.; Medrano, K.; Gonzales, H.; Ding, L.; Yu, G.; Yoshikawa, K. Co-gasification reactivity and synergy of banana residue hydrochar and anthracite coal blends. Appl. Energy 2019, 250, 92–97. [Google Scholar] [CrossRef]
- Chang, S.; Zhao, Z.; Zheng, A.; Li, X.; Wang, X.; Huang, Z.; He, F.; Li, H. Effect of hydrothermal pretreatment on properties of bio-oil produced from fast pyrolysis of eucalyptus wood in a fluidized bed reactor. Bioresour. Technol. 2013, 138, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Wu, Y.; Han, Y.; Qin, C.; Nie, S.; Liu, S.; Wang, S.; Yao, S. Effect of hydrothermal pretreatment on the demineralization and thermal degradation behavior of eucalyptus. Bioresour. Technol. 2020, 307, 123246. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bai, Z.-Q.; Bai, J.; Zhao, B.-B.; Li, P.; Han, Y.-N.; Kong, L.-X.; Li, W. Effect of Ca2+ species with different modes of occurrence on direct liquefaction of a calcium-rich lignite. Fuel Processing Technol. 2015, 133, 161–166. [Google Scholar] [CrossRef]
- Yao, S.; Nie, S.; Zhu, H.; Wang, S.; Song, X.; Qin, C. Extraction of hemicellulose by hot water to reduce adsorbable organic halogen formation in chlorine dioxide bleaching of bagasse pulp. Ind. Crops Prod. 2017, 96, 178–185. [Google Scholar] [CrossRef]
- Santos, H.M.; Coutinho, J.P.; Amorim, F.A.C.; Lôbo, I.P.; Moreira, L.S.; Nascimento, M.M.; de Jesus, R.M. Microwave-assisted digestion using diluted HNO3 and H2O2 for macro and microelements determination in guarana samples by ICP OES. Food Chemistry 2019, 273, 159–165. [Google Scholar] [CrossRef]
- Chauhan, G.; de Klerk, A. Dissolution Methods for the Quantification of Metals in Oil Sands Bitumen. Energy Fuels 2020, 34, 2870–2879. [Google Scholar] [CrossRef]
- Yang, Q.; Huo, D.; Si, C.; Fang, G.; Liu, Q.; Hou, Q.; Chen, X.; Zhang, F. Improving enzymatic saccharification of eucalyptus with a pretreatment process using MgCl2. Ind. Crops Prod. 2018, 123, 401–406. [Google Scholar] [CrossRef]
- Pettersson, A.; Åmand, L.-E.; Steenari, B.-M. Chemical fractionation for the characterisation of fly ashes from co-combustion of biofuels using different methods for alkali reduction. Fuel 2009, 88, 1758–1772. [Google Scholar] [CrossRef] [Green Version]
- Chan, Y.H.; Loh, S.K.; Chin, B.L.F.; Yiin, C.L.; How, B.S.; Cheah, K.W.; Wong, M.K.; Loy, A.C.M.; Gwee, Y.L.; Lo, S.L.Y.; et al. Fractionation and extraction of bio-oil for production of greener fuel and value-added chemicals: Recent advances and future prospects. Chem. Eng. J. 2020, 397, 125406. [Google Scholar] [CrossRef]
- An, L.; Si, C.; Bae, J.H.; Jeong, H.; Kim, Y.S. One-step silanization and amination of lignin and its adsorption of Congo red and Cu(II) ions in aqueous solution. Int. J. Biol. Macromol. 2020, 159, 222–230. [Google Scholar] [CrossRef]
- Kang, K.; Zhu, M.; Sun, G.; Qiu, L.; Guo, X.; Meda, V.; Sun, R. Codensification of Eucommia ulmoides Oliver stem with pyrolysis oil and char for solid biofuel: An optimization and characterization study. Appl. Energy 2018, 223, 347–357. [Google Scholar] [CrossRef]
- Cai, W.; Liu, R.; He, Y.; Chai, M.; Cai, J. Bio-oil production from fast pyrolysis of rice husk in a commercial-scale plant with a downdraft circulating fluidized bed reactor. Fuel Processing Technol. 2018, 171, 308–317. [Google Scholar] [CrossRef]
- Gu, J.; Fan, H.; Wang, Y.; Zhang, Y.; Yuan, H.; Chen, Y. Co-pyrolysis of xylan and high-density polyethylene: Product distribution and synergistic effects. Fuel 2020, 267, 116896. [Google Scholar] [CrossRef]
- Zhuang, X.; Zhan, H.; Song, Y.; He, C.; Huang, Y.; Yin, X.; Wu, C. Insights into the evolution of chemical structures in lignocellulose and non-lignocellulose biowastes during hydrothermal carbonization (HTC). Fuel 2019, 236, 960–974. [Google Scholar] [CrossRef]
- Bian, H.; Luo, J.; Wang, R.; Zhou, X.; Ni, S.; Shi, R.; Fang, G.; Dai, H. Recyclable and Reusable Maleic Acid for Efficient Production of Cellulose Nanofibrils with Stable Performance. ACS Sustain. Chem. Eng. 2019, 7, 20022–20031. [Google Scholar] [CrossRef]
- Yao, S.; Nie, S.; Yuan, Y.; Wang, S.; Qin, C. Efficient extraction of bagasse hemicelluloses and characterization of solid remainder. Bioresour. Technol. 2015, 185, 21–27. [Google Scholar] [CrossRef]
- Karley, A.J.; White, P.J. Moving cationic minerals to edible tissues: Potassium, magnesium, calcium. Curr. Opin. Plant Biol. 2009, 12, 291–298. [Google Scholar] [CrossRef]
- Phyo, P.; Gu, Y.; Hong, M. Impact of acidic pH on plant cell wall polysaccharide structure and dynamics: Insights into the mechanism of acid growth in plants from solid-state NMR. Cellulose 2019, 26, 291–304. [Google Scholar] [CrossRef]
- Huang, L.; Yang, Z.; Li, M.; Liu, Z.; Qin, C.; Nie, S.; Yao, S. Effect of Pre-Corrected pH on the Carbohydrate Hydrolysis of Bamboo during Hydrothermal Pretreatment. Polymers 2020, 12, 612. [Google Scholar] [CrossRef] [Green Version]
- Kratky, L.; Jirout, T. The effect of process parameters during the thermal-expansionary pretreatment of wheat straw on hydrolysate quality and on biogas yield. Renew. Energy 2015, 77, 250–258. [Google Scholar] [CrossRef]
- Karnowo; Zahara, Z.F.; Kudo, S.; Norinaga, K.; Hayashi, J.-I. Leaching of Alkali and Alkaline Earth Metallic Species from Rice Husk with Bio-oil from Its Pyrolysis. Energy Fuels 2014, 28, 6459–6466. [Google Scholar] [CrossRef]
- Hwang, H.; Lee, J.-H.; Moon, J.; Kim, U.-J.; Choi, I.-G.; Choi, J.W. Influence of K and Mg Concentration on the Storage Stability of Bio-Oil. ACS Sustain. Chem. Eng. 2016, 4, 4346–4353. [Google Scholar] [CrossRef]
- Wu, L.; Yang, Y.; Yan, T.; Wang, Y.; Zheng, L.; Qian, K.; Hong, F. Sustainable design and optimization of co-processing of bio-oil and vacuum gas oil in an existing refinery. Renew. Sustain. Energy Rev. 2020, 130, 109952. [Google Scholar] [CrossRef]
- Hosoya, T.; Kawamoto, H.; Saka, S. Cellulose–hemicellulose and cellulose–lignin interactions in wood pyrolysis at gasification temperature. J. Anal. Appl. Pyrolysis 2007, 80, 118–125. [Google Scholar] [CrossRef]
- Giudicianni, P.; Cardone, G.; Ragucci, R. Cellulose, hemicellulose and lignin slow steam pyrolysis: Thermal decomposition of biomass components mixtures. J. Anal. Appl. Pyrolysis 2013, 100, 213–222. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, Z.; Gong, H.; Chen, L.; Yu, H.; Wu, W. Characteristics of dehydration during rice husk pyrolysis and catalytic mechanism of dehydration reaction with NiO/γ-Al2O3 as catalyst. Fuel 2019, 245, 131–138. [Google Scholar] [CrossRef]
- Jiang, L.; Zheng, A.; Zhao, Z.; He, F.; Li, H. Comprehensive utilization of glycerol from sugarcane bagasse pretreatment to fermentation. Bioresour. Technol. 2015, 196, 194–199. [Google Scholar] [CrossRef]
- Collard, F.-X.; Blin, J. A review on pyrolysis of biomass constituents: Mechanisms and composition of the products obtained from the conversion of cellulose, hemicelluloses and lignin. Renew. Sustain. Energy Rev. 2014, 38, 594–608. [Google Scholar] [CrossRef]
- Wang, S.; Ru, B.; Lin, H.; Sun, W. Pyrolysis behaviors of four O-acetyl-preserved hemicelluloses isolated from hardwoods and softwoods. Fuel 2015, 150, 243–251. [Google Scholar] [CrossRef]
- Wang, S.; Li, Z.; Bai, X.; Yi, W.; Fu, P. Influence of inherent hierarchical porous char with alkali and alkaline earth metallic species on lignin pyrolysis. Bioresour. Technol. 2018, 268, 323–331. [Google Scholar] [CrossRef]
- He, Y.; Gao, X.; Qiao, Y.; Xu, M. Occurrence forms of key ash-forming elements in defatted microalgal biomass. Fuel 2017, 200, 182–185. [Google Scholar] [CrossRef]
- Wang, W.-L.; Ren, X.-Y.; Chang, J.-M.; Cai, L.-P.; Shi, S.Q. Characterization of bio-oils and bio-chars obtained from the catalytic pyrolysis of alkali lignin with metal chlorides. Fuel Processing Technol. 2015, 138, 605–611. [Google Scholar] [CrossRef]
- Wang, D.; Xiao, R.; Zhang, H.; He, G. Comparison of catalytic pyrolysis of biomass with MCM-41 and CaO catalysts by using TGA–FTIR analysis. J. Anal. Appl. Pyrolysis 2010, 89, 171–177. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Y.; Yang, H.; Chen, W.; Wang, X.; Chen, H. Fast pyrolysis of cotton stalk biomass using calcium oxide. Bioresour. Technol. 2017, 233, 15–20. [Google Scholar] [CrossRef]

| Code | Response Factor | ||
|---|---|---|---|
| Temperature (x1) °C | Time (x2) min | pH (x3) | |
| −1 | 160 | 50 | 3 |
| 0 | 170 | 60 | 4 |
| 1 | 180 | 70 | 5 |
| Run | Factor | Response | ||
|---|---|---|---|---|
| x1 (°C) | x2 (min) | x3 | Total Removal Rate Y (%) | |
| 1 | 170 | 60 | 4 | 43.10 |
| 2 | 180 | 70 | 4 | 35.99 |
| 3 | 170 | 50 | 5 | 32.81 |
| 4 | 180 | 60 | 3 | 47.42 |
| 5 | 170 | 60 | 4 | 44.44 |
| 6 | 160 | 70 | 4 | 32.57 |
| 7 | 180 | 50 | 4 | 37.62 |
| 8 | 170 | 60 | 4 | 44.26 |
| 9 | 170 | 50 | 3 | 43.18 |
| 10 | 180 | 60 | 5 | 35.31 |
| 11 | 170 | 70 | 3 | 45.68 |
| 12 | 160 | 60 | 3 | 40.46 |
| 13 | 170 | 70 | 5 | 33.66 |
| 14 | 170 | 60 | 4 | 43.51 |
| 15 | 170 | 60 | 4 | 44.32 |
| 16 | 160 | 50 | 4 | 36.65 |
| 17 | 160 | 60 | 5 | 29.9 |
| Samples | Bio-Oil Yield (%) | Biochar Yield (%) | Non-Condensable Gas Yield (%) | Bio-Oil Moisture (%) | Bio-Oil Viscosity (mPa·s) |
|---|---|---|---|---|---|
| Raw material | 49.56 | 18.24 | 26.43 | 27.08 | 53.17 |
| Acid leaching | 59.59 | 14.31 | 20.46 | 21.14 | 95.25 |
| Hydrothermal | 65.87 | 12.89 | 15.65 | 19.54 | 89.51 |
| Samples | Cellulose (%) | Hemicellulose (%) | Lignin (%) |
|---|---|---|---|
| Raw material | 49.55 | 14.93 | 32.53 |
| Acid leaching | 37.55 | 9.68 | 16.54 |
| Hydrothermal pretreatment | 47.02 | 6.24 | 33.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Bao, Y.; Lv, L.; Zeng, F.; Du, D.; Liang, C.; Ge, J.; Wang, S.; Yao, S. Optimization of Demineralization and Pyrolysis Performance of Eucalyptus Hydrothermal Pretreatment. Polymers 2022, 14, 1333. https://doi.org/10.3390/polym14071333
Zhu J, Bao Y, Lv L, Zeng F, Du D, Liang C, Ge J, Wang S, Yao S. Optimization of Demineralization and Pyrolysis Performance of Eucalyptus Hydrothermal Pretreatment. Polymers. 2022; 14(7):1333. https://doi.org/10.3390/polym14071333
Chicago/Turabian StyleZhu, Jiatian, Yuqi Bao, Luxiong Lv, Fanyan Zeng, Dasong Du, Chen Liang, Jiayan Ge, Shuangfei Wang, and Shuangquan Yao. 2022. "Optimization of Demineralization and Pyrolysis Performance of Eucalyptus Hydrothermal Pretreatment" Polymers 14, no. 7: 1333. https://doi.org/10.3390/polym14071333
APA StyleZhu, J., Bao, Y., Lv, L., Zeng, F., Du, D., Liang, C., Ge, J., Wang, S., & Yao, S. (2022). Optimization of Demineralization and Pyrolysis Performance of Eucalyptus Hydrothermal Pretreatment. Polymers, 14(7), 1333. https://doi.org/10.3390/polym14071333






