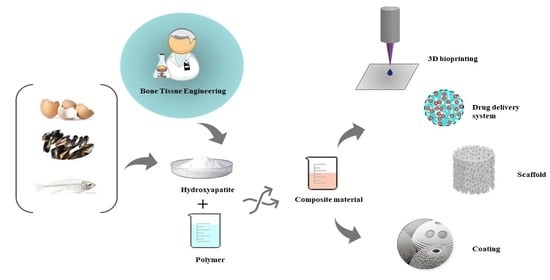
Novel Trends into the Development of Natural Hydroxyapatite-Based Polymeric Composites for Bone Tissue Engineering
Abstract
:1. Introduction
2. Polymers Applied in BTE
2.1. Natural Polymers
2.2. Synthetic Polymers
3. HA-Based on Natural Sources Used in BTE
3.1. Recent Strategies for Obtaining HA from Natural Sources
3.2. HA Obtained from Eggshells
3.3. HA Obtained from Seashells
3.4. HA Obtained from Fish Bone
4. Composite Materials: Polymers and HA Based from Natural Sources
4.1. Properties of Composite Materials for BTE
4.2. Applications of HA-Based Composites in BTE
5. Future Perspectives and Challenges in BTE
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sharma, A.; Sharma, P.K.; Malviya, R. Stimuli-responsive supramolecules for bone tissue engineering. Biointerface Res. Appl. Chem. 2020, 10, 5122–5127. [Google Scholar] [CrossRef]
- Oladapo, B.I.; Zahedi, S.A.; Ismail, S.O.; Olawade, D.B. Recent advances in biopolymeric composite materials: Future sustainability of bone-implant. Renew. Sustain. Energy Rev. 2021, 150, 111505. [Google Scholar] [CrossRef]
- Adithya, S.P.; Sidharthan, D.S.; Abhinandan, R.; Balagangadharan, K.; Selvamurugan, N. Nanosheets-incorporated bio-composites containing natural and synthetic polymers/ceramics for bone tissue engineering. Int. J. Biol. Macromol. 2020, 164, 1960–1972. [Google Scholar] [CrossRef]
- Adzila, A.R.N.M.I.S. Synthesis of Calcium Phosphate Extracted from Eggshell Waste through Precipitation Method. Biointerface Res. Appl. Chem. 2021, 11, 15058–15067. [Google Scholar] [CrossRef]
- Shuai, C.; Yu, L.; Feng, P.; Gao, C.; Peng, S. Interfacial reinforcement in bioceramic/biopolymer composite bone scaffold: The role of coupling agent. Colloids Surf. B Biointerfaces 2020, 193, 111083. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi Zerankeshi, M.; Bakhshi, R.; Alizadeh, R. Polymer/metal composite 3D porous bone tissue engineering scaffolds fabricated by additive manufacturing techniques: A review. Bioprinting 2022, 25, e00191. [Google Scholar] [CrossRef]
- Cao, S.; Zhao, Y.; Hu, Y.; Zou, L.; Chen, J. New perspectives: In-situ tissue engineering for bone repair scaffold. Compos. Part B Eng. 2020, 202, 108445. [Google Scholar] [CrossRef]
- Jain, S.; Gujjala, R.; Abdul Azeem, P.; Ojha, S.; Samudrala, R.K. A review on mechanical and In-vitro studies of polymer reinforced bioactive glass-scaffolds and their fabrication techniques. Ceram. Int. 2021, 48, 5908–5921. [Google Scholar] [CrossRef]
- Pu’ad, N.A.S.M.; Chuan, L.T.; Salman, N.S.; Selimin, M.A.; Latif, A.F.A.; Muhamad, M.S.; Abdullah, H.Z.; Idris, M.I.; Mustapha, S.N.H. Synthesis of bioactive calcium phosphate from cockle shell for biomedical applications. Biointerface Res. Appl. Chem. 2020, 10, 5787–5791. [Google Scholar] [CrossRef]
- Goh, K.W.; Wong, Y.H.; Ramesh, S.; Chandran, H.; Krishnasamy, S.; Ramesh, S.; Sidhu, A.; Teng, W.D. Effect of pH on the properties of eggshell-derived hydroxyapatite bioceramic synthesized by wet chemical method assisted by microwave irradiation. Ceram. Int. 2020, 47, 8879–8887. [Google Scholar] [CrossRef]
- Sobczak-Kupiec, A.; Pluta, K.; Drabczyk, A.; Włoś, M.; Tyliszczak, B. Synthesis and characterization of ceramic—Polymer composites containing bioactive synthetic hydroxyapatite for biomedical applications. Ceram. Int. 2018, 44, 13630–13638. [Google Scholar] [CrossRef]
- Terzioğlu, P.; Öğüt, H.; Kalemtaş, A. Natural calcium phosphates from fish bones and their potential biomedical applications. Mater. Sci. Eng. C 2018, 91, 899–911. [Google Scholar] [CrossRef]
- Shi, H.; Zhou, Z.; Li, W.; Fan, Y.; Li, Z.; Wei, J. Hydroxyapatite Based Materials for Bone Tissue Engineering: A Brief and Comprehensive Introduction. Crystals 2021, 11, 149. [Google Scholar] [CrossRef]
- Han, K.-S.; Sathiyaseelan, A.; Saravanakumar, K.; Wang, M.-H. Wound healing efficacy of biocompatible hydroxyapatite from bovine bone waste for bone tissue engineering application. J. Environ. Chem. Eng. 2022, 10, 106888. [Google Scholar] [CrossRef]
- Sanchez, A.G.; Prokhorov, E.; Luna-Barcenas, G.; Hernández-Vargas, J.; Román-Doval, R.; Mendoza, S.; Rojas-Chávez, H. Chitosan-hydroxyapatite-MWCNTs nanocomposite patch for bone tissue engineering applications. Mater. Today Commun. 2021, 28, 102615. [Google Scholar] [CrossRef]
- Sridevi, S.; Sutha, S.; Kavitha, L.; Gopi, D. Physicochemical and biological behaviour of biogenic derived hydroxyapatite and carboxymethyl cellulose/sodium alginate biocomposite coating on Ti6Al4V alloy for biomedical applications. Mater. Chem. Phys. 2020, 254, 123455. [Google Scholar] [CrossRef]
- Sathiyavimal, S.; Vasantharaj, S.; LewisOscar, F.; Selvaraj, R.; Brindhadevi, K.; Pugazhendhi, A. Natural organic and inorganic–hydroxyapatite biopolymer composite for biomedical applications. Prog. Org. Coat. 2020, 147, 105858. [Google Scholar] [CrossRef]
- Oladapo, B.I.; Zahedi, S.A.; Omigbodun, F.T. A systematic review of polymer composite in biomedical engineering. Eur. Polym. J. 2021, 154, 110534. [Google Scholar] [CrossRef]
- Gritsch, L.; Perrin, E.; Chenal, J.-M.; Fredholm, Y.; Maçon, A.L.B.; Chevalier, J.; Boccaccini, A.R. Combining bioresorbable polyesters and bioactive glasses: Orthopedic applications of composite implants and bone tissue engineering scaffolds. Appl. Mater. Today 2021, 22, 100923. [Google Scholar] [CrossRef]
- Dwivedi, R.; Kumar, S.; Pandey, R.; Mahajan, A.; Nandana, D.; Katti, D.S.; Mehrotra, D. Polycaprolactone as biomaterial for bone scaffolds: Review of literature. J. Oral Biol. Craniofacial Res. 2020, 10, 381–388. [Google Scholar] [CrossRef]
- Bharadwaz, A.; Jayasuriya, A.C. Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration. Mater. Sci. Eng. C 2020, 110, 110698. [Google Scholar] [CrossRef] [PubMed]
- ul Hassan, N.; Chaudhery, I.; ur.Rehman, A.; Ahmed, N. Chapter 7—Polymeric nanoparticles used in tissue engineering. In Advances in Polymeric Nanomaterials for Biomedical Applications; Bajpai, A.K., Saini, R.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 191–224. [Google Scholar]
- Guo, Z.; Poot, A.A.; Grijpma, D.W. Advanced polymer-based composites and structures for biomedical applications. Eur. Polym. J. 2021, 149, 110388. [Google Scholar] [CrossRef]
- Ciocca, L.; Lesci, I.G.; Ragazzini, S.; Gioria, S.; Valsesia, A.; Parrilli, A.; Spadari, A.; Dozza, B.; Mora, P.; Piattelli, A.; et al. Nanostructured surface bioactive composite scaffold for filling of bone defects. Biointerface Res. Appl. Chem. 2020, 10, 5038–5047. [Google Scholar] [CrossRef]
- Blidi, O.; El Omari, N.; Balahbib, A.; Ghchime, R.; Ibrahimi, A.; Bouyahya, A.; Chokairi, O.; Barkiyou, M. Extraction Methods, Characterization and Biomedical Applications of Collagen: A Review. Biointerface Res. Appl. Chem. 2021, 11, 13587–13613. [Google Scholar]
- Hogan, K.J.; Mikos, A.G. Biodegradable thermoresponsive polymers: Applications in drug delivery and tissue engineering. Polymer 2020, 211, 123063. [Google Scholar] [CrossRef]
- Gupta, P.S.; Wasnik, K.; Patra, S.; Pareek, D.; Singh, M.; Maity, S.; Pandey, M.; Paik, P. A Review on Biodegradable Polymeric Materials for Bone Tissue Engineering (BTE) Applications. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Iglesias-Mejuto, A.; García-González, C.A. 3D-printed alginate-hydroxyapatite aerogel scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2021, 131, 112525. [Google Scholar] [CrossRef]
- Asadi, N.; Del Bakhshayesh, A.R.; Davaran, S.; Akbarzadeh, A. Common biocompatible polymeric materials for tissue engineering and regenerative medicine. Mater. Chem. Phys. 2020, 242, 122528. [Google Scholar] [CrossRef]
- Zhai, P.; Peng, X.; Li, B.; Liu, Y.; Sun, H.; Li, X. The application of hyaluronic acid in bone regeneration. Int. J. Biol. Macromol. 2020, 151, 1224–1239. [Google Scholar] [CrossRef]
- Li, S.; Panda, A.K.; Liu, X.; Lin, Y.-C.; Huang, W.-Y.; Lin, C.; Zhao, G.; Chung, R.-J. Preparation and biocompatibility studies of Collagen/Hyaluronic Acid/Oligomeric proanthocyanidins composites. Mater. Chem. Phys. 2021, 272, 124959. [Google Scholar] [CrossRef]
- Dovedytis, M.; Liu, Z.J.; Bartlett, S. Hyaluronic acid and its biomedical applications: A review. Eng. Regen. 2020, 1, 102–113. [Google Scholar] [CrossRef]
- Hickey, R.J.; Pelling, A.E. Cellulose Biomaterials for Tissue Engineering. Front. Bioeng. Biotechnol. 2019, 7, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- del Valle, L.J.; Puiggalí, J. Hydroxyapatite Based Polymer Composites for Regenerative Medicine Applications. In Encyclopedia of Materials: Composites; Brabazon, D., Ed.; Elsevier: Oxford, UK, 2021; pp. 785–803. [Google Scholar]
- Guo, L.; Liang, Z.; Yang, L.; Du, W.; Yu, T.; Tang, H.; Li, C.; Qiu, H. The role of natural polymers in bone tissue engineering. J. Control. Release 2021, 338, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Pina, S.; Reis, R.L.; Oliveira, J.M. Chapter 3—Natural polymeric biomaterials for tissue engineering. In Tissue Engineering Using Ceramics and Polymers, 3rd ed.; Boccaccini, A.R., Ma, P.X., Liverani, L., Eds.; Woodhead Publishing: Cambridge, UK, 2022; pp. 75–110. [Google Scholar]
- Kakarla, A.B.; Kong, I.; Turek, I.; Kong, C.; Irving, H. Printable gelatin, alginate and boron nitride nanotubes hydrogel-based ink for 3D bioprinting and tissue engineering applications. Mater. Des. 2022, 213, 110362. [Google Scholar] [CrossRef]
- El-Zawahry, M.M.; Hassabo, A.G.; Abdelghaffar, F.; Abdelghaffar, R.A.; Hakeim, O.A. Preparation and Use of Aqueous Solutions Magnetic Chitosan/Nanocellulose Aerogels for the Sorption of Reactive Black 5. Biointerface Res. Appl. Chem. 2021, 11, 12380–12402. [Google Scholar] [CrossRef]
- Shakir, M.; Zia, I.; Rehman, A.; Ullah, R. Fabrication and characterization of nanoengineered biocompatible n-HA/chitosan-tamarind seed polysaccharide: Bio-inspired nanocomposites for bone tissue engineering. Int. J. Biol. Macromol. 2018, 111, 903–916. [Google Scholar] [CrossRef] [PubMed]
- Cunha, C.S.; Castro, P.J.; Sousa, S.C.; Pullar, R.C.; Tobaldi, D.M.; Piccirillo, C.; Pintado, M.M. Films of chitosan and natural modified hydroxyapatite as effective UV-protecting, biocompatible and antibacterial wound dressings. Int. J. Biol. Macromol. 2020, 159, 1177–1185. [Google Scholar] [CrossRef]
- Olmo, J.A.; Ruiz-Rubio, L.; Pérez-Alvarez, L.; Sáez-Martínez, V.; Vilas-Vilela, J.L. Antibacterial Coatings for Improving the Performance of Biomaterials. Coatings 2020, 10, 139. [Google Scholar] [CrossRef] [Green Version]
- Lavanya, K.; Chandran, S.V.; Balagangadharan, K.; Selvamurugan, N. Temperature- and pH-responsive chitosan-based injectable hydrogels for bone tissue engineering. Mater. Sci. Eng. C 2020, 111, 110862. [Google Scholar] [CrossRef]
- Rethinam, S.; Wilson Aruni, A.; Vijayan, S.; Munusamy, C.; Gobi, N. Enhanced bone regeneration using an electrospun nanofibrous membrane—A novel approach. J. Drug Deliv. Sci. Technol. 2019, 53, 101163. [Google Scholar] [CrossRef]
- Marques, C.F.; Diogo, G.S.; Pina, S.; Oliveira, J.M.; Silva, T.H.; Reis, R.L. Collagen-based bioinks for hard tissue engineering applications: A comprehensive review. J. Mater. Sci. Mater. Med. 2019, 30, 32. [Google Scholar] [CrossRef]
- Elkhoury, K.; Morsink, M.; Sanchez-Gonzalez, L.; Kahn, C.; Tamayol, A.; Arab-Tehrany, E. Biofabrication of natural hydrogels for cardiac, neural, and bone Tissue engineering Applications. Bioact. Mater. 2021, 6, 3904–3923. [Google Scholar] [CrossRef] [PubMed]
- Echave, M.C.; Pimenta-Lopes, C.; Pedraz, J.L.; Mehrali, M.; Dolatshahi-Pirouz, A.; Ventura, F.; Orive, G. Enzymatic crosslinked gelatin 3D scaffolds for bone tissue engineering. Int. J. Pharm. 2019, 562, 151–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luetchford, K.A.; Chaudhuri, J.B.; De Bank, P.A. Silk fibroin/gelatin microcarriers as scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2020, 106, 110116. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Varshney, R.; Das, N.; Sircar, D.; Roy, P. Synthesis and characterization of gelatin-PVP polymer composite scaffold for potential application in bone tissue engineering. Eur. Polym. J. 2019, 119, 155–168. [Google Scholar] [CrossRef]
- Rajabnejad keleshteri, A.; Moztarzadeh, F.; Farokhi, M.; Mehrizi, A.A.; Basiri, H.; Mohseni, S.S. Preparation of microfluidic-based pectin microparticles loaded carbon dots conjugated with BMP-2 embedded in gelatin-elastin-hyaluronic acid hydrogel scaffold for bone tissue engineering application. Int. J. Biol. Macromol. 2021, 184, 29–41. [Google Scholar] [CrossRef]
- Cui, N.; Qian, J.; Liu, T.; Zhao, N.; Wang, H. Hyaluronic acid hydrogel scaffolds with a triple degradation behavior for bone tissue engineering. Carbohydr. Polym. 2015, 126, 192–198. [Google Scholar] [CrossRef]
- Sionkowska, A. Collagen blended with natural polymers: Recent advances and trends. Prog. Polym. Sci. 2021, 122, 101452. [Google Scholar] [CrossRef]
- Fang, Y.; Shi, L.; Duan, Z.; Rohani, S. Hyaluronic acid hydrogels, as a biological macromolecule-based platform for stem cells delivery and their fate control: A review. Int. J. Biol. Macromol. 2021, 189, 554–566. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, D.; Ren, Y.; Guo, S.; Li, J.; Ma, S.; Yao, M.; Guan, F. Injectable hyaluronic acid hydrogel loaded with BMSC and NGF for traumatic brain injury treatment. Mater. Today Bio 2022, 13, 100201. [Google Scholar] [CrossRef]
- Abdollahiyan, P.; Oroojalian, F.; Hejazi, M.; de la Guardia, M.; Mokhtarzadeh, A. Nanotechnology, and scaffold implantation for the effective repair of injured organs: An overview on hard tissue engineering. J. Control. Release 2021, 333, 391–417. [Google Scholar] [CrossRef]
- Ferreira, F.V.; Otoni, C.G.; Lopes, J.H.; de Souza, L.P.; Mei, L.H.I.; Lona, L.M.F.; Lozano, K.; Lobo, A.O.; Mattoso, L.H.C. Ultrathin polymer fibers hybridized with bioactive ceramics: A review on fundamental pathways of electrospinning towards bone regeneration. Mater. Sci. Eng. C 2021, 123, 111853. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Ning, F.; Wang, Y. Additive manufacturing of biodegradable iron-based particle reinforced polylactic acid composite scaffolds for tissue engineering. J. Mater. Processing Technol. 2021, 289, 116952. [Google Scholar] [CrossRef]
- Arslan, A.; Steiger, W.; Roose, P.; Van den Bergen, H.; Gruber, P.; Zerobin, E.; Gantner, F.; Guillaume, O.; Ovsianikov, A.; Van Vlierberghe, S.; et al. Polymer architecture as key to unprecedented high-resolution 3D-printing performance: The case of biodegradable hexa-functional telechelic urethane-based poly-ε-caprolactone. Mater. Today 2021, 44, 25–39. [Google Scholar] [CrossRef]
- Wang, J.-Z.; You, M.-L.; Ding, Z.-Q.; Ye, W.-B. A review of emerging bone tissue engineering via PEG conjugated biodegradable amphiphilic copolymers. Mater. Sci. Eng. C 2019, 97, 1021–1035. [Google Scholar] [CrossRef] [PubMed]
- Yeo, T.; Ko, Y.-G.; Kim, E.J.; Kwon, O.K.; Chung, H.Y.; Kwon, O.H. Promoting bone regeneration by 3D-printed poly(glycolic acid)/hydroxyapatite composite scaffolds. J. Ind. Eng. Chem. 2021, 94, 343–351. [Google Scholar] [CrossRef]
- Thangprasert, A.; Tansakul, C.; Thuaksubun, N.; Meesane, J. Mimicked hybrid hydrogel based on gelatin/PVA for tissue engineering in subchondral bone interface for osteoarthritis surgery. Mater. Des. 2019, 183, 108113. [Google Scholar] [CrossRef]
- Kim, H.; Yang, G.H.; Kim, G. Three-dimensional gelatin/PVA scaffold with nanofibrillated collagen surface for applications in hard-tissue regeneration. Int. J. Biol. Macromol. 2019, 135, 21–28. [Google Scholar] [CrossRef]
- Bi, S.; Pang, J.; Huang, L.; Sun, M.; Cheng, X.; Chen, X. The toughness chitosan-PVA double network hydrogel based on alkali solution system and hydrogen bonding for tissue engineering applications. Int. J. Biol. Macromol. 2020, 146, 99–109. [Google Scholar] [CrossRef]
- Fu, Y.; Ding, Y.; Zhang, L.; Zhang, Y.; Liu, J.; Yu, P. Poly ethylene glycol (PEG)-Related controllable and sustainable antidiabetic drug delivery systems. Eur. J. Med. Chem. 2021, 217, 113372. [Google Scholar] [CrossRef]
- Xue, X.; Hu, Y.; Wang, S.; Chen, X.; Jiang, Y.; Su, J. Fabrication of physical and chemical crosslinked hydrogels for bone tissue engineering. Bioact. Mater. 2021. [Google Scholar] [CrossRef]
- Zhang, L.; Dou, S.; Li, Y.; Yuan, Y.; Ji, Y.; Wang, Y.; Yang, Y. Degradation and compatibility behaviors of poly(glycolic acid) grafted chitosan. Mater. Sci. Eng. C 2013, 33, 2626–2631. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Zhu, T.; Li, J.; Cui, L.; Zhang, Z.; Zhuang, X.; Ding, J. Poly(lactic-co-glycolic acid)-based composite bone-substitute materials. Bioact. Mater. 2021, 6, 346–360. [Google Scholar] [CrossRef] [PubMed]
- Ng, H.-M.; Bee, S.-T.; Sin, L.T.; Ratnam, C.T.; Rahmat, A.R. Effect of electron beam irradiation sterilization on biomedical polylactic acid composite filled with Scomberomorus Guttatus-derived hydroxyapatite. Compos. Part B Eng. 2019, 176, 107273. [Google Scholar] [CrossRef]
- Bal, Z.; Korkusuz, F.; Ishiguro, H.; Okada, R.; Kushioka, J.; Chijimatsu, R.; Kodama, J.; Tateiwa, D.; Ukon, Y.; Nakagawa, S.; et al. A novel nano-hydroxyapatite/synthetic polymer/bone morphogenetic protein-2 composite for efficient bone regeneration. Spine J. 2021, 21, 865–873. [Google Scholar] [CrossRef]
- Kumar, P.; Dehiya, B.S.; Sindhu, A. Synthesis and characterization of nHA-PEG and nBG-PEG scaffolds for hard tissue engineering applications. Ceram. Int. 2019, 45, 8370–8379. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, H.; Luo, H.; Chen, D.; Zhou, Z.; Cao, X. High strength HA-PEG/NAGA-Gelma double network hydrogel for annulus fibrosus rupture repair. Smart Mater. Med. 2022, 3, 128–138. [Google Scholar] [CrossRef]
- Bose, S.; Sarkar, N.; Banerjee, D. Effects of PCL, PEG and PLGA polymers on curcumin release from calcium phosphate matrix for in vitro and in vivo bone regeneration. Mater. Today Chem. 2018, 8, 110–120. [Google Scholar] [CrossRef]
- Dethe, M.R.; Prabakaran, A.; Ahmed, H.; Agrawal, M.; Roy, U.; Alexander, A. PCL-PEG copolymer based injectable thermosensitive hydrogels. J. Control. Release 2022, 343, 217–236. [Google Scholar] [CrossRef]
- Bai, J.; Wang, H.; Gao, W.; Liang, F.; Wang, Z.; Zhou, Y.; Lan, X.; Chen, X.; Cai, N.; Huang, W.; et al. Melt electrohydrodynamic 3D printed poly (ε-caprolactone)/polyethylene glycol/roxithromycin scaffold as a potential anti-infective implant in bone repair. Int. J. Pharm. 2020, 576, 118941. [Google Scholar] [CrossRef]
- Etminanfar, M.R.; Sheykholeslami, S.O.R.; Khalili, V.; Mahdavi, S. Biocompatibility and drug delivery efficiency of PEG-b-PCL/hydroxyapatite bilayer coatings on Nitinol superelastic alloy. Ceram. Int. 2020, 46, 12711–12717. [Google Scholar] [CrossRef]
- Kumar Saini, R.; Prasad Bagri, L.; Bajpai, A.K. Nano-silver hydroxyapatite based antibacterial 3D scaffolds of gelatin/alginate/poly (vinyl alcohol) for bone tissue engineering applications. Colloids Surf. B Biointerfaces 2019, 177, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Chen, N.; Wang, Q. Fabrication and properties of poly(vinyl alcohol)/β-tricalcium phosphate composite scaffolds via fused deposition modeling for bone tissue engineering. Compos. Sci. Technol. 2019, 172, 17–28. [Google Scholar] [CrossRef]
- Salim, S.A.; Loutfy, S.A.; El-Fakharany, E.M.; Taha, T.H.; Hussien, Y.; Kamoun, E.A. Influence of chitosan and hydroxyapatite incorporation on properties of electrospun PVA/HA nanofibrous mats for bone tissue regeneration: Nanofibers optimization and in-vitro assessment. J. Drug Deliv. Sci. Technol. 2021, 62, 102417. [Google Scholar] [CrossRef]
- Dong, Q.; Zhang, M.; Zhou, X.; Shao, Y.; Li, J.; Wang, L.; Chu, C.; Xue, F.; Yao, Q.; Bai, J. 3D-printed Mg-incorporated PCL-based scaffolds: A promising approach for bone healing. Mater. Sci. Eng. C 2021, 129, 112372. [Google Scholar] [CrossRef] [PubMed]
- Maharjan, B.; Kaliannagounder, V.K.; Jang, S.R.; Awasthi, G.P.; Bhattarai, D.P.; Choukrani, G.; Park, C.H.; Kim, C.S. In-situ polymerized polypyrrole nanoparticles immobilized poly(ε-caprolactone) electrospun conductive scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2020, 114, 111056. [Google Scholar] [CrossRef]
- Wang, Q.; Ye, W.; Ma, Z.; Xie, W.; Zhong, L.; Wang, Y.; Rong, Q. 3D printed PCL/β-TCP cross-scale scaffold with high-precision fiber for providing cell growth and forming bones in the pores. Mater. Sci. Eng. C 2021, 127, 112197. [Google Scholar] [CrossRef]
- Gatto, M.L.; Furlani, M.; Giuliani, A.; Bloise, N.; Fassina, L.; Visai, L.; Mengucci, P. Biomechanical performances of PCL/HA micro- and macro-porous lattice scaffolds fabricated via laser powder bed fusion for bone tissue engineering. Mater. Sci. Eng. C 2021, 128, 112300. [Google Scholar] [CrossRef]
- Cestari, F.; Petretta, M.; Yang, Y.; Motta, A.; Grigolo, B.; Sglavo, V.M. 3D printing of PCL/nano-hydroxyapatite scaffolds derived from biogenic sources for bone tissue engineering. Sustain. Mater. Technol. 2021, 29, e00318. [Google Scholar] [CrossRef]
- Soleymani Eil Bakhtiari, S.; Bakhsheshi-Rad, H.R.; Karbasi, S.; Fauzi Ismail, A.; Sharif, S.; Seifalian, A.; Savoji, H.; Berto, F. Poly(methyl methacrylate)-Based Composite Bone Cements With Different Types of Reinforcement Agents. In Encyclopedia of Materials: Composites; Brabazon, D., Ed.; Elsevier: Oxford, UK, 2021; pp. 867–886. [Google Scholar]
- Pahlevanzadeh, F.; Bakhsheshi-Rad, H.R.; Kharaziha, M.; Kasiri-Asgarani, M.; Omidi, M.; Razzaghi, M.; Ismail, A.F.; Sharif, S.; RamaKrishna, S.; Berto, F. CNT and rGO reinforced PMMA based bone cement for fixation of load bearing implants: Mechanical property and biological response. J. Mech. Behav. Biomed. Mater. 2021, 116, 104320. [Google Scholar] [CrossRef]
- Sas, A.; Helgason, B.; Ferguson, S.J.; van Lenthe, G.H. Mechanical and morphological characterization of PMMA/bone composites in human femoral heads. J. Mech. Behav. Biomed. Mater. 2021, 115, 104247. [Google Scholar] [CrossRef]
- Bardelcik, A.; Yang, S.; Alderson, F.; Gadsden, A. The effect of wash treatment on the mechanical properties and energy absorption potential of a 3D printed polymethyl methacrylate (PMMA). Mater. Today Commun. 2021, 26, 101728. [Google Scholar] [CrossRef]
- Al-Sherify, Z.F.; Dawood, N.M.; Khulief, Z.T. Corrosion behavior of AZ31 magnesium alloys coated with PMMA/HA as biodegradable implants. Mater. Today Proc. 2021, in press. [Google Scholar] [CrossRef]
- Atila, D.; Hasirci, V.; Tezcaner, A. Coaxial electrospinning of composite mats comprised of core/shell poly(methyl methacrylate)/silk fibroin fibers for tissue engineering applications. J. Mech. Behav. Biomed. Mater. 2022, 128, 105105. [Google Scholar] [CrossRef] [PubMed]
- Deb, P.; Barua, E.; Das Lala, S.; Deoghare, A.B. Synthesis of hydroxyapatite from Labeo rohita fish scale for biomedical application. Mater. Today Proc. 2019, 15, 277–283. [Google Scholar] [CrossRef]
- Pal, A.; Paul, S.; Choudhury, A.R.; Balla, V.K.; Das, M.; Sinha, A. Synthesis of hydroxyapatite from Lates calcarifer fish bone for biomedical applications. Mater. Lett. 2017, 203, 89–92. [Google Scholar] [CrossRef]
- Sathiyavimal, S.; Vasantharaj, S.; Shanmugavel, M.; Manikandan, E.; Nguyen-Tri, P.; Brindhadevi, K.; Pugazhendhi, A. Facile synthesis and characterization of hydroxyapatite from fish bones: Photocatalytic degradation of industrial dyes (crystal violet and Congo red). Prog. Org. Coat. 2020, 148, 105890. [Google Scholar] [CrossRef]
- Liu, K.S.; Tian, D.L.; Jiang, L. Chapter 24—Frontier of Inorganic Synthesis and Preparative Chemistry (I) Biomimetic Synthesis. In Modern Inorganic Synthetic Chemistry, 2nd ed.; Xu, R., Xu, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 687–721. [Google Scholar]
- Wu, S.-C.; Hsu, H.-C.; Hsu, S.-K.; Tseng, C.-P.; Ho, W.-F. Preparation and characterization of hydroxyapatite synthesized from oyster shell powders. Adv. Powder Technol. 2017, 28, 1154–1158. [Google Scholar] [CrossRef]
- Vinoth Kumar, K.C.; Jani Subha, T.; Ahila, K.G.; Ravindran, B.; Chang, S.W.; Mahmoud, A.H.; Mohammed, O.B.; Rathi, M.A. Spectral characterization of hydroxyapatite extracted from Black Sumatra and Fighting cock bone samples: A comparative analysis. Saudi J. Biol. Sci. 2020, 28, 840–846. [Google Scholar] [CrossRef]
- Nga, N.K.; Thuy Chau, N.T.; Viet, P.H. Facile synthesis of hydroxyapatite nanoparticles mimicking biological apatite from eggshells for bone-tissue engineering. Colloids Surf. B Biointerfaces 2018, 172, 769–778. [Google Scholar] [CrossRef]
- Behera, R.R.; Das, A.; Pamu, D.; Pandey, L.M.; Sankar, M.R. Mechano-tribological properties and in vitro bioactivity of biphasic calcium phosphate coating on Ti-6Al-4V. J. Mech. Behav. Biomed. Mater. 2018, 86, 143–157. [Google Scholar] [CrossRef]
- Behera, R.R.; Das, A.; Hasan, A.; Pamu, D.; Pandey, L.M.; Sankar, M.R. Deposition of biphasic calcium phosphate film on laser surface textured Ti–6Al–4V and its effect on different biological properties for orthopedic applications. J. Alloy. Compd. 2020, 842, 155683. [Google Scholar] [CrossRef]
- Nawaz, A.; Li, E.; Irshad, S.; Hhm, H.; Liu, J.; Shahbaz, H.M.; Ahmed, W.; Regenstein, J.M. Improved effect of autoclave processing on size reduction, chemical structure, nutritional, mechanical and in vitro digestibility properties of fish bone powder. Adv. Powder Technol. 2020, 31, 2513–2520. [Google Scholar] [CrossRef]
- Hernández-Cocoletzi, H.; Salinas, R.A.; Águila-Almanza, E.; Rubio-Rosas, E.; Chai, W.S.; Chew, K.W.; Mariscal-Hernández, C.; Show, P.L. Natural hydroxyapatite from fishbone waste for the rapid adsorption of heavy metals of aqueous effluent. Environ. Technol. Innov. 2020, 20, 101109. [Google Scholar] [CrossRef]
- Zhu, Q.; Ablikim, Z.; Chen, T.; Cai, Q.; Xia, J.; Jiang, D.; Wang, S. The preparation and characterization of HA/β-TCP biphasic ceramics from fish bones. Ceram. Int. 2017, 43, 12213–12220. [Google Scholar] [CrossRef]
- Zanelato, C.B.; Pires, A.F.; da Silva, S.N.; Galdino, A.G.S. Development of biphasic bone cement obtained from chicken eggshell. J. Mater. Res. Technol. 2020, 9, 7297–7304. [Google Scholar] [CrossRef]
- Shafiei, S.; Omidi, M.; Nasehi, F.; Golzar, H.; Mohammadrezaei, D.; Rezai Rad, M.; Khojasteh, A. Egg shell-derived calcium phosphate/carbon dot nanofibrous scaffolds for bone tissue engineering: Fabrication and characterization. Mater. Sci. Eng. C 2019, 100, 564–575. [Google Scholar] [CrossRef]
- Kolmas, J.; Kaflak, A.; Zima, A.; Ślósarczyk, A. Alpha-tricalcium phosphate synthesized by two different routes: Structural and spectroscopic characterization. Ceram. Int. 2015, 41, 5727–5733. [Google Scholar] [CrossRef]
- Roopavath, U.K.; Sah, M.K.; Panigrahi, B.B.; Rath, S.N. Mechanochemically synthesized phase stable and biocompatible β-tricalcium phosphate from avian eggshell for the development of tissue ingrowth system. Ceram. Int. 2019, 45, 12910–12919. [Google Scholar] [CrossRef]
- Oladele, I.O.; Agbabiaka, O.G.; Adediran, A.A.; Akinwekomi, A.D.; Balogun, A.O. Structural performance of poultry eggshell derived hydroxyapatite based high density polyethylene bio-composites. Heliyon 2019, 5, e02552. [Google Scholar] [CrossRef] [Green Version]
- Horta, M.; Aguilar, M.; Moura, F.; Campos, J.; Ramos, V.; Quizunda, A. Synthesis and characterization of green nanohydroxyapatite from hen eggshell by precipitation method. Mater. Today Proc. 2019, 14, 716–721. [Google Scholar] [CrossRef]
- Vidhya, G.; Kumar, G.S.; Kattimani, V.S.; Girija, E.K. Comparative study of hydroxyapatite prepared from eggshells and synthetic precursors by microwave irradiation method for medical applications. Mater. Today Proc. 2019, 15, 344–352. [Google Scholar] [CrossRef]
- Núñez, D.; Elgueta, E.; Varaprasad, K.; Oyarzún, P. Hydroxyapatite nanocrystals synthesized from calcium rich bio-wastes. Mater. Lett. 2018, 230, 64–68. [Google Scholar] [CrossRef]
- Alif, M.F.; Aprillia, W.; Arief, S. A hydrothermal synthesis of natural hydroxyapatite obtained from Corbicula moltkiana freshwater clams shell biowaste. Mater. Lett. 2018, 230, 40–43. [Google Scholar] [CrossRef]
- Zainol, I.; Adenan, N.H.; Rahim, N.A.; Jaafar, C.N.A. Extraction of natural hydroxyapatite from tilapia fish scales using alkaline treatment. Mater. Today Proc. 2019, 16, 1942–1948. [Google Scholar] [CrossRef]
- Jyotsna; Vijayakumar, P. Synthesis and characterization of hydroxyapatite nanoparticles and their cytotoxic effect on a fish vertebra derived cell line. Biocatal. Agric. Biotechnol. 2020, 25, 101612. [Google Scholar] [CrossRef]
- Paul, S.; Pal, A.; Choudhury, A.R.; Bodhak, S.; Balla, V.K.; Sinha, A.; Das, M. Effect of trace elements on the sintering effect of fish scale derived hydroxyapatite and its bioactivity. Ceram. Int. 2017, 43, 15678–15684. [Google Scholar] [CrossRef]
- Pon-On, W.; Suntornsaratoon, P.; Charoenphandhu, N.; Thongbunchoo, J.; Krishnamra, N.; Tang, I.M. Hydroxyapatite from fish scale for potential use as bone scaffold or regenerative material. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 62, 183–189. [Google Scholar] [CrossRef]
- Varadavenkatesan, T.; Vinayagam, R.; Pai, S.; Kathirvel, B.; Pugazhendhi, A.; Selvaraj, R. Synthesis, biological and environmental applications of hydroxyapatite and its composites with organic and inorganic coatings. Prog. Org. Coat. 2021, 151, 106056. [Google Scholar] [CrossRef]
- Luna-Domínguez, J.H.; Téllez-Jiménez, H.; Hernández-Cocoletzi, H.; García-Hernández, M.; Melo-Banda, J.A.; Nygren, H. Development and in vivo response of hydroxyapatite/whitlockite from chicken bones as bone substitute using a chitosan membrane for guided bone regeneration. Ceram. Int. 2018, 44, 22583–22591. [Google Scholar] [CrossRef]
- Nam, P.V.; Hoa, N.V.; Trung, T.S. Properties of hydroxyapatites prepared from different fish bones: A comparative study. Ceram. Int. 2019, 45, 20141–20147. [Google Scholar] [CrossRef]
- Cestari, F.; Agostinacchio, F.; Galotta, A.; Chemello, G.; Motta, A.; Sglavo, V.M. Nano-Hydroxyapatite Derived from Biogenic and Bioinspired Calcium Carbonates: Synthesis and In Vitro Bioactivity. Nanomaterials 2021, 11, 264. [Google Scholar] [CrossRef] [PubMed]
- Mohd Pu’ad, N.A.S.; Koshy, P.; Abdullah, H.Z.; Idris, M.I.; Lee, T.C. Syntheses of hydroxyapatite from natural sources. Heliyon 2019, 5, e01588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komalakrishna, H.; Shine Jyoth, T.G.; Kundu, B.; Mandal, S. Low Temperature Development of Nano-Hydroxyapatite from Austromegabalanus psittacus, Star fish and Sea urchin. Mater. Today Proc. 2017, 4, 11933–11938. [Google Scholar] [CrossRef]
- Mohd Pu’ad, N.A.S.; Abdul Haq, R.H.; Mohd Noh, H.; Abdullah, H.Z.; Idris, M.I.; Lee, T.C. Nano-size hydroxyapatite extracted from tilapia scale using alkaline heat treatment method. Mater. Today Proc. 2020, 29, 218–222. [Google Scholar] [CrossRef]
- Rahavi, S.; Ghaderi, O.; Monshi, A.; Fathi, M. A comparative study on physicochemical properties of hydroxyapatite powders derived from natural and synthetic sources. Russ. J. Non-Ferr. Met. 2017, 58, 276–286. [Google Scholar] [CrossRef]
- Khalid, H.; Chaudhry, A.A. 4—Basics of hydroxyapatite—Structure, synthesis, properties, and clinical applications. In Handbook of Ionic Substituted Hydroxyapatites; Khan, A.S., Chaudhry, A.A., Eds.; Woodhead Publishing: Cambridge, UK, 2020; pp. 85–115. [Google Scholar]
- Gibson, I.R. 1.3.4A—Natural and Synthetic Hydroxyapatites. In Biomaterials Science, 4th ed.; Wagner, W.R., Sakiyama-Elbert, S.E., Zhang, G., Yaszemski, M.J., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 307–317. [Google Scholar]
- Martinez, M.; Bayne, C.; Aiello, D.; Julian, M.; Gaume, R.; Baudelet, M. Multi-elemental matrix-matched calcium hydroxyapatite reference materials for laser ablation: Evaluation on teeth by laser-induced breakdown spectroscopy. Spectrochim. Acta Part B At. Spectrosc. 2019, 159, 105650. [Google Scholar] [CrossRef]
- Boutinguiza, M.; Pou, J.; Comesaña, R.; Lusquiños, F.; de Carlos, A.; León, B. Biological hydroxyapatite obtained from fish bones. Mater. Sci. Eng. C 2012, 32, 478–486. [Google Scholar] [CrossRef]
- Obada, D.O.; Dauda, E.T.; Abifarin, J.K.; Dodoo-Arhin, D.; Bansod, N.D. Mechanical properties of natural hydroxyapatite using low cold compaction pressure: Effect of sintering temperature. Mater. Chem. Phys. 2020, 239, 122099. [Google Scholar] [CrossRef]
- Shi, P.; Liu, M.; Fan, F.; Yu, C.; Lu, W.; Du, M. Characterization of natural hydroxyapatite originated from fish bone and its biocompatibility with osteoblasts. Mater. Sci. Eng. C 2018, 90, 706–712. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, M.; Ji, S.; Zhang, L.; Cao, W.; Wang, H.; Wang, S. Preparation and application of hydroxyapatite extracted from fish scale waste using deep eutectic solvents. Ceram. Int. 2020, 47, 9366–9372. [Google Scholar] [CrossRef]
- Wu, S.-C.; Tsou, H.-K.; Hsu, H.-C.; Hsu, S.-K.; Liou, S.-P.; Ho, W.-F. A hydrothermal synthesis of eggshell and fruit waste extract to produce nanosized hydroxyapatite. Ceram. Int. 2013, 39, 8183–8188. [Google Scholar] [CrossRef]
- Karunakaran, G.; Cho, E.-B.; Kumar, G.S.; Kolesnikov, E.; Janarthanan, G.; Pillai, M.M.; Rajendran, S.; Boobalan, S.; Sudha, K.G.; Rajeshkumar, M.P. Mesoporous Mg-doped hydroxyapatite nanorods prepared from bio-waste blue mussel shells for implant applications. Ceram. Int. 2020, 46, 28514–28527. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, S.; Zhao, Y.; Zeng, M. Synthesis and characterization of hydroxyapatite nano-rods from oyster shell with exogenous surfactants. Mater. Sci. Eng. C 2019, 105, 110102. [Google Scholar] [CrossRef]
- Yang, Y.; Yao, Q.; Pu, X.; Hou, Z.; Zhang, Q. Biphasic calcium phosphate macroporous scaffolds derived from oyster shells for bone tissue engineering. Chem. Eng. J. 2011, 173, 837–845. [Google Scholar] [CrossRef]
- Morris, J.P.; Wang, Y.; Backeljau, T.; Chapelle, G. Biomimetic and bio-inspired uses of mollusc shells. Mar. Genom. 2016, 27, 85–90. [Google Scholar] [CrossRef]
- Haslinda Shariffuddin, J.; Chee Yean, W.; Shariah Ghazali, S. Investigating the catalytic properties of calcium compounds derived from marine based shell waste for wastewater treatment. Mater. Today Proc. 2018, 5, 21718–21727. [Google Scholar] [CrossRef]
- Lala, S.; Satpati, B.; Kar, T.; Pradhan, S.K. Structural and microstructural characterizations of nanocrystalline hydroxyapatite synthesized by mechanical alloying. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 2891–2898. [Google Scholar] [CrossRef]
- Hembrick-Holloman, V.; Samuel, T.; Mohammed, Z.; Jeelani, S.; Rangari, V.K. Ecofriendly production of bioactive tissue engineering scaffolds derived from egg- and sea-shells. J. Mater. Res. Technol. 2020, 9, 13729–13739. [Google Scholar] [CrossRef]
- Oliveira, D.A.; Benelli, P.; Amante, E.R. A literature review on adding value to solid residues: Egg shells. J. Clean. Prod. 2013, 46, 42–47. [Google Scholar] [CrossRef]
- Ramesh, S.; Natasha, A.N.; Tan, C.Y.; Bang, L.T.; Ramesh, S.; Ching, C.Y.; Chandran, H. Direct conversion of eggshell to hydroxyapatite ceramic by a sintering method. Ceram. Int. 2016, 42, 7824–7829. [Google Scholar] [CrossRef]
- Baláž, M. Ball milling of eggshell waste as a green and sustainable approach: A review. Adv. Colloid Interface Sci. 2018, 256, 256–275. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-C.; Hsu, H.-C.; Hsu, S.-K.; Chang, Y.-C.; Ho, W.-F. Synthesis of hydroxyapatite from eggshell powders through ball milling and heat treatment. J. Asian Ceram. Soc. 2016, 4, 85–90. [Google Scholar] [CrossRef]
- Ho, W.-F.; Hsu, H.-C.; Hsu, S.-K.; Hung, C.-W.; Wu, S.-C. Calcium phosphate bioceramics synthesized from eggshell powders through a solid state reaction. Ceram. Int. 2013, 39, 6467–6473. [Google Scholar] [CrossRef]
- Alhussary, B.N.; Taqa, G.A.; Taqa, A.A.A. Preparation And Characterization Of Natural Nano Hydroxyapatite From Eggshell And Seashell And Its Effect On Bone Healing. J. Appl. Vet. Sci. 2020, 5, 25–32. [Google Scholar] [CrossRef] [Green Version]
- Waheed, M.; Yousaf, M.; Shehzad, A.; Inam-Ur-Raheem, M.; Khan, M.K.I.; Khan, M.R.; Ahmad, N.; Abdullah; Aadil, R.M. Channelling eggshell waste to valuable and utilizable products: A comprehensive review. Trends Food Sci. Technol. 2020, 106, 78–90. [Google Scholar] [CrossRef]
- Correlo, V.M.; Oliveira, J.M.; Mano, J.F.; Neves, N.M.; Reis, R.L. CHAPTER 32—Natural Origin Materials for Bone Tissue Engineering—Properties, Processing, and Performance. In Principles of Regenerative Medicine, 4th ed.; Atala, A., Lanza, R., Thomson, J.A., Nerem, R., Eds.; Academic Press: San Diego, CA, USA, 2011; pp. 557–586. [Google Scholar]
- Ağaoğulları, D.; Kel, D.; Gökçe, H.; Duman, I.; Öveçoğlu, L.; Akarsubasi, A.; Bilgiç Alkaya, D.; Oktar, F. Bioceramic Production from Sea Urchins. Acta Phys. Pol. A 2012, 121, 23–26. [Google Scholar] [CrossRef]
- Shavandi, A.; Bekhit, A.E.-D.A.; Ali, A.; Sun, Z. Synthesis of nano-hydroxyapatite (nHA) from waste mussel shells using a rapid microwave method. Mater. Chem. Phys. 2015, 149–150, 607–616. [Google Scholar] [CrossRef]
- Mitran, V.; Ion, R.; Miculescu, F.; Necula, M.G.; Mocanu, A.-C.; Stan, G.E.; Antoniac, I.V.; Cimpean, A. Osteoblast Cell Response to Naturally Derived Calcium Phosphate-Based Materials. Materials 2018, 11, 1097. [Google Scholar] [CrossRef] [Green Version]
- Maidaniuc, A.; Miculescu, F.; Ciocoiu, R.C.; Butte, T.M.; Pasuk, I.; Stan, G.E.; Voicu, S.I.; Ciocan, L.T. Effect of the processing parameters on surface, physico-chemical and mechanical features of bioceramics synthesized from abundant carp fish bones. Ceram. Int. 2020, 46, 10159–10171. [Google Scholar] [CrossRef]
- Lalzawmliana, V.; Anand, A.; Mukherjee, P.; Chaudhuri, S.; Kundu, B.; Nandi, S.K.; Thakur, N.L. Marine organisms as a source of natural matrix for bone tissue engineering. Ceram. Int. 2019, 45, 1469–1481. [Google Scholar] [CrossRef]
- Venkatesan, J.; Qian, Z.J.; Ryu, B.; Thomas, N.V.; Kim, S.K. A comparative study of thermal calcination and an alkaline hydrolysis method in the isolation of hydroxyapatite from Thunnus obesus bone. Biomed. Mater. 2011, 6, 035003. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.; Nishikawa, M.; Tagaya, M. Preparation of gold/hydroxyapatite hybrids using natural fish scale template and their effective albumin interactions. Adv. Powder Technol. 2018, 29, 1198–1203. [Google Scholar] [CrossRef]
- Buraiki, N.S.S.A.; Ali Albadri, B.; Alsheriqi, S.; Alshabibi, B.; Al-Mammari, S.; Premkumar, S.; Sah, M.K.; Sudhakar, M.S. Characterization of Catla catla and Oreochromis niloticus fish scales derived hydroxyapatite scaffolds for regenerative medicine. Mater. Today Proc. 2020, 27, 2609–2616. [Google Scholar] [CrossRef]
- Piccirillo, C.; Silva, M.F.; Pullar, R.C.; Braga da Cruz, I.; Jorge, R.; Pintado, M.M.E.; Castro, P.M.L. Extraction and characterisation of apatite- and tricalcium phosphate-based materials from cod fish bones. Mater. Sci. Eng. C 2013, 33, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Sasaki, K. Synthesis of morphologically controlled hydroxyapatite from fish bone by urea-assisted hydrothermal treatment and its Sr2+ sorption capacity. Powder Technol. 2016, 292, 314–322. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, C.; Zhang, R.; Jiang, D.; Zhu, Q.; Wang, S. Extraction and characterization of HA/β-TCP biphasic calcium phosphate from marine fish. Mater. Lett. 2019, 236, 680–682. [Google Scholar] [CrossRef]
- Akpan, E.S.; Dauda, M.; Kuburi, L.S.; Obada, D.O.; Dodoo-Arhin, D. A comparative study of the mechanical integrity of natural hydroxyapatite scaffolds prepared from two biogenic sources using a low compaction pressure method. Results Phys. 2020, 17, 103051. [Google Scholar] [CrossRef]
- Popescu-Pelin, G.; Ristoscu, C.; Duta, L.; Pasuk, I.; Stan, G.E.; Stan, M.S.; Popa, M.; Chifiriuc, M.C.; Hapenciuc, C.; Oktar, F.N.; et al. Fish Bone Derived Bi-Phasic Calcium Phosphate Coatings Fabricated by Pulsed Laser Deposition for Biomedical Applications. Mar. Drugs 2020, 18, 623. [Google Scholar] [CrossRef]
- Bas, M.; Daglilar, S.; Kuskonmaz, N.; Kalkandelen, C.; Erdemir, G.; Kuruca, S.E.; Tulyaganov, D.; Yoshioka, T.; Gunduz, O.; Ficai, D.; et al. Mechanical and Biocompatibility Properties of Calcium Phosphate Bioceramics Derived from Salmon Fish Bone Wastes. Int. J. Mol. Sci. 2020, 21, 8082. [Google Scholar] [CrossRef]
- Begam, H.; Nandi, S.K.; Chanda, A.; Kundu, B. Effect of bone morphogenetic protein on Zn-HAp and Zn-HAp/collagen composite: A systematic in vivo study. Res. Vet. Sci. 2017, 115, 1–9. [Google Scholar] [CrossRef]
- Wang, N.; Thameem Dheen, S.; Fuh, J.Y.H.; Senthil Kumar, A. A review of multi-functional ceramic nanoparticles in 3D printed bone tissue engineering. Bioprinting 2021, 23, e00146. [Google Scholar] [CrossRef]
- Su, X.; Wang, T.; Guo, S. Applications of 3D printed bone tissue engineering scaffolds in the stem cell field. Regen. Ther. 2021, 16, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.D.N.; Park, K.D.; Ching, Y.C.; Huynh, C.; Nguyen, D.H. A comprehensive review on polymeric hydrogel and its composite: Matrices of choice for bone and cartilage tissue engineering. J. Ind. Eng. Chem. 2020, 89, 58–82. [Google Scholar] [CrossRef]
- Deb, P.; Barua, E.; Deoghare, A.B.; Lala, S.D. Development of bone scaffold using Puntius conchonius fish scale derived hydroxyapatite: Physico-mechanical and bioactivity evaluations. Ceram. Int. 2019, 45, 10004–10012. [Google Scholar] [CrossRef]
- Pon-On, W.; Suntornsaratoon, P.; Charoenphandhu, N.; Thongbunchoo, J.; Krishnamra, N.; Tang, I.M. Synthesis and investigations of mineral ions-loaded apatite from fish scale and PLA/chitosan composite for bone scaffolds. Mater. Lett. 2018, 221, 143–146. [Google Scholar] [CrossRef]
- Nawang, R.; Hussein, M.Z.; Matori, K.A.; Che Abdullah, C.A.; Hashim, M. Physicochemical properties of hydroxyapatite/montmorillonite nanocomposite prepared by powder sintering. Results Phys. 2019, 15, 102540. [Google Scholar] [CrossRef]
- Hakobyan, S.; Roohpour, N.; Gautrot, J.E. Modes of adsorption of polyelectrolytes to model substrates of hydroxyapatite. J. Colloid Interface Sci. 2019, 543, 237–246. [Google Scholar] [CrossRef]
- Stewart, C.; Akhavan, B.; Wise, S.G.; Bilek, M.M.M. A review of biomimetic surface functionalization for bone-integrating orthopedic implants: Mechanisms, current approaches, and future directions. Prog. Mater. Sci. 2019, 106, 100588. [Google Scholar] [CrossRef]
- Morakul, S.; Otsuka, Y.; Ohnuma, K.; Tagaya, M.; Motozuka, S.; Miyashita, Y.; Mutoh, Y. Enhancement effect on antibacterial property of gray titania coating by plasma-sprayed hydroxyapatite-amino acid complexes during irradiation with visible light. Heliyon 2019, 5, e02207. [Google Scholar] [CrossRef] [Green Version]
- Lu, A.; Gao, Y.; Jin, T.; Luo, X.; Zeng, Q.; Shang, Z. Effects of surface roughness and texture on the bacterial adhesion on the bearing surface of bio-ceramic joint implants: An in vitro study. Ceram. Int. 2020, 46, 6550–6559. [Google Scholar] [CrossRef]
- Sopchenski, L.; Cogo, S.; Dias-Ntipanyj, M.F.; Elifio-Espósito, S.; Popat, K.C.; Soares, P. Bioactive and antibacterial boron doped TiO2 coating obtained by PEO. Appl. Surf. Sci. 2018, 458, 49–58. [Google Scholar] [CrossRef]
- Fordham, W.R.; Redmond, S.; Westerland, A.; Cortes, E.G.; Walker, C.; Gallagher, C.; Medina, C.J.; Waecther, F.; Lunk, C.; Ostrum, R.F.; et al. Silver as a Bactericidal Coating for Biomedical Implants. Surf. Coat. Technol. 2014, 253, 52–57. [Google Scholar] [CrossRef]
- Barberia-Roque, L.; Obidi, O.F.; Gámez-Espinosa, E.; Viera, M.; Bellotti, N. Hygienic coatings with bioactive nano-additives from Senna occidentalis-mediated green synthesis. NanoImpact 2019, 16, 100184. [Google Scholar] [CrossRef]
- Kaczmarek, B.; Sionkowska, A.; Osyczka, A.M. Physicochemical properties of scaffolds based on mixtures of chitosan, collagen and glycosaminoglycans with nano-hydroxyapatite addition. Int. J. Biol. Macromol. 2018, 118, 1880–1883. [Google Scholar] [CrossRef]
- Anita Lett, J.; Sagadevan, S.; Fatimah, I.; Hoque, M.E.; Lokanathan, Y.; Léonard, E.; Alshahateet, S.F.; Schirhagl, R.; Oh, W.C. Recent advances in natural polymer-based hydroxyapatite scaffolds: Properties and applications. Eur. Polym. J. 2021, 148, 110360. [Google Scholar] [CrossRef]
- Echave, M.C.; Sánchez, P.; Pedraz, J.L.; Orive, G. Progress of gelatin-based 3D approaches for bone regeneration. J. Drug Deliv. Sci. Technol. 2017, 42, 63–74. [Google Scholar] [CrossRef]
- Zhu, G.; Zhang, T.; Chen, M.; Yao, K.; Huang, X.; Zhang, B.; Li, Y.; Liu, J.; Wang, Y.; Zhao, Z. Bone physiological microenvironment and healing mechanism: Basis for future bone-tissue engineering scaffolds. Bioact. Mater. 2021, 6, 4110–4140. [Google Scholar] [CrossRef]
- Vukajlovic, D.; Parker, J.; Bretcanu, O.; Novakovic, K. Chitosan based polymer/bioglass composites for tissue engineering applications. Mater. Sci. Eng. C 2019, 96, 955–967. [Google Scholar] [CrossRef]
- Jodati, H.; Yılmaz, B.; Evis, Z. A review of bioceramic porous scaffolds for hard tissue applications: Effects of structural features. Ceram. Int. 2020, 46, 15725–15739. [Google Scholar] [CrossRef]
- Mondal, S.; Pal, U. 3D hydroxyapatite scaffold for bone regeneration and local drug delivery applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101131. [Google Scholar] [CrossRef]
- Khurshid, Z.; Zafar, M.S.; Hussain, S.; Fareed, A.; Yousaf, S.; Sefat, F. 10—Silver-substituted hydroxyapatite. In Handbook of Ionic Substituted Hydroxyapatites; Khan, A.S., Chaudhry, A.A., Eds.; Woodhead Publishing: Cambridge, UK, 2020; pp. 237–257. [Google Scholar]
- Das, A.; Pamu, D. A comprehensive review on electrical properties of hydroxyapatite based ceramic composites. Mater. Sci. Eng. C 2019, 101, 539–563. [Google Scholar] [CrossRef] [PubMed]
- Agbeboh, N.I.; Oladele, I.O.; Daramola, O.O.; Adediran, A.A.; Olasukanmi, O.O.; Tanimola, M.O. Environmentally sustainable processes for the synthesis of hydroxyapatite. Heliyon 2020, 6, e03765. [Google Scholar] [CrossRef]
- Szcześ, A.; Hołysz, L.; Chibowski, E. Synthesis of hydroxyapatite for biomedical applications. Adv. Colloid Interface Sci. 2017, 249, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Hoang, G.; Manivasagan, P.; Moorthy, M.S.; Kim, H.H.; Vy Phan, T.T.; Oh, J. Comparative characterization of biogenic and chemical synthesized hydroxyapatite biomaterials for potential biomedical application. Mater. Chem. Phys. 2019, 228, 344–356. [Google Scholar] [CrossRef]
- Mondal, S.; Pal, U.; Dey, A. Natural origin hydroxyapatite scaffold as potential bone tissue engineering substitute. Ceram. Int. 2016, 42, 18338–18346. [Google Scholar] [CrossRef]
- Athinarayanan, J.; Periasamy, V.S.; Alshatwi, A.A. Simultaneous fabrication of carbon nanodots and hydroxyapatite nanoparticles from fish scale for biomedical applications. Mater. Sci. Eng. C 2020, 117, 111313. [Google Scholar] [CrossRef]
- Mahmoud, E.M.; Sayed, M.; El-Kady, A.M.; Elsayed, H.; Naga, S.M. In vitro and in vivo study of naturally derived alginate/hydroxyapatite bio composite scaffolds. Int. J. Biol. Macromol. 2020, 165, 1346–1360. [Google Scholar] [CrossRef]
- Chai, Y.; Okuda, M.; Otsuka, Y.; Ohnuma, K.; Tagaya, M. Comparison of two fabrication processes for biomimetic collagen/hydroxyapatite hybrids. Adv. Powder Technol. 2019, 30, 1419–1423. [Google Scholar] [CrossRef]
- Prasad, A.; Mohan Bhasney, S.; Sankar, M.R.; Katiyar, V. Fish Scale Derived Hydroxyapatite reinforced Poly (Lactic acid) Polymeric Bio-films: Possibilities for Sealing/locking the Internal Fixation Devices. Mater. Today Proc. 2017, 4, 1340–1349. [Google Scholar] [CrossRef]
- Kara, A.; Tamburaci, S.; Tihminlioglu, F.; Havitcioglu, H. Bioactive fish scale incorporated chitosan biocomposite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2019, 130, 266–279. [Google Scholar] [CrossRef]
- Humayun, A.; Luo, Y.; Mills, D.K. Electrophoretic Deposition of Gentamicin-Loaded ZnHNTs-Chitosan on Titanium. Coatings 2020, 10, 944. [Google Scholar] [CrossRef]
- Kang, M.; Kim, S.; Kim, H.; Song, Y.; Jung, D.; Kang, S.; Seo, J.-H.; Nam, S.; Lee, Y. Calcium-Binding Polymer-Coated Poly(lactide-co-glycolide) Microparticles for Sustained Release of Quorum Sensing Inhibitors to Prevent Biofilm Formation on Hydroxyapatite Surfaces. ACS Appl. Mater. Interfaces 2019, 11, 7686–7694. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Song, G.; Qiao, H.; Lan, J.; Wang, B.; Yang, H.; Ma, L.; Wang, S.; Wang, Z.; Lin, H.; et al. Novel ternary vancomycin/strontium doped hydroxyapatite/graphene oxide bioactive composite coatings electrodeposited on titanium substrate for orthopedic applications. Colloids Surf. A Physicochem. Eng. Asp. 2020, 603, 125223. [Google Scholar] [CrossRef]
- Li, Z.; Du, T.; Ruan, C.; Niu, X. Bioinspired mineralized collagen scaffolds for bone tissue engineering. Bioact. Mater. 2021, 6, 1491–1511. [Google Scholar] [CrossRef]
- Lowe, B.; Venkatesan, J.; Anil, S.; Shim, M.S.; Kim, S.-K. Preparation and characterization of chitosan-natural nano hydroxyapatite-fucoidan nanocomposites for bone tissue engineering. Int. J. Biol. Macromol. 2016, 93, 1479–1487. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Y.; Li, R.; Bai, H.; Zhu, Z.; Zhu, L.; Zhu, C.; Che, Z.; Liu, H.; Wang, J.; et al. Collagen-based biomaterials for bone tissue engineering. Mater. Des. 2021, 210, 110049. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, M.; Zhang, W.; Zhang, X. Homology of selenium (Se) and tellurium (Te) endow the functional similarity of Se-doped and Te-doped mesoporous bioactive glass nanoparticles in bone tissue engineering. Ceram. Int. 2022, 48, 3729–3739. [Google Scholar] [CrossRef]
- Murugan, S.S.; Dalavi, P.A.; Devi, G.V.Y.; Chatterjee, K.; Venkatesan, J. Natural and Synthetic Biopolymeric Biomaterials for Bone Tissue Engineering Applications. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Eftekhari, A.; Maleki Dizaj, S.; Sharifi, S.; Salatin, S.; Rahbar Saadat, Y.; Zununi Vahed, S.; Samiei, M.; Ardalan, M.; Rameshrad, M.; Ahmadian, E.; et al. The Use of Nanomaterials in Tissue Engineering for Cartilage Regeneration; Current Approaches and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 536. [Google Scholar] [CrossRef] [Green Version]
- Fang, J.; Wang, D.; Hu, F.; Li, X.; Zou, X.; Xie, J.; Zhou, Z. Strontium mineralized silk fibroin porous microcarriers with enhanced osteogenesis as injectable bone tissue engineering vehicles. Mater. Sci. Eng. C 2021, 128, 112354. [Google Scholar] [CrossRef]
- Shirzaei Sani, I.; Rezaei, M.; Baradar Khoshfetrat, A.; Razzaghi, D. Preparation and characterization of polycaprolactone/chitosan-g-polycaprolactone/hydroxyapatite electrospun nanocomposite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2021, 182, 1638–1649. [Google Scholar] [CrossRef]
- Panda, S.; Biswas, C.K.; Paul, S. A comprehensive review on the preparation and application of calcium hydroxyapatite: A special focus on atomic doping methods for bone tissue engineering. Ceram. Int. 2021, 47, 28122–28144. [Google Scholar] [CrossRef]
- Yang, L.; Jin, S.; Shi, L.; Ullah, I.; Yu, K.; Zhang, W.; Bo, L.; Zhang, X.; Guo, X. Cryogenically 3D printed biomimetic scaffolds containing decellularized small intestinal submucosa and Sr2+/Fe3+ co-substituted hydroxyapatite for bone tissue engineering. Chem. Eng. J. 2022, 431, 133459. [Google Scholar] [CrossRef]
- Wan, Z.; Zhang, P.; Liu, Y.; Lv, L.; Zhou, Y. Four-dimensional bioprinting: Current developments and applications in bone tissue engineering. Acta Biomater. 2020, 101, 26–42. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Boschetto, F.; Yagi, S.; Marin, E.; Adachi, T.; Chen, X.; Pezzotti, G.; Sakurai, S.; Yamane, H.; Xu, H. Design and manufacturing of 3D high-precision micro-fibrous poly (l-lactic acid) scaffold using melt electrowriting technique for bone tissue engineering. Mater. Des. 2021, 210, 110063. [Google Scholar] [CrossRef]
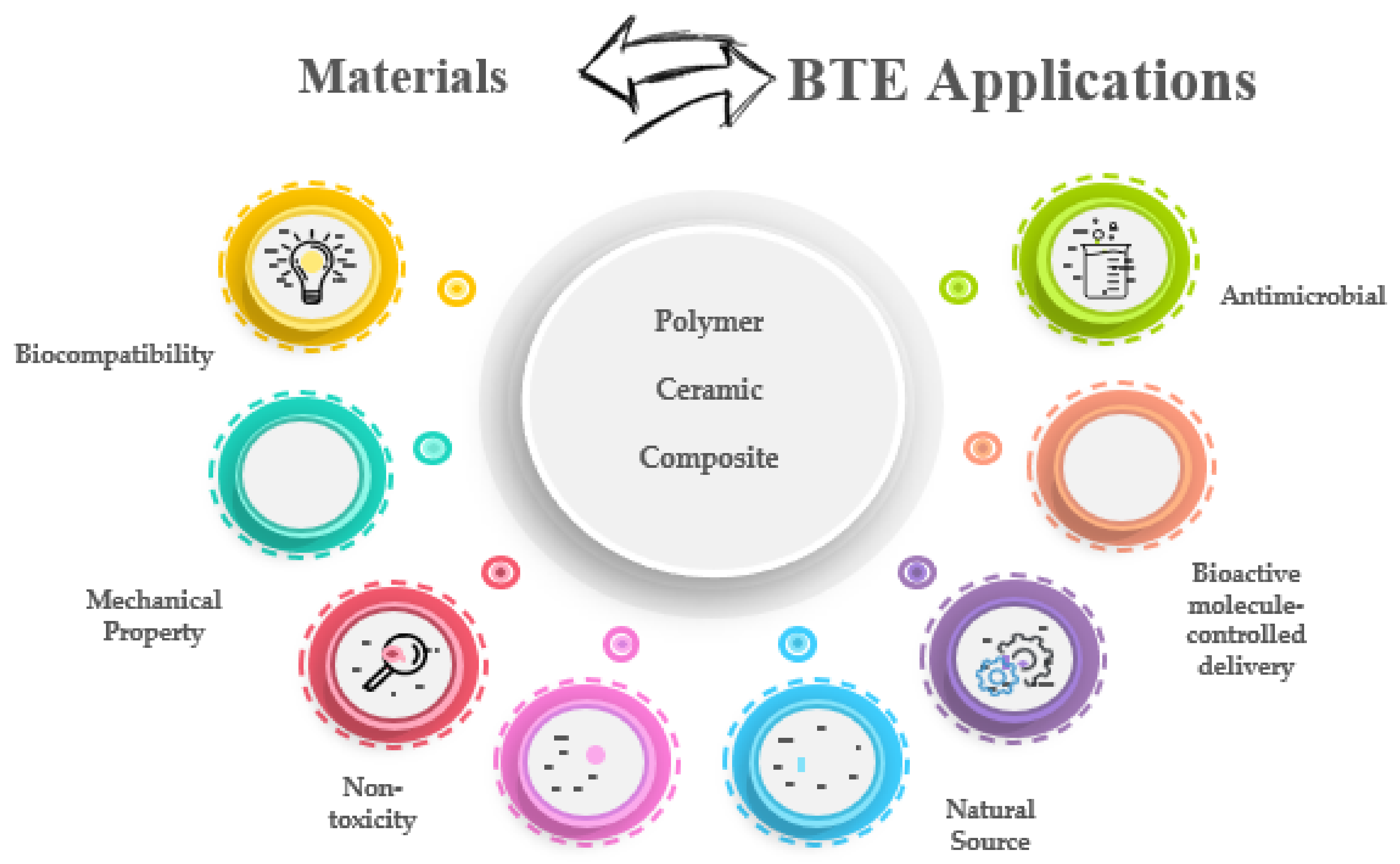
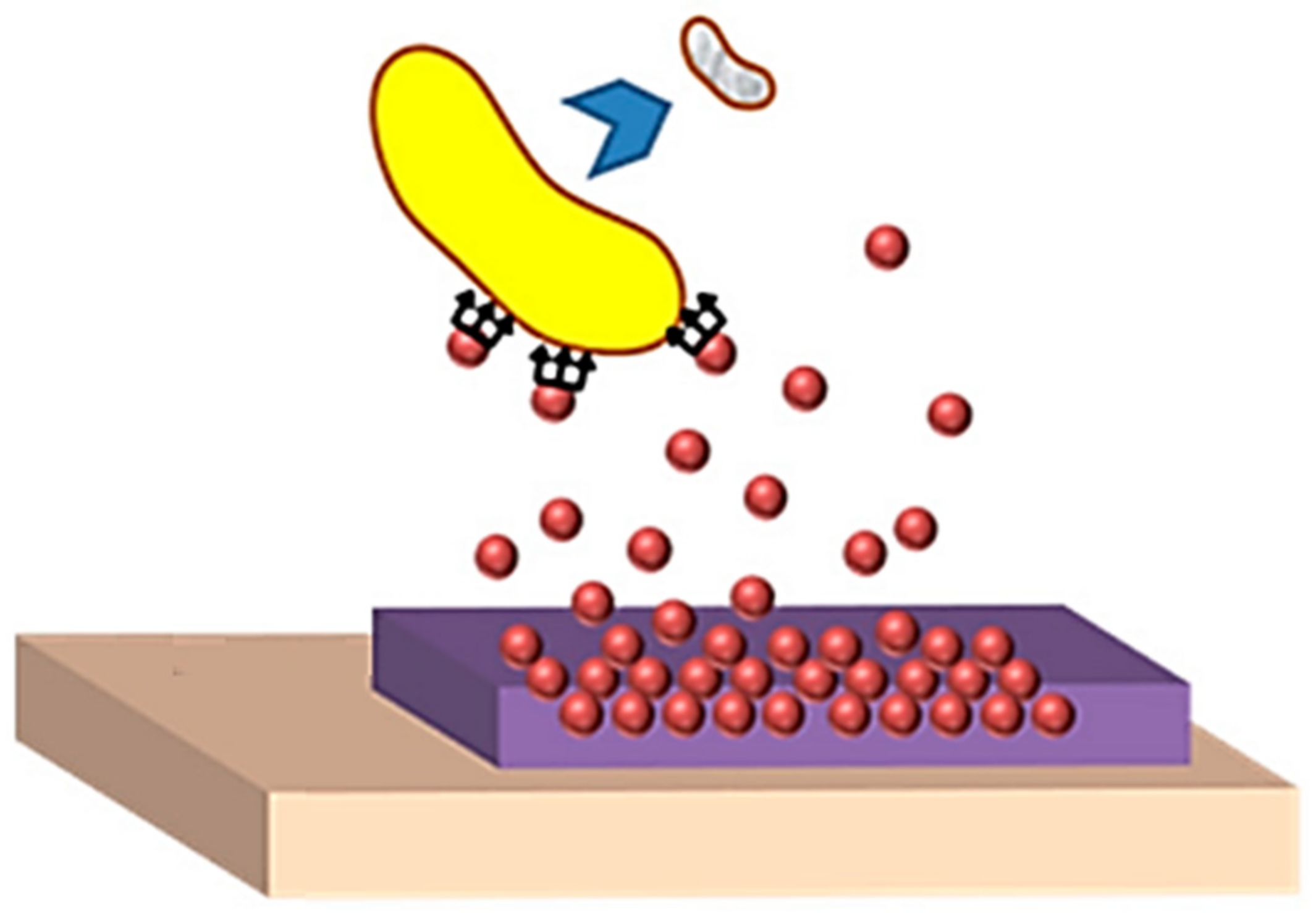




| Polymer | Properties | Applications | References | |
|---|---|---|---|---|
| Advantages | Disadvantages | |||
| Collagen | Great biocompatibility, biodegradability, cytocompatibility, non-toxicity | Poor mechanical strength | Scaffolds, drug delivery systems, 3D printing | [26] |
| Alginate | Biodegradability, biocompatibility, bioresorbability, non-toxicity, presenting synergic effects with bioactive components | Poor mechanical strength and bioactivity | Bone tissue applications | [27,28] |
| Chitosan | Superior biocompatibility; biodegradability, anti-inflammatory | Poor stability, mechanical strength | Hydrogels, scaffolds, microspheres | [29] |
| Hyaluronic Acid | Great biocompatibility, biodegradability, cell adhesion, proliferation, and differentiation | Poor mechanical properties, high degradation rate | Scaffolds, hydrogel | [30,31,32] |
| Bacterial cellulose | Good water absorption, mechanical strength and structural properties, good cell adhesion and biocompatibility, continuous structural support | Low biodegradability in the human body and biological activity | 3D scaffolds, bone tissue replacements | [33,34] |
| Silk fibroin | Increased flexibility, biocompatibility, with good mechanical strength | Reduced biodegradation rate | Scaffolds | [35] |
| Gelatin | Great biocompatibility, biodegradability, non-toxicity, improved cell adhesion, and proliferation | Poor mechanical properties, high biodegradation rate | Scaffolds for hard tissue engineering | [26,36,37] |
| Polymer | Properties | Applications | References | |
|---|---|---|---|---|
| Advantages | Disadvantages | |||
| Polylactic acid (PLA) | Superior tensile strength, elongation, and modulus, biodegradability, and minimal inflammatory response | Low toughness, mechanical support, insufficient biocompatibility | Load bearing applications, orthopedic repair, suture anchors, scaffolds | [27,55,56] |
| Poly(ε-caprolactone) (PCL) | Good biodegradability, biocompatibility, low Young’s modulus, tailorable physical properties, reduced degradation rate | Poor cell adhesion, hydrophobic nature | Scaffolds, BTE, 3D bioprinting | [55,57,58] |
| Poly(glycolic acid) (PGA) | High crystallinity; great mechanical strength, good cell adhesion, proliferation, and differentiation | Hydrophobic nature | Scaffolds, BTE | [58,59] |
| Poly(vinyl alcohol) (PVA) | Biocompatibility, biodegradability, good compressive mechanical and elastic strength | Low bioactivity, decreased cell attachment | Scaffolds, drug delivery systems | [60,61,62] |
| Poly(ethylene glycol) (PEG) | Biocompatibility, hydrophilicity, able to improve degradation, non-toxicity, and non-immunogenicity combined with different polymers, enhanced enzymatic stability | Limited tailorable mechanical property and rheological behavior, reduced bioactivity | Scaffolds, BTE, 3D bioprinting, orthopedic implant | [58,63,64] |
| Poly(lactic-co-glycolic acid) (PLGA) | Excellent biocompatibility, processability, good mechanical strength, adjustable degradation rate, and minimal inflammatory response | Possible inflammatory response, low bioactivity | Scaffolds, orthopedic implants, drug delivery systems | [65,66] |
| Poly(methyl methacrylate) (PMMA) | Processability, durability | Non-degradability | Scaffolds | [66] |
| Natural Source | Crystalline Phase | Morphology | Application | References |
|---|---|---|---|---|
| Fishbone | Hydroxyapatite | Laminar and irregular structure, 149–325 nm | Surface coating; nutrition | [96,97,98,99] |
| Biphasic calcium phosphate | 30–100 nm, as nanorods | Scaffolds | [100] | |
| Eggshells | Biphasic calcium phosphate | Spherical structure | Orthopedic and dental applications | [101] |
| α-Tricalcium phosphate | Compact and agglomerated structure | Scaffolds; dental reconstruction | [102,103] | |
| ꞵ-Tricalcium phosphate | Round shape, with dimensions between 150 nm–2 µm | Scaffold in dental and orthopedic reconstruction | [104] | |
| Hydroxyapatite | Irregularly shaped, with sizes between 10–18 µm | Reinforcing filler; biomedical devices | [105,106] | |
| Hydroxyapatite | Flower-like, with the aspect of hexagonal rods and dimensions between 200–300 nm | Biomedical applications | [107] | |
| Seashells | Hydroxyapatite | Nano-rods, with sizes between 20–90 nm | BTE; drug delivery; dentalApplications; coating | [108,109] |
| Fish scales | Hydroxyapatite | Dimensions between 20–60 nm, in the form of agglomerations or nano-rods | Coating; dental applications; bone graft; filler | [110,111,112,113] |
| Property | Required Characteristics | References |
|---|---|---|
| Biodegradability | The material should possess a prearranged biodegradability to improve the composition of different tissue. In this manner, the biodegradable matrices offer temporary scaffolds within defects into the bone tissues to improve their regeneration. | [174] |
| Biocompatibility | The composite material must perform with a suitable host response in the regeneration of bone tissue. This ability must be in synchronization with osseous tissue without producing damaging changes. | [175] |
| Mechanical Properties | Surface roughness enhances cell attachment, differentiation, and maturation. Moreover, scaffolds’ mechanical stability supports their adhesion to the neighboring tissue. These properties enhance the adsorption of adhesive proteins (e.g., fibrin), leading to an improved osteogenic cell attachment, proliferation, and differentiation into osteoblasts, to further bone production integrated within the scaffold. | [55,176] |
| Porosity | Needs be tuned, as the initial porosity must be low or else the scaffold resorbs very fast, incapacitating the mechanical support to further affect novel tissue growth. On the other hand, materials with a low degradation rate can possess high porosity, optimizing the degradation of the scaffold. | [177] |
| Bioactivity | This characteristic is essential to improve ECM development through the stimulation of cellular behavior andcan contribute to the cells the molecular signals. | [174,176] |
| Processability | The composite material should be easily processed to design various formulations and configurations such as nanometric, 3D scaffolds, micro-metric particles, and/or injectable formulations. | [175] |
| Immune response and toxicity | The obtained materials must be non-cytotoxic and allow cell attachment to function properly, proliferate and differentiate. Moreover, they must possess non-inflammatory properties and induce a minimal immune response. | [178] |
| Controlled Delivery | To deliver biomolecules in BTE applications, it is mandatory to develop scaffolds as a drug delivery system. Additionally, the biological activities of these biomolecules and interaction among surrounding cells in the bone-healing process are the foundation for the fabrication of BTE scaffolds. | [176] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radulescu, D.-E.; Neacsu, I.A.; Grumezescu, A.-M.; Andronescu, E. Novel Trends into the Development of Natural Hydroxyapatite-Based Polymeric Composites for Bone Tissue Engineering. Polymers 2022, 14, 899. https://doi.org/10.3390/polym14050899
Radulescu D-E, Neacsu IA, Grumezescu A-M, Andronescu E. Novel Trends into the Development of Natural Hydroxyapatite-Based Polymeric Composites for Bone Tissue Engineering. Polymers. 2022; 14(5):899. https://doi.org/10.3390/polym14050899
Chicago/Turabian StyleRadulescu, Diana-Elena, Ionela Andreea Neacsu, Alexandru-Mihai Grumezescu, and Ecaterina Andronescu. 2022. "Novel Trends into the Development of Natural Hydroxyapatite-Based Polymeric Composites for Bone Tissue Engineering" Polymers 14, no. 5: 899. https://doi.org/10.3390/polym14050899
APA StyleRadulescu, D.-E., Neacsu, I. A., Grumezescu, A.-M., & Andronescu, E. (2022). Novel Trends into the Development of Natural Hydroxyapatite-Based Polymeric Composites for Bone Tissue Engineering. Polymers, 14(5), 899. https://doi.org/10.3390/polym14050899