A Novel Approach to Charcoal Fine Waste: Sustainable Use as Filling of Polymeric Matrices
Abstract
1. Introduction
2. Materials and Methods
2.1. Biomass Characterization and Charcoal Production
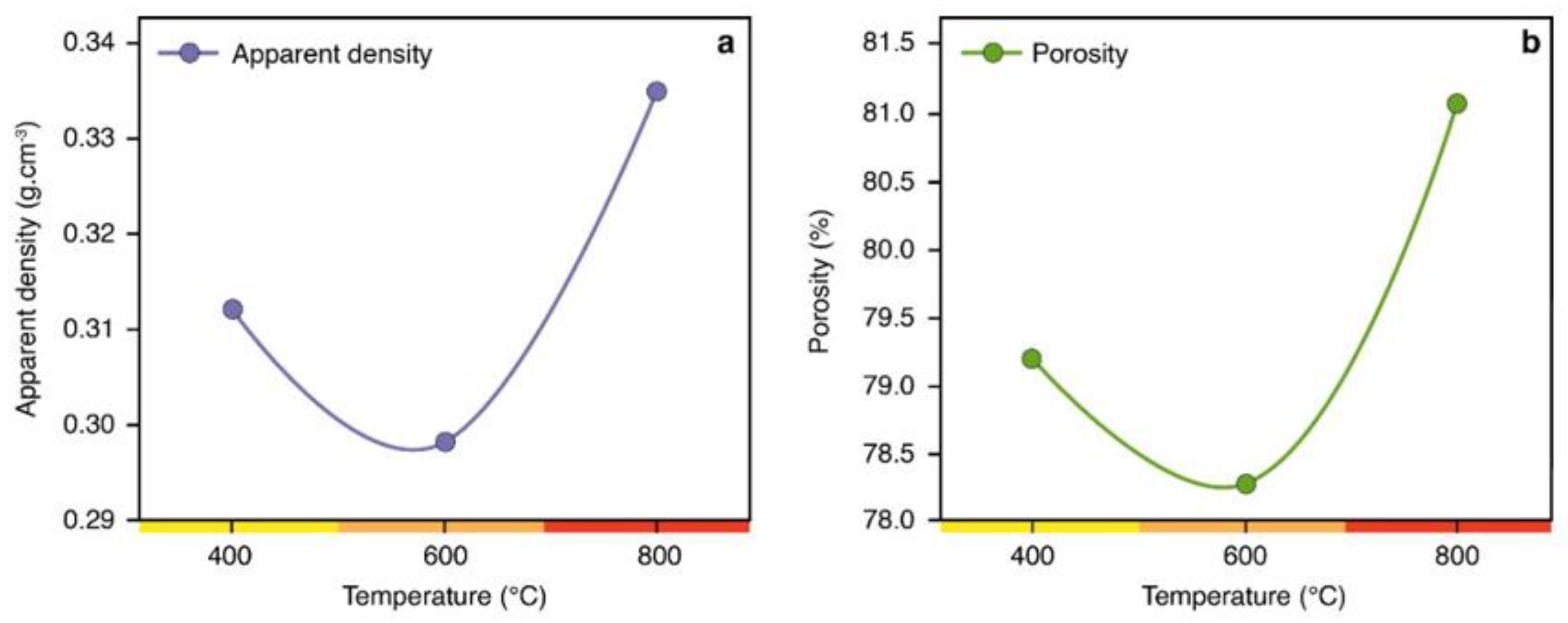
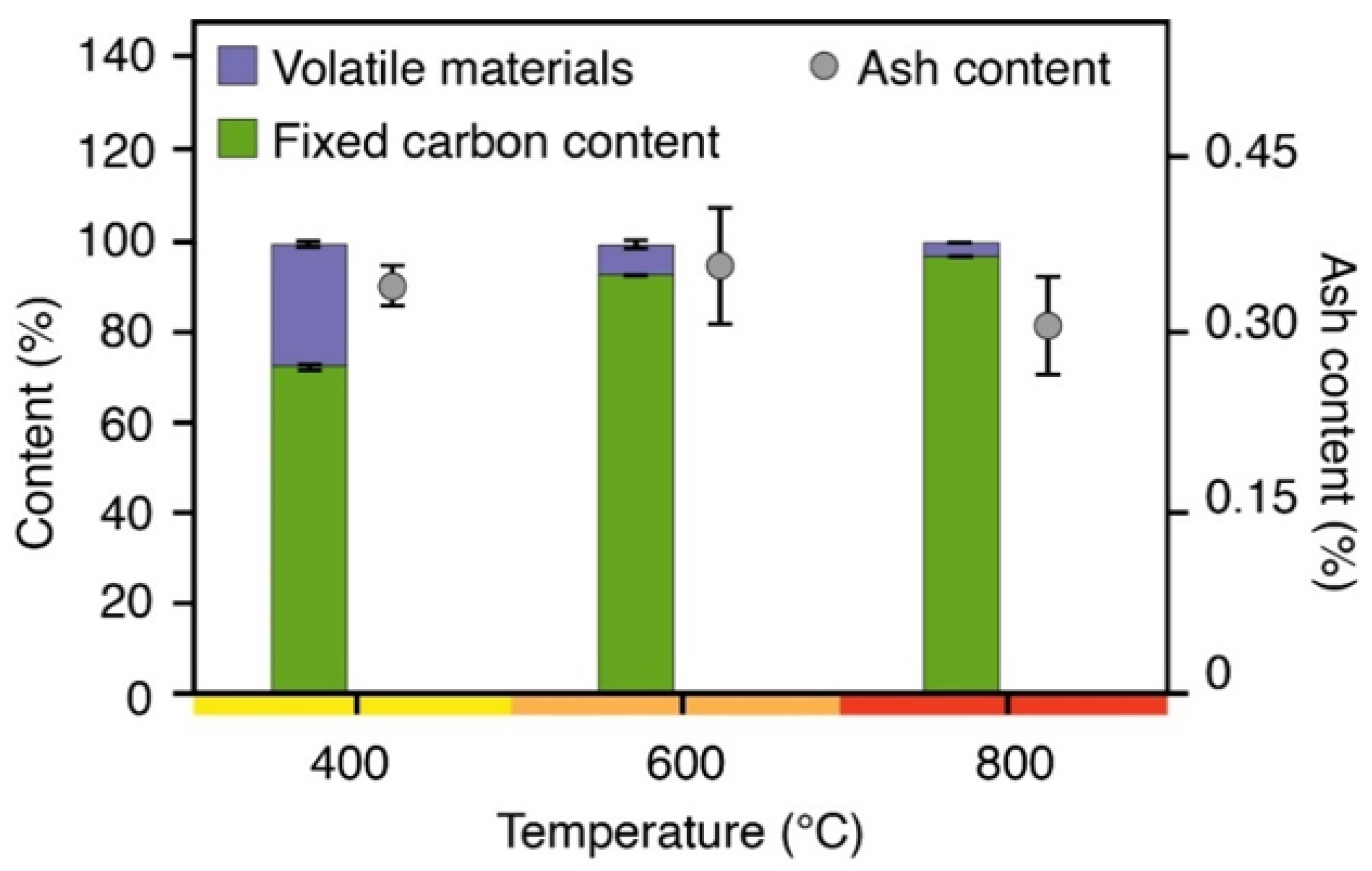
2.1.1. Charcoal Fine Characterization
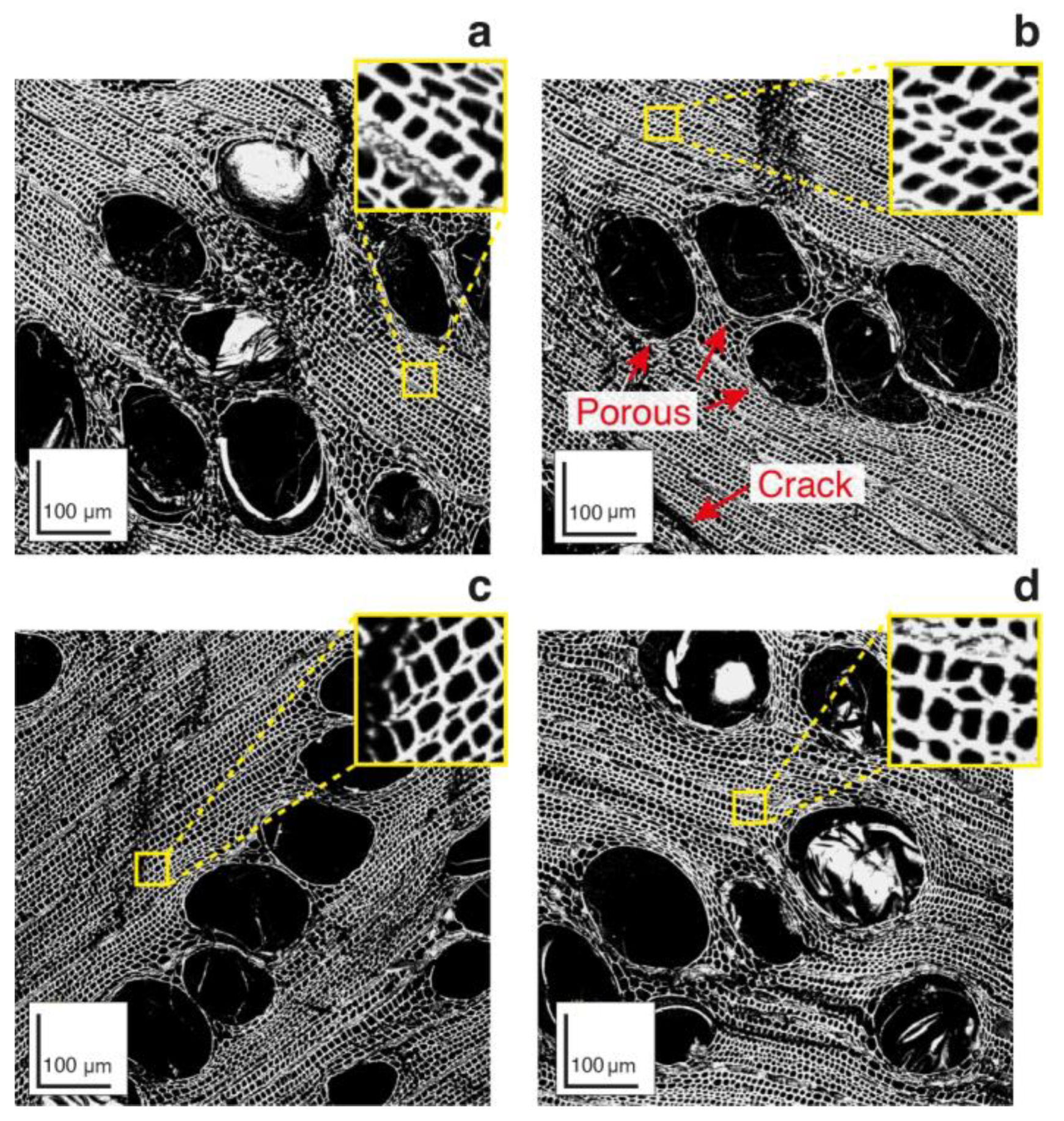
2.1.2. Visual Analysis of Charcoal Microstructures
2.1.3. Fourier-Transform Infrared Spectroscopy (FTIR)
2.1.4. Charcoal Fine X-ray Photoelectron Spectroscopy
2.2. Production and Characterization of Biocomposites
2.2.1. Mechanical Tests
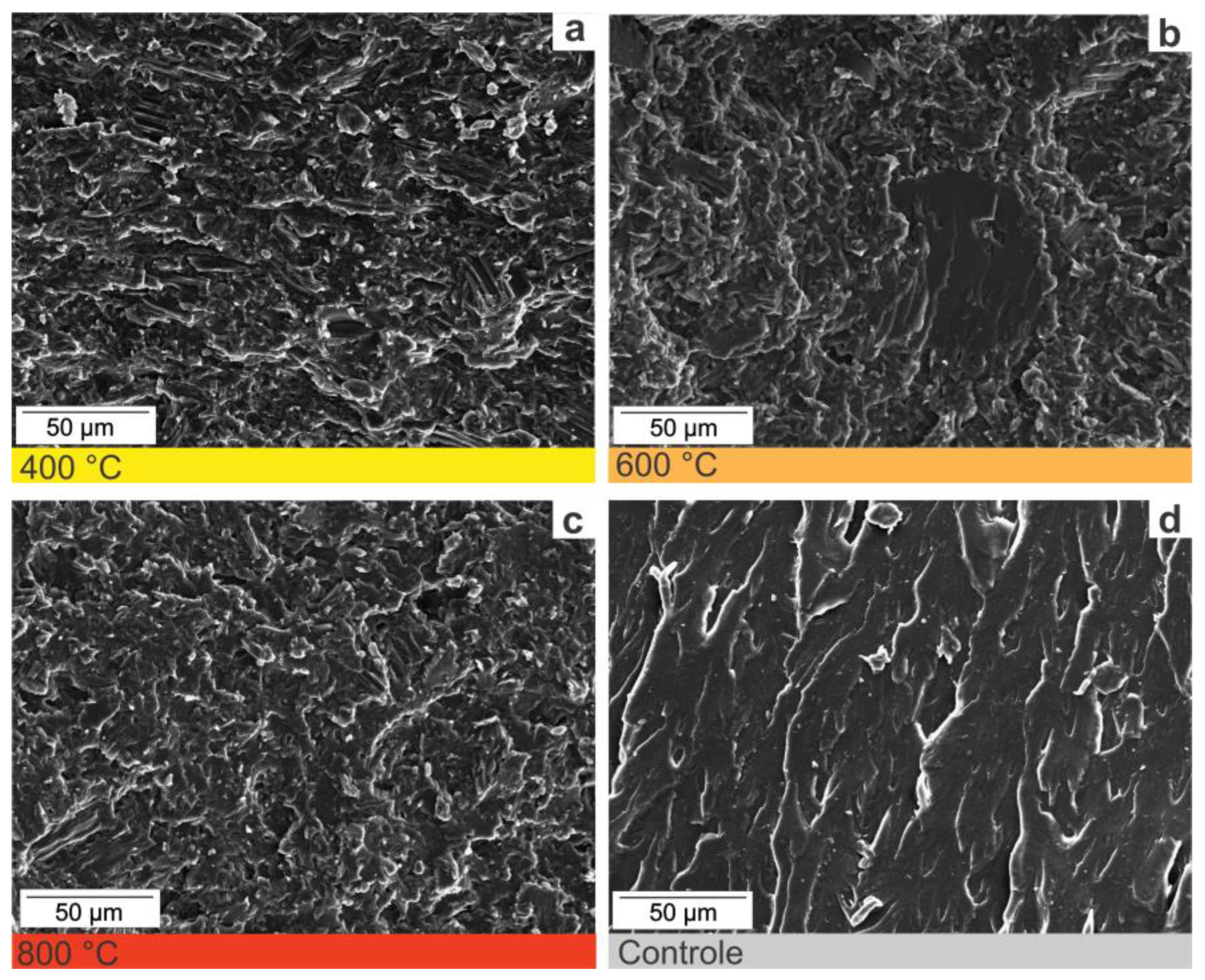
2.2.2. Fractographic Analyses
2.3. Data Analysis
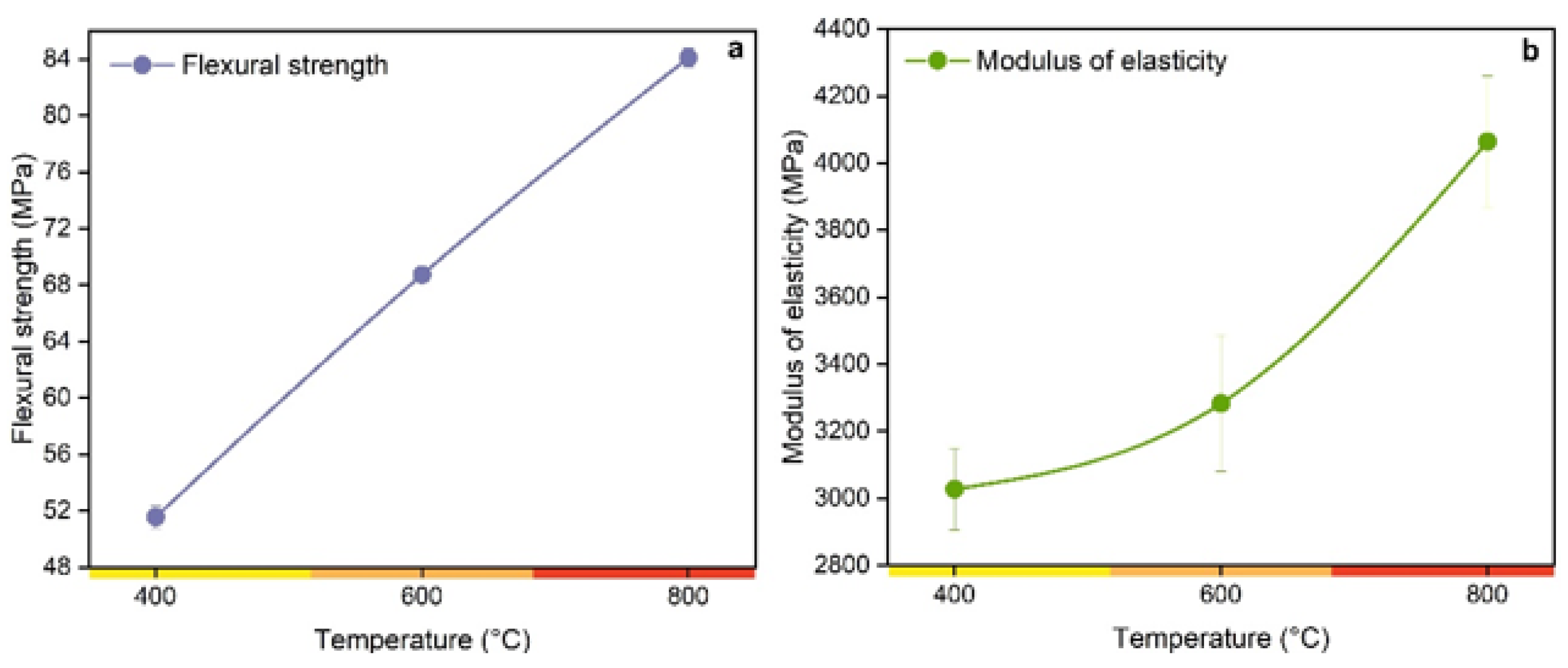
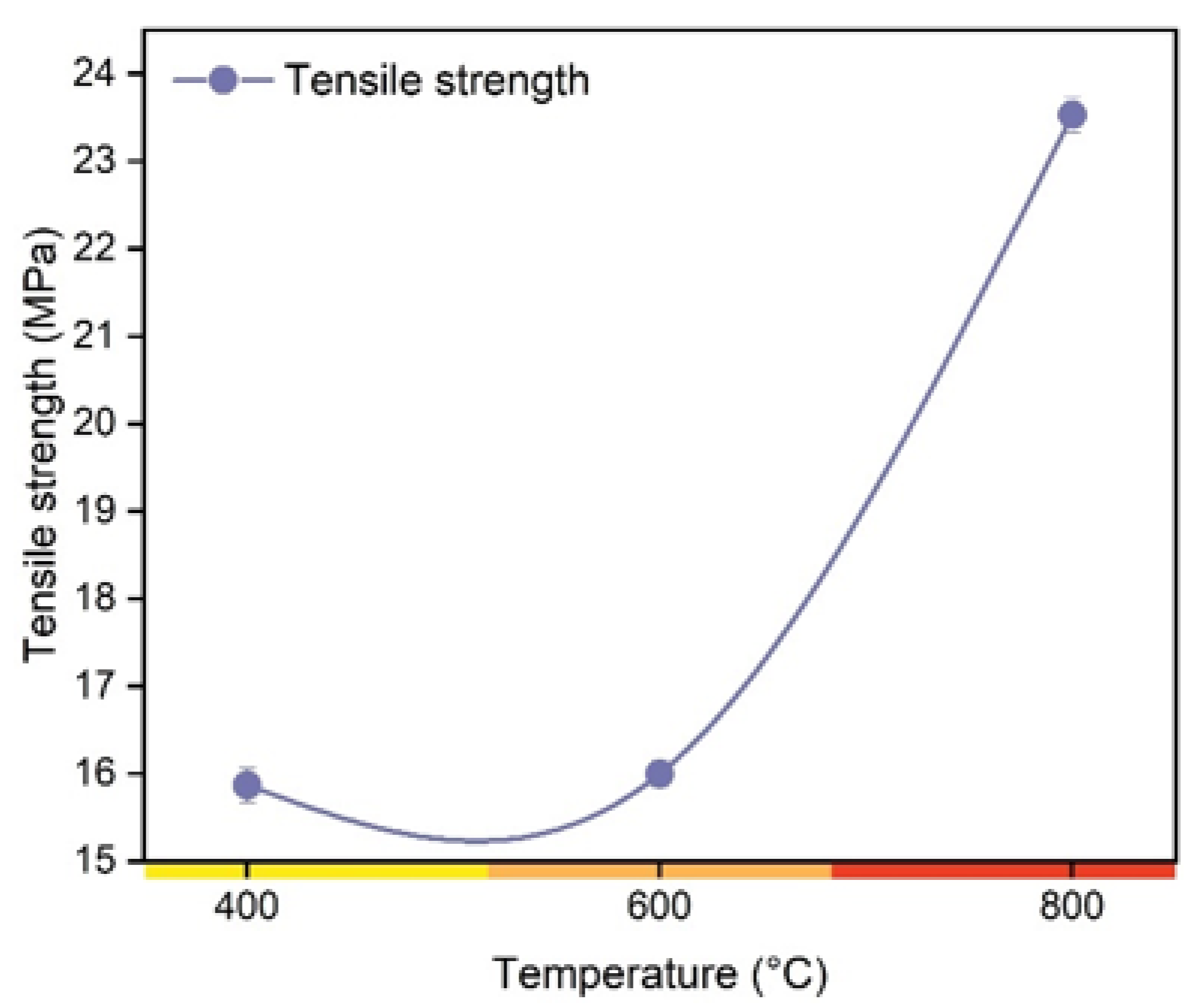
3. Results and Discussion
3.1. Insights about the Use Charcoal in Polymerics Biocomposites
3.2. Practical and Policy Implications and Future Perspectives
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A



References
- Delatorre, F.M.; Cupertino, G.F.M.; Junior, A.J.d.S.; Da Silva, Á.M.; Júnior, A.F.D.; Silveira, M.P.R. Insights Acerca Do Uso de Finos de Carvão Vegetal Para Geração de Bioenergia. Agropec. Cient. Semiárido 2020, 16, 138. [Google Scholar] [CrossRef]
- Akaluzia, R.O.; Edoziuno, F.O.; Adediran, A.A.; Odoni, B.U.; Edibo, S.; Olayanju, T.M.A. Evaluation of the Effect of Reinforcement Particle Sizes on the Impact and Hardness Properties of Hardwood Charcoal Particulate-Polyester Resin Composites. Mater. Today Proc. 2021, 38, 570–577. [Google Scholar] [CrossRef]
- Polok-Rubiniec, M.; Włodarczyk-Fligier, A. Polypropylene Matrix Composite with Charcoal Filler. J. Achiev. Mater. Manuf. Eng. 2020, 2, 60–66. [Google Scholar] [CrossRef]
- Dias Júnior, A.F.; Anuto, R.B.; Andrade, C.R.; De Souza, N.D.; Takeshita, S.; Brito, J.O.; Nolasco, A.M. Influence of Eucalyptus Wood Addition to Urban Wood Waste During Combustion. Cerne 2017, 23, 455–464. [Google Scholar] [CrossRef]
- Aktar, S.; Hossain, M.A.; Rathnayake, N.; Patel, S.; Gasco, G.; Mendez, A.; de Figueiredo, C.; Surapaneni, A.; Shah, K.; Paz-Ferreiro, J. Effects of Temperature and Carrier Gas on Physico-Chemical Properties of Biochar Derived from Biosolids. J. Anal. Appl. Pyrolysis 2022, 164, 105542. [Google Scholar] [CrossRef]
- Das, O.; Bhattacharyya, D.; Hui, D.; Lau, K.-T. Mechanical and Flammability Characterisations of Biochar/Polypropylene Biocomposites. Compos. B Eng. 2016, 106, 120–128. [Google Scholar] [CrossRef]
- Holmes, M. Biocomposites Take Natural Step Forward. Reinf. Plast. 2019, 63, 194–201. [Google Scholar] [CrossRef]
- Kumar, S.; Das, J. Carbon Nanotubes, Nanochains and Quantum Dots Synthesized through the Chemical Treatment of Charcoal Powder. J. Mol. Struct. 2021, 1227, 129419. [Google Scholar] [CrossRef]
- NBR 11941; Brasileira de Normas Técnicas—Madeira: Determinação da Densidade Básica. Associação Brasileira de Normas Técnicas: Sao Paulo, Brazil, 2003; 6p.
- TAPPI 222 05-74; Industry Lignin in Wood. Technical Association of the Pulp and Paper Industry: Peachtree Corners, GA, USA, 1974; 12p.
- TAPPI T-12 05-75; Industry Preparation of Wood for Chemical Analysis (Including Procedures for Removal of Extractives an Determination of Moisture Content). Technical Association of the Pulp and Paper Industry: Peachtree Corners, GA, USA, 1975; 21p.
- ASTM D1102-84; Standad Test Methods for Ash in Wood. ASTM International: West Conshohocken, PA, USA, 2021.
- NBR 9165; Carvão Vegetal: Determinação da Densidade Relativa Aparente, Relativa Verdadeira e Porosidade. ABNT NBR: Sao Paulo, Brazil, 1985.
- ASTM D1762-84; Standard Test Method for Chemical Analysis of Wood Charcoal. ASTM International: West Conshohocken, PA, USA, 2021.
- ASTM D7264M-21; Standard Test Method for Flexural Properties of Polymer Matrix Composite Materials. ASTM International: West Conshohocken, PA, USA, 2021.
- ASTM D 3039-17; Standard Test Method for Tensile Properties of Polymer Matrix Composite Materials. ASTM International: West Conshohocken, PA, USA, 2021.
- Ge, S.; Zuo, S.; Zhang, M.; Luo, Y.; Yang, R.; Wu, Y.; Zhang, Y.; Li, J.; Xia, C. Utilization of Decayed Wood for Polyvinyl Chloride/Wood Flour Composites. J. Mater. Res. Technol. 2021, 12, 862–869. [Google Scholar] [CrossRef]
- Ren, K.; Xia, Q.; Liu, Y.; Cheng, W.; Zhu, Y.; Liu, Y.; Yu, H. Wood/Polyimide Composite via a Rapid Substitution Compositing Method for Extreme Temperature Conditions. Compos. Sci. Technol. 2021, 207, 108698. [Google Scholar] [CrossRef]
- Ho, M.P.; Lau, K.T.; Wang, H.; Hui, D. Improvement on the Properties of Polylactic Acid (PLA) Using Bamboo Charcoal Particles. Compos. B Eng. 2015, 81, 14–25. [Google Scholar] [CrossRef]
- Somerville, M.; Jahanshahi, S. The Effect of Temperature and Compression during Pyrolysis on the Density of Charcoal Made from Australian Eucalypt Wood. Renew. Energy 2015, 80, 471–478. [Google Scholar] [CrossRef]
- Assis, M.R.; Brancheriau, L.; Napoli, A.; Trugilho, P.F. Factors Affecting the Mechanics of Carbonized Wood: Literature Review. Wood Sci. Technol. 2016, 50, 519–536. [Google Scholar] [CrossRef]
- Weber, K.; Quicker, P. Properties of Biochar. Fuel 2018, 217, 240–261. [Google Scholar] [CrossRef]
- Brewer, C.E.; Chuang, V.J.; Masiello, C.A.; Gonnermann, H.; Gao, X.; Dugan, B.; Driver, L.E.; Panzacchi, P.; Zygourakis, K.; Davies, C.A. New Approaches to Measuring Biochar Density and Porosity. Biomass Bioenergy 2014, 66, 176–185. [Google Scholar] [CrossRef]
- Gao, X.; Driver, L.E.; Kasin, I.; Masiello, C.A.; Pyle, L.A.; Dugan, B.; Ohlson, M. Effect of Environmental Exposure on Charcoal Density and Porosity in a Boreal Forest. Sci. Total Environ. 2017, 592, 316–325. [Google Scholar] [CrossRef]
- Chen, H.; Chen, X.; Qin, Y.; Wei, J.; Liu, H. Effect of Torrefaction on the Properties of Rice Straw High Temperature Pyrolysis Char: Pore Structure, Aromaticity and Gasification Activity. Bioresour. Technol. 2017, 228, 241–249. [Google Scholar] [CrossRef]
- Elyounssi, K.; Blin, J.; Halim, M. High-Yield Charcoal Production by Two-Step Pyrolysis. J. Anal. Appl. Pyrolysis 2010, 87, 138–143. [Google Scholar] [CrossRef]
- Das, S.C.; Ashek-E-Khoda, S.; Sayeed, M.A.; Suruzzaman; Paul, D.; Dhar, S.A.; Grammatikos, S.A. On the Use of Wood Charcoal Filler to Improve the Properties of Natural Fiber Reinforced Polymer Composites. Mater. Today Proc. 2021, 44, 926–929. [Google Scholar] [CrossRef]
- Das, O.; Sarmah, A.K.; Bhattacharyya, D. Biocomposites from Waste Derived Biochars: Mechanical, Thermal, Chemical, and Morphological Properties. Waste Manag. 2016, 49, 560–570. [Google Scholar] [CrossRef]
- Mullani, N.; Ali, I.; Dongale, T.D.; Kim, G.H.; Choi, B.J.; Basit, M.A.; Park, T.J. Improved Resistive Switching Behavior of Multiwalled Carbon Nanotube/TiO2 Nanorods Composite Film by Increased Oxygen Vacancy Reservoir. Mater. Sci. Semicond. Process. 2020, 108, 104907. [Google Scholar] [CrossRef]
- Batista, E.M.C.C.; Shultz, J.; Matos, T.T.S.; Fornari, M.R.; Ferreira, T.M.; Szpoganicz, B.; de Freitas, R.A.; Mangrich, A.S. Effect of Surface and Porosity of Biochar on Water Holding Capacity Aiming Indirectly at Preservation of the Amazon Biome. Sci. Rep. 2018, 8, 10677. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.A.; Joseph, K.; Mathew, G.D.G.; Pothen, L.A.; Thomas, S. Influence of Polarity Parameters on the Mechanical Properties of Composites from Polypropylene Fiber and Short Banana Fiber. Compos. Part A Appl. Sci. Manuf. 2010, 41, 1380–1387. [Google Scholar] [CrossRef]
- Li, J.; Dou, B.; Zhang, H.; Zhang, H.; Chen, H.; Xu, Y.; Wu, C. Pyrolysis Characteristics and Non-Isothermal Kinetics of Waste Wood Biomass. Energy 2021, 226, 120358. [Google Scholar] [CrossRef]
- Zhang, Z.; Qu, J.; Feng, Y.; Feng, W. Assembly of Graphene-Aligned Polymer Composites for Thermal Conductive Applications. Compos. Commun. 2018, 9, 33–41. [Google Scholar] [CrossRef]
- Zielke, U.; Hüttinger, K.J.; Hoffman, W.P. Surface-Oxidized Carbon Fibers: I. Surface Structure and Chemistry. Carbon 1996, 34, 983–998. [Google Scholar] [CrossRef]
- Azargohar, R.; Nanda, S.; Kozinski, J.A.; Dalai, A.K.; Sutarto, R. Effects of Temperature on the Physicochemical Characteristics of Fast Pyrolysis Bio-Chars Derived from Canadian Waste Biomass. Fuel 2014, 125, 90–100. [Google Scholar] [CrossRef]
- Wen, B.; Xia, W.; Niu, C. Comparison of Pyrolysis and Oxidation Actions on Chemical and Physical Property of Anthracite Coal Surface. Adv. Powder Technol. 2020, 31, 2447–2455. [Google Scholar] [CrossRef]
- Jiang, Z.; Liu, G. Microencapsulation of Ammonium Polyphosphate with Melamine-Formaldehyde-Tris(2-Hydroxyethyl)Isocyanurate Resin and Its Flame Retardancy in Polypropylene. RSC Adv. 2015, 5, 88445–88455. [Google Scholar] [CrossRef]
- Wu, K.; Zhang, Y.; Hu, W.; Lian, J.; Hu, Y. Influence of Ammonium Polyphosphate Microencapsulation on Flame Retardancy, Thermal Degradation and Crystal Structure of Polypropylene Composite. Compos. Sci. Technol. 2013, 81, 17–23. [Google Scholar] [CrossRef]
- Luo, L.; Yang, Y.; Xiao, M.; Bian, L.; Yuan, B.; Liu, Y.; Jiang, F.; Pan, X. A Novel Biotemplated Synthesis of TiO2/Wood Charcoal Composites for Synergistic Removal of Bisphenol A by Adsorption and Photocatalytic Degradation. Chem. Eng. J. 2015, 262, 1275–1283. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, T.; Xiong, Y.; Dong, Q. Effects of Wet Torrefaction on the Physicochemical Properties and Pyrolysis Product Properties of Rice Husk. Energy Convers. Manag. 2017, 141, 403–409. [Google Scholar] [CrossRef]
- Pappu, A.; Pickering, K.L.; Thakur, V.K. Manufacturing and Characterization of Sustainable Hybrid Composites Using Sisal and Hemp Fibres as Reinforcement of Poly (Lactic Acid) via Injection Moulding. Ind. Crops Prod. 2019, 137, 260–269. [Google Scholar] [CrossRef]
- Demartini, M.; Tonelli, F.; Govindan, K. An Investigation into Modelling Approaches for Industrial Symbiosis: A Literature Review and Research Agenda. Clean. Logist. Supply Chain. 2022, 3, 100020. [Google Scholar] [CrossRef]
- Chertow, M.R. Industrial Symbiosis: Literature and Taxonomy. Annu. Rev. Energy Environ. 2000, 25, 313–337. [Google Scholar] [CrossRef]
- Lombardi, D.R.; Laybourn, P. Redefining Industrial Symbiosis. J. Ind. Ecol. 2012, 16, 28–37. [Google Scholar] [CrossRef]
- Casa Civil. LEI N° 12.187, de 29 de Dezembro de 2009; Institui a Política Nacional Sobre Mudança do Clima—PNMC e dá Outras Providências: Brasília, Brazil, 2009. [Google Scholar]
- Casa Civil. Lei N° 12.305, de 2 de Agosto de 2009; Institui a Política Nacional Sobre Mudança do Clima—PNMC e dá Outras Providências: Brasília, Brazil, 2010. [Google Scholar]

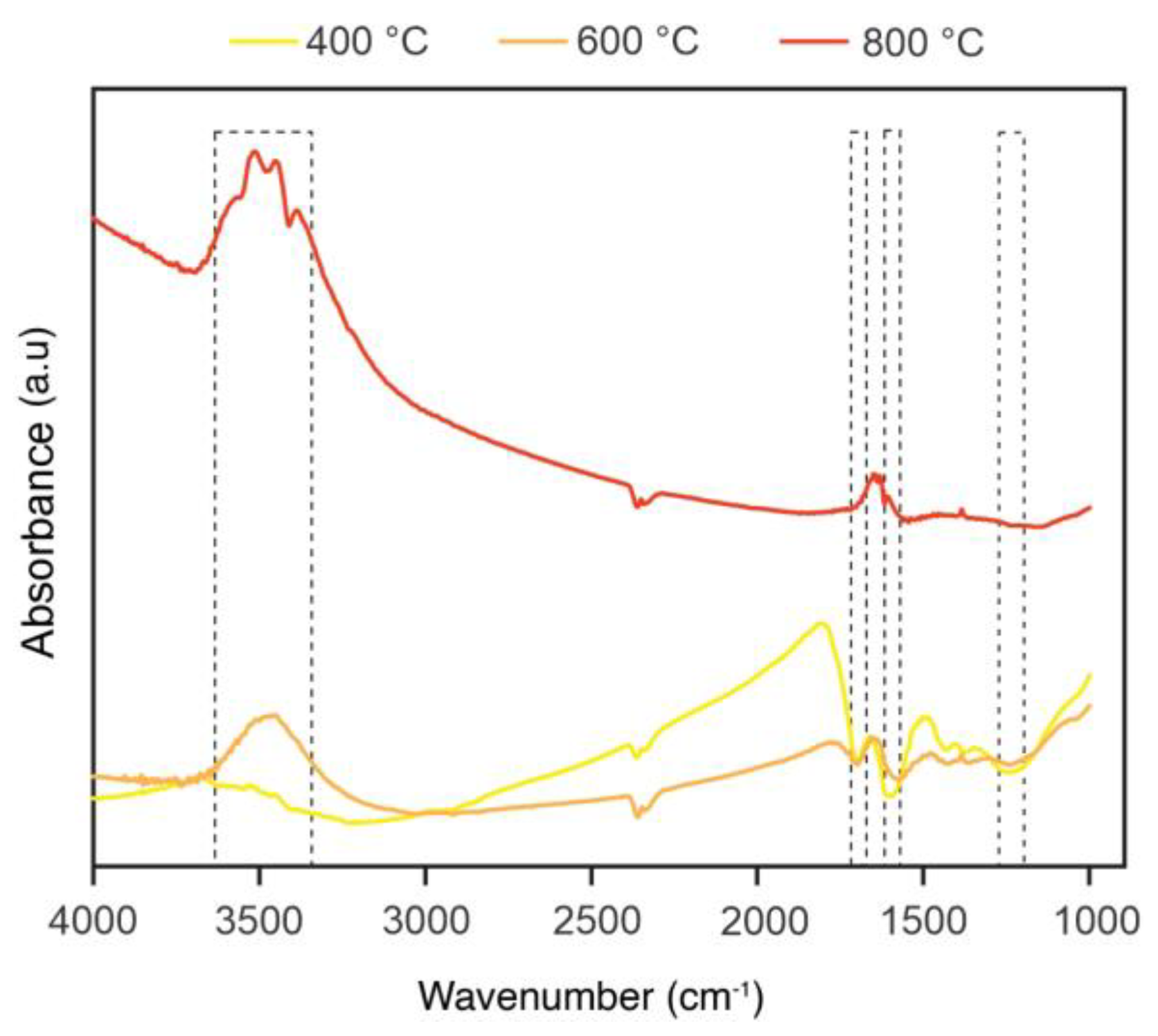

| Sample | C=C, C−C, C−H (Aromatic, Aliphatic Carbon) | C−O, C−O−C, C−OH (Hydroxyl, Ether, Phenol) | C−O (Ketone) | C=O (Carbonyl) | O−C=O (Carboxylic Acid, Ester) |
|---|---|---|---|---|---|
| CV 400 | 62.1 ± 1.9 | 19.3 ± 0.9 | 10.8 ± 0.7 | 3.9 ± 0.3 | 3.95 ± 0.08 |
| CV 600 | 72.1 ± 1.7 | 12.7 ± 2.5 | 7.5 ± 0.5 | 3.4 ± 0.2 | 4.3 ± 0.1 |
| CV 800 | 70.4 ± 1.8 | 12.5 ± 1.5 | 7.7 ± 0.8 | 3.8 ± 0.2 | 5.7 ± 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delatorre, F.M.; Cupertino, G.F.M.; Oliveira, M.P.; da Silva Gomes, F.; Profeti, L.P.R.; Profeti, D.; Júnior, M.G.; de Azevedo, M.G.; Saloni, D.; Júnior, A.F.D. A Novel Approach to Charcoal Fine Waste: Sustainable Use as Filling of Polymeric Matrices. Polymers 2022, 14, 5525. https://doi.org/10.3390/polym14245525
Delatorre FM, Cupertino GFM, Oliveira MP, da Silva Gomes F, Profeti LPR, Profeti D, Júnior MG, de Azevedo MG, Saloni D, Júnior AFD. A Novel Approach to Charcoal Fine Waste: Sustainable Use as Filling of Polymeric Matrices. Polymers. 2022; 14(24):5525. https://doi.org/10.3390/polym14245525
Chicago/Turabian StyleDelatorre, Fabíola Martins, Gabriela Fontes Mayrinck Cupertino, Michel Picanço Oliveira, Felipe da Silva Gomes, Luciene Paula Roberto Profeti, Demetrius Profeti, Mário Guimarães Júnior, Márcia Giardinieri de Azevedo, Daniel Saloni, and Ananias Francisco Dias Júnior. 2022. "A Novel Approach to Charcoal Fine Waste: Sustainable Use as Filling of Polymeric Matrices" Polymers 14, no. 24: 5525. https://doi.org/10.3390/polym14245525
APA StyleDelatorre, F. M., Cupertino, G. F. M., Oliveira, M. P., da Silva Gomes, F., Profeti, L. P. R., Profeti, D., Júnior, M. G., de Azevedo, M. G., Saloni, D., & Júnior, A. F. D. (2022). A Novel Approach to Charcoal Fine Waste: Sustainable Use as Filling of Polymeric Matrices. Polymers, 14(24), 5525. https://doi.org/10.3390/polym14245525









