Pineapple Agro-Industrial Biomass to Produce Biomedical Applications in a Circular Economy Context in Costa Rica
Abstract
1. Introduction
2. Discussion
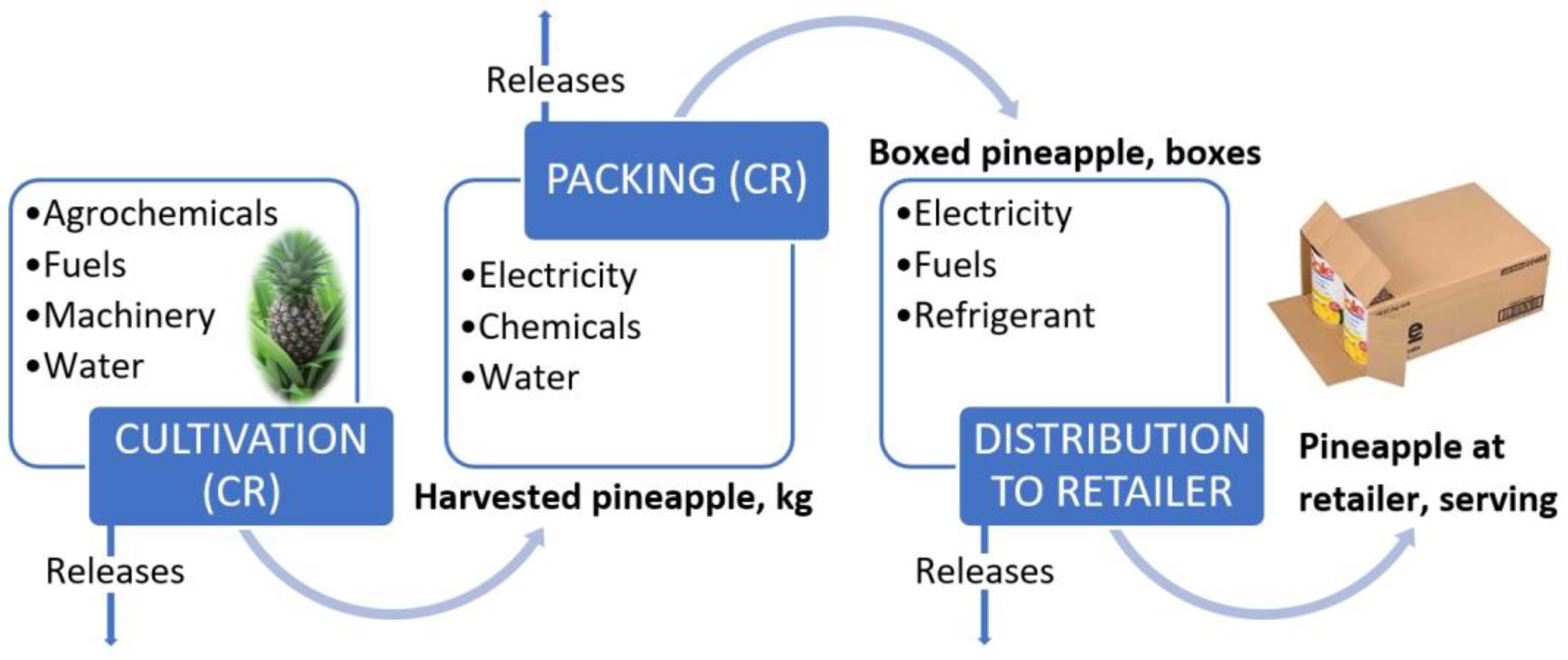
2.1. Pineapple Production
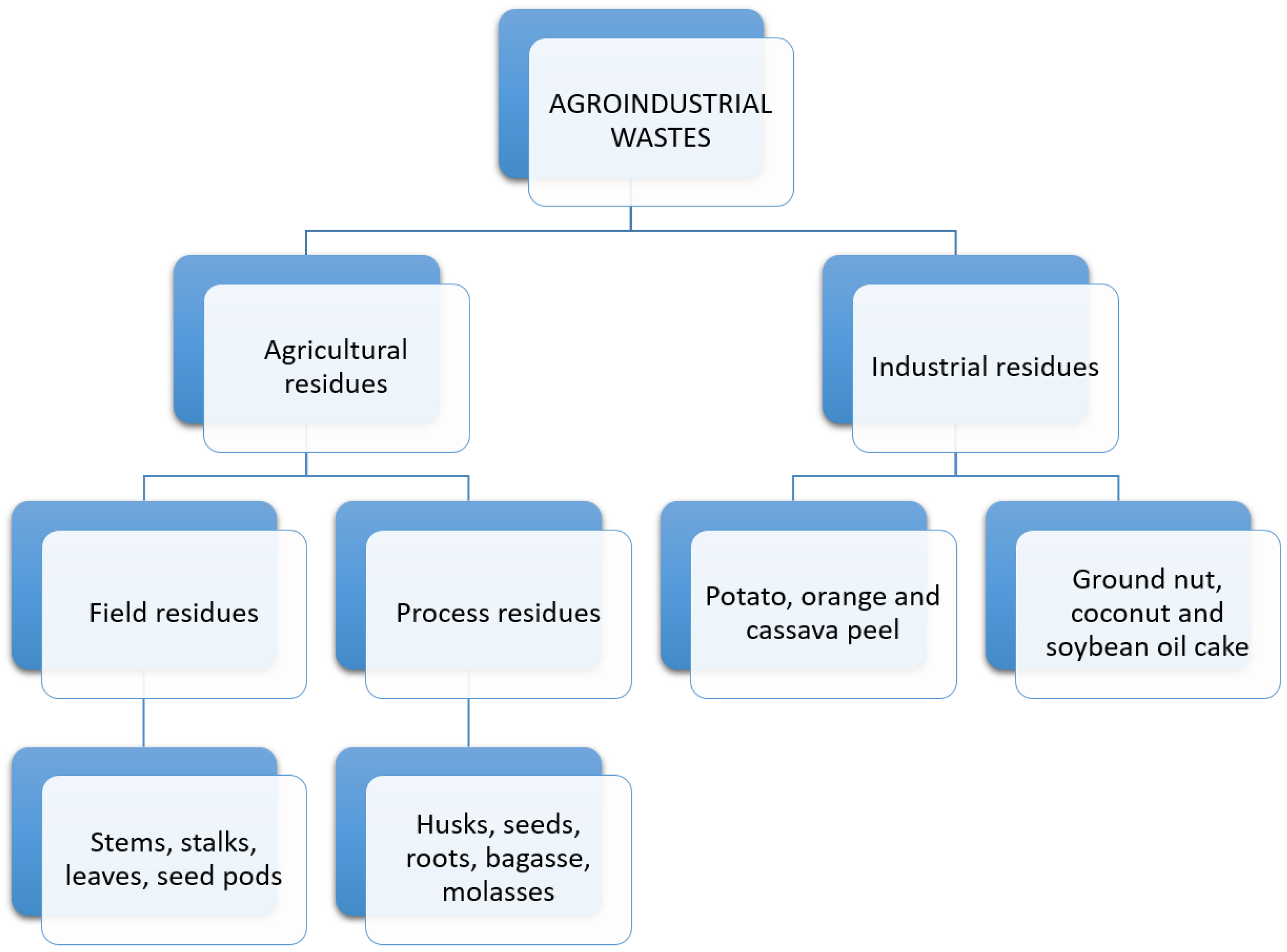
2.2. Agricultural Waste
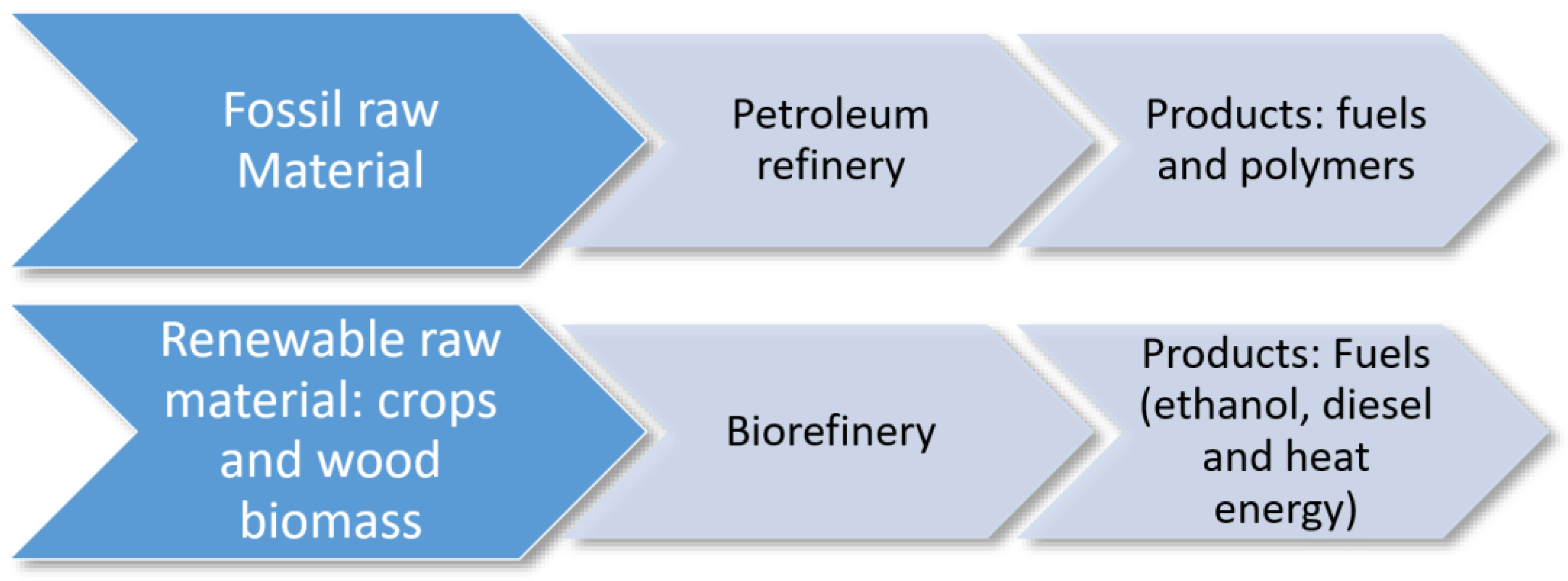
2.3. Biorefinery and the Importance Thereof for Costa Rica and Other Pineapple Producing Countries
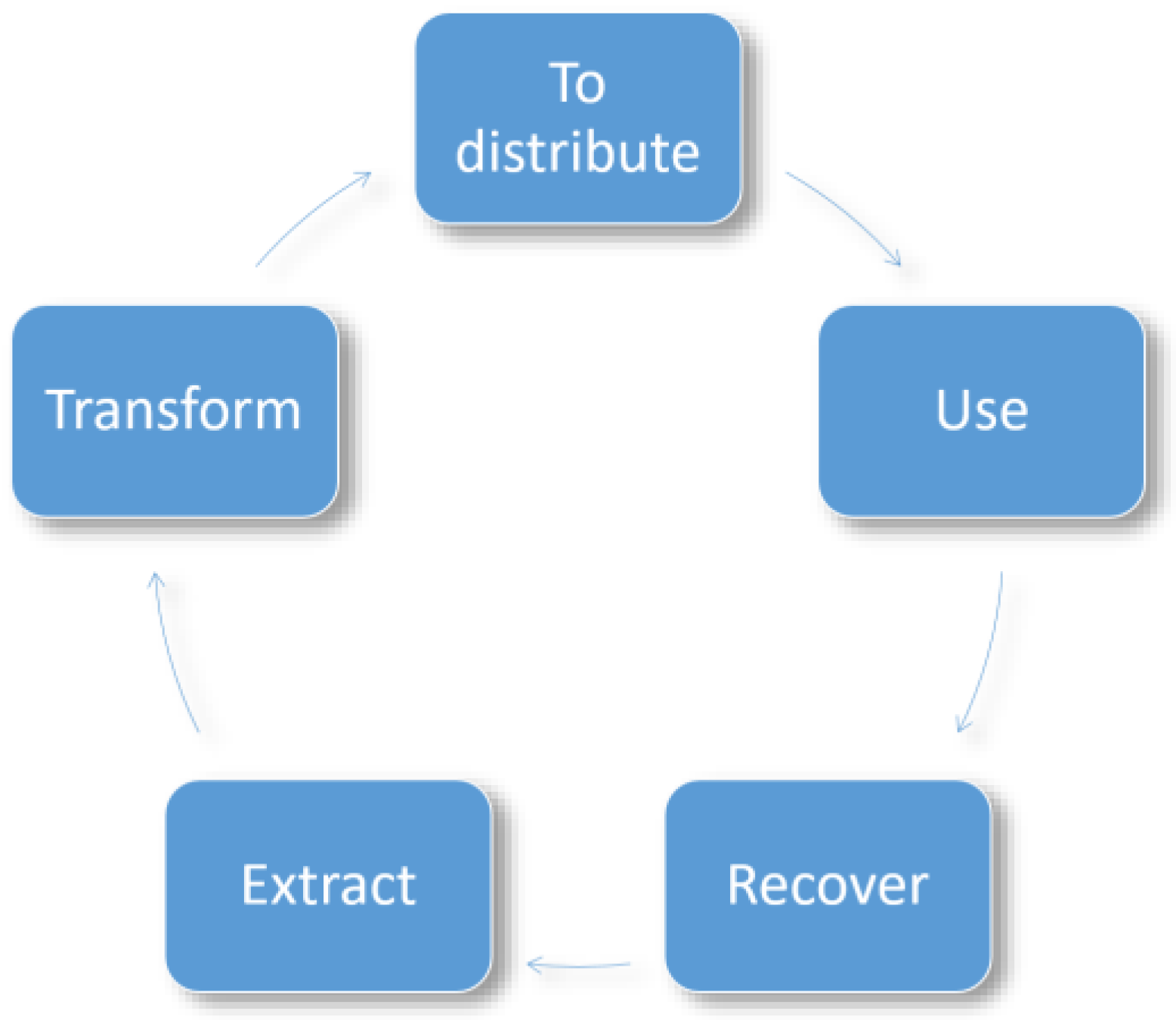
2.4. Circular Economy
2.5. Main Raw Materials from Pineapple Biomass
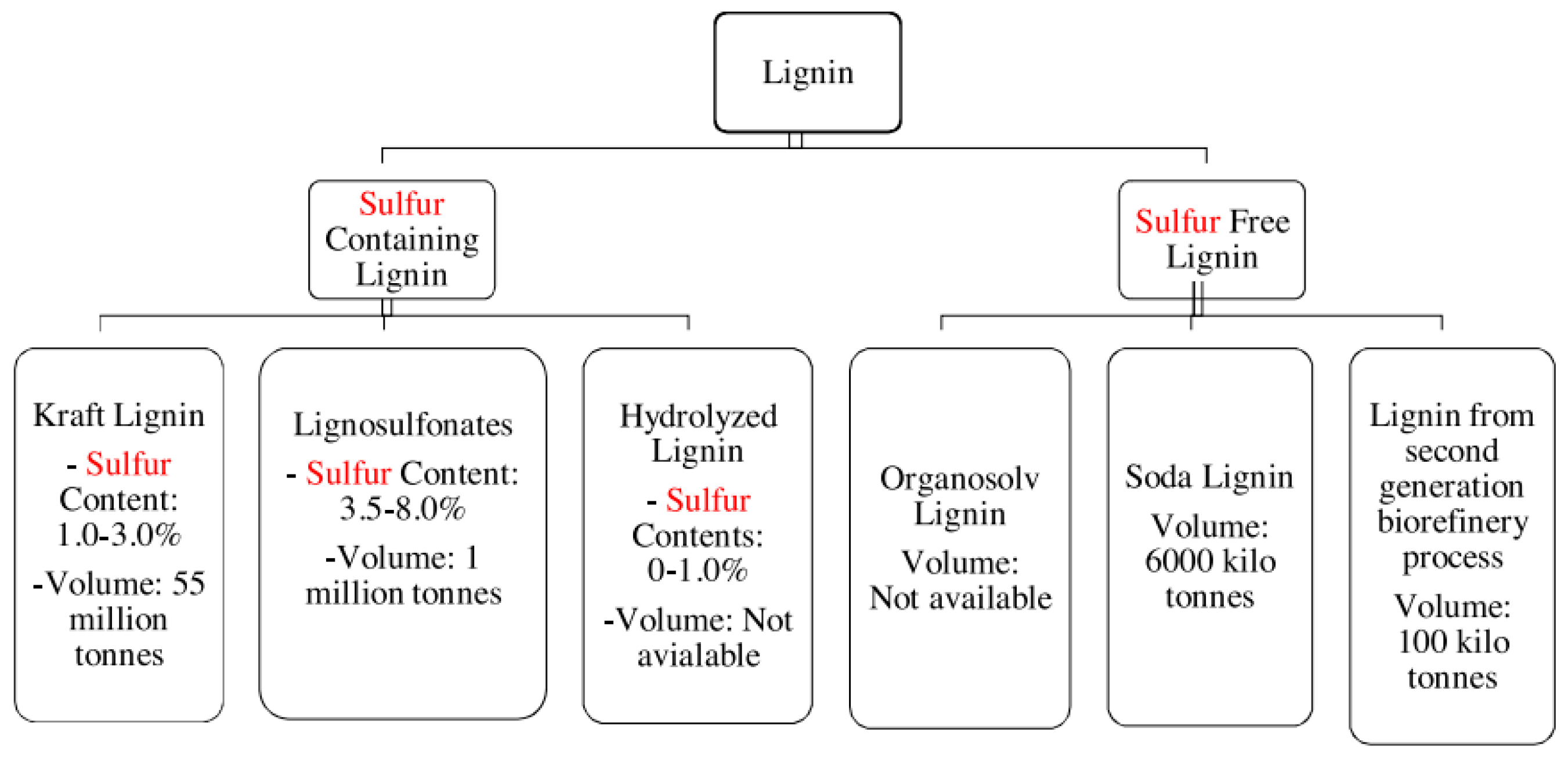
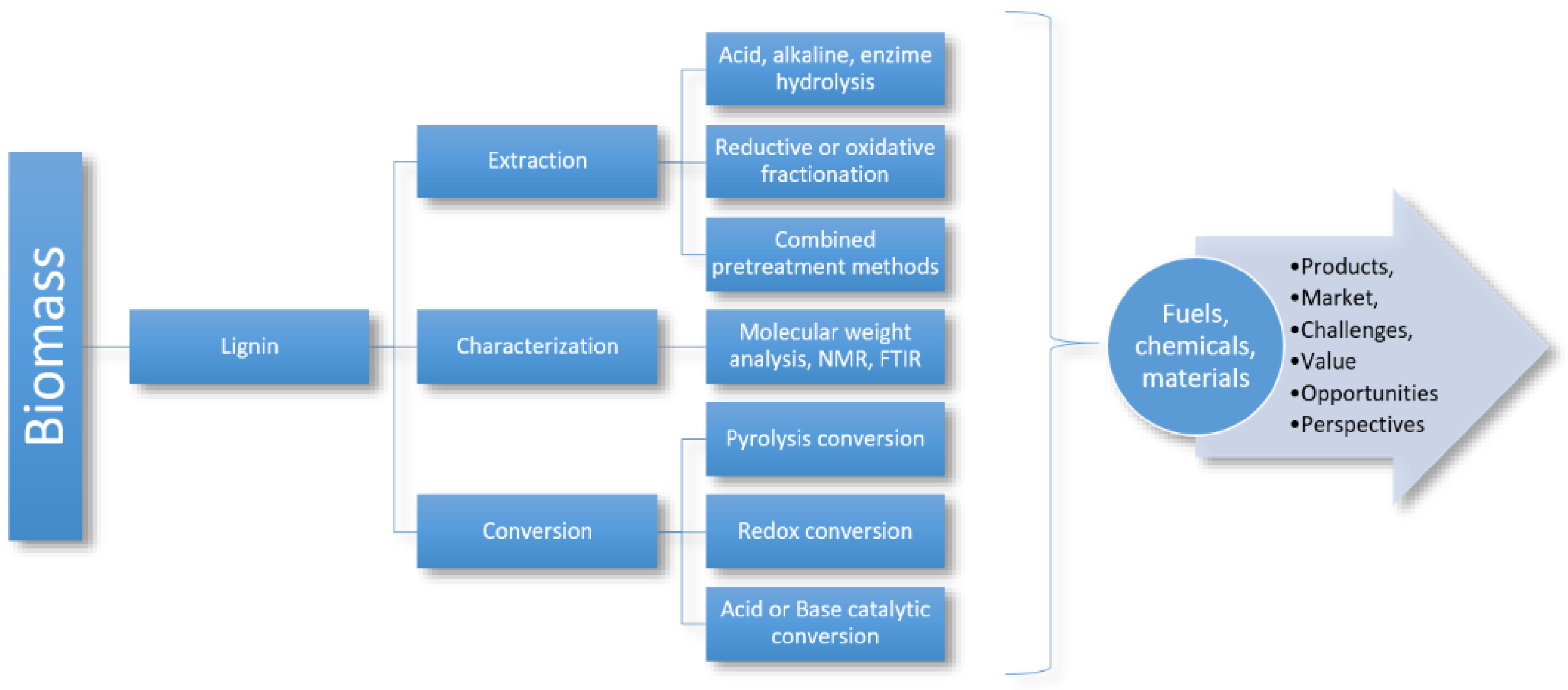
2.5.1. Lignin
2.5.2. Cellulose
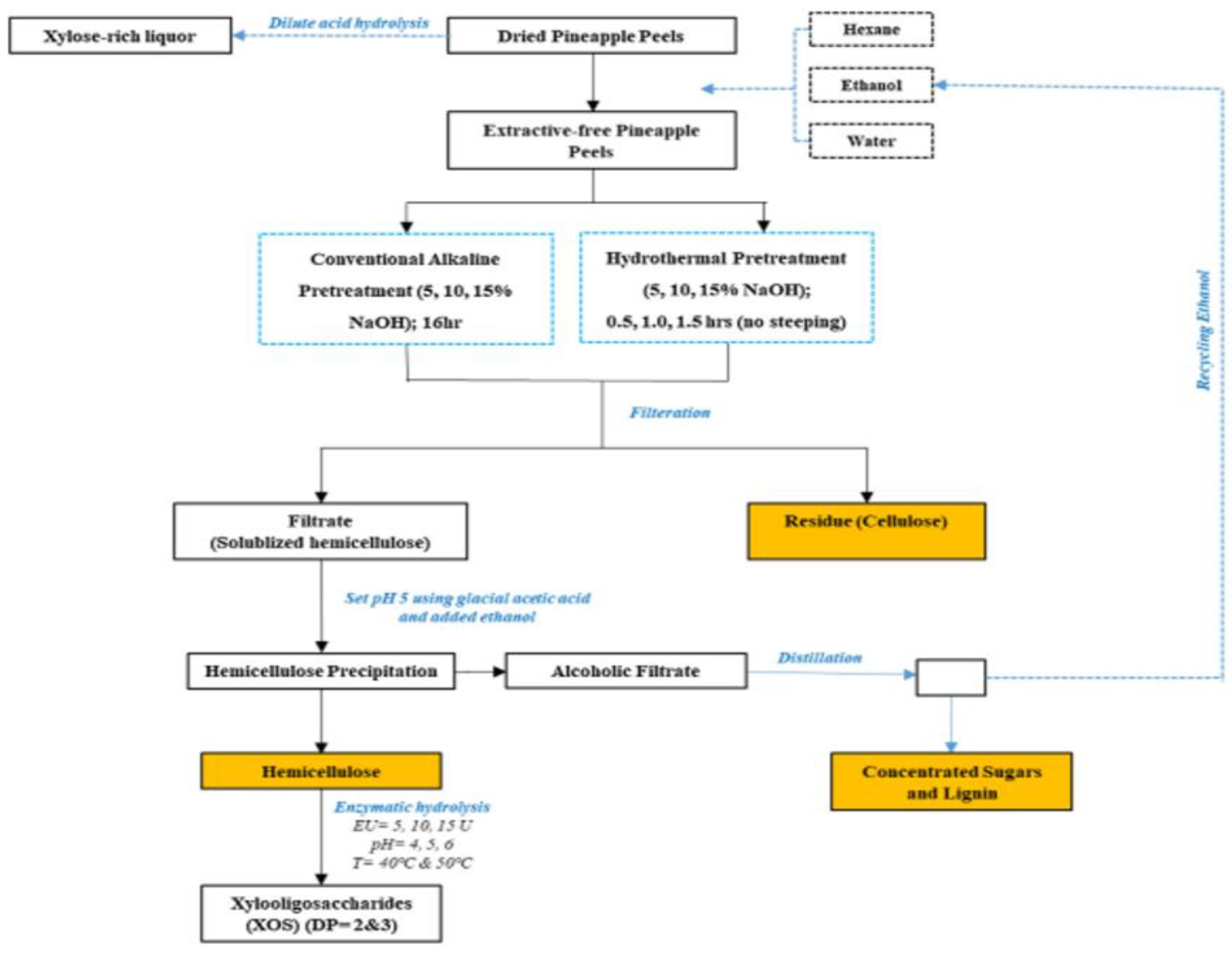
2.5.3. Hemicellulose
2.6. Medical Applications
2.6.1. Hydrogels Force Cellulose Sources
2.6.2. Enzymatic Products
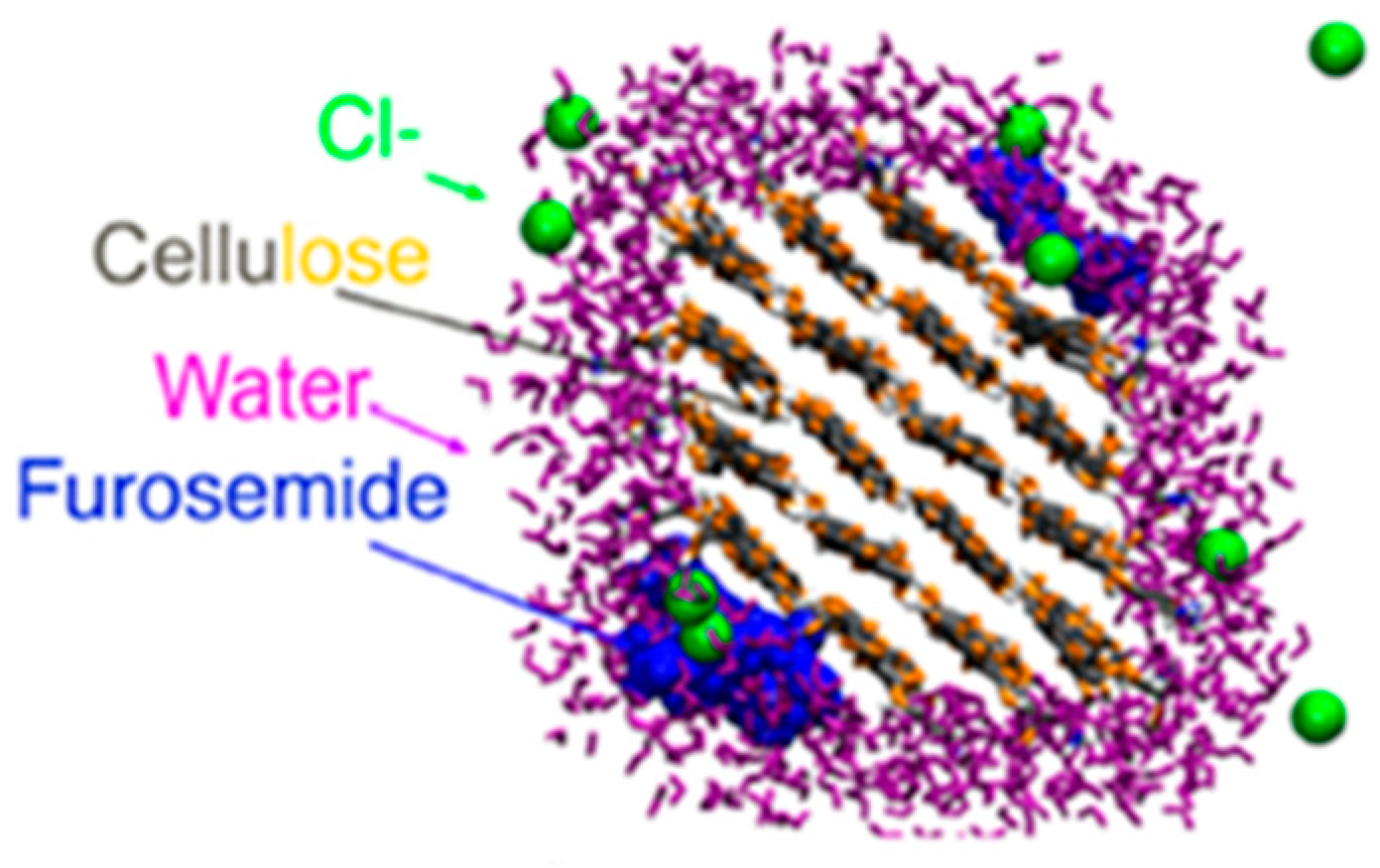
2.6.3. Drug Delivery
2.6.4. Antimicrobial Products
2.7. Modeling and Simulation Studies of Pineapple Derivates
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cámara Nacional de Productores y Exportadores de Piña—CANAPEP. Available online: https://bit.ly/3CCeY5R (accessed on 26 August 2021).
- Maglianesi-Sandoz, M.A. Development of pineapple trees in Costa Rica and its impacts on natural and agro-urban ecosystems. Biocenosis 2013, 27, 1–2. [Google Scholar]
- Morales-Abarca, L.F. Production and yield of the pineapple (Ananas comosus) crop in Costa Rica, 1984–2014 period. Agronegocios 2018, 4, 2. [Google Scholar]
- Costa Rican Foreign Trade Promoter (PROCOMER) Data. Available online: https://www.procomer.com/exportador/documentos/anuario-estadistico-2020/ (accessed on 20 March 2022).
- Ingwersen, W.W. Life cycle assessment of fresh pineapple from Costa Rica. J. Clean. Prod. 2012, 35, 152–163. [Google Scholar] [CrossRef]
- Hernández-Chaverri, R.A.; Prado-Barragán, L.A. Impacto y oportunidades de biorrefinería de los desechos agrícolas del cultivo de piña (Ananas comosus) en Costa Rica. Cuad. Investig. UNED 2018, 10, 455–468. [Google Scholar] [CrossRef][Green Version]
- Sadh, P.K.; Duhan, S.; Duhan, J.S. Agro-industrial wastes and their utilization using solid-state fermentation: A review. Bioresour. Bioprocess. 2018, 5, 1. [Google Scholar] [CrossRef]
- Morales-Ramos, R. Universidad Nacional de Costa Rica. Available online: https://repositorio.una.ac.cr/handle/11056/18733 (accessed on 24 May 2022).
- Conferencia de las Naciones Unidas sobre Comercio y Desarrollo-UNCTAD. Available online: https://bit.ly/2W0jbkh (accessed on 30 August 2021).
- Cerrato, I. CEDIA. Available online: https://bit.ly/3lOwtcu (accessed on 30 August 2021).
- Cámara Nacional de Productores y Exportadores de Piña—CANAPEP. Available online: https://bit.ly/3lOQ3VY (accessed on 7 September 2021).
- Food and Agriculture Organization—FAO. Available online: http://www.fao.org/3/cb0834es/CB0834ES.pdf (accessed on 31 August 2021).
- Red Latinoamericana de Investigadores en Cadenas Globales de Mercancías—REDILACG. Available online: http://www.redilacg.org/pina-cr (accessed on 30 August 2021).
- Vinyet, N. PINDECO. Available online: https://bit.ly/3o2DZ6a (accessed on 31 August 2021).
- Vargas-Bolaños, C.; Miller-Granados, C.; Arguedas-González, C. CENAT. Available online: https://hdl.handle.net/20.500.12337/7892 (accessed on 31 August 2021).
- Vargas-Céspedes, A.; Morales, M.; Watler, A.; Vignola, R. MAG. Available online: http://www.mag.go.cr/bibliotecavirtual/F01-8166.pdf (accessed on 24 May 2022).
- PROCOMER. Available online: https://bit.ly/3o0v1Xr (accessed on 30 August 2021).
- Fernández-Vicente, G. Soluciones Verdes. Available online: https://bit.ly/3zT2wha (accessed on 7 September 2021).
- Miranda-Durán, S.; Porras-Reyes, L.; Schmidt-Durán, A. Evaluation of agro-industrial residues produced in Costa Rica for a low-cost culture medium using Bacillus subtilis 168. Rev. Tecnol. Marcha 2020, 33, 15–25. [Google Scholar] [CrossRef]
- INEC. Available online: https://bit.ly/3lKrvgR (accessed on 6 September 2021).
- Cury, K.; Aguas, Y.; Martinez, A.; Olivero, R.; Ch, L.C. Residuos agroindustriales su impacto, manejo y aprovechamiento. Rev. Colomb. Cienc. Anim.-RECIA 2017, 9, 122–132. [Google Scholar] [CrossRef]
- Blanco-Obando, E.E. Cultivo de piña y conflictos socio-ambientales en la región Atlántico/Caribe, Costa Ri-ca, 1990–2017. Athenea Digit. 2020, 20, 3. [Google Scholar]
- Salas-Murillo, O. Universidad de Costa Rica. Available online: https://bit.ly/2Vnr9nm (accessed on 6 September 2021).
- Vargas-Castro, E. Dirección de Gestión de Calidad Ambiental DIGECA. Available online: https://bit.ly/3zORauG (accessed on 6 September 2021).
- Díaz-Porras, R.A.; Monge-Gutiérrez, M.J. Centro Internacional de Política Económica para el Desarrollo Sostenible—CINPE. Available online: https://repositorio.una.ac.cr/bitstream/handle/11056/15266/Cuaderno%20de%20Politica%20Econ%20002-2019.pdf?sequence=1&isAllowed=y (accessed on 24 May 2022).
- López-Herrera, M.; WingChing-Jones, R.; Rojas-Bourrillón, A. Meta-análisis de los subproductos de piña (Ananas comosus) para la alimentación animal. Agron. Mesoam. 2014, 25, 383–391. [Google Scholar] [CrossRef][Green Version]
- Clauser, N.M. Estudio Técnico-Económico de la Biorrefinería de Los Residuos de Industrialización Primaria de la Madera y Agroindustriales. Doctoral Dissertation, Universidad Nacional de Misiones, Puerto Iguazú, Argentina, 2019. [Google Scholar]
- Carrillo-González, G.; Torres-Bustillos, L.G. Biorrefinerías y Economía Circular, 1st ed.; División de Ciencias Sociales y Humanidades: Leon, Mexico, 2019. [Google Scholar]
- Chávez-Sifontes, M. La Biomasa–Fuente Alternativa de Combustibles y Compuestos Químicos. An. Quím. RSEQ 2019, 115, 399. [Google Scholar]
- Ministerio de Ciencia, Innovación, Tecnología y Telecomunicaciones—MICITT. Available online: https://eurocamaracr.com/costa-rica-lanza-estrategia-nacional-de-bioeconomia/# (accessed on 24 May 2022).
- SCIJ. Available online: https://bit.ly/3AtoNCM (accessed on 20 September 2021).
- Fundación para la Economía Circular. Available online: https://bit.ly/3nRK5qd (accessed on 20 September 2021).
- Alvarado-Alcázar, D. Gestión de Proyectos de Construcción bajo una perspectiva de Economía Circular. Master Dissertation, Instituto Tecnológico de Costa Rica, Cartago, Costa Rica, 2019. [Google Scholar]
- Prieto-Sandoval, V.; Jaca-García, C.; Ormazabal-Goenaga, M. Economía circular: Relación con la evolución del concepto de sostenibilidad y estrategias para su implementación. Mem. Investig. Ing. 2017, 15, 85–95. [Google Scholar]
- Alcubilla, L. El País. Available online: https://bit.ly/3EHbvFe (accessed on 20 September 2021).
- Morató, J.; Tollin, N.; Jiménez, L. Situación y Evolución de la Economía Circular en España, 1st ed.; Fundación COTEC para la Innovación: Madrid, Spain, 2017. [Google Scholar]
- Chávez-Sifontes, M.; Domine, M.E. Lignina, estructura y aplicaciones: Métodos de despolimerización para la obtención de derivados aromáticos de interés industrial. Av. Cienc. Ing. 2013, 4, 15–46. [Google Scholar]
- Tobimatsu, Y.; Schuetz, M. Lignin polymerization: How do plants manage the chemistry so well? Curr. Opin. Biotechnol. 2019, 56, 75–81. [Google Scholar] [CrossRef]
- Wang, H.; Pu, Y.; Ragauskas, A.; Yang, B. From lignin to valuable products–strategies, challenges, and prospects. Bioresour. Technol. 2019, 271, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Gillet, S.; Aguedo, M.; Petitjean, L.; Morais, A.R.C.; da Costa Lopes, A.M.; Łukasik, R.M.; Anastas, P.T. Lignin transformations for high value applications: Towards targeted modifications using green chemistry. Green Chem. 2017, 19, 4200–4233. [Google Scholar] [CrossRef]
- Bajwa, D.S.; Pourhashem, G.; Ullah, A.H.; Bajwa, S.G. A concise review of current lignin production, applications, products and their environmental impact. Ind. Crops Prod. 2019, 139, 111526. [Google Scholar] [CrossRef]
- Iram, A.; Berenjian, A.; Demirci, A. A Review on the Utilization of Lignin as a Fermentation Substrate to Produce Lignin-Modifying Enzymes and Other Value-Added Products. Molecules 2021, 26, 2960. [Google Scholar] [CrossRef]
- Ye, K.; Liu, Y.; Wu, S.; Zhuang, J. A review for lignin valorization: Challenges and perspectives in catalytic hydrogenolysis. Ind. Crops Prod. 2021, 172, 114008. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Y.; Li, X.; Chen, L.; Li, G.; Li, X. Lignin valorization: A novel in situ catalytic hydrogenolysis method in alkaline aqueous solution. Energy Fuels 2018, 32, 7643–7651. [Google Scholar] [CrossRef]
- Qi, S.-C.; Hayashi, J.-I.; Kudo, S.; Zhang, L. Catalytic hydrogenolysis of kraft lignin to monomers at high yield in alkaline water. Green Chem. 2017, 19, 2636–2645. [Google Scholar] [CrossRef]
- Zhang, J.-W.; Lu, G.-P.; Cai, C. Self-hydrogen transfer hydrogenolysis of β-O-4 linkages in lignin catalyzed by MIL-100 (Fe) supported Pd–Ni BMNPs. Green Chem. 2017, 19, 4538–4543. [Google Scholar] [CrossRef]
- Barta, K.; Matson, T.D.; Fettig, M.L.; Scott, S.L.; Iretskii, A.V.; Ford, P.C. Catalytic disassembly of an organosolv lignin via hydrogen transfer from supercritical methanol. Green Chem. 2010, 12, 1640–1647. [Google Scholar] [CrossRef]
- Shu, R.; Zhang, Q.; Ma, L.; Xu, Y.; Chen, P.; Wang, C.; Wang, T. Insight into the solvent, temperature and time effects on the hydrogenolysis of hydrolyzed lignin. Bioresour. Technol. 2016, 221, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Park, J.; Kim, U.J.; Choi, J.W. Conversion of lignin to phenol-rich oil fraction under supercritical alcohols in the presence of metal catalysts. Energy Fuels 2015, 29, 5154–5163. [Google Scholar] [CrossRef]
- Kong, L.; Liu, C.; Gao, J.; Wang, Y.; Dai, L. Efficient and controllable alcoholysis of Kraft lignin catalyzed by porous zeolite-supported nickel-copper catalyst. Bioresour. Technol. 2019, 276, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Qi, Z.; Liu, Y.; Xia, H.; Li, L.; Huang, Q.; Li, C. Tungsten-based catalysts for lignin depolymerization: The role of tungsten species in C–O bond cleavage. Catal. Sci. Technol. 2019, 9, 2144–2151. [Google Scholar] [CrossRef]
- Shao, Y.; Xia, Q.; Dong, L.; Liu, X.; Han, X.; Parker, S.F.; Wang, Y. Selective production of arenes via direct lignin upgrading over a niobium-based catalyst. Nat. Commun. 2017, 8, 16104. [Google Scholar] [CrossRef]
- Xin, Y.; Dong, L.; Guo, Y.; Liu, X.; Hu, Y.; Wang, Y. Correlation of the catalytic performance with Nb2O5 surface properties in the hydrodeoxygenation of lignin model compound. J. Catal. 2019, 375, 202–212. [Google Scholar] [CrossRef]
- Korányi, T.I.; Huang, X.; Coumans, A.E.; Hensen, E.J. Synergy in lignin upgrading by a combination of Cu-based mixed oxide and Ni-phosphide catalysts in supercritical ethanol. ACS Sustain. Chem. Eng. 2017, 5, 3535–3543. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Kuhn, E.; Tucker, M.P.; Yang, B. Production of Jet Fuel-Range Hydrocarbons from Hydrodeoxygenation of Lignin over Super Lewis Acid Combined with Metal Catalysts. ChemSusChem 2018, 11, 285–291. [Google Scholar] [CrossRef]
- Gañán, P.; Zuluaga, R.; Castro, C.; Restrepo-Osorio, A.; Cock, J.V.; Osorio, M.; Montoya, Ú.; Vélez, L.; Álvarez, C.; Correa, C.; et al. Celulosa: Un polímero de siempre con mucho futuro. Rev. Colomb. Mater. 2017, 11, 11. [Google Scholar]
- Keller, S. American Chemical Society—ACS. Available online: https://bit.ly/3vhNf8i (accessed on 15 October 2021).
- Kargarzadeh, H.; Ioelovich, M.; Ahmad, I.; Thomas, S.; Dufresne, A. Methods for extraction of nanocellulose from various sources. In Handbook of Nanocellulose and Cellulose Nanocomposites; John Wiley & Sons: Hoboken, NJ, USA, 2017; Volume 1, pp. 1–51. [Google Scholar]
- Gupta, V.K.; Carrott, P.J.M.; Singh, R.; Chaudhary, M.; Kushwaha, S. Cellulose: A review as natural, modified and activated carbon adsorbent. Bioresour. Technol. 2016, 216, 1066–1076. [Google Scholar]
- Zhao, X.-Q.; Wahid, F.; Cui, J.-X.; Wang, Y.-Y.; Zhong, C. Cellulose-based special wetting materials for oil/water separation: A review. Int. J. Biol. Macromol. 2021, 185, 890–906. [Google Scholar] [CrossRef]
- Morales-Vázquez, J.G.; López-Zamora, L.; Aguilar-Uscanga, M.G. Ethanol Production from Pineapple Waste. Ph.D. Thesis, Instituto Tecnológico de Orizaba, Orizaba, Mexico, 2020. [Google Scholar]
- Sánchez-Pardo, M.E.; Ramos-Cassellis, M.E.; Mora-Escobedo, R.; Jiménez-García, E. Chemical characterization of the industrial residues of the pineapple (Ananas comosus). J. Agric. Chem. Environ. 2014, 3, 53–56. [Google Scholar]
- Segura, A.; Manriquez, A.; Santos, D.; Ambriz, E.; Casas, P.; Muñoz, A.S. Obtención de bioetanol a partir de residuos de cascara de piña (Ananas comosus). Jovenes Cienc. 2020, 8, 1–8. [Google Scholar]
- Camacho, M.; Ureña, Y.R.C.; Lopretti, M.; Carballo, L.B.; Moreno, G.; Alfaro, B.; Baudrit, J.R.V. Synthesis and characterization of nanocrystalline cellulose derived from pineapple peel residues. J. Renew. Mater. 2017, 5, 271–279. [Google Scholar] [CrossRef]
- Rambabu, N.; PAnthapulakkal, S.; Sain, M.; Dalai, A.K. Production of nanocellulose fibers from pinecone biomass: Evaluation and optimization of chemical and mechanical treatment conditions on mechanical properties of nanocellulose films. Ind. Crops Prod. 2016, 83, 746–754. [Google Scholar] [CrossRef]
- Mahardika, M.; Abral, H.; Kasim, A.; Arief, S.; Asrofi, M. Production of nanocellulose from pineapple leaf fibers via high-shear homogenization and ultrasonication. Fibers 2018, 6, 28. [Google Scholar] [CrossRef]
- Moon, R.J.; Schueneman, G.T.; Simonsen, J. Overview of cellulose nanomaterials, their capabilities and applications. JOM 2016, 68, 2383–2394. [Google Scholar] [CrossRef]
- Rivas-Siota, S. Biomass Hemicellulose Valorization. Doctoral Dissertation, Universidade de Vigo, Vigo, Spain, 2014. [Google Scholar]
- Dutta, S.K.; Chakraborty, S. Multiscale dynamics of hemicellulose hydrolysis for biofuel production. Ind. Eng. Chem. Res. 2019, 58, 8963–8978. [Google Scholar] [CrossRef]
- Sun, S.C.; Wang, P.F.; Cao, X.F.; Sun, S.N.; Wen, J.L. An integrated pretreatment for accelerating the enzymatic hydrolysis of poplar and improving the isolation of co-produced hemicelluloses. Ind. Crops Prod. 2021, 173, 114101. [Google Scholar] [CrossRef]
- Banerjee, S.; Patti, A.F.; Ranganathan, V.; Arora, A. Hemicellulose based biorefinery from pineapple peel waste: Xylan extraction and its conversion into xylooligosaccharides. Food Bioprod. Process. 2019, 117, 38–50. [Google Scholar] [CrossRef]
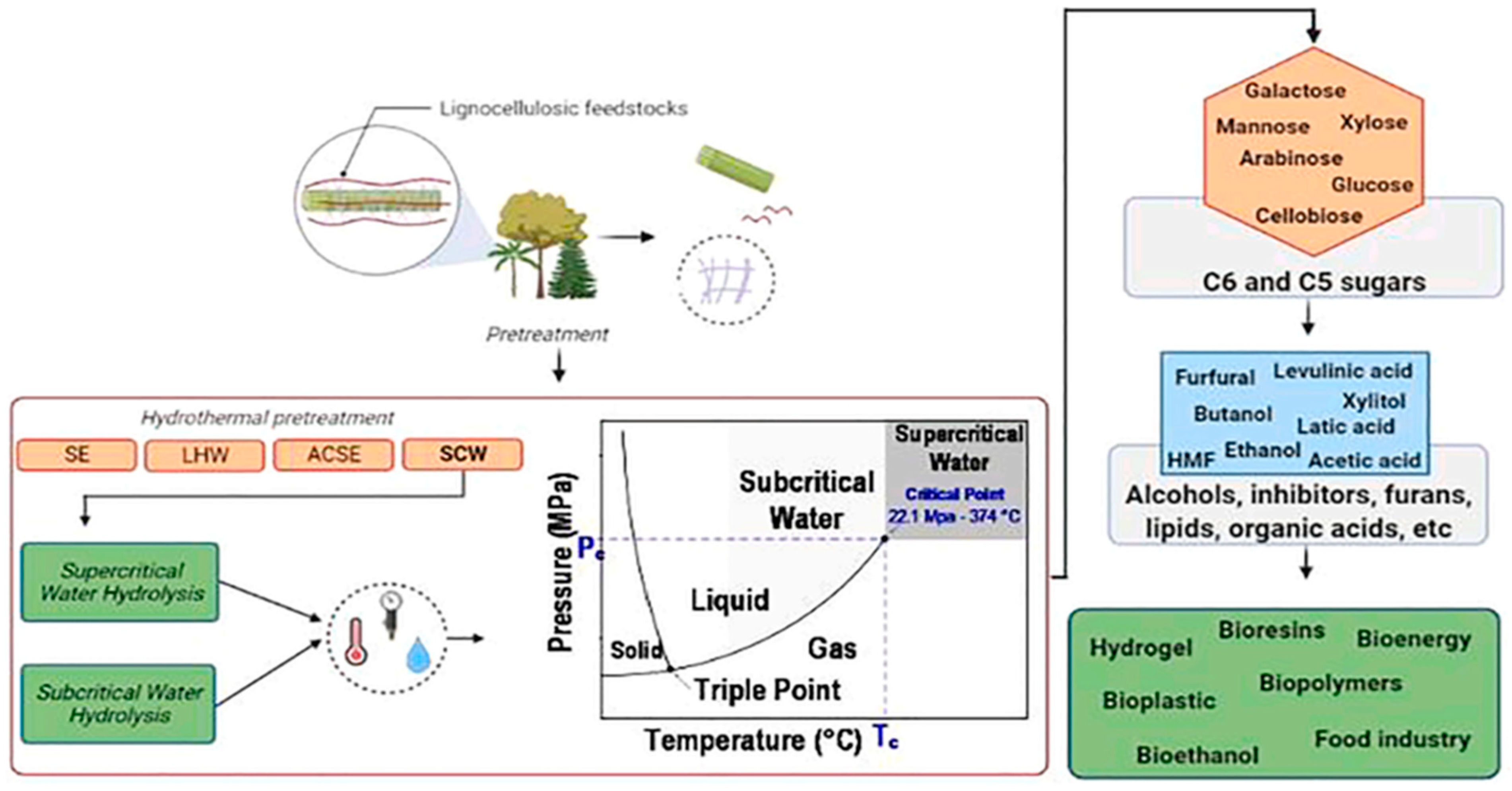
- Scapini, T.; dos Santos, M.S.; Bonatto, C.; Wancura, J.H.; Mulinari, J.; Camargo, A.F.; Klanovicz, N.; Zabot, G.L.; Tres, M.V.; Fongaro, G.; et al. Hydrothermal pretreatment of lignocellulosic biomass for hemicellulose recovery. Bioresour. Technol. 2021, 342, 126033. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Zou, Y.; Arno, M.C.; Chen, S.; Wang, T.; Gao, J.; Dove, A.P.; Du, J. Hydrogel scaffolds for differentiation of adipose-derived stem cells. Chem. Soc. Rev. 2017, 46, 6255–6275. [Google Scholar] [CrossRef]
- Bai, T.; Li, J.; Sinclair, A.; Imren, S.; Merriam, F.; Sun, F.; O’Kelly, M.B.; Nourigat, C.; Jain, P.; Delrow, J.J.; et al. Expansion of primitive human hematopoietic stem cells by culture in a zwitterionic hydrogel. Nat. Med. 2019, 25, 1566–1575. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in biology and medicine: From molecular principles to bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Xu, C.; Liu, L.; Renneckar, S.; Jiang, F. Chemically and physically crosslinked lignin hydrogels with antifouling and antimicrobial properties. Ind. Crops Prod. 2021, 170, 113759. [Google Scholar] [CrossRef]
- Sun, X.F.; Zhang, T.; Wang, H.H. Hemicelluloses-based hydrogels. In Plant and Algal Hydrogels for Drug Delivery and Regenerative Medicine; Woodhead Publishing: Thorston, UK, 2021; pp. 181–216. [Google Scholar]
- Liu, X.; Luan, S.; Li, W. Utilization of waste hemicelluloses lye for superabsorbent hydrogel synthesis. Int. J. Biol. Macromol. 2019, 132, 954–962. [Google Scholar] [CrossRef]
- Kumar, A.; Han, S.S. Efficacy of Bacterial Nanocellulose in Hard Tissue Regeneration: A Review. Materials 2021, 14, 4777. [Google Scholar] [CrossRef]
- An, S.-J.; Lee, S.-H.; Huh, J.-B.; Jeong, S.I.; Park, J.-S.; Gwon, H.-J.; Kang, E.-S.; Jeong, C.-M.; Lim, Y.-M. Preparation and characterization of resorbable bacterial cellulose membranes treated by electron beam irradiation for guided bone regeneration. Int. J. Mol. Sci. 2017, 18, 2236. [Google Scholar] [CrossRef]
- Bassi, A.P.F.; Bizelli, V.F.; Brasil, L.F.D.M.; Pereira, J.C.; Al-Sharani, H.M.; Momesso, G.A.C.; Faverani, L.P.; Lucas, F.D.A. Is the Bacterial Cellulose Membrane Feasible for Osteopromotive Property? Membranes 2020, 10, 230. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Li, Y.; Sun, J.; Zhang, H.; Chen, L.; Chen, B.; Yang, H.; Wang, Z. The osteogenesis of bacterial cellulose scaffold loaded with bone morphogenetic protein-2. Biomaterials 2012, 33, 6644–6649. [Google Scholar] [CrossRef] [PubMed]
- Noh, Y.K.; Da Costa, A.D.S.; Park, Y.S.; Du, P.; Kim, I.H.; Park, K. Fabrication of bacterial cellulose-collagen composite scaffolds and their osteogenic effect on human mesenchymal stem cells. Carbohydr. Polym. 2019, 219, 210–218. [Google Scholar] [CrossRef]
- Cai, Z.; Kim, J. Preparation and characterization of novel bacterial cellulose/gelatin scaffold for tissue regeneration using bacterial cellulose hydrogel. J. Nanotechnol. Eng. Med. 2010, 1, 021002. [Google Scholar] [CrossRef]
- Kheiry, E.V.; Parivar, K.; Baharara, J.; Bazzaz, B.S.F.; Iranbakhsh, A. The osteogenesis of bacterial cellulose scaffold loaded with fisetin. Iran. J. Basic Med. Sci. 2018, 21, 965–971. [Google Scholar]
- Hong, L.; Wang, Y.L.; Jia, S.R.; Huang, Y.; Gao, C.; Wan, Y.Z. Hydroxyapatite/bacterial cellulose composites synthesized via a biomimetic route. Mater. Lett. 2006, 60, 1710–1713. [Google Scholar] [CrossRef]
- Liu, J.; Chinga-Carrasco, G.; Cheng, F.; Xu, W.; Willför, S.; Syverud, K.; Xu, C. Hemicellulose-reinforced nanocellulose hydrogels for wound healing application. Cellulose 2016, 23, 3129–3143. [Google Scholar] [CrossRef]
- Angueta-López, N.O. Diseño de Una Planta Para la Obtención de Bromelina a Partir de Residuos de Piña. Bachelor’s Thesis, Escuela Politécnica Nacional, Quito, Ecuador, 2019. [Google Scholar]
- Chakraborty, A.J.; Mitra, S.; Tallei, T.E.; Tareq, A.M.; Nainu, F.; Cicia, D.; Dhama, K.; Emran, T.B.; Simal-Gandara, J.; Capasso, R. Bromelain a potential bioactive compound: A comprehensive overview from a pharmacological perspective. Life 2021, 11, 317. [Google Scholar] [CrossRef]
- Juhasz, B.; Thirunavukkarasu, M.; Pant, R.; Zhan, L.; Penumathsa, S.V.; Secor, E.R., Jr.; Srivastava, S.; Raychaudhuri, U.; Menon, V.P.; Otani, H.; et al. Bromelain induces cardioprotection against ischemia-reperfusion injury through Akt/FOXO pathway in rat myocardium. Am. J. Physiol.-Heart Circ. Physiol. 2008, 294, 1365–1370. [Google Scholar] [CrossRef]
- Mahato, D.; Sharma, H.P. Phytochemical profiling and antioxidant activity of Leea macrophylla Roxb. ex Hornem.-in vitro study. Indian J. Tradit. Knowl. (IJTK) 2019, 18, 493–499. [Google Scholar]
- López-Rodríguez, M. Uso en Quemaduras de Bromelina, un Complejo Enzimático Obtenido de Ananas comosus. Diploma Thesis, Universidad de Sevilla, Sevilla, Spain, 2020. [Google Scholar]
- Brito, A.M.; Oliveira, V.; Icimoto, M.Y.; Nantes-Cardoso, I.L. Collagenase Activity of Bromelain Immobilized at Gold Nanoparticle Interfaces for Therapeutic Applications. Pharmaceutics 2021, 13, 1143. [Google Scholar] [CrossRef] [PubMed]
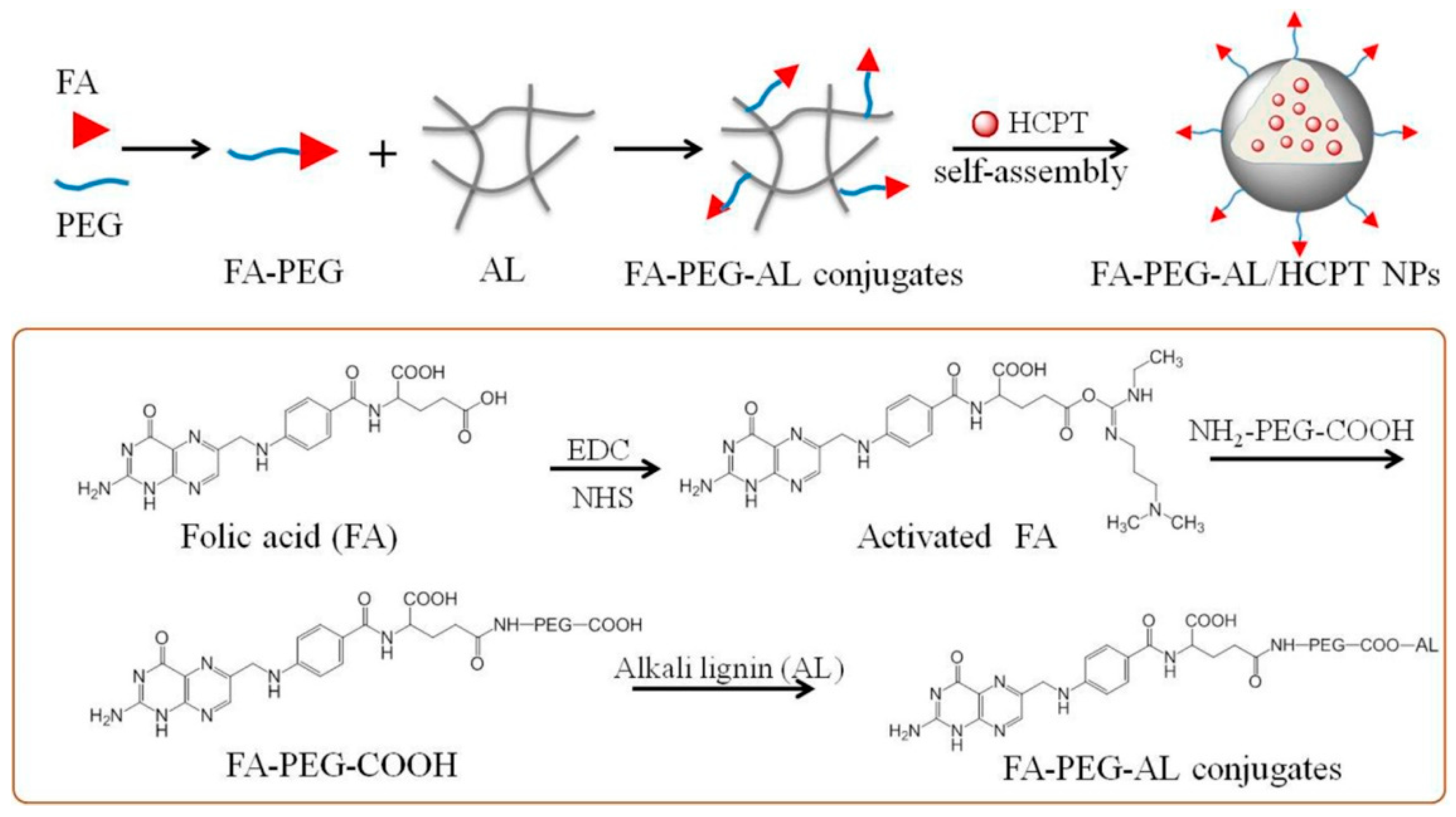
- Liu, K.; Zheng, D.; Lei, H.; Liu, J.; Lei, J.; Wang, L.; Ma, X. Development of novel lignin-based targeted polymeric nanoparticle platform for efficient delivery of anticancer drugs. ACS Biomater. Sci. Eng. 2018, 4, 1730–1737. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Dempere, L.A.; Tong, Z. Synthesis of pH-responsive lignin-based nanocapsules for controlled release of hydrophobic molecules. ACS Sustain. Chem. Eng. 2016, 4, 5204–5211. [Google Scholar] [CrossRef]
- Cui, Y.; Lawoko, M.; Svagan, A.J. High value use of technical lignin. fractionated lignin enables facile synthesis of microcapsules with various shapes: Hemisphere, bowl, mini-tablets, or spheres with single holes. ACS Sustain. Chem. Eng. 2020, 8, 13282–13291. [Google Scholar] [CrossRef]
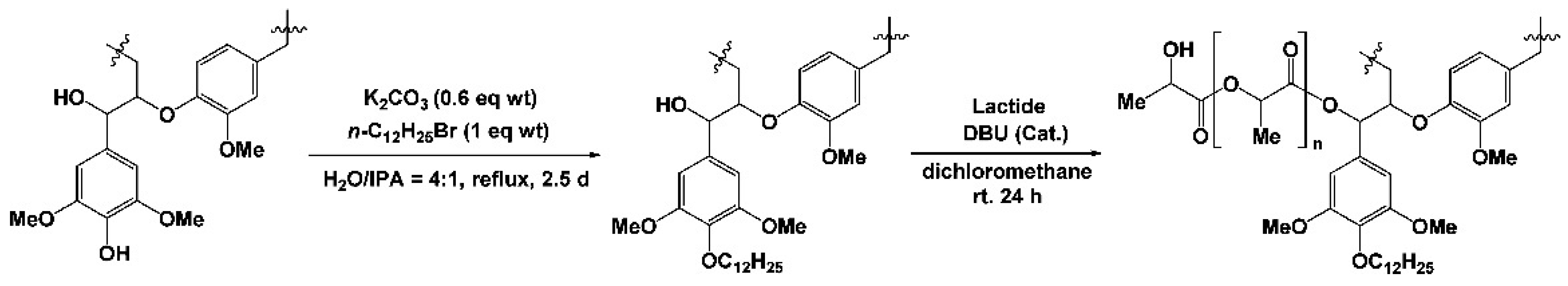
- Kai, D.; Ren, W.; Tian, L.; Chee, P.L.; Liu, Y.; Ramakrishna, S.; Loh, X.J. Engineering poly (lactide)–lignin nanofibers with antioxidant activity for biomedical application. ACS Sustain. Chem. Eng. 2016, 4, 5268–5276. [Google Scholar] [CrossRef]
- Edgar, K.J.; Heinze, T.; Buchanan, C.M. Polysaccharide Materials: Performance by Design, 1st ed.; American Chemical Society: Washington, DC, USA, 2009. [Google Scholar]
- Hansen, K.; Kim, G.; Desai, K.-G.H.; Patel, H.; Olsen, K.F.; Curtis-Fisk, J.; Tocce, E.; Jordan, S.; Schwendeman, S.P. Feasibility investigation of cellulose polymers for mucoadhesive nasal drug delivery applications. Mol. Pharm. 2015, 12, 2732–2741. [Google Scholar] [CrossRef]
- Zharfan, R.S.; Purwono, P.B.; Mustika, A. Antimicrobial activity of pineapple (Ananas comosus L. Merr) extract against multidrug-resistant of Pseudomonas aeruginosa: An in vitro study. Indones. J. Trop. Infect. Dis. 2017, 6, 118–123. [Google Scholar] [CrossRef]
- Hasan, A.; Saha, T.; Ahmed, T. Antibaterial activity of the extracts of pineapple and pomelo against five different pathogenic bacterial isolates. Stamford J. Microbiol. 2021, 11, 1–6. [Google Scholar] [CrossRef]
- Istiqomah, N.; Ramadhani, A.; Ningrum, R.; Purwat, E. Ethanol extract analysis of steam pineapple (Ananas comosus. L) and its application as antibacterial agent: In vitro and silico studies. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021; Volume 886, p. 012019. [Google Scholar]
- Castro, M.; Vázquez, M.; Cordero, J.; Benavides, M.; González, J.; López, M.; Vega, J.; Corrales, Y. Bacterial anti-adhesive films of PDMS coated with microstructures of biogenic silica rosettes extracted from pineapple peels residues. Surf. Interfaces 2022, 20, 101881. [Google Scholar] [CrossRef]
- Trentin, L.N.; Pereira, C.S.; Silveira, R.L.; Hill, S.; Sorieul, M.; Skaf, M.S. Nanoscale Wetting of Crystalline Cellulose. Biomacromolecules 2021, 22, 4251–4261. [Google Scholar] [CrossRef]
- Mehandzhiyski, A.; Rolland, N.; Garg, M.; Wohlert, J.; Linares, M.; Zozoulenko, I. A novel supra coarse-grained model for cellulose. Cellulose 2020, 27, 4221–4234. [Google Scholar] [CrossRef]
- Dong, R.Y.; Dong, Y.; Li, Q.; Wan, C. Ballistic-diffusive phonon transport in cellulose nanocrystals by ReaxFF molecular dynamics simulations. Int. J. Heat Mass Transf. 2020, 148, 119155. [Google Scholar] [CrossRef]
- Lopez-Sanchez, P.; Rincon, M.; Wang, D.; Brulhart, S.; Stokes, J.R.; Gidley, M.J. Micromechanics and poroelasticity of hydrated cellulose networks. Biomacromolecules 2014, 15, 2274–2284. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, S.; Chen, P.; Larsson, P.A.; Thielemans, W.; Wohlert, J.; Svagan, A.J. Toward Improved Understanding of the Interactions between Poorly Soluble Drugs and Cellulose Nanofibers. Langmuir 2018, 34, 5464–5473. [Google Scholar] [CrossRef]
- Ning, L.; Ma, H.; Shi, Q.; Li, X.; Ren, J. Effects of Nanocellulose on the Structure of Collagen: Insights from Molecular Dynamics Simulation and Umbrella Sampling. Res. Sq. 2022, 1–16. [Google Scholar] [CrossRef]
- Lee, K.K.; Low, D.Y.S.; Foo, M.L.; Yu, L.J.; Choong, T.S.Y.; Tang, S.Y.; Tan, K.W. Molecular Dynamics Simulation of Nanocellulose-Stabilized Pickering Emulsions. Polymers 2021, 13, 668. [Google Scholar] [CrossRef]
- Vilela, C.; Freire, C.S.R.; Araujo, C.; Rudic, S.; Silvestre, A.J.D.; Vaz, P.D.; Ribeiro-Claro, P.J.A.; Nolasco, M.M. Understanding the Structure and Dynamics of Nanocellulose-Based Composites with Neutral and ionic Poly(methacrylate) Derivatives using Inelastic Neutron Scattering and DFT Calculations. Molecules 2020, 25, 1689. [Google Scholar] [CrossRef]
- Zarei, S.; Niad, M.; Raanaei, H. The removal of mercury ion pollution by using Fe3O4-nanocellulose: Synthesis, characterizations and DFT studies. J. Hazard. Mater. 2018, 344, 258–273. [Google Scholar] [CrossRef]
- Chen, P.; Lo Re, G.; Berglund, L.; Wohlert, J. Surface modification effects on nanocellulose—Molecular dynamics simulations using umbrella sampling and computational alchemy. J. Mater. Chem. A 2020, 8, 23617–23627. [Google Scholar] [CrossRef]
- Pang, W.C.; Ramli, A.N.M.; Hamid, A.A.A. Comparative modelling studies of fruit bromelain using molecular dynamics simulation. J. Mol. Model. 2020, 26, 142. [Google Scholar] [CrossRef]
- Rani, A.; Taha, M.; Venkatesu, P.; Lee, M.J. Coherent Experimental and Simulation Approach To Explore the Underlying Mechanism of Denaturation of Stem Bromelain in Osmolytes. J. Phys. Chem. B 2017, 121, 6456–6470. [Google Scholar] [CrossRef] [PubMed]
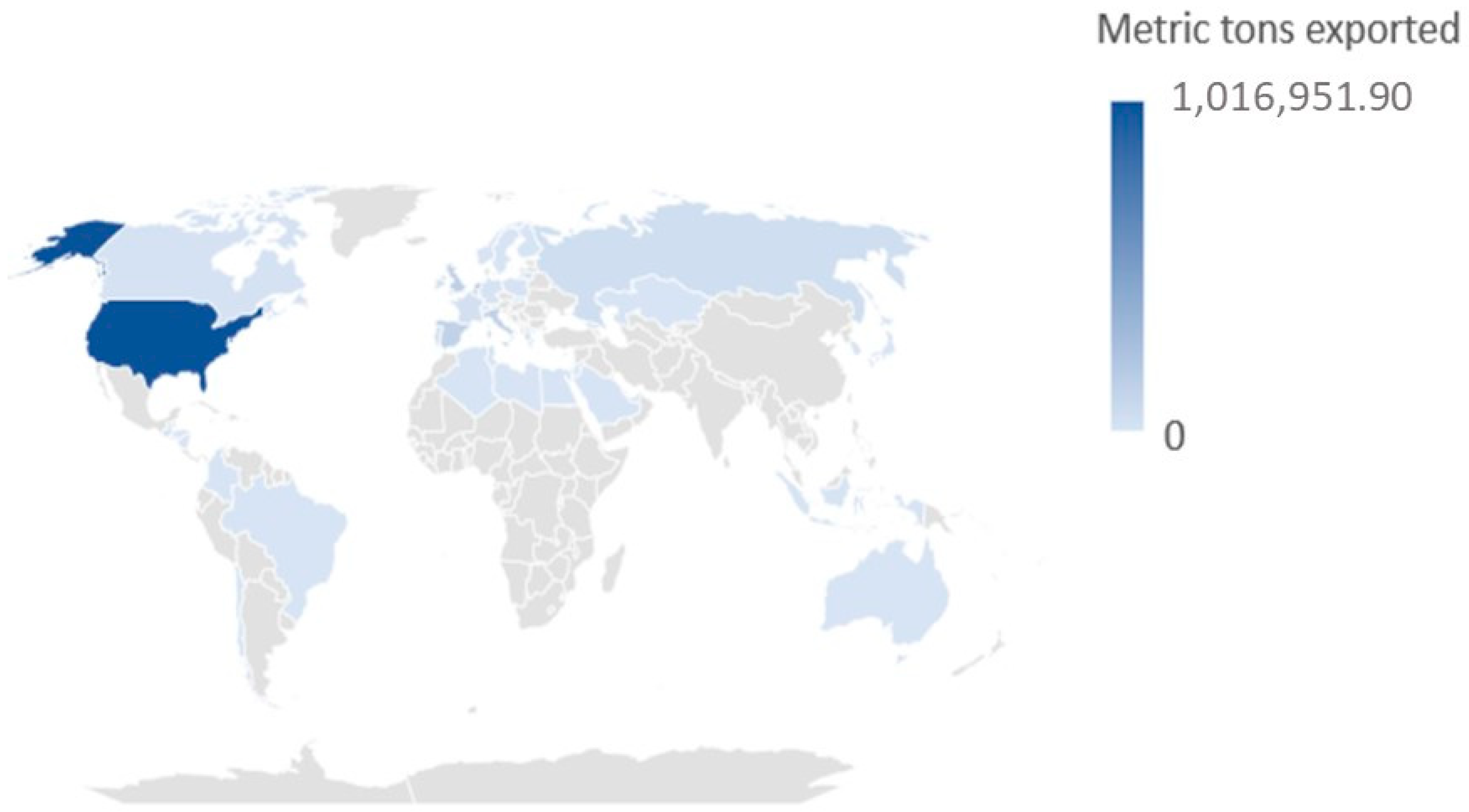
| Destination | Metric Tons | Percentage |
|---|---|---|
| USA | 1,016,951.89 | 51.01 |
| European Union | 715,785.82 | 35.90 |
| United Kingdom | 142,386.54 | 7.14 |
| Russia | 45,673.77 | 2.29 |
| Turkey | 28,414.61 | 1.43 |
| Other countries | 44,554.93 | 2.23 |
| Total | 1,993,767.56 | 100.00 |
| Feed | Solvent | Catalyst | Reaction Conditions | Major Product | Major Product Yield (%) | Ref. | |
|---|---|---|---|---|---|---|---|
| Temp. (°C) | Time (h) | ||||||
| Poplar wood sawdust | Alkaline aqueous solution | NiAl alloy | 220 | 3 | Aromatic monomers | 76 | [44] |
| Kraft lignin | Alkaline water | Ni/ZSM-5 zeolite | 200 | 4 | Monomers | 17 | [45] |
| Birch sawdust | water | Pd1Ni4/MIL-100(Fe) | 130–180 | 6 | Phenol + acetophenone | 56.3 | [46] |
| Organosolv lignin | Methanol | Cu20PMO | 300 | 24 | Liquid phase products | 26.3 | [47] |
| Hydrolyzed lignin | Methanol | PD/C, CrCl3 | 300 | 4 | Monomers | 60–86 | [48] |
| Asian lignin | Ethanol | Pt/C | 350 | 2/3 | Lignin-oil | 77.4 | [49] |
| Kraft lignin | Isopropanol | Ni-Cu/H-Beta | 330 | 3 | cycloalkanes | 40.39 | [50] |
| beech dioxasolv lignin | Methanol | W/C | 200 | 6 | Lignin. Oil | 56.4 | [51] |
| Birch lignin | Aqueous solution | Ru/Nb2O5 | 250 | 20 | Liquid hydrocarbons | 35.5 | [52] |
| Enzymatic lignin | Water | Ru/Nb2O5 | 250 | 20 | Hydrocarbons | 99.6 | [53] |
| Alkali lignin | Ethanol | Ni2P/SiO2 + CuMgAl2O3 | 340 | 4 | Monomers | 53 | [54] |
| Corn stover lignin | N-octane | Ru/Al2O3 + Hf (OTf) | 250 | 4 | Hydrocarbons | >30 | [55] |
| Lignocellulosic Material | Cellulose (%) | Hemicellulose (%) | Lignin (%) |
|---|---|---|---|
| Pineapple crown (leaves) | 45.53 ± 1.17 | 21.88 ± 0.22 | 13.88 ± 1.70 |
| Pineapple peel | 40.55 ± 1.02 | 28.69 ± 0.35 | 10.01 ± 0.38 |
| Pineapple core | 24.53 ± 1.68 | 28.53 ± 1.37 | 5.78 ± 0.429 |
| Pineapple stubble | 32.2 | 21 | 2.83 |
| Composition | Scaffold Form | Cell/Drug/Biomolecule | Features | Ref |
|---|---|---|---|---|
| BNC | Membrane | NIH-3T3 fibroblast cells | Suitable biocompatibility and enhanced cell viability, remarkable formation of large new bone area | [80] |
| BNC | Membrane | - | Low biocompatibility and large amount of mature connective tissue in filling the defect (adult male rat) | [81] |
| BNC | Nanofibrous | BMP-2, C2C12 cells | Suitable biocompatibility and osteogenic differentiation of fibroblast-like cells, and BNC scaffold with BMP-2 exhibited more bone formation and higher calcium content than that of BNC only | [82] |
| BNC/collagen | Fibrous | UCB-MSCs and NIH3T3 cells, BMP-2, dexamethasone | Favorable cell adhesion and growth, more up-regulated osteogenic markers and remarkably uplifted proteins and calcium deposition, and positive signals (-smooth muscle actin) for neovascularization | [83] |
| BNC/Gel | Nanofibrous | NIH-3T3 fibroblast cells | Decreased crystallinity and improved thermal stability, Enhanced Young’s modulus and decreased tensile strength, and excellent biocompatibility | [84] |
| BNC/fisetin | Nanofibrous | BMSCs | Decreased crystallinity and improved thermal stability, Enhanced Young’s modulus and decreased tensile strength, and excellent biocompatibility | [85] |
| BNC/HAp | Nanofibrous | - | 3D porous network with homogenous precipitation of carbonated-HAp crystals on BC fibers | [86] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amores-Monge, V.; Goyanes, S.; Ribba, L.; Lopretti, M.; Sandoval-Barrantes, M.; Camacho, M.; Corrales-Ureña, Y.; Vega-Baudrit, J.R. Pineapple Agro-Industrial Biomass to Produce Biomedical Applications in a Circular Economy Context in Costa Rica. Polymers 2022, 14, 4864. https://doi.org/10.3390/polym14224864
Amores-Monge V, Goyanes S, Ribba L, Lopretti M, Sandoval-Barrantes M, Camacho M, Corrales-Ureña Y, Vega-Baudrit JR. Pineapple Agro-Industrial Biomass to Produce Biomedical Applications in a Circular Economy Context in Costa Rica. Polymers. 2022; 14(22):4864. https://doi.org/10.3390/polym14224864
Chicago/Turabian StyleAmores-Monge, Valeria, Silvia Goyanes, Laura Ribba, Mary Lopretti, Manuel Sandoval-Barrantes, Melissa Camacho, Yendry Corrales-Ureña, and José Roberto Vega-Baudrit. 2022. "Pineapple Agro-Industrial Biomass to Produce Biomedical Applications in a Circular Economy Context in Costa Rica" Polymers 14, no. 22: 4864. https://doi.org/10.3390/polym14224864
APA StyleAmores-Monge, V., Goyanes, S., Ribba, L., Lopretti, M., Sandoval-Barrantes, M., Camacho, M., Corrales-Ureña, Y., & Vega-Baudrit, J. R. (2022). Pineapple Agro-Industrial Biomass to Produce Biomedical Applications in a Circular Economy Context in Costa Rica. Polymers, 14(22), 4864. https://doi.org/10.3390/polym14224864






