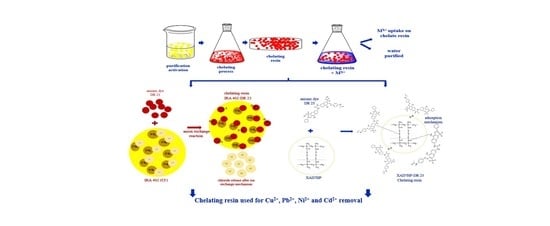
New Chelate Resins Prepared with Direct Red 23 for Cd2+, Ni2+, Cu2+ and Pb2+ Removal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Equipment
2.3. Preparation of Working Solution
2.4. Analytical Method Used for DR 23 and M2+ Determination
2.4.1. UV-Vis Determination of DR 23
2.4.2. AAS Method for M2+ Determination
2.5. Procedure for Determination of the Interaction Time for Optimal Adsorption of DR 23 in IRA 402 (Cl−) and XAD7HP Resins
2.6. Procedure for Studies the Influence of pH
2.7. Procedure for Obtained Chelate IRA 402 (Cl−) Resin
2.8. Procedure for Obtained Chelate Amberlite XAD7HP Resin
2.9. Procedure for Evaluate the Stability of Chelate Resins
2.10. Procedure for Studies on the Influence of Contact Time between Cd2+, Ni2+, Cu2+ and Pb2+ and Chelate Resins
2.11. Procedure for Cd2+, Ni2+, Cu2+ and Pb2+ Removal Using Chelate Resins
2.12. Procedure for M2+ Recovery from IRA 402-DR 23-M2+ and XAD7HP-DR 23-M2+
3. Results and Discussions
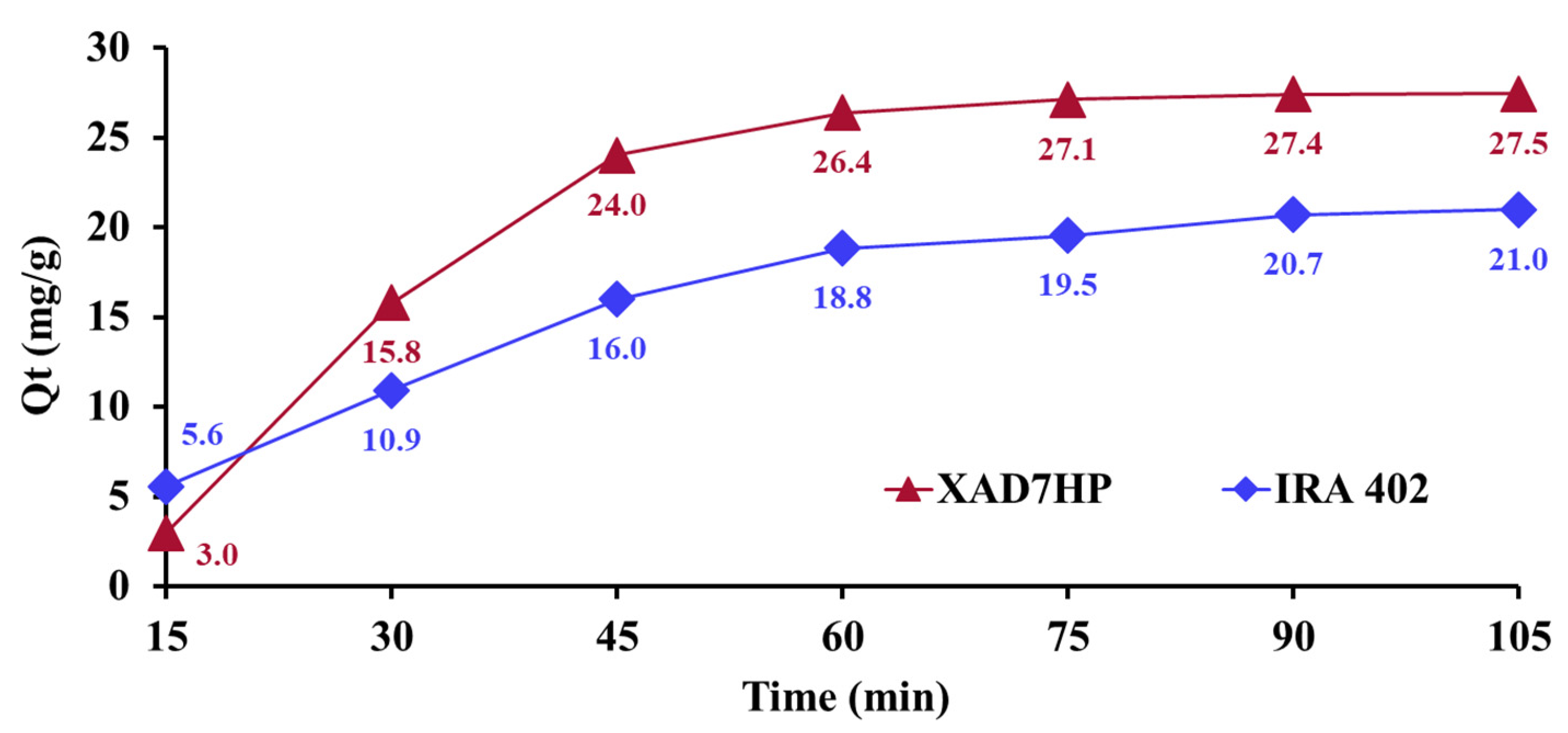
3.1. Effect of Interaction Time (DR 23-Resin)
3.2. The Influence of pH
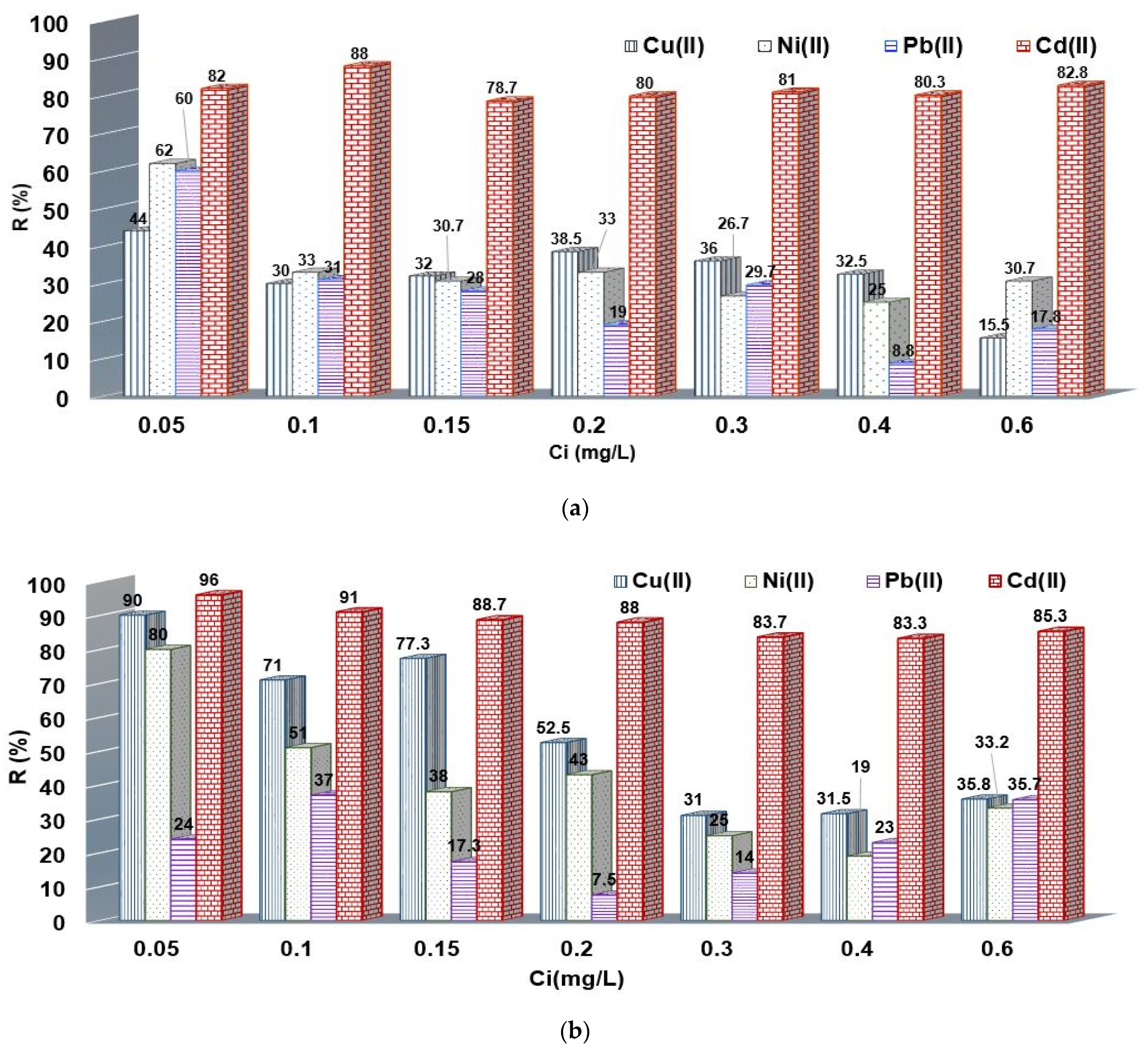
3.3. Influence of Chelate Agent Concentration
3.4. Effect of Desorption Agent
3.5. Adsorption Kinetics
3.6. Application of Chelating Resin toward M2+ Removal
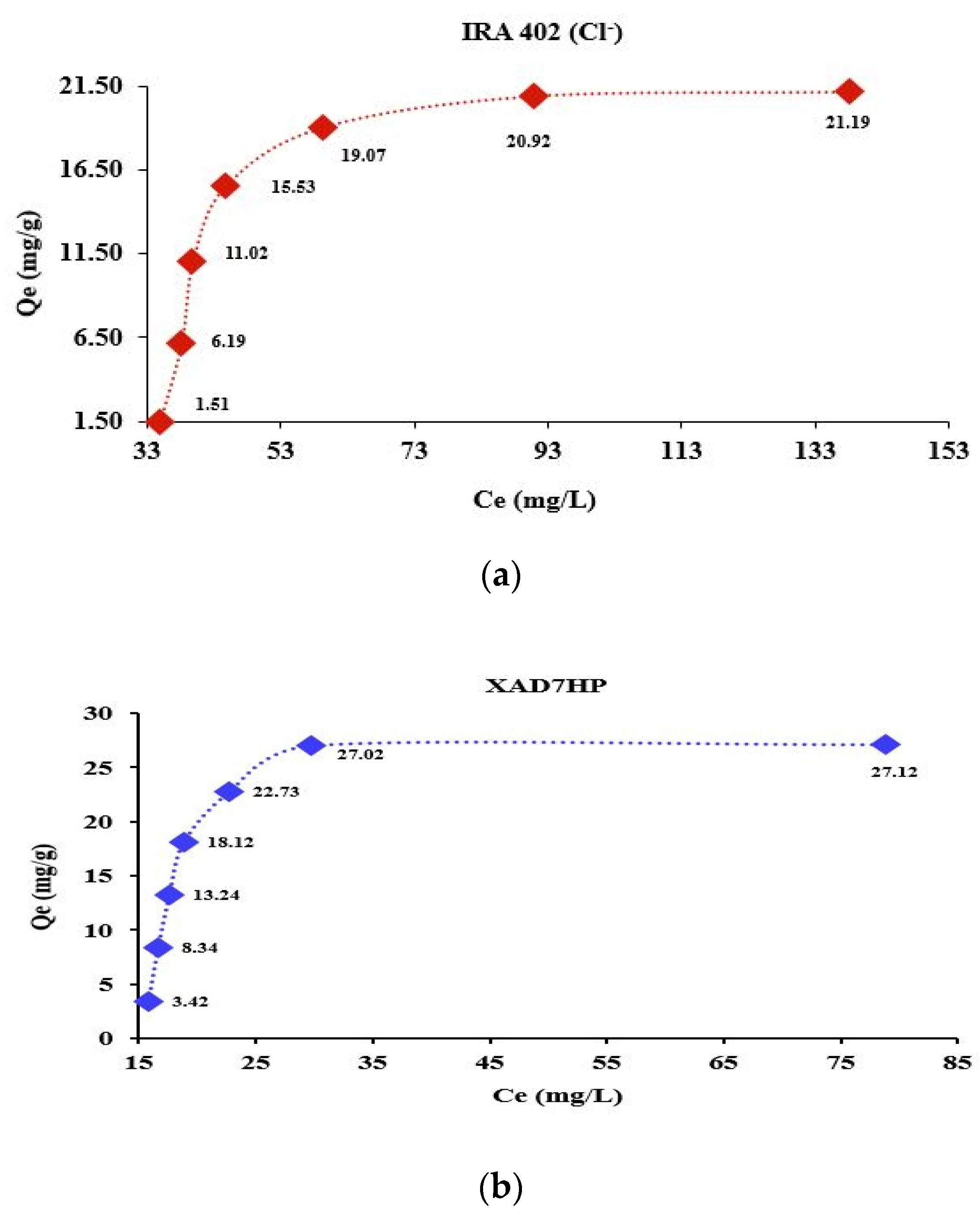
3.7. Adsorption Isotherms
3.8. Effect of the Desorption Agent from the Chelating Resins Loaded with M2+
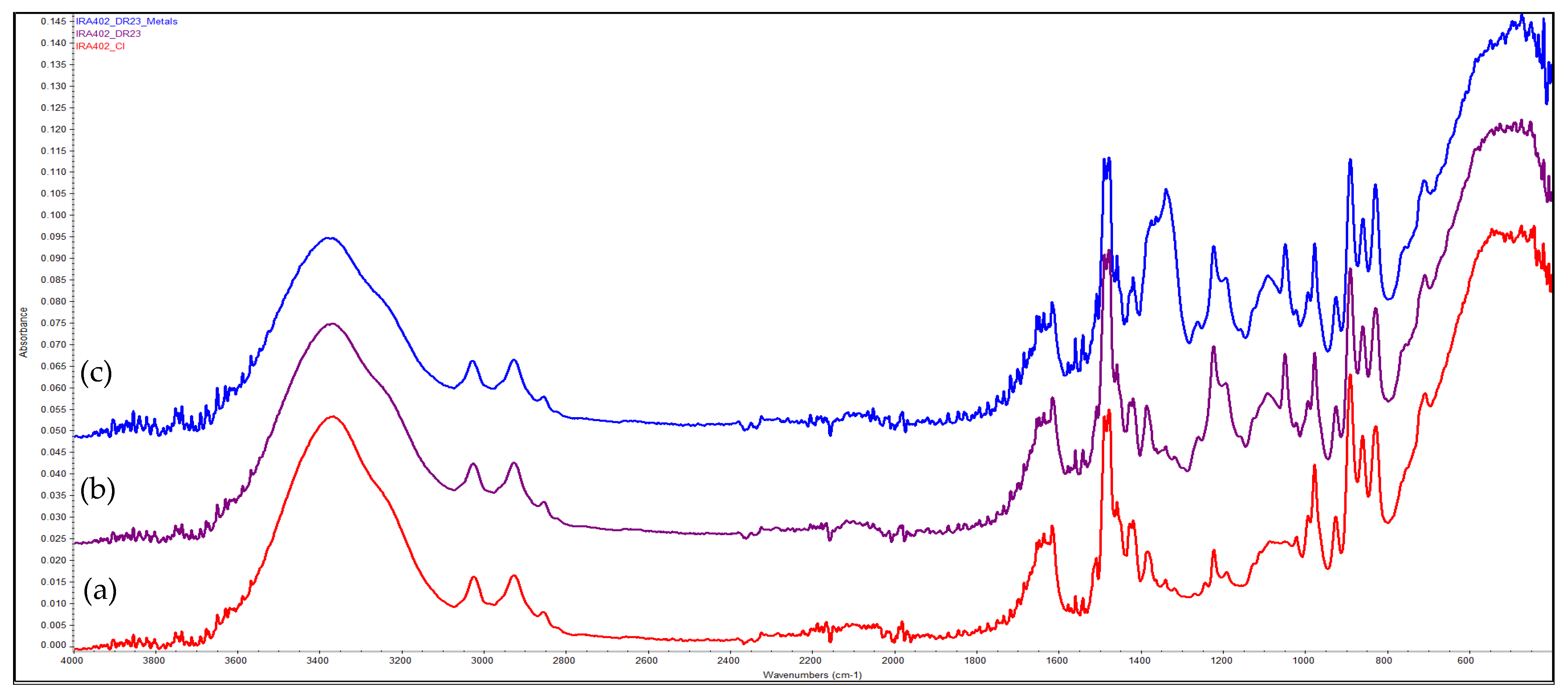
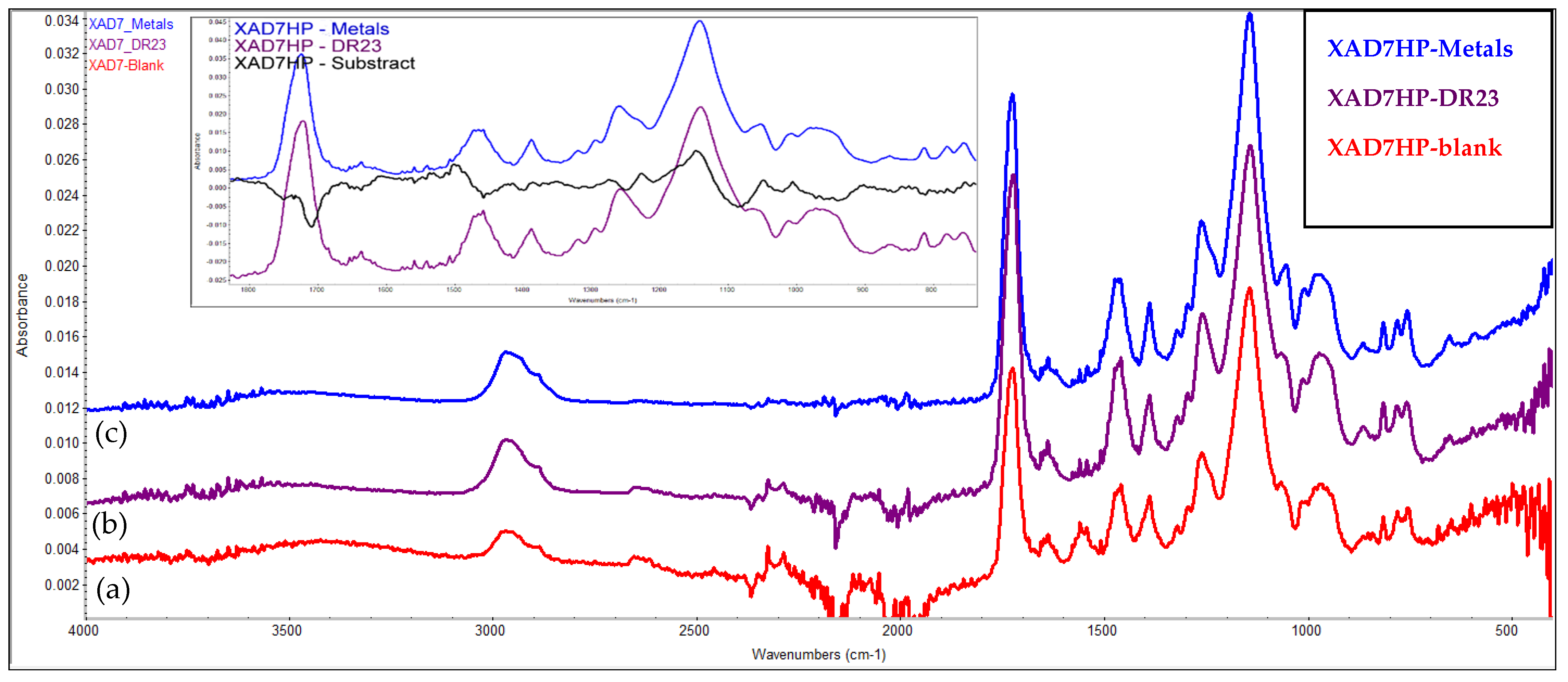
3.9. FTIR Evaluation of the Chelating Resin–Metal Interaction
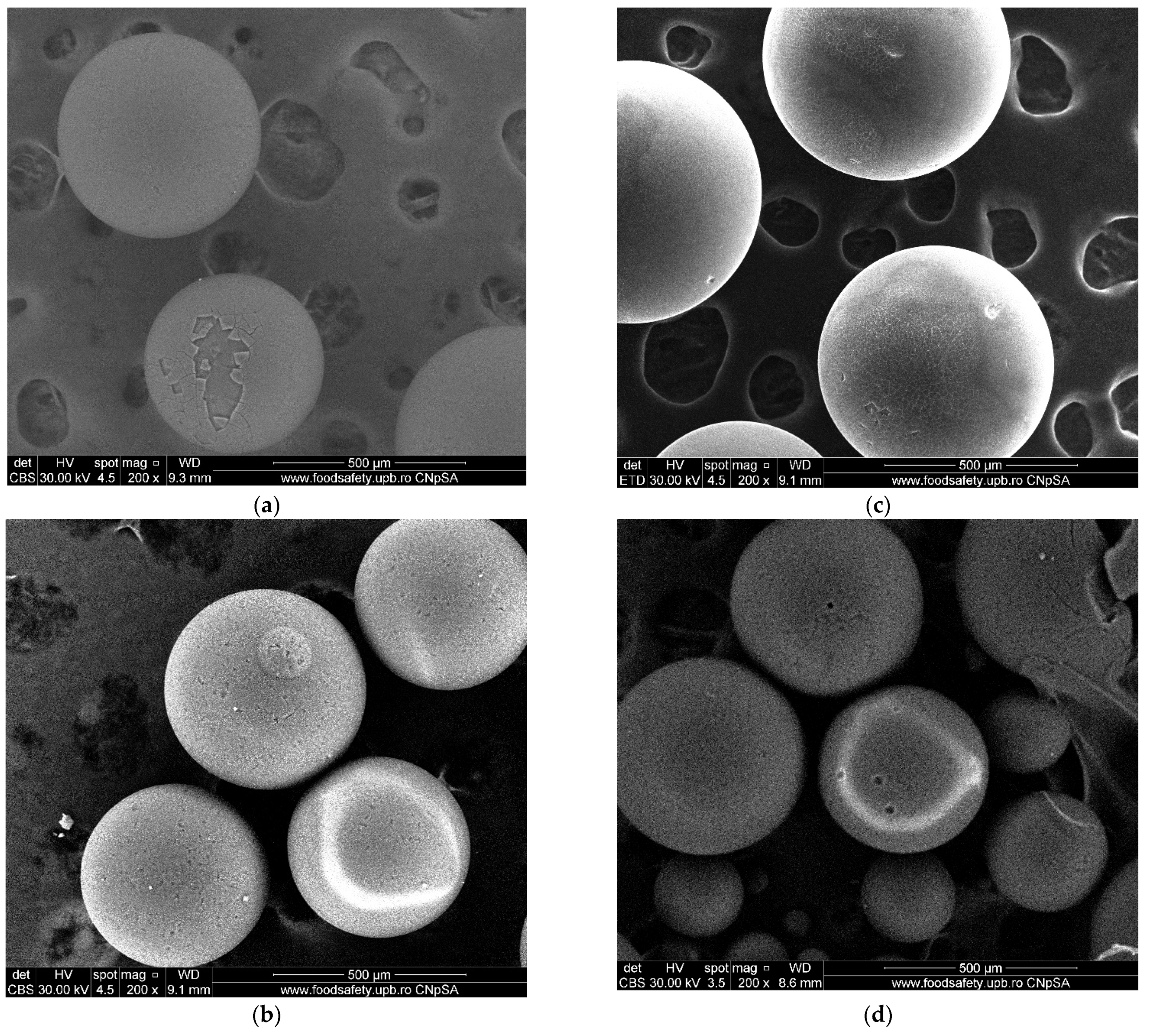
3.10. SEM Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmad, A.; Siddique, J.A.; Laskar, M.A.; Kumar, R.; Mohd-Setapar, S.H.; Khatoon, A.; Shiekh, R.A. New generation Amberlite XAD resin for the removal of metal ions: A review. J. Environ. Sci. 2015, 31, 104–123. [Google Scholar] [CrossRef] [PubMed]
- Baby, R.; Hussein, M.Z.; Abdullah, A.H.; Zainal, Z. Nanomaterials for the treatment of heavy metal contaminated water. Polymers 2022, 14, 583. [Google Scholar] [CrossRef] [PubMed]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic mechanisms of five heavy metals: Mercury, lead, chromium, cadmium, and arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef] [PubMed]
- Popescu, F.; Trumić, M.; Cioabla, A.E.; Vujić, B.; Stoica, V.; Trumić, M.; Opris, C.; Bogdanović, G.; Trif-Tordai, G. Analysis of Surface Water Quality and Sediments Content on Danube Basin in Djerdap-Iron Gate Protected Areas. Water 2022, 14, 2991. [Google Scholar] [CrossRef]
- Chu, S.; Feng, X.; Liu, C.; Wu, H.; Liu, X. Advances in Chelating Resins for Adsorption of Heavy Metal Ions. Ind. Eng. Chem. Res. 2022, 61, 11309–11328. [Google Scholar] [CrossRef]
- Zafar, M.N.; Aslam, I.; Nadeem, R.; Munir, S.; Rana, U.A.; Khan, S.U.-D. Characterization of chemically modified biosorbents from rice bran for biosorption of Ni(II). J. Taiwan Inst. Chem. Eng. 2015, 46, 82–88. [Google Scholar] [CrossRef]
- Nadeem, R.; Zafar, M.N.; Afzal, A.; Hanif, M.A.; Saeed, R. Potential of NaOH pretreated Mangifera indica waste biomass for the mitigation of Ni(II) and Co(II) from aqueous solutions. J. Taiwan Inst. Chem. Eng. 2014, 45, 967–972. [Google Scholar] [CrossRef]
- Zafar, M.N.; Parveen, A.; Nadeem, R. A pretreated green biosorbent based on Neem leaves biomass for the removal of lead from wastewater. Desalination Water Treat. 2013, 51, 4459–4466. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, J.; Zou, B.; Ran, L.; Zhu, L.; Zhang, H.; Ye, Z.; Zhou, L. Synthesis of a novel bis Schiff base chelating resin for adsorption of heavy metal ions and catalytic reduction of 4-NP. React. Funct. Polym. 2022, 180, 105409. [Google Scholar] [CrossRef]
- Suwannahong, K.; Sripirom, J.; Sirilamduan, C.; Thathong, V.; Kreetachart, T.; Panmuang, P.; Deepatana, A.; Punbut, S.; Wongcharee, S. Selective Chelating Resin for Copper Removal and Recovery in Aqueous Acidic Solution Generated from Synthetic Copper-Citrate Complexes from Bioleaching of E-waste. Adsorp. Sci. Technol. 2022, 2022, 5009124. [Google Scholar] [CrossRef]
- Benettayeb, A.; Ghosh, S.; Usman, M.; Seihoub, F.Z.; Sohoo, I.; Chia, C.H.; Sillanpää, M. Some Well-Known Alginate and Chitosan Modifications Used in Adsorption: A Review. Water 2022, 14, 1353. [Google Scholar] [CrossRef]
- Elbadawy, H.A.; Abdel-Salam, A.H.; Khalil, T.E. The impact of an Amberlite XAD-16-based chelating resin for the removal of aqueous Cd(II) and Pb(II) ions. Microchem. J. 2021, 165, 106097. [Google Scholar] [CrossRef]
- Kocaoba, S. Determination of some heavy metals from aqueous solutions using modified Amberlite XAD-4 resin by selective solid-phase extraction. J. Anal. Sci. Technol. 2022, 13, 15. [Google Scholar] [CrossRef]
- Cai, Y.; Wang, M.; Zeng, Y.; Hu, B.; Wang, Y.; Yuan, L.; Feng, W. Efficient and selective lanthanide recovery from highly acidic solutions by using a porous pillar[5]arene-based diglycolamide impregnated resin. Hydrometallurgy 2022, 211, 105867. [Google Scholar] [CrossRef]
- Van Nguyen, N.; Lee, J.-C.; Jeong, J.; Pandey, B. Enhancing the adsorption of chromium(VI) from the acidic chloride media using solvent impregnated resin (SIR). Chem. Eng. J. 2013, 219, 174–182. [Google Scholar] [CrossRef]
- Benmansour, Y.; Didi, M.A.; Abderarhim, O. Amberlite IRA-93 and modified Amberlite IRA-93 resins for the uranyl ions extraction: Optimization through factorial design methodology. Desalination Water Treat. 2022, 249, 281–296. [Google Scholar] [CrossRef]
- Sharaf, M.; Yoshida, W.; Kubota, F.; Goto, M. A novel binary-extractant-impregnated resin for selective recovery of scandium. J. Chem. Eng. Jpn. 2019, 52, 49–55. [Google Scholar] [CrossRef] [Green Version]
- Marin, N.M. Natural and Synthetic Polymers Modified with Acid Blue 113 for Removal of Cr3+, Zn2+ and Mn2+. Polymers 2022, 14, 2139. [Google Scholar] [CrossRef]
- Marin, N.M.; Stanculescu, I. Removal of procainamide and lidocaine on Amberlite XAD7HP resin and of As(V), Pb(II) and Cd(II) on the impregnated resin for water treatment. Mater. Chem. Phys. 2022, 277, 125582. [Google Scholar] [CrossRef]
- Ghanbarizadeh, P.; Parivazh, M.M.; Abbasi, M.; Osfouri, S.; Dianat, M.J.; Rostami, A.; Dibaj, M.; Akrami, M. Performance Enhancement of Specific Adsorbents for Hardness Reduction of Drinking Water and Groundwater. Water 2022, 14, 2749. [Google Scholar] [CrossRef]
- Alswieleh, A.M. Efficient Removal of Dyes from Aqueous Solution by Adsorption on L-Arginine-Modified Mesoporous Silica Nanoparticles. Processes 2022, 10, 1079. [Google Scholar] [CrossRef]
- Mosoarca, G.; Popa, S.; Vancea, C.; Dan, M.; Boran, S. Removal of Methylene Blue from Aqueous Solutions Using a New Natural Lignocellulosic Adsorbent—Raspberry (Rubus idaeus) Leaves Powder. Polymers 2022, 14, 1966. [Google Scholar] [CrossRef] [PubMed]
- Kali, A.; Amar, A.; Loulidi, I.; Hadey, C.; Jabri, M.; Alrashdi, A.A.; Lgaz, H.; Sadoq, M.; El-kordy, A.; Boukhlifi, F. Efficient Adsorption Removal of an Anionic Azo Dye by Lignocellulosic Waste Material and Sludge Recycling into Combustible Briquettes. Colloids Interfaces 2022, 6, 22. [Google Scholar] [CrossRef]
- Marin, N.M. Maize Stalk Obtained after Acid Treatment and Its Use for Simultaneous Removal of Cu2+, Pb2+, Ni2+, Cd2+, Cr3+ and Fe3+. Polymers 2022, 14, 3141. [Google Scholar] [CrossRef]
- Marin, N.M.; Stanculescu, I. Application of amberlite IRA 402 resin adsorption and laccase treatment for acid blue 113 removal from aqueous media. Polymers 2021, 13, 3991. [Google Scholar] [CrossRef]
- Marin, N.M.; Pascu, L.F.; Demba, A.; Nita-Lazar, M.; Badea, I.A.; Aboul-Enein, H. Removal of the Acid Orange 10 by ion exchange and microbiological methods. Int. J. Environ. Sci. Technol. 2019, 16, 6357–6366. [Google Scholar] [CrossRef]
- Awasthi, A.; Datta, D. Application of Amberlite XAD-7HP resin impregnated with Aliquat 336 for the removal of Reactive Blue-13 dye: Batch and fixed-bed column studies. J. Environ. Chem. Eng. 2019, 7, 103502. [Google Scholar] [CrossRef]
- Ion Exchange Capacity. Available online: http://dardel.info/IX/capacity.html (accessed on 1 October 2022).
- Yavuz, E.; Tokalıoğlu, Ş.; Erkılıç, H.; Soykan, C. Novel chelating resin for solid-phase extraction of metals in certified reference materials and waters. Anal. Lett. 2017, 50, 364–378. [Google Scholar] [CrossRef]
- Jiang, J.; Ma, X.-S.; Xu, L.-Y.; Wang, L.-H.; Liu, G.-Y.; Xu, Q.-F.; Lu, J.-M.; Zhang, Y. Applications of chelating resin for heavy metal removal from wastewater. e-Polymers 2015, 15, 161–167. [Google Scholar] [CrossRef]
- Goodwin, W.E.; Rao, R.R.; Chatt, A. Reversed-phase extraction chromatography-neutron activation analysis (RPEC-NAA) for copper in natural waters using Amberlite XAD-4 resin coated with 1-(2-thiazolylazo)-2-naphthol. J. Radioanal. Nucl. Chem. 2013, 296, 489–494. [Google Scholar] [CrossRef]
- Moniri, E.; Ahmad Panahi, H.; Karimi, M.; Ahmad Rajabi, N.; Faridi, M.; Manoochehri, M. Modification and characterization of amberlite XAD-2 with calcein blue for preconcentration and determination of copper(II) from environmental samples by atomic absorption spectroscopy. Korean J. Chem. Eng. 2011, 28, 1523–1531. [Google Scholar] [CrossRef]
- Allam, E.M.; Lashen, T.A.; Abou El-Enein, S.A.; Hassanin, M.A.; Sakr, A.K.; Hanfi, M.Y.; Sayyed, M.; Al-Otaibi, J.S.; Cheira, M.F. Cetylpyridinium Bromide/Polyvinyl Chloride for Substantially Efficient Capture of Rare Earth Elements from Chloride Solution. Polymers 2022, 14, 954. [Google Scholar] [CrossRef] [PubMed]
- Ibrahium, H.; Abdel Aal, M.; Awwad, N.; Atia, B.; Ali, H.; Gado, M.; Hakami, R.; Cheira, M. Solid-liquid separation of V(V) from aqueous medium by 3-(2-hydroxy phenyl)-imino-1-phenyl butan-1-one Schiff base immobilized XAD-2 resin. Int. J. Environ. Sci. Technol. 2022, 1–18. [Google Scholar] [CrossRef]
- Shi, X.; Fu, L.; Wu, Y.; Zhao, H.; Zhao, S.; Xu, S. Functionalized dithiocarbamate chelating resin for the removal of Co2+ from simulated wastewater. Appl. Water Sci. 2017, 7, 4351–4360. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Xu, L.; Liu, Y.; Zhou, W. Synthesis of citric acid-modified resins and their adsorption properties towards metal ions. R. Soc. Open Sci. 2018, 5, 171667. [Google Scholar] [CrossRef] [Green Version]
- Simonescu, C.M.; Lavric, V.; Musina, A.; Antonescu, O.M.; Culita, D.C.; Marinescu, V.; Tardei, C.; Oprea, O.; Pandele, A.M. Experimental and modeling of cadmium ions removal by chelating resins. J. Mol. Liq. 2020, 307, 112973. [Google Scholar] [CrossRef]
- Fatah, A.; Elashry, S.M. La(III) Separation by Tri Octyl Phosphine Oxide (Cyanex 921) Based on Amberlite Xad-4 Chelating Resin. J. Inorg. Organomet. Polym. 2022, 32, 2793–2805. [Google Scholar] [CrossRef]
- Suwannahong, K.; Sirilamduan, C.; Deepatana, A.; Kreetachat, T.; Wongcharee, S. Characterization and Optimization of Polymeric Bispicolamine Chelating Resin: Performance Evaluation via RSM Using Copper in Acid Liquors as a Model Substrate through Ion Exchange Method. Molecules 2022, 27, 7210. [Google Scholar] [CrossRef]




| Chelating Resins | IRA402-DR 23 | XAD7HP-DR 23 | ||||||
|---|---|---|---|---|---|---|---|---|
| Kinetic Models | Cd2+ | Cu2+ | Ni2+ | Pb2+ | Cd2+ | Cu2+ | Ni2+ | Pb2+ |
| Pseudo-first-order model | ||||||||
| k1 (min−1) | 0.040 | 0.058 | 0.023 | 0.046 | 0.060 | 0.026 | 0.050 | 0.057 |
| Qe calc. (mg/g) | 21.7 | 49.3 | 28.0 | 70.1 | 10.3 | 31.2 | 25.0 | 18.3 |
| * Qe exp. (mg/g) | 0.49 | 0.009 | 0.019 | 0.011 | 0.050 | 0.024 | 0.020 | 0.022 |
| R2 | 0.950 | 0.953 | 0.700 | 0.900 | 0.920 | 0.958 | 0.953 | 0.861 |
| Pseudo-second-order | ||||||||
| k2 (g/(mg∙min)) | 0.240 | 0.230 | 0.005 | 0.004 | 0.014 | 0.010 | 0.050 | 0.048 |
| Qe calc (mg/g) | 0.006 | 0.078 | 0.150 | 0.170 | 0.340 | 0.30 | 0.096 | 0.06 |
| R2 | 0.886 | 0.886 | 0.200 | 0.100 | 0.908 | 0.910 | 0.250 | 0.201 |
| Matrices | Chelating Agents | Adsorption Capacities (mg/g) | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cd2+ | Co2+ | Cr3+ | Cu2+ | Mn2+ | Ni2+ | Pb2+ | Zn2+ | |||
| Amberlite IRA 402 (Cl−) | DR 23 | 0.050 | - | - | 0.009 | - | 0.0018 | 0.011 | - | This study |
| Amberlite XAD7HP | DR 23 | 0.051 | - | - | 0.022 | - | 0.020 | 0.021 | - | This study |
| Poly(diacetonitrile methacrylamide-coinylimidazole) | Polyvinyl- imidazole | 29.3 | 31.6 | 29.3 | 27.3 | 35.5 | 31.7 | 39.8 | 32.3 | [29] |
| Glycidyl methacrylate | N,N-methylene biscarylamid | 369 | 73 | 40.6 | 151 | 94.1 | 99.2 | 136.8 | 92.2 | [30] |
| Amberlite XAD4 | 1-(2-thiazolylazo) -2-naphthol | - - | - | 8 | - | - | - | - | [31] | |
| Amberlite XAD2 | Calcein blue | - | - | - | 27 | - | - | -- | [32] | |
| Chelating Resins | IRA402-DR 23 | XAD7HP-DR 23 | ||||||
|---|---|---|---|---|---|---|---|---|
| Isotherm Models | Cd2+ | Cu2+ | Ni2+ | Pb2+ | Cd2+ | Cu2+ | Ni2+ | Pb2+ |
| Langmuir | ||||||||
| Q0 (mg/g) | 0.324 | 0.013 | 0.029 | 0.008 | 0.069 | 0.018 | 0.015 | 0.168 |
| b (L/mg) | 1.478 | 7.732 | 2.296 | 9.701 | 16.813 | 17.043 | 9.238 | 0.122 |
| R2 | 0.160 | 0.775 | 0.401 | 0.455 | 0.674 | 0.754 | 0.555 | 0.204 |
| RL | 0.576 | 0.582 | 0.703 | 0.491 | 0.104 | 0.214 | 0.352 | 0.974 |
| Freundlich | ||||||||
| KF | 2.429 | 1.879 | 1.746 | 1.363 | 1.821 | 1.310 | 1.379 | 2.365 |
| 1/n | 0.888 | 0.631 | 0.558 | 0.310 | 0.600 | 0.270 | 1.321 | 0.861 |
| n | 1.127 | 1.586 | 1.794 | 3.230 | 1.668 | 3.704 | 3.112 | 1.162 |
| R2 | 0.942 | 0.792 | 0.830 | 0.421 | 0.968 | 0.732 | 0.636 | 0.537 |
| Dubinin–Radushkevich | ||||||||
| qm (mg/g) | 13.448 | 70.0 | 85.151 | 152.0 | 19.101 | 66.622 | 94.332 | 100.0 |
| β (mol2/kJ2) | 0.2 × 10−7 | 0.3 × 10−7 | 0.2 × 10−7 | 0.1 × 10−7 | 0.1 × 10−7 | 0.7 × 10−8 | 0.9 × 10−8 | 0.3 × 10−7 |
| E (KJ/mol) | 5000 | 4082 | 5000 | 7071 | 7071 | 8452 | 7454 | 4083 |
| R2 | 0.914 | 0.838 | 0.656 | 0.344 | 0.906 | 0.7060 | 0.515 | 0.445 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marin, N.M.; Ficai, A.; Constantin, L.A.; Motelica, L.; Trusca, R. New Chelate Resins Prepared with Direct Red 23 for Cd2+, Ni2+, Cu2+ and Pb2+ Removal. Polymers 2022, 14, 5523. https://doi.org/10.3390/polym14245523
Marin NM, Ficai A, Constantin LA, Motelica L, Trusca R. New Chelate Resins Prepared with Direct Red 23 for Cd2+, Ni2+, Cu2+ and Pb2+ Removal. Polymers. 2022; 14(24):5523. https://doi.org/10.3390/polym14245523
Chicago/Turabian StyleMarin, Nicoleta Mirela, Anton Ficai, Lucian Alexandru Constantin, Ludmila Motelica, and Roxana Trusca. 2022. "New Chelate Resins Prepared with Direct Red 23 for Cd2+, Ni2+, Cu2+ and Pb2+ Removal" Polymers 14, no. 24: 5523. https://doi.org/10.3390/polym14245523
APA StyleMarin, N. M., Ficai, A., Constantin, L. A., Motelica, L., & Trusca, R. (2022). New Chelate Resins Prepared with Direct Red 23 for Cd2+, Ni2+, Cu2+ and Pb2+ Removal. Polymers, 14(24), 5523. https://doi.org/10.3390/polym14245523