A Polysiloxane Delivery Vehicle of Cyclic N-Halamine for Biocidal Coating of Cellulose in Supercritical CO2
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
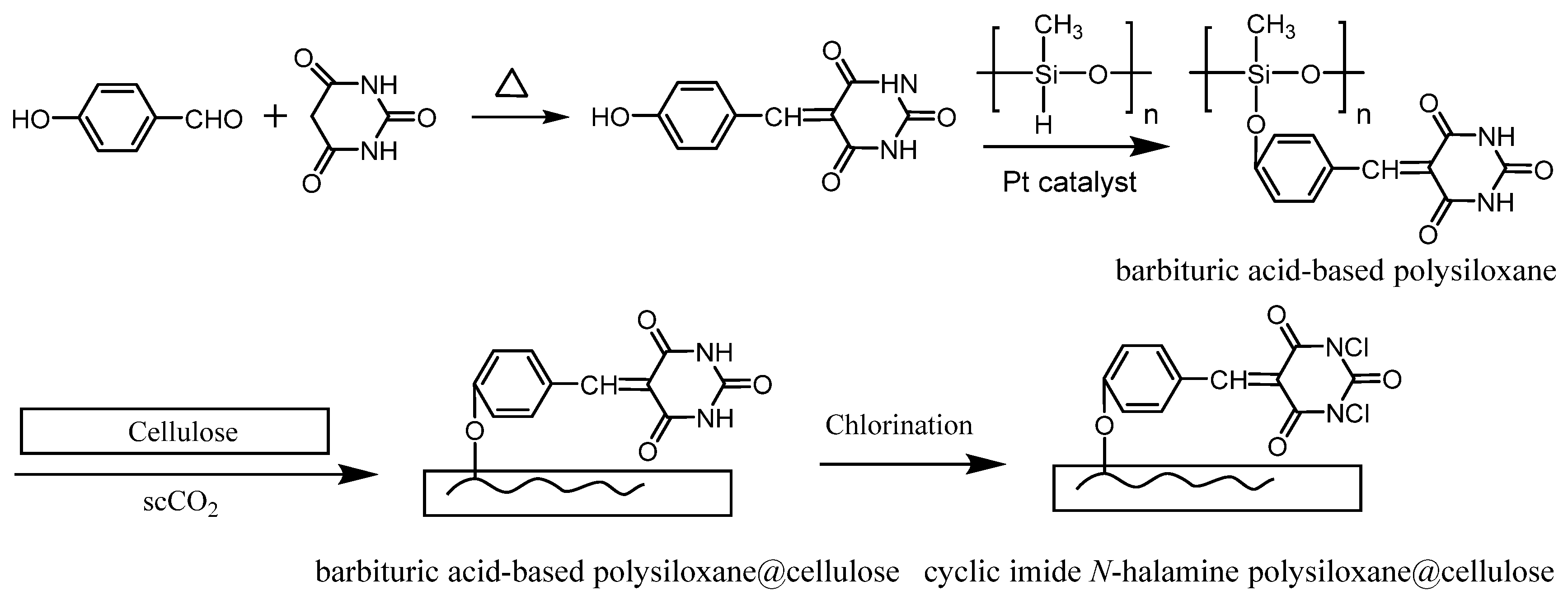
2.3. Synthesis of Barbituric Acid-Based Polysiloxane That Bears Cyclic Imide N−H Sites
2.4. Interpenetration of Cellulose with Barbituric Acid-Based Polysiloxane in scCO2
2.5. Chlorination of Cyclic Imide N−H Sites of Barbituric Acid-Based Polysiloxane
2.6. Titration of Biocidal Chlorine
2.7. Antibacterial Kinetics of Cyclic Imide N-Halamine Polysiloxane@Cellulose Fabrics
2.8. Testing of Biocidal Stability and Rechargeability
3. Results and Discussion
3.1. Synthesis of Barbituric Acid-Based Polysiloxane
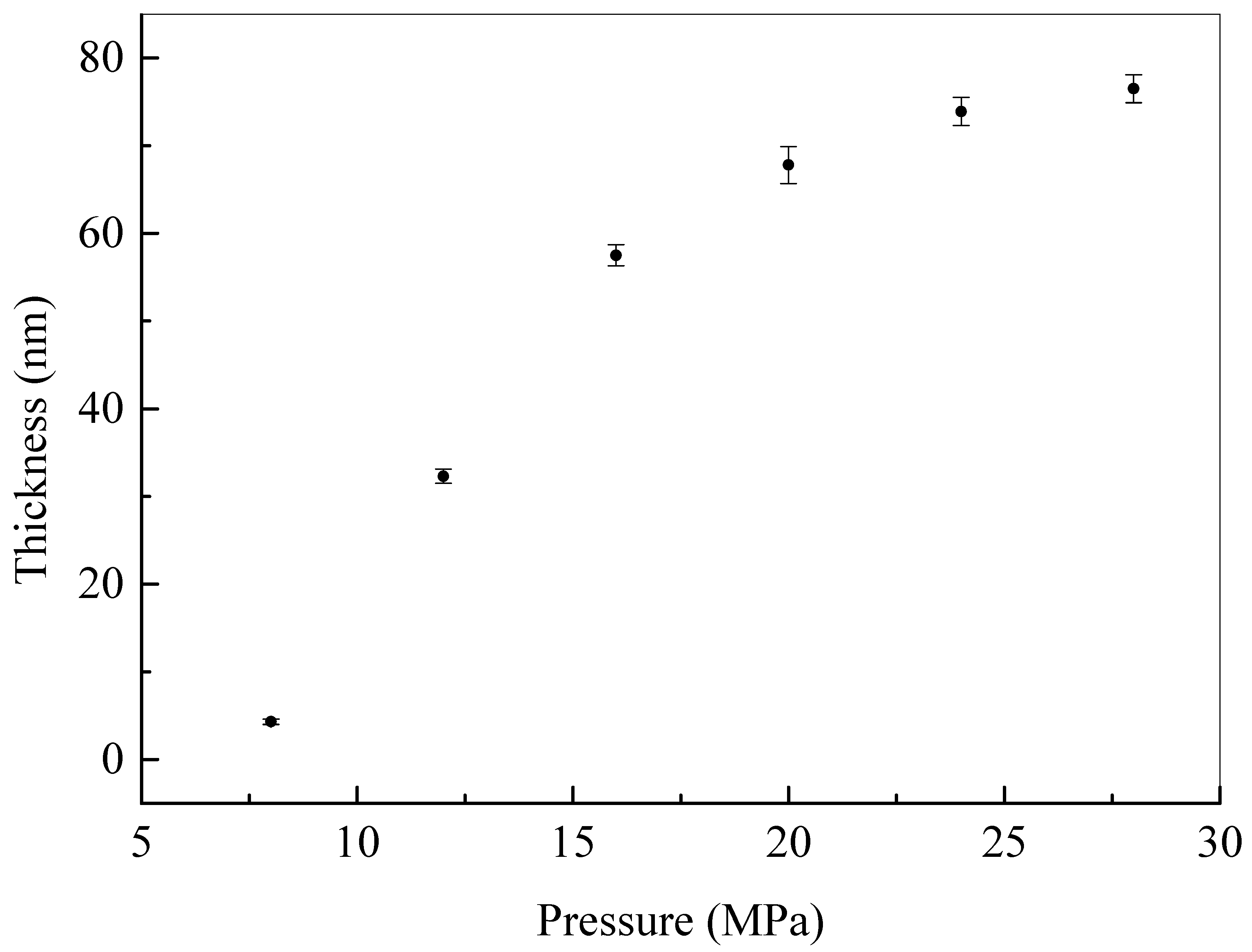
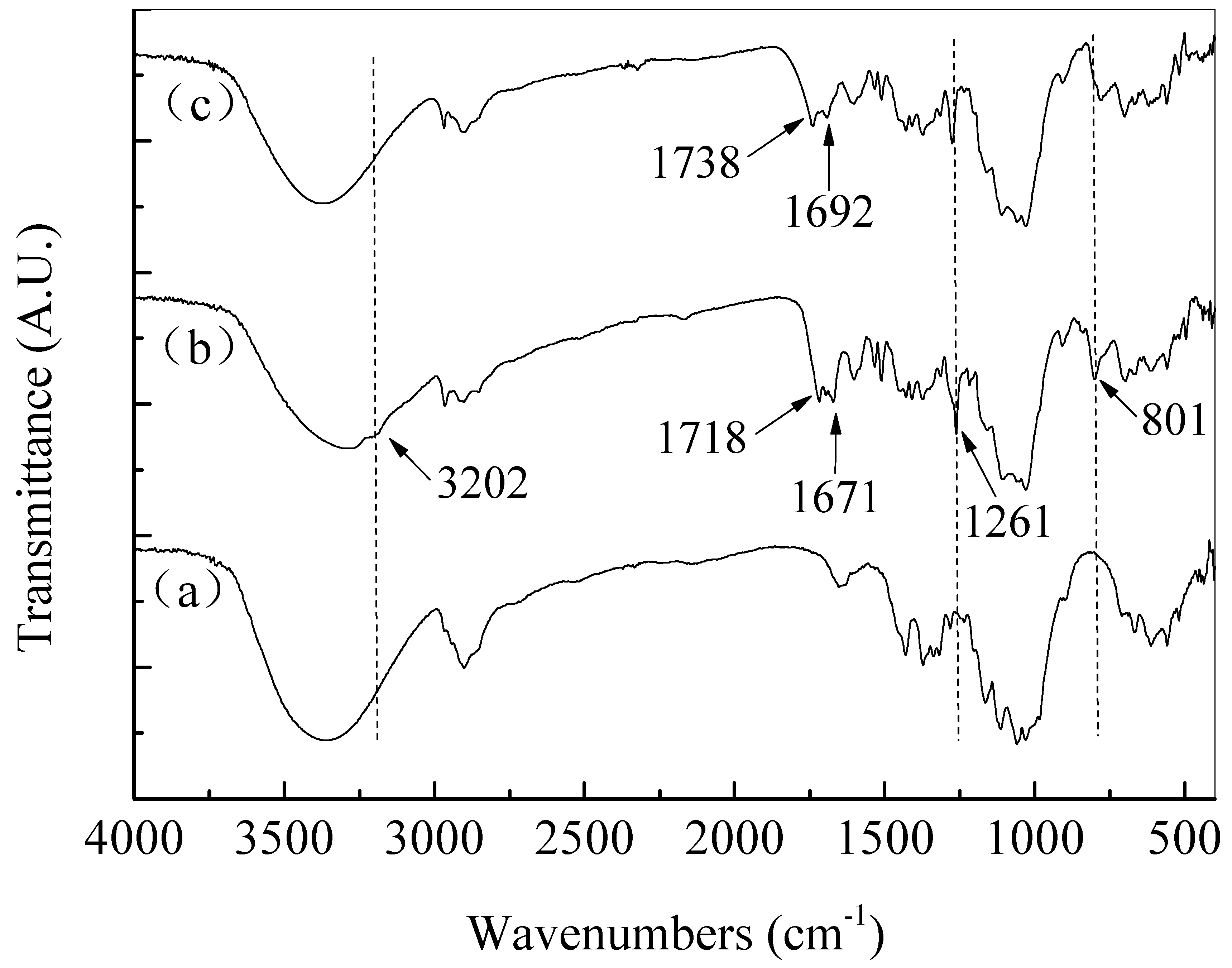
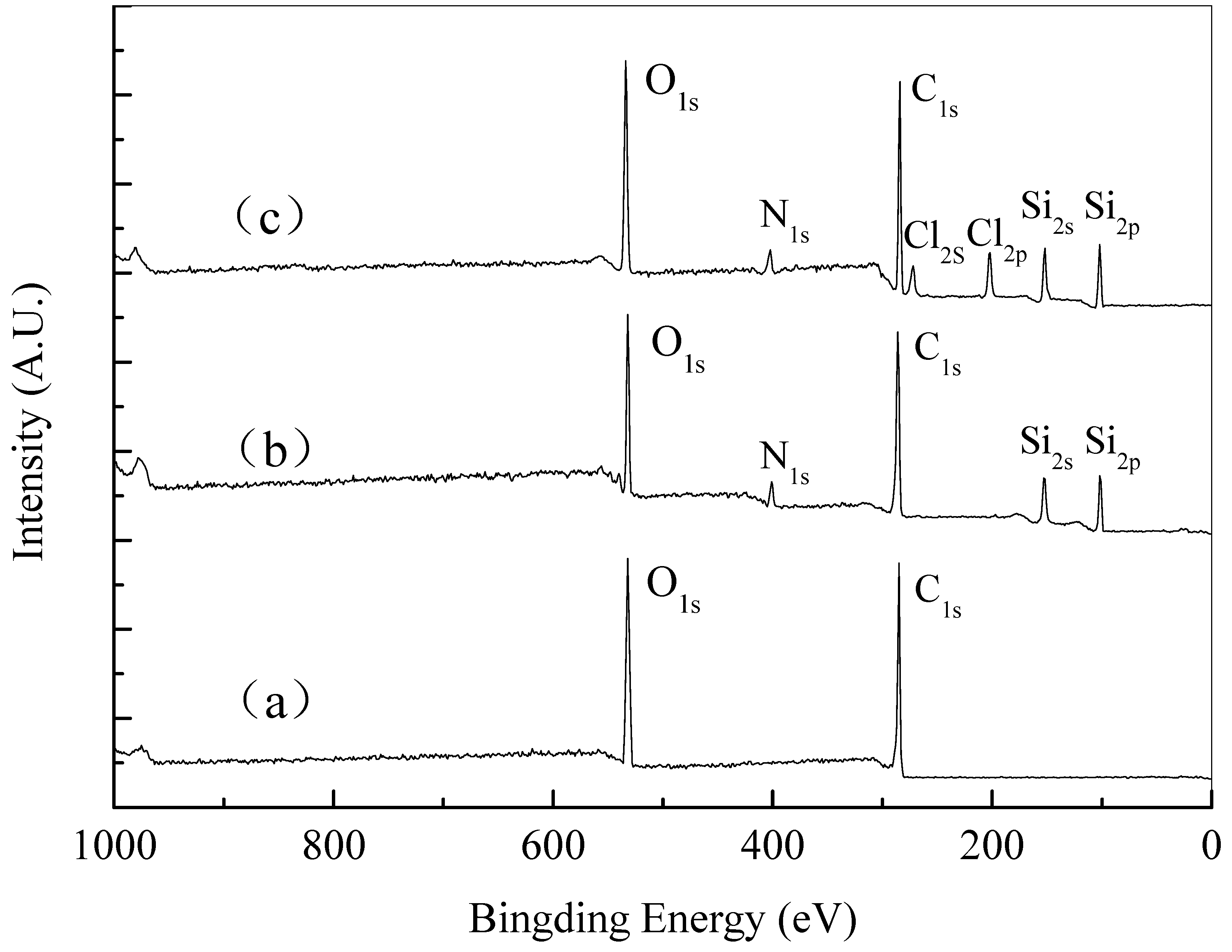
3.2. Construction and Characterizations of Biocidal Polysiloxane Layer on Cellulose
3.3. Antibacterial Testing
3.4. Biocidal Stability and Rechargeability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mitchell, A.; Spencer, M.; Edmiston, C. Role of healthcare apparel and other healthcare textiles in the transmission of pathogens: A review of the literature. J. Hosp. Infect. 2015, 90, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Kinali-Demirci, S. Cross-Linked Polymer Brushes Containing N-Halamine Groups for Antibacterial Surface Applications. Polymers 2021, 13, 1269. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wisuthiphaet, N.; Bolt, H.; Nitin, N.; Zhao, Q.; Wang, D.; Pourdeyhimi, B.; Grondin, P.; Sun, G. N-Halamine Polypropylene Nonwoven Fabrics with Rechargeable Antibacterial and Antiviral Functions for Medical Applications. ACS Biomater. Sci. Eng. 2021, 7, 2329–2336. [Google Scholar] [CrossRef] [PubMed]
- Cerkez, I.; Kocer, H.B.; Worley, S.D.; Broughton, R.M.; Huang, T.S. N-halamine biocidal coatings via a layer-by-layer assembly technique. Langmuir 2011, 27, 4091–4097. [Google Scholar] [CrossRef] [PubMed]
- Cheung, Y.H.; Ma, K.; van Leeuwen, H.C.; Wasson, M.C.; Wang, X.; Idrees, K.B.; Gong, W.; Cao, R.; Mahle, J.J.; Islamoglu, T.; et al. Immobilized Regenerable Active Chlorine within a Zirconium-Based MOF Textile Composite to Eliminate Biological and Chemical Threats. J. Am. Chem. Soc. 2021, 143, 16777–16785. [Google Scholar] [CrossRef] [PubMed]
- Qian, F.Y.; Zheng, Y.X.; Pan, N.Y.; Li, L.; Li, R.; Ren, X.H. Synthesis of polysiloxane and its co-application with nano-SiO2 for antibacterial and hydrophobic cotton fabrics. Cellulose 2021, 28, 3169–3181. [Google Scholar] [CrossRef]
- Wang, Q.; Zhu, X.; Zhu, P.; Jiang, Z. N-halamine Antibacterial Cellulose Fabrics Functionalized with Copoly(acrylamide-maleic anhydride). Fibers Polym. 2019, 20, 906–912. [Google Scholar] [CrossRef]
- Hussain, S.; Joo, J.; Kang, J.; Kim, B.; Braun, G.B.; She, Z.G.; Kim, D.; Mann, A.P.; Molder, T.; Teesalu, T.; et al. Antibiotic-loaded nanoparticles targeted to the site of infection enhance antibacterial efficacy. Nat. Biomed. Eng. 2018, 2, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Ye, Y.; Qian, L.; Zhao, G.; He, B.; Xiao, H. Antibacterial modification of cellulose fibers by grafting β-cyclodextrin and inclusion with ciprofloxacin. Cellulose 2014, 21, 1921–1932. [Google Scholar] [CrossRef]
- Hu, B.; Chen, X.; Zuo, Y.; Liu, Z.; Xing, X. Dual action bactericides: Quaternary ammonium/N-halamine-functionalized cellulose fiber. J. Appl. Polym. Sci. 2014, 131, 40070. [Google Scholar] [CrossRef]
- Przybylak, M.; Maciejewski, H.; Dudkiewicz, A.; Walentowska, J.; Foksowicz-Flaczyk, J. Development of multifunctional cotton fabrics using difunctional polysiloxanes. Cellulose 2018, 25, 1483–1497. [Google Scholar] [CrossRef]
- Li, L.; Chi, X.; Gai, F.; Hao, Z.; Zhang, F.; Zhao, Z. Synthesis of novel pyridinium N-chloramine precursors and its antimicrobial application on cotton fabrics. J. Appl. Polym. Sci. 2017, 134, 45323. [Google Scholar] [CrossRef]
- Tang, P.; Zhang, Z.; El-Moghazy, A.Y.; Wisuthiphaet, N.; Nitin, N.; Sun, G. Daylight-Induced Antibacterial and Antiviral Cotton Cloth for Offensive Personal Protection. ACS Appl. Mater. Interfaces 2020, 12, 49442–49451. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.; El-Moghazy, A.Y.; Ji, B.; Nitin, N.; Sun, G. Unique “posture” of rose Bengal for fabricating personal protective equipment with enhanced daylight-induced biocidal efficiency. Mater. Adv. 2021, 2, 3569–3578. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Li, X.; Li, Y.; Lyu, B.; Ren, J.; Ma, J. Long-acting antibacterial activity on the cotton fabric. Cellulose 2021, 28, 1221–1240. [Google Scholar] [CrossRef]
- Smiechowicz, E.; Niekraszewicz, B.; Kulpinski, P.; Dzitko, K. Antibacterial composite cellulose fibers modified with silver nanoparticles and nanosilica. Cellulose 2018, 25, 3499–3517. [Google Scholar] [CrossRef]
- Khafaga, M.R.; Ali, H.E.; El-Naggar, A.W.M. Antimicrobial finishing of cotton fabrics based on gamma irradiated carboxymethyl cellulose/poly(vinyl alcohol)/TiO2 nanocomposites. J. Text. Inst. 2015, 107, 766–773. [Google Scholar] [CrossRef]
- Cao, Z.; Sun, X.; Yao, J.; Sun, Y. Silver sulfadiazine–immobilized celluloses as biocompatible polymeric biocides. J. Bioact. Compat. Polym. 2013, 28, 398–410. [Google Scholar] [CrossRef]
- Cao, Z.; Sun, Y. N-halamine-based chitosan: Preparation, characterization, and antimicrobial function. J. Biomed. Mater. Res. Part A 2008, 85, 99–107. [Google Scholar] [CrossRef]
- Akdag, A.; Okur, S.; McKee, M.L.; Worley, S.D. The stabilities of N-Cl bonds in biocidal materials. J. Chem. Theory Comput. 2006, 2, 879–884. [Google Scholar] [CrossRef]
- Dong, A.; Zhang, Q.; Wang, T.; Wang, W.; Liu, F.; Gao, G. Immobilization of cyclic N-Halamine on polystyrene-functionalized silica nanoparticles: Synthesis, characterization, and biocidal activity. J. Phys. Chem. C 2010, 114, 17298–17303. [Google Scholar] [CrossRef]
- Luo, J.; Sun, Y. Acyclic N-halamine coated Kevlar fabric materials: Preparation and biocidal functions. Ind. Eng. Chem. Res. 2008, 47, 5291–5297. [Google Scholar] [CrossRef]
- Kocer, H.B.; Worley, S.D.; Broughton, R.M.; Huang, T.S. A novel N-halamine acrylamide monomer and its copolymers for antimicrobial coatings. React. Funct. Polym. 2011, 71, 561–568. [Google Scholar] [CrossRef]
- Krishnan, S.; Ward, R.J.; Hexemer, A.; Sohn, K.E.; Lee, K.L.; Angert, E.R.; Fischer, D.A.; Kramer, E.J.; Ober, C.K. Surfaces of fluorinated pyridinium block copolymers with enhanced antibacterial activity. Langmuir 2006, 22, 11255–11266. [Google Scholar] [CrossRef] [PubMed]
- Dong, A.; Sun, Y.; Lan, S.; Wang, Q.; Cai, Q.; Qi, X.; Zhang, Y.; Gao, G.; Liu, F.; Harnoode, C. Barbituric acid-based magnetic N-Halamine nanoparticles as recyclable antibacterial agents. ACS Appl. Mater. Interfaces 2013, 5, 8125–8133. [Google Scholar] [CrossRef]
- Dong, A.; Xue, M.; Lan, S.; Wang, Q.; Zhao, Y.; Wang, Y.; Zhang, Y.; Gao, G.; Liu, F.; Harnoode, C. Bactericidal evaluation of N-halamine-functionalized silica nanoparticles based on barbituric acid. Colloids Surf. B Biointerfaces 2014, 113, 450–457. [Google Scholar] [CrossRef]
- Kalita, S.J.; Mecadon, H.; Deka, D.C. FeCl3·6H2O catalyzed aqueous media domino synthesis of 5-monoalkylbarbiturates: Water as both reactant and solvent. RSC Adv. 2014, 4, 10402–10411. [Google Scholar] [CrossRef]
- Gomes, A.P.; Mano, J.F.; Queiroz, J.A.; Gouveia, I.C. Layer-by-layer assembly for biofunctionalization of cellulosic fibers with emergent antimicrobial agents. Adv. Polym. Sci. 2015, 2016, 225–240. [Google Scholar]
- Luo, G.; Xi, G.; Wang, X.; Qin, D.; Zhang, Y.; Fu, F.; Liu, X. Antibacterial N-halamine coating on cotton fabric fabricated using mist polymerization. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Ren, X.; Huang, T. Antimicrobial cotton containing N-halamine and quaternary ammonium groups by grafting copolymerization. Appl. Surf. Sci. 2014, 296, 231–236. [Google Scholar] [CrossRef]
- Hong, K.H.; Liu, N.; Sun, G. UV-induced graft polymerization of acrylamide on cellulose by using immobilized benzophenone as a photo-initiator. Eur. Polym. J. 2009, 45, 2443–2449. [Google Scholar] [CrossRef]
- Kocer, H.B.; Cerkez, I.; Worley, S.D.; Broughton, R.M.; Huang, T.S. Polymeric antimicrobial N-halamine epoxides. ACS Appl. Mater. Interfaces 2011, 3, 2845–2850. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, Y.; Feng, C.; He, Q.; Chen, Q.; Wang, Z.; Han, Q. Novel quat/di-N-halamines silane unit with enhanced synergism polymerized on cellulose for development of superior biocidability. Int. J. Biol. Macromol. 2020, 154, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Kou, L.; Liang, J.; Worley, S.D.; Tzou, Y.-M.; Huang, T. Antimicrobial efficacy and light stability of N-halamine siloxanes bound to cotton. Cellulose 2008, 15, 593–598. [Google Scholar] [CrossRef]
- Kou, L.; Liang, J.; Ren, X.; Kocer, H.B.; Worley, S.; Broughton, R.; Huang, T. Novel N-halamine silanes. Colloids Surf. A Physicochem. Eng. Asp. 2009, 345, 88–94. [Google Scholar] [CrossRef]
- O’Neill, M.L.; Cao, Q.; Fang, M.; Johnston, K.P.; Wilkinson, S.P.; Smith, C.D.; Kerschner, J.L.; Jureller, S.H. Solubility of homopolymers and copolymers in carbon dioxide. Ind. Eng. Chem. Res. 1998, 37, 3067–3079. [Google Scholar] [CrossRef]
- Sarbu, T.; Styranec, T.; Beckman, E.J. Non-fluorous polymers with very high solubility in supercritical CO2 down to low pressures. Nature 2000, 405, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Kiran, E. Miscibility, density and viscosity of poly(dimethylsiloxane) in supercritical carbon dioxide. Polymer 1995, 36, 4817–4826. [Google Scholar] [CrossRef]
- Teeter, H.M.; Bell, E.W. tert-Butyl hypochlorite. Org. Synth. 1952, 32, 20–21. [Google Scholar]
- Ma, Y.; Yi, J.; Pan, B.; Nitin, N.; Sun, G. Chlorine Rechargeable Biocidal N-Halamine Nanofibrous Membranes Incorporated with Bifunctional Zwitterionic Polymers for Efficient Water Disinfection Applications. ACS Appl. Mater. Interfaces 2020, 12, 51057–51068. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, G. Novel refreshable N-halamine polymeric biocides: N-chlorination of aromatic polyamides. Ind. Eng. Chem. Res. 2004, 43, 5015–5020. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, G. Durable and refreshable polymeric N-halamine biocides containing 3-(4′-vinylbenzyl)-5,5-dimethylhydantoin. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 3348–3355. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.Y.; Wang, Z.D.; Wang, Y.W.; Han, Y.J.; Zhang, Q.; Han, Q.X. Engineering of super bactericidal cotton using pyridinium/di-N-chloramine siloxane with intensified synergism. Cellulose 2021, 28, 6713–6725. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Q.; Han, Q.; Mi, Y.; Sun, S.; Feng, C.; Xiao, H.; Yu, P.; Yang, C. Synthesis of polysiloxane with 5,5-dimethylhydantoin-based N-halamine pendants for biocidal functionalization of polyethylene by supercritical impregnation. J. Appl. Polym. Sci. 2017, 134, 44721. [Google Scholar]
- Chen, Y.; Wang, Y.; Zhang, Q.; Yang, C.; Han, Q. Preparation of silicone containing 2,2,6,6-tetramethyl-4-piperidinolbased N-chloramine for antibacterial polyethylene via interpenetration in supercritical carbon dioxide. J. Appl. Polym. Sci. 2019, 136, 47614. [Google Scholar] [CrossRef]
- Liang, J.; Wu, R.; Wang, J.W.; Barnes, K.; Worley, S.D.; Cho, U.; Lee, J.; Broughton, R.M.; Huang, T.S. N-halamine biocidal coatings. J. Ind. Microbiol. Biotechnol. 2007, 34, 157–163. [Google Scholar] [CrossRef]
- Ouyang, M.; Muisener, R.J.; Boulares, A.; Koberstein, J.T. UV-ozone induced growth of a SiOx surface layer on a cross-linked polysiloxane film: Characterization and gas separation properties. J. Membr. Sci. 2000, 177, 177–187. [Google Scholar] [CrossRef]

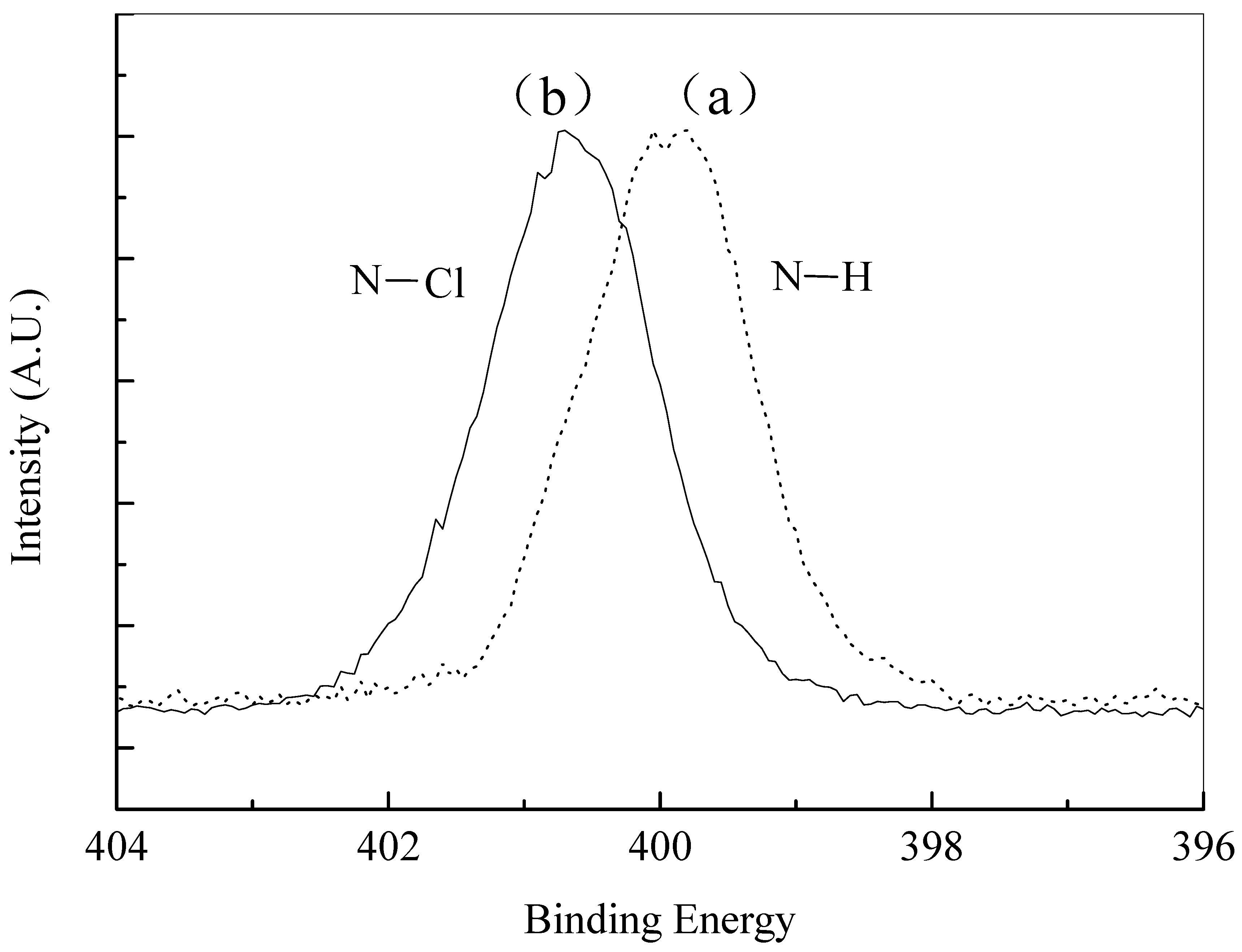
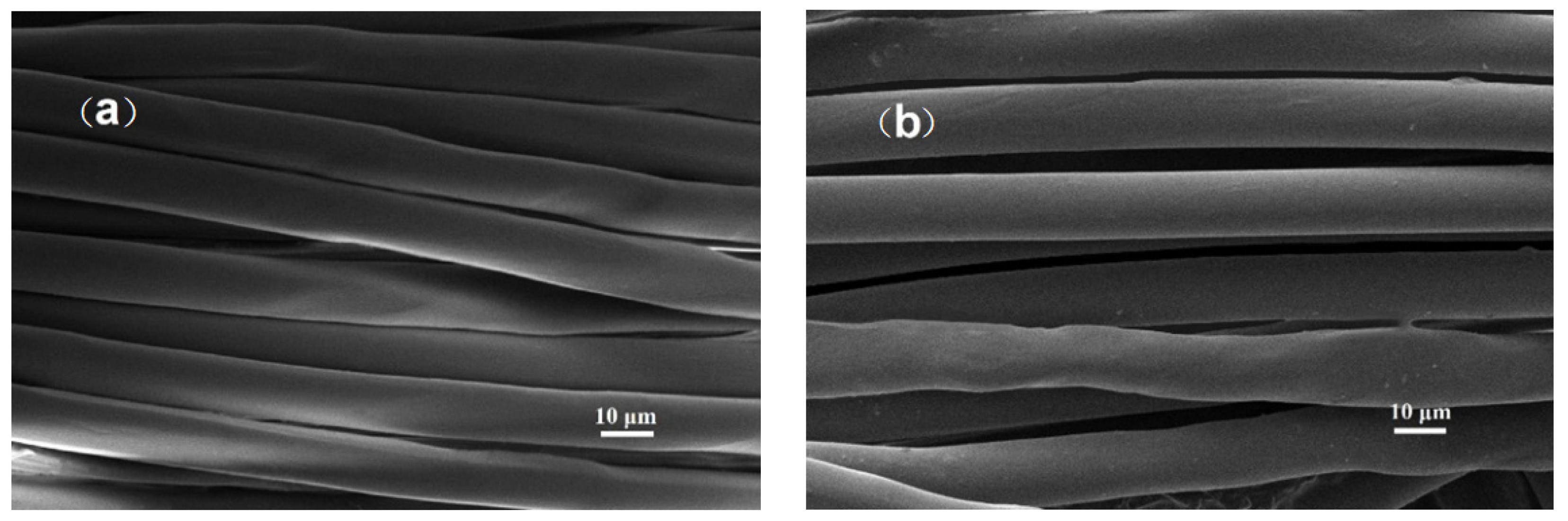
| Material | Contact Time (min) | Log Reduction of E. coli | Log Reduction of S. aureus |
|---|---|---|---|
| Pristine control | 5 | 0.13 ± 0.07 | 0.14 ± 0.08 |
| 10 | 0.18 ± 0.08 | 0.19 ± 0.06 | |
| 15 | 0.25 ± 0.06 | 0.27 ± 0.06 | |
| 20 | 0.37 ± 0.05 | 0.36 ± 0.06 | |
| Cyclic imide N-halamine polysiloxane@cellulose | 5 | 3.96 ± 0.07 | 3.42 ± 0.06 |
| 10 | 5.22 ± 0.07 | 4.76 ± 0.07 | |
| 15 | 6.78 ± 0.06 | 6.12 ± 0.06 | |
| 20 | 7.11 ± 0.00 | 7.08 ± 0.00 |
| No. of Washing Cycles | Remained Chlorine (wt% Cl+) | Recovered Chlorine after Rechlorination (wt% Cl+) |
|---|---|---|
| 1 | 0.18 ± 0.01 | 0.24 ± 0.02 |
| 3 | 0.13 ± 0.02 | 0.17 ± 0.01 |
| 5 | 0.10 ± 0.01 | 0.15 ± 0.02 |
| 10 | 0.06 ± 0.01 | 0.10 ± 0.01 |
| Exposure Time | 1 h | 2 h | 4 h | 8 h | 12 h | 24 h | 7 d |
|---|---|---|---|---|---|---|---|
| Remained chlorine (wt%) | 0.18 ± 0.01 | 0.14 ± 0.02 | 0.09 ± 0.01 | 0.06 ± 0.01 | 0.03 ± 0.01 | 0.02 ± 0.01 | 0 ± 0.00 |
| Recovered chlorine (wt%) | 0.26 ± 0.02 | 0.24 ± 0.01 | 0.23 ± 0.02 | 0.21 ± 0.01 | 0.20 ± 0.01 | 0.19 ± 0.01 | 0.17 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Xin, Y.; Wu, F.; Lyu, X.; Yao, Q.; Yin, X.; Zhang, Q.; Shan, W.; Chen, Y.; Han, Q. A Polysiloxane Delivery Vehicle of Cyclic N-Halamine for Biocidal Coating of Cellulose in Supercritical CO2. Polymers 2022, 14, 5080. https://doi.org/10.3390/polym14235080
Li L, Xin Y, Wu F, Lyu X, Yao Q, Yin X, Zhang Q, Shan W, Chen Y, Han Q. A Polysiloxane Delivery Vehicle of Cyclic N-Halamine for Biocidal Coating of Cellulose in Supercritical CO2. Polymers. 2022; 14(23):5080. https://doi.org/10.3390/polym14235080
Chicago/Turabian StyleLi, Leixuan, Yan Xin, Fengze Wu, Xiangrong Lyu, Qiyuan Yao, Xiaoting Yin, Qiang Zhang, Wenjuan Shan, Yong Chen, and Qiuxia Han. 2022. "A Polysiloxane Delivery Vehicle of Cyclic N-Halamine for Biocidal Coating of Cellulose in Supercritical CO2" Polymers 14, no. 23: 5080. https://doi.org/10.3390/polym14235080
APA StyleLi, L., Xin, Y., Wu, F., Lyu, X., Yao, Q., Yin, X., Zhang, Q., Shan, W., Chen, Y., & Han, Q. (2022). A Polysiloxane Delivery Vehicle of Cyclic N-Halamine for Biocidal Coating of Cellulose in Supercritical CO2. Polymers, 14(23), 5080. https://doi.org/10.3390/polym14235080




