A Salt-Resistant Sodium Carboxymethyl Cellulose Modified by the Heterogeneous Process of Oleate Amide Quaternary Ammonium Salt
Abstract
:1. Introduction
2. Experimental Materials and Methods
2.1. Material
2.2. Experimental Methods
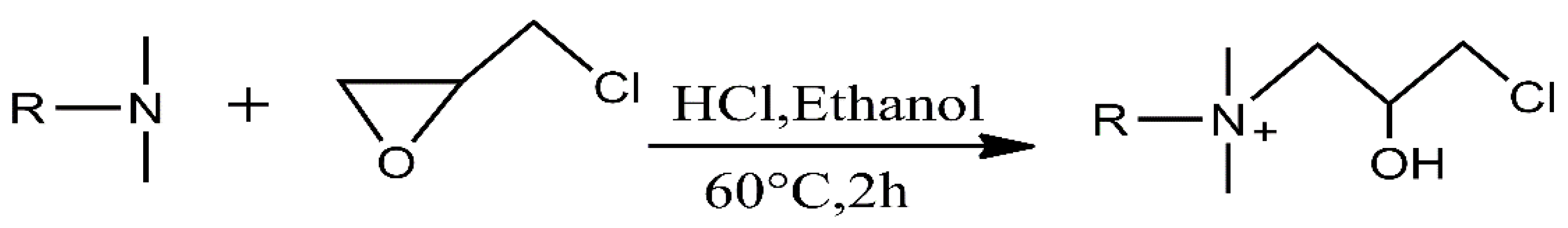
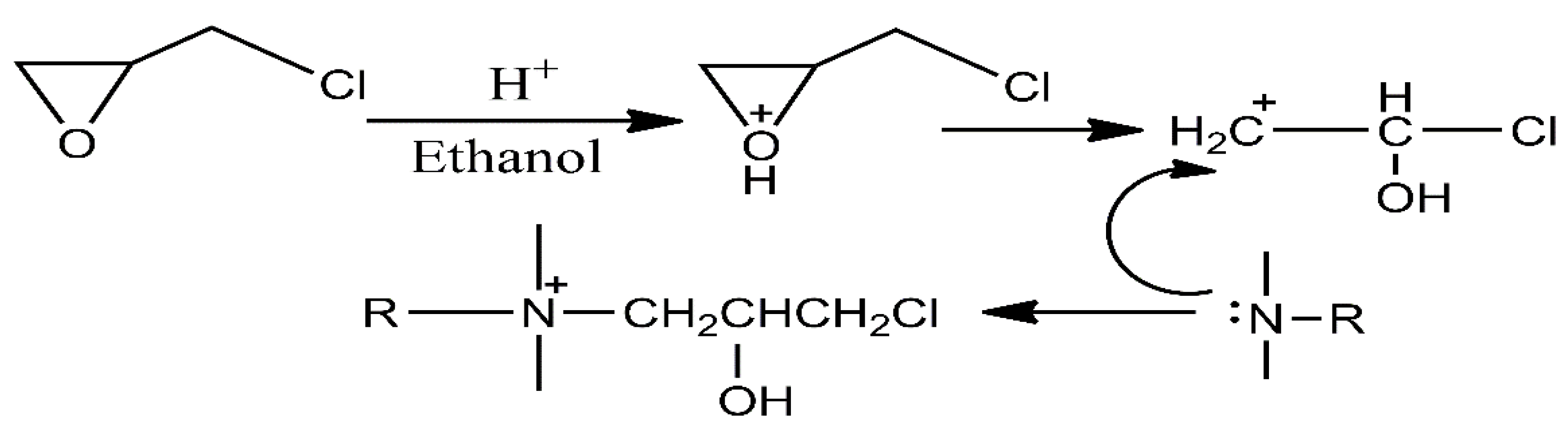
2.2.1. Synthesis of Hydrophobic Chain Intermediates of Quaternary Ammonium Oleate
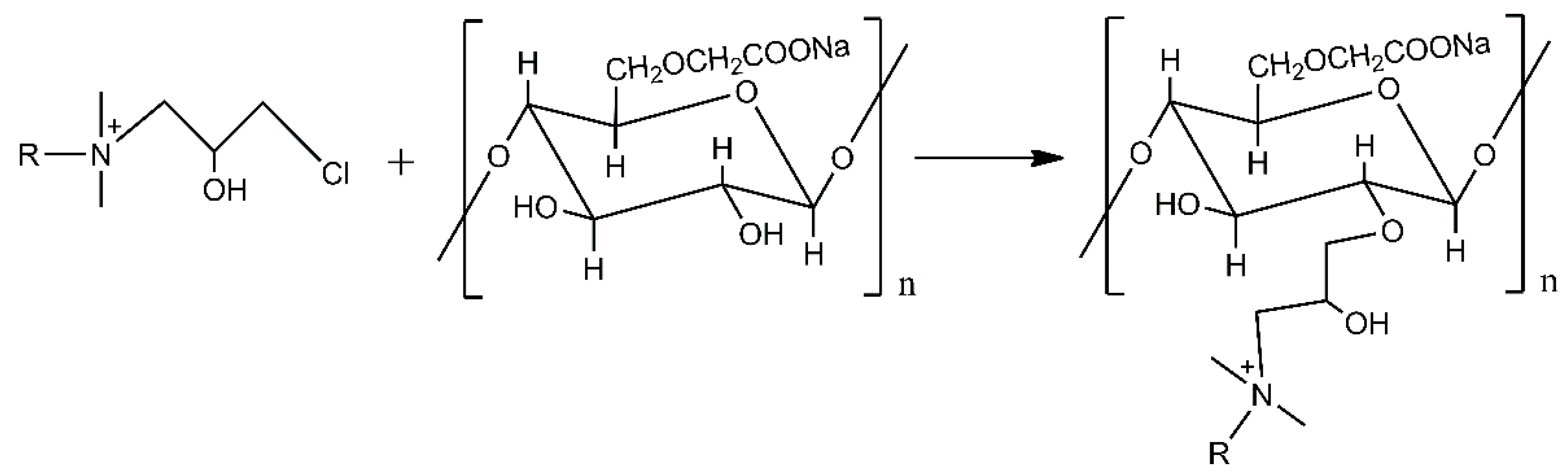
2.2.2. Modification of the CMC
2.3. Characterization Analysis
2.3.1. Characterization of Rheology
2.3.2. Characterization of IR
2.3.3. SEM Scanning
2.3.4. Drag Reduction Rate Characterization
2.3.5. XPS Characterization
2.3.6. HNMR
3. Results and Characterization
3.1. Preparation of Modified CMC
- (1)
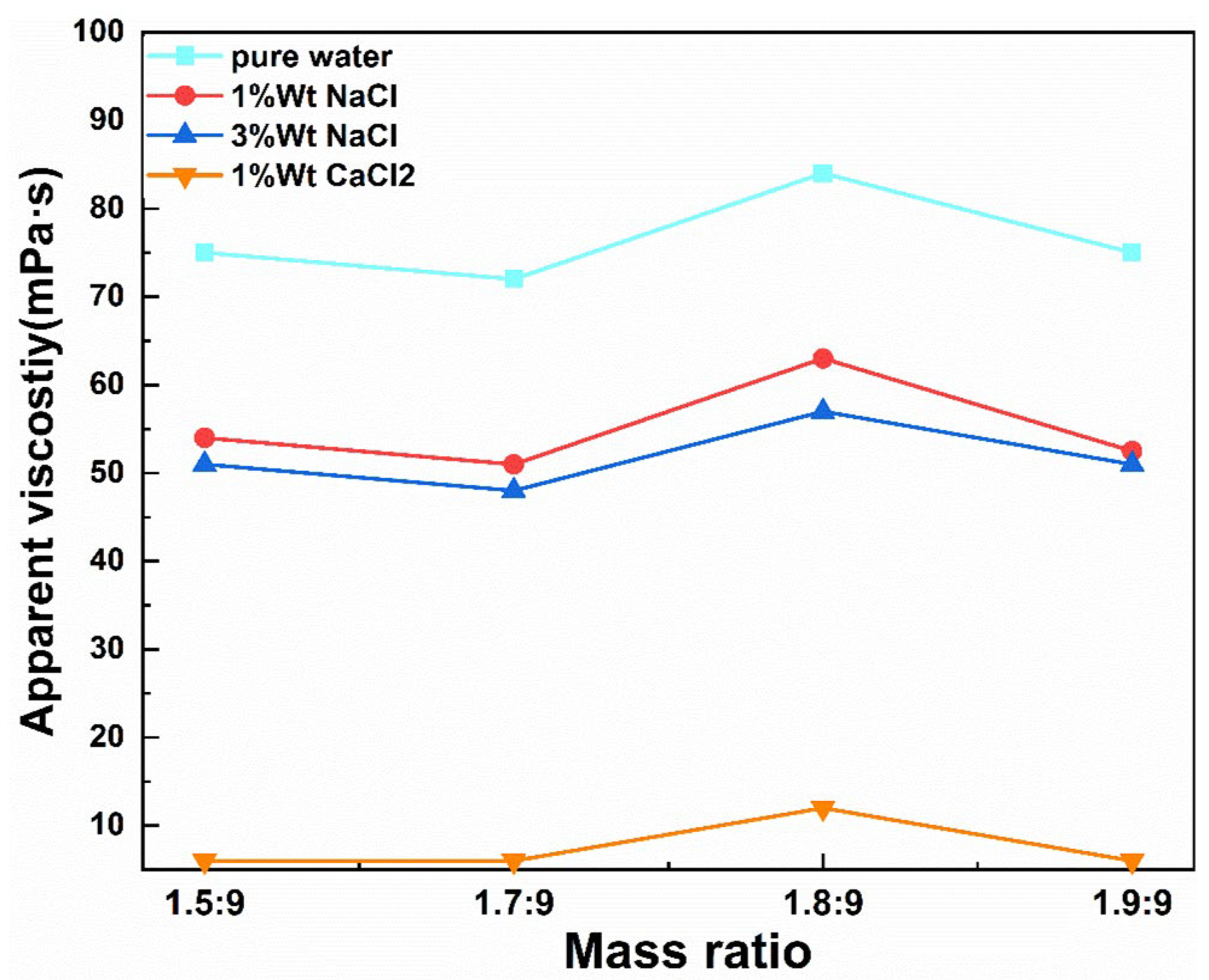
- Influence of mass ratio of intermediate to CMC on product viscosity
- (2)
- The influence of reaction time
- (3)
- The influence of reaction temperature
- (4)
- The effect of the amount of NaOH solution
3.2. Characterization Results of Modified CMC
3.2.1. Infrared Spectrogram
3.2.2. SEM
3.2.3. XPS
3.3. Performance Evaluation of Modified and Unmodified CMC
3.3.1. Apparent Viscosity
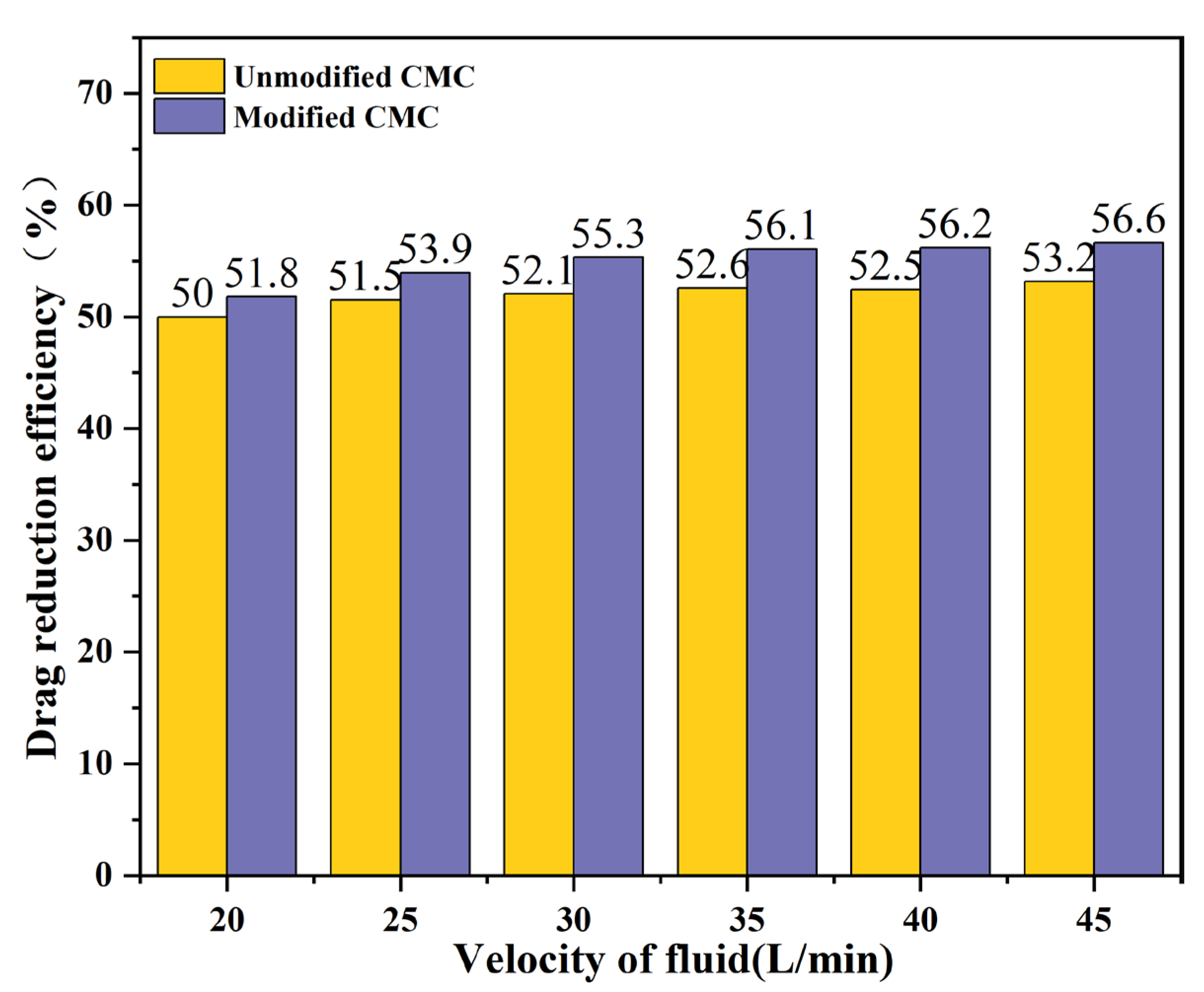
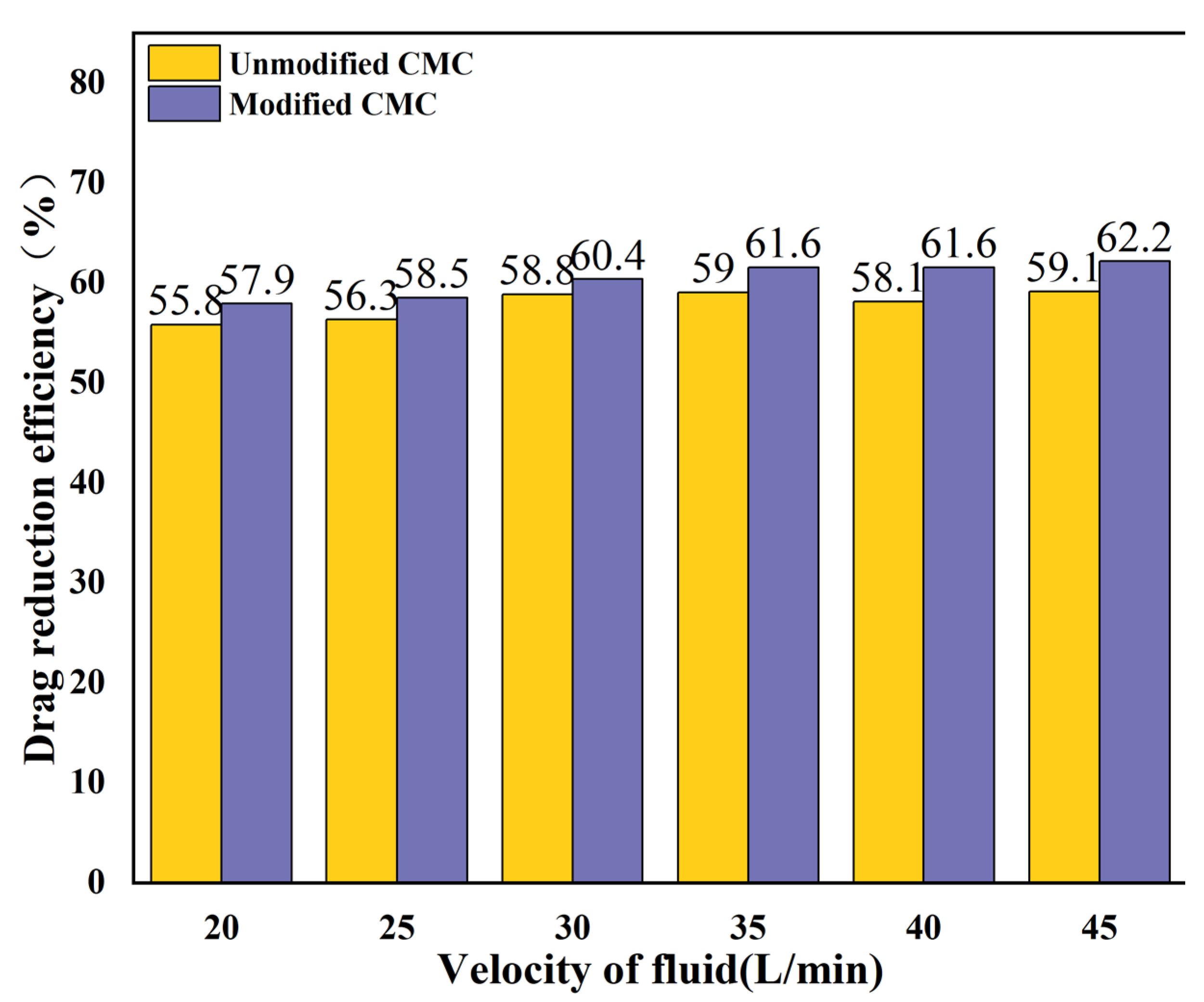
3.3.2. Drag Reduction Rates
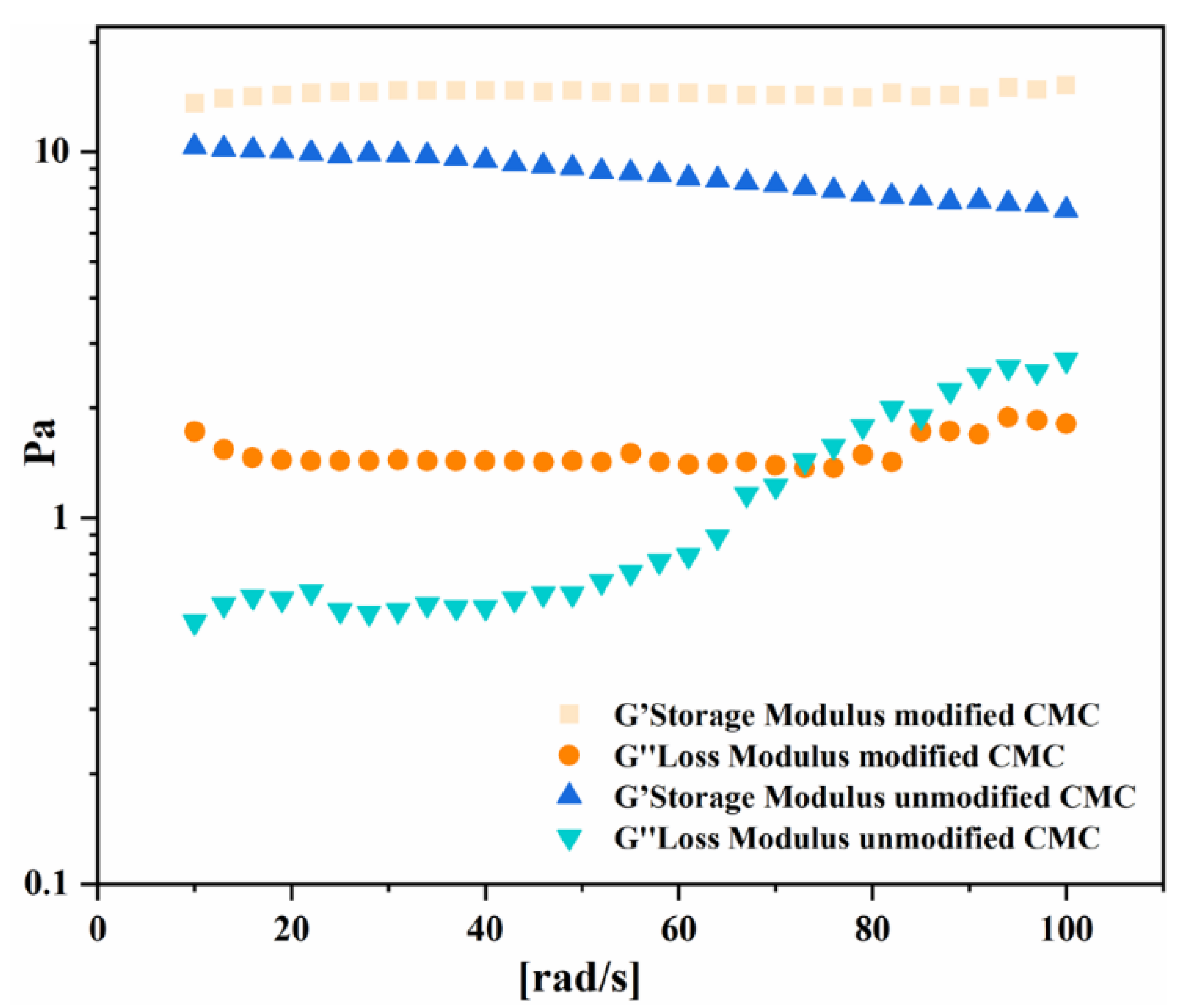
3.3.3. Comparison of Rheological Properties before and after Modification
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, G.; Wang, R. Review on Key Technologies of shale gas Exploration and Development. Explor. Eng. Geotech. Drill. Excav. Eng. 2013, 40, 3–8. [Google Scholar]
- Gudmundsson, A.; Brenner, S.L. How hydrofractures become arrested. Terra Nova 2001, 13, 456–462. [Google Scholar] [CrossRef]
- Guo, B.; Lyons, W.C.; Ghalambor, A. Matrix Acidizing. Pet. Prod. Eng. 2007, 36, 243–249. [Google Scholar]
- Palisch, T.T.; Vincent, M.; Handren, P.J. Slickwater Fracturing: Food for Thought. In Proceedings of the SPE Annual Technical Conference and Exhibition, Denver, CO, USA, 21–24 September 2008; pp. 327–344. [Google Scholar]
- Liu, K.; Du, H.; Zheng, T.; Liu, H.; Zhang, M.; Zhang, R.; Li, H.; Xie, H.; Zhang, X.; Ma, M.; et al. Recent advances in Cellulose and its derivatives for oilfield applications. Carbohydr. Polym. 2021, 259, 117740. [Google Scholar] [CrossRef]
- Langan, P.; Nishiyama, Y.; Wada, M.; Sugiyama, J.; Chanzy, H. Crystal structure and hydrogen-bonding system in cellulose from neutron fiber diffraction. Fibre Diffr. Rev. 1999, 219, U262. [Google Scholar]
- Glasser, W.G.; Atalla, R.H.; Blackwell, J.; Malcolm Brown, R.; Burchard, W.; French, A.D.; Klemm, D.O.; Nishiyama, Y. About the structure of Cellulose: Debating the Lindman hypothesis. Cellulose 2012, 19, 589–598. [Google Scholar] [CrossRef]
- Fatima, A.; Yasir, S.; Khan, M.S.; Manan, S.; Ullah, M.W.; Ul-Islam, M. Plant extract-loaded bacterial Cellulose composite membrane for potential biomedical applications. J. Bioresour. Bioprod. 2021, 6, 26–32. [Google Scholar] [CrossRef]
- Zou, Y.; Zhao, J.; Zhu, J.; Guo, X.; Chen, P.; Duan, G.; Liu, X.; Li, Y. A mussel-inspired polydopamine-filled Cellulose aerogel for solar-enabled water remediation. ACS Appl. Mater. Interfaces 2021, 13, 7617–7624. [Google Scholar] [CrossRef]
- Deeksha, B.; Sadanand, V.; Hariram, N.; Rajulu, A.V. Preparation and properties of Cellulose nanocomposite fabrics with in situ generated silver nanoparticles by bioreduction method. J. Bioresour. Bioprod. 2021, 6, 75–81. [Google Scholar] [CrossRef]
- Esmaeilirad, N.; White, S.; Terry, C.; Prior, A.; Carlson, K. Influence of inorganic ions in recycled produced water on gel-based hydraulic fracturing fluid viscosity. J. Pet. Sci. Eng. 2016, 139, 104–111. [Google Scholar] [CrossRef]
- Yaoyao, D.; Hua, M.; Dongmei, D.A.I. Application of Cellulose fracturing fluid in Sulige Gas Field. Spec. Oil Gas Reserv. 2014, 21, 123–125. [Google Scholar]
- Cao, Y.; Tan, H. Effects of cellulase on the modification of Cellulose. Carbohydr. Res. 2002, 337, 1291–1296. [Google Scholar] [CrossRef]
- Selvakumar, S.; Chandrasekar, N.; Kumar, G. Hydrogeochemical characteristics and groundwater contamination in the rapid urban development areas of Coimbatore, India. Water Resour. Ind. 2017, 17, 26–33. [Google Scholar] [CrossRef]
- McCormick, C.L.; Dawsey, T.R. Preparation of Cellulose derivatives via ring-opening reactions with cyclic reagents in lithium chloride/N, N-dimethylacetamide. Macromolecules 1990, 23, 3606–3610. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Langan, P.; Chanzy, H. Crystal structure and hydrogen-bonding system in Cellulose Iβ from synchrotron X-ray and neutron fiber diffraction. J. Am. Chem. Soc. 2002, 124, 9074–9082. [Google Scholar] [CrossRef] [PubMed]
- Hedlund, A.; Germgård, U. Some aspects on the kinetics of etherification in the preparation of CMC. Cellulose 2007, 14, 161–169. [Google Scholar] [CrossRef]
- Sun, R.; Fang, B.; Lu, Y.; Qiu, X.; Du, W.; Han, X.; Zhou, Q.; Qiu, Y. Rheological properties of hexadecyl dimethyl amine modified carboxymethyl hydroxyethyl Cellulose solutions and its gelling process. J. Dispers. Sci. Technol. 2018, 39, 138–142. [Google Scholar] [CrossRef]
- Al-Muntasheri G, A. A Critical Review of Hydraulic Fracturing Fluids over the Last Decade. In Proceedings of the SPE Western North American and Rocky Mountain Joint Meeting, Denver, CO, USA, 16–18 April 2014. [Google Scholar]
- Es-said, A.; El Moussaouiti, M.; Bchitou, R. Esterification optimization of Cellulose with p-Iodobenzoyl chloride using experimental design method. J. Polym. Res. 2019, 26, 237. [Google Scholar] [CrossRef]
- Pettignano, A.; Charlot, A.; Fleury, E. Carboxyl-functionalized derivatives of carboxymethyl Cellulose: Towards advanced biomedical applications. Polym. Rev. 2019, 59, 510–560. [Google Scholar] [CrossRef]
- Tijsen, C.J.; Kolk, H.J.; Stamhuis, E.J.; Beenackers, A.A. An experimental study on the carboxymethylation of granular potato starch in non-aqueous media. Carbohydr. Polym. 2001, 45, 219–226. [Google Scholar] [CrossRef]
- Hu, D. Preparation, Characterization and Application of Two Kinds of Seasonal Cellulose; Northeast Forestry University: Harbin, China, 2017. [Google Scholar]
- Dorris, G.M. The suface analysis of paper and wood fiber by ESCA, 1: Application to Cellulose and lignin. Cellul. Chem. Technol 1978, 12, 9–23. [Google Scholar]
- Dorris, G.M. The surface analysis of paper and wood fiber by ESCA, 2: Surface composition of mechanical pulps. Cellul. Chem. Technol. 1978, 12, 721–734. [Google Scholar]
- Keyes, D.E.; Abernathy, F.H. A model for the dynamics of polymers in laminar shear flows. J. Fluid Mech. 1987, 185, 503–522. [Google Scholar] [CrossRef]
- Mao, J.; Zhang, H.; Zhang, W.; Fan, J.; Zhang, C.; Zhao, J. Dissymmetric beauty: A novel design of heterogemini viscoelastic surfactant for the clean fracturing fluid. J. Ind. Eng. Chem. 2018, 60, 133–142. [Google Scholar] [CrossRef]
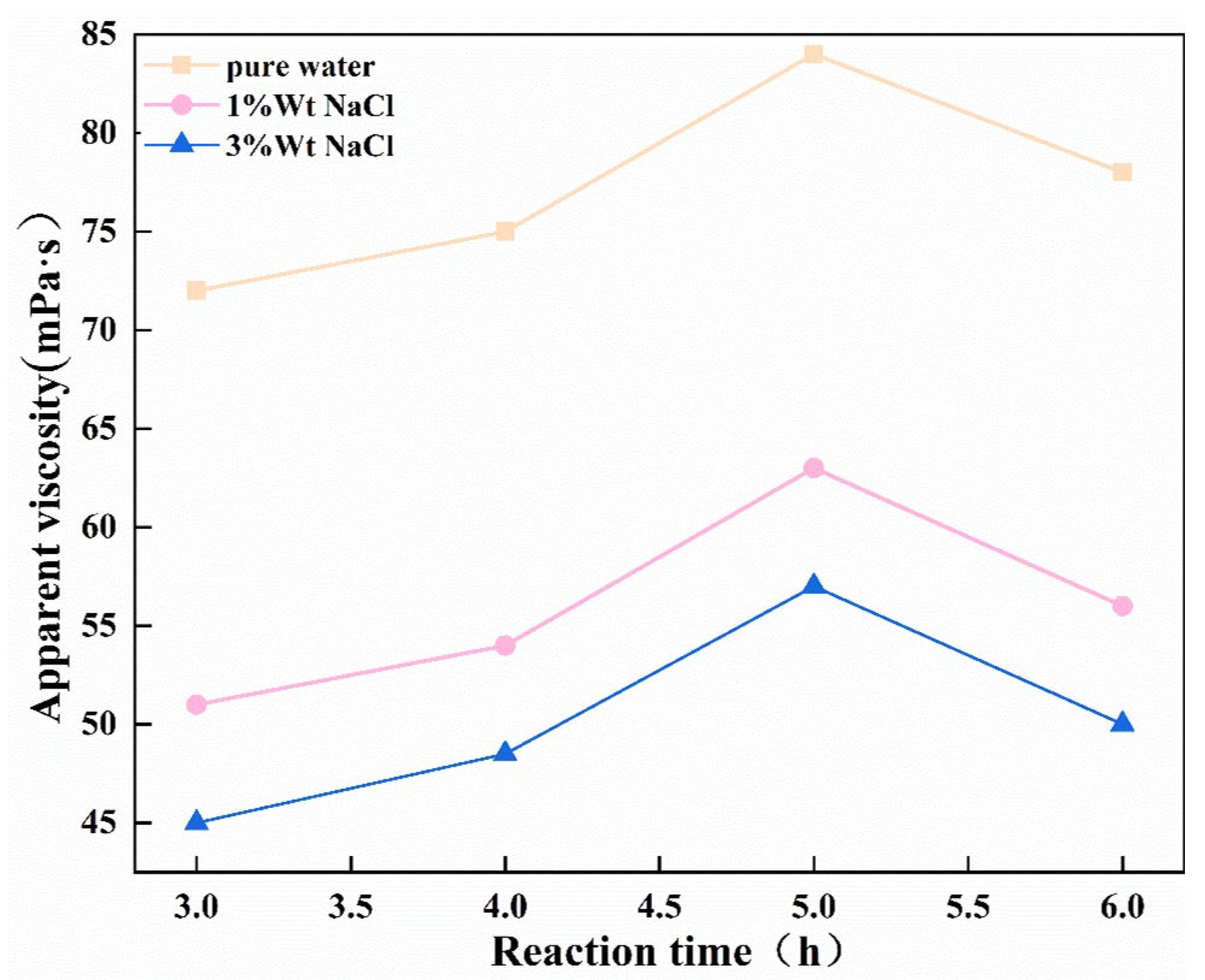
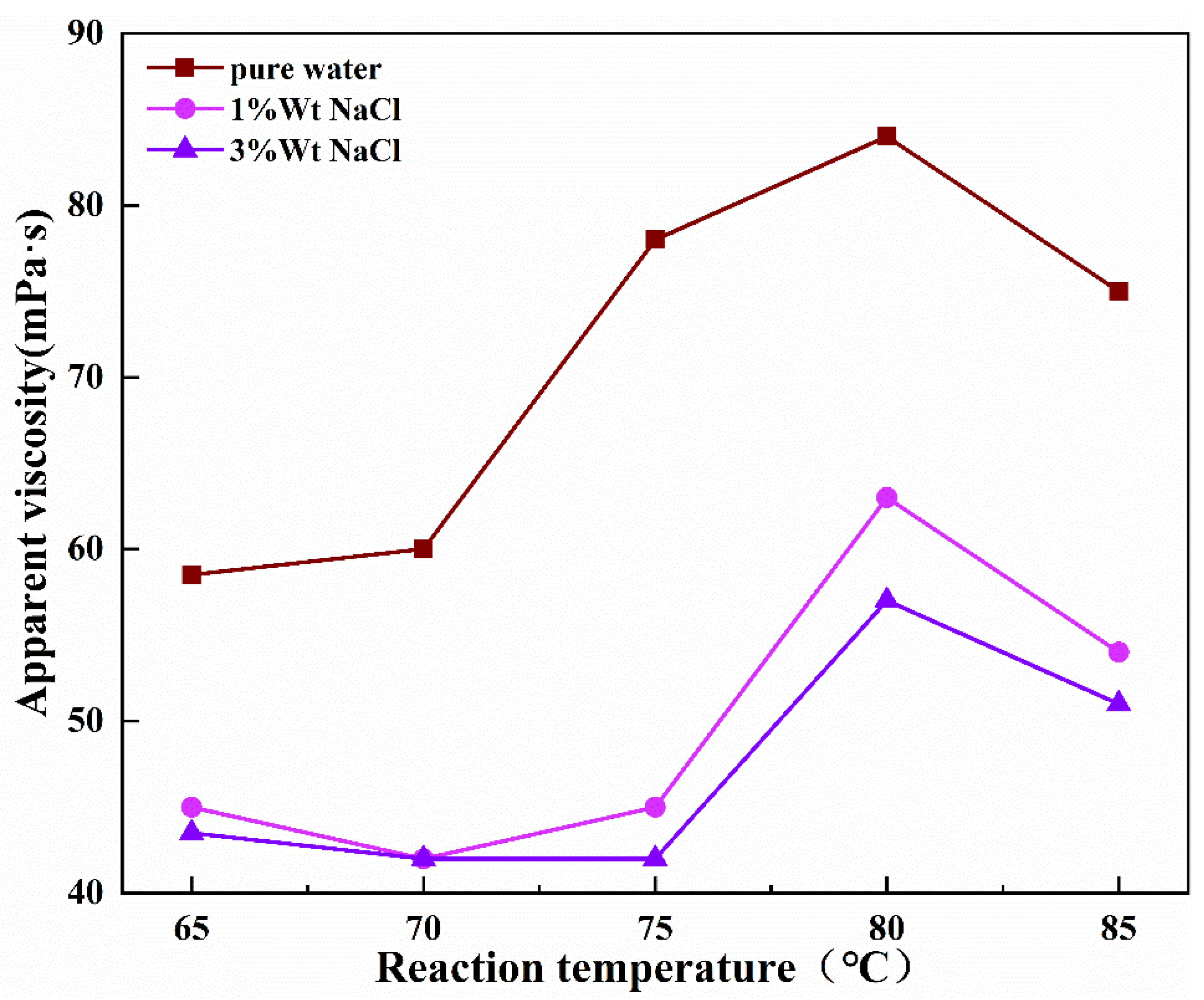





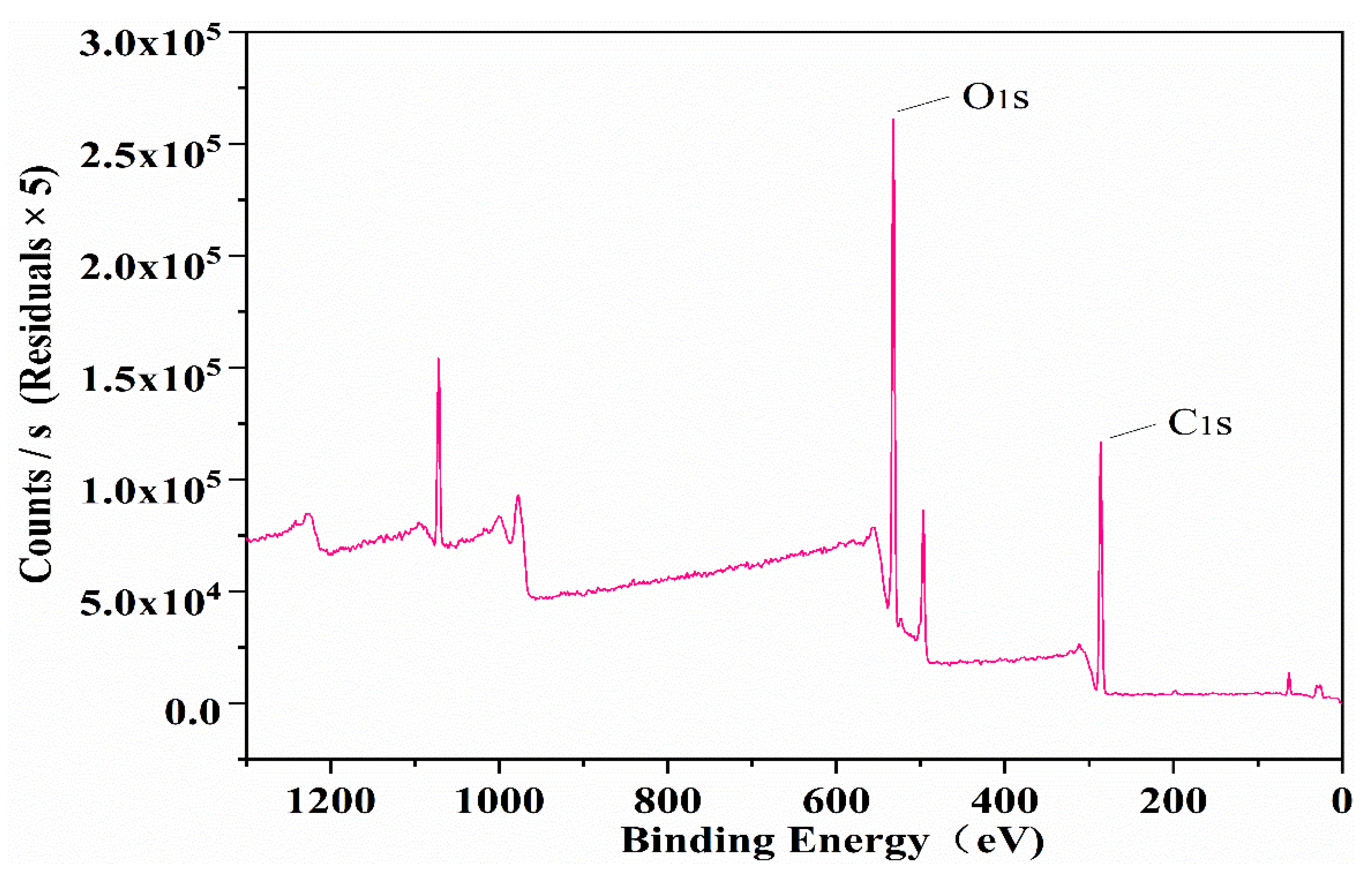
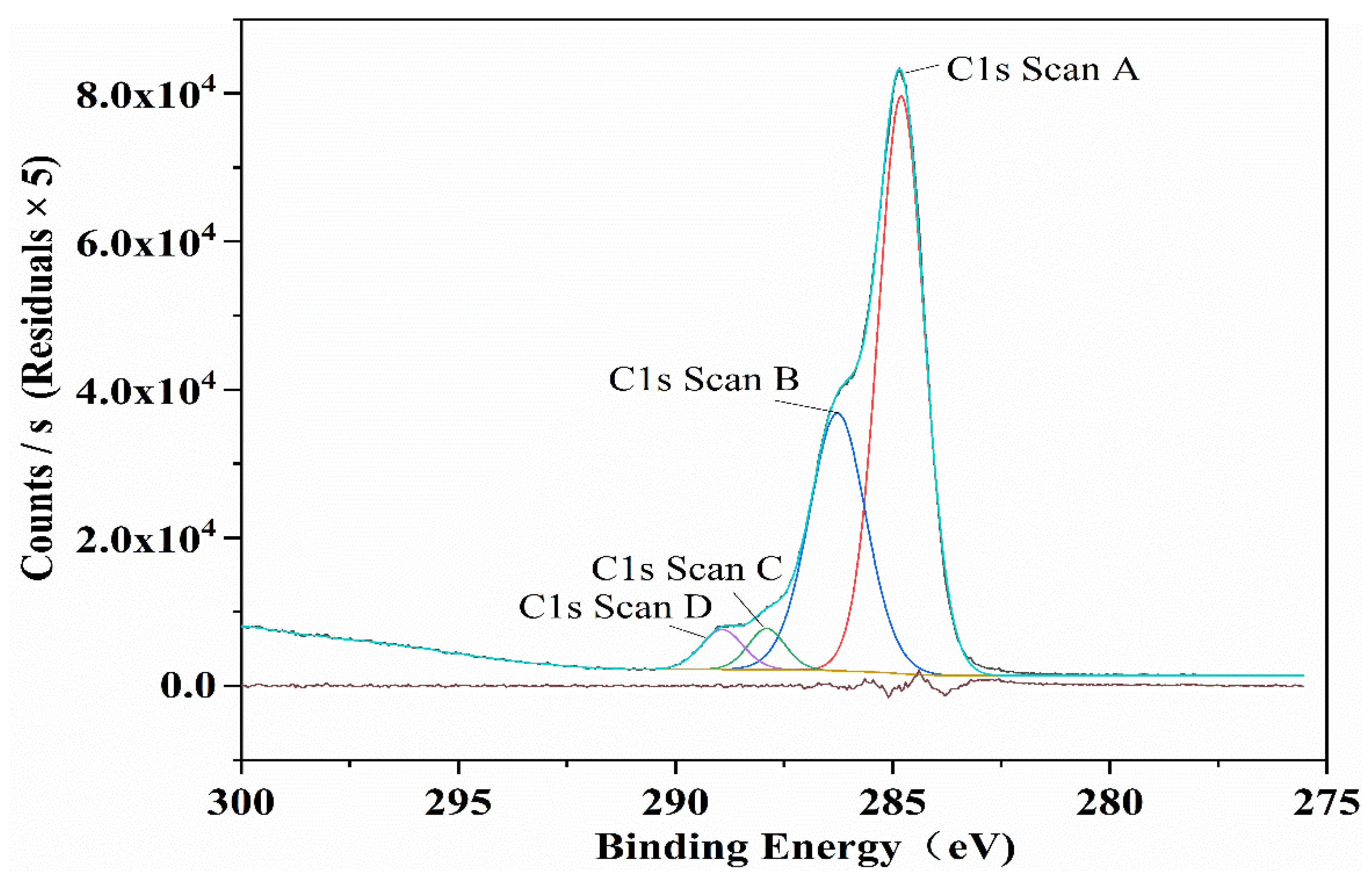

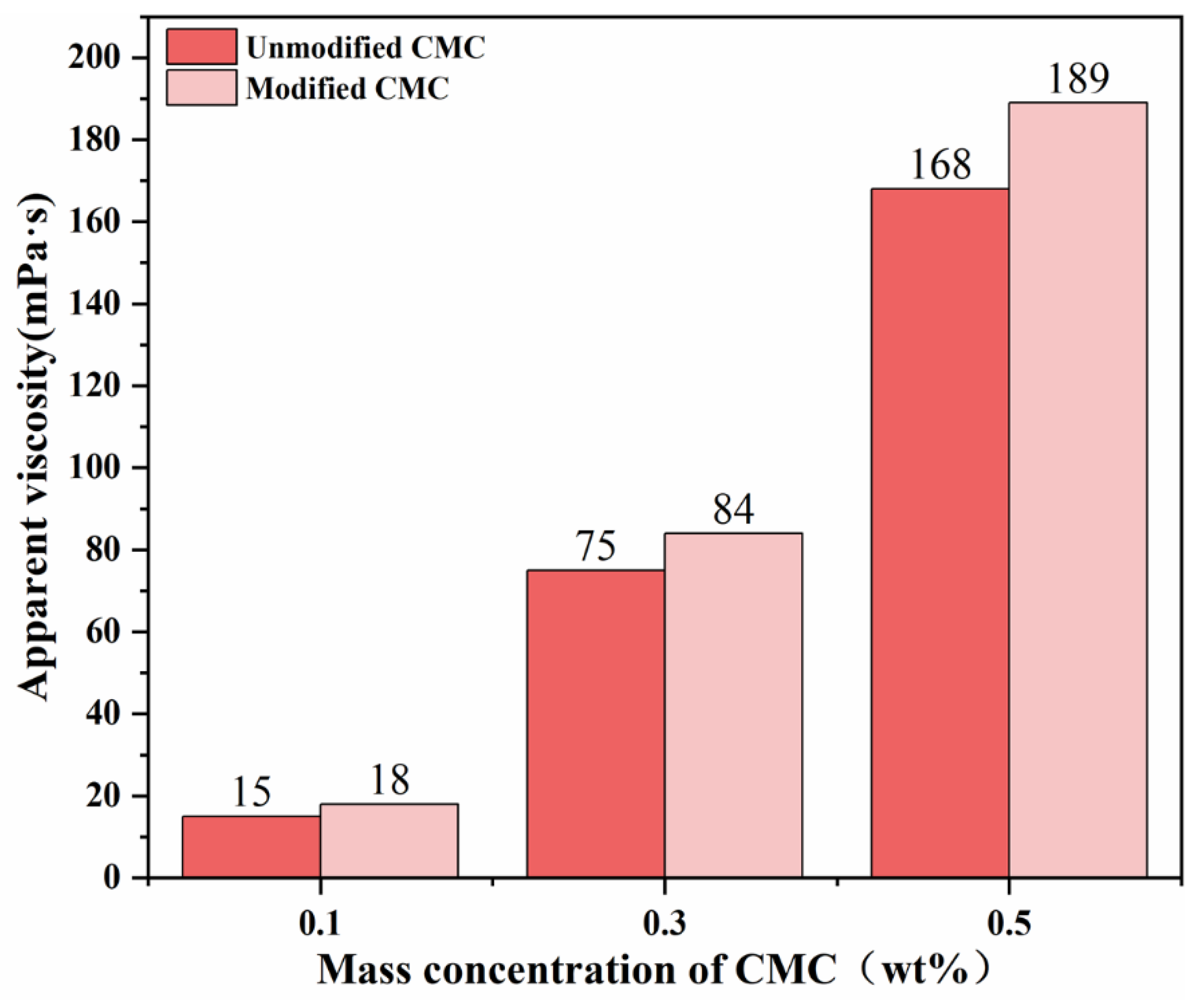
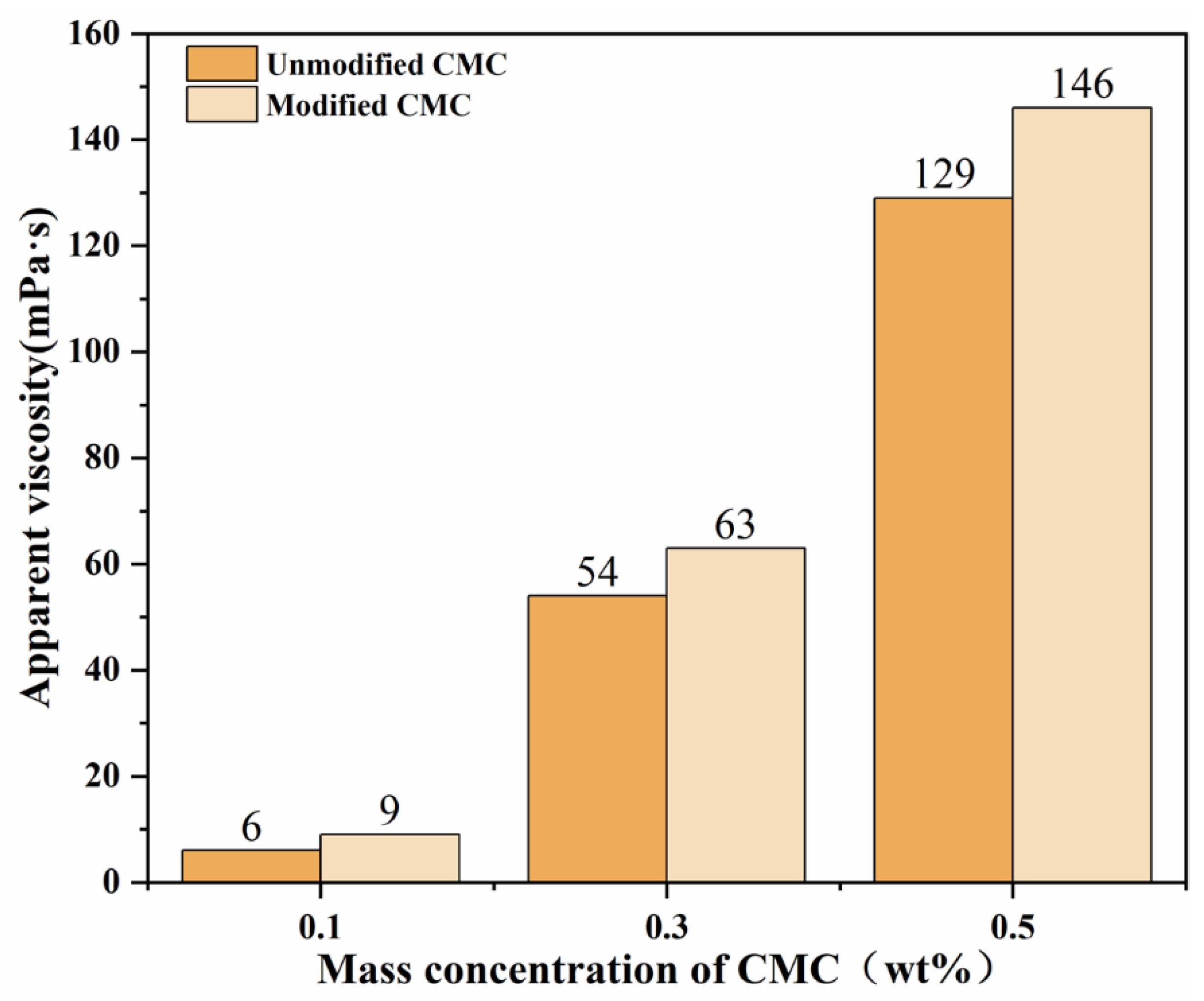
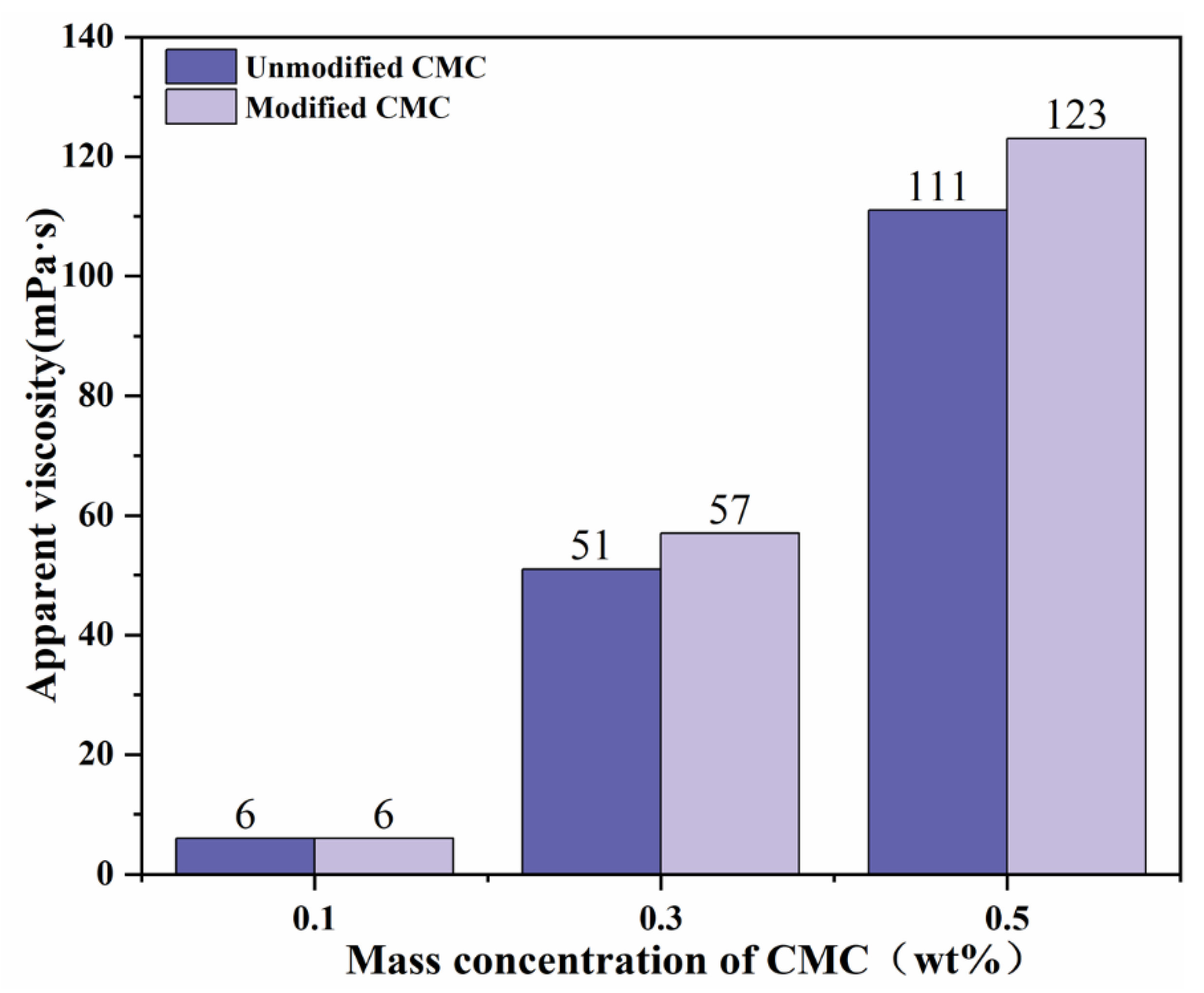
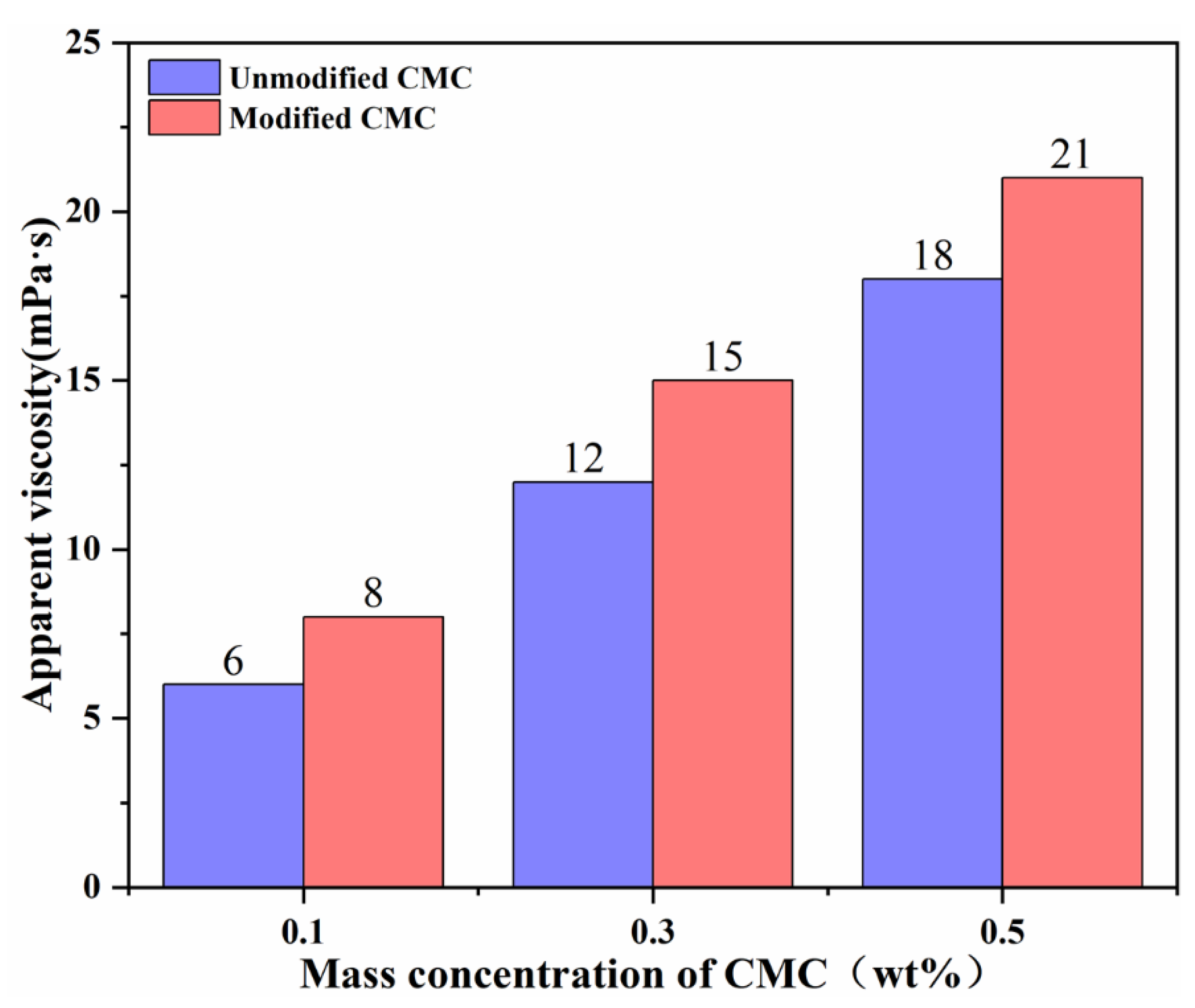
| Sample Code | Mass Ratio | Reaction Time (h) | Reaction Temperature (°C) | Amount of NaOH (mol) |
|---|---|---|---|---|
| 1 | 1.5:9 | 5 | 80 | 5 × 10−4 |
| 2 | 1.7:9 | 5 | 80 | 5 × 10−4 |
| 3 | 1.9:9 | 5 | 80 | 5 × 10−4 |
| 4 | 1.8:9 | 3 | 80 | 5 × 10−4 |
| 5 | 1.8:9 | 4 | 80 | 5 × 10−4 |
| 6 | 1.8:9 | 6 | 80 | 5 × 10−4 |
| 7 | 1.8:9 | 5 | 65 | 5 × 10−4 |
| 8 | 1.8:9 | 5 | 70 | 5 × 10−4 |
| 9 | 1.8:9 | 5 | 75 | 5 × 10−4 |
| 10 | 1.8:9 | 5 | 85 | 5 × 10−4 |
| 11 | 1.8:9 | 5 | 80 | 3 × 10−4 |
| 12 | 1.8:9 | 5 | 80 | 5 × 10−4 |
| 13 | 1.8:9 | 5 | 80 | 7 × 10−4 |
| 14 | 1.8:9 | 5 | 80 | 9 × 10−4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, Z.; Zuo, C.; Cai, H.; Li, X.; Su, X.; Yin, J.; Zhang, W. A Salt-Resistant Sodium Carboxymethyl Cellulose Modified by the Heterogeneous Process of Oleate Amide Quaternary Ammonium Salt. Polymers 2022, 14, 5012. https://doi.org/10.3390/polym14225012
Jia Z, Zuo C, Cai H, Li X, Su X, Yin J, Zhang W. A Salt-Resistant Sodium Carboxymethyl Cellulose Modified by the Heterogeneous Process of Oleate Amide Quaternary Ammonium Salt. Polymers. 2022; 14(22):5012. https://doi.org/10.3390/polym14225012
Chicago/Turabian StyleJia, Zhenfu, Chengwei Zuo, Huishan Cai, Xiaojiang Li, Xiaodong Su, Jierui Yin, and Wenlong Zhang. 2022. "A Salt-Resistant Sodium Carboxymethyl Cellulose Modified by the Heterogeneous Process of Oleate Amide Quaternary Ammonium Salt" Polymers 14, no. 22: 5012. https://doi.org/10.3390/polym14225012
APA StyleJia, Z., Zuo, C., Cai, H., Li, X., Su, X., Yin, J., & Zhang, W. (2022). A Salt-Resistant Sodium Carboxymethyl Cellulose Modified by the Heterogeneous Process of Oleate Amide Quaternary Ammonium Salt. Polymers, 14(22), 5012. https://doi.org/10.3390/polym14225012







