Synthesis and Biocompatibility Evaluation of PCL Electrospun Membranes Coated with MTA/HA for Potential Application in Dental Pulp Capping
Abstract
1. Introduction
2. Materials and Methods
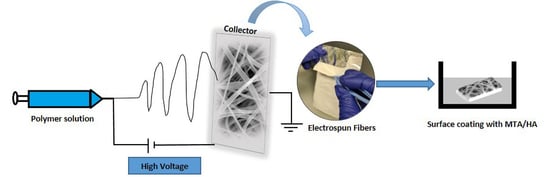
2.1. Fiber Preparation by Electrospinning
2.2. Field Emission Scanning Electron Microscopy (FE-SEM)/Energy Dispersive X-ray (EDX) Analysis
2.3. X-ray Diffraction Analysis (XRD)
2.4. Raman Spectroscopy
2.5. Contact Angle Measurement
2.6. Stability of the Coating
2.7. Human Dental Pulp Stem Cells (hDPSCs) Culture
2.8. Cell Viability Studies on PCL Membranes
2.9. Cell Adhesion and Spreading Studies by Field Emission Scanning Electron Microscopy (FE-SEM) and Confocal Microscopy
2.10. Wound Healing Assay-Scratch Test
2.11. Statistical Analysis
3. Results
3.1. FE-SEM/EDX Analysis
3.2. XRD Analysis
3.3. Raman Spectroscopy
3.4. Water Contact Angle
3.5. HA and MTA Coating Stability
3.6. Cell Viability
3.7. Cell Adhesion and Spreading Behavior on the Electrospun Membranes
3.8. Wound Healing Assay
4. Discussion
5. Conclusions
6. Limitation of the Study and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elamin, A.; Garemo, M.; Gardner, A. Dental Caries and Their Association with Socioeconomic Characteristics, Oral Hygiene Practices and Eating Habits among Preschool Children in Abu Dhabi, United Arab Emirates-the NOPLAS Project. BMC Oral Health 2018, 18, 104. [Google Scholar] [CrossRef] [PubMed]
- Global Burden of Disease Collaborative Network Global Burden of Disease Study 2019 (GBD 2019) Relative Risk; United States Institute for Health Metrics and Evaluation: Seattle, WA, USA, 2020.
- Huang, G.T.-J. Dental Pulp and Dentin Tissue Engineering and Regeneration: Advancement and Challenge. Front. Biosci. 2011, 3, 788–800. [Google Scholar] [CrossRef] [PubMed]
- Yavorek, A. The Influence of Full Coverage Restorations on Pulp Vitality: A Ten-Year Retrospective Study. Master’s Thesis, Marquette University, Milwaukee, WI, USA, 2019. [Google Scholar]
- Hilton, T.J. Keys to Clinical Success with Pulp Capping: A Review of the Literature. Oper. Dent. 2009, 34, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M. Dental Pulp Therapies: Indirect and Direct Capping and Pulp Regeneration. J. Clin. Med. Res. 2022, 3, 1–11. [Google Scholar] [CrossRef]
- Schröder, U. Effects Calcium Hydroxide-Containing Pulp-Capping Agents on Pup Cell Migration, Proliferation, and Differentiation. J. Dent. Res. 1985, 64, 541–548. [Google Scholar] [CrossRef]
- Takita, T.; Hayashi, M.; Takeichi, O.; Ogiso, B.; Suzuki, N.; Otsuka, K.; Ito, K. Effect of Mineral Trioxide Aggregate on Proliferation of Cultured Human Dental Pulp Cells. Int. Endod. J. 2006, 39, 415–422. [Google Scholar] [CrossRef]
- Daniele, L. Mineral Trioxide Aggregate (MTA) Direct Pulp Capping: 10 Years Clinical Results. G. Ital. Endod. 2017, 31, 48–57. [Google Scholar] [CrossRef]
- Agnes, A.; Long, A.; Best, S.; Lobner, D. Pulp Capping Materials Alter the Toxicity and Oxidative Stress Induced by Composite Resins in Dental Pulp Culture. Eur. Endod. J. 2017, 2, 1–6. [Google Scholar] [CrossRef]
- Onat Altan, H. The setting mechanism of mineral trioxide aggregate. J. Istanb. Univ. Fac. Dent. 2016, 50, 65–72. [Google Scholar] [CrossRef]
- Tsai, C.L.; Ke, M.C.; Chen, Y.H.; Kuo, H.K.; Yu, H.J.; Chen, C.T.; Tseng, Y.C.; Chuang, P.C.; Wu, P.C. Mineral Trioxide Aggregate Affects Cell Viability and Induces Apoptosis of Stem Cells from Human Exfoliated Deciduous Teeth. BMC Pharmacol. Toxicol. 2018, 19, 21. [Google Scholar] [CrossRef]
- Lee, L.W.; Hsiao, S.H.; Hung, W.C.; Lin, Y.H.; Chen, P.Y.; Chiang, C.P. Clinical Outcomes for Teeth Treated with Electrospun Poly(ε-Caprolactone) Fiber Meshes/Mineral Trioxide Aggregate Direct Pulp Capping. J. Endod. 2015, 41, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Oh, J.H.; Park, J.C.; Shin, H.I.; Baek, J.H.; Ryoo, H.M.; Woo, K.M. Performance of Electrospun Poly(-Caprolactone) Fiber Meshes Used with Mineral Trioxide Aggregates in a Pulp Capping Procedure. Acta Biomater. 2012, 8, 2986–2995. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Lv, S.; Lu, J.; Jiang, S.; Lin, L. Characterization of Polycaprolactone/Collagen Fibrous Scaffolds by Electrospinning and Their Bioactivity. Int. J. Biol. Macromol. 2015, 76, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, L.; Zhou, Z.; Lai, H.; Xu, P.; Liao, L.; Wei, J. Biodegradable Polymer Membranes Applied in Guided Bone/Tissue Regeneration: A Review. Polymers 2016, 8, 115. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.J.; Kim, S.E.; Hyun, Y.T.; Kim, D.H.; Lee, H.M.; Hwang, Y.M.; Park, S.A.; Shin, J.W. In Vitro Evaluation of Poly ε-Caprolactone/Hydroxyapatite Composite as Scaffolds for Bone Tissue Engineering with Human Bone Marrow Stromal Cells. Key Eng. Mater. 2007, 342–343, 369–372. [Google Scholar] [CrossRef]
- Petretta, M.; Gambardella, A.; Boi, M.; Berni, M.; Cavallo, C.; Marchiori, G.; Maltarello, M.C.; Bellucci, D.; Fini, M.; Baldini, N.; et al. Composite Scaffolds for Bone Tissue Regeneration Based on Pcl and Mg-Containing Bioactive Glasses. Biology 2021, 10, 398. [Google Scholar] [CrossRef]
- Dziadek, M.; Zagrajczuk, B.; Menaszek, E.; Cholewa-Kowalska, K. A New Insight into in vitro Behaviour of Poly(ε-Caprolactone)/Bioactive Glass Composites in Biologically Related Fluids. J. Mater. Sci. 2018, 53, 3939–3958. [Google Scholar] [CrossRef]
- Stastna, E.; Castkova, K.; Rahel, J. Influence of Hydroxyapatite Nanoparticles and Surface Plasma Treatment on Bioactivity of Polycaprolactone Nanofibers. Polymers 2020, 12, 1877. [Google Scholar] [CrossRef]
- Alipour, M.; Aghazadeh, Z.; Hassanpour, M.; Ghorbani, M.; Salehi, R.; Aghazadeh, M. MTA-Enriched Polymeric Scaffolds Enhanced the Expression of Angiogenic Markers in Human Dental Pulp Stem Cells. Stem Cells Int. 2022, 2022, 7583489. [Google Scholar] [CrossRef]
- Babaki, D.; Amoako, K.; Bahrami, A.R.; Yaghoubi, S.; Mirahmadi, M.; Matin, M.M. Mta Enhances the Potential of Adipose-Derived Mesenchymal Stem Cells for Dentin–Pulp Complex Regeneration. Materials 2020, 13, 5712. [Google Scholar] [CrossRef]
- Li, W.J.; Laurencin, C.T.; Caterson, E.J.; Tuan, R.S.; Ko, F.K. Electrospun Nanofibrous Structure: A Novel Scaffold for Tissue Engineering. J. Biomed. Mater. Res. 2002, 60, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Meireles, A.B.; Corrêa, D.K.; da Silveira, J.V.W.; Millás, A.L.G.; Bittencourt, E.; de Brito-Melo, G.E.A.; González-Torres, L.A. Trends in Polymeric Electrospun Fibers and Their Use as Oral Biomaterials. Exp. Biol. Med. 2018, 243, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, M.A.; Hutmacher, D.W. The Return of a Forgotten Polymer-Polycaprolactone in the 21st Century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Manoukian, O.S.; Arul, M.R.; Sardashti, N.; Stedman, T.; James, R.; Rudraiah, S.; Kumbar, S.G. Biodegradable Polymeric Injectable Implants for Long-Term Delivery of Contraceptive Drugs. J. Appl. Polym. Sci. 2018, 135, 46068. [Google Scholar] [CrossRef]
- Ang, L.P.K.; Zi, Y.C.; Beuerman, R.W.; Swee, H.T.; Zhu, X.; Tan, D.T.H. The Development of a Serum-Free Derived Bioengineered Conjunctival Epithelial Equivalent Using an Ultrathin Poly(ε-Caprolactone) Membrane Substrate. Investig. Opthalmology Vis. Sci. 2006, 47, 105–112. [Google Scholar] [CrossRef]
- Obregon, N.; Agubra, V.; Pokhrel, M.; Campos, H.; Flores, D.; De la Garza, D.; Mao, Y.; Macossay, J.; Alcoutlabi, M. Effect of Polymer Concentration, Rotational Speed, and Solvent Mixture on Fiber Formation Using Forcespinning®. Fibers 2016, 4, 20. [Google Scholar] [CrossRef]
- Gopinath, V.K.; Soumya, S.; Yogeshwar Chakrapani, V.; Sampath Kumar, T.S. Odontogenic Differentiation of Inflamed Dental Pulp Stem Cells (Idpscs) on Polycaprolactone (Pcl) Nanofiber Blended with Hydroxyapatite. Dent. Mater. J. 2021, 40, 312–321. [Google Scholar] [CrossRef]
- Feng, P.; Liu, M.; Peng, S.; Bin, S.; Zhao, Z.; Shuai, C. Polydopamine Modified Polycaprolactone Powder for Fabrication Bone Scaffold Owing Intrinsic Bioactivity. J. Mater. Res. Technol. 2021, 15, 3375–3385. [Google Scholar] [CrossRef]
- Parirokh, M.; Torabinejad, M. Mineral Trioxide Aggregate: A Comprehensive Literature Review-Part I: Chemical, Physical, and Antibacterial Properties. J. Endod. 2010, 36, 16–27. [Google Scholar] [CrossRef]
- Alipour, M.; Firouzi, N.; Aghazadeh, Z.; Samiei, M.; Montazersaheb, S.; Khoshfetrat, A.B.; Aghazadeh, M. The Osteogenic Differentiation of Human Dental Pulp Stem Cells in Alginate-Gelatin/Nano-Hydroxyapatite Microcapsules. BMC Biotechnol. 2021, 21, 6. [Google Scholar] [CrossRef]
- Guven, Y.; Tuna, E.B.; Dincol, M.E.; Aktoren, O. X-ray Diffraction Analysis of MTA-plus, MTA-Angelus and Diaroot Bioaggregate. Eur. J. Dent. 2014, 8, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Abou Neel, E.; Rani, K.G.A.; Samsudin, A.R. Mineralized Nodule Formation in Primary Osteoblasts Culture in Titanium Doped Phosphate Glass and in-House Prepared Freeze Dried Demineralized Bone Extracts. Mater. Chem. Phys. 2022, 276, 125425. [Google Scholar] [CrossRef]
- Lourenço, B.N.; Marchioli, G.; Song, W.; Reis, R.L.; van Blitterswijk, C.A.; Karperien, M.; van Apeldoorn, A.; Mano, J.F. Wettability Influences Cell Behavior on Superhydrophobic Surfaces with Different Topographies. Biointerphases 2012, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Liu, Z.; Feng, B.; Hu, R.; He, X.; Wang, H.; Yin, M.; Huang, H.; Zhang, H.; Wang, W. Electrospun Gelatin/PCL and Collagen/PLCL Scaffolds for Vascular Tissue Engineering. Int. J. Nanomed. 2014, 9, 2335–2344. [Google Scholar] [CrossRef]
- Chiu, Y.C.; Fang, H.Y.; Hsu, T.T.; Lin, C.Y.; Shie, M.Y. The Characteristics of Mineral Trioxide Aggregate/Polycaprolactone 3-Dimensional Scaffold with Osteogenesis Properties for Tissue Regeneration. J. Endod. 2017, 43, 923–929. [Google Scholar] [CrossRef]
- Alipour, M.; Aghazadeh, M.; Akbarzadeh, A.; Vafajoo, Z.; Aghazadeh, Z.; Raeisdasteh Hokmabad, V. Towards Osteogenic Differentiation of Human Dental Pulp Stem Cells on PCL-PEG-PCL/Zeolite Nanofibrous Scaffolds. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3431–3437. [Google Scholar] [CrossRef]
- Wu, Y.; Azmi, D.F.; Rosa, V.; Fawzy, A.S.; Fuh, J.Y.; Wong, Y.S.; Lu, W.F. Fabrication of Dentin-like Scaffolds through Combined 3D Printing and Bio-Mineralisation. Cogent Eng. 2016, 3, 1222777. [Google Scholar] [CrossRef]
- Bhargav, A.; Min, K.S.; Wen Feng, L.; Fuh, J.; Rosa, V. Taguchi’s methods to optimize the properties and bioactivity of 3D printed polycaprolactone/mineral trioxide aggregate scaffold: Theoretical predictions and experimental validation. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 629–637. [Google Scholar] [CrossRef]
- Lee, L.W.; Hsiao, S.H.; Lin, Y.H.; Chen, P.Y.; Lee, Y.L.; Hung, W.C. Outcomes of necrotic immature open-apex central incisors treated by MTA apexification using poly(ε-caprolactone) fiber mesh as an apical barrier. J. Formos. Med. Assoc. 2019, 118, 362–370. [Google Scholar] [CrossRef]
- Tsai, S.W.; Yu, W.X.; Hwang, P.A.; Hsu, Y.W.; Hsu, F.Y. Fabrication and Characteristics of PCL Membranes Containing Strontium-Substituted Hydroxyapatite Nanofibers for Guided Bone Regeneration. Polymers 2019, 11, 1761. [Google Scholar] [CrossRef]
- Xu, H.; Xu, F.; Zhao, J.; Zhou, C.; Liu, J. Platelet-Rich Plasma Induces Autophagy and Promotes Regeneration in Human Dental Pulp Cells. Front. Bioeng. Biotechnol. 2021, 9, 659742. [Google Scholar] [CrossRef] [PubMed]
- Manaspon, C.; Jongwannasiri, C.; Chumprasert, S.; Sa-Ard-Iam, N.; Mahanonda, R.; Pavasant, P.; Porntaveetus, T.; Osathanon, T. Human Dental Pulp Stem Cell Responses to Different Dental Pulp Capping Materials. BMC Oral Health 2021, 21, 209. [Google Scholar] [CrossRef] [PubMed]
- D’Antò, V.; Di Caprio, M.P.; Ametrano, G.; Simeone, M.; Rengo, S.; Spagnuolo, G. Effect of Mineral Trioxide Aggregate on Mesenchymal Stem Cells. J. Endod. 2010, 36, 1839–1843. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, J.; Luo, T.; Shen, Y.; Zou, L. Effects of Two Fast-Setting Pulp-Capping Materials on Cell Viability and Osteogenic Differentiation in Human Dental Pulp Stem Cells: An in vitro Study. Arch. Oral Biol. 2019, 100, 100–105. [Google Scholar] [CrossRef]
- Farrugia, C.; Baca, P.; Camilleri, J.; Arias Moliz, M.T. Antimicrobial activity of ProRoot MTA in contact with blood. Sci. Rep. 2017, 7, 41359. [Google Scholar] [CrossRef] [PubMed]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheela, S.; AlGhalban, F.M.; Khalil, K.A.; Laoui, T.; Gopinath, V.K. Synthesis and Biocompatibility Evaluation of PCL Electrospun Membranes Coated with MTA/HA for Potential Application in Dental Pulp Capping. Polymers 2022, 14, 4862. https://doi.org/10.3390/polym14224862
Sheela S, AlGhalban FM, Khalil KA, Laoui T, Gopinath VK. Synthesis and Biocompatibility Evaluation of PCL Electrospun Membranes Coated with MTA/HA for Potential Application in Dental Pulp Capping. Polymers. 2022; 14(22):4862. https://doi.org/10.3390/polym14224862
Chicago/Turabian StyleSheela, Soumya, Fatma Mousa AlGhalban, Khalil Abdelrazek Khalil, Tahar Laoui, and Vellore Kannan Gopinath. 2022. "Synthesis and Biocompatibility Evaluation of PCL Electrospun Membranes Coated with MTA/HA for Potential Application in Dental Pulp Capping" Polymers 14, no. 22: 4862. https://doi.org/10.3390/polym14224862
APA StyleSheela, S., AlGhalban, F. M., Khalil, K. A., Laoui, T., & Gopinath, V. K. (2022). Synthesis and Biocompatibility Evaluation of PCL Electrospun Membranes Coated with MTA/HA for Potential Application in Dental Pulp Capping. Polymers, 14(22), 4862. https://doi.org/10.3390/polym14224862