Biodegradable Active Packaging Material Containing Grape Seed Ethanol Extract and Corn Starch/κ-Carrageenan Composite Film
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
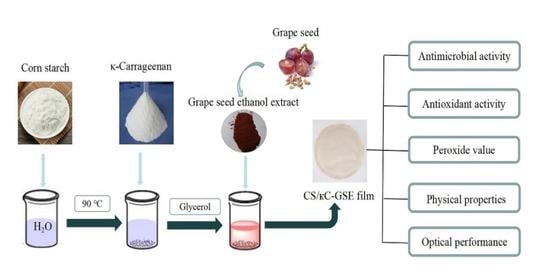
2.2. Extraction of GSE
2.3. Preparation of Films
2.4. Characterization of the CS/κC-GSE Films
2.4.1. Mechanical Property
2.4.2. Water Vapor Permeability (WVP)
2.4.3. Analysis of Film Color and Transparency
2.4.4. Scanning Electron Microscopy (SEM)
2.4.5. Fourier Transform Infrared (FT-IR) Spectroscopy
2.4.6. X-ray Diffraction (XRD)
2.4.7. Differential Scanning Calorimetry (DSC) Analysis
2.5. Assay of the Antioxidant and Antibacterial Activity of Films
2.5.1. Total Phenol Content
2.5.2. DPPH Free Radical Scavenging Activity of Films
2.5.3. ABTS Free Radical Scavenging Activity of Films
2.5.4. Antimicrobial Activity
2.5.5. Determination of Peroxide Value
2.6. Statistical Analysis
3. Results and Discussion
3.1. Physical Properties of the CS/κC-GSE Films
3.1.1. Mechanical Properties
3.1.2. Water Vapor Permeability (WVP)
3.1.3. Color, opacity, and light transmittance of the film
3.2. Scanning Electron Microscopy (SEM)
3.3. Fourier Transform Infrared (FTIR) Spectroscopy
3.4. X-ray Diffraction (XRD)
3.5. Differential Scanning Calorimetry (DSC) Analysis
3.6. Biological Activity of the CS/κC-GSE Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cui, H.Y.; Surendhiran, D.; Li, C.Z.; Lin, L. Biodegradable zein active film containing chitosan nanoparticle encapsulated with pomegranate peel extract for food packaging. Food Packag. Shelf Life 2020, 24, 100511. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, Y.; Yong, H.; Liu, J.; Zhang, X.; Liu, J. Preparation and characterization of active and intelligent packaging films based on cassava starch and anthocyanins from Lycium ruthenicum murr. Int. J. Biol. Macromol. 2019, 134, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Prietto, L.; Mirapalhete, T.C.; Pinto, V.Z.; Hoffmann, J.F.; Vanier, N.L.; Lim, L.T.; Dias, A.R.G.; Zavareze, E.D.R. pH-sensitive films containing anthocyanins extracted from black bean seed coat and red cabbage. LWT Food Sci. Technol. 2017, 80, 492–500. [Google Scholar] [CrossRef]
- Marvizadeh, M.M.; Tajik, A.; Moosavian, V.; Oladzadabbasabadi, N.; Nafchi, A.M. Fabrication of cassava starch/mentha piperita essential oil biodegradable film with enhanced antibacterial properties. J. Chem. Health Risks 2021, 11, 23–29. [Google Scholar] [CrossRef]
- Riaz, A.; Lagnika, C.; Luo, H.; Dai, Z.; Nie, M.; Hashim, M.M.; Liu, C.; Song, J.; Li, D. Chitosan-based biodegradable active food packaging film containing Chinese chive (Allium tuberosum) root extract for food application. Int. J. Biol. Macromol. 2020, 150, 595–604. [Google Scholar] [CrossRef]
- Perez, F.P.; Resa, C.P.O.; Gerschenson, L.N.; Jagus, R.J. Addition of zein for the improvement of physicochemical properties of antimicrobial tapioca starch edible film. Food Bioprocess Technol. 2021, 14, 262–271. [Google Scholar] [CrossRef]
- Evangelho, J.; Dannenberg, G.; Biduski, B.; Halal, S.; Kringel, D.H.; Gularte, M.A.; Fiorentini, A.M.; Zavareze, E. Antibacterial activity, optical, mechanical, and barrier properties of corn starch films containing orange essential oil. Carbohydr. Polym. 2019, 222, 114981. [Google Scholar] [CrossRef]
- Trongchuen, K.; Ounkaew, A.; Kasemsiri, P.; Hiziroglu, S.; Mongkolthanaruk, W.; Wannasutta, R.; Pongsa, U.; Chindaprasirt, P. Bioactive starch foam composite enriched with natural antioxidants from spent coffee ground and essential oil. Starch 2017, 70, 1700238. [Google Scholar] [CrossRef]
- Abdin, M.; El-Beltagy, A.E.; Naeem, M.A. Characterization, rheological properties, and immunomodulatory efficiency of corn silk polysaccharides. Int. J. Food Sci. Technol. 2022. [Google Scholar] [CrossRef]
- Kong, R.Q.; Wang, J.; Cheng, M.; Lu, W.Q.; Chen, M.L.; Zhang, R.F.; Wang, X.Y. Development and characterization of corn starch/pva active films incorporated with carvacrol nanoemulsions. Int. J. Biol. Macromol. 2020, 164, 1631–1639. [Google Scholar] [CrossRef]
- Na, Y.G.; Huh, H.W.; Kim, M.K.; Byeon, J.J.; Han, M.G.; Lee, H.K.; Cho, C.W. Development and evaluation of a film-forming system hybridized with econazole-loaded nanostructured lipid carriers for enhanced antifungal activity against dermatophytes. ActaBiomater. 2020, 101, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Hamdan, M.A.; Ramli, N.A.; Othman, N.A.; Amin, K.N.M.; Adam, F. Characterization and property investigation of microcrystalline cellulose (MCC) and carboxymethyl cellulose (CMC) filler on the carrageenan-based biocomposite film. Mater. Today Proc. 2021, 42, 56–62. [Google Scholar] [CrossRef]
- Sandhu, K.S.; Sharma, L.; Kaur, M.; Kaur, R. Physical, structural and thermal properties of composite edible films prepared from pearl millet starch and carrageenan gum: Process optimization using response surface methodology. Int. J. Biol. Macromol. 2020, 143, 704–713. [Google Scholar] [CrossRef]
- Yang, C.L.; Shang, K.; Lin, C.C.; Wang, H.; Shi, X.Q.; Wang, H.; Li, H. Processing technologies, phytochemical constituents, and biological activities of grape seed oil (GSO): A review. Trends Food Sci. Technol. 2021, 116, 1074–1083. [Google Scholar] [CrossRef]
- Monteiro, G.C.; Minatel, I.O.; Junior, A.P.; Gomez-Gomez, H.A.; de Camargo, J.P.C.; Diamante, M.S.; Basílio, L.S.P.; Tecchio, M.A.; Lima, G.P.P. Bioactive compounds and antioxidant capacity of grape pomace flours. LWT 2021, 135, 110053. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Sapuan, S.M.; Sanyang, M.L.; Ishak, M.R.; Zainudin, E.S. Nanocrystalline cellulose as reinforcement for polymeric matrix nanocomposites and its potential applications: A review. Curr. Anal. Chem. 2017, 264, 203–225. [Google Scholar] [CrossRef]
- Gungor, E.; Altop, A.; Erener, G. Effect of raw and fermented grape seed on growth performance, antioxidant capacity, and cecal microflora in broiler chickens. Animal 2021, 15, 100194. [Google Scholar] [CrossRef]
- Moro, K.I.B.; Bender, A.B.B.; Da Silva, L.P.; Penna, N.G. Green extraction methods and microencapsulation technologies of phenolic compounds from grape pomace: A review. Food Bioprocess Technol. 2021, 14, 1407–1431. [Google Scholar] [CrossRef]
- Guo, Y.C.; Huang, J.C.; Chen, Y.R.; Hou, Q.; Huang, M. Effect of grape seed extract combined with modified atmosphere packaging on the quality of roast chicken. Poult. Sci. 2020, 99, 1598–1605. [Google Scholar] [CrossRef]
- Gomaa, M.M.; Fadly, E.E.; Salama, M.A.; Abdin, M. Production of bio-composite films from gum Arabic and Galangal extract to prolong the shelf life of Agaricus bisporus. J. Polym. Environ. 2022, 30, 4787–4799. [Google Scholar] [CrossRef]
- Abdin, M.; El-Beltagy, A.E.; El-Sayed, M.E.; Naeem, M.A. Production and characterization of sodium alginate/Gum Arabic based films enriched with Syzygium cumini seeds extracts for food application. J. Polym. Environ. 2022, 30, 1615–1626. [Google Scholar] [CrossRef]
- Gao, Z.M.; Wang, C.T.; Li, Z.Y. Effect of ethanol extract of black soybean coat on physicochemical properties and biological activities of chitosan packaging film. Food Sci. Biotechnol. 2021, 30, 1369–1381. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.Y.; Chen, M.Y.; Zhou, Y.Q.; Li, Y.; Hu, Y.Q. Functional characteristics improvement by structural modification of hydroxypropyl methylcellulose modified polyvinyl alcohol films incorporating roselle anthocyanins for shrimp freshness monitoring. Int. J. Biol. Macromol. 2020, 162, 1250–1261. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Rhim, J.W. Fabrication of carboxymethyl cellulose/agar-based functional film hybridized with alizarin and grapefruit seed extract. ACS Appl. Bio Mater. 2021, 4, 4470–4478. [Google Scholar] [CrossRef]
- Wang, X.C.; Yong, H.M.; Gao, L.; Li, L.; Jin, M.; Liu, J. Preparation and characterization of antioxidant and pH-sensitive films based on chitosan and black soybean seed coat extract. Food Hydrocoll. 2018, 89, 56–66. [Google Scholar] [CrossRef]
- Chilczuk, B.; Marciniak, B.; Stochmal, A.; Pecio, U.; Kontek, R.; Jackowska, L.; Materska, M. Anticancer potential and capsianosides identification in lipophilic fraction of sweet pepper (Capsicum annuum L.). Molecules 2020, 25, 3097. [Google Scholar] [CrossRef]
- Riaz, A.; Lagnika, C.; Abdin, M.; Hashim, M.M.; Ahmed, W. Preparation and characterization of chitosan/gelatin-based active food packaging films containing apple peel nanoparticles. J. Polym. Environ. 2019, 28, 411–420. [Google Scholar] [CrossRef]
- Söğüt, E.; Seydim, A.C. The effects of chitosan and grape seed extract-based edible films on the quality of vacuum packaged chicken breast fillets. Food Packag. Shelf Life 2018, 18, 13–20. [Google Scholar] [CrossRef]
- Wadaugsorn, K.; Panrong, T.; Wongphan, P.; Harnkarnsujarit, N. Plasticized hydroxypropyl cassava starch blended PBAT for improved clarity blown films: Morphology and properties. Ind. Crop. Prod. 2022, 176, 114311. [Google Scholar] [CrossRef]
- Basch, C.Y.; Jagus, R.J.; Flores, S.K. Physical and Antimicrobial Properties of Tapioca Starch-HPMC Edible Films Incorporated with Nisin and/or Potassium Sorbate. Food Bioprocess Technol. 2013, 6, 2419–2428. [Google Scholar] [CrossRef]
- Chen, C.-H.; Kuo, W.-S.; Lai, L.-S. Development of Tapioca Starch/Decolorized Hsian-Tsao Leaf Gum-Based Antimicrobial Films: Physical Characterization and Evaluation Against Listeria monocytogenes. Food Bioprocess Technol. 2013, 6, 1516–1525. [Google Scholar] [CrossRef]
- Gutierrez, T.J.; Gonzalez, G. Effects of Exposure to Pulsed Light on Surface and Structural Properties of Edible Films Made from Cassava and Taro Starch. Food Bioprocess Technol. 2016, 9, 1812–1824. [Google Scholar] [CrossRef]
- Motta, J.F.G.; de Souza, A.R.; Goncalves, S.M.; Mandella, D.K.S.F.; de Carvalho, C.W.P.; Vitorazi, L.; de Melo, N.R. Development of active films based on modified starches incorporating the antimicrobial agent lauroyl arginate (LAE) for the food industry. Food Bioprocess Technol. 2020, 13, 2082–2093. [Google Scholar] [CrossRef]
- Bof, M.J.; Jiménez, A.; Locaso, D.E.; García, M.A.; Chiralt, A. Grapefruit seed extract and lemon essential oil as active agents in corn starch-chitosan blend films. Food Bioprocess Technol. 2016, 9, 2033–2045. [Google Scholar] [CrossRef]
- Moradi, M.; Tajik, H.; Rohani, S.M.R.; Oromiehie, A.R.; Malekinejad, H.; Aliakbarlu, J.; Hadian, M. Characterization of antioxidant chitosan film incorporated with Zataria multiflora Boiss essential oil and grape seed extract. LWT Food Sci. Technol. 2012, 46, 477–484. [Google Scholar] [CrossRef]
- Luchese, C.L.; Uranga, J.; Spada, J.C.; Tessaro, I.C.; de la Caba, K. Valorisation of blueberry waste and use of compression to manufacture sustainable starch films with enhanced properties. Int. J. Biol. Macromol. 2018, 115, 955–960. [Google Scholar] [CrossRef]
- Shahbazi, Y. The properties of chitosan and gelatin films incorporated with ethanolic red grape seed extract and Ziziphora clinopodioides essential oil as biodegradable materials for active food packaging. Int. J. Biol. Macromol. 2017, 99, 746–753. [Google Scholar] [CrossRef]
- Rubilar, J.F.; Cruz, R.M.S.; Silva, H.D.; Vicente, A.A.; Khmelinskii, I.; Vieira, M.C. Physico-mechanical properties of chitosan films with carvacrol and grape seed extract. J. Food Eng. 2013, 115, 466–474. [Google Scholar] [CrossRef]
- Söğüt, E.; Seydim, A.C. Characterization of cyclic olefin copolymer-coated chitosan bilayer films containing nanocellulose and grape seed extract. Packag. Technol. Sci. 2018, 31, 499–508. [Google Scholar] [CrossRef]
- Promsorn, J.; Harnkarnsujarit, N. Oxygen absorbing food packaging made by extrusion compounding of thermoplastic cassava starch with gallic acid. Food Control 2022, 142, 109273. [Google Scholar] [CrossRef]
- Wu, Z.G.; Deng, W.J.; Luo, J.W.; Deng, D.Y. Multifunctional nano-cellulose composite films with grape seed extracts and immobilized silver nanoparticles. Carbohydr. Polym. 2018, 205, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Tavares, K.M.; Campos, A.D.; Luchesi, B.R.; Resende, A.A.; de Oliveira, J.E.; Marconcini, J.M. Effect of carboxymethyl cellulose concentration on mechanical and water vapor barrier properties of corn starch films. Carbohydr. Polym. 2020, 246, 116521. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, S.; Wu, Q.Q.; Gu, Y.Y.; Kan, J.; Jin, C.H. Effect of protocatechuic acid incorporation on the physical, mechanical, structural and antioxidant properties of chitosan film. Food Hydrocoll. 2017, 73, 90–100. [Google Scholar] [CrossRef]
- Ghelejlu, S.B.; Esmaiili, M.; Almasi, H. Characterization of chitosan-nanoclay bionanocomposite active films containing milk thistle extract. Int. J. Biol. Macromol. 2016, 86, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Promsorn, J.; Harnkarnsujarit, N. Pyrogallol loaded thermoplastic cassava starch based films as bio-based oxygen scavengers. Ind. Crop. Prod. 2022, 186, 115226. [Google Scholar] [CrossRef]
- Phothisarattana, D.; Harnkarnsujarit, N. Migration, aggregations and thermal degradation behaviors of TiO2 and ZnO incorporated PBAT/TPS nanocomposite blown films. Food Packag. Shelf Life 2022, 33, 100901. [Google Scholar] [CrossRef]
- Naghdi, S.; Rezaei, M.; Abdollahi, M. A starch-based pH-sensing and ammonia detector film containing betacyanin of paperflower for application in intelligent packaging of fish. Int. J. Biol. Macromol. 2021, 191, 161–170. [Google Scholar] [CrossRef]
- Jayakumar, A.; Heera, K.V.; Sumi, T.S.; Joseph, M.; Mathew, S.; Praveen, G.; Indu, C.N.; Radhakrishnan, E.K. Starch-pva composite films with zinc-oxide nanoparticles and phytochemicals as intelligent pH sensing wraps for food package application. Int. J. Biol. Macromol. 2019, 136, 395–403. [Google Scholar] [CrossRef]
- Vostrejs, P.; Adamcová, D.; Vaverková, M.D.; Enev, V.; Kalina, M.; Machovsky, M.; Šourková, M.; Marova, I.; Kovalcik, A. Active biodegradable packaging films modified with grape seeds lignin. RSC Adv. 2020, 10, 29202–29213. [Google Scholar] [CrossRef]
- Goiana, M.L.; de Brito, E.S.; Filho, E.G.A.; Miguel, E.D.C.; Fernandes, F.A.N.; de Azeredo, H.M.C.; Rosa, M. Corn starch based films treated by dielectric barrier discharge plasma. Int. J. Biol. Macromol. 2021, 183, 2009–2016. [Google Scholar] [CrossRef]
- Liu, D.A.; Dang, S.; Zhang, L.; Munsop, K.; Li, X.X. Corn starch/polyvinyl alcohol based films incorporated with curcumin-loaded pickering emulsion for application in intelligent packaging. Int. J. Biol. Macromol. 2021, 188, 974–982. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Huang, R.L.; Zha, X.Q.; Li, Q.M.; Pan, L.H.; Luo, J.P. Encapsulation and sustained release of curcumin by a composite hydrogel of lotus root amylopectin and chitosan. Carbohydr. Polym. 2019, 232, 115810. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, L.; Zhang, C.; Show, P.L.; Du, A.; Fu, J.C.; Veeramuthu, A. Preparation and characterization of curdlan/polyvinyl alcohol/ thyme essential oil blending film and its application to chilled meat preservation. Carbohydr. Polym. 2022, 247, 116670. [Google Scholar] [CrossRef]
- Yuan, G.Q.; Jia, Y.N.; Pan, Y.X.; Li, W.W.; Wang, C.; Wang, C.; Xu, L.; Chen, H.X. Preparation and characterization of shrimp shell waste protein-based films modified with oolong tea, corn silk and black soybean seed coat extracts. Polym. Test. 2019, 81, 106235. [Google Scholar] [CrossRef]
- Jiang, G.; Hou, X.; Zeng, X.; Zhang, C.; Wu, H.; Shen, G.; Li, S.; Luo, Q.; Li, M.; Liu, X.; et al. Preparation and characterization of indicator films from carboxymethyl-cellulose/starch and purple sweet potato (Ipomoea batatas (L.) lam) anthocyanins for monitoring fish freshness. Int. J. Biol. Macromol. 2020, 143, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Aslaner, G.; Sumnu, G.; Sahin, S. Encapsulation of grape seed extract in rye flour and whey protein-based electrospun nanofibers. Food Bioprocess Technol. 2021, 14, 1118–1131. [Google Scholar] [CrossRef]
- Niazi, M.B.K.; Broekhuis, A.A. Surface photo-crosslinking of plasticized thermoplastic starch films. Eur. Polym. J. 2015, 64, 229–243. [Google Scholar] [CrossRef]
- Kowalczyk, D.; Szymanowska, U.; Skrzypek, T.; Cembala, M.B.; Materska, M.; Łupina, K. Corn starch and methylcellulose edible films incorporated with fireweed (Chamaenerion angustifolium L.) extract: Comparison of physicochemical and antioxidant properties. Int. J. Biol. Macromol. 2021, 190, 969–977. [Google Scholar] [CrossRef]
- Wang, W.; Wang, K.; Xiao, J.; Liu, Y.; Zhao, Y.; Liu, A. Performance of high amylose starch-composited gelatin films influenced by gelatinization and concentration. Int. J. Biol. Macromol. 2017, 94, 258–265. [Google Scholar] [CrossRef]
- Pichayakorn, W.; Panrat, K.; Suksaeree, J.; Taweepreda, W. Propranolol hydrochloride film coated tablets using natural rubber latex blends as film former. J. Polym. Environ. 2021, 30, 925–927. [Google Scholar] [CrossRef]
- Maroufi, L.Y.; Ghorbani, M.; Tabibiazar, M. A gelatin-based film reinforced by covalent interaction with oxidized guar gum containing green tea extract as an active food packaging system. Food Bioprocess Technol. 2020, 13, 1633–1644. [Google Scholar] [CrossRef]
- Bi, F.Y.; Zhang, X.; Bai, R.Y.; Liu, Y.P.; Liu, J.; Liu, J. Preparation and characterization of antioxidant and antimicrobial packaging films based on chitosan and proanthocyanidins. Int. J. Biol. Macromol. 2019, 134, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.L.; Huang, J.Y.; Ying, Y.B.; Hu, L.P.; Hu, Y.Q. Ph-sensitive and antibacterial films developed by incorporating anthocyanins extracted from purple potato or roselle into chitosan/polyvinyl alcohol/nano-zno matrix: Comparative study. Int. J. Biol. Macromol. 2021, 178, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Simona, J.; Dani, D.; Petr, S.; Marcela, N.; Jakub, T.; Bohuslava, T. Edible films from carrageenan/orange essential oil/trehalose—Structure, optical properties, and antimicrobial activity. Polymers 2021, 13, 332. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.X.; Song, R.; Zhang, X.H.; Zhang, D.W. Enhanced antimicrobial activity and pH-responsive sustained release of chitosan/poly (vinyl alcohol)/graphene oxide nanofibrous membrane loading with allicin. Int. J. Biol. Macromol. 2020, 161, 1405–1413. [Google Scholar] [CrossRef]
- Lim, J.Y.; Lee, C.L.; Kim, G.H.; Bang, Y.J.; Rhim, J.W.; Yoon, K.S. Using lactic acid bacteria and packaging with grapefruit seed extract for controlling listeria monocytogenes growth in fresh soft cheese. J. Dairy Sci. 2020, 103, 8761–8770. [Google Scholar] [CrossRef]







| Films Sample | Tensile Strength (MPa) | Elongation at Break (%) | WVP (g·mm/m2·d kPa) |
|---|---|---|---|
| CS/κC film | 9.07 ± 0.45 a | 22.37 ± 0.98 c | 1.08 ± 0.04 c |
| 1% CS/κC-GSE film | 5.34 ± 0.31 b | 25.94 ± 0.97 bc | 1.25 ± 0.02 bc |
| 3% CS/κC-GSE film | 4.27 ± 0.41 bc | 29.92 ± 1.94 b | 1.38 ± 0.04 b |
| 5% CS/κC-GSE film | 3.50 ± 0.27 c | 36.87 ± 2.08 a | 1.58 ± 0.03 a |
| Film Sample | L* | a* | b* | Opaqueness s/% | Picture |
|---|---|---|---|---|---|
| CS/κC film | 90.24 ± 0.33 a | −1.22 ± 0.04 d | −0.18 ± 0.08 d | 1.06 ± 0.13 d |  |
| 1% CS/κC-GSE film | 85.74 ± 0.50 b | 1.70 ± 0.20 c | 3.64 ± 0.22 c | 2.25 ± 0.43 c |  |
| 3% CS/κC-GSE film | 81.22 ± 0.73 c | 4.18 ± 0.32 b | 6.96 ± 0.35 b | 3.03 ± 0.14 b |  |
| 5% CS/κC-GSE film | 77.08 ± 1.05 d | 6.74 ± 0.48 a | 10.90 ± 0.64 a | 3.76 ± 0.18 a |  |
| Extract Concentration | Diameter of the Bacteriostatic Circle (mm) | |
|---|---|---|
| Escherichia coli | Staphylococcus aureus | |
| CS/κC film | 7.06 ± 0.03 d | 7.02 ± 0.05 d |
| 1% CS/κC-GSE film | 9.62 ± 0.12 c | 10.04 ± 0.10 c |
| 3% CS/κC-GSE film | 10.96 ± 0.28 b | 11.02 ± 0.22 b |
| 5% CS/κC-GSE film | 12.78 ± 0.42 a | 13.52 ± 0.66 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; An, X.; Lu, Y.; Li, Z.; Gao, Z.; Tian, S. Biodegradable Active Packaging Material Containing Grape Seed Ethanol Extract and Corn Starch/κ-Carrageenan Composite Film. Polymers 2022, 14, 4857. https://doi.org/10.3390/polym14224857
Wang C, An X, Lu Y, Li Z, Gao Z, Tian S. Biodegradable Active Packaging Material Containing Grape Seed Ethanol Extract and Corn Starch/κ-Carrageenan Composite Film. Polymers. 2022; 14(22):4857. https://doi.org/10.3390/polym14224857
Chicago/Turabian StyleWang, Cuntang, Xuanzhe An, Yueyi Lu, Ziyu Li, Zengming Gao, and Shengxin Tian. 2022. "Biodegradable Active Packaging Material Containing Grape Seed Ethanol Extract and Corn Starch/κ-Carrageenan Composite Film" Polymers 14, no. 22: 4857. https://doi.org/10.3390/polym14224857
APA StyleWang, C., An, X., Lu, Y., Li, Z., Gao, Z., & Tian, S. (2022). Biodegradable Active Packaging Material Containing Grape Seed Ethanol Extract and Corn Starch/κ-Carrageenan Composite Film. Polymers, 14(22), 4857. https://doi.org/10.3390/polym14224857