The Preparation of Monomer Casting Polyamide 6/Thermotropic Liquid Crystalline Polymer Composite Materials with Satisfactory Miscibility
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
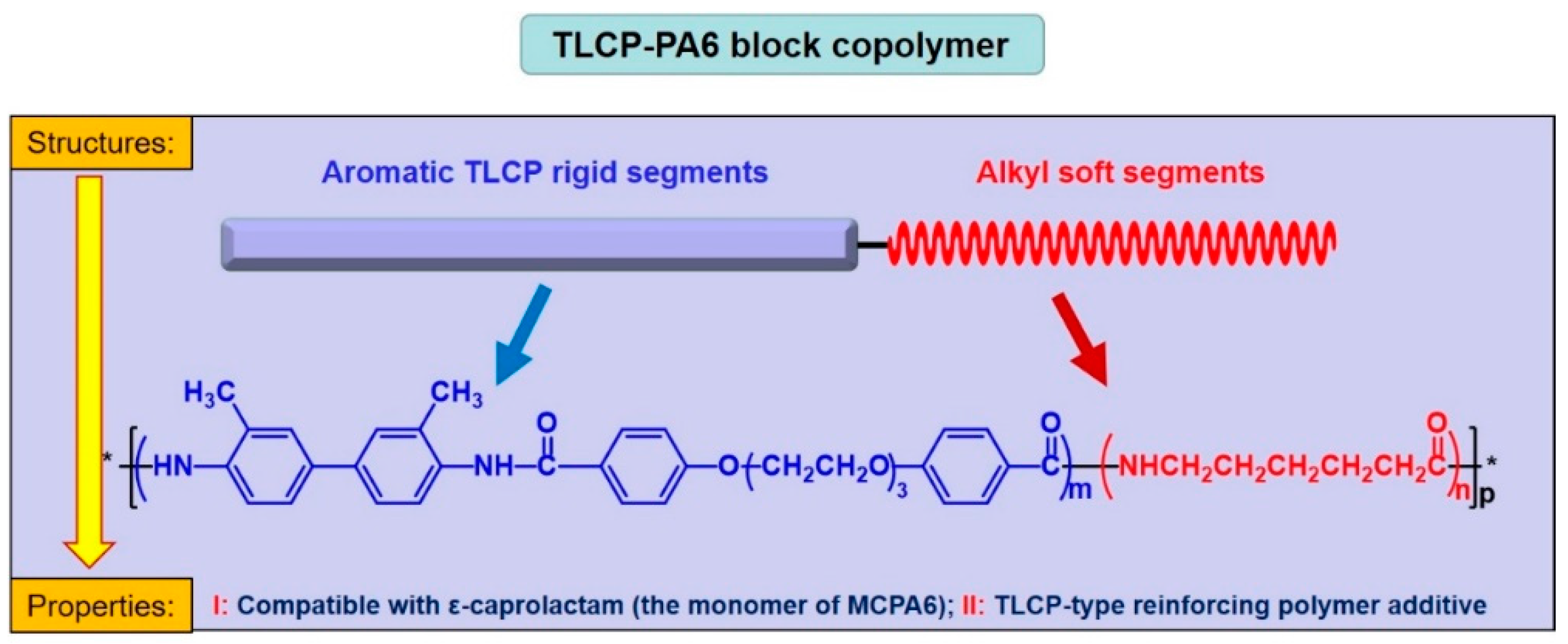
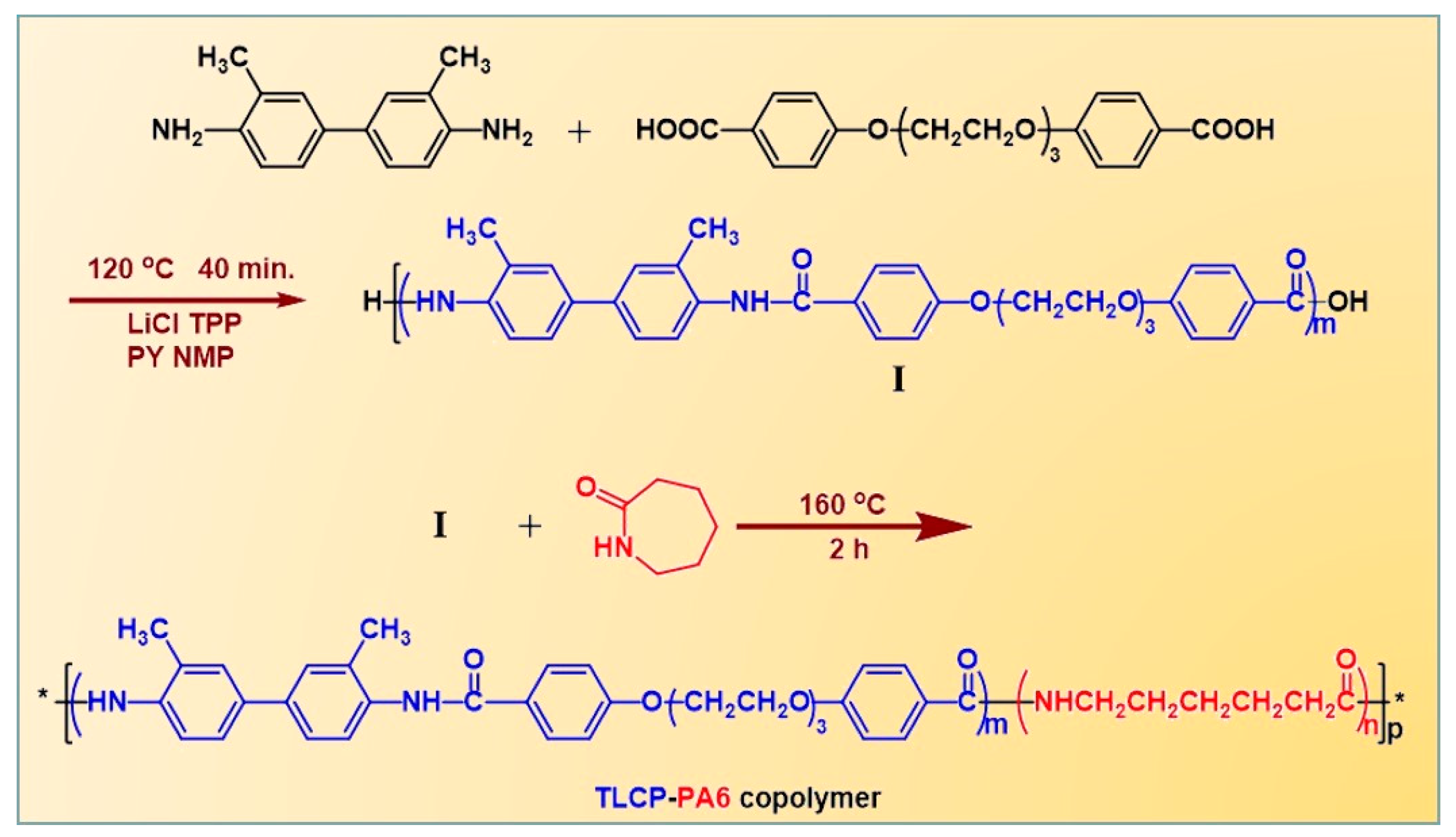
2.2. Synthesis of Thermotropic Polyamide Liquid Crystalline Block Copolymers (TLCP-PA6)
2.3. Preparation of MCPA6/TLCP-PA6 Blends
2.4. Measurement
3. Results
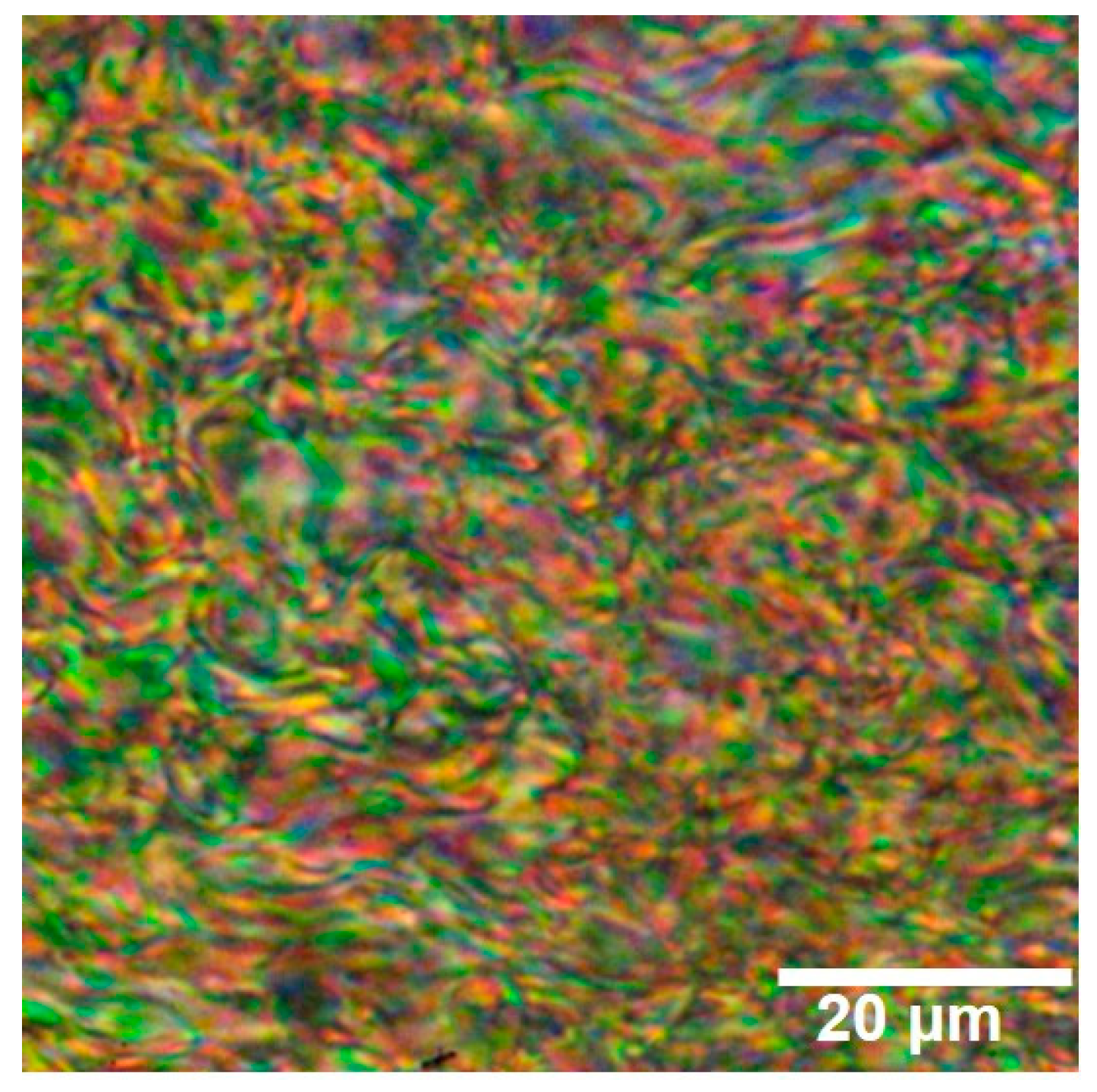
3.1. Structure and Liquid Crystalline Analysis of TLCP-PA6
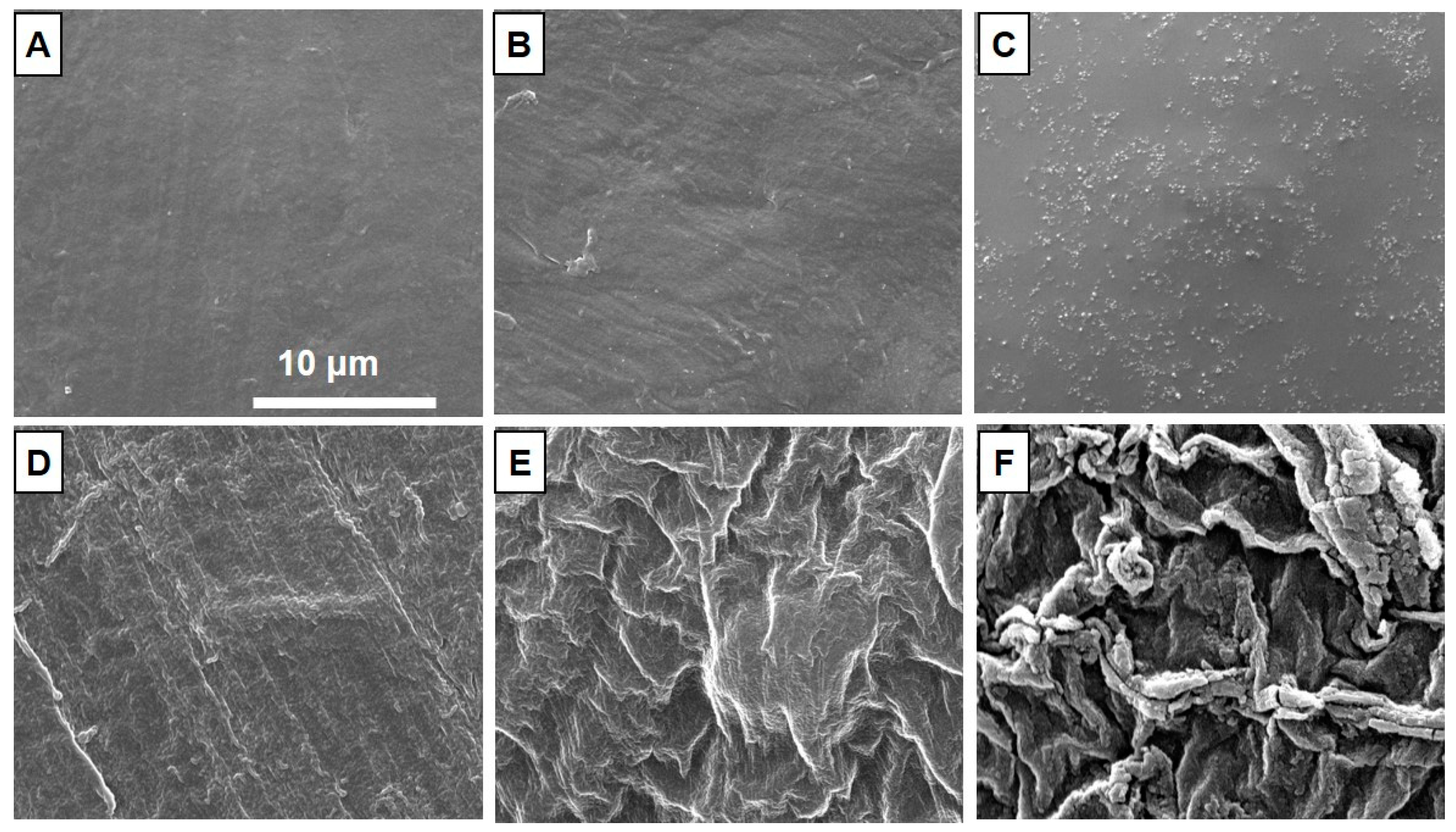
3.2. Morphology
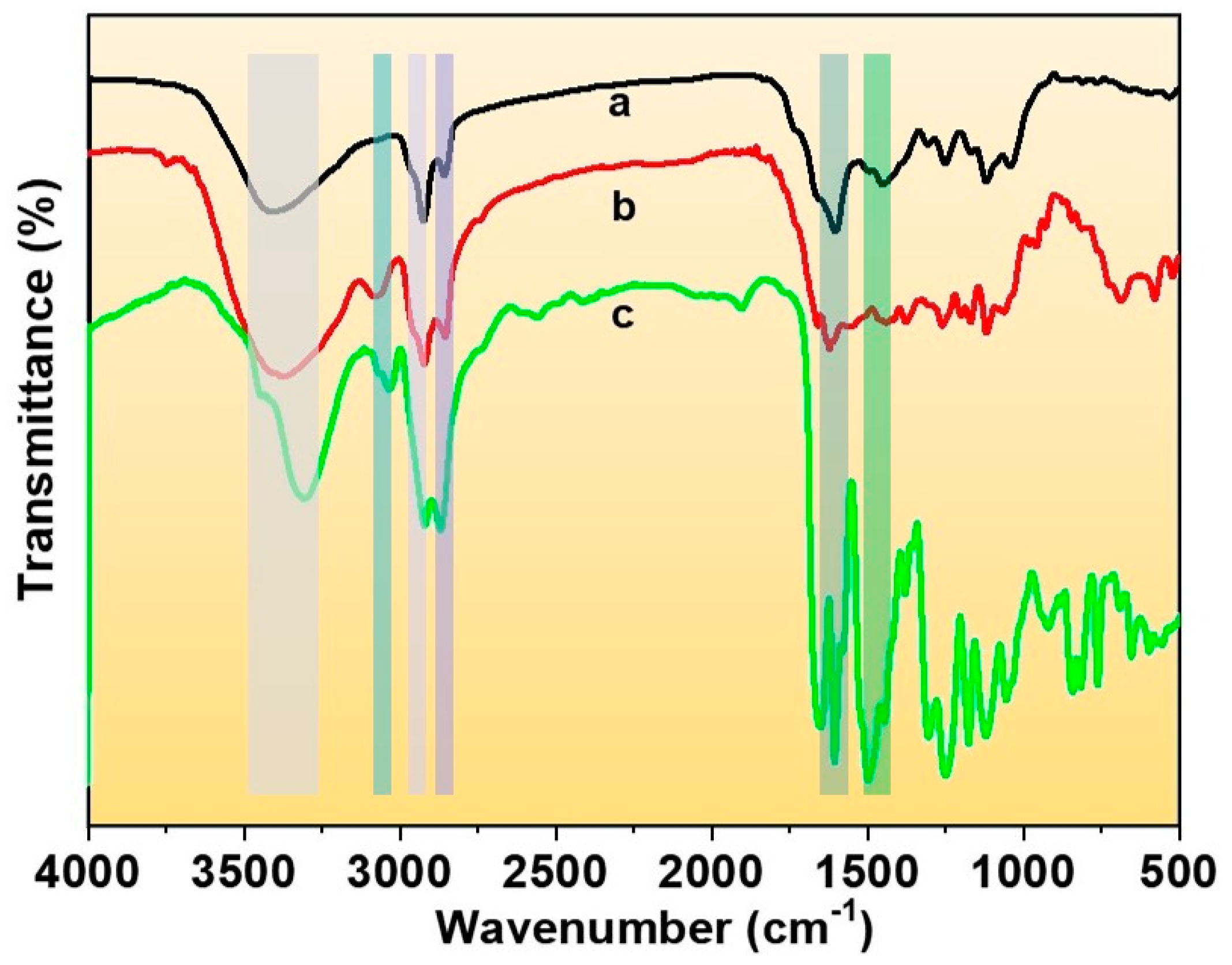
3.3. FTIR Analysis
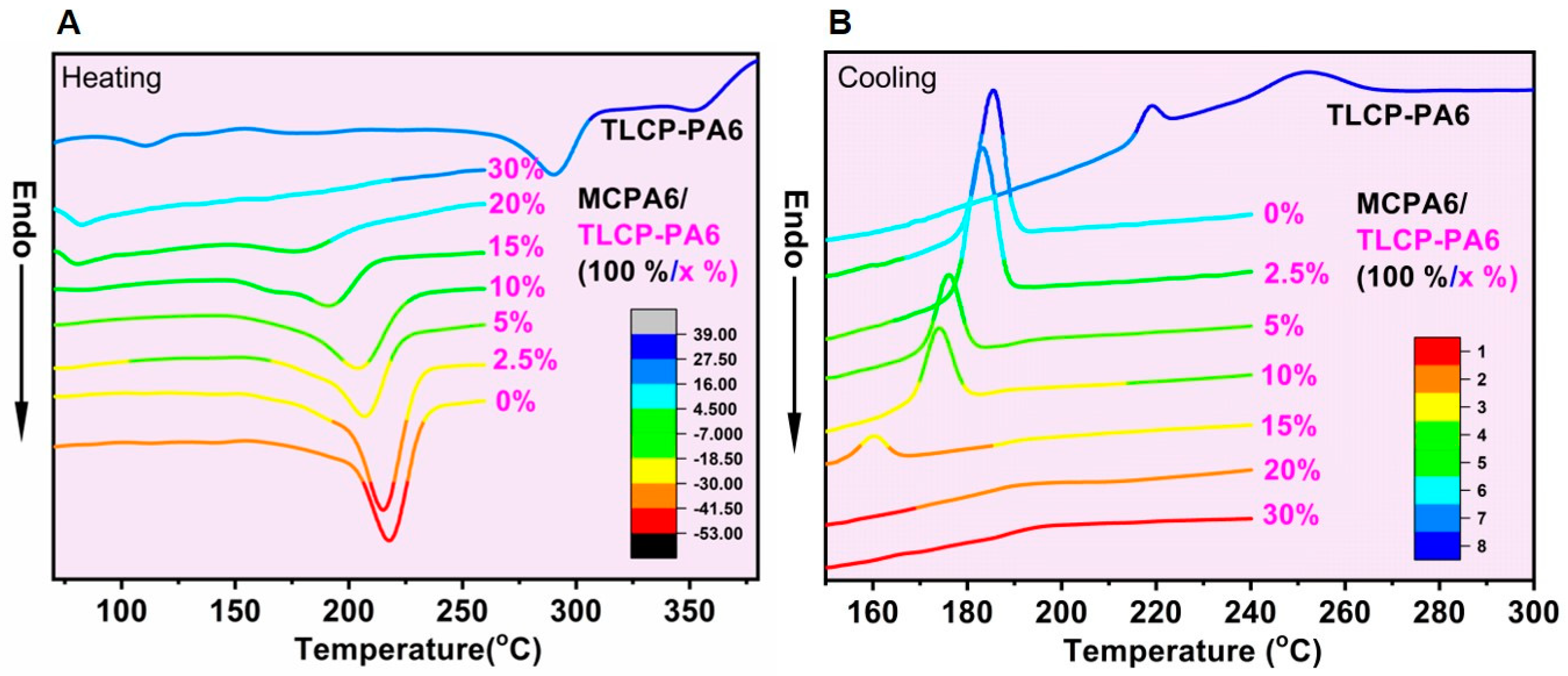
3.4. Thermal Properties
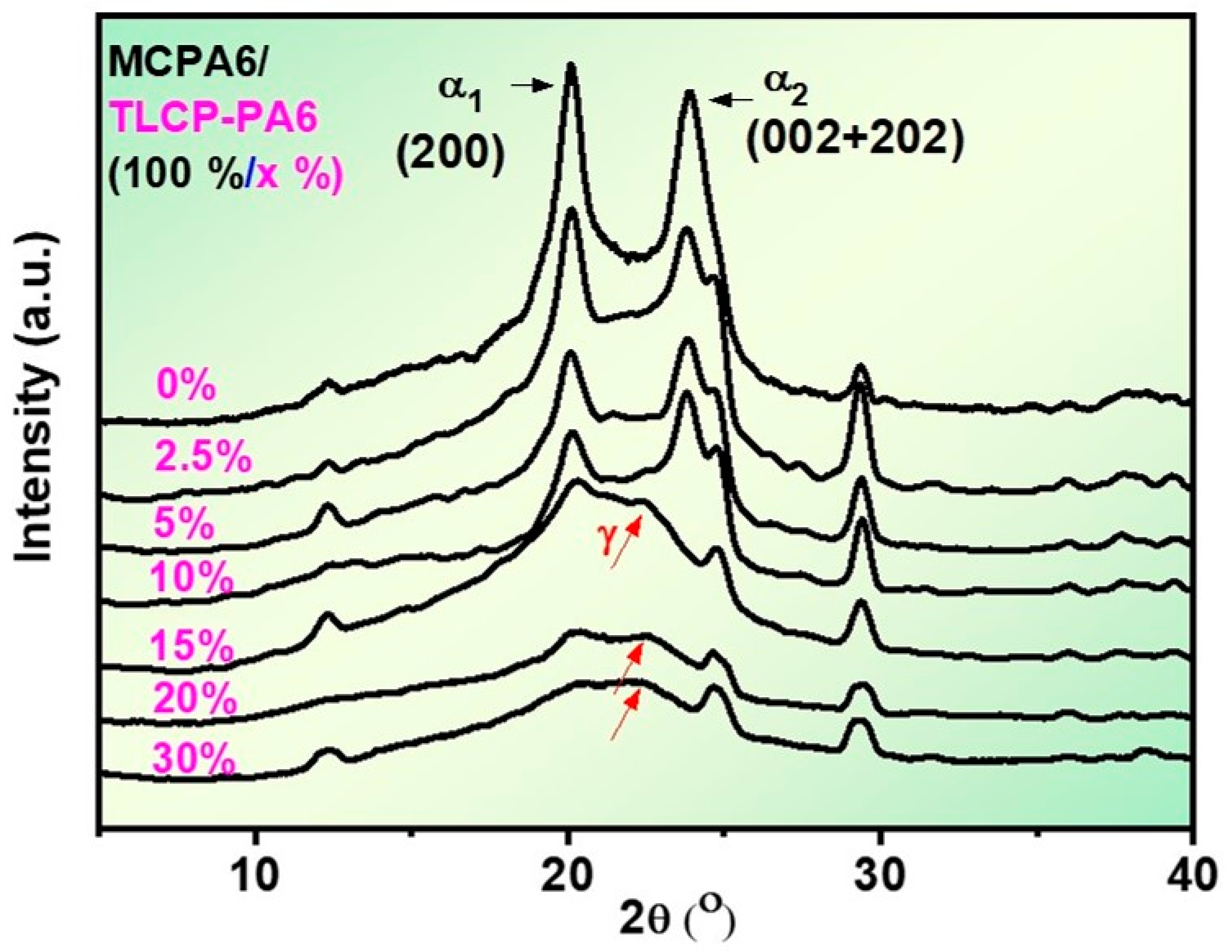
3.5. XRD Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abd Elbary, A.M.; Tammam, M.T. Physical and mechanical properties of polyamide 6/polystyrene (PA6/PS) reinforced by PbO2 composites for X-ray shielding. J. Thermoplast. Compos. Mater. 2021, 34, 1701–1719. [Google Scholar] [CrossRef]
- Fu, X.; Yao, C.; Yang, G. Recent advances in graphene/polyamide 6 composites: A review. RSC Adv. 2015, 5, 61688–61702. [Google Scholar] [CrossRef]
- Ghanta, T.S.; Aparna, S.; Verma, N.; Purnima, D. Review on nano-and microfiller-based polyamide 6 hybrid composite: Effect on mechanical properties and morphology. Polym. Eng. Sci. 2020, 60, 1717–1759. [Google Scholar] [CrossRef]
- Nirmala, R.; Navamathavan, R.; Park, S.-J.; Kim, H.Y. Recent Progress on the Fabrication of Ultrafine Polyamide-6 Based Nanofibers Via Electrospinning: A Topical Review. Nano-Micro Lett. 2014, 6, 89–107. [Google Scholar] [CrossRef]
- Soltani, R.; Pishnamazi, M.; Pelalak, R.; Rezakazemi, M.; Marjani, A.; Dinari, M.; Sarkar, S.M.; Shirazian, S. Preparation of COOH-KCC-1/polyamide 6 composite by in situ ring-opening polymerization: Synthesis, characterization, and Cd (II) adsorption study. J. Environ. Chem. Eng. 2021, 9, 104683. [Google Scholar] [CrossRef]
- Chen, H.; Tang, M.; Yang, X.; Tsang, Y.F.; Wu, Y.; Wang, D.; Zhou, Y. Polyamide 6 microplastics facilitate methane production during anaerobic digestion of waste activated sludge. Chem. Eng. J. 2021, 408, 127251. [Google Scholar] [CrossRef]
- Liang, Y.; Zhu, Y.; Liu, C.; Lee, K.-R.; Hung, W.-S.; Wang, Z.; Li, Y.; Elimelech, M.; Jin, J.; Lin, S. Polyamide nanofiltration membrane with highly uniform sub-nanometre pores for sub-1 angstrom precision separation. Nat. Commun. 2020, 11, 2015. [Google Scholar] [CrossRef]
- Gao, S.; Zhu, Y.; Gong, Y.; Wang, Z.; Fang, W.; Jin, J. Ultrathin Polyamide Nanofiltration Membrane Fabricated on Brush-Painted Single-Walled Carbon Nanotube Network Support for Ion Sieving. ACS Nano 2019, 13, 5278–5290. [Google Scholar] [CrossRef]
- Tan, Z.; Chen, S.; Peng, X.; Zhang, L.; Gao, C. Polyamide membranes with nanoscale Turing structures for water purification. Science 2018, 360, 518–521. [Google Scholar] [CrossRef]
- Ez-Zahraoui, S.; Kassab, Z.; Ablouh, E.-h.; Sehaqui, H.; Bouhfid, R.; Alami, J.; El Achaby, M.; Qaiss, A.E.K. Effect of fly ash and coupling agent on the structural, morphological, thermal, and mechanical properties of polyamide 6/acrylonitrile-butadiene-styrene blend. Polym. Compos. 2021, 42, 3518–3538. [Google Scholar] [CrossRef]
- Babaei, A.; Arefazar, A. Phase Structure of Polyamide 6/Poly(styrene-co-acrylonitrile) and Poly(styrene-b-(ethylene-co-butylene)-b-styrene) or Poly (maleated Styrene/Ethylene-co-Butylene/Styrene) Ternary Blends. J. Macromol. Sci. Part B-Phys. 2014, 53, 1377–1393. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Zhao, S.; Xie, L.; Sheng, K. Compatibility effect of radiation-grafting-functionalized styrene-butadiene-styrene on polyamide 6/styrene-butadiene-styrene blends. J. Appl. Polym. Sci. 2008, 108, 1029–1036. [Google Scholar] [CrossRef]
- Pourali, M.; Peterson, A.M. A tale of two polyamides: Comparing the crystallization kinetics of a hot-melt adhesive and a PA 6/66 copolymer. Thermochim. Acta 2022, 710, 179176. [Google Scholar] [CrossRef]
- Han, S.; Jiang, C.; Mi, J.; Wang, Y.; Chen, S.; Wang, X. Influence of fibrillated poly(tetrafluoroethylene) on microcellular foaming of polyamide 6 in the presence of supercritical CO2. J. Appl. Polym. Sci. 2021, 138, e51258. [Google Scholar] [CrossRef]
- Romo-Uribe, A.; Reyes-Mayer, A.; Rodriguez, M.C.; Sarmiento-Bustos, E. On the influence of thermal annealing on molecular relaxations and structure in thermotropic liquid crystalline polymer. Polymer 2022, 240, 124506. [Google Scholar] [CrossRef]
- Choi, N.S.; Cho, N.; Takahashi, K.; Kurokawa, M. Layered morphology and bending fracture behavior of moulded composites of thermotropic liquid crystalline polymer and polyamide 6 containing epoxy component. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2002, 323, 467–477. [Google Scholar] [CrossRef]
- Choi, N.S.; Takahashi, K. Microstructural morphology of molded thin composites of thermotropic liquid crystalline polymer and polyamide 6. In Fracture and Strength of Solids, Pts 1 and 2; Hwang, W., Han, K.S., Eds.; Trans. Tech. Publications Ltd.: Zurich-Uetikon, Switzerland, 2000; Volume 183-1, pp. 1081–1086. [Google Scholar]
- Choi, N.S.; Takahashi, K. Orientation of the skin layer in molded blends of thermotropic liquid crystalline polymer and polyamide 6. J. Mater. Sci. Lett. 2000, 19, 1435–1438. [Google Scholar] [CrossRef]
- Kurokawa, M.; Nagai, S. Reinforcement of polyamide 6 with thermotropic liquid crystalline polymer. Polym. Eng. Sci. 1999, 39, 872–880. [Google Scholar] [CrossRef]
- Wang, M.; Xin, Y.; Su, C.; Xue, F.; Xu, F.; Li, T. Morphology evolution of a thermotropic liquid-crystalline polymer in a polyamide 6,6 matrix regulated by graphene. J. Appl. Polym. Sci. 2016, 133, 43735. [Google Scholar] [CrossRef]
- Tjong, S.C.; Meng, Y.Z. Morphology and mechanical characteristics of compatibilized polyamide 6-liquid crystalline polymer composites. Polymer 1997, 38, 4609–4615. [Google Scholar] [CrossRef]
- Meng, Y.Z.; Tjong, S.C. Rheology and morphology of compatibilized polyamide 6 blends containing liquid crystalline copolyesters. Polymer 1998, 39, 99–107. [Google Scholar] [CrossRef]
- Shin, B.Y.; Chung, I.J. Polymer blend containing a thermotropic polyester with long flexible spacer in the main chain. Polym. Eng. Sci. 1990, 30, 22–29. [Google Scholar] [CrossRef]
- Castellano, M.; Nebbia, D.; Turturro, A.; Valenti, B.; Costa, G.; Falqui, L. Reactive blending of polyamide 6,6 and vectra A, 2. Role of a bifunctional epoxy coupler. Macromol. Chem. Phys. 2002, 203, 1614–1624. [Google Scholar] [CrossRef]
- Pawlikowski, G.T.; Weiss, R.A.; Huang, S.J. Synthesis and Characterization of Segmented Copolymers of a Methylated Polyamide and a Thermotropic Liquid Crystalline Polyester. MRS Online Proc. Libr. 1989, 171, 299–304. [Google Scholar] [CrossRef]
- Higashi, F.; Nakajima, K.; Watabiki, M.; Zhang, W.X. Thermotropic liquid crystalline polyamides from polyethyleneglycol bis (4-carboxyphenyl) ethers. J. Polym. Sci. Pol. Chem. 1993, 31, 2929–2933. [Google Scholar] [CrossRef]
- Higashi, F.; Zhang, W.X.; Nakajima, K. Thermotropic copolyamides from triethyleneglycol bis (4-carboxyphenyl) ether and two kinds of aromatic diamines. J. Polym. Sci. Pol. Chem. 1994, 32, 89–95. [Google Scholar] [CrossRef]
- Li, Y.J.; Shimizu, H. Co-continuous polyamide 6 (PA6)/acrylonitrile-cutadiene-styrene (ABS) nanocomposites. Macromol. Rapid Commun. 2005, 26, 710–715. [Google Scholar] [CrossRef]
- Ishii, Y.; Ryan, A.J. Processing of poly (2,6-dimethyl-1,4-phenylene ether) with epoxy resin. 1. Reaction-induced phase separation. Macromolecules 2000, 33, 158–166. [Google Scholar] [CrossRef]
- Tseng, F.P.; Lin, J.J.; Tseng, C.R.; Chang, F.C. Poly(oxypropylene)-amide grafted polypropylene as novel compatibilizer for PP and PA6 blends. Polymer 2001, 42, 713–725. [Google Scholar] [CrossRef]
- Tjong, S.C.; Li, R.K.Y.; Xie, X.L. Compatibilizing effect of styrene–maleic anhydride copolymer on the properties of polyamide-6/liquid crystalline copolyester composites. J. Appl. Polym. Sci. 2000, 77, 1964–1974. [Google Scholar] [CrossRef]
- Zhao, Z.D.; Yu, W.X.; Liu, Y.H.; Zhang, J.Q.; Shao, Z.J. Isothermal crystallization behaviors of nylon-6 and nylon-6/montmorillonite nanocomposite. Mater. Lett. 2004, 58, 802–806. [Google Scholar] [CrossRef]
- Holmes, D.R.; Bunn, C.W.; Smith, D.J. The crystal structure of polycaproamide: Nylon 6. J. Polym. Sci. 1955, 17, 159–177. [Google Scholar] [CrossRef]
- Malta, V.; Cojazzi, G.; Fichera, A.; Ajò, D.; Zannetti, R. A re-examination of the crystal structure and molecular packing of α-nylon 6. Eur. Polym. J. 1979, 15, 765–770. [Google Scholar] [CrossRef]
- Ho, J.C.; Wei, K.H. Induced gamma -> alpha crystal transformation in blends of polyamide 6 and liquid crystalline copolyester. Macromolecules 2000, 33, 5181–5186. [Google Scholar] [CrossRef]
- Kim, G.-M.; Michler, G.H.; Ania, F.; Calleja, F.J.B. Temperature dependence of polymorphism in electrospun nanofibres of PA6 and PA6/clay nanocomposite. Polymer 2007, 48, 4814–4823. [Google Scholar] [CrossRef]
- Murthy, N.S.; Bray, R.G.; Correale, S.T.; Moore, R.A.F. Drawing and annealing of nylon-6 fibres: Studies of crystal growth, orientation of amorphous and crystalline domains and their influence on properties. Polymer 1995, 36, 3863–3873. [Google Scholar] [CrossRef]
| Samples | [η] (dl.g−1) | DSC (First Heating) | |||
|---|---|---|---|---|---|
| Tg (°C) | Tm (°C) | Ti (°C) | Hm (J∙g−1) | ||
| TLCP | 1.30 | 120.0 | 311.4 | 371.7 | 54.8 |
| TLCP-PA6 | 4.04 | 110.8 | 291.9 | 352.3 | 91.1 |
| Vibration Mode | TLCP-PA6 | MCPA6/TLCP-PA6 (100 wt. %/15 wt. %) | MCPA6 |
|---|---|---|---|
| Hydrogen-bonded NH stretching | 3316 | 3378 | 3413 |
| NH stretching connected to amide II | 3035 | 3080 | - |
| CH2 asymmetrical stretching | 2922 | 2923 | 2923 |
| CH2 symmetrical stretching | - | 2855 | 2858 |
| Amide I | 1652 | 1623 | 1603 |
| Amide II | 1499 | 1452 | 1444 |
| MCPA6/ TLCP-PA6 (wt. %/wt. %) | Heating (2nd) | Cooling | |||||
|---|---|---|---|---|---|---|---|
| Tg (°C) | Tm (°C) | ΔHf (J∙g−1 PA6) | Xc(DSC) (%) | ΔHC (J∙g−1 PA6) | Tc,m (°C) | Tc,0 (°C) | |
| 100/0 | - | 217.3 | −104.6 | 48.5 | 116.9 | 185.3 | 192.3 |
| 100/2.5 | - | 215.4 | −93.6 | 41.7 | 112.6 | 183.0 | 190.3 |
| 100/5 | - | 206.9 | −74.7 | 34.1 | 98.4 | 175.9 | 182.4 |
| 100/10 | - | 204.0 | −70.9 | 33.9 | 88.5 | 174.0 | 181.4 |
| 100/15 | - | 190.9 | −50.0 | 20.5 | 45.3 | 160.1 | 165.8 |
| 100/20 | 80.2 | - | - | - | - | - | - |
| 100/30 | 82.0 | - | - | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Qiu, J.; Yue, Y.; Liu, J.; Zhang, B. The Preparation of Monomer Casting Polyamide 6/Thermotropic Liquid Crystalline Polymer Composite Materials with Satisfactory Miscibility. Polymers 2022, 14, 4355. https://doi.org/10.3390/polym14204355
Li M, Qiu J, Yue Y, Liu J, Zhang B. The Preparation of Monomer Casting Polyamide 6/Thermotropic Liquid Crystalline Polymer Composite Materials with Satisfactory Miscibility. Polymers. 2022; 14(20):4355. https://doi.org/10.3390/polym14204355
Chicago/Turabian StyleLi, Mingmin, Jiahao Qiu, Yifei Yue, Jingbing Liu, and Baohua Zhang. 2022. "The Preparation of Monomer Casting Polyamide 6/Thermotropic Liquid Crystalline Polymer Composite Materials with Satisfactory Miscibility" Polymers 14, no. 20: 4355. https://doi.org/10.3390/polym14204355
APA StyleLi, M., Qiu, J., Yue, Y., Liu, J., & Zhang, B. (2022). The Preparation of Monomer Casting Polyamide 6/Thermotropic Liquid Crystalline Polymer Composite Materials with Satisfactory Miscibility. Polymers, 14(20), 4355. https://doi.org/10.3390/polym14204355







