Novel Design of Superhydrophobic and Anticorrosive PTFE and PAA + β − CD Composite Coating Deposited by Electrospinning, Spin Coating and Electrospraying Techniques
Abstract
1. Introduction
2. Experimental Procedure
2.1. Materials and Reagents
2.2. Fabrication Techniques
2.2.1. Solution and Dispersion Preparation
2.2.2. Electrospinning (ES), Electrospraying (SP) and Spin-Coating (SC) Processes
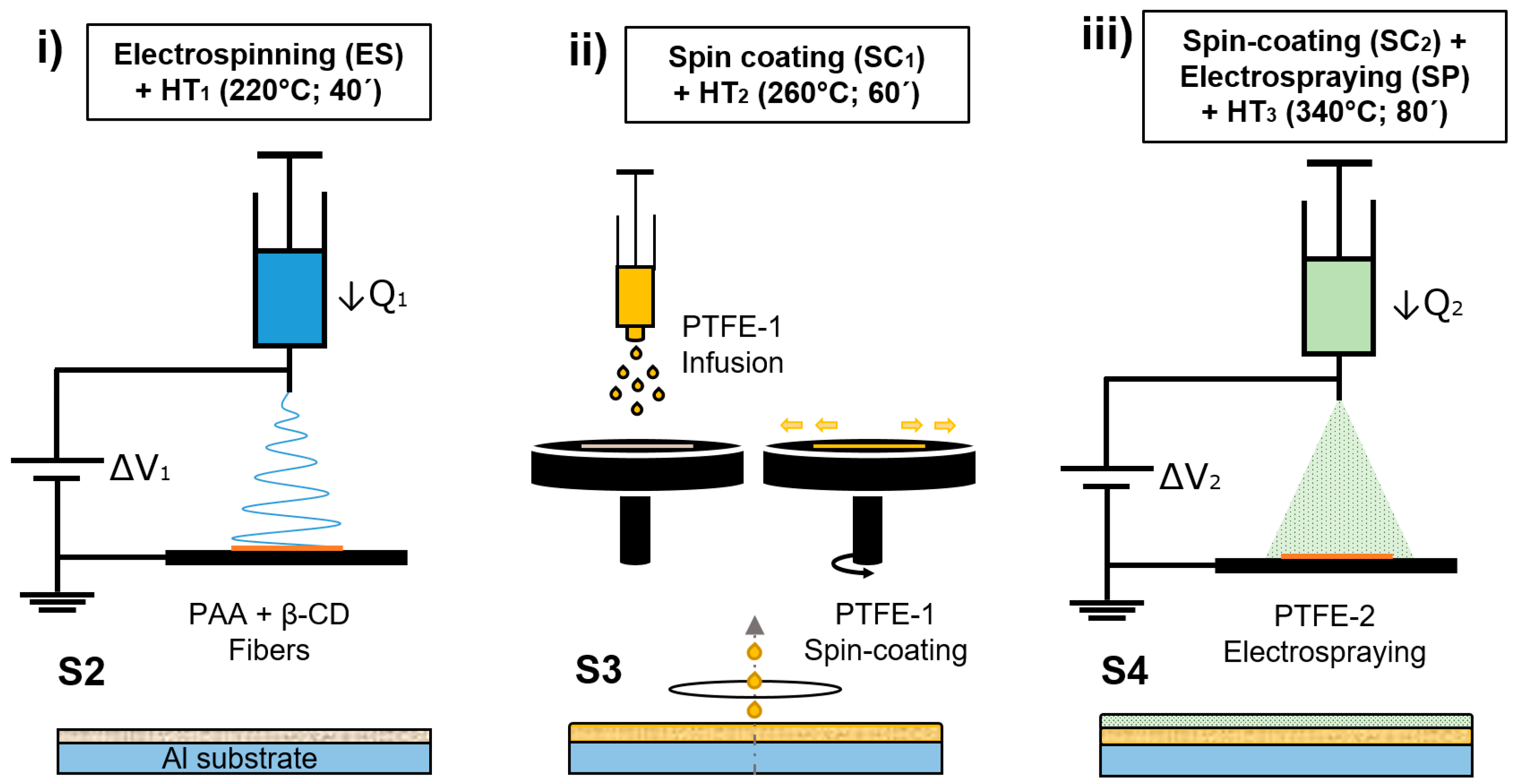
2.2.3. Coating Fabrication Process
- (i)
- Electrospinning + HT1
- (ii)
- Spin Coating + HT2
- (iii)
- Spin Coating + Electrospraying + HT3
2.3. Characterization Techniques
3. Results and Discussion
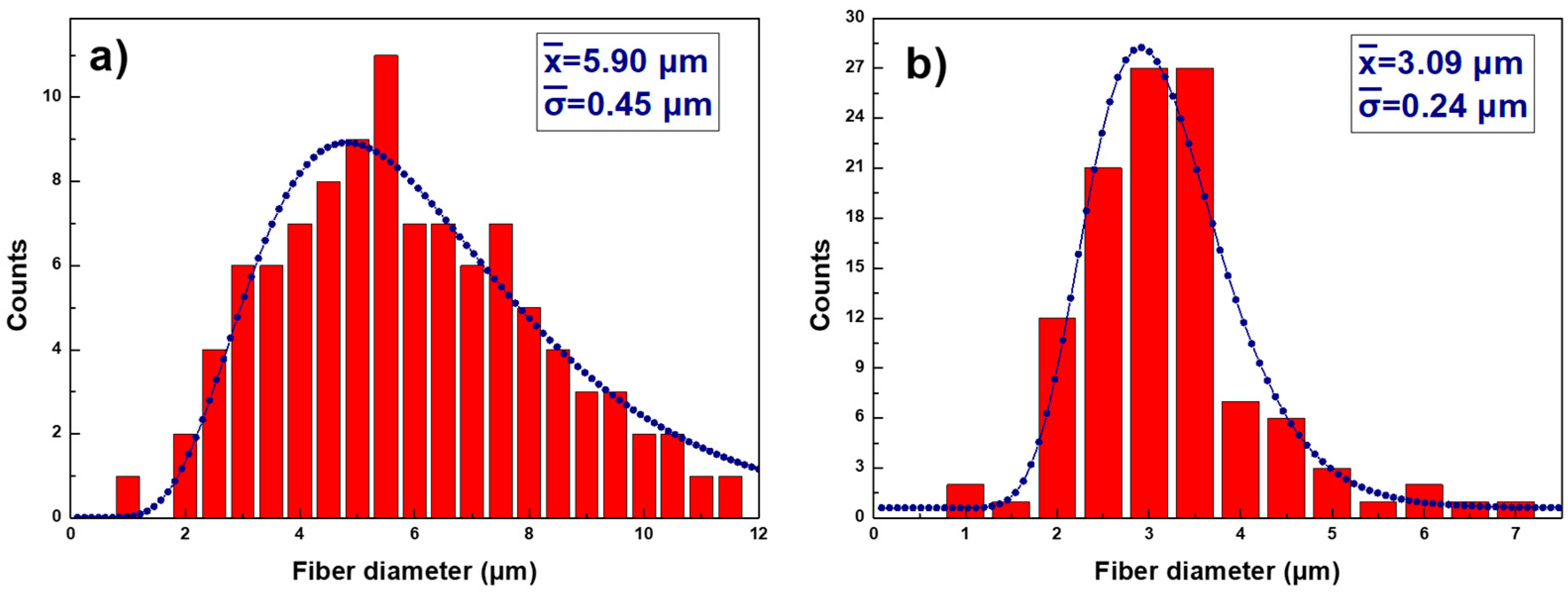
3.1. Sample Thickness and Surface Morphology
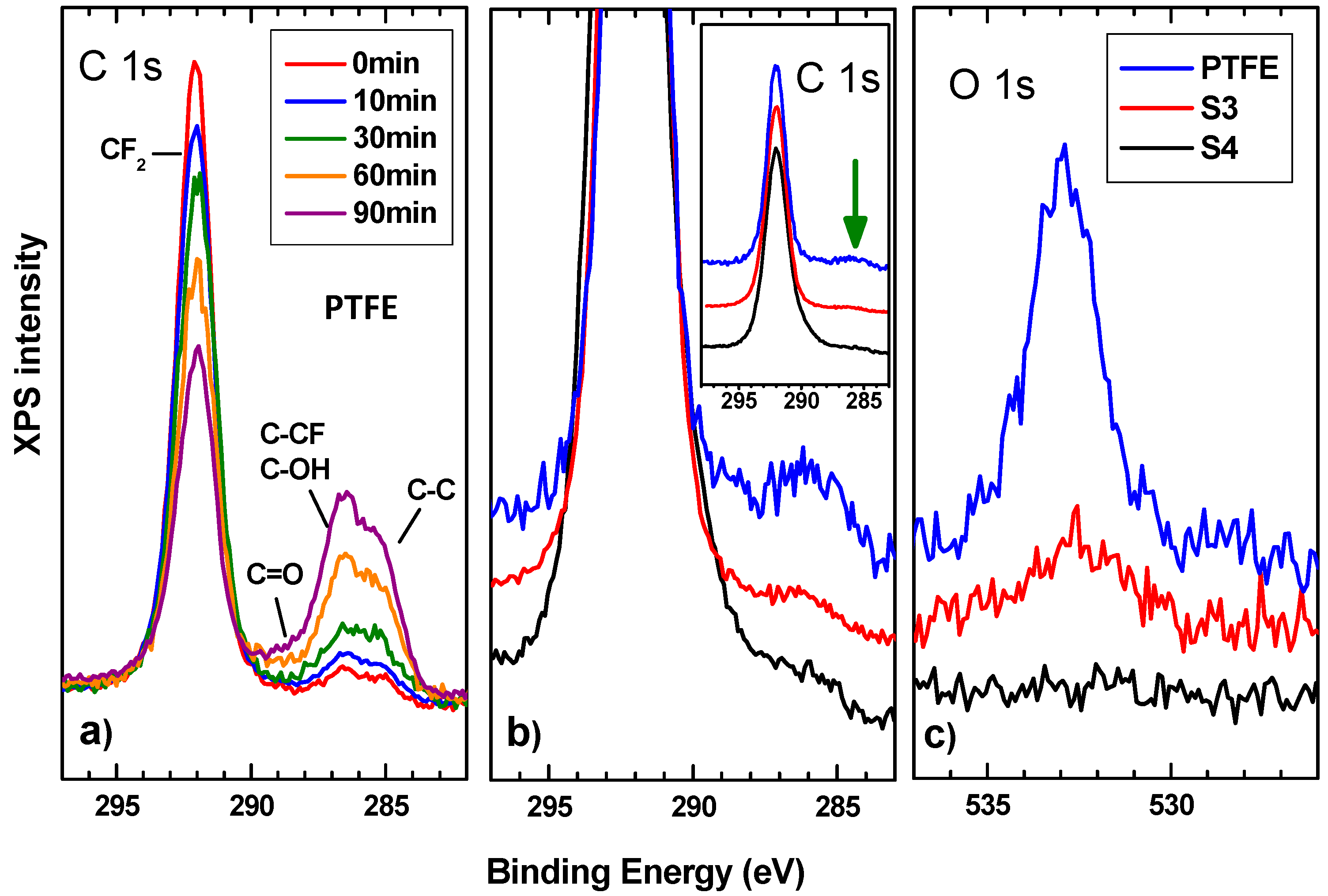
3.2. Chemical Composition
3.3. Coating Adhesion Study by Scratch Test
3.4. Wetting Properties
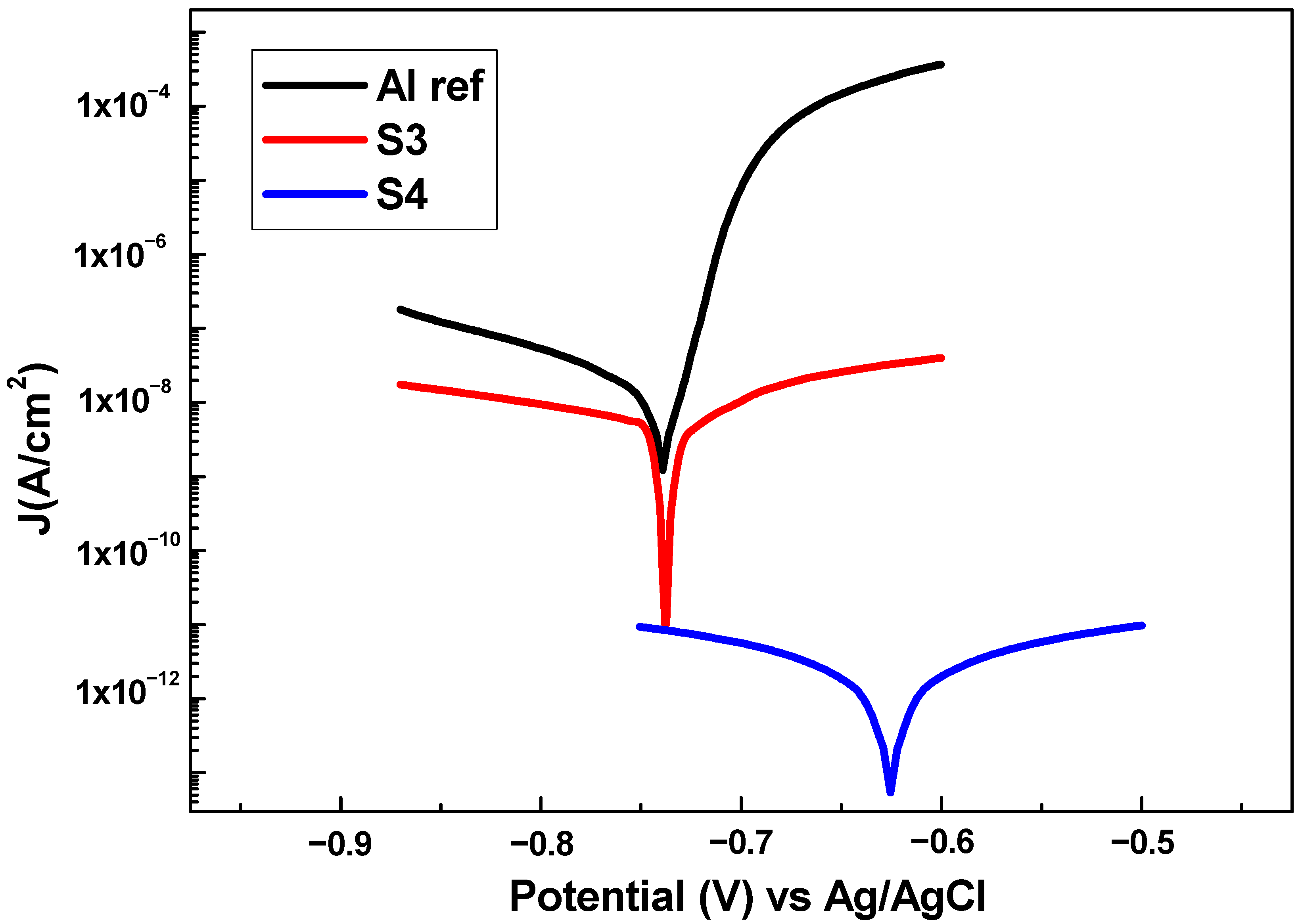
3.5. Anticorrosion Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shirtcliffe, N.J.; McHale, G.; Newton, M.I. The superhydrophobicity of polymer surfaces: Recent developments. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 1203–1217. [Google Scholar] [CrossRef]
- Kundu, C.K.; Wang, X.; Song, L.; Hu, Y. Borate cross-linked layer-by-layer assembly of green polyelectrolytes on polyamide 66 fabrics for flame-retardant treatment. Prog. Org. Coat. 2018, 121, 173–181. [Google Scholar] [CrossRef]
- Saffar, M.A.; Eshaghi, A.; Dehnavi, M.R. Fabrication of superhydrophobic, self-cleaning and anti-icing ZnO/PTFE-SiO2 nano-composite thin film. Mater. Chem. Phys. 2021, 259, 124085. [Google Scholar] [CrossRef]
- Zhang, Y.-Y.; Ge, Q.; Yang, L.-L.; Shi, X.-J.; Li, J.-J.; Yang, D.-Q.; Sacher, E. Durable superhydrophobic PTFE films through the introduction of micro- and nanostructured pores. Appl. Surf. Sci. 2015, 339, 151–157. [Google Scholar] [CrossRef]
- Jafari, R.; Momen, G.; Farzaneh, M. Durability enhancement of icephobic fluoropolymer film. J. Coat. Technol. Res. 2016, 13, 405–412. [Google Scholar] [CrossRef]
- Shi, L.; Hu, J.; Lin, X.; Fang, L.; Wu, F.; Xie, J.; Meng, F. A robust superhydrophobic PPS-PTFE/SiO2 composite coating on AZ31 Mg alloy with excellent wear and corrosion resistance properties. J. Alloys Compd. 2017, 721, 157–163. [Google Scholar] [CrossRef]
- Basu, B.J.; Kumar, V.D. Fabrication of Superhydrophobic Nanocomposite Coatings Using Polytetrafluoroethylene and Silica Nanoparticles. ISRN Nanotechnol. 2011, 2011, 803910. [Google Scholar] [CrossRef]
- Price, D.M.; Jarratt, M. Thermal conductivity of PTFE and PTFE composites. Thermochim. Acta 2002, 392–393, 231–236. [Google Scholar] [CrossRef]
- Subodh, G.; Pavithran, C.; Mohanan, P.; Sebastian, M. PTFE/Sr2Ce2Ti5O16 polymer ceramic composites for electronic packaging applications. J. Eur. Ceram. Soc. 2007, 27, 3039–3044. [Google Scholar] [CrossRef]
- McCook, N.; Boesl, B.; Burris, D.; Sawyer, W. Epoxy, ZnO, and PTFE nanocomposite: Friction and wear optimization. Tribol. Lett. 2006, 22, 253–257. [Google Scholar] [CrossRef]
- Qian, H.; Zhu, Y.; Wang, H.; Song, H.; Wang, C.; Liu, Z.; Li, H. Preparation and Antiscaling Performance of Superhydrophobic Poly(phenylene sulfide)/Polytetrafluoroethylene Composite Coating. Ind. Eng. Chem. Res. 2017, 56, 12663–12671. [Google Scholar] [CrossRef]
- Li, H.; Yu, S.; Han, X.; Zhao, Y. A stable hierarchical superhydrophobic coating on pipeline steel surface with self-cleaning, anticorrosion, and anti-scaling properties. Colloids Surfaces A Physicochem. Eng. Asp. 2016, 503, 43–52. [Google Scholar] [CrossRef]
- Bangar, G.Y.; Ghule, D.; Singh, R.; Kandasubramanian, B. Thermally triggered transition of fluid atomized micro- and nanotextured multiscale rough surfaces. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 549, 212–220. [Google Scholar] [CrossRef]
- Erturk, A.T.; Vatansever, F.; Yarar, E.; Karabay, S. Machining behavior of multiple layer polymer composite bearing with using different drill bits. Compos. Part B Eng. 2019, 176, 107318. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; Jiang, P.; Yan, F. Hydrostatic pressure-dependent corrosion behaviour of polytetrafluoroethylene composites in the deep sea. Corros. Sci. 2018, 139, 289–300. [Google Scholar] [CrossRef]
- Haq, M.I.U.; Raina, A.; Vohra, K.; Kumar, R.; Anand, A. An Assessment of Tribological Characteristics of Different Materials under Sea Water Environment. Mater. Today Proc. 2018, 5, 3602–3609. [Google Scholar] [CrossRef]
- Drobny, J.G. Applications of Fluoropolymer Films: Properties, Processing, and Products; William Andrew: Norwich, UK, 2020. [Google Scholar]
- Ebnesajjad, S. Introduction to Fluoropolymers: MATERIALS, Technology, and Applications; William Andrew: Norwich, UK, 2020. [Google Scholar]
- Dhanumalayan, E.; Joshi, G.M. Performance properties and applications of polytetrafluoroethylene (PTFE)—A review. Adv. Compos. Hybrid Mater. 2018, 1, 247–268. [Google Scholar] [CrossRef]
- Burkarter, E.; Saul, C.; Thomazi, F.; Cruz, N.; Roman, L.; Schreiner, W. Superhydrophobic electrosprayed PTFE. Surf. Coat. Technol. 2007, 202, 194–198. [Google Scholar] [CrossRef]
- Ma, L.; Wang, J.; Zhang, Z.; Kang, Y.; Sun, M.; Ma, L. Preparation of a superhydrophobic TiN/PTFE composite film toward self-cleaning and corrosion protection applications. J. Mater. Sci. 2021, 56, 1413–1425. [Google Scholar] [CrossRef]
- Kato, S.; Sato, A. Micro/nanotextured polymer coatings fabricated by UV curing-induced phase separation: Creation of superhydrophobic surfaces. J. Mater. Chem. 2012, 22, 8613–8621. [Google Scholar] [CrossRef]
- Guo, X.-J.; Xue, C.-H.; Sathasivam, S.; Page, K.; He, G.; Guo, J.; Promdet, P.; Heale, F.L.; Carmalt, C.J.; Parkin, I.P. Fabrication of robust superhydrophobic surfaces via aerosol-assisted CVD and thermo-triggered healing of superhydrophobicity by recovery of roughness structures. J. Mater. Chem. A 2019, 7, 17604–17612. [Google Scholar] [CrossRef]
- Nilsson, M.A.; Daniello, R.J.; Rothstein, J.P. A novel and inexpensive technique for creating superhydrophobic surfaces using Teflon and sandpaper. J. Phys. D. Appl. Phys. 2010, 43, 045301. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Kido, H.; Ukita, Y.; Kishihara, M.; Utsumi, Y. Anisotropic pyrochemical microetching of poly (tetrafluoroethylene) initiated by synchrotron radiation-induced scission of molecule bonds. Appl. Phys. Lett. 2016, 108, 51610. [Google Scholar] [CrossRef]
- Fiedorowicz, H.; Bartnik, A.; Bittner, M.; Juha, L.; Krása, J.; Kubát, P.; Mikolajczyk, J.; Rakowski, R. Micromachining of organic polymers by direct photo-etching using a laser plasma X-ray source. Microelectron. Eng. 2004, 73–74, 336–339. [Google Scholar] [CrossRef]
- Yong, J.; Fang, Y.; Chen, F.; Huo, J.; Yang, Q.; Bian, H.; Du, G.; Hou, X. Femtosecond laser ablated durable superhydrophobic PTFE films with micro-through-holes for oil/water separation: Separating oil from water and corrosive solutions. Appl. Surf. Sci. 2016, 389, 1148–1155. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, M.-J.; Ouyang, X.; Zhang, A.P.; Tam, H.-Y. 3D μ-printing of polytetrafluoroethylene microstructures: A route to superhydrophobic surfaces and devices. Appl. Mater. Today 2020, 19, 100580. [Google Scholar] [CrossRef]
- Qin, Z.; Ai, J.; Du, Q.; Liu, J.; Zeng, X. Superhydrophobic polytetrafluoroethylene surfaces with accurately and continuously tunable water adhesion fabricated by picosecond laser direct ablation. Mater. Des. 2019, 173, 107782. [Google Scholar] [CrossRef]
- Gao, J.; Deng, Y.; Peng, L.; Yi, P.; Lin, Z. Water-repellent hierarchical microstructured PTFE films via micro powder hot embossing. J. Mater. Process. Technol. 2021, 297, 117261. [Google Scholar] [CrossRef]
- Qin, C.; Mulroney, A.T.; Gupta, M.C. Anti-icing epoxy resin surface modified by spray coating of PTFE Teflon particles for wind turbine blades. Mater. Today Commun. 2020, 22, 100770. [Google Scholar] [CrossRef]
- Kianfar, P.; Bongiovanni, R.; Ameduri, B.; Vitale, A. Electrospinning of Fluorinated Polymers: Current State of the Art on Processes and Applications. Polym. Rev. 2022, 106, 1–73. [Google Scholar] [CrossRef]
- Eykens, L.; De Sitter, K.; Dotremont, C.; Pinoy, L.; Van der Bruggen, B. Membrane synthesis for membrane distillation: A review. SePurif. Technol. 2017, 182, 36–51. [Google Scholar] [CrossRef]
- Nthunya, L.N.; Gutierrez, L.; Derese, S.; Nxumalo, E.N.; Verliefde, A.R.; Mamba, B.; Mhlanga, S.D. A review of nanoparticle-enhanced membrane distillation membranes: Membrane synthesis and applications in water treatment. J. Chem. Technol. Biotechnol. 2019, 94, 2757–2771. [Google Scholar] [CrossRef]
- Silva, L.M.G.; Leocádio, G.; de Souza, R.; Mierzwa, J.; Duong, A.; Venancio, E.; Neto, A. New approach by electrospray technique to prepare a gas diffusion layer for the proton exchange membrane fuel cell anode. Mater. Today Adv. 2021, 12, 100161. [Google Scholar] [CrossRef]
- Haider, S.; Haider, A. Electrospinning and Electrospraying—Techniques and Applications; IntechOpen: London, UK, 2019. [Google Scholar]
- Liu, M.; Hou, Y.; Li, J.; Tie, L.; Guo, Z. Transparent slippery liquid-infused nanoparticulate coatings. Chem. Eng. J. 2018, 337, 462–470. [Google Scholar] [CrossRef]
- Ruan, M.; Zhan, Y.; Wu, Y.; Wang, X.; Li, W.; Chen, Y.; Wei, M.; Wang, X.; Deng, X. Preparation of PTFE/PDMS superhydrophobic coating and its anti-icing performance. RSC Adv. 2017, 7, 41339–41344. [Google Scholar] [CrossRef]
- Rivero, P.J.; Urrutia, A.; Goicoechea, J.; Rodríguez, Y.; Corres, J.M.; Arregui, F.J.; Matías, I.R. An antibacterial submicron fiber mat with in situ synthesized silver nanoparticles. J. Appl. Polym. Sci. 2012, 126, 1228–1235. [Google Scholar] [CrossRef]
- Albistur, A.; Rivero, P.; Esparza, J.; Rodríguez, R. Evaluation of the Photocatalytic Activity and Anticorrosion Performance of Electrospun Fibers Doped with Metallic Oxides. Polymers 2021, 13, 2011. [Google Scholar] [CrossRef]
- Sandua, X.; Rivero, P.J.; Esparza, J.; Fernández-Palacio, J.; Conde, A.; Rodríguez, R.J. Design of Photocatalytic Functional Coatings Based on the Immobilization of Metal Oxide Particles by the Combination of Electrospinning and Layer-by-Layer Deposition Techniques. Coatings 2022, 12, 862. [Google Scholar] [CrossRef]
- Huang, Q.-L.; Xiao, C.-F.; Hu, X.-Y.; Li, X.-F. Study on the effects and properties of hydrophobic poly (tetrafluoroethylene) membrane. Desalination 2011, 277, 187–192. [Google Scholar] [CrossRef]
- Huang, Y.; Huang, Q.-L.; Liu, H.; Zhang, C.-X.; You, Y.-W.; Li, N.-N.; Xiao, C.-F. Preparation, characterization, and applications of electrospun ultrafine fibrous PTFE porous membranes. J. Memb. Sci. 2017, 523, 317–326. [Google Scholar] [CrossRef]
- Shi, Z.; Ju, J.; Liang, Y.; Huang, W.; Kang, W.; Cheng, B. A Comparative Study of Poly(tetrafluoroethylene) Ultrafine Fibrous Porous Membranes Prepared by Electrospinning, Solution Blowing Spinning, and Electroblown Spinning. Chem. Lett. 2017, 46, 131–134. [Google Scholar] [CrossRef]
- Su, C.; Li, Y.; Cao, H.; Lu, C.; Li, Y.; Chang, J.; Duan, F. Novel PTFE hollow fiber membrane fabricated by emulsion electrospinning and sintering for membrane distillation. J. Memb. Sci. 2019, 583, 200–208. [Google Scholar] [CrossRef]
- Li, M.; Chen, F.; Liu, C.; Qian, J.; Wu, Z.; Chen, Z. Electrospun Fibrous PTFE Supported ZnO for Oil–Water Separation. J. Inorg. Organomet. Polym. Mater. 2019, 29, 1738–1745. [Google Scholar] [CrossRef]
- Zhou, T.; Zhong, Q.; Li, J.; Yao, Y.; Xiang, R.; Zhu, P. Superhydrophobic polytetrafluoroethylene nanofiber membranes prepared by vacuum sintering and their application in vacuum membrane distillation. J. Appl. Polym. Sci. 2020, 137, 49060. [Google Scholar] [CrossRef]
- Gil-Herrera, L.K.; Gallego-Gómez, F.; Torres-Pardo, A.; González-Calbet, J.M.; Palomares, F.J.; Blanco, A.; Juárez, B.H.; López, C. Silicon-Based Photonic Architectures from Hierarchically Porous Carbon Opals. Part. Part. Syst. Charact. 2020, 37, 1900396. [Google Scholar] [CrossRef]
- Vicente, A.; Rivero, P.J.; Palacio, J.F.; Rodríguez, R. The Role of the Fiber/Bead Hierarchical Microstructure on the Properties of PVDF Coatings Deposited by Electrospinning. Polymers 2021, 13, 464. [Google Scholar] [CrossRef]
- Yuan, R.; Wu, S.; Yu, P.; Wang, B.; Mu, L.; Zhang, X.; Zhu, Y.; Wang, B.; Wang, H.; Zhu, J. Superamphiphobic and Electroactive Nanocomposite toward Self-Cleaning, Antiwear, and Anticorrosion Coatings. ACS Appl. Mater. Interfaces 2016, 8, 12481–12493. [Google Scholar] [CrossRef]
- Vicente, A.; Rivero, P.J.; García, P.; Mora, J.; Carreño, F.; Palacio, J.F.; Rodríguez, R. Icephobic and Anticorrosion Coatings Deposited by Electrospinning on Aluminum Alloys for Aerospace Applications. Polymers 2021, 13, 4164. [Google Scholar] [CrossRef]
- Shih, T.-W.; Hsu, C.-L.; Chen, L.-Y.; Huang, Y.-C.; Chen, C.-J.; Inoue, Y.; Sugiyama, T. Optical Trapping-Induced New Polymorphism of β-Cyclodextrin in Unsaturated Solution. Cryst. Growth Des. 2021, 21, 6913–6923. [Google Scholar] [CrossRef]
- Shao, K.; Yuan, Q. Inclusion of inorganic polyoxomolybdate molecules into β-cyclodextrin: By slow crystallization or rapid crystallization. J. Cryst. Growth 2013, 363, 145–149. [Google Scholar] [CrossRef]
- Shao, K.; Wang, H.; Peng, A. Inclusion of CdS quantum DoT into beta-cyclodextrin crystal by simple rapid crystallization. J. Cryst. Growth 2015, 409, 10–13. [Google Scholar] [CrossRef]
- Ishiguro, T.; Hirayama, F.; Iohara, D.; Arima, H.; Uekama, K. Crystallization and polymorphic transitions of chlorpropamide in aqueous 2-hydroxybutyl-β-cyclodextrin solution. Eur. J. Pharm. Sci. 2010, 39, 248–255. [Google Scholar] [CrossRef]
- Zhao, B.; Sun, S.; Luo, Y.; Cheng, Y. Fabrication of Polytetrafluoroethylene Coated Micron Aluminium with Enhanced Oxidation. Materials 2020, 13, 3384. [Google Scholar] [CrossRef]
- Girardeaux, C.; Pireaux, J.-J. Analysis of Poly(tetrafluoroethylene) (PTFE) by XPS. Surf. Sci. Spectra 1996, 4, 138–141. [Google Scholar] [CrossRef]
- Gutiérrez, M.C.; Picó, F.; Rubio, F.; Amarilla, J.M.; Palomares, F.J.; Ferrer, M.L.; del Monte, F.; Rojo, J.M. PPO15-PEO22-PPO15 block copolymer assisted synthesis of monolithic macro- and microporous carbon aerogels exhibiting high conductivity and remarkable capacitance. J. Mater. Chem. 2009, 19, 1236–1240. [Google Scholar] [CrossRef]
- Cozens, E.J.; Kong, D.; Roohpour, N.; Gautrot, J.E. The physico-chemistry of adhesions of protein resistant and weak polyelectrolyte brushes to cells and tissues. Soft Matter 2019, 16, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Martinez, J.F.; Kakar, E.; Erkselius, S.; Rehnberg, N.; Sotres, J. The role of cross-linking in the scratch resistance of organic coatings: An investigation using Atomic Force Microscopy. Wear 2018, 418–419, 151–159. [Google Scholar] [CrossRef]
- Jothi, V.; Adesina, A.Y.; Kumar, A.M.; Al-Aqeeli, N.; Ram, J.N. Influence of an anodized layer on the adhesion and surface protective performance of organic coatings on AA2024 aerospace Al alloy. Prog. Org. Coat. 2020, 138, 105396. [Google Scholar] [CrossRef]
- Rivero, P.J.; Yurrita, D.; Berlanga, C.; Palacio, J.F.; Rodríguez, R. Functionalized Electrospun Fibers for the Design of Novel Hydrophobic and Anticorrosive Surfaces. Coatings 2018, 8, 300. [Google Scholar] [CrossRef]
- Tsibouklis, J.; Nevell, T.G. Ultra-Low Surface Energy Polymers: The Molecular Design Requirements. Adv. Mater. 2003, 15, 647–650. [Google Scholar] [CrossRef]
- Shirtcliffe, N.J.; McHale, G.; Atherton, S.; Newton, M.I. An introduction to superhydrophobicity. Adv. Colloid Interface Sci. 2010, 161, 124–138. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Tieu, A.K.; Wan, S.; Zhu, H.; Pham, S.T.; Johnston, B. Surface characteristics and wettability of superhydrophobic silanized inorganic glass coating surfaces textured with a picosecond laser. Appl. Surf. Sci. 2021, 537, 147808. [Google Scholar] [CrossRef]
- Kim, H.; Noh, K.; Choi, C.; Khamwannah, J.; Villwock, D.; Jin, S. Extreme Superomniphobicity of Multiwalled 8 nm TiO2 Nanotubes. Langmuir 2011, 27, 10191–10196. [Google Scholar] [CrossRef]
- Shirtcliffe, N.J.; McHale, G.; Newton, M.I.; Chabrol, G.; Perry, C.C. Dual-Scale Roughness Produces Unusually Water-Repellent Surfaces. Adv. Mater. 2004, 16, 1929–1932. [Google Scholar] [CrossRef]
- Li, J.; Wu, R.; Jing, Z.; Yan, L.; Zha, F.; Lei, Z. One-Step Spray-Coating Process for the Fabrication of Colorful Superhydrophobic Coatings with Excellent Corrosion Resistance. Langmuir 2015, 31, 10702–10707. [Google Scholar] [CrossRef] [PubMed]
- Qing, Y.; Yang, C.; Yu, N.; Shang, Y.; Sun, Y.; Wang, L.; Liu, C. Superhydrophobic TiO2/polyvinylidene fluoride composite surface with reversible wettability switching and corrosion resistance. Chem. Eng. J. 2016, 290, 37–44. [Google Scholar] [CrossRef]
- Su, F.; Yao, K. Facile fabrication of superhydrophobic surface with excellent mechanical abrasion and corrosion resistance on copper substrate by a novel method. ACS Appl. Mater. Interfaces 2014, 6, 8762–8770. [Google Scholar] [CrossRef]
- Lv, Z.; Yu, S.; Song, K.; Zhou, X.; Yin, X. A two-step method fabricating a hierarchical leaf-like superamphiphobic PTFE/CuO coating on 6061Al. Prog. Org. Coat. 2020, 147, 105723. [Google Scholar] [CrossRef]
- Akram, W.; Rafique, A.F.; Maqsood, N.; Khan, A.; Badshah, S.; Khan, R.U. Characterization of PTFE Film on 316L Stainless Steel Deposited through Spin Coating and Its Anticorrosion Performance in Multi Acidic Mediums. Materials 2020, 13, 388. [Google Scholar] [CrossRef]




| Sample (Sx) | Composition | Procedures | Heat Treatment (HT) | ||
|---|---|---|---|---|---|
| S1 | PAA + β-CD | ES | Temp (°C) | Time (min) | |
| S2 | PAA + β-CD | S1 + HT1 | HT1 | 220 | 40 |
| S3 | PAA + β-CD + PTFE-1 | S2 + SC1 + HT2 | HT2 | 260 | 60 |
| S4 | PAA + β-CD + PTFE-1,2 | S3 + SC2 + SP + HT3 | HT3 | 340 | 80 |
| Parameters | ES | SP |
|---|---|---|
| Applied voltage (needle/collector) (KV) | 3.55/−3.05 | 8.90/−3.00 |
| Flow rate (mL/h) | 1.5 | 0.5 |
| Deposition time/sample (min) | 30 | 25 |
| Distance | 15 | 15 |
| Sample (Sx) | Thickness (µm) | Df (µm) | Ra (µm) |
|---|---|---|---|
| S1 | 37 ± 3 | 5.90 ± 0.45 | 1.05 ± 0.20 |
| S2 | 64 ± 4 | 3.09 ± 0.24 | 6.87 ± 0.99 |
| S3 | 81 ± 3 | ―― | 0.97 ± 0.25 |
| S4 | 111 ± 5 | ―― | 5.58 ± 0.91 |
| Sample (Sx) | Scratch Resistance (N) |
|---|---|
| S1 | ―― |
| S2 | 1.0 ± 0.1 |
| S3 | 3.3 ± 0.1 |
| S4 | 8.2 ± 0.2 |
| Angles | S3 | S4 |
|---|---|---|
| Sample | Jcorr (nA/cm2) | Ecorr (V) | Corrosion Rate (nm/year) | βa (V/dec) | βc (V/dec) | η (%) |
|---|---|---|---|---|---|---|
| Al ref | 217.320 | −0.76 | 2427.20 | 0.02 | 0.13 | 0 |
| S3 | 50.468 | −0.73 | 563.66 | 0.11 | 0.16 | 76.77 |
| S4 | 0.040 | −0.62 | 0.45 | 0.30 | 0.29 | 99.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vicente, A.; Rivero, P.J.; Urdiroz, U.; García, P.; Mora, J.; Palacio, J.F.; Palomares, F.J.; Rodríguez, R. Novel Design of Superhydrophobic and Anticorrosive PTFE and PAA + β − CD Composite Coating Deposited by Electrospinning, Spin Coating and Electrospraying Techniques. Polymers 2022, 14, 4356. https://doi.org/10.3390/polym14204356
Vicente A, Rivero PJ, Urdiroz U, García P, Mora J, Palacio JF, Palomares FJ, Rodríguez R. Novel Design of Superhydrophobic and Anticorrosive PTFE and PAA + β − CD Composite Coating Deposited by Electrospinning, Spin Coating and Electrospraying Techniques. Polymers. 2022; 14(20):4356. https://doi.org/10.3390/polym14204356
Chicago/Turabian StyleVicente, Adrián, Pedro J. Rivero, Unai Urdiroz, Paloma García, Julio Mora, José F. Palacio, F. Javier Palomares, and Rafael Rodríguez. 2022. "Novel Design of Superhydrophobic and Anticorrosive PTFE and PAA + β − CD Composite Coating Deposited by Electrospinning, Spin Coating and Electrospraying Techniques" Polymers 14, no. 20: 4356. https://doi.org/10.3390/polym14204356
APA StyleVicente, A., Rivero, P. J., Urdiroz, U., García, P., Mora, J., Palacio, J. F., Palomares, F. J., & Rodríguez, R. (2022). Novel Design of Superhydrophobic and Anticorrosive PTFE and PAA + β − CD Composite Coating Deposited by Electrospinning, Spin Coating and Electrospraying Techniques. Polymers, 14(20), 4356. https://doi.org/10.3390/polym14204356









