Lignin Distribution on Cell Wall Micro-Morphological Regions of Fibre in Developmental Phyllostachys pubescens Culms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bamboo Samples
2.2. Sectioning and Microscopy
2.3. Mäule Reaction, Wiesner Reaction and Visible-Light Spectrophotometry
3. Results and Discussion
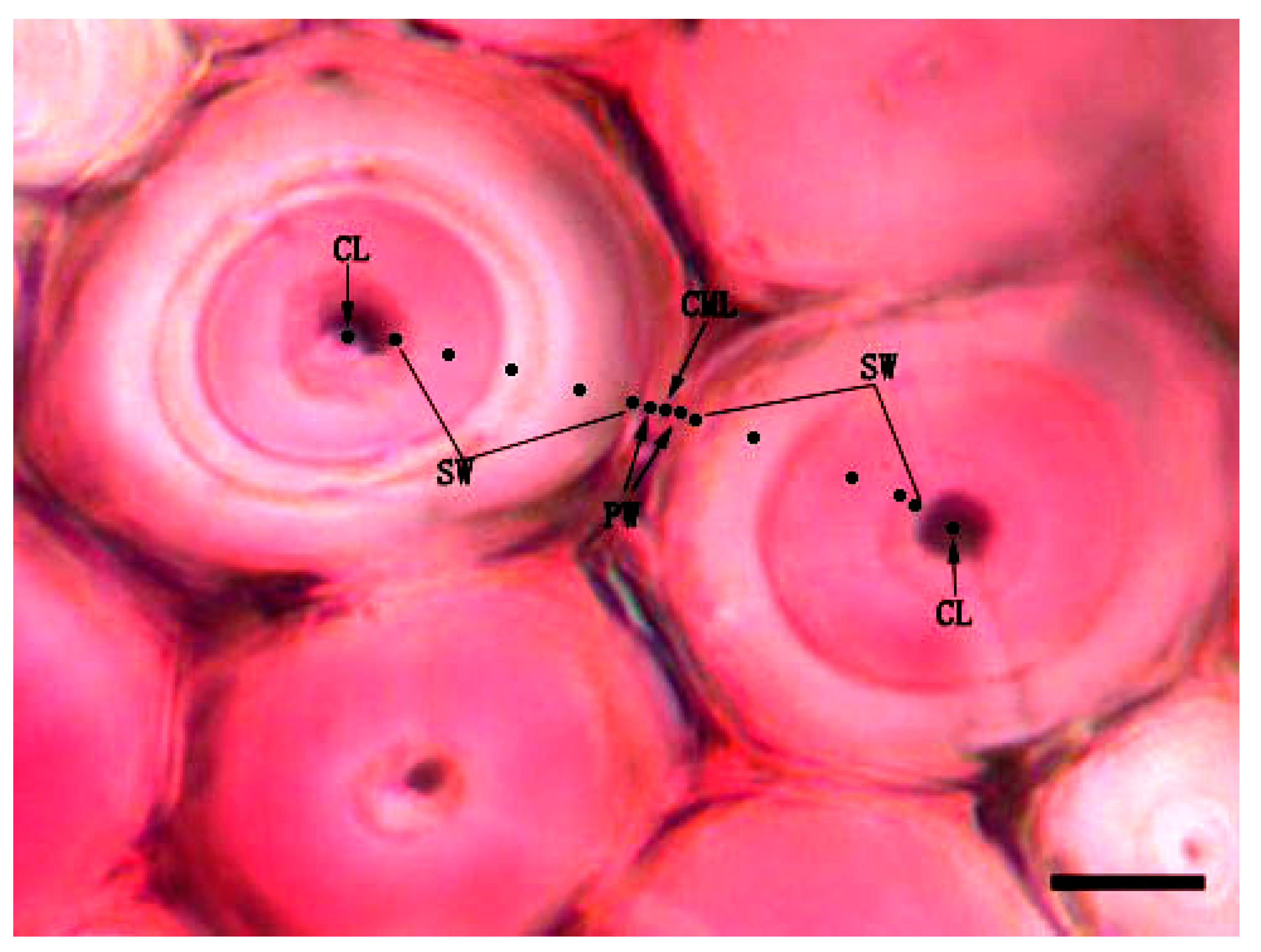
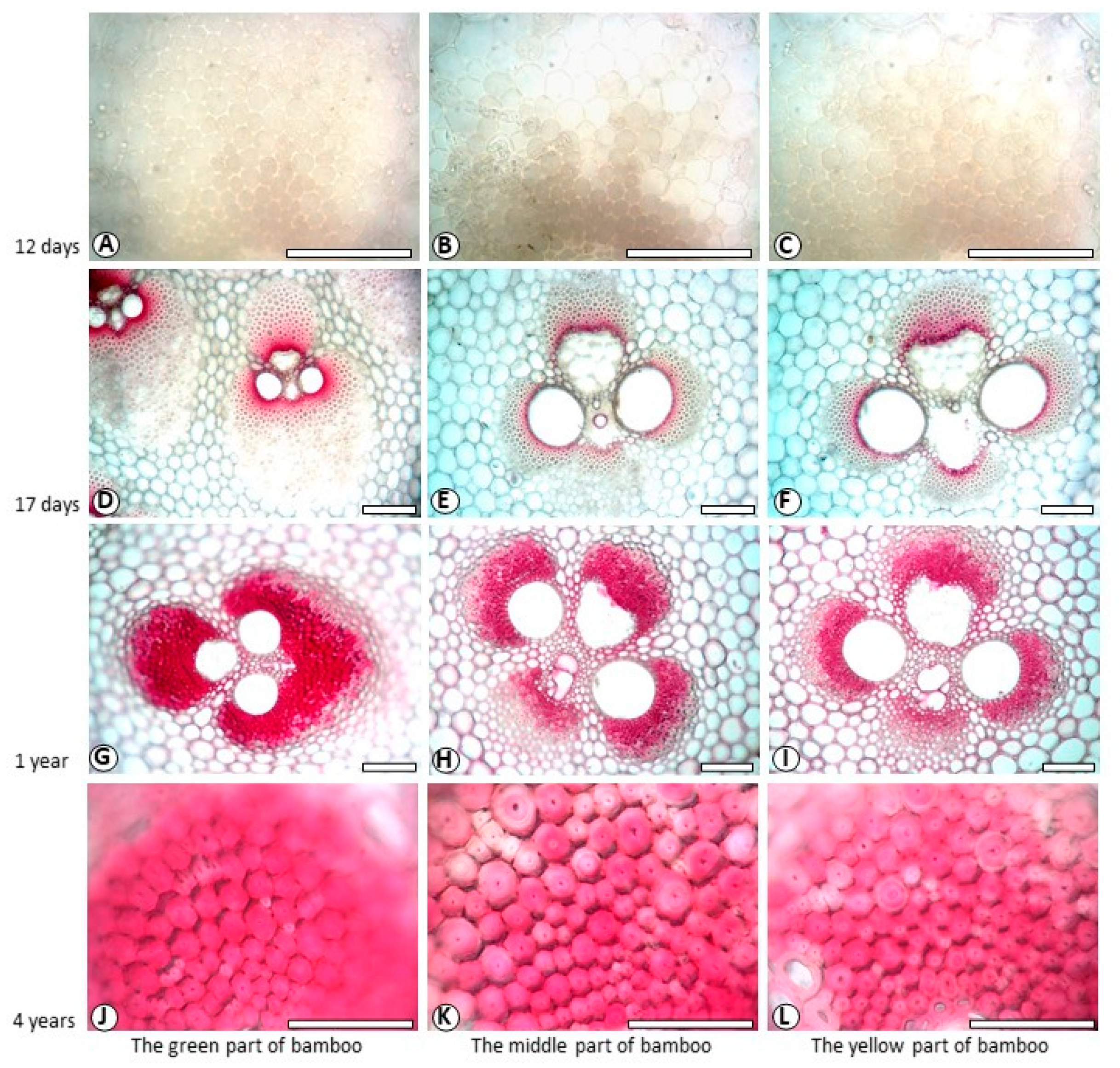
3.1. Histochemical Staining of the Cell Walls and Determination of Lignin Components
3.2. Visible-Light Absorption Spectra
3.3. Variation of Lignin Content of SW and CC in Different Ages
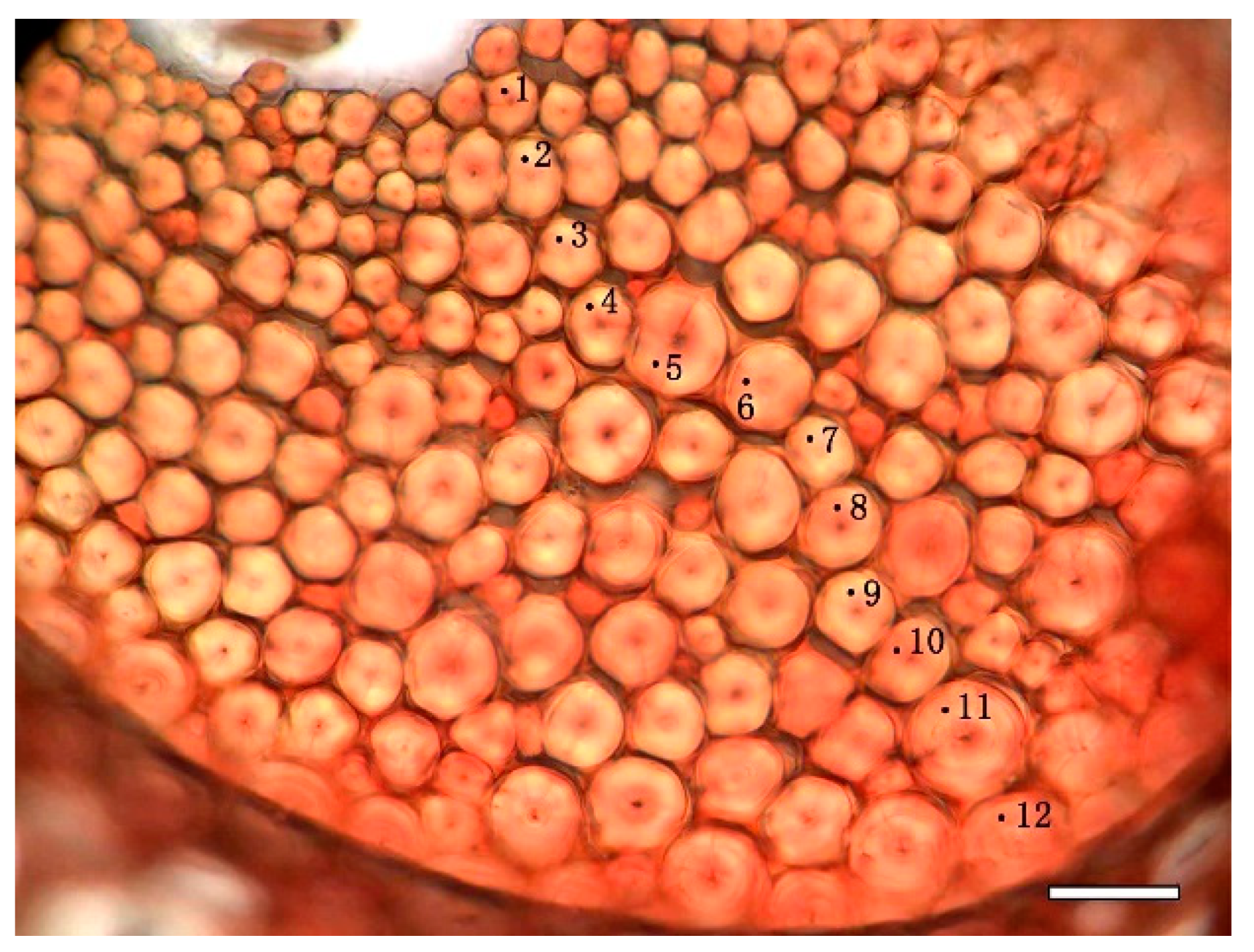
3.4. Variation of Lignin Content of SW and CC in Different Radial Location
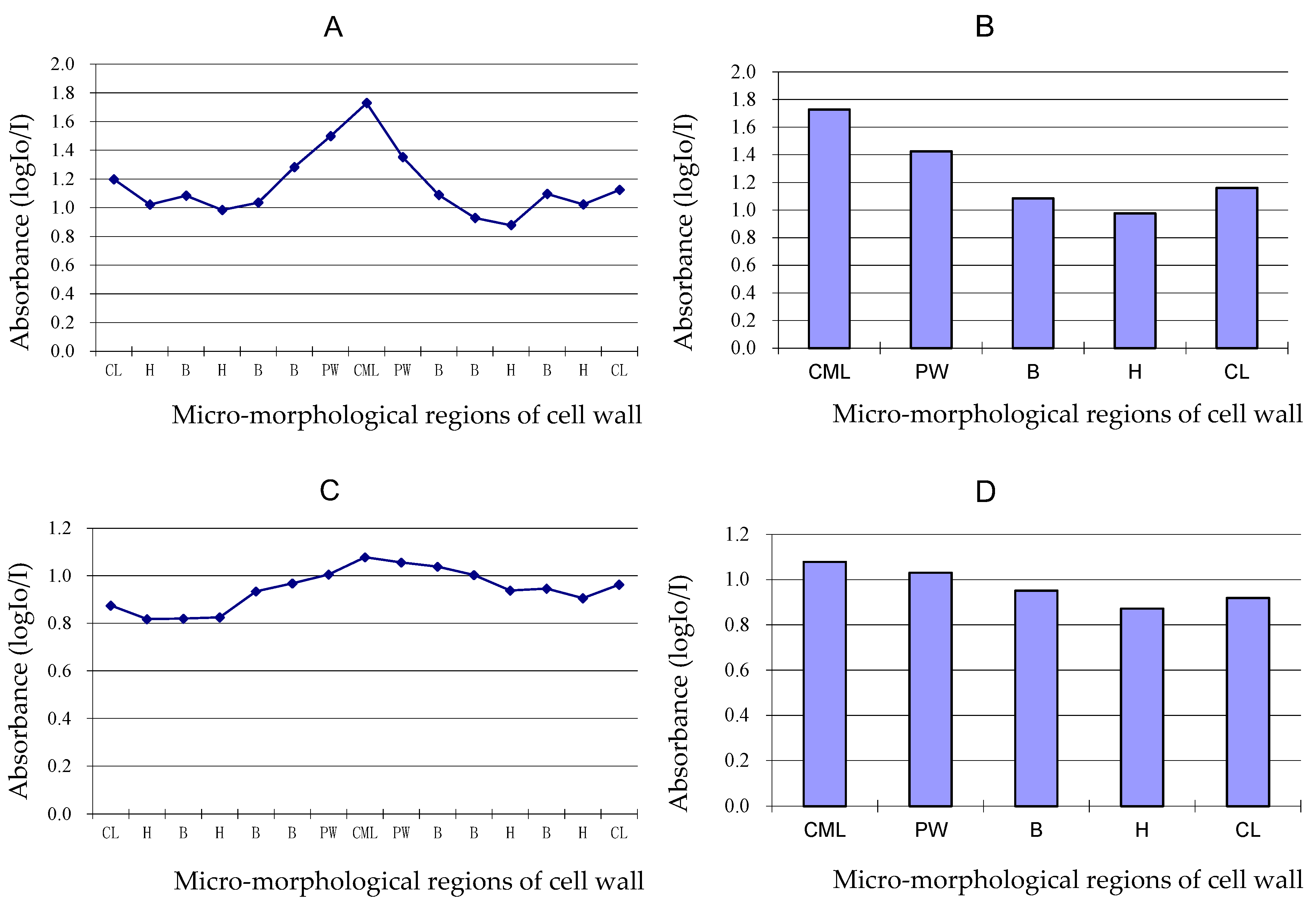
3.5. Variation of Lignin Content of Different Cell Wall Micro-Morphological Regions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, Y.X.; Ji, Y.H.; Yu, W.J. Development of bamboo scrimber: A literature review. J. Wood Sci. 2019, 65, 25. [Google Scholar] [CrossRef]
- Huang, S.S.; Jiang, Q.F.; Yu, B.; Nie, Y.J.; Ma, Z.Q.; Ma, L.F. Combined chemical modifification of bamboo material prepared using vinyl acetate and methyl methacrylate: Dimensional stability, chemical structure, and dynamic mechanical properties. Polymers 2019, 11, 1651. [Google Scholar] [CrossRef] [Green Version]
- Nirmala, C.; Bisht, M.S.; Bajwa, H.K.; Santosh, O. Bamboo: A rich source of natural antioxidants and its applications in the food and pharmaceutical industry. Trends Food Sci. Technol. 2018, 77, 91–99. [Google Scholar] [CrossRef]
- Xu, G.F.; Shi, Z.J.; Zhao, Y.H.; Deng, J.; Dong, M.Y.; Liu, C.T.; Vignesh, M.; Mai, X.M.; Guo, Z. Structural characterization of lignin and its carbohydrate complexes isolated from bamboo (Dendrocalamus sinicus). Int. J. Biol. Macromol. 2019, 126, 376–384. [Google Scholar] [CrossRef]
- Verma, S.; Hashmi, S.A.R.; Mili, M.; Hada, V.; Prashant, N.; Naik, A.; Rathore, S.K.S.; Srivastava, A.K. Extraction and applications of lignin from bamboo: A critical review. Eur. J. Wood Wood Prod. 2021, 79, 1341–1357. [Google Scholar] [CrossRef]
- Kaur, P.J.; Kardam, V.; Pant, K.K.; Naik, S.N.; Satya, S. Characterization of commercially important Asian bamboo species. Eur. J. Wood Wood Prod. 2016, 74, 137–139. [Google Scholar] [CrossRef]
- Figueiredo, P.; Lintinen, K.; Hirvonen, J.T.; Kostiainen, M.A.; Santos, H.A. Properties and chemical modifcations of lignin: Towards lignin-based nanomaterials for biomedical applications. Prog. Mater. Sci. 2018, 93, 233–269. [Google Scholar] [CrossRef]
- Salmén, L.; Burgert, I. Cell wall features with regard to mechanical performance. A review COST Action E35 2004-2008: Wood machining micromechanics and fracture. Holzforschung 2009, 63, 121–129. [Google Scholar] [CrossRef]
- Lybeer, B.; Koch, G. Lignin distribution in the tropical bamboo species Gigantochloa levis. IAWA J. 2005, 26, 443–456. [Google Scholar] [CrossRef]
- Yang, S.M.; Liu, X.E.; Shang, L.L.; Ma, J.F.; Tian, G.L.; Jiang, Z.H. The Characteristics and Representation Methods of Lignin for Bamboo. Polym. Polym. Matrix Compos. 2020, 34, 7177–7182. [Google Scholar]
- Igor, C. Unraveling the regulatory network of bamboo lignification. Plant Physiol. 2021, 187, 673–675. [Google Scholar]
- Jin, K.X.; Ling, Z.; Jin, Z.; Ma, J.F.; Yang, S.M.; Liu, X.E.; Jiang, Z.H. Local Variations in Carbohydrates and Matrix Lignin in Mechanically Graded Bamboo Culms. Polymers 2022, 14, 143. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T. Lignification of bamboo (Phyllostachys heterocycla Mitf.) during its growth. Holzforschung 1990, 44, 191–200. [Google Scholar] [CrossRef]
- Qu, C.; Shinjiro, O.; Takao, K. Characterization of immature bamboo (Phyllostachys nigra) component changes with its growth via heteronuclear single-quantum coherence nuclear magnetic resonance spectroscopy. J. Agric. Food Chem. 2020, 68, 9896–9905. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.K.; Huang, J.W.; Wang, K.L.; Wang, B.; Sun, S.L.; Lin, X.C.; Song, L.L.; Wu, A.M.; Li, H.L. Characterization of Lignin Structures in Phyllostachys edulis (Moso Bamboo) at Different Ages. Polymers 2020, 12, 187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, K.; Itoh, T. The changes in cell wall architecture during lignification of bamboo, Phyllostachys aurea Carr. Trees 2001, 15, 137–147. [Google Scholar] [CrossRef]
- Lin, J.X.; He, X.Q.; Hu, Y.X.; Kuang, T.Y.; Ceulemans, R. Lignification and lignin heterogeneity for various age classes of bamboo (Phyllostachys pubescens) stems. Physiol. Plant. 2002, 144, 296–302. [Google Scholar] [CrossRef]
- Gan, X.H.; Ding, Y.L. Investigation on the Variation of Fiber Wall in Phyllostachys edulis Culms. J. For. Res. 2006, 19, 457–462. [Google Scholar]
- Liu, B. Study on the Formation of Cell Wall during the Development of Phyllostachys pubescens. Ph.D. Thesis, Chinese Academy of Forestry, Beijing, China, 2008. [Google Scholar]
- Zhang, X.X.; Yu, Z.X.; Yu, Y.; Wang, H.K.; Li, J.H. Axial compressive behavior of Moso Bamboo and its components with respect to fiber-reinforced composite structure. J. For. Res. 2019, 30, 2371–2377. [Google Scholar] [CrossRef]
- Watanabe, Y.; Kojima, Y.; Ona, T.; Asada, T. Histochemical study on heterogeneity of lignin in Eucalyptus species II. The distribution of lignins and polyphenols in the walls of various cell types. IAWA J. 2004, 25, 283–295. [Google Scholar] [CrossRef] [Green Version]
- Parameswaran, N.; Liese, W. On the fine structure of bamboo fibres. Wood Sci. Technol. 1976, 10, 231–246. [Google Scholar]
- Lybeer, B.; Koch, G. A top chemical and semi quantitative study of the signification during ageing of bamboo culms (phyllostachys viridiglaucescens). IAWA J. 2005, 26, 99–109. [Google Scholar] [CrossRef]
- He, X.Q. Histo- and Cytological Studies on the Lignification of Bamboo Stem (Phyllostachys pubescens Mazel). Ph.D. Thesis, The Chinese Academy of Sciences, Beijing, China, 1999. [Google Scholar]
- Lu, F.; Ralph, J.; Agric, J. The DFRC method for lignin analysis. 2. Monomers from isolated lignins. Food Chem. 1998, 46, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.Y.; Ding, Z.F.; Li, Y.F. Mediacy growth and innernode growth of bamboo. Sci. Silvae Sin. 1980, 16, 81–89. [Google Scholar]
- Huang, Y.H.; Fei, B.H. Comparison of the mechanical characteristics of fifibers and cell walls from moso bamboo and wood. BioResources 2017, 12, 8230–8239. [Google Scholar] [CrossRef]
- Antonova, G.F.; Varaksina, T.N.; Zheleznichenko, T.V.; Stasova, V.V. Lignin deposition during earlywood and latewood formation in Scots pine stems. Wood Sci. Technol. 2014, 48, 919–936. [Google Scholar] [CrossRef]
- Fukushima, K.; Terashima, N. Hererogeneity in formation of lignin. 15. Formation and structure of lignin in comparession wood of pinus-thundergii studied by microautoradiography. Wood Sci. Technol. 1991, 25, 371–381. [Google Scholar]
- Wang, X.Q.; Ren, H.Q.; Zhang, B.; Fei, B.H.; Burgert, I. Cell wall structure and formation of maturing fifibres of moso bamboo (Phyllostachys pubescens) increase buckling resistance. J. R. Soc. Interface 2012, 9, 988–996. [Google Scholar] [CrossRef] [Green Version]

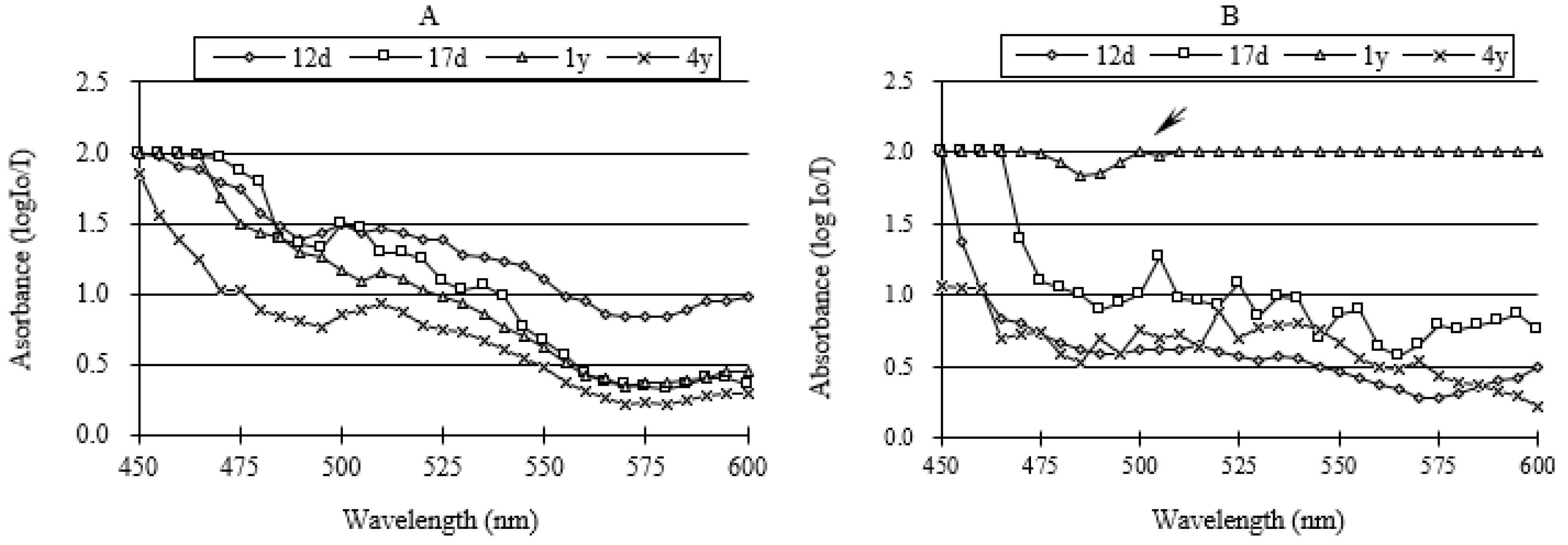

| Type of Reaction | Absorption Peak of Spectra (nm) | |||
|---|---|---|---|---|
| 12-Day-Old | 17-Day-Old | 1-Year-Old | 4-Year-Old | |
| Mäule reaction | 500 | 500 | 510 | 510 |
| Wiesner reaction | 515 | 505 | 500 | 520 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Tang, L.; Chen, Q.; Zhu, L.; Zou, X.; Li, B.; Zhou, Q.; Fu, Y.; Lu, Y. Lignin Distribution on Cell Wall Micro-Morphological Regions of Fibre in Developmental Phyllostachys pubescens Culms. Polymers 2022, 14, 312. https://doi.org/10.3390/polym14020312
Liu B, Tang L, Chen Q, Zhu L, Zou X, Li B, Zhou Q, Fu Y, Lu Y. Lignin Distribution on Cell Wall Micro-Morphological Regions of Fibre in Developmental Phyllostachys pubescens Culms. Polymers. 2022; 14(2):312. https://doi.org/10.3390/polym14020312
Chicago/Turabian StyleLiu, Bo, Lina Tang, Qian Chen, Liming Zhu, Xianwu Zou, Botao Li, Qin Zhou, Yuejin Fu, and Yun Lu. 2022. "Lignin Distribution on Cell Wall Micro-Morphological Regions of Fibre in Developmental Phyllostachys pubescens Culms" Polymers 14, no. 2: 312. https://doi.org/10.3390/polym14020312
APA StyleLiu, B., Tang, L., Chen, Q., Zhu, L., Zou, X., Li, B., Zhou, Q., Fu, Y., & Lu, Y. (2022). Lignin Distribution on Cell Wall Micro-Morphological Regions of Fibre in Developmental Phyllostachys pubescens Culms. Polymers, 14(2), 312. https://doi.org/10.3390/polym14020312








