Encapsulation of Bioactive Compounds for Food and Agricultural Applications
Abstract
1. Introduction
2. Polymers Used for Encapsulation of Bioactive Compounds
2.1. Chitosan
| Polymer | Material | Encapsulated Bioactive Compound | Objective | References |
|---|---|---|---|---|
| Alginate | Ca alginate hydrogel granules | Reishi medicinal mushroom extract; Probiotic Lactobacillus acidophilus | Mask the bitter taste of extract and protect bioactive substances in oral administration to prolong cell viability under simulated gastrointestinal conditions and to protect the bioactive ingredients of Reishi mushroom along the storage | [16] |
| Alginate | Macrospheres | Pseudomonas sp. DN18 | Effective protection against diseases caused by Eclerotium rolfsii | [28] |
| Alginate | Hydrogel | Jujube extract (Ziziphus spp.) | Effect of encapsulation on antioxidant activity and protection of bioactive compounds | [29] |
| Gum Arabic | Adhesive membrane | Cinnamon extract | Present an active food packaging material with more control over its pungent smell and quick release | [13] |
| Gum Arabic | Nanocapsule | Savory essential oil | Alternative control method to the pre-emergence herbicide Metribuzin (Sencor®) | [30] |
| Gum Arabic + Chitosan | Nanocapsule | Saffron (Crocus sativus L.) | Increase bioavailability and protection of bioactive compounds through nanoencapsulation | [31] |
| Cellulose | Powder particles | Vitamin A | Evaluate the emulsifying properties of cellulose particles and the ability to encapsulate with vitamin A | [17] |
| Cellulose | Edible films | Probiotic bacteria (Lactobacillus rhamnosus GG) | Search for new applications of coatings and films based on edible cellulose as carriers of various probiotics | [32] |
| Celulose | Cryogels | Tebuconazole fungicide | Controlled release of Tebuconazole fungicide | [33] |
| Chitosan + Cellulose | Nanoformulations | Citronella essential oil (Cymbopogon nardus (L.)) | Control of Spodoptera littoralis | [34] |
| Chitosan | Nanoparticles | Peppermint oil (Mentha piperita (L.)) | A nanoinsecticide to control Tribolium castaneum (Herbst) and Sitophilus oryzae (L.) | [35] |
| Chitosan | Nanoliposomes | Caffeine | Retention and release of caffeine in the digestive system | [36] |
| Pectin | Hydrogels | Lactase | Lactase encapsulated in pectin-based hydrogels | [37] |
| Pectin | Film | Beetroot extract encapsulated in pectin from watermelon peel | Monitor the freshness of packaged chilled beef by developing a pH indicator film | [15] |
| Pectin | Edible coating | Carvacrol/2-hydroxypropyl-β-cyclodextrin (CAR/HPβCD-IC) | Fungal inhibition against Botrytis cinerea and Alternaria alternata | [38] |
| Shellac | Gels | Riboflavin Lactobacillus acidophilus amylase | Form shellac-based gels and oat protein at neutral pH as a carrier to entrap and deliver active substances | [19] |
| Shellac + Chitin | Composite microspheres | Yeast alcohol dehydrogenase (YADH) | Enzymatic immobilization by adsorption | [39] |
| Shellac + Zein | Composite capsules | Curcumin | Controlled release of curcumin | [40] |
| Xanthan gum + sodium alginate | Gels | Debranched pea starch | Improve the performance of debranched pea starch gels | [41] |
| Xanthan gum | Edible films | Xanthan Gum solutions with glycerol acid | Increase lotus root storage stability | [42] |
2.2. Cellulose
2.3. Starch
2.4. Alginate
2.5. Shellac
2.6. Pectin
2.7. Gum Arabic
2.8. Xanthan Gum
2.9. Dextran
2.10. Milk Proteins
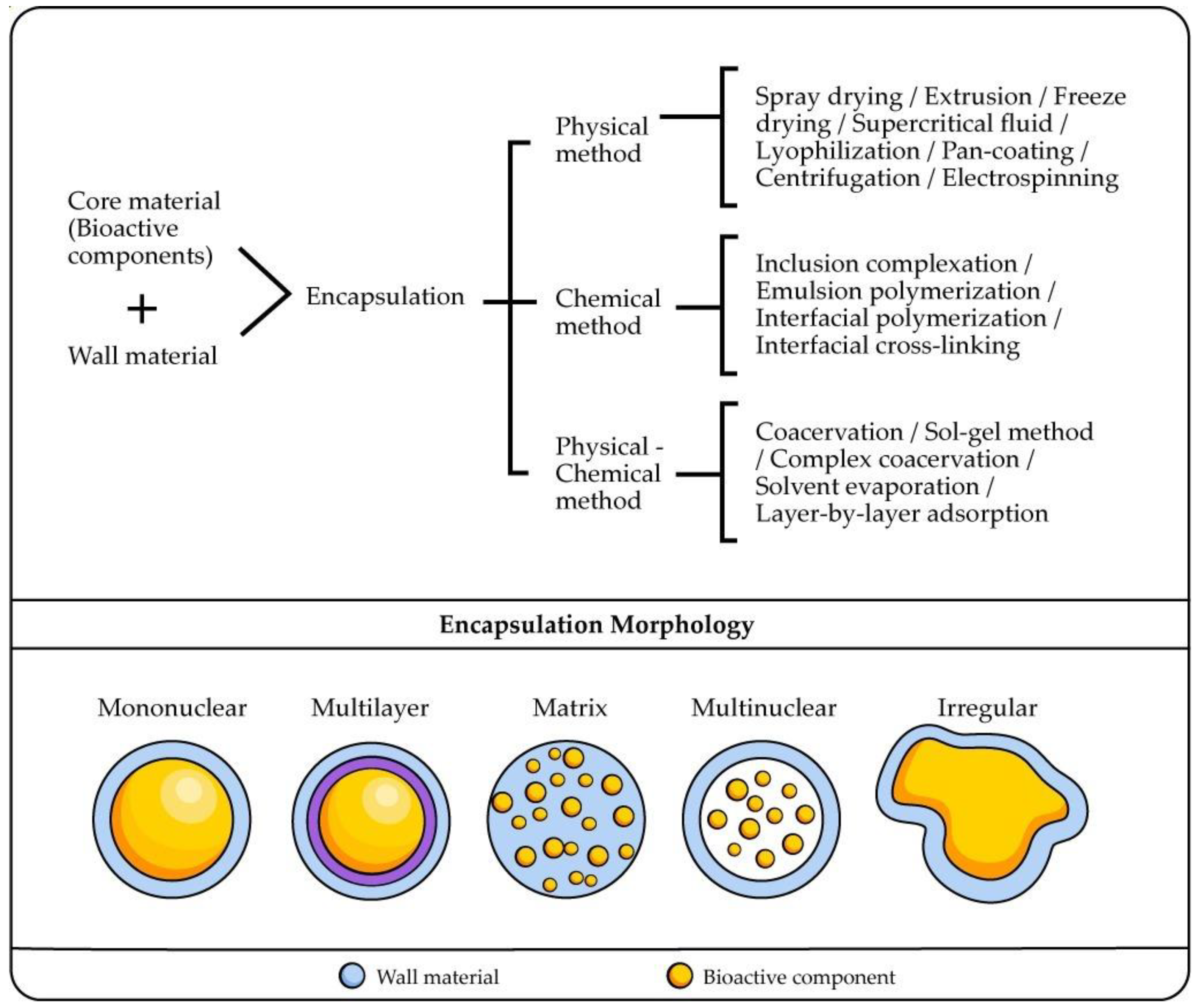
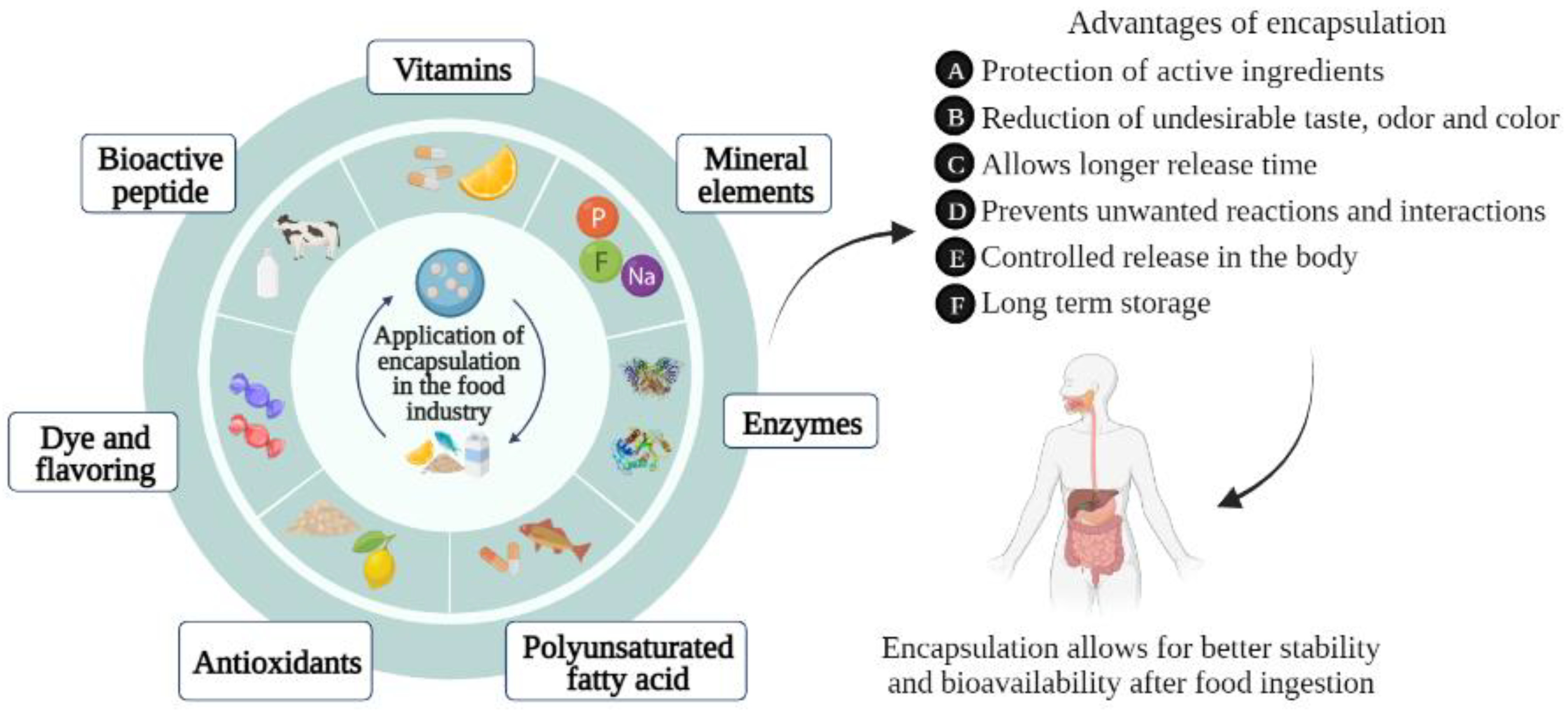
3. Encapsulation of Bioactive Compounds
3.1. Bioactive Peptides
3.2. Fatty Acids from Vegetable Oils
3.3. Vitamins
| Bioactive Compounds | Sources | Encapsulation Material | Encapsulation Method | Process Conditions | Objective | Results | References |
|---|---|---|---|---|---|---|---|
| Bioactive peptides | Milk casein hydrolysate | Pullulan | Electrospinning (encapsulation of various bioactive compounds in the form of nanosized fibers) | Pullulan at 100, 120, and 140 g kg−1 | Improve the stability, and low bioavailability, masking the bitter taste | Production of clean bead-free peptides-loaded pullulan nanofibres at 120 and 140 g kg−1 with an encapsulation efficiency of 72–86% and a mean diameter of 60–133 nm | [107] |
| Antioxidant peptides | Fish hydrolyzed collagen | Liposome | Freeze-dried | The highest encapsulation efficiency was found in SPC-CHO-0.5% HC (p < 0.05) (85%); liposome stabilized with glycerol presented the highest efficiency (75%) | Improve stability and bioactivities | Lyophilized SPC-CHO-0.5% HC presented higher stability than lyophilized SPC-GLY-0.25% HC during storage for 28 days at 25 °C | [108] |
| Antioxidant peptides | Fish protein hydrolysate | Not described | Spray dried | Enzymatic hydrolysis and chemical methods; spray dried at 180 °C inlet temperature to obtain powder; stored at −18 °C | Retention of antioxidant properties and microstructure | Visceral protein hydrolysate prepared with pepsin had better quality regarding antioxidant characteristics and papain in nutritional aspect | [109] |
| Antihypertensive peptide | Whey protein hydrolysate <3 kDa | Alginate-collagen, alginate-gum Arabic, and alginate–gelatin | Extrusion method | Sonication for 15 min | Released antihypertensive peptides during gastrointestinal digestion | The highest efficiency was obtained in capsules of alginate—gum Arabic (95%); the released peptides incremented their ACE activity (85%) | [110] |
| Antioxidant peptides | Flaxseed protein | Maltodextrin | Spray drying | Hydrolysate to maltodextrin (MD) to ratios (1:1, 1:2 and 1:3, w/w); alcalase enzyme was used to produce hydrolysates | Retention of antioxidant properties and microstructure | Samples powders obtained by 1:3 ratio presented the highest radical scavenging activity for and ABTS+ (86%) and DPPH (69%); analysis of chemical structure indicated that hydrolysates were coated and dispersed within maltodextrin | [91] |
| Antioxidant peptides | Milk Casein hydrolysate | Maltodextrin | Spray drying | Spraying was carried out by a pressure nozzle, compressed air flow rate of 0.54 m3 h−1, flow rate of 5 mL min−1, and inlet air temperature and outlet air temperature of 130 ± 1 °C and 70.0 ± 0.5 °C | Reduction of hygroscopicity and retention of antioxidant properties | Antioxidant activities were 90–99%, 77–92%, 77–93%, 95–99%, and 77–98% after the spray-drying process; hygroscopicity was reduced by microencapsulation (p < 0.05) | [111] |
| Antioxidant peptides | Pink peach meat protein hydrolysate | Gum Arabic and maltodextrin | Emulsions-spray drying | Inlet air at 160 °C, outlet at 80 °C, nozzle diameter of 0.5 mm, air at 0.4 MPa, and spray flow feed rate of 15–20 mL min−1 | Retention of antioxidant properties; improvement of sensory properties | Antioxidant activity was improved; sensory properties were improved in a concentration of up to 3% | [112] |
| Bioactive peptides | Azocasein | Not applicable | Double emulsions water-in-oil-in-water | Enzymatic hydrolysis | Improved bioavailability | Encapsulation efficiency of casein peptides was 93% | [113] |
| Antioxidant peptides | Casein hydrolysate | Gum Arabic and maltodextrin | Freeze-dried | Enzymatic hydrolysis, coating material (10:0, 8:2, 6:4); ultrasonication at 40 kHz, 750 W, 12 mm diameter tip and with 50% pulse for 20 min | Improve the antioxidant and sensorial properties | Reduced bitterness if compared to the casein hydrolysate; maintenance of antioxidant activity (93%) | [114] |
| Polyunsaturated fatty acids | Tea oil | Maltodextrin/Xanthan gum/Lysozyme nanoparticles | Pickering emulsion | Tea oil plus composite solutions at oil-water volume ratio of 1:5; homogenization for 3 min at 18,000 rpm to obtain the tea oil Pickering emulsion | Reduce lipid oxidation on tea oil powder | Encapsulation efficiency of 66% when 50% maltodextrin and 4% Xanthan gum/Xanthan gum/lysozyme nanoparticles was used; the surface of tea oil powder presented a relatively smooth porous microstructure | [115] |
| Omega-3 fatty acid | Flaxseed oil | Maltodextrin | Coacervation | - | Maintaining stability | - | [116] |
| Omega-3 and omega-6 fatty acids | Linum usitatissimum | Gum Arabic/whey protein/modified starch/sodium caseinate | Drying (spray drying, freeze drying)/supercritical emulsification/emulsion/coacervation | - | Prevents the oxidation of fatty acids | Microcapsules prepared with spray- and freeze-drying ranged between 10–400 and 20–5000 µm | [117] |
| Unsaturated fatty acids | Drumstick oil (Moringa oleifera) | Maltodextrin/gum Arabic (25:75); (oil to wall ratio 1:4) | Spray drying | Inlet air at 180 °C, outlet at 85 °C, air pressure of 0.06 MPa and air flow rate of 73 m3 h−1 | To evaluate the protection of the encapsulating compound in drumstick oil | The range of emulsion droplet mean diameters was 1.94 to 4.92 µm; encapsulation efficiency of 91.05% with lower water activity; good oxidative stability; peroxide value was 7.63 to 8.07 meq of peroxide/kg of oil after 30 days of storage at 45 °C; particles size was 22.56 ± 0.63 µm;showed larger smooth-surfaced particles, which may indicate that viscosity of the emulsions and emulsifying capacity are higher. | [83] |
| Omega-3 fatty acid | Chia seed oil | Soy protein microparticles | Supercritical CO2-assisted impregnation | 16 MPa impregnation pressure with ethanol as cosolvent (0.1-ethanol:oil ratio, w/w), temperature 40 °C and 4 h of contact time | Protect bioactive compounds through microencapsulation and increase bioavailability | Encapsulation efficiency of 95% and a retention efficiency of 35%, showing excellent oxidative stability; microcapsules ranged between 1 and 10 μm, having a spherical form with occasional depressions but no pores or fissures; 95.69 ± 4.28% of the encapsulated oil is released upon exposure to gastrointestinal conditions and becomes available for absorption. | [118] |
| Omega-3 fatty acid | Chia, camelina and echium oilseeds and wet microalgal lipids | Sodium caseinate and lactose (oil to wall ratio 1:4 (w/w)) | Spray drying | Inlet air at 170 °C, compressed air pressure of 0.5 MPa, air flow of 700 L min−1 and aspiration 70% | Produce microencapsulated lipid extracts from sources of omega-3 | Particles size ranged between 1.5 and 30 μm and they presented a spherical shape and a smooth surface without cracks; the chia fatty acid ethyl esters microcapsules had the best microencapsulation efficiency of 76.9%, while the echium microcapsules had the highest payload of 142 mg/g. | [14] |
| Omega-3 fatty acid | Flaxseed oil | 4% gum Arabic and 16% soy protein isolate | Spray drying | Inlet air at 150 °C, outlet at 80–85 °C and flow rate of 4 mL min−1 | Evaluate the effect of flaxseed oil nano-encapsulation on stability, physical, color, rheological, textural, and organoleptic properties in egg-free cake | Nanoencapsulated flaxseed oil used as an egg replacer in cakes; had the greatest percentage of omega-3 fatty acids (30%); particle size less than 100 nm; encapsulation efficiency of 72%; moisture content of 4% and peroxide value of 1.1 meq/kg. | [119] |
| Polyunsaturated fatty acids | Walnut oil | Fructooligosaccharide/soybean protein isolate (20% w/w) | Freeze drying | Temperature − 46 °C, pressure 4.1 Pa for 48 h | Evaluate the fructooligosaccharide/soy isolate protein | Microcapsules ranged between 121.51 and 162.02 µm; fructooligosaccharide reduces particle size and increases viscosity; microencapsulated walnut oil had a peroxide value of 26.84 meq/kg after 8 days of storage, compared to the walnut oil which reached a peroxide value of 74.56 meq/kg. | [120] |
| Alpha-linolenic acid | Perilla oil; (Perilla frutescens) | γ-cyclodextrin | Inclusion complex | The formation of pseudorotaxane complexes that precipitate in aqueous media | Evaluate the thermal stability and bioavailability α-linolenic acid from perilla oil | The complexes may serve as an effective supply of α-linolenic acid to raise plasma omega-3 fatty acid levels | [121] |
| Cinnamaldehy | Cinnamon essential oil | Carboxymethyl cellulose and polyvinyl alcohol | Pickering emulsions by in situ hydrophobization | Use oleic acid as a hydrophobic compound | Increase the shelf life of the bread | No fungal growth at 25 °C for 15 days; controlled release of cinnamon essential oil; fungal inhibition against P. digitatum in films containing 1.5 and 3% CEO. | [122] |
| Flavonoid karanjin | Pongamia pinnata L. seed oil | Polyuria | Interfacial polymerization | 400–500 rpm slow mixing | Evaluate the insecticidal activity of microencapsulated P. pinnata oil | High encapsulation efficiency of 87.41%; release kinetics was y = −0.0042 x + 6.4205; effective protection against Aphis gossypii (71.8%) and Bemisia tabaci (74.7%). | [123] |
| Monoterpene α-pinene | Juniper berry essential oil | Gum Arabic/maltodextrin (1:1); (oil to wall ratio 1:4, w/w) | Spray drying | Inlet air at 120 °C, outlet at 80 °C and 3.2 cm3 min−1 of feed flow rate | Evaluate properties of microcapsules | Particle size was 10.83 µm ± 1.86; encapsulation efficiency of 70.07% and a retention efficiency of 82.66%; powder has the following characteristics: 4.92% moisture, 10.18% hygroscopicity, 63.80% solubility, 72.83% porosity, and 3.23 min of dissolving time; depending on the kind of wall material, it took between 15 and 45 min for the oil to completely discharge; presents antimicrobial and antifungal activity; it can be used as a food preservative. | [124] |
| Eugenol | Clove essential oil | Chitosan nanoparticles (oil to wall ratio 0.5:1) | Emulsification (o/w) and ionic gelation | Homogenize at 13,000 rpm for 10 min in ice bath conditions; for ionic gelation of the chitosan, sodium tripolyphosphate was added and agitated for 40 min | Improved antioxidant and antimicrobial activity by nanoencapsulation of clove essential oil | Particle size of 295.8 ± 45.6 nm; high retention rate (73.4%); high in vitro antimicrobial activity against Listeria monocytogenes, Staphylococcus aureus, Salmonella typhi and E. coli (a 4.80 to 4.78 cm inhibitory halo). | [125] |
| Alpha-tocopherol | Wheat germ oil | 1.5% Sodium alginate and 2% pectin | Air atomization | O/W emulsion dropped; 5% (w/v) calcium chloride solution agitated for 30 min | Increase antioxidant activity and thus shelf life and nutritional value of cookies | Encapsulation efficiency of 55.97%; maximum antioxidant activity of 41.1%; improved storage stability and shelf life of cookies; microencapsulated α-tocopherol can serve as an antioxidant to avoid autoxidation in fat-based bakery products. | [104] |
| Alpha-tocopherol | Palm oil | Maltodextrin and sodium caseinate; (Core to wall ratio 1) | Spray drying | 1.5 mm nozzle diameter, 10 mL min−1 of feed flow rate, 55 kgf cm2 air pressure, 20,000–25,000 rpm atomization speed, inlet air at 110 °C and outlet at 90 °C | Demonstrate the encapsulating and protective capacity of the wall material for the microencapsulation of vitamin E | Encapsulation efficiency of 59.9 ± 0.017 to 71.5 ± 0.027%; particle size from 13 to 29 µm; moisture content from 4.5 to 4.98%, microcapsule considered soluble due to the short solubility time of 178 to 251 s. | [126] |
| Alpha-tocopherol | Palm fatty acid distillate | Galactomannan and gum acacia | Spray drying | Inlet air at 180–200 °C and outlet at 90 °C | Evaluate emulsion and oxidative stability | Encapsulation efficiency between 60.68 and 70.01%; microcapsules ranged between 16 µm and 11 µm; a yield between 53.15 and 64.09%; moisture content from 3.40 to 3.08%; microencapsulation improved oxidative stability and absorption of vitamin E. | [127] |
| Alpha-tocopherol | Vitamin E | Whey protein isolate; (Core to wall ratio 1:3) | (1) Spray drying; (2) Freeze-drying; (3) Spray freeze-drying | (1) Inlet temperature 100 °C, outlet temperature 80 °C and 4 mL min−1 feed flow rate; (2) Temperature − 25 °C for 2 h and 7.6 × 102 Torr to 0.8 Torr vacuum; shelf temperature between-25 °C and 20 °C for 16–18 h, after that 25 °C for 2 h; | Evaluate the effect of the three techniques of vitamin E microencapsulation | Encapsulation efficiencies and particle size of 90% and 195.8 µm for spray dried microcapsules, 86% and 279 µm for freeze-dried microcapsules, 89% and 145.3 µm for spray freeze-dried microcapsules, respectively; the rats showed plasma vitamin E concentrations of 7.35 at 4 h, 7.69 at 4 h, 9.45 µg/mL at 3 h; area | [103] |
| (3) The temperature was kept between −25 °C and −10 °C with a vacuum of 0.8 torr, and then brought to 10 °C with a vacuum of 0.3 torr | under the curve were 109.84, 104.38 and 124.46 µg/(mL × h); spray freeze-drying microencapsulation improved the oral bioavailability by 1.13 and 1.19-fold compared to other techniques. | ||||||
| Vitamin E | Palm oil | Maltodextrin/Sodium caseinate (3:2:1) | Freeze drying | Temperature −41 °C and pressure 4 × 10−4 mbar | Effect on vitamin E encapsulation with selenomethionine | Encapsulated vitamin E with 5.6 mg selenomethionine improves solubility and bioavailability; particle size was 3.00 µm ± 0.55; release rates of vitamin E after 30 min in simulated gastric fluid solution and simulated intestinal fluid solution were 87% and 42%, respectively. | [105] |
| Alpha-tocopherol | Vitamin E | Polycaprolactone | Supercritical fluid extraction of emulsions | Pressure 8 MPa and temperature 40 °C; CO2 flow rate of 7.2 kg h−1 kg−1 emulsion and acetone as solvent | Demonstrate the feasibility of supercritical fluid technique in the nano-encapsulation of liquid lipophilic compounds | High encapsulation efficiency of 90%; particle size was from 8 and 276 nm; spherical, core-shell, and non-aggregated nanocapsules were formed, according to morphological analyses; higher storage stability between 6 and 12 months. | [128] |
| Alpha-tocopherol | Vitamin E | Polycaprolactone | Nanoprecipitation | At 30 °C in an ultrasonic bath, PCL was dissolved in acetone, lecithin, acetone-methanol mixture (60 to 40%, v/v) and α-tocopherol | Improve the carboxymethylcellulose film in the production of active packaging with α-tocopherol nanocapsules | High encapsulation efficiency of 88.43–99.66%; particle size ranged between 201.6 and 230.2 nm; alpha-tocopherol nanocapsules’ release behavior from CMC films might be best described by the Higuchi kinetic model; the maximum radical scavenging activity (68.85%) was found in films with 70% nanocapsules. | [129] |
| Alpha-tocoferol | Vitamin E | Acid hydrolysis-carboxymethyl starch (H-CMS) and xanthan gum (XG) | Spray-drying | Inlet air temperature 190 ± 5 °C and outlet air temperature 80 ± 5 °C | Improved bioavailability | Microcapsules produced with substitution grades of 0.44 and a ratio of 1:20 (H-CMS/XG) demonstrated higher specific delivery in the small intestine, releasing 38.32% and 61.68% of vitamin E into simulated gastric and intestinal fluids, respectively | [130] |
| Alpha-tocoferol | Vitamin E | Gelatin and gum Arabic | Complex coacervation | Adjust the pH with acetic acid (10% v/v) to 4–4.5 (isoelectric point of gelatin and gum arabic), at a speed of 1500 rpm, temperature 45 °C for 90 min | Optimize by response surface methodology the conditions for vitamin E microencapsulation | High encapsulation efficiency (93.42%), when the core material is 4 g and surfactant is 0.5% (%w/v); particle size was from 4 to 80 µm. | [18] |
| Alpha-tocopherol | Vitamin E | Nano-hydroxyapatite as a Pickering stabilizer | Pickering emulsions | Uses a mixer with continuous mode; O/W ratio of 20/80 (v/v) | Improved bioavailability, bioaccessibility and stability | Particle sizes were 7.53, 11.56 and 17.72 μm; improved bioaccessibility of vitamin E by 10.87 ± 1.04% for gelatin and 18.07 ± 2.90% for fortified milk | [131] |
3.4. Polyphenols
3.5. Carotenoids
3.6. Pigments
3.7. Encapsulating Efficiency
3.8. Release Characteristics and Kinetics of Microcapsules of Bioactive Components
4. Novel Technologies for Encapsulation of Bioactive Compounds
4.1. Supercritical Microencapsulation
| Microencapsulation Technique | Method | Applications | References |
|---|---|---|---|
| Physical | Spray-drying | Phenolic acids, carotenoids | [122] |
| Spray chilling | Pigments | ||
| Spray coating | Pigments | ||
| Supercritical microencapsulation—micronization | Carotenoids | ||
| Ionic gelation | Nutraceutical | ||
| Cocrystallization | Food ingredients | ||
| Freeze-drying | Carotenoids, Pigments | ||
| Fluidized bed coating | Carotenoids | ||
| Centrifugal extrusion | Food ingredients | ||
| Chemical | Interfacial polymerization | Food | [138,166,168,169] |
| Molecular inclusion | Nutraceutical | ||
| In situ polymerization | Nutraceutical | ||
| Physical-chemical | Coacervation | Volatile flavor oils | [138,166,168,169] |
| Complex coacervation | Lycopene | ||
| Emulsion-solvent evaporation | Food ingredients | ||
| Solidification emulsion | Food ingredients | ||
| Liposomes | Food ingredients, nutraceuticals |
4.2. Complex Coacervation
4.3. Spray Chilling
4.4. Other Encapsulation Techniques
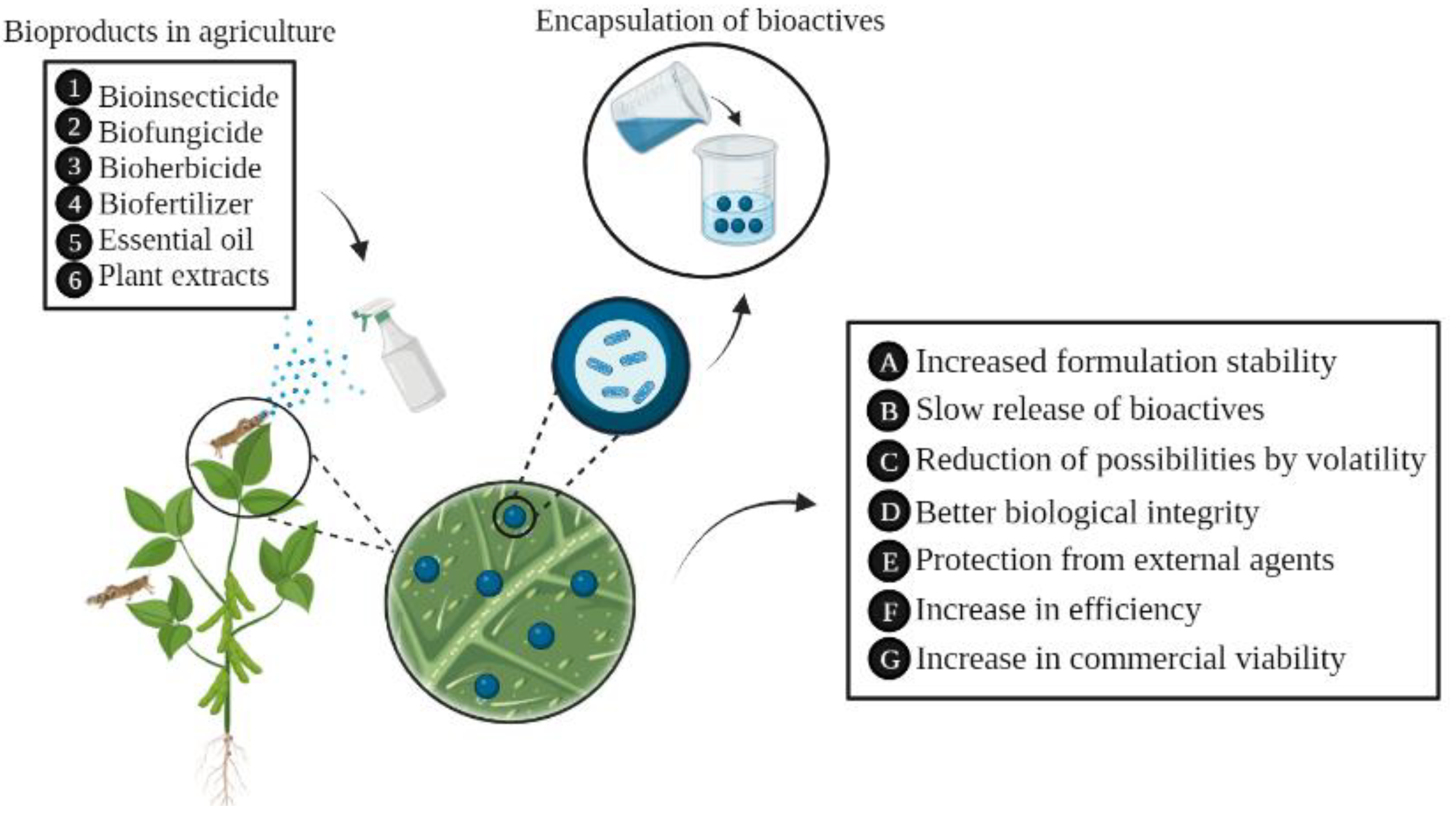
5. Applications of Encapsulated Products
6. Concluding Remarks and Future Trends
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Genovese, A.; Balivo, A.; Salvati, A.; Sacchi, R. Functional Ice Cream Health Benefits and Sensory Implications. Food Res. Int. 2022, 161, 111858. [Google Scholar] [CrossRef]
- Riley, S.; Lale, A.; Nguyen, V.; Xi, H.; Wilkinson, K.; Searle, I.R.; Fisk, I. Volatile Profiles of Commercial Vetch Prepared via Different Processing Methods. Food Chem. 2022, 395, 133569. [Google Scholar] [CrossRef]
- Mafra, J.F.; de Santana, T.S.; Cruz, A.I.C.; Ferreira, M.A.; Miranda, F.M.; Araújo, F.M.; Ribeiro, P.R.; Evangelista-Barreto, N.S. Influence of Red Propolis on the Physicochemical, Microbiological and Sensory Characteristics of Tilapia (Oreochromis niloticus) Salami. Food Chem. 2022, 394, 133502. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yuan, B.; Yu, P.; Jia, Y.; Zhou, Q.; Sun, J. Flavor Characteristics of Peanut Butter Pretreated by Radio Frequency Heating, Explosion Puffing, Microwave, and Oven Heating. Food Chem. 2022, 394, 133487. [Google Scholar] [CrossRef] [PubMed]
- Zabot, G.L.; Silva, E.K.; Emerick, L.B.; Felisberto, M.H.F.; Clerici, M.T.P.S.; Meireles, M.A.A. Physicochemical, Morphological, Thermal and Pasting Properties of a Novel Native Starch Obtained from Annatto Seeds. Food Hydrocoll. 2019, 89, 321–329. [Google Scholar] [CrossRef]
- Riseh, R.S.; Ebrahimi-Zarandi, M.; Tamanadar, E.; Pour, M.M.; Thakur, V.K. Salinity Stress: Toward Sustainable Plant Strategies and Using Plant Growth-Promoting Rhizobacteria Encapsulation for Reducing It. Sustainability 2021, 13, 12758. [Google Scholar] [CrossRef]
- Do Nascimento Junior, D.R.; Tabernero, A.; Cabral Albuquerque, E.C.d.M.; Vieira de Melo, S.A.B. Biopesticide Encapsulation Using Supercritical CO2: A Comprehensive Review and Potential Applications. Molecules 2021, 26, 4003. [Google Scholar] [CrossRef]
- De Oliveira, J.L.; Fraceto, L.F.; Bravo, A.; Polanczyk, R.A. Encapsulation Strategies for Bacillus thuringiensis: From Now to the Future. J. Agric. Food Chem. 2021, 69, 4564–4577. [Google Scholar] [CrossRef]
- Vassilev, N.; Vassileva, M.; Martos, V.; Garcia del Moral, L.F.; Kowalska, J.; Tylkowski, B.; Malusá, E. Formulation of Microbial Inoculants by Encapsulation in Natural Polysaccharides: Focus on Beneficial Properties of Carrier Additives and Derivatives. Front. Plant Sci. 2020, 11, 270. [Google Scholar] [CrossRef]
- Bae, M.; Lewis, A.; Liu, S.; Arcot, Y.; Lin, Y.-T.; Bernal, J.S.; Cisneros-Zevallos, L.; Akbulut, M. Novel Biopesticides Based on Nanoencapsulation of Azadirachtin with Whey Protein to Control Fall Armyworm. J. Agric. Food Chem. 2022, 70, 7900–7910. [Google Scholar] [CrossRef]
- Kadmiri, I.M.; El Mernissi, N.; Azaroual, S.E.; Mekhzoum, M.E.M.; Qaiss, A.E.K.; Bouhfid, R. Bioformulation of Microbial Fertilizer Based on Clay and Alginate Encapsulation. Curr. Microbiol. 2021, 78, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Riseh, R.S.; Skorik, Y.A.; Thakur, V.K.; Pour, M.M.; Tamanadar, E.; Noghabi, S.S. Encapsulation of Plant Biocontrol Bacteria with Alginate as a Main Polymer Material. Int. J. Mol. Sci. 2021, 22, 11165. [Google Scholar] [CrossRef] [PubMed]
- Ali, E.A.; Nada, A.A.; Al-Moghazy, M. Self-Stick Membrane Based on Grafted Gum Arabic as Active Food Packaging for Cheese Using Cinnamon Extract. Int. J. Biol. Macromol. 2021, 189, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Castejón, N.; Luna, P.; Señoráns, F.J. Microencapsulation by Spray Drying of Omega-3 Lipids Extracted from Oilseeds and Microalgae: Effect on Polyunsaturated Fatty Acid Composition. LWT 2021, 148, 111789. [Google Scholar] [CrossRef]
- Guo, Z.; Ge, X.; Li, W.; Yang, L.; Han, L.; Yu, Q. Active-Intelligent Film Based on Pectin from Watermelon Peel Containing Beetroot Extract to Monitor the Freshness of Packaged Chilled Beef. Food Hydrocoll. 2021, 119, 106751. [Google Scholar] [CrossRef]
- Mirmazloum, I.; Ladányi, M.; Omran, M.; Papp, V.; Ronkainen, V.-P.; Pónya, Z.; Papp, I.; Némedi, E.; Kiss, A. Co-Encapsulation of Probiotic Lactobacillus acidophilus and Reishi Medicinal Mushroom (Ganoderma lingzhi) Extract in Moist Calcium Alginate Beads. Int. J. Biol. Macromol. 2021, 192, 461–470. [Google Scholar] [CrossRef]
- Sodeinde, K.O.; Ojo, A.M.; Olusanya, S.O.; Ayanda, O.S.; Adeoye, A.O.; Dada, T.M.; Lawal, O.S. Cellulose Isolated from Delonixregia Pods: Characterisation and Application in the Encapsulation of Vitamin, A. Ind. Crops Prod. 2021, 160, 113138. [Google Scholar] [CrossRef]
- Köksal, E.; Bayram, O.; Göde, F.; Aktaş, A.H. Microencapsulation of Vitamin E: Optimization and Characterization of Complex Coacervation Conditions Using Response Surface Methodology. Sak. Univ. J. Sci. 2021, 25, 906–913. [Google Scholar] [CrossRef]
- Yang, C.; Wang, Y.; Lu, L.; Unsworth, L.; Guan, L.L.; Chen, L. Oat Protein-Shellac Beads: Superior Protection and Delivery Carriers for Sensitive Bioactive Compounds. Food Hydrocoll. 2018, 77, 754–763. [Google Scholar] [CrossRef]
- Guía-García, J.L.; Charles-Rodríguez, A.V.; Reyes-Valdés, M.H.; Ramírez-Godina, F.; Robledo-Olivo, A.; García-Osuna, H.T.; Cerqueira, M.A.; Flores-López, M.L. Micro and Nanoencapsulation of Bioactive Compounds for Agri-Food Applications: A Review. Ind. Crops Prod. 2022, 186, 115198. [Google Scholar] [CrossRef]
- Marcillo-Parra, V.; Tupuna-Yerovi, D.S.; González, Z.; Ruales, J. Encapsulation of Bioactive Compounds from Fruit and Vegetable By-Products for Food Application—A Review. Trends Food Sci. Technol. 2021, 116, 11–23. [Google Scholar] [CrossRef]
- Díaz-Torres, R.D.C.; Alonso-Castro, A.J.; Carrillo-Inungaray, M.L.; Carranza-Alvarez, C. Bioactive Compounds Obtained from Plants, Their Pharmacological Applications and Encapsulation. In Phytomedicine; Academic Press: Cambridge, MA, USA, 2021; pp. 181–205. ISBN 978-0-12-824109-7. [Google Scholar]
- Rehman, A.; Ahmad, T.; Aadil, R.M.; Spotti, M.J.; Bakry, A.M.; Khan, I.M.; Zhao, L.; Riaz, T.; Tong, Q. Pectin Polymers as Wall Materials for the Nano-Encapsulation of Bioactive Compounds. Trends Food Sci. Technol. 2019, 90, 35–46. [Google Scholar] [CrossRef]
- Ashter, S.A. Chemistry of Cellulosic Polymers. In Technology and Applications of Polymers Derived from Biomass; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 57–74. ISBN 978-0-323-51115-5. [Google Scholar]
- Hosseinnejad, M.; Jafari, S.M. Evaluation of Different Factors Affecting Antimicrobial Properties of Chitosan. Int. J. Biol. Macromol. 2016, 85, 467–475. [Google Scholar] [CrossRef]
- Shah, P. Polymers in Food. In Polymer Science and Innovative Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 567–592. ISBN 978-0-12-816808-0. [Google Scholar]
- Hossain, F.; Follett, P.; Salmieri, S.; Vu, K.D.; Fraschini, C.; Lacroix, M. Antifungal Activities of Combined Treatments of Irradiation and Essential Oils (EOs) Encapsulated Chitosan Nanocomposite Films in in Vitro and in Situ Conditions. Int. J. Food Microbiol. 2019, 295, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Panichikkal, J.; Prathap, G.; Nair, R.A.; Krishnankutty, R.E. Evaluation of Plant Probiotic Performance of Pseudomonas Sp. Encapsulated in Alginate Supplemented with Salicylic Acid and Zinc Oxide Nanoparticles. Int. J. Biol. Macromol. 2021, 166, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Farahani, Z.K.; Mousavi, M.; Ardebili, S.M.S.; Bakhoda, H. Modification of Sodium Alginate by Octenyl Succinic Anhydride to Fabricate Beads for Encapsulating Jujube Extract. Curr. Res. Food Sci. 2022, 5, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Taban, A.; Saharkhiz, M.J.; Kavoosi, G. Development of Pre-Emergence Herbicide Based on Arabic Gum-Gelatin, Apple Pectin and Savory Essential Oil Nano-Particles: A Potential Green Alternative to Metribuzin. Int. J. Biol. Macromol. 2021, 167, 756–765. [Google Scholar] [CrossRef]
- Rajabi, H.; Jafari, S.M.; Rajabzadeh, G.; Sarfarazi, M.; Sedaghati, S. Chitosan-Gum Arabic Complex Nanocarriers for Encapsulation of Saffron Bioactive Components. Colloids Surf. Physicochem. Eng. Asp. 2019, 578, 123644. [Google Scholar] [CrossRef]
- Singh, P.; Magalhães, S.; Alves, L.; Antunes, F.; Miguel, M.; Lindman, B.; Medronho, B. Cellulose-Based Edible Films for Probiotic Entrapment. Food Hydrocoll. 2019, 88, 68–74. [Google Scholar] [CrossRef]
- El-Naggar, M.E.; Hasanin, M.; Youssef, A.M.; Aldalbahi, A.; El-Newehy, M.H.; Abdelhameed, R.M. Hydroxyethyl Cellulose/Bacterial Cellulose Cryogel Dopped Silver@titanium Oxide Nanoparticles: Antimicrobial Activity and Controlled Release of Tebuconazole Fungicide. Int. J. Biol. Macromol. 2020, 165, 1010–1021. [Google Scholar] [CrossRef]
- Ibrahim, S.S.; Abou-Elseoud, W.S.; Elbehery, H.H.; Hassan, M.L. Chitosan-Cellulose Nanoencapsulation Systems for Enhancing the Insecticidal Activity of Citronella Essential Oil against the Cotton Leafworm Spodoptera Littoralis. Ind. Crops Prod. 2022, 184, 115089. [Google Scholar] [CrossRef]
- Rajkumar, V.; Gunasekaran, C.; Paul, C.A.; Dharmaraj, J. Development of Encapsulated Peppermint Essential Oil in Chitosan Nanoparticles: Characterization and Biological Efficacy against Stored-Grain Pest Control. Pestic. Biochem. Physiol. 2020, 170, 104679. [Google Scholar] [CrossRef] [PubMed]
- Seyedabadi, M.M.; Rostami, H.; Jafari, S.M.; Fathi, M. Development and Characterization of Chitosan-Coated Nanoliposomes for Encapsulation of Caffeine. Food Biosci. 2021, 40, 100857. [Google Scholar] [CrossRef]
- Cargnin, M.A.; Gasparin, B.C.; dos Santos Rosa, D.; Paulino, A.T. Performance of Lactase Encapsulated in Pectin-Based Hydrogels during Lactose Hydrolysis Reactions. LWT 2021, 150, 111863. [Google Scholar] [CrossRef]
- Sun, C.; Cao, J.; Wang, Y.; Huang, L.; Chen, J.; Wu, J.; Zhang, H.; Chen, Y.; Sun, C. Preparation and Characterization of Pectin-Based Edible Coating Agent Encapsulating Carvacrol/HPβCD Inclusion Complex for Inhibiting Fungi. Food Hydrocoll. 2022, 125, 107374. [Google Scholar] [CrossRef]
- Mei, S.; Han, P.; Wu, H.; Shi, J.; Tang, L.; Jiang, Z. One-Pot Fabrication of Chitin-Shellac Composite Microspheres for Efficient Enzyme Immobilization. J. Biotechnol. 2018, 266, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Xu, C.; Mao, L.; Wang, D.; Yang, J.; Gao, Y. Preparation, Characterization and Stability of Curcumin-Loaded Zein-Shellac Composite Colloidal Particles. Food Chem. 2017, 228, 656–667. [Google Scholar] [CrossRef]
- Wang, K.; Sui, J.; Gao, W.; Yu, B.; Yuan, C.; Guo, L.; Cui, B.; Abd El-Aty, A.M. Effects of Xanthan Gum and Sodium Alginate on Gelatinization and Gels Structure of Debranched Pea Starch by Pullulanase. Food Hydrocoll. 2022, 130, 107733. [Google Scholar] [CrossRef]
- Lara, G.; Yakoubi, S.; Villacorta, C.M.; Uemura, K.; Kobayashi, I.; Takahashi, C.; Nakajima, M.; Neves, M.A. Spray Technology Applications of Xanthan Gum-Based Edible Coatings for Fresh-Cut Lotus Root (Nelumbo nucifera). Food Res. Int. 2020, 137, 109723. [Google Scholar] [CrossRef]
- Akbari-Alavijeh, S.; Shaddel, R.; Jafari, S.M. Encapsulation of Food Bioactives and Nutraceuticals by Various Chitosan-Based Nanocarriers. Food Hydrocoll. 2020, 105, 105774. [Google Scholar] [CrossRef]
- Raza, Z.A.; Khalil, S.; Ayub, A.; Banat, I.M. Recent Developments in Chitosan Encapsulation of Various Active Ingredients for Multifunctional Applications. Carbohydr. Res. 2020, 492, 108004. [Google Scholar] [CrossRef] [PubMed]
- Ambaye, T.G.; Vaccari, M.; Prasad, S.; van Hullebusch, E.D.; Rtimi, S. Preparation and Applications of Chitosan and Cellulose Composite Materials. J. Environ. Manage. 2022, 301, 113850. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Han, Y.; Jian, L.; Liao, W.; Zhang, Y.; Gao, Y. Fabrication, Characterization, Physicochemical Stability of Zein-Chitosan Nanocomplex for Co-Encapsulating Curcumin and Resveratrol. Carbohydr. Polym. 2020, 236, 116090. [Google Scholar] [CrossRef] [PubMed]
- Bayer, J.; Granda, L.A.; Méndez, J.A.; Pèlach, M.A.; Vilaseca, F.; Mutjé, P. Cellulose Polymer Composites (WPC). In Advanced High Strength Natural Fibre Composites in Construction; Woodhead Publishing Series in Composites Science and Engineering; Woodhead Publishing, Ltd.: Sawston, UK, 2017; pp. 115–139. ISBN 978-0-08-100411-1. [Google Scholar]
- Keshk, S.M.A.S.; El-Kott, A.F. Natural Bacterial Biodegradable Medical Polymers: Bacterial Cellulose. In Science and Principles of Biodegradable and Bioresorbable Medical Polymers: Materials and Properties; Woodhead Publishing Series in Biomaterials; Woodhead Publishing, Ltd.: Sawston, UK, 2017; pp. 295–319. ISBN 978-0-08-100372-5. [Google Scholar]
- Moura, I.G.; Sá, A.V.; Abreu, A.S.L.; Machado, A.V.A. Bioplastics from Agro-Wastes for Food Packaging Applications. In Food Packaging; Nanotechnology in the Agri-Food Industry; Academic Press: Cambridge, MA, USA, 2017; pp. 223–263. ISBN 978-0-12-804302-8. [Google Scholar]
- Bagheriasl, D.; Carreau, P.J. Polymer-Cellulose Nanocrystal (CNC) Nanocomposites. In Processing of Polymer Nanocomposites; Hanser: Minhen, Germany, 2019; pp. 371–393. ISBN 978-1-56990-635-4. [Google Scholar]
- Avérous, L.; Halley, P.J. Starch Polymers: From the Field to Industrial Products. In Starch Polymers: From Genetic Engineering to Green Applications; Elsevier: Amsterdam, The Netherlands, 2014; pp. 3–10. ISBN 978-0-444-53730-0. [Google Scholar]
- Prasathkumar, M.; Sadhasivam, S. Chitosan/Hyaluronic Acid/Alginate and an Assorted Polymers Loaded with Honey, Plant, and Marine Compounds for Progressive Wound Healing—Know-How. Int. J. Biol. Macromol. 2021, 186, 656–685. [Google Scholar] [CrossRef]
- Park, J.; Nam, J.; Yun, H.; Jin, H.-J.; Kwak, H.W. Aquatic Polymer-Based Edible Films of Fish Gelatin Crosslinked with Alginate Dialdehyde Having Enhanced Physicochemical Properties. Carbohydr. Polym. 2021, 254, 117317. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Li, M.; Zhai, X.; Zhao, L.; Tahir, H.E.; Shi, J.; Zou, X.; Huang, X.; Li, Z.; Xiao, J. Development and Characterization of Sodium Alginate/Tea Tree Essential Oil Nanoemulsion Active Film Containing TiO2 Nanoparticles for Banana Packaging. Int. J. Biol. Macromol. 2022, 213, 145–154. [Google Scholar] [CrossRef]
- Flamminii, F.; Paciulli, M.; Di Michele, A.; Littardi, P.; Carini, E.; Chiavaro, E.; Pittia, P.; Di Mattia, C.D. Alginate-Based Microparticles Structured with Different Biopolymers and Enriched with a Phenolic-Rich Olive Leaves Extract: A Physico-Chemical Characterization. Curr. Res. Food Sci. 2021, 4, 698–706. [Google Scholar] [CrossRef]
- Thombare, N.; Kumar, S.; Kumari, U.; Sakare, P.; Yogi, R.K.; Prasad, N.; Sharma, K.K. Shellac as a Multifunctional Biopolymer: A Review on Properties, Applications and Future Potential. Int. J. Biol. Macromol. 2022, 215, 203–223. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, X.; Pan, Z.; Xue, Q.; Wu, Y.; Li, Y.; Li, B.; Li, L. Improving the Properties of Chitosan Films by Incorporating Shellac Nanoparticles. Food Hydrocoll. 2021, 110, 106164. [Google Scholar] [CrossRef]
- Ma, J.; Zhou, Z.; Li, K.; Li, K.; Liu, L.; Zhang, W.; Xu, J.; Tu, X.; Du, L.; Zhang, H. Novel Edible Coating Based on Shellac and Tannic Acid for Prolonging Postharvest Shelf Life and Improving Overall Quality of Mango. Food Chem. 2021, 354, 129510. [Google Scholar] [CrossRef]
- Soradech, S.; Nunthanid, J.; Limmatvapirat, S.; Luangtana-anan, M. Utilization of Shellac and Gelatin Composite Film for Coating to Extend the Shelf Life of Banana. Food Control 2017, 73, 1310–1317. [Google Scholar] [CrossRef]
- Yabe, T. New Understanding of Pectin as a Bioactive Dietary Fiber: A Review. J. Food Bioact. 2018, 3, 95–100. [Google Scholar] [CrossRef]
- Lin, L.; Xu, W.; Liang, H.; He, L.; Liu, S.; Li, Y.; Li, B.; Chen, Y. Construction of PH-Sensitive Lysozyme/Pectin Nanogel for Tumor Methotrexate Delivery. Colloids Surf. B Biointerfaces 2015, 126, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Noreen, A.; Nazli, Z.-H.; Akram, J.; Rasul, I.; Mansha, A.; Yaqoob, N.; Iqbal, R.; Tabasum, S.; Zuber, M.; Zia, K.M. Pectins Functionalized Biomaterials; a New Viable Approach for Biomedical Applications: A Review. Int. J. Biol. Macromol. 2017, 101, 254–272. [Google Scholar] [CrossRef]
- Neufeld, L.; Bianco-Peled, H. Pectin–Chitosan Physical Hydrogels as Potential Drug Delivery Vehicles. Int. J. Biol. Macromol. 2017, 101, 852–861. [Google Scholar] [CrossRef]
- Da, S.; Gulão, E.; de Souza, C.J.F.; Andrade, C.T.; Garcia-Rojas, E.E. Complex Coacervates Obtained from Peptide Leucine and Gum Arabic: Formation and Characterization. Food Chem. 2016, 194, 680–686. [Google Scholar] [CrossRef]
- Riseh, R.S.; Tamanadar, E.; Pour, M.M.; Thakur, V.K. Novel Approaches for Encapsulation of Plant Probiotic Bacteria with Sustainable Polymer Gums: Application in the Management of Pests and Diseases. Adv. Polym. Technol. 2022, 2022, 4419409. [Google Scholar] [CrossRef]
- Mirhosseini, H.; Tan, C.P.; Hamid, N.S.A.; Yusof, S. Effect of Arabic Gum, Xanthan Gum and Orange Oil Contents on ζ-Potential, Conductivity, Stability, Size Index and PH of Orange Beverage Emulsion. Colloids Surf. Physicochem. Eng. Asp. 2008, 315, 47–56. [Google Scholar] [CrossRef]
- Sworn, G. Xanthan Gum. In Handbook of Hydrocolloids; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead: Sawston, UK, 2021; pp. 833–853. ISBN 978-0-12-820104-6. [Google Scholar]
- Noor, I.S.M.; Majid, S.R.; Arof, A.K.; Djurado, D.; Claro Neto, S.; Pawlicka, A. Characteristics of Gellan Gum–LiCF3SO3 Polymer Electrolytes. Solid State Ion. 2012, 225, 649–653. [Google Scholar] [CrossRef]
- Lopes, B.D.; Lessa, V.L.; Silva, B.M.; Carvalho, M.A.D.; Schnitzler, E.; Lacerda, L.G. Xanthan Gum: Properties, Production Conditions, Quality and Economic Perspective. J. Food Nutr. Res. 2015, 54, 185–194. [Google Scholar]
- Kaewprapan, K.; Baros, F.; Marie, E.; Inprakhon, P.; Durand, A. Macromolecular Surfactants Synthesized by Lipase-Catalyzed Transesterification of Dextran with Vinyl Decanoate. Carbohydr. Polym. 2012, 88, 313–320. [Google Scholar] [CrossRef]
- Broaders, K.E.; Grandhe, S.; Fréchet, J.M.J. A Biocompatible Oxidation-Triggered Carrier Polymer with Potential in Therapeutics. J. Am. Chem. Soc. 2011, 133, 756–758. [Google Scholar] [CrossRef] [PubMed]
- Fathi, M.; Martín, Á.; McClements, D.J. Nanoencapsulation of Food Ingredients Using Carbohydrate Based Delivery Systems. Trends Food Sci. Technol. 2014, 39, 18–39. [Google Scholar] [CrossRef]
- Chen, H.; Wang, J.; Cheng, Y.; Wang, C.; Liu, H.; Bian, H.; Pan, Y.; Sun, J.; Han, W. Application of Protein-Based Films and Coatings for Food Packaging: A Review. Polymers 2019, 11, 2039. [Google Scholar] [CrossRef]
- Rashidinejad, A.; Tarhan, O.; Rezaei, A.; Capanoglu, E.; Boostani, S.; Khoshnoudi-Nia, S.; Samborska, K.; Garavand, F.; Shaddel, R.; Akbari-Alavijeh, S.; et al. Addition of Milk to Coffee Beverages; the Effect on Functional, Nutritional, and Sensorial Properties. Crit. Rev. Food Sci. Nutr. 2022, 62, 6132–6152. [Google Scholar] [CrossRef]
- Du, X.; Jing, H.; Wang, L.; Huang, X.; Mo, L.; Bai, X.; Wang, H. PH-Shifting Formation of Goat Milk Casein Nanoparticles from Insoluble Peptide Aggregates and Encapsulation of Curcumin for Enhanced Dispersibility and Bioactivity. LWT 2022, 154, 112753. [Google Scholar] [CrossRef]
- Garavand, F.; Jafarzadeh, S.; Cacciotti, I.; Vahedikia, N.; Sarlak, Z.; Tarhan, Ö.; Yousefi, S.; Rouhi, M.; Castro-Muñoz, R.; Jafari, S.M. Different Strategies to Reinforce the Milk Protein-Based Packaging Composites. Trends Food Sci. Technol. 2022, 123, 1–14. [Google Scholar] [CrossRef]
- Tang, C. Assembled Milk Protein Nano-Architectures as Potential Nanovehicles for Nutraceuticals. Adv. Colloid Interface Sci. 2021, 292, 102432. [Google Scholar] [CrossRef]
- Daniloski, D.; Petkoska, A.T.; Lee, N.A.; Bekhit, A.E.-D.; Carne, A.; Vaskoska, R.; Vasiljevic, T. Active Edible Packaging Based on Milk Proteins: A Route to Carry and Deliver Nutraceuticals. Trends Food Sci. Technol. 2021, 111, 688–705. [Google Scholar] [CrossRef]
- Nollet, M.; Laurichesse, E.; Besse, S.; Soubabère, O.; Schmitt, V. Determination of Formulation Conditions Allowing Double Emulsions Stabilized by PGPR and Sodium Caseinate to Be Used as Capsules. Langmuir 2018, 34, 2823–2833. [Google Scholar] [CrossRef]
- Sittipummongkol, K.; Chuysinuan, P.; Techasakul, S.; Pisitsak, P.; Pechyen, C. Core Shell Microcapsules of Neem Seed Oil Extract Containing Azadirachtin and Biodegradable Polymers and Their Release Characteristics. Polym. Bull. 2019, 76, 3803–3817. [Google Scholar] [CrossRef]
- Shishir, M.R.I.; Xie, L.; Sun, C.; Zheng, X.; Chen, W. Advances in Micro and Nano-Encapsulation of Bioactive Compounds Using Biopolymer and Lipid-Based Transporters. Trends Food Sci. Technol. 2018, 78, 34–60. [Google Scholar] [CrossRef]
- Daneshniya, M.; Nezhad, H.J.; Maleki, M.H.; Jalali, V.; Behrouzian, M. A Review of Encapsulation of Bioactive Peptides with Antimicrobial and Antioxidant Activity. Int. J. Acad. Eng. Res. 2020, 4, 7. [Google Scholar]
- Premi, M.; Sharma, H.K. Effect of Different Combinations of Maltodextrin, Gum Arabic and Whey Protein Concentrate on the Encapsulation Behavior and Oxidative Stability of Spray Dried Drumstick (Moringa oleifera) Oil. Int. J. Biol. Macromol. 2017, 105, 1232–1240. [Google Scholar] [CrossRef]
- Corrêa-Filho, L.; Moldão-Martins, M.; Alves, V. Advances in the Application of Microcapsules as Carriers of Functional Compounds for Food Products. Appl. Sci. 2019, 9, 571. [Google Scholar] [CrossRef]
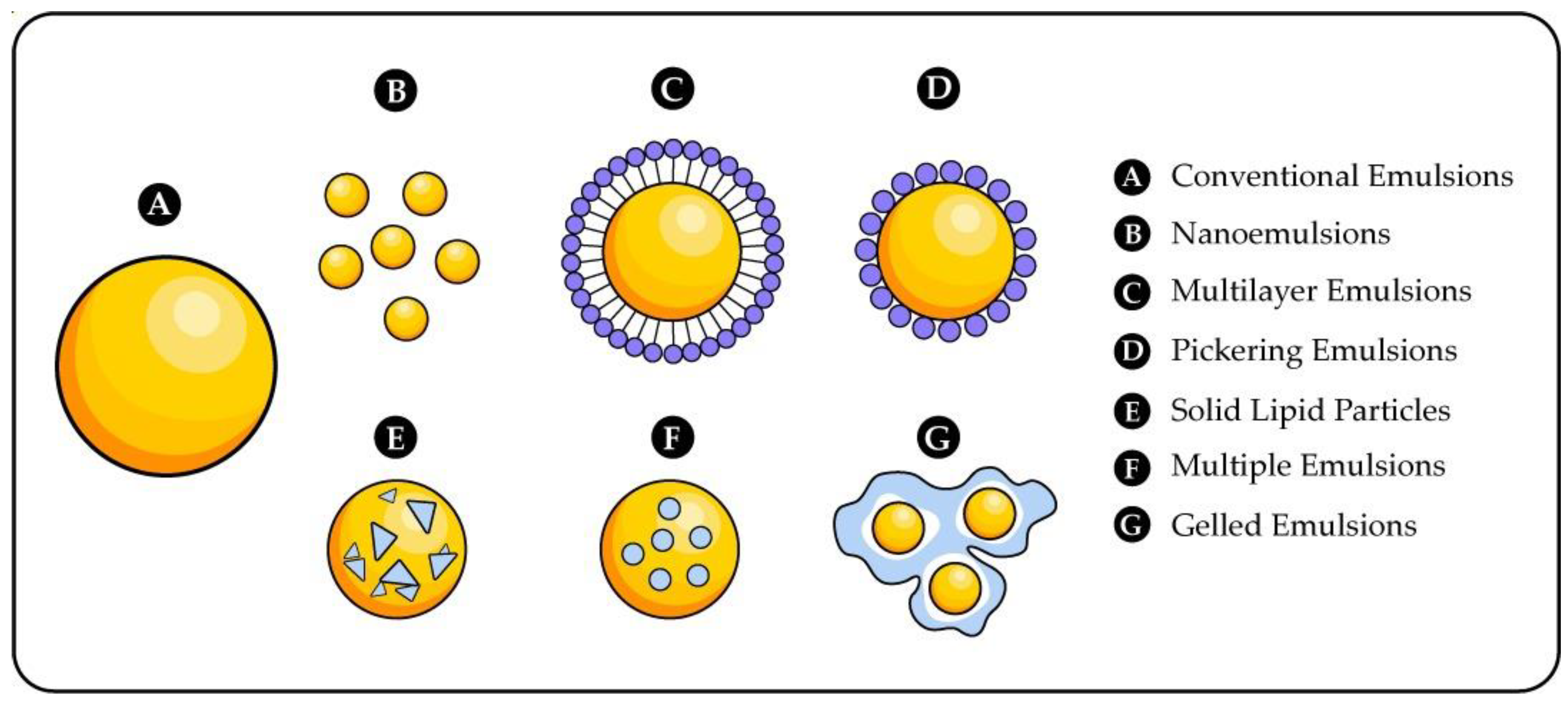
- Yang, Y.; Fang, Z.; Chen, X.; Zhang, W.; Xie, Y.; Chen, Y.; Liu, Z.; Yuan, W. An Overview of Pickering Emulsions: Solid-Particle Materials, Classification, Morphology, and Applications. Front. Pharmacol. 2017, 8, 287. [Google Scholar] [CrossRef]
- Li, K.-Y.; Zhou, Y.; Huang, G.-Q.; Li, X.-D.; Xiao, J.-X. Preparation of Powdered Oil by Spray Drying the Pickering Emulsion Stabilized by Ovalbumin—Gum Arabic Polyelectrolyte Complex. Food Chem. 2022, 391, 133223. [Google Scholar] [CrossRef]
- Klettenhammer, S.; Ferrentino, G.; Morozova, K.; Scampicchio, M. Novel Technologies Based on Supercritical Fluids for the Encapsulation of Food Grade Bioactive Compounds. Foods 2020, 9, 1395. [Google Scholar] [CrossRef]
- Olivera-Montenegro, L.; Best, I.; Gil-Saldarriaga, A. Effect of Pretreatment by Supercritical Fluids on Antioxidant Activity of Protein Hydrolyzate from Quinoa (Chenopodium quinoa Willd.). Food Sci. Nutr. 2021, 9, 574–582. [Google Scholar] [CrossRef]
- Akbarian, M.; Khani, A.; Eghbalpour, S.; Uversky, V.N. Bioactive Peptides: Synthesis, Sources, Applications, and Proposed Mechanisms of Action. Int. J. Mol. Sci. 2022, 23, 1445. [Google Scholar] [CrossRef]
- Olivera-Montenegro, L.; Bugarin, A.; Marzano, A.; Best, I.; Zabot, G.L.; Romero, H. Production of Protein Hydrolysate from Quinoa (Chenopodium quinoa Willd.): Economic and Experimental Evaluation of Two Pretreatments Using Supercritical Fluids’ Extraction and Conventional Solvent Extraction. Foods 2022, 11, 1015. [Google Scholar] [CrossRef] [PubMed]
- Akbarbaglu, Z.; Mahdi Jafari, S.; Sarabandi, K.; Mohammadi, M.; Khakbaz Heshmati, M.; Pezeshki, A. Influence of Spray Drying Encapsulation on the Retention of Antioxidant Properties and Microstructure of Flaxseed Protein Hydrolysates. Colloids Surf. B Biointerfaces 2019, 178, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Toalá, J.E.; Quintanar-Guerrero, D.; Liceaga, A.M.; Zambrano-Zaragoza, M.L. Encapsulation of Bioactive Peptides: A Strategy to Improve the Stability, Protect the Nutraceutical Bioactivity and Support Their Food Applications. RSC Adv. 2022, 12, 6449–6458. [Google Scholar] [CrossRef] [PubMed]
- Sarabandi, K.; Gharehbeglou, P.; Jafari, S.M. Spray-Drying Encapsulation of Protein Hydrolysates and Bioactive Peptides: Opportunities and Challenges. Dry. Technol. 2020, 38, 577–595. [Google Scholar] [CrossRef]
- Delshadi, R.; Bahrami, A.; Tafti, A.G.; Barba, F.J.; Williams, L.L. Micro and Nano-Encapsulation of Vegetable and Essential Oils to Develop Functional Food Products with Improved Nutritional Profiles. Trends Food Sci. Technol. 2020, 104, 72–83. [Google Scholar] [CrossRef]
- Angane, M.; Swift, S.; Huang, K.; Butts, C.A.; Quek, S.Y. Essential Oils and Their Major Components: An Updated Review on Antimicrobial Activities, Mechanism of Action and Their Potential Application in the Food Industry. Foods 2022, 11, 464. [Google Scholar] [CrossRef]
- Hammoud, Z.; Ben Abada, M.; Greige-Gerges, H.; Elaissari, A.; Mediouni Ben Jemâa, J. Insecticidal Effects of Natural Products in Free and Encapsulated Forms: An Overview. J. Nat. Pestic. Res. 2022, 1, 100007. [Google Scholar] [CrossRef]
- Ribeiro-Santos, R.; Andrade, M.; Sanches-Silva, A. Application of Encapsulated Essential Oils as Antimicrobial Agents in Food Packaging. Curr. Opin. Food Sci. 2017, 14, 78–84. [Google Scholar] [CrossRef]
- Niki, E.; Abe, K. Vitamin E: Structure, Properties and Functions. In Food Chemistry, Function and Analysis; Chapter 1; Niki, E., Ed.; Royal Society of Chemistry: Cambridge, UK, 2019; pp. 1–11. ISBN 978-1-78801-240-9. [Google Scholar]
- Samanta, S. Fat-Soluble Vitamins. In Nutrition and Functional Foods in Boosting Digestion, Metabolism and Immune Health; Elsevier: Amsterdam, The Netherlands, 2022; pp. 329–364. ISBN 978-0-12-821232-5. [Google Scholar]
- Ribeiro, A.M.; Estevinho, B.N.; Rocha, F. Improvement of Vitamin E Microencapsulation and Release Using Different Biopolymers as Encapsulating Agents. Food Bioprod. Process. 2021, 130, 23–33. [Google Scholar] [CrossRef]
- Carella, A.M.; Benvenuto, A.; Lagattolla, V.; Arbaiza, T.; De Luca, P.; Ciavarrella, G.; Modola, G.; Di Pumpo, M.; Ponziano, E.; Benvenuto, M. Vitamin Supplements in the Era of SARS-Cov2 Pandemic. GSC Biol. Pharm. Sci. 2020, 11, 7–19. [Google Scholar] [CrossRef]
- Trela, A.; Szymańska, R. Less Widespread Plant Oils as a Good Source of Vitamin E. Food Chem. 2019, 296, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathi, S.; Anandharamakrishnan, C. Enhancement of Oral Bioavailability of Vitamin E by Spray-Freeze Drying of Whey Protein Microcapsules. Food Bioprod. Process. 2016, 100, 469–476. [Google Scholar] [CrossRef]
- Kaur, K.; Singh, J.; Singh, V. Effect of Encapsulated Vitamin E on Physical, Storage and Retention Parameters in Cookies. J. Food Sci. Technol. 2020, 57, 3509–3517. [Google Scholar] [CrossRef] [PubMed]
- Lazim, N.A.M.; Muhamad, I.I. Encapsulation of Vitamin e Using Maltodextrin/Sodium Caseinate/Selenomethionine and Its Release Study. Chem. Eng. Trans. 2017, 56, 1951–1956. [Google Scholar] [CrossRef]
- Galli, F.; Azzi, A.; Birringer, M.; Cook-Mills, J.M.; Eggersdorfer, M.; Frank, J.; Cruciani, G.; Lorkowski, S.; Özer, N.K. Vitamin E: Emerging Aspects and New Directions. Free Radic. Biol. Med. 2017, 102, 16–36. [Google Scholar] [CrossRef]
- Rajanna, D.; Pushpadass, H.A.; Emerald, F.M.E.; Padaki, N.V.; Nath, B.S. Nanoencapsulation of casein-derived Peptides within Electrospun Nanofibres. J. Sci. Food Agric. 2022, 102, 1684–1698. [Google Scholar] [CrossRef]
- Chotphruethipong, L.; Battino, M.; Benjakul, S. Effect of Stabilizing Agents on Characteristics, Antioxidant Activities and Stability of Liposome Loaded with Hydrolyzed Collagen from Defatted Asian Sea Bass Skin. Food Chem. 2020, 328, 127127. [Google Scholar] [CrossRef]
- Hassan, M.A.; Xavier, M.; Gupta, S.; Nayak, B.B.; Balange, A.K. Antioxidant Properties and Instrumental Quality Characteristics of Spray Dried Pangasius Visceral Protein Hydrolysate Prepared by Chemical and Enzymatic Methods. Environ. Sci. Pollut. Res. 2019, 26, 8875–8884. [Google Scholar] [CrossRef]
- Alvarado, Y.; Muro, C.; Illescas, J.; Díaz, M.d.C.; Riera, F. Encapsulation of Antihypertensive Peptides from Whey Proteins and Their Releasing in Gastrointestinal Conditions. Biomolecules 2019, 9, 164. [Google Scholar] [CrossRef]
- Sarabandi, K.; Sadeghi Mahoonak, A.; Hamishekar, H.; Ghorbani, M.; Jafari, S.M. Microencapsulation of Casein Hydrolysates: Physicochemical, Antioxidant and Microstructure Properties. J. Food Eng. 2018, 237, 86–95. [Google Scholar] [CrossRef]
- Murthy, L.N.; Phadke, G.G.; Mohan, C.O.; Chandra, M.V.; Annamalai, J.; Visnuvinayagam, S.; Unnikrishnan, P.; Ravishankar, C.N. Characterization of Spray-Dried Hydrolyzed Proteins from Pink Perch Meat Added with Maltodextrin and Gum Arabic. J. Aquat. Food Prod. Technol. 2017, 26, 913–928. [Google Scholar] [CrossRef]
- Giroux, H.J.; Robitaille, G.; Britten, M. Controlled Release of Casein-Derived Peptides in the Gastrointestinal Environment by Encapsulation in Water-in-Oil-in-Water Double Emulsions. LWT-Food Sci. Technol. 2016, 69, 225–232. [Google Scholar] [CrossRef]
- Rao, P.S.; Bajaj, R.K.; Mann, B.; Arora, S.; Tomar, S.K. Encapsulation of Antioxidant Peptide Enriched Casein Hydrolysate Using Maltodextrin–Gum Arabic Blend. J. Food Sci. Technol. 2016, 53, 3834–3843. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Sun, H.; Li, H.; Li, Z.; Zheng, S.; Luo, D.; Ning, Y.; Wang, Y.; Shah, B.R. Preparation and Characterization of Tea Oil Powder with High Water Solubility Using Pickering Emulsion Template and Vacuum Freeze-Drying. LWT 2022, 160, 113330. [Google Scholar] [CrossRef]
- Kouamé, K.J.E.-P.; Bora, A.F.M.; Li, X.; Sun, Y.; Liu, L. Novel Trends and Opportunities for Microencapsulation of Flaxseed Oil in Foods: A Review. J. Funct. Foods 2021, 87, 104812. [Google Scholar] [CrossRef]
- Yakdhane, A.; Labidi, S.; Chaabane, D.; Tolnay, A.; Nath, A.; Koris, A.; Vatai, G. Microencapsulation of Flaxseed Oil—State of Art. Processes 2021, 9, 295. [Google Scholar] [CrossRef]
- Gañan, N.; Bordón, M.G.; Ribotta, P.D.; González, A. Study of Chia Oil Microencapsulation in Soy Protein Microparticles Using Supercritical CO2-Assisted Impregnation. J. CO2 Util. 2020, 40, 101221. [Google Scholar] [CrossRef]
- Murugkar, D.A.; Zanwar, A.A.; Shrivastava, A. Effect of Nano-Encapsulation of Flaxseed Oil on the Stability, Characterization and Incorporation on the Quality of Eggless Cake. Appl. Food Res. 2021, 1, 100025. [Google Scholar] [CrossRef]
- Lin, D.; Xiao, L.; Li, S.; Qin, W.; Loy, D.A.; Chen, H.; Zhang, Q. Effects of Fructooligosaccharide and Soybean Protein Isolate in the Microencapsulation of Walnut Oil. Ind. Crops Prod. 2022, 177, 114431. [Google Scholar] [CrossRef]
- Yoshikiyo, K.; Yoshioka, Y.; Narumiya, Y.; Oe, S.; Kawahara, H.; Kurata, K.; Shimizu, H.; Yamamoto, T. Thermal Stability and Bioavailability of Inclusion Complexes of Perilla Oil with γ-Cyclodextrin. Food Chem. 2019, 294, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Fasihi, H.; Noshirvani, N.; Hashemi, M.; Fazilati, M.; Salavati, H.; Coma, V. Antioxidant and Antimicrobial Properties of Carbohydrate-Based Films Enriched with Cinnamon Essential Oil by Pickering Emulsion Method. Food Packag. Shelf Life 2019, 19, 147–154. [Google Scholar] [CrossRef]
- Purkait, A.; Mukherjee, A.; Hazra, D.K.; Roy, K.; Biswas, P.K.; Kole, R.K. Encapsulation, Release and Insecticidal Activity of Pongamia pinnata (L.) Seed Oil. Heliyon 2021, 7, e06557. [Google Scholar] [CrossRef] [PubMed]
- Bajac, J.; Nikolovski, B.; Lončarević, I.; Petrović, J.; Bajac, B.; Đurović, S.; Petrović, L. Microencapsulation of Juniper Berry Essential Oil (Juniperus communis L.) by Spray Drying: Microcapsule Characterization and Release Kinetics of the Oil. Food Hydrocoll. 2022, 125, 107430. [Google Scholar] [CrossRef]
- Hadidi, M.; Pouramin, S.; Adinepour, F.; Haghani, S.; Jafari, S.M. Chitosan Nanoparticles Loaded with Clove Essential Oil: Characterization, Antioxidant and Antibacterial Activities. Carbohydr. Polym. 2020, 236, 116075. [Google Scholar] [CrossRef]
- Selamat, S.N.; Mohamad, S.N.H.; Muhamad, I.I.; Khairuddin, N.; Md Lazim, N.A. Characterization of Spray-Dried Palm Oil Vitamin E Concentrate. Arab. J. Sci. Eng. 2018, 43, 6165–6169. [Google Scholar] [CrossRef]
- Tarigan, J.B.; Kaban, J.; Zulmi, R. Microencapsulation of Vitamin e from Palm Fatty Acid Distillate with Galactomannan and Gum Acacia Using Spray Drying Method. IOP Conf. Ser. Mater. Sci. Eng. 2018, 309, 12095. [Google Scholar] [CrossRef]
- Prieto, C.; Calvo, L. Supercritical Fluid Extraction of Emulsions to Nanoencapsulate Vitamin E in Polycaprolactone. J. Supercrit. Fluids 2017, 119, 274–282. [Google Scholar] [CrossRef]
- Mirzaei-Mohkam, A.; Garavand, F.; Dehnad, D.; Keramat, J.; Nasirpour, A. Optimisation, Antioxidant Attributes, Stability and Release Behaviour of Carboxymethyl Cellulose Films Incorporated with Nanoencapsulated Vitamin E. Prog. Org. Coat. 2019, 134, 333–341. [Google Scholar] [CrossRef]
- Jiang, M.; Hong, Y.; Gu, Z.; Cheng, L.; Li, Z.; Li, C. Preparation of a Starch-Based Carrier for Oral Delivery of Vitamin E to the Small Intestine. Food Hydrocoll. 2019, 91, 26–33. [Google Scholar] [CrossRef]
- Ribeiro, A.; Gonçalves, R.F.S.; Pinheiro, A.C.; Manrique, Y.A.; Barreiro, M.F.; Lopes, J.C.B.; Dias, M.M. In Vitro Digestion and Bioaccessibility Studies of Vitamin E-Loaded Nanohydroxyapatite Pickering Emulsions and Derived Fortified Foods. LWT 2022, 154, 112706. [Google Scholar] [CrossRef]
- Zhang, Z.; Qiu, C.; Li, X.; McClements, D.J.; Jiao, A.; Wang, J.; Jin, Z. Advances in Research on Interactions between Polyphenols and Biology-Based Nano-Delivery Systems and Their Applications in Improving the Bioavailability of Polyphenols. Trends Food Sci. Technol. 2021, 116, 492–500. [Google Scholar] [CrossRef]
- Costa, M.; Sezgin-Bayindir, Z.; Losada-Barreiro, S.; Paiva-Martins, F.; Saso, L.; Bravo-Díaz, C. Polyphenols as Antioxidants for Extending Food Shelf-Life and in the Prevention of Health Diseases: Encapsulation and Interfacial Phenomena. Biomedicines 2021, 9, 1909. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.; Sang, S.; McClements, D.J.; Chen, L.; Long, J.; Jiao, A.; Jin, Z.; Qiu, C. Polyphenols as Plant-Based Nutraceuticals: Health Effects, Encapsulation, Nano-Delivery, and Application. Foods 2022, 11, 2189. [Google Scholar] [CrossRef] [PubMed]
- Akbarbaglu, Z.; Peighambardoust, S.H.; Sarabandi, K.; Jafari, S.M. Spray Drying Encapsulation of Bioactive Compounds within Protein-Based Carriers; Different Options and Applications. Food Chem. 2021, 359, 129965. [Google Scholar] [CrossRef]
- Guan, T.; Zhang, Z.; Li, X.; Cui, S.; McClements, D.J.; Wu, X.; Chen, L.; Long, J.; Jiao, A.; Qiu, C.; et al. Preparation, Characteristics, and Advantages of Plant Protein-Based Bioactive Molecule Delivery Systems. Foods 2022, 11, 1562. [Google Scholar] [CrossRef] [PubMed]
- Milani, A.; Basirnejad, M.; Shahbazi, S.; Bolhassani, A. Carotenoids: Biochemistry, Pharmacology and Treatment. Br. J. Pharmacol. 2017, 174, 1290–1324. [Google Scholar] [CrossRef]
- Janiszewska-Turak, E. Carotenoids Microencapsulation by Spray Drying Method and Supercritical Micronization. Food Res. Int. 2017, 99, 891–901. [Google Scholar] [CrossRef]
- Gul, K.; Tak, A.; Singh, A.K.; Singh, P.; Yousuf, B.; Wani, A.A. Chemistry, Encapsulation, and Health Benefits of β-Carotene—A Review. Cogent Food Agric. 2015, 1, 1018696. [Google Scholar] [CrossRef]
- Eun, J.-B.; Maruf, A.; Das, P.R.; Nam, S.-H. A Review of Encapsulation of Carotenoids Using Spray Drying and Freeze Drying. Crit. Rev. Food Sci. Nutr. 2020, 60, 3547–3572. [Google Scholar] [CrossRef]
- Chen, B.-H.; Stephen Inbaraj, B. Nanoemulsion and Nanoliposome Based Strategies for Improving Anthocyanin Stability and Bioavailability. Nutrients 2019, 11, 1052. [Google Scholar] [CrossRef]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef] [PubMed]
- Nayak, B.; Dahmoune, F.; Moussi, K.; Remini, H.; Dairi, S.; Aoun, O.; Khodir, M. Comparison of Microwave, Ultrasound and Accelerated-Assisted Solvent Extraction for Recovery of Polyphenols from Citrus Sinensis Peels. Food Chem. 2015, 187, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Sadowska-Bartosz, I.; Bartosz, G. Biological Properties and Applications of Betalains. Molecules 2021, 26, 2520. [Google Scholar] [CrossRef] [PubMed]
- Madadi, E.; Mazloum-Ravasan, S.; Yu, J.S.; Ha, J.W.; Hamishehkar, H.; Kim, K.H. Therapeutic Application of Betalains: A Review. Plants 2020, 9, 1219. [Google Scholar] [CrossRef] [PubMed]
- Castro-Enríquez, D.D.; Montaño-Leyva, B.; Del Toro-Sánchez, C.L.; Juaréz-Onofre, J.E.; Carvajal-Millan, E.; Burruel-Ibarra, S.E.; Tapia-Hernández, J.A.; Barreras-Urbina, C.G.; Rodríguez-Félix, F. Stabilization of Betalains by Encapsulation—A Review. J. Food Sci. Technol. 2020, 57, 1587–1600. [Google Scholar] [CrossRef]
- Gandía-Herrero, F.; García-Carmona, F. Biosynthesis of Betalains: Yellow and Violet Plant Pigments. Trends Plant Sci. 2013, 18, 334–343. [Google Scholar] [CrossRef]
- Ravichandran, K.; Palaniraj, R.; Saw, N.M.M.T.; Gabr, A.M.M.; Ahmed, A.R.; Knorr, D.; Smetanska, I. Effects of Different Encapsulation Agents and Drying Process on Stability of Betalains Extract. J. Food Sci. Technol. 2014, 51, 2216–2221. [Google Scholar] [CrossRef]
- Piacentini, E. Encapsulation Efficiency. In Encyclopedia of Membranes; Drioli, E., Giorno, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 706–707. ISBN 978-3-662-44323-1. [Google Scholar]
- Mehran, M.; Masoum, S.; Memarzadeh, M. Improvement of Thermal Stability and Antioxidant Activity of Anthocyanins of Echium Amoenum Petal Using Maltodextrin/Modified Starch Combination as Wall Material. Int. J. Biol. Macromol. 2020, 148, 768–776. [Google Scholar] [CrossRef]
- Alvarenga, D.; Victória de Barros, R.; Vilela, S. Chapter 12: Microencapsulation of Essential Oils Using Spray Drying Technology. In Microencapsulation and Microspheres for Food Applications; Sagis, L.M.C., Ed.; Academic Press: Cambridge, MA, USA, 2015; p. 245. ISBN 978-0-12-800350-3. [Google Scholar]
- Potdar, S.B.; Landge, V.K.; Barkade, S.S.; Potoroko, I.; Sonawane, S.H. Flavor Encapsulation and Release Studies in Food. In Encapsulation of Active Molecules and Their Delivery System; Elsevier: Waltham, MA, USA, 2020; p. 296. ISBN 978-0-12-819363-1. [Google Scholar]
- Akhavan Mahdavi, S.; Jafari, S.M.; Assadpour, E.; Ghorbani, M. Storage Stability of Encapsulated Barberry’s Anthocyanin and Its Application in Jelly Formulation. J. Food Eng. 2016, 181, 59–66. [Google Scholar] [CrossRef]
- Samborska, K.; Jedlińska, A. Spray Drying Encapsulation of Anthocyanins. In Spray Drying Encapsulation of Bioactive Materials; Advances in Drying Sciences and Technology; CRC Press: Boca Raton, FL, USA, 2021; p. 107. ISBN 978-0-367-36646-9. [Google Scholar]
- Flores, F.P.; Kong, F. In Vitro Release Kinetics of Microencapsulated Materials and the Effect of the Food Matrix. Annu. Rev. Food Sci. Technol. 2017, 8, 237–259. [Google Scholar] [CrossRef]
- Vinceković, M.; Jurić, S.; Đermić, E.; Topolovec-Pintarić, S. Kinetics and Mechanisms of Chemical and Biological Agents Release from Biopolymeric Microcapsules. J. Agric. Food Chem. 2017, 65, 9608–9617. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, J.; Siepmann, F. Mathematical Modeling of Drug Delivery. Int. J. Pharm. 2008, 364, 328–343. [Google Scholar] [CrossRef] [PubMed]
- Rawat, A.; Burgess, D.J. USP Apparatus 4 Method for in Vitro Release Testing of Protein Loaded Microspheres. Int. J. Pharm. 2011, 409, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Dolinina, E.S.; Akimsheva, E.Y.; Parfenyuk, E.V. Silica Microcapsules as Containers for Protein Drugs: Direct and Indirect Encapsulation. J. Mol. Liq. 2019, 287, 110938. [Google Scholar] [CrossRef]
- Sonawane, S.H.; Bhanvase, B.A.; Sivakumar, M. (Eds.) Encapsulation of Active Molecules and Their Delivery System, 1st ed.; Elsevier: Waltham, MA, USA, 2020; ISBN 978-0-12-819363-1. [Google Scholar]
- Pradhane, A.; Barai, D.; Bhanvase, B.; Sonawane, S. Mathematical Modeling and Simulation of the Release of Active Agents from Nanocontainers/Microspheres. In Encapsulation of Active Molecules and Their Delivery System; Elsevier: Waltham, MA, USA, 2020; pp. 257–291. ISBN 978-0-12-819363-1. [Google Scholar]
- Mehta, N.; Kumar, P.; Verma, A.K.; Umaraw, P.; Kumar, Y.; Malav, O.P.; Sazili, A.Q.; Domínguez, R.; Lorenzo, J.M. Microencapsulation as a Noble Technique for the Application of Bioactive Compounds in the Food Industry: A Comprehensive Review. Appl. Sci. 2022, 12, 1424. [Google Scholar] [CrossRef]
- Yun, P.; Devahastin, S.; Chiewchan, N. Microstructures of Encapsulates and Their Relations with Encapsulation Efficiency and Controlled Release of Bioactive Constituents: A Review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1768–1799. [Google Scholar] [CrossRef]
- Mahdavi, S.A.; Jafari, S.M.; Ghorbani, M.; Assadpoor, E. Spray-Drying Microencapsulation of Anthocyanins by Natural Biopolymers: A Review. Dry. Technol. 2014, 32, 509–518. [Google Scholar] [CrossRef]
- Santos, D.T.; Albarelli, J.Q.; Beppu, M.M.; Meireles, M.A.A. Stabilization of Anthocyanin Extract from Jabuticaba Skins by Encapsulation Using Supercritical CO2 as Solvent. Food Res. Int. 2013, 50, 617–624. [Google Scholar] [CrossRef]
- De Freitas Santos, P.D.; Rubio, F.T.V.; Da Silva, M.P.; Pinho, L.S.; Favaro-Trindade, C.S. Microencapsulation of Carotenoid-Rich Materials: A Review. Food Res. Int. 2021, 147, 110571. [Google Scholar] [CrossRef]
- Tabernero, A.; Martín del Valle, E.M.; Galán, M.A. Supercritical Fluids for Pharmaceutical Particle Engineering: Methods, Basic Fundamentals and Modelling. Chem. Eng. Process. Process Intensif. 2012, 60, 9–25. [Google Scholar] [CrossRef]
- Gheonea, I.; Aprodu, I.; Cîrciumaru, A.; Râpeanu, G.; Bahrim, G.E.; Stănciuc, N. Microencapsulation of Lycopene from Tomatoes Peels by Complex Coacervation and Freeze-Drying: Evidences on Phytochemical Profile, Stability and Food Applications. J. Food Eng. 2021, 288, 110166. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Adhikari, R.; Barrow, C.J.; Adhikari, B. Physicochemical and Functional Properties of Protein Isolate Produced from Australian Chia Seeds. Food Chem. 2016, 212, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Marques da Silva, T.; Jacob Lopes, E.; Codevilla, C.F.; Cichoski, A.J.; de Moraes Flores, É.M.; Motta, M.H.; de Bonada Silva, C.; Grosso, C.R.F.; de Menezes, C.R. Development and Characterization of Microcapsules Containing Bifidobacterium Bb-12 Produced by Complex Coacervation Followed by Freeze Drying. LWT 2018, 90, 412–417. [Google Scholar] [CrossRef]
- Sabir, M.; Naseem, Z.; Ahmad, W.; Usman, M.; Nadeem, F.; Saifullah; Ahmad, H.R. Alleviation of Adverse Effects of Nickel on Growth and Concentration of Copper and Manganese in Wheat through Foliar Application of Ascorbic Acid. Int. J. Phytoremediation 2022, 24, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Yaseen, S.; Amjad, S.F.; Mansoora, N.; Kausar, S.; Shahid, H.; Alamri, S.A.M.; Alrumman, S.A.; Eid, E.M.; Ansari, M.J.; Danish, S.; et al. Supplemental Effects of Biochar and Foliar Application of Ascorbic Acid on Physio-Biochemical Attributes of Barley (Hordeum vulgare L.) under Cadmium-Contaminated Soil. Sustainability 2021, 13, 9128. [Google Scholar] [CrossRef]
- Khazaei, Z.; Estaji, A. Effect of Foliar Application of Ascorbic Acid on Sweet Pepper (Capsicum annuum) Plants under Drought Stress. Acta Physiol. Plant. 2020, 42, 118. [Google Scholar] [CrossRef]
- Ghahremani, Z.; Mikaealzadeh, M.; Barzegar, T.; Ranjbar, M.E. Foliar Application of Ascorbic Acid and Gamma Aminobutyric Acid Can Improve Important Properties of Deficit Irrigated Cucumber Plants (Cucumis sativus Cv. Us). Gesunde Pflanz. 2021, 73, 77–84. [Google Scholar] [CrossRef]
- Allahveran, A.; Farokhzad, A.; Asghari, M.; Sarkhosh, A. Foliar Application of Ascorbic and Citric Acids Enhanced ‘Red Spur’ Apple Fruit Quality, Bioactive Compounds and Antioxidant Activity. Physiol. Mol. Biol. Plants 2018, 24, 433–440. [Google Scholar] [CrossRef]
- Hassan, A.; Amjad, S.F.; Saleem, M.H.; Yasmin, H.; Imran, M.; Riaz, M.; Ali, Q.; Joyia, F.A.; Mobeen; Ahmed, S.; et al. Foliar Application of Ascorbic Acid Enhances Salinity Stress Tolerance in Barley (Hordeum vulgare L.) through Modulation of Morpho-Physio-Biochemical Attributes, Ions Uptake, Osmo-Protectants and Stress Response Genes Expression. Saudi J. Biol. Sci. 2021, 28, 4276–4290. [Google Scholar] [CrossRef]
- Dos Santos Carvalho, J.D.; Oriani, V.B.; de Oliveira, G.M.; Hubinger, M.D. Characterization of Ascorbic Acid Microencapsulated by the Spray Chilling Technique Using Palm Oil and Fully Hydrogenated Palm Oil. LWT 2019, 101, 306–314. [Google Scholar] [CrossRef]
- Dos Santos, J.D.; Oriani, V.B.; Oliveira, G.M.; Hubinger, M.D. Solid Lipid Microparticles Loaded with Ascorbic Acid: Release Kinetic Profile during Thermal Stability. J. Food Process. Preserv. 2021, 45, e15557. [Google Scholar] [CrossRef]
- Jahanian, H.; Kahkeshani, N.; Sanei-Dehkordi, A.; Isman, M.B.; Saeedi, M.; Khanavi, M. Rosmarinus Officinalis as a Natural Insecticide: A Review. Int. J. Pest Manag. 2022. [Google Scholar] [CrossRef]
- Shawer, R.; El-Shazly, M.M.; Khider, A.M.; Baeshen, R.S.; Hikal, W.M.; Kordy, A.M. Botanical Oils Isolated from Simmondsia Chinensis and Rosmarinus Officinalis Cultivated in Northern Egypt: Chemical Composition and Insecticidal Activity against Sitophilus oryzae (L.) and Tribolium castaneum (Herbst). Molecules 2022, 27, 4383. [Google Scholar] [CrossRef]
- Trombin de Souza, M.; Trombin de Souza, M.; Bernardi, D.; da Oliveira, D.C.; Morais, M.C.; de Melo, D.J.; Richardi, V.S.; Zarbin, P.H.G.; Zawadneak, M.A.C. Essential Oil of Rosmarinus officinalis Ecotypes and Their Major Compounds: Insecticidal and Histological Assessment Against Drosophila suzukii and Their Impact on a Nontarget Parasitoid. J. Econ. Entomol. 2022, 115, 955–966. [Google Scholar] [CrossRef]
- Souza, C.R.F.; Baldim, I.; Bankole, V.O.; da Ana, R.; Durazzo, A.; Lucarini, M.; Cicero, N.; Santini, A.; Souto, E.B.; Oliveira, W.P. Spouted Bed Dried Rosmarinus Officinalis Extract: A Novel Approach for Physicochemical Properties and Antioxidant Activity. Agriculture 2020, 10, 349. [Google Scholar] [CrossRef]
- Benelli, L.; Oliveira, W.P. Fluidized Bed Coating of Inert Cores with a Lipid-Based System Loaded with a Polyphenol-Rich Rosmarinus Officinalis Extract. Food Bioprod. Process. 2019, 114, 216–226. [Google Scholar] [CrossRef]
- Dono, D.; Widayani, N.S.; Ishmayana, S.; Hidayat, Y.; Widiantini, F.; Nasahi, C. Resistance of Nilaparvata Lugens to Fenobucarb and Imidacloprid and Susceptibility to Neem Oil Insecticides. HAYATI J. Biosci. 2022, 29, 234–244. [Google Scholar] [CrossRef]
- Pascoli, M.; de Albuquerque, F.P.; Calzavara, A.K.; Tinoco-Nunes, B.; Oliveira, W.H.C.; Gonçalves, K.C.; Polanczyk, R.A.; Vechia, J.F.D.; de Matos, S.T.S.; de Andrade, D.J.; et al. The Potential of Nanobiopesticide Based on Zein Nanoparticles and Neem Oil for Enhanced Control of Agricultural Pests. J. Pest Sci. 2020, 93, 793–806. [Google Scholar] [CrossRef]
- Bagle, A.V.; Jadhav, R.S.; Gite, V.V.; Hundiwale, D.G.; Mahulikar, P.P. Controlled Release Study of Phenol Formaldehyde Microcapsules Containing Neem Oil as an Insecticide. Int. J. Polym. Mater. 2013, 62, 421–425. [Google Scholar] [CrossRef]
- Pascoli, M.; Jacques, M.T.; Agarrayua, D.A.; Avila, D.S.; Lima, R.; Fraceto, L.F. Neem Oil Based Nanopesticide as an Environmentally-Friendly Formulation for Applications in Sustainable Agriculture: An Ecotoxicological Perspective. Sci. Total Environ. 2019, 677, 57–67. [Google Scholar] [CrossRef]
- Luiz De Oliveira, J.; Ramos Campos, E.V.; Fraceto, L.F. Recent Developments and Challenges for Nanoscale Formulation of Botanical Pesticides for Use in Sustainable Agriculture. J. Agric. Food Chem. 2018, 66, 8898–8913. [Google Scholar] [CrossRef] [PubMed]
- De Souza, M.T.; Porsani, M.V.; Bach, R.P.; De Souza, M.T. Encapsulamento de Moléculas Como Oportunidade Emergente Na Agricultura. Pesqui. Agropecuária Pernambucana 2021, 26, 1–5. [Google Scholar]
- Maruyama, C.R.; Bilesky-José, N.; de Lima, R.; Fraceto, L.F. Encapsulation of Trichoderma Harzianum Preserves Enzymatic Activity and Enhances the Potential for Biological Control. Front. Bioeng. Biotechnol. 2020, 8, 225. [Google Scholar] [CrossRef] [PubMed]
- Peil, S.; Beckers, S.J.; Fischer, J.; Wurm, F.R. Biodegradable, Lignin-Based Encapsulation Enables Delivery of Trichoderma Reesei with Programmed Enzymatic Release against Grapevine Trunk Diseases. Mater. Today Bio 2020, 7, 100061. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, G.O.; dos Santos, G.F.; Botelho, P.S.; Finkler, C.L.L.; Bueno, L.A. Development of Trichoderma Sp. Formulations in Encapsulated Granules (CG) and Evaluation of Conidia Shelf-Life. Biol. Control 2018, 117, 21–29. [Google Scholar] [CrossRef]
- Yaakov, N.; Ananth Mani, K.; Felfbaum, R.; Lahat, M.; Da Costa, N.; Belausov, E.; Ment, D.; Mechrez, G. Single Cell Encapsulation via Pickering Emulsion for Biopesticide Applications. ACS Omega 2018, 3, 14294–14301. [Google Scholar] [CrossRef]
- Yaakov, N.; Kottakota, C.; Mani, K.A.; Naftali, S.M.; Zelinger, E.; Davidovitz, M.; Ment, D.; Mechrez, G. Encapsulation of Bacillus Thuringiensis in an Inverse Pickering Emulsion for Pest Control Applications. Colloids Surf. B Biointerfaces 2022, 213, 112427. [Google Scholar] [CrossRef]
- Pour, M.M.; Saberi-Riseh, R.; Mohammadinejad, R.; Hosseini, A. Investigating the Formulation of Alginate- Gelatin Encapsulated Pseudomonas Fluorescens (VUPF5 and T17-4 Strains) for Controlling Fusarium Solani on Potato. Int. J. Biol. Macromol. 2019, 133, 603–613. [Google Scholar] [CrossRef]
- Amar Feldbaum, R.; Yaakov, N.; Ananth Mani, K.; Yossef, E.; Metbeev, S.; Zelinger, E.; Belausov, E.; Koltai, H.; Ment, D.; Mechrez, G. Single Cell Encapsulation in a Pickering Emulsion Stabilized by TiO2 Nanoparticles Provides Protection against UV Radiation for a Biopesticide. Colloids Surf. B Biointerfaces 2021, 206, 111958. [Google Scholar] [CrossRef]
- De Araújo, J.S.F.; de Souza, E.L.; Oliveira, J.R.; Gomes, A.C.A.; Kotzebue, L.R.V.; da Silva Agostini, D.L.; de Oliveira, D.L.V.; Mazzetto, S.E.; da Silva, A.L.; Cavalcanti, M.T. Microencapsulation of Sweet Orange Essential Oil (Citrus aurantium Var. Dulcis) by Liophylization Using Maltodextrin and Maltodextrin/Gelatin Mixtures: Preparation, Characterization, Antimicrobial and Antioxidant Activities. Int. J. Biol. Macromol. 2020, 143, 991–999. [Google Scholar] [CrossRef]
- Nyari, N.; Paulazzi, A.; Zamadei, R.; Steffens, C.; Zabot, G.L.; Tres, M.V.; Zeni, J.; Venquiaruto, L.; Dallago, R.M. Synthesis of Isoamyl Acetate by Ultrasonic System Using Candida antarctica Lipase B Immobilized in Polyurethane. J. Food Process Eng. 2018, 41, e12812. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Haque, M.A.; Adhikari, B. Encapsulation in the Food Industry: A Brief Historical Overview to Recent Developments. Food Nutr. Sci. 2020, 11, 481–508. [Google Scholar] [CrossRef]
- Pavoni, L.; Benelli, G.; Maggi, F.; Bonacucina, G. Green Nanoemulsion Interventions for Biopesticide Formulations; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780128158296. [Google Scholar]
- Maes, C.; Bouquillon, S.; Fauconnier, M.L. Encapsulation of Essential Oils for the Development of Biosourced Pesticides with Controlled Release: A Review. Molecules 2019, 24, 2539. [Google Scholar] [CrossRef] [PubMed]
- Taban, A.; Saharkhiz, M.J.; Naderi, R. A Natural Post-Emergence Herbicide Based on Essential Oil Encapsulation by Cross-Linked Biopolymers: Characterization and Herbicidal Activity. Environ. Sci. Pollut. Res. 2020, 27, 45844–45858. [Google Scholar] [CrossRef]
- Karaaslan, M.; Şengün, F.; Cansu, Ü.; Başyiğit, B.; Sağlam, H.; Karaaslan, A. Gum Arabic/Maltodextrin Microencapsulation Confers Peroxidation Stability and Antimicrobial Ability to Pepper Seed Oil. Food Chem. 2021, 337. [Google Scholar] [CrossRef]
- Arango-Ruiz, Á.; Martin, Á.; Cosero, M.J.; Jiménez, C.; Londoño, J. Encapsulation of Curcumin Using Supercritical Antisolvent (SAS) Technology to Improve Its Stability and Solubility in Water. Food Chem. 2018, 258, 156–163. [Google Scholar] [CrossRef]
- Rosa, M.T.M.G.; Alvarez, V.H.; Albarelli, J.Q.; Santos, D.T.; Meireles, M.A.A.; Saldaña, M.D.A. Supercritical Anti-Solvent Process as an Alternative Technology for Vitamin Complex Encapsulation Using Zein as Wall Material: Technical-Economic Evaluation. J. Supercrit. Fluids 2020, 159, 104499. [Google Scholar] [CrossRef]
- Sakata, G.S.B.; Ribas, M.M.; Dal Magro, C.; Santos, A.E.; Aguiar, G.P.S.; Oliveira, J.V.; Lanza, M. Encapsulation of Trans-Resveratrol in Poly(ε-Caprolactone) by GAS Antisolvent. J. Supercrit. Fluids 2021, 171, 105164. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, F.; Li, A.; Wang, J.; Wang, D.; Chen, J. Synthesis of Curcumin-loaded Shellac Nanoparticles via Co-precipitation in a Rotating Packed Bed for Food Engineering. J. Appl. Polym. Sci. 2022, 139, e52421. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zabot, G.L.; Schaefer Rodrigues, F.; Polano Ody, L.; Vinícius Tres, M.; Herrera, E.; Palacin, H.; Córdova-Ramos, J.S.; Best, I.; Olivera-Montenegro, L. Encapsulation of Bioactive Compounds for Food and Agricultural Applications. Polymers 2022, 14, 4194. https://doi.org/10.3390/polym14194194
Zabot GL, Schaefer Rodrigues F, Polano Ody L, Vinícius Tres M, Herrera E, Palacin H, Córdova-Ramos JS, Best I, Olivera-Montenegro L. Encapsulation of Bioactive Compounds for Food and Agricultural Applications. Polymers. 2022; 14(19):4194. https://doi.org/10.3390/polym14194194
Chicago/Turabian StyleZabot, Giovani Leone, Fabiele Schaefer Rodrigues, Lissara Polano Ody, Marcus Vinícius Tres, Esteban Herrera, Heidy Palacin, Javier S. Córdova-Ramos, Ivan Best, and Luis Olivera-Montenegro. 2022. "Encapsulation of Bioactive Compounds for Food and Agricultural Applications" Polymers 14, no. 19: 4194. https://doi.org/10.3390/polym14194194
APA StyleZabot, G. L., Schaefer Rodrigues, F., Polano Ody, L., Vinícius Tres, M., Herrera, E., Palacin, H., Córdova-Ramos, J. S., Best, I., & Olivera-Montenegro, L. (2022). Encapsulation of Bioactive Compounds for Food and Agricultural Applications. Polymers, 14(19), 4194. https://doi.org/10.3390/polym14194194








