Recycling of Thermoset Materials and Thermoset-Based Composites: Challenge and Opportunity
Abstract
1. Introduction
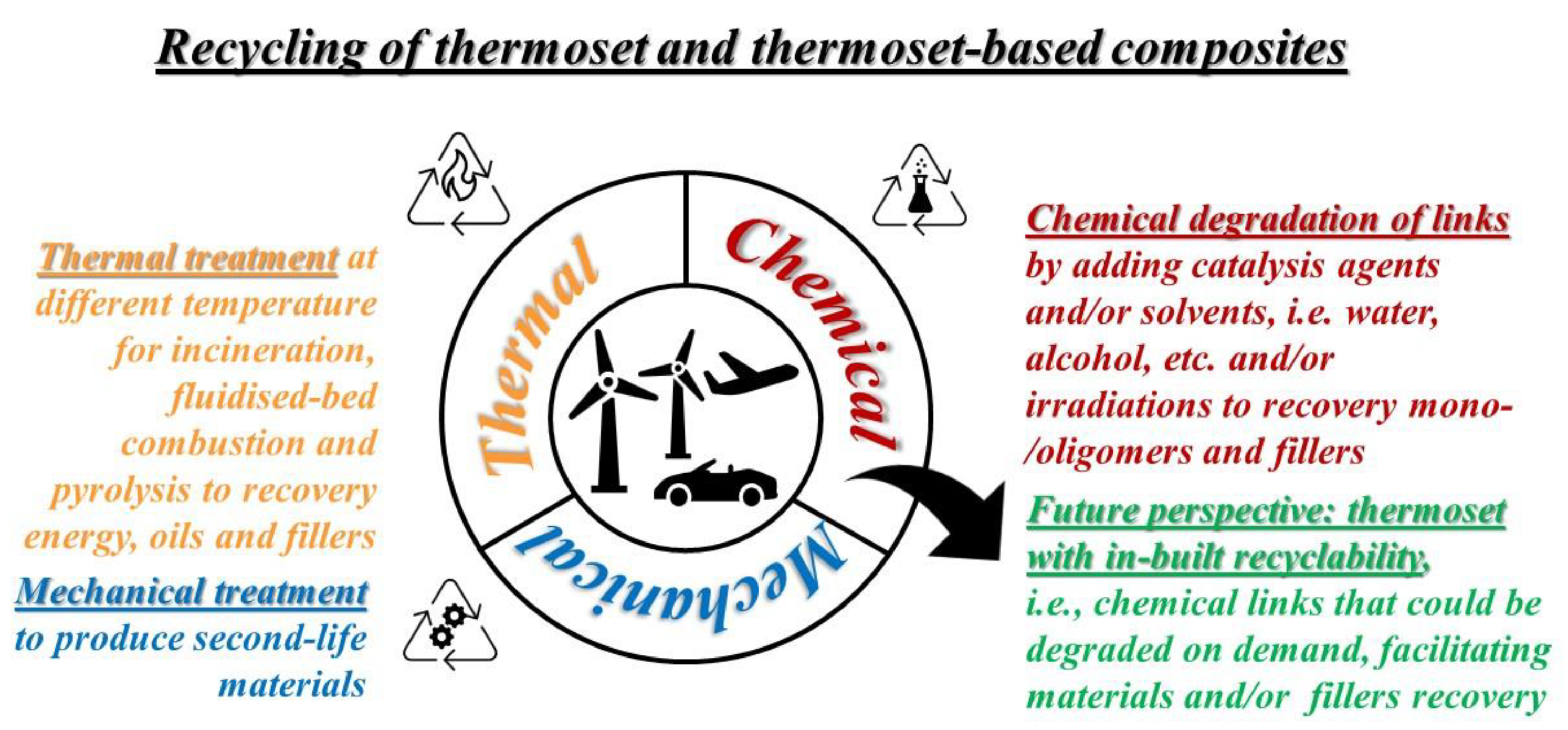
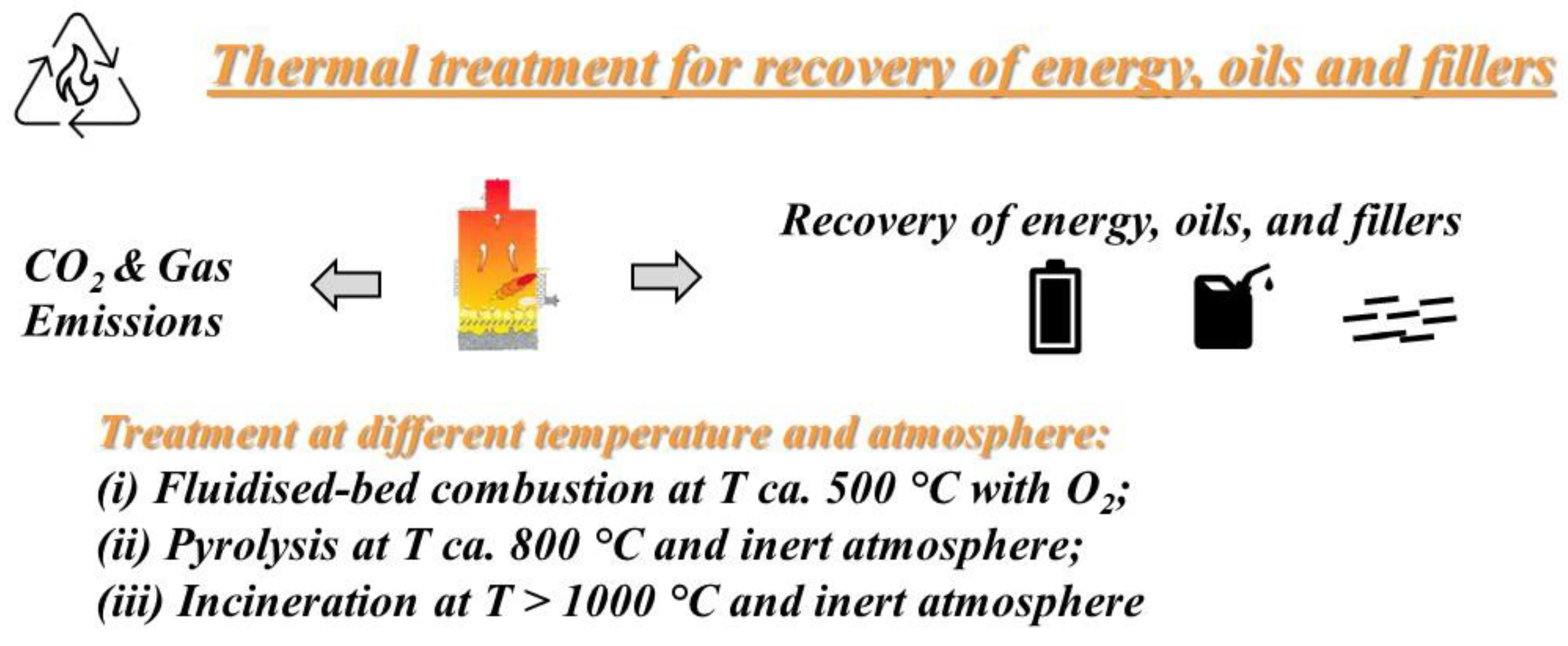
2. Thermal Recycling
2.1. Incineration
2.2. Fluidised-Bed Combustion
2.3. Pyrolysis
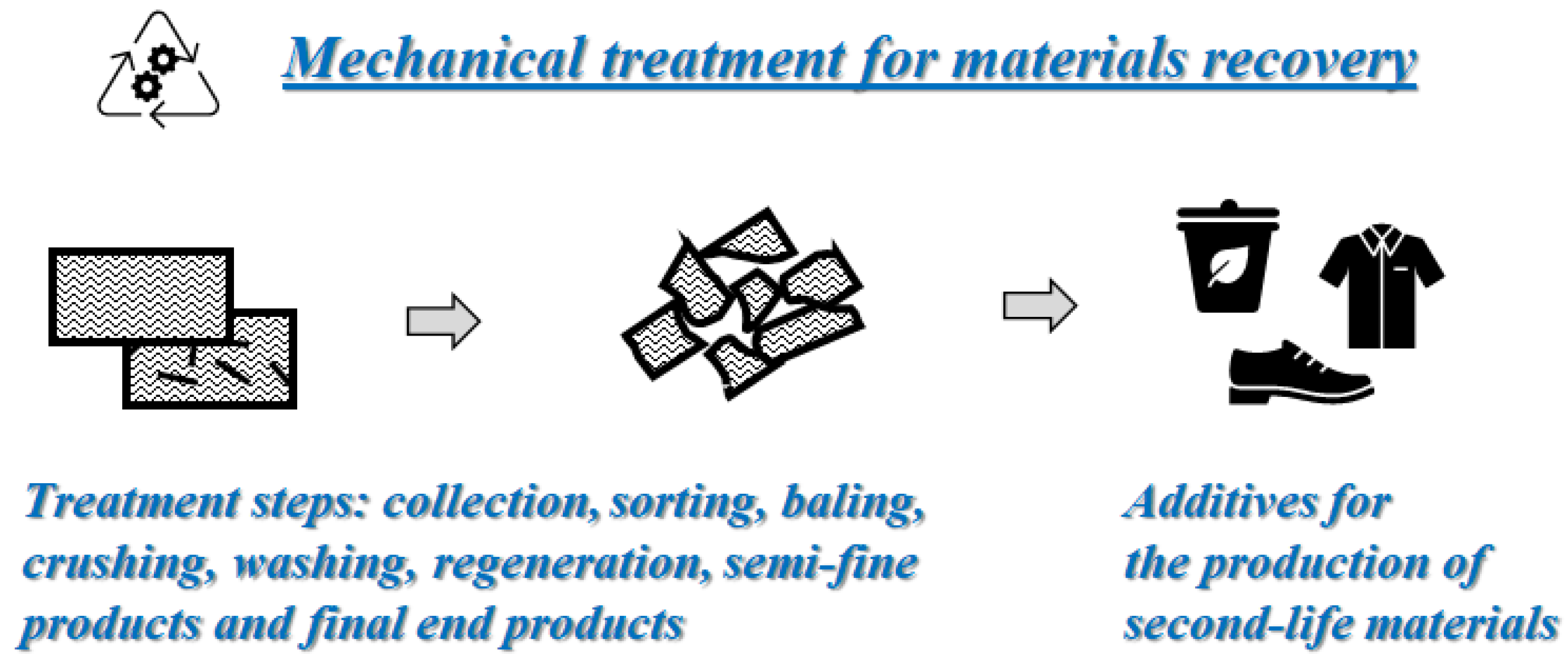
3. Mechanical Recycling
4. Chemical Recycling
5. Thermoset with Built-in Recyclability
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ratna, D. Chapter 2—Properties and processing of thermoset resin. In Recent Advances and Applications of Thermoset Resins, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 173–292. [Google Scholar]
- Hay, J.N.; O’Gara, P. Recent developments in thermoset curing methods. Proc. Inst. Mech. Eng. G J. Aerosp. Eng. 2006, 220, 187–195. [Google Scholar] [CrossRef]
- Boquillon, N.; Fringant, C. Polymer networks derived from curing of epoxidised linseed oil: Influence of different catalysts and anhydride hardeners. Polymer 2000, 41, 8603–8613. [Google Scholar] [CrossRef]
- Walczyk, D.; Kuppers, J. Thermal press curing of advanced thermoset composite laminate parts. Compos. Part A Appl. Sci. Manuf. 2012, 43, 635–646. [Google Scholar] [CrossRef]
- Fan-Long, J.; Park, S.J. Preparation and Characterization of Carbon Fiber-Reinforced Thermosetting Composites: A Review. Carbon Lett. 2015, 16, 67–77. [Google Scholar]
- Caydamli, Y.; Heudorfer, K.; Take, J.; Podjaski, F.; Middendorf, P.; Buchmeiser, M.R. Transparent Fiber-Reinforced Composites Based on a Thermoset Resin Using Liquid Composite Molding (LCM) Techniques. Materials 2021, 14, 6087. [Google Scholar] [CrossRef]
- Gore, P.M.; Kandasubramanian, B. Functionalized Aramid Fibers and Composites for Protective Applications: A Review. Ind. Eng. Chem. Res. 2018, 57, 16537–16563. [Google Scholar] [CrossRef]
- Blanco, D.; Rubio, E.M.; Lorente-Pedreille, R.M.; Sáenz-Nuño, M.A. Lightweight Structural Materials in Open Access: Latest Trends. Materials. 2021, 14, 577. [Google Scholar] [CrossRef]
- Hagnell, M.; Kumaraswamy, S.; Nyman, T.; Åkermo, M. From aviation to automotive—A study on material selection and its implication on cost and weight efficient structural composite and sandwich designs. Heliyon 2020, 6, e03716. [Google Scholar] [CrossRef]
- Mishnaevsky, L.; Branner, K.; Petersen, H.N.; Beauson, J.; McGugan, M.; Sørensen, B.F. Materials for Wind Turbine Blades: An Overview. Materials 2017, 10, 1285. [Google Scholar] [CrossRef]
- Timmis, A.; Hodzic, A.; Koh, L.; Bonner, M.; Soutis, C.; Schäfer, A.; Dray, L. Environmental impact assessment of aviation emission reduction through the implementation of composite materials. Int. J. Life Cycle Assess. 2015, 20, 233–243. [Google Scholar] [CrossRef]
- Naheed, S.; Jawaid, M. Epoxy resin based hybrid polymer composites. In Hybrid Polymer Composite Materials; Thakur, V.K., Thakur, M.K., Pappu, A., Eds.; Woodhead Publishing: Kidlington, UK, 2017; pp. 57–82. [Google Scholar]
- Gama, N.V.; Ferreira, A.; Barros-Timmons, A. Polyurethane Foams: Past, Present, and Future. Materials 2018, 11, 1841. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yuan, L.; Guan, Q.; Liang, G.; Gu, A. Synergistically building flame retarding thermosetting composites with high toughness and thermal stability through unique phosphorus and silicone hybridized graphene oxide. Compos. Part A Appl. Sci. Manuf. 2017, 98, 174–183. [Google Scholar] [CrossRef]
- Xue, X.; Liu, S.Y.; Zhang, Z.Y.; Wang, Q.Z.; Xiao, C.Z. A technology review of recycling methods for fiber-reinforced thermosets. J. Reinf. Plast. Compos. 2022, 41, 459–480. [Google Scholar] [CrossRef]
- Leszczyñski, S.; Brzychczyk, B. Waste management of half-finished products and thermosetting wastes. Polish J. Chem. Technol. 2007, 9, 122–126. [Google Scholar] [CrossRef]
- Morici, E.; Carroccio, S.C.; Bruno, E.; Scarfato, P.; Filippone, G.; Dintcheva, N.T. Recycled (Bio)Plastics and (Bio)Plastic Composites: A Trade Opportunity in a Green Future. Polymers 2022, 14, 2038. [Google Scholar] [CrossRef]
- Marson, A.; Masiero, M.; Modesti, M.; Scipioni, A.; Manzardo, A. Life Cycle Assessment of Polyurethane Foams from Polyols Obtained through Chemical Recycling. ACS Omega 2021, 6, 1718–1724. [Google Scholar] [CrossRef]
- La Rosa, A.D.; Blanco, I.; Banatao, D.R.; Pastine, S.J.; Björklund, A.; Cicala, G. Innovative Chemical Process for Recycling Thermosets Cured with Recyclamines® by Converting Bio-Epoxy Composites in Reusable Thermoplastic-An LCA Study. Materials 2018, 11, 353. [Google Scholar] [CrossRef]
- Pimenta, S.; Pinho, S.T. Recycling carbon fibre reinforced polymers for structural applications: Technology review and market outlook. Waste Manag. 2011, 31, 378–392. [Google Scholar] [CrossRef]
- Vieira, D.R.; Vieira, R.K.; Chain, M.C. Strategy and management for the recycling of carbon fiber-reinforced polymers (CFRPs) in the aircraft industry: A critical review. Int. J. Sustain. Dev. World Ecol. 2017, 24, 214–223. [Google Scholar] [CrossRef]
- Meng, F.; Olivetti, E.A.; Zhao, Y.; Chang, J.C.; Pickering, S.J.; McKechnie, J. Comparing Life Cycle Energy and Global Warming Potential of Carbon Fiber Composite Recycling Technologies and Waste Management Options. ACS Sustain. Chem. Eng. 2018, 6, 9854–9865. [Google Scholar] [CrossRef]
- Joshi, R.; Ahmed, S. Status and challenges of municipal solid waste management in India: A review. Cogent Environ Sci. 2016, 2, 1139434. [Google Scholar] [CrossRef]
- Gonçalves, R.M.; Martinho, A.; Oliveira, J.P. Recycling of Reinforced Glass Fibers Waste: Current Status. Materials 2022, 15, 1596. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.; Liu, K.C.; Chen, J.F.; Teng, J.G. Concrete reinforced with macro fibres recycled from waste GFRP. Constr. Build. Mater. 2021, 310, 125063. [Google Scholar] [CrossRef]
- Utekar, S.; Suriya, V.K.; More, N.; Rao, A. Comprehensive study of recycling of thermosetting polymer composites—Driving force, challenges and methods. Compos. Part B 2021, 207, 108596. [Google Scholar] [CrossRef]
- Pickering, S.J.; Kelly, R.M.; Kennerley, J.R.; Rudd, C.; Fenwick, N.J. Fluidized-bed process for the recovery of glass fibres from scrap thermoset composites. Compos. Sci. Technol. 2000, 60, 509–523. [Google Scholar] [CrossRef]
- Torres, A.; de Marco, I.; Caballero, B.M.; Laresgoiti, M.F.; Legarreta, J.A.; Cabrero, M.A.; González, A.; Chomón, M.J.; Gondra, K. Recycling by pyrolysis of thermoset composites: Characteristics of the liquid and gaseous fuels obtained. Fuel 2000, 79, 897–902. [Google Scholar] [CrossRef]
- Vasile, C.; Brebu, M.; Karayildirim, T.; Yanik, J.; Darie, H. Feedstock recycling from plastic and thermoset fractions of used computers (I): Pyrolysis. J. Mater. Cycles Waste Manag. 2006, 8, 99–108. [Google Scholar] [CrossRef]
- Ginder, R.S.; Ozcan, S. Recycling of Commercial E-glass Reinforced Thermoset Composites via Two Temperature Step Pyrolysis to Improve Recovered Fiber Tensile Strength and Failure Strain. Recycling. 2019, 4, 24. [Google Scholar] [CrossRef]
- Hao, S.; He, L.; Liu, J.; Liu, Y.; Rudd, C.; Liu, X. Recovery of Carbon Fibre from Waste Prepreg via Microwave Pyrolysis. Polymers 2021, 13, 1231. [Google Scholar] [CrossRef]
- Dorigato, A. Recycling of thermosetting composites for wind blade application. Adv. Ind. Eng. Polym. Res. 2021, 4, 116–132. [Google Scholar] [CrossRef]
- Conroy, A.; Halliwell, S.; Reynolds, T. Composite recycling in the construction industry. Compos. Part A Appl. Sci. Manuf. 2006, 37, 1216–1222. [Google Scholar] [CrossRef]
- Haider, M.M.; Nassiri, S.; Englund, K.; Li, H.; Chen, Z. Exploratory Study of Flexural Performance of Mechanically Recycled Glass Fiber Reinforced Polymer Shreds as Reinforcement in Cement mortar. Transp. Res. Rec. 2021, 2675, 1254–1267. [Google Scholar] [CrossRef]
- Shuaib, N.A.; Mativenga, P.T. Energy demand in mechanical recycling of glass fibre reinforced thermoset plastic composites. J. Clean. Prod. 2016, 120, 198–206. [Google Scholar] [CrossRef]
- Rahimizadeh, A.; Kalman, J.; Henri, R.; Fayazbakhsh, K.; Lessard, L. Recycled Glass Fiber Composites from Wind Turbine Waste for 3D Printing Feedstock: Effects of Fiber Content and Interface on Mechanical Performance. Materials 2019, 12, 3929. [Google Scholar] [CrossRef]
- Zhang, Y.; Pontikes, Y.; Lessard, L.; Willem van Vuure, A. Recycling and valorization of glass fibre thermoset composite waste by cold incorporation into a sustainable inorganic polymer matrix. Compos. B Eng. 2021, 223, 109120. [Google Scholar] [CrossRef]
- Okajima, I.; Hiramatsu, M.; Shimamura, Y.; Awaya, T.; Sako, T. Chemical recycling of carbon fiber reinforced plastic using supercritical methanol. J. Supercrit. Fluids 2014, 91, 68–76. [Google Scholar] [CrossRef]
- Yu, H.; Potter, K.D.; Wisnom, M.R. A novel manufacturing method for aligned discontinuous fibre composites (High Performance-Discontinuous Fibre method). Compos. Part A Appl. Sci. Manuf. 2014, 65, 175–185. [Google Scholar] [CrossRef]
- Ning, H.; Lu, N.; Hassen, A.A.; Chawla, K.; Selim, M.; Pillay, S. A review of Long fibre thermoplastic (LFT) composites. Int. Mater. Rev. 2020, 65, 164–188. [Google Scholar] [CrossRef]
- Kuroyanagi, M.; Yamaguchi, A.; Hashimoto, T.; Urushisaki, M.; Sakaguchi, T.; Kawabe, K. Novel degradable acetal-linkage-containing epoxy resins with high thermal stability: Synthesis and application in carbon fiber-reinforced plastics. Polym. J. 2022, 54, 313–322. [Google Scholar] [CrossRef]
- Oliveux, G.; Dandy, L.O.; Leeke, G.A. Current Status of Recycling of Fibre Reinforced Polymers: Review of Technologies, Reuse and Resulting Properties. Prog. Mater. Sci. 2015, 72, 61–99. [Google Scholar] [CrossRef]
- Ma, S.; Webster, D.C. Degradable thermosets based on labile bonds or linkages: A review. Prog. Polym. Sci. 2018, 76, 65–110. [Google Scholar] [CrossRef]
- Bapat, A.P.; Sumerlin, B.S.; Sutti, A. Bulk network polymers with dynamic B–O bonds: Healable and reprocessable materials. Mater. Horiz. 2020, 7, 694–714. [Google Scholar] [CrossRef]
- Zhang, G.; Tian, C.; Feng, H.; Tan, T.; Wang, R.; Zhang, L. Thermal Reprocessing and Closed-Loop Chemical Recycling of Styrene-Butadiene Rubber Enabled by Exchangeable and Cleavable Acetal Linkages. Macromol. Rapid Commun. 2022, 43, 2100887. [Google Scholar] [CrossRef] [PubMed]
- Post, W.; Susa, A.; Blaauw, R.; Molenveld, K.; Knoop, R.J.I. A Review on the Potential and Limitations of Recyclable Thermosets for Structural Applications. Polym Rev. 2020, 60, 359–388. [Google Scholar] [CrossRef]
- Overcash, M.; Twomey, J.; Asmatulu, E.; Vozzola, E.; Griffing, E. Thermoset Composite Recycling—Driving Forces, Development, and Evolution of New Opportunities. J. Compos. Mater. 2018, 52, 1033–1043. [Google Scholar] [CrossRef]
- Chen, J.S.; Ober, C.K.; Poliks, M.D.; Zhang, Y.M.; Wiesner, U.; Cohen, C. Controlled degradation of epoxy networks: Analysis of crosslink density and glass transition temperature changes in thermally reworkable thermosets. Polymer 2004, 45, 1939–1950. [Google Scholar] [CrossRef]
- Liu, T.; Guo, X.; Liu, W.; Hao, C.; Wang, L.; Hiscox, W.C.; Liu, C.; Jin, C.; Xina, J.; Zhang, J. Selective cleavage of ester linkages of anhydride-cured epoxy using a benign method and reuse of the decomposed polymer in new epoxy preparation. Green Chem. 2017, 19, 4364–4372. [Google Scholar] [CrossRef]
- Niu, W.; O’Sullivan, C.; Rambo, B.M.; Smith, M.D.; Lavigne, J.J. Self-repairing polymers: Poly(dioxaborolane)s containing trigonal planar boron. Chem. Commun. 2005, 34, 4342–4344. [Google Scholar] [CrossRef]
- Bao, C.; Zhang, X.; Yu, P.; Li, Q.; Qina, Y.; XinJ, Z. Facile fabrication of degradable polyurethane thermosets with high mechanical strength and toughness via the cross-linking of triple boron–urethane bonds. Mater. Chem. A. 2021, 9, 22410–22417. [Google Scholar] [CrossRef]
- Buchwalter, S.L.; Kuczynski, J.P.; Stephanie, J.G. Cleavable Diepoxide for Removable Epoxy Compositions; International Business Machines Corporation: Armonk, NY, USA, 1999. [Google Scholar]
- Shirai, M. Reworkable UV curing materials. Prog. Org. Coat. 2007, 58, 158–165. [Google Scholar] [CrossRef]
- Memon, H.; Wei, Y. Welding and reprocessing of disulfide-containing thermoset epoxy resin exhibiting behavior reminiscent of a thermoplastic. J. Appl. Polym. Sci. 2020, 137, 49541–49550. [Google Scholar] [CrossRef]
- de Luzuriaga, A.R.; Martin, R.; Markaide, N.; Rekondo, A.; Cabañero, G.; Rodríguez, J.; Odriozola, I. Epoxy Resin with Exchangeable Disulfide Crosslinks to Obtain Reprocessable, Repairable and Recyclable Fiber-Reinforced Thermoset Composites. Mater. Horiz. 2016, 3, 241–247. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, R.; Wu, Q.; Chen, T.; Sun, P. Bio-Inspired High-Performance and Recyclable Cross-Linked Polymers. Adv. Mater. 2013, 25, 4912–4917. [Google Scholar] [CrossRef] [PubMed]
- Amamoto, Y.; Kamada, J.M.; Otsuka, H.; Takahara, A.; Matyjaszewski, K. Repeatable photoinduced self-healing of covalently cross-linked polymers through reshuffling of trithiocarbonate units. Angew. Chem. Int. Ed. 2011, 50, 1660–1663. [Google Scholar] [CrossRef] [PubMed]
- Bowman, C.N.; Kloxin, C.J. Covalent adaptable networks: Reversible bond structures incorporated in polymer networks. Angew. Chem. Int. Ed. 2012, 51, 4272–4274. [Google Scholar] [CrossRef]
- Rekondo, A.; Martin, R.; Ruiz de Luzuriaga, A.; Cabanero, G.; Grande, H.J.; Odriozola, I. Catalyst-free room-temperature self-healing elastomers based on aromatic disulfide metathesis. Mater. Horiz. 2014, 1, 237–240. [Google Scholar] [CrossRef]
- Winne, J.M.; Leibler, L.; Du Prez, F.E. Dynamic covalent chemistry in polymer networks: A mechanistic perspective. Polym. Chem. 2019, 10, 6091–6108. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, Z.; Wang, B.; Li, P.; Zhua, J.; Ma, S. Closed-loop chemical recycling of thermosetting polymers and their applications: A review. Green Chem. 2022, 24, 5691–5708. [Google Scholar] [CrossRef]
- Peng, W.L.; You, Y.; Xie, P.; Rong, M.Z.; Zhang, M.Q. Adaptable interlocking macromolecular networks with homogeneous architecture made from immiscible single networks. Macromolecules 2020, 53, 584–593. [Google Scholar] [CrossRef]
- Hammer, L.; Van Zee, N.J.; Nicolaÿ, R. Dually Crosslinked Polymer Networks Incorporating Dynamic Covalent Bonds. Polymers 2021, 13, 396. [Google Scholar] [CrossRef]
- Liang, R.; Zhang, H.; Wang, Y.; Ye, J.; Guo, L.; He, L.; Li, X.; Qiu, T.; Tuo, X. Dual dynamic network system constructed by waterborne polyurethane for improved and recoverable performances. Chem. Eng. J. 2022, 442, 136204. [Google Scholar] [CrossRef]
- Shieh, P.; Zhang, W.; Husted, K.E.L.; Kristufek, S.L.; Xiong, B.; Lundberg, D.J.; Lem, J.; Veysset, D.; Sun, Y.; Nelson, K.A.; et al. Cleavable comonomers enable degradable, recyclable thermoset plastics. Nature 2020, 583, 542–547. [Google Scholar] [CrossRef] [PubMed]


| Recycling Process | (+) Advantages and (−) Disadvantages of Different Recycling Processes |
|---|---|
| Thermal recycling | (+) Recovery of energy, oils and fillers (+) No use of solvent and catalysts agents (−) Reduced dimensions and properties of recovered materials (−) Production of emissions and CO2 |
| Mechanical recycling | (+) Recovery of fillers and matrix that can be used as additives to produce second-life materials (−) Long time pre-treatments to recover the materials (−) Low mechanical performance of recovered materials (−) Impossibility to re-manufacture the materials |
| Chemical recycling | (+) Recovery of mono-oligomers and fillers (+) Recovered fillers with unchanged shape, dimensions, composition and mechanical properties (−) Use of solvents and catalysts agents (−) Difficult to implement the process at a larger scale |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morici, E.; Dintcheva, N.T. Recycling of Thermoset Materials and Thermoset-Based Composites: Challenge and Opportunity. Polymers 2022, 14, 4153. https://doi.org/10.3390/polym14194153
Morici E, Dintcheva NT. Recycling of Thermoset Materials and Thermoset-Based Composites: Challenge and Opportunity. Polymers. 2022; 14(19):4153. https://doi.org/10.3390/polym14194153
Chicago/Turabian StyleMorici, Elisabetta, and Nadka Tz. Dintcheva. 2022. "Recycling of Thermoset Materials and Thermoset-Based Composites: Challenge and Opportunity" Polymers 14, no. 19: 4153. https://doi.org/10.3390/polym14194153
APA StyleMorici, E., & Dintcheva, N. T. (2022). Recycling of Thermoset Materials and Thermoset-Based Composites: Challenge and Opportunity. Polymers, 14(19), 4153. https://doi.org/10.3390/polym14194153








