A Review on Fully Bio-Based Materials Development from Polylactide and Cellulose Nanowhiskers
Abstract
:1. Introduction
2. Synthesis and Properties of PLA and CNWs
2.1. Polylactide (PLA)
2.2. Cellulose Nano-Whiskers (CNW)
2.3. PLA and CNW Compatibility
3. PLA–CNW Nanocomposites
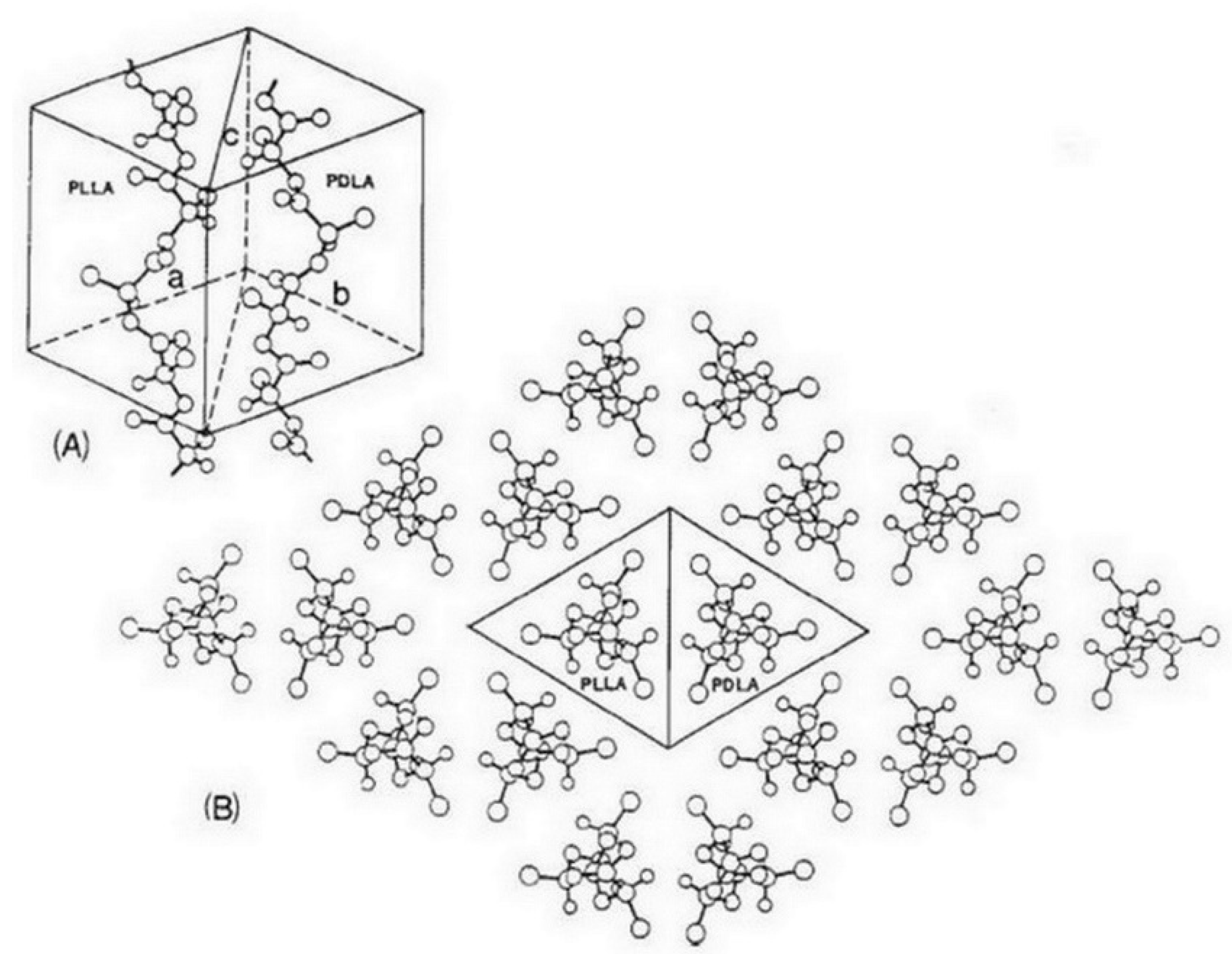
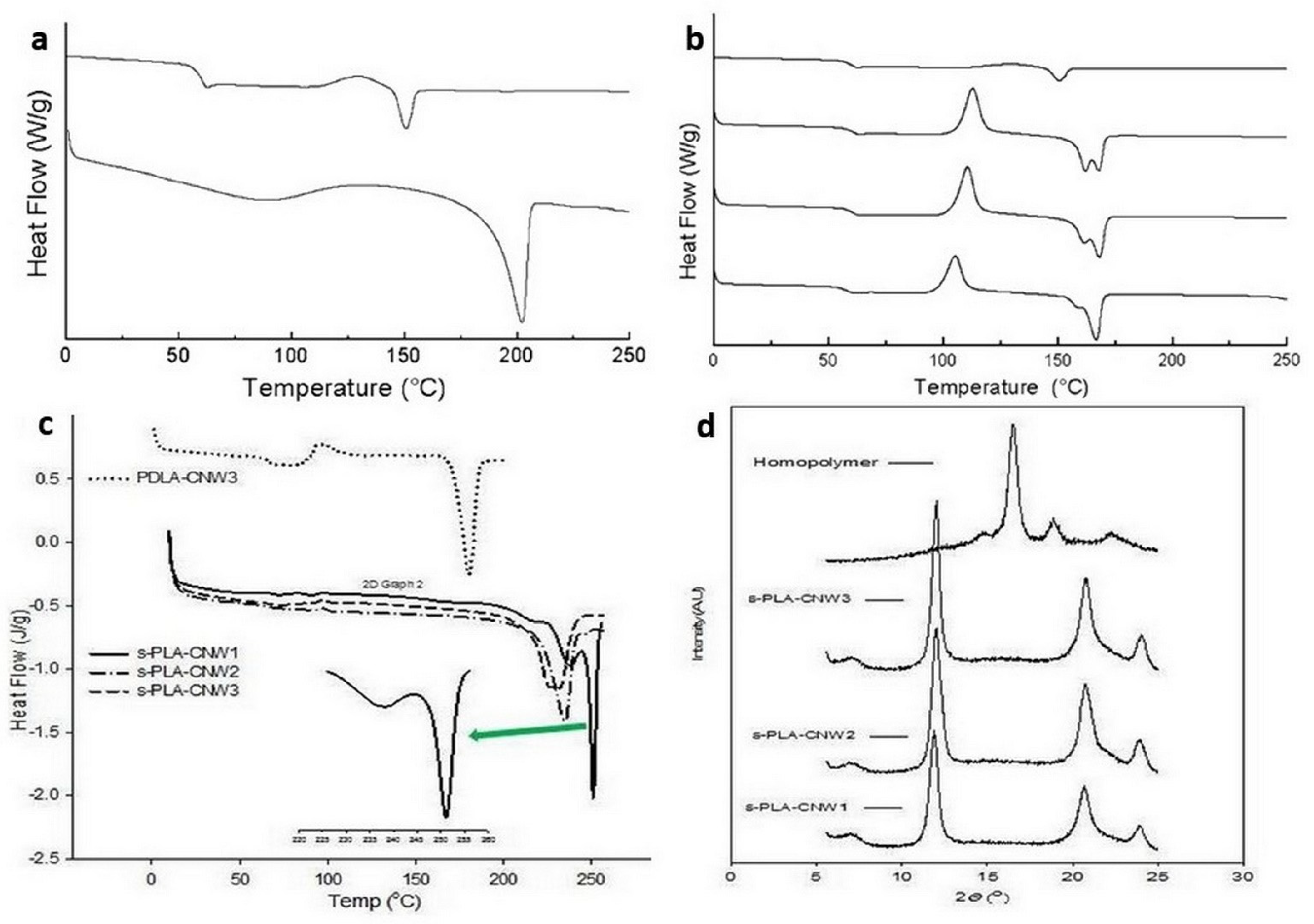
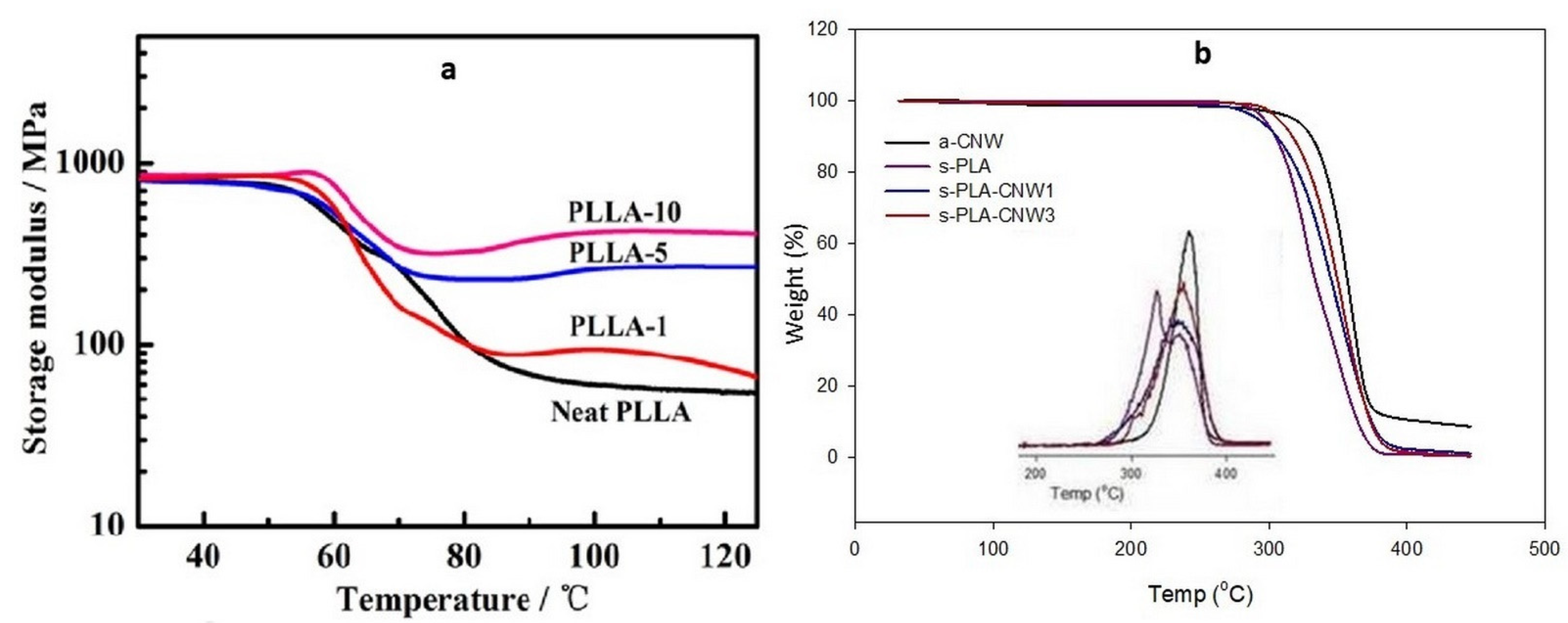
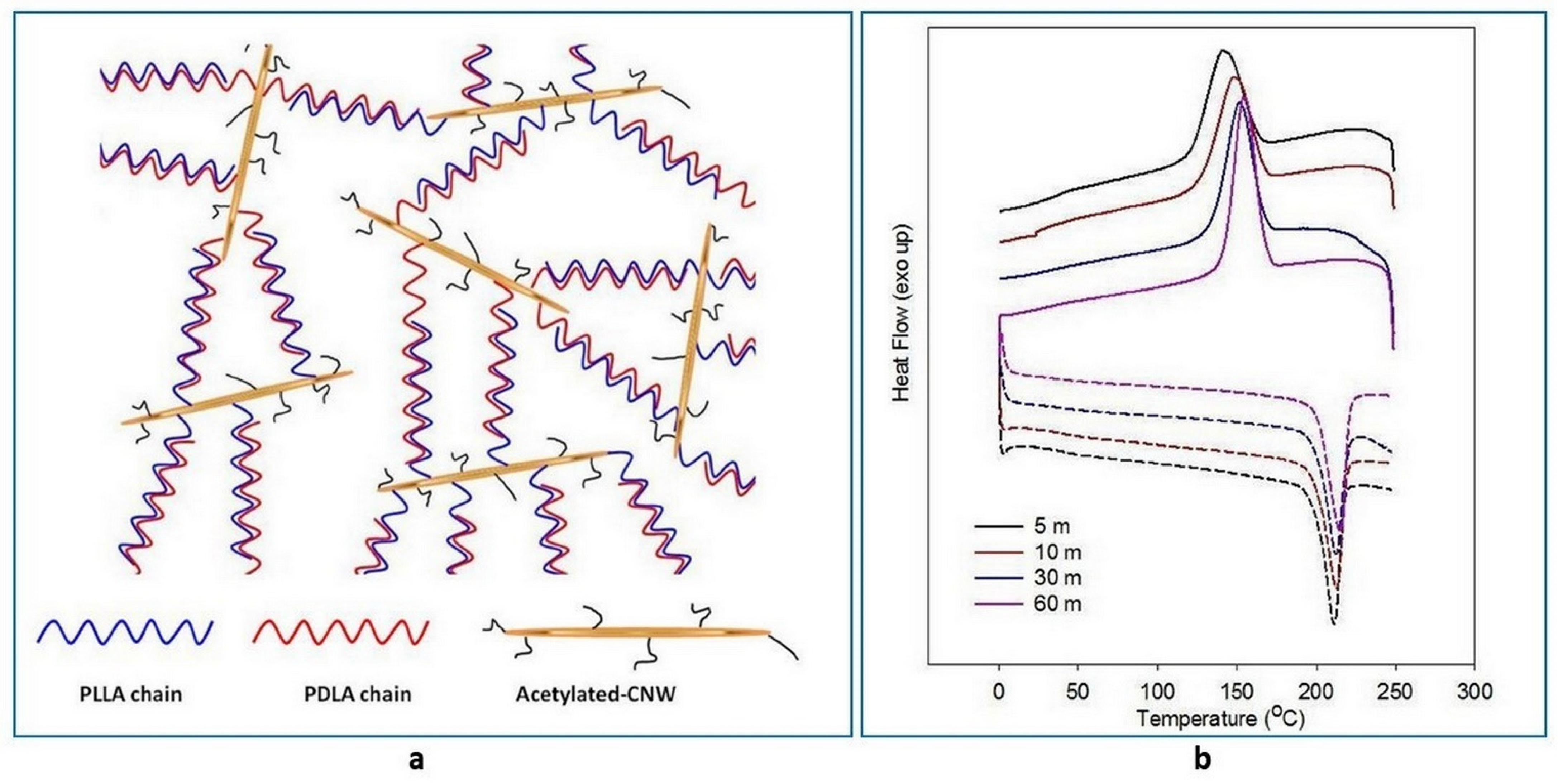
4. Bio-Stereo-Nanocomposites PLA–CNW
5. Conclusions and Prospective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Babu, R.P.; O’Connor, K.; Seeram, R. Current progress on bio-based polymers and their future trends. Prog. Biomater. 2013, 2, 8. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.K.; Hillmyer, M.A. Polymer from renewable resources: A perspective for a special issue of polymer reviews. Polym. Rev. 2008, 45, 1–10. [Google Scholar] [CrossRef]
- Garlotta, D. A literature review of poly(lactic acid). Polym. Environ. 2001, 9, 63–84. [Google Scholar] [CrossRef]
- Ikada, Y.; Tsuji, H. Biodegradable polyesters for medical and ecological applications. Macromol. Rapid Commun. 2000, 21, 117–132. [Google Scholar] [CrossRef]
- Wu, D.; Yuan, L.; Laredo, E.; Zhang, M.; Zhou, W. Interfacial properties, viscoelasticity, and thermal behaviours of poly(butylene succinate)/polylactide blend. Ind. Eng. Chem. Res. 2012, 51, 2290–2298. [Google Scholar] [CrossRef]
- Noroozi, N.; Schafer, L.L.; Hatzikiriakos, S.G. Thermorheological properties of poly(ε-caprolactone)/polylactide blends. Polym. Eng. Sci. 2012, 52, 2348–2359. [Google Scholar] [CrossRef]
- Ojijo, V.; Ray, S.S. Super toughened biodegradable polylactide blends with non-linear copolymer interfacial architechture obtained via facile in-situ reactive compatibilization. Polymer 2015, 80, 1–17. [Google Scholar] [CrossRef]
- Ikada, Y.; Jamshidi, K.; Tsuji, H.; Hyon, S.-H. Stereocomplex formation between enantiomeric poly(lactides). Macromolecules 1987, 20, 904–906. [Google Scholar] [CrossRef]
- Okihara, T.; Tsuji, M.; Kawaguchi, A.; Katayama, K.; Tsuji, H.; Hyon, S.-H.; Ikada, Y. Crystal structure of stereocomplex of poly(L-lactide) and poly(D-lactide). J. Macromol. Sci. B Phys. 1991, B30, 119–140. [Google Scholar] [CrossRef]
- Brochu, S.; Prud’homme, R.E.; Barakat, I.; Jerome, R. Stereocomplexation and Morphology of Polylactides. Macromolecules 1995, 28, 5230–5239. [Google Scholar] [CrossRef]
- Zhang, J.; Sato, H.; Tsuji, H.; Noda, I.; Ozaki, Y. Infrared spectroscopic study of CH3---O = C interaction during poly(L-lactide)/poly(D-lactide) stereocomplex formation. Macromolecules 2005, 38, 1822–1828. [Google Scholar] [CrossRef]
- Fukushima, K.; Kimura, Y. Stereocomplexed polylactides (Neo-PLA) as high-performance bio-based polymers: Their formation, properties, and applications. Polym. Int. 2006, 55, 626–642. [Google Scholar] [CrossRef]
- Purnama, P.; Kim, S.H. Stereocomplex formation of high-molecular-weight polylactide using supercritical fluid. Macromolecules 2010, 43, 1137–1142. [Google Scholar] [CrossRef]
- Purnama, P.; Jung, Y.; Kim, S.H. Stereocomplexation of poly(L-lactide) and Random Copolymer Poly(D-lactide-co-ε-caprolactone) to enhance melt stability. Macromolecules 2012, 45, 4012–4014. [Google Scholar] [CrossRef]
- Darder, M.; Aranda, P.; Ruiz-Hitzky, E. Bionanocomposites: A new concept of ecological, bioinspired, and functional hybrid materials. Adv. Mater. 2007, 19, 1309–1319. [Google Scholar] [CrossRef]
- Darder, M.; Aranda, P.; Ferrer, M.L.; Gutierrez, M.C.; del Monte, F.; Ruiz-Hitzky, E. Progress in bionanocomposite and bioinspired foams. Adv. Mater. 2011, 23, 5262–5267. [Google Scholar] [CrossRef]
- Bitinis, N.; Hernandez, M.; Verdejo, R.; Kenny, J.M.; Lopez-Manchado, M.A. Recent advances in clay/polymer nanocomposites. Adv. Mater. 2011, 23, 5229–5236. [Google Scholar] [CrossRef]
- Braun, B.; Dorgan, J.R.; Hollingsworth, L.O. Supra-molecular ecobionanocomposites based on polylactide and cellulosic nanowhiskers: Synthesis and properties. Biomacromolecules 2012, 13, 2013–2019. [Google Scholar] [CrossRef]
- Park, W.I.; Kang, M.; Kim, H.S.; Jin, H.J. Electrospinning of poly (ethylene oxide) with bacterial cellulose whiskers. Macromol. Symp. 2007, 249, 289–294. [Google Scholar] [CrossRef]
- Peresin, M.S.; Habibi, Y.; Zoppe, J.O.; Pawlak, J.; Rojas, O.J. Nanofiber composites of polyvinyl alcohol and cellulose nanocrystals: Manufacture and characterization. Biomacromolecules 2010, 11, 674–681. [Google Scholar] [CrossRef]
- Zoppe, J.O.; Peresin, M.S.; Habibi, Y.; Venditi, R.; Rojas, O.J. Reinforcing poly (ε-caprolactone) nanofibers with cellulose nanocrystals. Appl. Mater. Interfaces 2009, 1, 1996–2004. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.F.; Zhou, C.J.; Yue, Y.Y.; Guo, W.; Wu, Y.; Wu, Q. Mechanical properties and in vitro degradation of electrospun bio-nanocomposite mats from PLA and cellulose nanocrystals. Carbohyd. Polym. 2012, 90, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Zhao, L.; Jiang, X.; Wang, H.; Gao, W. Poly(lactic acid)-based biocomposites reinforced with modified cellulose nanocrystals. Cellulose 2017, 24, 4773–4784. [Google Scholar] [CrossRef]
- Mokhena, T.C.; Sefadi, J.S.; Sadiku, E.R.; John, M.J.; Mochane, M.J.; Mtibe, A. Thermmoplastic processing of PLA/Cellulose nanomaterials composites. Polymers 2018, 10, 1363. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, S.H.; Kim, S.H. Surface modification of cellulose nanowishkers and their reinforcing effect in polylactide. Macromol. Res. 2014, 22, 424–430. [Google Scholar] [CrossRef]
- Sanchez-Garcia, M.D.; Lagaron, J.M. On the use of plant cellulose nanowishkers to enhance the barrier properties of polylactic acid. Cellulose 2010, 17, 987–1004. [Google Scholar] [CrossRef]
- Liu, W.; Dong, Y.; Liu, D.; Bai, X.; Lu, X. Polylactid acid (PLA)/cellulose nanowishkers (CNWs) composite nanofibers: Microstructural and properties analysis. J. Compos. Sci. 2018, 2, 4. [Google Scholar] [CrossRef]
- Lizundia, E.; Fortunati, E.; Dominici, F.; Vilas, J.L.; Leon, L.M.; Armentano, I.; Torre, L.; Kenny, J.M. PLLA-grafted cellulose nanocrystals: Role of the CNC content and grafting on the PLA bionanocomposite film properties. Carbohydr. Polym. 2016, 142, 105–113. [Google Scholar] [CrossRef]
- Purnama, P.; Kim, S.H. Synergism of cellulosic nanowhiskers and graft structure in stereocomplex-based materials: Formation in solution and a stereocomplex memory study. Cellulose 2014, 21, 2539–2548. [Google Scholar] [CrossRef]
- Purnama, P.; Kim, S.H. Bio-based composite of stereocomplex polylactide and cellulose nanowhiskers. Polym. Degrad. Stab. 2014, 109, 430–435. [Google Scholar] [CrossRef]
- Drumright, R.E.; Gruber, P.R.; Henton, D.E. Polylactic acid technology. Adv. Mater. 2000, 12, 1841. [Google Scholar] [CrossRef]
- Lunt, J. Large-scale production, properties and commercial applications of polylactic acid polymers. Polym. Degrad. Stab. 1998, 59, 145–152. [Google Scholar] [CrossRef]
- Enomoto, K.; Ajioka, M.; Yamaguchi, A. Polyhydroxycarboxylic Acid and Preparation Process Thereof. U.S. Patent 5,310,865, 10 May 1994. [Google Scholar]
- Gruber, P.R.; Hall, E.S.; Kolstad, J.J.; Iwen, M.L.; Benson, R.D.; Borchardt, R.L. Continuous Process for Manufacture of Lactide polymers with Controlled Optical Purity. U.S. Patent 5,142,023, 25 August 1992. [Google Scholar]
- Gruber, P.; O’Brien, M. Biopolymer. In Polylactides: NatureWorks® PLA; Wiley-VCH: Weinheim, Germany, 2001; Volume 6, Chapter 8. [Google Scholar]
- Kricheldorf, H.R. Syntheses and application of polylactides. Chemosphere 2001, 43, 49. [Google Scholar] [CrossRef]
- Kowalski, A.; Duda, A.; Penczek, S. Kinetics and mechanicm of cyclic esters polymerization initiated with tin(II) octoate. 3. Polymerization L,L-lactide. Macromolecules 2000, 33, 7359–7370. [Google Scholar] [CrossRef]
- Urbanczyk, L.; Noundjo, F.; Alexandre, M.; Jérôme, C.; Detrembleur, C.; Calberg, C. Synthesis of polylactide/clay nanocomposites by in situ intercalative polymerization in supercritical carbon dioxide. Eur. Polym. J. 2009, 45, 643–648. [Google Scholar] [CrossRef]
- Purnama, P.; Jung, Y.; Hong, C.H.; Han, D.S.; Kim, S.H. Synthesis of poly(D-lactide) with different molecular weight via melt-polymerization. Macromol. Res. 2012, 20, 515–519. [Google Scholar] [CrossRef]
- Dubois, P.; Jacobs, C.; Jérôme, R.; Teyssié, P. Macromolecular engineering of polylactones and polylactides. 4. Mechanism and kinetics of lactide homopolymerization by aluminum isopropoxide. Macromolecules 1991, 24, 2266–2270. [Google Scholar] [CrossRef]
- Mecerreyes, D.; Jerome, R. From living to controlled aluminium alkoxide mediated ring-opening polymerization of (di)lactones, a powerful tool for the macromolecular engineering of aliphatic polyesters. Macromol. Chem. Phys. 1999, 200, 2581. [Google Scholar] [CrossRef]
- Stevels, W.M.; Dijkstra, P.J.; Feijen, J. New initiators for the ring-opening polymerization of cyclic esters. Trends Polym. Sci. 1997, 5, 300. [Google Scholar]
- O’Keefe, B.J.; Monnier, S.M.; Hillmyer, M.A.; Tolman, W.B. Rapid and controlled polymerization of lactide by structurally characterized ferric alkoxides. J. Am. Chem. Soc. 2001, 123, 339. [Google Scholar] [CrossRef]
- Vink, E.T.H.; Rabago, K.R.; Glassner, D.A.; Gruber, P.R. Applications of life cycle assessment to NatureWorksTM polylactide (PLA) production. Polym. Degrad. Stab. 2003, 80, 403–419. [Google Scholar] [CrossRef]
- Anderson, K.S.; Schreck, K.M.; Hillmyer, M.A. Toughening polylactide. Polym. Rev. 2008, 48, 85–108. [Google Scholar] [CrossRef]
- Kaawashima, N.; Ogawa, S.; Obuchi, S.; Matsuo, M.; Yagi, T. Polylactic acid LACEA. In Biopolymers; Doi, Y., Steinbuchel, A., Eds.; Wiley Online Library: Hoboken, NJ, USA, 2002; pp. 251–2744. [Google Scholar]
- Auras, R.; Harte, B.; Slke, S. An overview of polylactide as packaging materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef]
- Bouapao, L.; Tsuji, H.; Tashiro, K.; Zhang, J.; Hanesaka, M. Crystallization, spherulite growth, and structure of blends of crystalline and amorphous poly(lactide)s. Polymer 2009, 50, 4007–4017. [Google Scholar] [CrossRef]
- Klemm, D.; Heinze, T.; Heinze, U.; Edgar, K.J.; Philip, B.; Zugenmaier, P. Comprehensive Cellulose Chemistry, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2009. [Google Scholar]
- Dufresne, A. Nanocellulose: A new ageless biomaterials. Mater. Today 2013, 16, 220–227. [Google Scholar] [CrossRef]
- Gousse, C.; Chanzy, H.; Excoffier, G.; Soubeyrand, L.; Fleury, E. Stable suspension of partially silylated cellulose whiskers dispersed in organic solvents. Polymer 2002, 43, 2645–2651. [Google Scholar] [CrossRef]
- Grunert, M.; Winter, W.T. Nanocomposites of cellulose acetate butyrate reinforced with cellulose Nanocrystals. J. Polym. Environ. 2002, 10, 27–30. [Google Scholar] [CrossRef]
- Habibi, Y.; Goffin, A.L.; Schiltz, N.; Duquesne, E.; Dubois, P.; Dufresne, A. Bionanocomposites based on poly(ε-caprolactone)-grafted cellulose nanocrystals by ring-opening polymerization. J. Mater. Chem. 2008, 18, 5002–5010. [Google Scholar] [CrossRef]
- Azizi Samir, M.A.S.; Alloin, F.; Dufresne, A. Review of recent research into cellulosic whiskers, their properties and their application in nanocomposite field. Biomacromolecules 2005, 6, 612–626. [Google Scholar] [CrossRef]
- Bondeson, D.; Mathew, A.; Oksman, K. Optimization of the isolation of nanocrystals from microcrystalline cellulose by acid hydrolysis. Cellulose 2006, 13, 171–180. [Google Scholar] [CrossRef]
- Beck-Candanedo, S.; Roman, M.; Gray, D.G. Effect of reaction conditions on the properties and behavior of wood cellulose nanocrystal suspensions. Biomacromolecules 2005, 6, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Elazzouzi-Hafraoui, S.; Nishiyama, Y.; Putaux, J.L.; Heux, L.; Dubreuil, F.; Rochas, C. The shape and size distribution nanoparticles prepared by acid hydrolysis of native cellulose. Biomacromolecules 2008, 9, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Goffin, A.-L.; Raquez, J.-M.; Duquesne, E.; Siqueira, G.; Habibi, Y.; Dufresne, A.; Dubois, P. From interfacial ring-opening polymerization to melt processing of cellulose nanowhiskers-filled polylactide-based nanocomposites. Biomacromolecules 2011, 12, 2456–2465. [Google Scholar] [CrossRef] [PubMed]
- Sakurada, I.; Nukushina, Y.; Ito, T. Experimental determination of the elastic modulus of crystalline regions in oriented polymers. J. Polym. Sci. 1962, 57, 651–660. [Google Scholar] [CrossRef]
- Nishino, T.; Takano, K.; Nakamae, K. Acetylation of plant cellulose fiber in supercritical carbon dioxide. J. Polym. Sci. Part B Polym. Phys. 1995, 33, 1647–1651. [Google Scholar] [CrossRef]
- Sturcova, A.; Davies, G.R.; Eichhorn, S.J. Elastic modulus and stress-transfer properties of tunicate cellulose whiskers. Biomacromolecules 2005, 6, 1055–1061. [Google Scholar] [CrossRef]
- Rusli, R.; Eichhorn, S.J. Determination of the stiffness of cellulose nanowhiskers and the fiber-matrix interface in a nanocomposite using raman spectroscopy. Appl. Phys. Lett. 2008, 93, 331111–331113. [Google Scholar] [CrossRef]
- Araki, J.; Wada, M.; Kuga, S. Steric stabilization of a cellulose microcrystal suspension by poly(ethylene glycol) grafting. Langmuir 2001, 17, 21–27. [Google Scholar] [CrossRef]
- Zhu, L.; Qiu, J.; Liu, W.; Sakai, E. Mechanical and thermal properties of rice Straw/PLA modified by nano attapulgite/PLA interfacial layer. Compos. Commun. 2019, 13, 18–21. [Google Scholar] [CrossRef]
- Dufresne, A. Interfacial phenomena in nanocomposites based on polysaccharide nanocrystals. Compos. Interfaces 2003, 10, 369–387. [Google Scholar] [CrossRef]
- Ren, Z.; Guo, R.; Bi, H.; Jia, X.; Xu, M.; Cai, L. Interfacial Adhesion of Polylactic Acid on Cellulose Surface: A Molecular Dynamics Study. ACS Appl. Mater. Interfaces 2020, 12, 3236–3244. [Google Scholar] [CrossRef] [PubMed]
- Eichhorn, S.J. Cellulose nanowhiskers: Promising materials for advanced applications. Soft Matter 2011, 7, 303–315. [Google Scholar] [CrossRef]
- Tomé, L.C.; Pinto, R.J.B.; Trovatti, E.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P.; Gandini, A. Transparent bionanocomposites with improved properties prepared from acetylated bacterial cellulose and poly(lactic acid) through a simple approach. Green Chem. 2011, 13, 419–427. [Google Scholar] [CrossRef]
- Siqueira, G.; Bras, J.; Dufresne, A. New process of chemical grafting of cellulose nanoparticles with a long chain isocyanate. Langmuir 2010, 26, 402–411. [Google Scholar] [CrossRef]
- Tingaut, P.; Zimmermann, T.; Lopez-Suevos, F. Syntheis and characterization of bionanocomposites with tunable properties from poly(lactic acid) and acetylated microfibrillated cellulose. Biomacromolecules 2010, 11, 454–464. [Google Scholar] [CrossRef]
- Braun, B.; Dorgan, J.R. Single-step method for the isolation and surface functionalization of cellulosic nanowhiskers. Biomacromolecules 2009, 2, 334–341. [Google Scholar] [CrossRef]
- Bondeson, D.; Oksman, K. Polylactic acid/cellulose whisker nanocomposites modified by polyvinyl alcohol. Compos. Part A 2007, 38, 2486–2492. [Google Scholar] [CrossRef]
- Shojaeiarani, J.; Bajwa, D.S.; Stark, N.M. Green esterification: A new approach to improve thermal and mechanical properties of poly(lactic acid) composites reinforced by cellulose nanocrystals. J. Appl. Polym. Sci. 2018, 135, 46468. [Google Scholar] [CrossRef]
- Shojaeiarani, J.; Bajwa, D.S.; Hartman, K. Esterified cellulose nanocrystals as reinforcement in poly(lactic acid) nanocomposites. Cellulose 2019, 26, 2349–2362. [Google Scholar] [CrossRef]
- Wu, H.; Nagarajan, S.; Shu, J.; Zhang, T.; Zhou, L.; Duan, Y.; Zhang, J. Green and facile surface modification of cellulose nanocrystal as the route to produce poly(lactic acid) nanocomposites with improved properties. Carbohydr. Polym. 2018, 197, 204–214. [Google Scholar] [CrossRef]
- Zhou, L.; Ke, K.; Yang, M.-B.; Yang, W. Recent progress on chemical modification of cellulose for high mechanical-performance Poly(lactic acid)/Cellulose composite: A short review. Compos. Commun. 2020, 23, 100548. [Google Scholar] [CrossRef]
- Riedel, U.; Nickel, J. Natural fibre-reinforced biopolymers as construction materials-new discoveries. Die Angew. Macromol. Chem. 1999, 272, 34–40. [Google Scholar] [CrossRef]
- Oksman, K.; Skrifvars, M.; Selin, J.F. Natural fiber as reinforcement in polylactic acid (PLA) composites. Compos. Sci. Technol. 2003, 9, 1317–1324. [Google Scholar] [CrossRef]
- Braun, B.; Dorgan, J.R.; Knauss, D.M. Reactively compatibilized cellulosic polylactide microcomposites. J. Polym. Environ. 2006, 1, 49–58. [Google Scholar] [CrossRef]
- Favier, V.; Cavaille, J.Y.; Canova, G.R.; Shrivastava, S.C. Mechanical percolation in cellulose whisker nanocomposites. Polym. Eng. Sci. 1997, 10, 1732–1739. [Google Scholar] [CrossRef]
- De Paula, E.L.; Mano, V.; Pereira, F.V. Influence of cellulose nanowhiskers on the hydrolytic degradation behavior of poly(D,L-lactide). Polym. Degrad. Stab. 2011, 96, 1631–1638. [Google Scholar] [CrossRef]
- Mukherjee, T.; Tobin, M.J.; Puskar, L.; Sani, M.-A.; Kao, N.; Gupta, R.K.; Pannirselvam, M.; Quazi, N.; Bhattacharya, S. Chemically imaging the interaction of acetylated nanocrystalline cellulose (NCC) with a polylactic acid (PLA) polymer matrix. Cellulose 2017, 24, 1717–1729. [Google Scholar] [CrossRef]
- Yu, H.Y.; Zhang, H.; Song, M.L.; Zhou, Y.; Yao, J.; Ni, Q.Q. From Cellulose Nanospheres, Nanorods to Nanofibers: Various Aspect Ratio Induced Nucleation/Reinforcing Effects on Polylactic Acid for Robust-Barrier Food Packaging. ACS Appl. Mater. Interfaces 2017, 9, 43920–43938. [Google Scholar] [CrossRef]
- Gwon, J.-G.; Cho, H.-J.; Chun, S.-J.; Lee, S.; Wu, Q.; Lee, S.-Y. Physiochemical, optical and mechanical properties of poly(lactic acid) nanocomposites filled with toluene diisocyanate grafted cellulose nanocrystals. RSC Adv. 2016, 6, 9438–9445. [Google Scholar] [CrossRef]
- Gwon, J.-G.; Cho, H.-J.; Chun, S.-J.; Lee, S.; Wu, Q.; Li, M.; Lee, S.-Y. Mechanical and thermal properties of toluene diisocyanate-modified cellulose nanocrystal nanocomposites using semi-crystalline poly(lactic acid) as base matrix. RSC Adv. 2016, 6, 73879–73886. [Google Scholar] [CrossRef]
- Yin, Y.; Zhao, L.; Jiang, X.; Wang, H.; Gao, W. Cellulose nanocrystals modified with a triazine derivative and their reinforcement of poly(lactic acid)-based bionanocomposites. Cellulose 2018, 25, 2965–2976. [Google Scholar] [CrossRef]
- Jin, K.; Tang, Y.; Zhu, X.; Zhou, Y. Polylactic acid based biocomposite films reinforced with silanized nanocrystalline cellulose. Int. J. Biol. Macromol. 2020, 162, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Dhar, P.; Tarafder, D.; Kumar, A.; Katiyar, V. Thermally recyclable polylactic acid/cellulose nanocrystal films through reactive extrusion process. Polymer 2016, 87, 268–282. [Google Scholar] [CrossRef]
- Dhar, P.; Gaur, S.S.; Soundararajan, N.; Gupta, A.; Bhasney, S.M.; Milli, M.; Kumar, A.; Katiyar, V. Reactive Extrusion of Polylactic Acid/Cellulose Nanocrystal Films for Food Packaging Applications: Influence of Filler Type on Thermomechanical, Rheological, and Barrier Properties. Ind. Eng. Chem. Res. 2017, 56, 4718–4735. [Google Scholar] [CrossRef]
- Miao, C.; Hamad, W.Y. In-situ polymerized cellulose nanocrystals (CNC)-poly(l-lactide) (PLLA) nanomaterials and applications in nanocomposite processing. Carbohydr. Polym. 2016, 153, 549–558. [Google Scholar] [CrossRef]
- Mujica-Garcia, A.; Hooshmand, S.; Skrifvars, M.; Kenny, J.M.; Oksman, K.; Peponi, L. Poly(lactic acid) melt-spun fibers reinforced with functionalized cellulose nanocrystals. RSC Adv. 2016, 6, 9221–9231. [Google Scholar] [CrossRef]
- Tsuji, H.; Ikada, Y. Stereocomplex formation between enantiomeric poly(lactic acid)s. 6. Binary blends from copolymers. Macromolecules 1992, 25, 5719–5723. [Google Scholar] [CrossRef]
- Yamane, H.; Sasai, K. Effect of the addition of poly(D-lactic acid) on the termal property of poly(L-actic acid). Polymer 2003, 44, 2569–2575. [Google Scholar] [CrossRef]
- Fukushima, K.; Chang, Y.H.; Kimura, Y. Enhanced stereocomplex formation of poly(L-lactic acid) and poly(D-lactic acid) in the presence of stereoblock poly(lactic acid). Macromol. Biosci. 2007, 7, 829–835. [Google Scholar] [CrossRef]
- Tsuji, H.; Ikada, Y. Stereocomplex formation between enantiomeric poly(lactic acid)s. 9. Stereocomplexation from the melt. Macromolecules 1993, 26, 6918–6926. [Google Scholar] [CrossRef]
- Fukushima, K.; Kimura, Y. An efficient solid-state polycondensation method for synthesizing stereocomplexed poly(lactic acid)s with high molecular weight. J. Polym. Sci. A Polym. Chem. 2008, 46, 3714–3722. [Google Scholar] [CrossRef]
- Anderson, K.S.; Hillmyer, M.A. Melt preparation and nucleation efficiency of polylactide stereocomplex crystallites. Polymer 2006, 47, 2030–2035. [Google Scholar] [CrossRef]
- Purnama, P.; Kim, S.H. Rapid stereocomplex formation of polylactide using supercritical fluid technology. Polym. Int. 2012, 61, 939–942. [Google Scholar] [CrossRef]
- Purnama, P.; Kim, S.H. Stereocomplex formation of polylactide using microwave irradiation. Polym. Int. 2014, 63, 741–745. [Google Scholar] [CrossRef]
- Tsuji, H.; Ikada, Y. Stereocomplex formation between enantiomeric poly(lactic acid)s. XI. Mechanical properties and morphology of solution-cast films. Polymer 1999, 40, 6699–6708. [Google Scholar] [CrossRef]
- Dorgan, J.R.; Braun, B. Polymer Composites Incorporating Stereocomplexation. US Patent 2011/0319509 A1, 29 December 2011. [Google Scholar]
- Habibi, Y.; Aouadi, S.; Raquez, J.-M.; Dubois, P. Effect of interfacial stereocomplexation in cellulose nanocrystal-filled polylactide nanocomposites. Cellulose 2013, 20, 2877–2885. [Google Scholar] [CrossRef]
- Wu, H.; Nagarajan, S.; Zhou, L.; Duan, Y.; Zhang, J. Synthesis and characterization of cellulose nanocrystal-graft-poly(D-lactide) and its nanocomposite with poly(L-lactide). Polymer 2016, 103, 365–375. [Google Scholar] [CrossRef]
- Cao, X.; Wang, Y.; Chen, H.; Hu, J.; Cui, L. Preparation of different morphologies cellulose nanocrystals from waste cotton fibers and its effect on PLLA/PDLA composites films. Compos. Part B 2021, 217, 108934. [Google Scholar] [CrossRef]
- Raquez, J.-M.; Murena, Y.; Goffin, A.-L.; Habibi, Y.; Ruelle, B.; DeBuyl, F.; Dubois, P. Surface-modification of cellulose nanowhiskers and their use as nanoreinforcers into polylactide: A sustainability-integrated approach. Compos. Sci. Technol. 2012, 72, 544–549. [Google Scholar] [CrossRef]
- Biela, T.; Duda, A.; Penczek, S. Enhanced melt stability of star-shaped stereocomplexes as compared with linear stereocomplexes. Macromolecules 2006, 39, 3710–3713. [Google Scholar] [CrossRef]
- Tsuji, H. Poly(lactide) stereocomplex: Formation, structure, properties, degradation, and applications. Macromol. Biosci. 2005, 5, 569–597. [Google Scholar] [CrossRef] [PubMed]
- Purnama, P.; Jung, Y.; Kim, S.H. Melt stability of 8-arms star-shaped stereocomplex polylactide with three-dimensional core structures. Polym. Degrad. Stab. 2013, 96, 1097–1101. [Google Scholar] [CrossRef]
- Oksman, K.; Mathew, A.P.; Sain, M. Novel bionanocomposites: Processing, properties and potential applications. Plast. Rubber Compos. 2009, 38, 396–405. [Google Scholar] [CrossRef]
- Spunella, S.; Samuel, C.; Raquez, J.-M.; McCallum, S.A.; Gross, R.; Dubois, P. Green and efficient sytnthesis of dispersible cellulose nanocrystals in biobased polyesters for engineering applications. ACS Sustain. Chem. Eng. 2016, 4, 2517–2527. [Google Scholar] [CrossRef]
- Sucinda, E.F.; Abdul Majid, M.S.; Ridzuan, M.J.M.; Cheng, E.M.; Alshahrani, H.A. Development and characterization of packaging film from napier cellulose nanowhisker reinforced polylactic acid (PLA) bionanocomposites. Int. J. Biol. Macromol. 2021, 18, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Pal, N.; Banerjee, S.; Roy, P.; Pal, K. Melt-blending of unmodified and modified cellulose nanocrystals with reduced graphene oxide into PLA matrix for biomedical application. Polym. Adv. Technol. 2019, 30, 3049–3060. [Google Scholar] [CrossRef]
- Pal, N.; Banerjee, S.; Roy, P.; Pal, K. Reduced graphene oxide and PEG-grafted TEMPO-oxidized cellulose nanocrystal reinforced poly-lactic acid nanocomposite film for biomedical application. Mater. Sci. Eng. C 2019, 104, 109956. [Google Scholar] [CrossRef]




| Properties | PLLA | PS | PET |
|---|---|---|---|
| Density (kg m−3) | 1.26 | 1.05 | 1.40 |
| Tensile strength (MPa) | 59 | 45 | 57 |
| Elastic modulus (GPa) | 3.8 | 3.2 | 2.8–4.1 |
| Elongation at break (%) | 4–7 | 3 | 300 |
| Notched izod (J m−1) | 26 | 21 | 59 |
| Heat deflection (°C) | 55 | 75 | 67 |
| Polymer Matrix | CNW Modifications/Synthesis | Nanocomposites Processing | Properties Improvement | Ref. (Year) |
|---|---|---|---|---|
| PLA (Mw = 100,000 g/mol) | Acid hydrolysis from cotton fabric | Solution casting in chloroform/N,N’dimethylformamide | Thermal stability, tensile strength, and Young’s modulus | [22] (2021) |
| PLA (Mn = 130,000 g/mol) | Acid hydrolyzed from cellulose | Solution casting in chloroform | Water permeability and oxygen permeability | [26] (2010) |
| PLA | Acid hydrolyzed from flax cellulose | Solution process in N,N’dimethylformamide to form nanofibrous mat | Crystallinity and water absorption | [27] (2018) |
| PDLLA | Acid hydrolyzed from eucalyptus kraft wood pulp | Solution casting in dimethylformamide | Hydrolytic degradation, thermal stability | [81] (2011) |
| PLA | Acetylation CNW | Solution polymerization Bulk Polymerization Solution Blending | Thermal stability and Crystallinity | [18] (2012) |
| PLA (Mw = 52,000 g/mol) | Acetylation using acetic anhydride | Solution casting in chloroform | Tensile strength, thermal stability, dimensional stability, and dynamic mechanical properties | [25] (2014) |
| PLA (Mw = 180,000 g/mol) | Acetylation CNW | Solution casting in dichloromethane | Stress transfer between CNW and PLA matrix | [82] (2017) |
| PLA (Mn = 1.0 × 105 g/mol) | Surface esterification by formic acid | Solution casting in chloroform | Barrier properties | [83] (2017) |
| PLA (Mw = 87,000 g/mol) | Grafted toluene diisocyanate | Solution casting in chloroform | Tensile strength | [84,85] (2016) |
| PLA (Mw = 100,000 g/mol) | Surface modification by triazine derivative | Hot compression process 170oC 40 MPa | Breaking strength, elongation, compatibility, and thermal properties | [23,86] (2017, 2018) |
| PLA (Mn = 98,000 g/mol; Mw = 199,590 g/mol) | Surface esterification by benzoic acid | Masterbatch followed by extrusion process | The Young’s modulus and ultimate tensile stress | [73] (2018) |
| PLA (Mn = 98,000 g/mol; Mw = 199,590 g/mol) | Surface esterification by valeric acid | Masterbatch followed by extrusion process | Thermal decomposition, mechanical properties, and crystallinity growth | [74] (2019) |
| PLA (Mw = 2.1 × 105 g/mol) | Graft modification by 3-aminopropyltriethoxysilane | Solution casting in dichloromethane | Air permeability, light resistance, thermal stability, and mechanical properties | [87] (2020) |
| PLA (Mn = 150,000 g/mol) | Addition radical initiator with dicumyl peroxide | Reactive extrusion by Twin-screw extruder | Mechanical properties, crystallinity. processability, melt-strength, rheological behavior | [88,89] (2016, 2017) |
| PLLA (Mw = 100,000 g/mol) | Grafted lactic acid | Solution casting in chloroform | Tensile strength and Young’s modulus | [75] (2018) |
| PLA (Mn = 95,000 g/mol) PLA (Mn = 130,000 g/mol) | Grafting PLLA by ring-opening polymerization in toluene | Melt-blending in mini extruder | Compatibility, thermal behavior, and mechanical properties | [58] (2011) |
| PLA | Grafting PLLA by ring-opening polymerization in toluene | Twin-screw extruder | Thermal, mechanical, optical, and morphological properties | [84] (2016) |
| PLA | Grafting PLLA by ring-opening polymerization in dimethyl sulfoxide | Solution casting and co-extrusion | Barrier and dynamic mechanical properties | [90] (2016) |
| PLA (Mn = 110,000 g/mol) | Grafting PLLA by ring-opening polymerization in toluene | Melt spinning using twin-screw micro-compounder | Thermal stability, degree of crystallinity, and mechanical properties | [91] (2016) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Purnama, P.; Samsuri, M.; Iswaldi, I. A Review on Fully Bio-Based Materials Development from Polylactide and Cellulose Nanowhiskers. Polymers 2022, 14, 4009. https://doi.org/10.3390/polym14194009
Purnama P, Samsuri M, Iswaldi I. A Review on Fully Bio-Based Materials Development from Polylactide and Cellulose Nanowhiskers. Polymers. 2022; 14(19):4009. https://doi.org/10.3390/polym14194009
Chicago/Turabian StylePurnama, Purba, Muhammad Samsuri, and Ihsan Iswaldi. 2022. "A Review on Fully Bio-Based Materials Development from Polylactide and Cellulose Nanowhiskers" Polymers 14, no. 19: 4009. https://doi.org/10.3390/polym14194009
APA StylePurnama, P., Samsuri, M., & Iswaldi, I. (2022). A Review on Fully Bio-Based Materials Development from Polylactide and Cellulose Nanowhiskers. Polymers, 14(19), 4009. https://doi.org/10.3390/polym14194009








