In Vivo Bone Tissue Engineering Strategies: Advances and Prospects
Abstract
1. Introduction
2. Recent Developments in Bone Tissue Engineering
2.1. Scaffolds in Bone Tissue Engineering
2.1.1. Natural Polymers
2.1.2. Synthetic Polymers
2.1.3. Bioceramic and Bioglass Scaffolds
2.1.4. Metallic Scaffolds
2.1.5. Biologic Scaffolds
2.1.6. Composite Scaffolds
2.2. Cellular Approach in BTE
2.3. Growth Factors in Bone Tissue Engineering
2.4. In Vitro Vascularization Strategies in Bone Tissue Engineering
2.5. Bioreactors for In Vitro Bone Tissue Engineering
3. In Vivo Bone Tissue Engineering Advances
3.1. Historical and Terminological Aspects of Flap Prefabrication
3.2. In Vivo Bioreactor Approach to BTE: Experimental Studies
3.2.1. Scaffolds for In Vivo BTE
3.2.2. In Vivo Vascularization and Prefabrication Strategies in BTE
3.3. In Vivo Bioreactor Approach to BTE: Clinical Application
4. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vidal, L.; Kampleitner, C.; Brennan, M.Á.; Hoornaert, A.; Layrolle, P. Reconstruction of Large Skeletal Defects: Current Clinical Therapeutic Strategies and Future Directions Using 3D Printing. Front. Bioeng. Biotechnol. 2020, 8, 61. [Google Scholar] [CrossRef]
- Toogood, P.; Miclau, T. Critical-Sized Bone Defects: Sequence and Planning. J. Orthop. Trauma 2017, 31 (Suppl. S5), S23–S26. [Google Scholar] [CrossRef]
- Nkenke, E.; Neukam, F.W. Autogenous bone harvesting and grafting in advanced jaw resorption: Morbidity, resorption and implant survival. Eur. J. Oral Implantol. 2014, 7 (Suppl. S2), S203–S217. [Google Scholar] [PubMed]
- Barone, A.; Ricci, M.; Mangano, F.; Covani, U. Morbidity associated with iliac crest harvesting in the treatment of maxillary and mandibular atrophies: A 10-year analysis. J. Oral Maxillofac. Surg. 2011, 69, 2298–2304. [Google Scholar] [CrossRef]
- Ritschl, L.M.; Mücke, T.; Hart, D.; Unterhuber, T.; Kehl, V.; Wolff, K.D.; Fichter, A.M. Retrospective analysis of complications in 190 mandibular resections and simultaneous reconstructions with free fibula flap, iliac crest flap or reconstruction plate: A comparative single centre study. Clin. Oral Investig. 2021, 25, 2905–2914. [Google Scholar] [CrossRef]
- Wu, C.C.; Lin, P.Y.; Chew, K.Y.; Kuo, Y.R. Free tissue transfers in head and neck reconstruction: Complications, outcomes and strategies for management of flap failure: Analysis of 2019 flaps in single institute. Microsurgery 2014, 34, 339–344. [Google Scholar] [CrossRef]
- Ling, X.F.; Peng, X. What is the price to pay for a free fibula flap? A systematic review of donor-site morbidity following free fibula flap surgery. Plast. Reconstr. Surg. 2012, 129, 657–674. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.F.; Chen, Y.M.; Gokavarapu, S.; Shen, Q.C.; Ji, T. Free flap reconstruction for patients aged 85 years and over with head and neck cancer: Clinical considerations for comprehensive care. Br. J. Oral Maxillofac. Surg. 2017, 55, 793–797. [Google Scholar] [CrossRef]
- Al-Moraissi, E.A.; Alkhutari, A.S.; Abotaleb, B.; Altairi, N.H.; Del Fabbro, M. Do osteoconductive bone substitutes result in similar bone regeneration for maxillary sinus augmentation when compared to osteogenic and osteoinductive bone grafts? A systematic review and frequentist network meta-analysis. Int. J. Oral Maxillofac. Surg. 2020, 49, 107–120. [Google Scholar] [CrossRef]
- Darwich, K.; Ismail, M.B.; Al-Mozaiek, M.Y.A.; Alhelwani, A. Reconstruction of mandible using a computer-designed 3D-printed patient-specific titanium implant: A case report. Oral Maxillofac. Surg. 2021, 25, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.D.; Khechoyan, D.Y.; Phillips, J.H.; Forrest, C.R. Custom CAD/CAM implants for complex craniofacial reconstruction in children: Our experience based on 136 cases. J. Plast. Reconstr. Aesthet. Surg. 2018, 71, 1609–1617. [Google Scholar] [CrossRef] [PubMed]
- Almansoori, A.A.; Choung, H.W.; Kim, B.; Park, J.Y.; Kim, S.M.; Lee, J.H. Fracture of Standard Titanium Mandibular Reconstruction Plates and Preliminary Study of Three-Dimensional Printed Reconstruction Plates. J. Oral Maxillofac. Surg. 2020, 78, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Hirohata, H.; Yanagawa, T.; Takaoka, S.; Yamagata, K.; Sasaki, K.; Shibuya, Y.; Uchida, F.; Fukuzawa, S.; Tabuchi, K.; Hasegawa, S.; et al. A small number of residual teeth after the mandibular resection of oral cancer is associated with titanium reconstruction plate exposure. Clin. Exp. Dent. Res. 2019, 5, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Tack, P.; Victor, J.; Gemmel, P.; Annemans, L. 3D-printing techniques in a medical setting: A systematic literature review. Biomed. Eng. Online 2016, 15, 115. [Google Scholar] [CrossRef]
- Thakeb, M.F.; Fayyad, T.A.; ElGebeily, M.A.; Diab, R.A.; El Zahlawy, H.; Sharafeldin, M.S.; Al Kersh, M.A. Bifocal Compression-Distraction for Combined Bone and Soft-Tissue Defects in Post-traumatic Tibial Nonunion. J. Orthop. Trauma 2019, 33, e372–e377. [Google Scholar] [CrossRef]
- Goldstein, J.A.; Paliga, J.T.; Taylor, J.A.; Bartlett, S.P. Complications in 54 frontofacial distraction procedures in patients with syndromic craniosynostosis. J. Craniofacial Surg. 2015, 26, 124–128. [Google Scholar] [CrossRef]
- Kumar, P.; Saini, M.; Dehiya, B.S.; Sindhu, A.; Kumar, V.; Kumar, R.; Lamberti, L.; Pruncu, C.I.; Thakur, R. Comprehensive Survey on Nanobiomaterials for Bone Tissue Engineering Applications. Nanomaterials 2020, 10, 2019. [Google Scholar] [CrossRef]
- Al-Harbi, N.; Mohammed, H.; Al-Hadeethi, Y.; Bakry, A.S.; Umar, A.; Hussein, M.A.; Abbassy, M.A.; Vaidya, K.G.; Berakdar, G.A.; Mkawi, E.M.; et al. Silica-Based Bioactive Glasses and Their Applications in Hard Tissue Regeneration: A Review. Pharmaceuticals 2021, 14, 75. [Google Scholar] [CrossRef]
- Shahabipour, F.; Ashammakhi, N.; Oskuee, R.K.; Bonakdar, S.; Hoffman, T.; Shokrgozar, M.A.; Khademhosseini, A. Key components of engineering vascularized 3-dimensional bioprinted bone constructs. Transl. Res. 2019, 216, 57–76. [Google Scholar] [CrossRef]
- Kohli, N.; Sharma, V.; Orera, A.; Sawadkar, P.; Owji, N.; Frost, O.G.; Bailey, R.J.; Snow, M.; Knowles, J.C.; Blunn, G.W.; et al. Pro-angiogenic and osteogenic composite scaffolds of fibrin, alginate and calcium phosphate for bone tissue engineering. J. Tissue Eng. 2021, 12, 20417314211005610. [Google Scholar] [CrossRef]
- Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Bone regeneration: Current concepts and future directions. BMC Med. 2011, 9, 66. [Google Scholar] [CrossRef]
- Fu, R.; Liu, C.; Yan, Y.; Li, Q.; Huang, R.L. Bone defect reconstruction via endochondral ossification: A developmental engineering strategy. J. Tissue Eng. 2021, 12, 20417314211004211. [Google Scholar] [CrossRef] [PubMed]
- Noori, A.; Ashrafi, S.J.; Vaez-Ghaemi, R.; Hatamian-Zaremi, A.; Webster, T.J. A review of fibrin and fibrin composites for bone tissue engineering. Int. J. Nanomed. 2017, 12, 4937–4961. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zhu, S.; Mei, D.; Li, J.; Zhang, J.; Yang, S.; Guan, S. Application of 3D Printing Technology in Bone Tissue Engineering: A Review. Curr. Drug Deliv. 2021, 18, 847–861. [Google Scholar] [CrossRef]
- Novais, A.; Chatzopoulou, E.; Chaussain, C.; Gorin, C. The Potential of FGF-2 in Craniofacial Bone Tissue Engineering: A Review. Cells 2021, 10, 932. [Google Scholar] [CrossRef]
- Black, C.R.; Goriainov, V.; Gibbs, D.; Kanczler, J.; Tare, R.S.; Oreffo, R.O. Bone Tissue Engineering. Curr. Mol. Biol. Rep. 2015, 1, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Shadjou, N.; Hasanzadeh, M.; Khalilzadeh, B. Graphene based scaffolds on bone tissue engineering. Bioengineered 2017, 9, 38–47. [Google Scholar] [CrossRef]
- Park, J.Y.; Park, S.H.; Kim, M.G.; Park, S.H.; Yoo, T.H.; Kim, M.S. Biomimetic Scaffolds for Bone Tissue Engineering. Adv. Exp. Med. Biol. 2018, 1064, 109–121. [Google Scholar] [CrossRef]
- Yin, S.; Zhang, W.; Zhang, Z.; Jiang, X. Recent Advances in Scaffold Design and Material for Vascularized Tissue-Engineered Bone Regeneration. Adv. Healthc. Mater. 2019, 8, e1801433. [Google Scholar] [CrossRef]
- Wubneh, A.; Tsekoura, E.K.; Ayranci, C.; Uludağ, H. Current state of fabrication technologies and materials for bone tissue engineering. Acta Biomater. 2018, 80, 1–30. [Google Scholar] [CrossRef]
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for Bone Tissue Engineering: State of the art and new perspectives. Mater. Sci. Eng. C 2017, 78, 1246–1262. [Google Scholar] [CrossRef] [PubMed]
- Bajuri, M.Y.; Selvanathan, N.; Dzeidee Schaff, F.N.; Abdul Suki, M.H.; Ng, A.M.H. Tissue-Engineered Hydroxyapatite Bone Scaffold Impregnated with Osteoprogenitor Cells Promotes Bone Regeneration in Sheep Model. Tissue Eng. Regen. Med. 2021, 18, 377–385. [Google Scholar] [CrossRef]
- Kruyt, M.C.; van Gaalen, S.M.; Oner, F.C.; Verbout, A.J.; de Bruijn, J.D.; Dhert, W.J. Bone tissue engineering and spinal fusion: The potential of hybrid constructs by combining osteoprogenitor cells and scaffolds. Biomaterials 2004, 25, 1463–1473. [Google Scholar] [CrossRef]
- Paschos, N.K.; Brown, W.E.; Eswaramoorthy, R.; Hu, J.C.; Athanasiou, K.A. Advances in tissue engineering through stem cell-based co-culture. J. Tissue Eng. Regen. Med. 2015, 9, 488–503. [Google Scholar] [CrossRef] [PubMed]
- Walmsley, G.G.; Ransom, R.C.; Zielins, E.R.; Leavitt, T.; Flacco, J.S.; Hu, M.S.; Lee, A.S.; Longaker, M.T.; Wan, D.C. Stem Cells in Bone Regeneration. Stem Cell Rev. Rep. 2016, 12, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Yin, G.; Huang, Z.; Liao, X.; Chen, X.; Yao, Y.; Pu, X. Localized delivery of growth factors for angiogenesis and bone formation in tissue engineering. Int. Immunopharmacol. 2013, 16, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Liu, X.; Zhu, M.; Luo, G.; Sun, T.; Peng, Q.; Zeng, Y.; Chen, T.; Wang, Y.; Liu, K.; et al. Hybrid use of combined and sequential delivery of growth factors and ultrasound stimulation in porous multilayer composite scaffolds to promote both vascularization and bone formation in bone tissue engineering. J. Biomed. Mater. Res. A 2016, 104, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Hsu, E.L.; Stock, S.R. Growth Factors, Carrier Materials, and Bone Repair. Handb. Exp. Pharmacol. 2020, 262, 121–156. [Google Scholar] [CrossRef] [PubMed]
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided bone regeneration: Materials and biological mechanisms revisited. Eur. J. Oral Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef]
- Oryan, A.; Alidadi, S.; Moshiri, A. Platelet-rich plasma for bone healing and regeneration. Expert Opin. Biol. Ther. 2016, 16, 213–232. [Google Scholar] [CrossRef]
- Simunovic, F.; Finkenzeller, G. Vascularization Strategies in Bone Tissue Engineering. Cells 2021, 10, 1749. [Google Scholar] [CrossRef] [PubMed]
- Filipowska, J.; Tomaszewski, K.A.; Niedźwiedzki, Ł.; Walocha, J.A.; Niedźwiedzki, T. The role of vasculature in bone development, regeneration and proper systemic functioning. Angiogenesis 2017, 20, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Barabaschi, G.D.; Manoharan, V.; Li, Q.; Bertassoni, L.E. Engineering Pre-vascularized Scaffolds for Bone Regeneration. Adv. Exp. Med. Biol. 2015, 881, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Wang, M.; He, J. A review of biomimetic scaffolds for bone regeneration: Toward a cell-free strategy. Bioeng. Transl. Med. 2020, 6, e10206. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.L.; Lee, S.S.; Rangasamy, J.; Hwang, N.S. Biomimetic Materials and Fabrication Approaches for Bone Tissue Engineering. Adv. Healthc. Mater. 2017, 6, 1700612. [Google Scholar] [CrossRef]
- Bose, S.; Roy, M.; Bandyopadhyay, A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012, 30, 546–554. [Google Scholar] [CrossRef]
- Pan, H.; Jiang, H.; Chen, W. The biodegradability of electrospun Dextran/PLGA scaffold in a fibroblast/macrophage co-culture. Biomaterials 2008, 29, 1583–1592. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Kawazoe, N. Porous Scaffolds for Regeneration of Cartilage, Bone and Osteochondral Tissue. Adv. Exp. Med. Biol. 2018, 1058, 171–191. [Google Scholar] [CrossRef] [PubMed]
- Poh, P.S.P.; Valainis, D.; Bhattacharya, K.; van Griensven, M.; Dondl, P. Optimization of Bone Scaffold Porosity Distributions. Sci. Rep. 2019, 9, 9170. [Google Scholar] [CrossRef] [PubMed]
- Koroleva, A.; Deiwick, A.; Nguyen, A.; Schlie-Wolter, S.; Narayan, R.; Timashev, P.; Popov, V.; Bagratashvili, V.; Chichkov, B. Osteogenic differentiation of human mesenchymal stem cells in 3-D Zr-Si organic-inorganic scaffolds produced by two-photon polymerization technique. PLoS ONE 2015, 10, e0118164. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Currier, T.; Edraki, M.; Liu, B.; Lynch, M.E.; Modarres-Sadeghi, Y. Flow inside a bone scaffold: Visualization using 3D phase contrast MRI and comparison with numerical simulations. J. Biomech. 2021, 126, 110625. [Google Scholar] [CrossRef]
- Garot, C.; Bettega, G.; Picart, C. Additive Manufacturing of Material Scaffolds for Bone Regeneration: Toward Application in the Clinics. Adv. Funct. Mater. 2020, 31, 2006967. [Google Scholar] [CrossRef]
- Dai, Z.; Ronholm, J.; Tian, Y.; Sethi, B.; Cao, X. Sterilization techniques for biodegradable scaffolds in tissue engineering applications. J. Tissue Eng. 2016, 7, 2041731416648810. [Google Scholar] [CrossRef]
- Santos-Rosales, V.; Magariños, B.; Starbird, R.; Suárez-González, J.; Fariña, J.B.; Alvarez-Lorenzo, C.; García-González, C.A. Supercritical CO2 technology for one-pot foaming and sterilization of polymeric scaffolds for bone regeneration. Int. J. Pharm. 2021, 605, 120801. [Google Scholar] [CrossRef]
- Arnold, A.M.; Holt, B.D.; Daneshmandi, L.; Laurencin, C.T.; Sydlik, S.A. Phosphate graphene as an intrinsically osteoinductive scaffold for stem cell-driven bone regeneration. Proc. Natl. Acad. Sci. USA 2019, 116, 4855–4860. [Google Scholar] [CrossRef]
- Kuttappan, S.; Mathew, D.; Nair, M.B. Biomimetic composite scaffolds containing bioceramics and collagen/gelatin for bone tissue engineering—A mini review. Int. J. Biol. Macromol. 2016, 93 Pt B, 1390–1401. [Google Scholar] [CrossRef]
- Xing, F.; Chi, Z.; Yang, R.; Xu, D.; Cui, J.; Huang, Y.; Zhou, C.; Liu, C. Chitin-hydroxyapatite-collagen composite scaffolds for bone regeneration. Int. J. Biol. Macromol. 2021, 184, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Dhivya, S.; Ajita, J.; Selvamurugan, N. Metallic Nanomaterials for Bone Tissue Engineering. J. Biomed. Nanotechnol. 2015, 11, 1675–1700. [Google Scholar] [CrossRef] [PubMed]
- Cockerill, I.; Su, Y.; Sinha, S.; Qin, Y.X.; Zheng, Y.; Young, M.L.; Zhu, D. Porous zinc scaffolds for bone tissue engineering applications: A novel additive manufacturing and casting approach. Mater. Sci. Eng. C 2020, 110, 110738. [Google Scholar] [CrossRef]
- Costa, A.; Naranjo, J.D.; Londono, R.; Badylak, S.F. Biologic Scaffolds. Cold Spring Harb. Perspect. Med. 2017, 7, a025676. [Google Scholar] [CrossRef] [PubMed]
- Freeman, F.E.; Browe, D.C.; Nulty, J.; Von Euw, S.; Grayson, W.L.; Kelly, D.J. Biofabrication of multiscale bone extracellular matrix scaffolds for bone tissue engineering. Eur. Cell Mater. 2019, 38, 168–187. [Google Scholar] [CrossRef]
- Fu, J.N.; Wang, X.; Yang, M.; Chen, Y.R.; Zhang, J.Y.; Deng, R.H.; Zhang, Z.N.; Yu, J.K.; Yuan, F.Z. Scaffold-Based Tissue Engineering Strategies for Osteochondral Repair. Front. Bioeng. Biotechnol. 2022, 9, 812383. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, G.; Liang, H.; Gao, C.; Peng, S.; Shen, L.; Shuai, C. Additive manufacturing of bone scaffolds. Int. J. Bioprint. 2018, 5, 148. [Google Scholar] [CrossRef]
- Zhou, Y.; Chyu, J.; Zumwalt, M. Recent Progress of Fabrication of Cell Scaffold by Electrospinning Technique for Articular Cartilage Tissue Engineering. Int. J. Biomater. 2018, 2018, 1953636. [Google Scholar] [CrossRef]
- Matai, I.; Kaur, G.; Seyedsalehi, A.; McClinton, A.; Laurencin, C.T. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials 2020, 226, 119536. [Google Scholar] [CrossRef] [PubMed]
- Bahraminasab, M. Challenges on optimization of 3D-printed bone scaffolds. Biomed. Eng. Online 2020, 19, 69. [Google Scholar] [CrossRef]
- Qian, Y.; Zhou, X.; Zhang, F.; Diekwisch, T.G.H.; Luan, X.; Yang, J. Triple PLGA/PCL Scaffold Modification Including Silver Impregnation, Collagen Coating, and Electrospinning Significantly Improve Biocompatibility, Antimicrobial, and Osteogenic Properties for Orofacial Tissue Regeneration. ACS Appl. Mater. Interfaces 2019, 11, 37381–37396. [Google Scholar] [CrossRef]
- Tang, D.; Tare, R.S.; Yang, L.-Y.; Williams, D.F.; Ou, K.-L.; Oreffo, R.O.C. Biofabrication of Bone Tissue: Approaches, Challenges and Translation for Bone Regeneration. Biomaterials 2016, 83, 363–382. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, D.M.; Vaezi, M.; Yang, S.; Oreffo, R.O. Hope versus Hype: What Can Additive Manufacturing Realistically Offer Trauma and Orthopedic Surgery? Regen. Med. 2014, 9, 535–549. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.F.; Sfeir, C.; Kumta, P.N. Novel synthesis strategies for natural polymer and composite biomaterials as potential scaffolds for tissue engineering. Philos. Trans. A Math. Phys. Eng. Sci. 2010, 368, 1981–1997. [Google Scholar] [CrossRef]
- Kozusko, S.D.; Riccio, C.; Goulart, M.; Bumgardner, J.; Jing, X.L.; Konofaos, P. Chitosan as a Bone Scaffold Biomaterial. J. Craniofacial Surg. 2018, 29, 1788–1793. [Google Scholar] [CrossRef]
- Nabavi, M.H.; Salehi, M.; Ehterami, A.; Bastami, F.; Semyari, H.; Tehranchi, M.; Nabavi, M.A.; Semyari, H. A collagen-based hydrogel containing tacrolimus for bone tissue engineering. Drug Deliv. Transl. Res. 2019, 10, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.M.; Gentile, P.; Chiono, V.; Ciardelli, G. Collagen for bone tissue regeneration. Acta Biomater. 2012, 8, 3191–3200. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Tao, Y.; Huang, Y.; Xu, T.; Niu, W. Applications of marine collagens in bone tissue engineering. Biomed. Mater. 2021, 16, 042007. [Google Scholar] [CrossRef] [PubMed]
- Alkildani, S.; Jung, O.; Barbeck, M. In Vitro Investigation of Jellyfish Collagen as a Tool in Cell Culture and (Bone) Tissue Engineering. Anticancer Res. 2021, 41, 707–717. [Google Scholar] [CrossRef] [PubMed]
- Glowacki, J.; Mizuno, S. Collagen Scaffolds for Tissue Engineering. Biopolymers 2008, 89, 338–344. [Google Scholar] [CrossRef]
- O’Brien, F.J. Biomaterials and scaffolds for tissue engineering. Mater. Today 2010, 14, 88–95. [Google Scholar] [CrossRef]
- Eyre, D.R.; Wu, J.J. Collagen cross-links. In Collagen; Brinckmann, J., Notbohm, H., Müller, P.K., Eds.; Topics in Current Chemistry 247; Springer: Berlin/Heidelberg, Germany, 2005; pp. 207–209. [Google Scholar]
- Yamauchi, M.; Sricholpech, M. Lysine post-translational modifications of collagen. Essays Biochem. 2012, 52, 113–133. [Google Scholar] [CrossRef]
- Orgel, J.P.; Wess, T.J.; Miller, A. The in situ conformation and axial location of the intermolecular cross-linked non-helical telopeptides of type I collagen. Structure 2000, 8, 137–142. [Google Scholar] [CrossRef]
- Bharadwaz, A.; Jayasuriya, A.C. Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration. Mater. Sci. Eng. C 2020, 110, 110698. [Google Scholar] [CrossRef]
- Rodríguez-Vázquez, M.; Vega-Ruiz, B.; Ramos-Zúñiga, R.; Saldaña-Koppel, D.A.; Quiñones-Olvera, L.F. Chitosan and Its Potential Use as a Scaffold for Tissue Engineering in Regenerative Medicine. BioMed Res. Int. 2015, 2015, e821279. [Google Scholar] [CrossRef] [PubMed]
- Örlygsson, G.; Laxdal, E.H.; Kárason, S.; Dagbjartsson, A.; Gunnarsson, E.; Ng, C.H.; Einarsson, J.M.; Gíslason, J.; Jónsson, H., Jr. Mineralization in a Critical Size Bone-Gap in Sheep Tibia Improved by a Chitosan-Calcium Phosphate-Based Composite as Compared to Predicate Device. Materials 2022, 15, 838. [Google Scholar] [CrossRef] [PubMed]
- Zhai, P.; Peng, X.; Li, B.; Liu, Y.; Sun, H.; Li, X. The application of hyaluronic acid in bone regeneration. Int. J. Biol. Macromol. 2020, 151, 1224–1239. [Google Scholar] [CrossRef] [PubMed]
- Issa, J.P.M.; Santos Neto, O.M.D.; Macedo, A.P.; Gonzaga, M.G.; Pereira, Y.C.L.; Feldman, S. Evaluation of tissue in repair with natural latex and / or hyaluronic acid in surgical bone defects. Braz. Dent. J. 2021, 32, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Lee, J. Enhanced bone healing by improved fibrin-clot formation via fibrinogen adsorption on biphasic calcium phosphate granules. Clin. Oral Implant. Res. 2014, 26, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- Murizan, N.I.S.; Mustafa, N.S.; Ngadiman, N.H.A.; Mohd Yusof, N.; Idris, A. Review on Nanocrystalline Cellulose in Bone Tissue Engineering Applications. Polymers 2020, 12, 2818. [Google Scholar] [CrossRef]
- Li, L.; He, Z.Y.; Wei, X.W.; Wei, Y.Q. Recent advances of biomaterials in biotherapy. Regen. Biomater. 2016, 3, 99–105. [Google Scholar] [CrossRef]
- Rao, S.H.; Harini, B.; Shadamarshan, R.P.K.; Balagangadharan, K.; Selvamurugan, N. Natural and synthetic polymers/bioceramics/bioactive compounds-mediated cell signalling in bone tissue engineering. Int. J. Biol. Macromol. 2018, 110, 88–96. [Google Scholar] [CrossRef]
- Januariyasa, I.K.; Ana, I.D.; Yusuf, Y. Nanofibrous poly(vinyl alcohol)/chitosan contained carbonated hydroxyapatite nanoparticles scaffold for bone tissue engineering. Mater. Sci. Eng. C 2020, 107, 110347. [Google Scholar] [CrossRef]
- Çetin, D.; Kahraman, A.S.; Gümüşderelioğlu, M. Novel scaffolds based on poly(2-hydroxyethyl methacrylate) superporous hydrogels for bone tissue engineering. J. Biomater. Sci. Polym. Ed. 2011, 22, 1157–1178. [Google Scholar] [CrossRef]
- Tamjid, E.; Bagheri, R.; Vossoughi, M.; Simchi, A. Effect of TiO2 morphology on in vitro bioactivity of polycaprolactone/TiO2 nanocomposites. Mater. Lett. 2011, 65, 2530–2533. [Google Scholar] [CrossRef]
- Gogolewski, S.; Mainil-Varlet, P. Effect of thermal treatment on sterility, molecular and mechanical properties of various polylactides. 2. Poly(L/D-lactide) and poly(L/DL-lactide). Biomaterials 1997, 18, 251–255. [Google Scholar] [CrossRef]
- Lyons, F.; Partap, S.; O’Brien, F.J. Part 1: Scaffolds and surfaces. Technol. Health Care 2008, 16, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Vyas, C.; Zhang, J.; Øvrebø, Ø.; Huang, B.; Roberts, I.; Setty, M.; Allardyce, B.; Haugen, H.; Rajkhowa, R.; Bartolo, P. 3D printing of silk microparticle reinforced polycaprolactone scaffolds for tissue engineering applications. Mater. Sci. Eng. C 2021, 118, 111433. [Google Scholar] [CrossRef]
- Ahmed, M.K.; Mansour, S.F.; Al-Wafi, R. Nanofibrous scaffolds ofϵ-polycaprolactone containing Sr/Se-hydroxyapatite/graphene oxide for tissue engineering applications. Biomed. Mater. 2020, 16, 045030. [Google Scholar] [CrossRef]
- Abudhahir, M.; Saleem, A.; Paramita, P.; Kumar, S.D.; Tze-Wen, C.; Selvamurugan, N.; Moorthi, A. Polycaprolactone fibrous electrospun scaffolds reinforced with copper doped wollastonite for bone tissue engineering applications. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 654–664. [Google Scholar] [CrossRef]
- Yoshida, M.; Turner, P.R.; Ali, M.A.; Cabral, J.D. Three-Dimensional Melt-Electrowritten Polycaprolactone/Chitosan Scaffolds Enhance Mesenchymal Stem Cell Behavior. ACS Appl. Bio. Mater. 2021, 4, 1319–1329. [Google Scholar] [CrossRef]
- Sordi, M.B.; da Cruz, A.C.C.; Aragones, Á.; Cordeiro, M.M.R.; de Souza Magini, R. PLGA+HA/βTCP Scaffold Incorporating Simvastatin: A Promising Biomaterial for Bone Tissue Engineering. J. Oral Implantol. 2021, 47, 93–101. [Google Scholar] [CrossRef]
- Sokolova, V.; Kostka, K.; Shalumon, K.T.; Prymak, O.; Chen, J.P.; Epple, M. Synthesis and characterization of PLGA/HAP scaffolds with DNA-functionalised calcium phosphate nanoparticles for bone tissue engineering. J. Mater. Sci. Mater. Med. 2020, 31, 102. [Google Scholar] [CrossRef]
- Rasoulianboroujeni, M.; Fahimipour, F.; Shah, P.; Khoshroo, K.; Tahriri, M.; Eslami, H.; Yadegari, A.; Dashtimoghadam, E.; Tayebi, L. Development of 3D-printed PLGA/TiO2 nanocomposite scaffolds for bone tissue engineering applications. Mater. Sci. Eng. C 2019, 96, 105–113. [Google Scholar] [CrossRef]
- Garg, T.; Singh, O.; Arora, S.; Murthy, R.S.R. Scaffold: A novel carrier for cell and drug delivery. Crit. Rev. Ther. Drug Carr. Syst. 2012, 29, 1–63. [Google Scholar] [CrossRef] [PubMed]
- Engebretson, B.; Sikavitsas, V.I. Long-term in vivo effect of PEG bone tissue engineering scaffolds. J. Long Term Eff. Med. Implant. 2012, 22, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tan, B.; Wu, Y.; Zhang, M.; Liao, J. A Review on Hydrogels with Photothermal Effect in Wound Healing and Bone Tissue Engineering. Polymers 2021, 13, 2100. [Google Scholar] [CrossRef] [PubMed]
- Rice, J.J.; Martino, M.M.; De Laporte, L.; Tortelli, F.; Briquez, P.S.; Hubbell, J.A. Engineering the regenerative microenvironment with biomaterials. Adv. Healthc. Mater. 2014, 2, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Dawson, J.I.; Kanczler, J.M.; Yang, X.B.; Attard, G.S.; Oreffo, R.O. Clay gels for the delivery of regenerative microenvironments. Adv. Mater. 2011, 23, 3304–3308. [Google Scholar] [CrossRef] [PubMed]
- Vega, S.L.; Kwon, M.Y.; Burdick, J.A. Recent advances in hydrogels for cartilage tissue engineering. Eur. Cell Mater. 2017, 33, 59–75. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, X.; Zhao, Q.; Tan, B.; Chen, X.; Liao, J. Role of Hydrogels in Bone Tissue Engineering: How Properties Shape Regeneration. J. Biomed. Nanotechnol. 2020, 16, 1667–1686. [Google Scholar] [CrossRef] [PubMed]
- Hasani-Sadrabadi, M.M.; Sarrion, P.; Pouraghaei, S.; Chau, Y.; Ansari, S.; Li, S.; Aghaloo, T.; Moshaverinia, A. An engineered cell-laden adhesive hydrogel promotes craniofacial bone tissue regeneration in rats. Sci. Transl. Med. 2020, 12, eaay6853. [Google Scholar] [CrossRef] [PubMed]
- Leukers, B.; Gülkan, H.; Irsen, S.H.; Milz, S.; Tille, C.; Schieker, M.; Seitz, H. Hydroxyapatite scaffolds for bone tissue engineering made by 3D printing. J. Mater. Sci. Mater. Med. 2005, 16, 1121–1124. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wang, G.; Hu, S.; Cui, J.; Ren, N.; Liu, D.; Liu, H.; Cao, C.; Wang, J.; Wang, Z. In vitro biomimetic construction of hydroxyapatite-porcine acellular dermal matrix composite scaffold for MC3T3-E1 preosteoblast culture. Tissue Eng. A 2011, 17, 765–776. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, C.; Huang, R.; Chen, K.; Huang, B.; Liu, Q.; Chen, Z.; Li, Z. Effects of fluorinated porcine hydroxyapatite on lateral ridge augmentation: An experimental study in the canine mandible. Am. J. Transl. Res. 2020, 12, 2473–2487. [Google Scholar] [PubMed]
- Allo, B.A.; Costa, D.O.; Dixon, S.J.; Mequanint, K.; Rizkalla, A.S. Bioactive and biodegradable nanocomposites and hybrid biomaterials for bone regeneration. J. Funct. Biomater. 2012, 3, 432–463. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Z.; Dai, G.; Wan, R.; Zhang, D.; Zhao, C.; Chen, C.; Li, J.; Gu, H.; Huang, W. Osteogenic and antibacterial dual functions of a novel levofloxacin loaded mesoporous silica microspheres/nano-hydroxyapatite/polyurethane composite scaffold. Genes Dis. 2019, 8, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Rustom, L.E.; Poellmann, M.J.; Johnson, A.J.W. Mineralization in micropores of calcium phosphate scaffolds. Acta Biomater. 2019, 83, 435–455. [Google Scholar] [CrossRef] [PubMed]
- Tarafder, S.; Balla, V.; Davies, N.M.; Bandyopadhyay, A.; Bose, S. Microwave-sintered 3D printed tricalcium phosphate scaffolds for bone tissue engineering. J. Tissue Eng. Regen. Med. 2013, 7, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, S.B.; Keong, T.K.; Cheng, C.H.; Saim, A.B.; Idrus, R.B. Tricalcium phosphate/hydroxyapatite (TCP-HA) bone scaffold as potential candidate for the formation of tissue engineered bone. Indian J. Med. Res. 2013, 137, 1093–1101. [Google Scholar] [PubMed]
- Seong, Y.J.; Kang, I.G.; Song, E.H.; Kim, H.E.; Jeong, S.H. Calcium Phosphate-Collagen Scaffold with Aligned Pore Channels for Enhanced Osteochondral Regeneration. Adv. Healthc. Mater. 2017, 6, 1700966. [Google Scholar] [CrossRef]
- Xu, W.; Tan, W.; Li, C.; Wu, K.; Zeng, X.; Xiao, L. Metformin-loaded β-TCP/CTS/SBA-15 composite scaffolds promote alveolar bone regeneration in a rat model of periodontitis. J. Mater. Sci. Mater. Med. 2021, 32, 145. [Google Scholar] [CrossRef]
- Bühring, J.; Voshage, M.; Schleifenbaum, J.H.; Jahr, H.; Schröder, K.U. Influence of Degradation Product Thickness on the Elastic Stiffness of Porous Absorbable Scaffolds Made from an Bioabsorbable Zn-Mg Alloy. Materials 2021, 14, 6027. [Google Scholar] [CrossRef]
- Cutolo, A.; Engelen, B.; Desmet, W.; van Hooreweder, B. Mechanical properties of diamond lattice Ti–6Al–4V structures produced by laser powder bed fusion: On the effect of the load direction. J. Mech. Behav. Biomed. Mater. 2020, 104, 103656. [Google Scholar] [CrossRef]
- Rabbani, M.; Zakian, N.; Alimoradi, N. Contribution of Physical Methods in Decellularization of Animal Tissues. J. Med. Signals Sens. 2021, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Zhao, Z.; Wu, X.; Shen, Q.; Wang, Z.; Kang, Y.; Xing, Z.; Zhang, T. Experimental Study on Chitosan/Allogeneic Bone Powder Composite Porous Scaffold to Repair Bone Defects in Rats. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2016, 30, 298–302. (In Chinese) [Google Scholar]
- Tao, M.; Ao, T.; Mao, X.; Yan, X.; Javed, R.; Hou, W.; Wang, Y.; Sun, C.; Lin, S.; Yu, T.; et al. Sterilization and disinfection methods for decellularized matrix materials: Review, consideration and proposal. Bioact. Mater. 2021, 6, 2927–2945. [Google Scholar] [CrossRef]
- Tsiklin, I.L.; Pugachev, E.I.; Kolsanov, A.V.; Timchenko, E.V.; Boltovskaya, V.V.; Timchenko, P.E.; Volova, L.T. Biopolymer Material from Human Spongiosa for Regenerative Medicine Application. Polymers 2022, 14, 941. [Google Scholar] [CrossRef] [PubMed]
- Kjalarsdóttir, L.; Dýrfjörd, A.; Dagbjartsson, A.; Laxdal, E.H.; Örlygsson, G.; Gíslason, J.; Einarsson, J.M.; Ng, C.H.; Jónsson, H., Jr. Bone remodeling effect of a chitosan and calcium phosphate-based composite. Regen. Biomater. 2019, 6, 241–247. [Google Scholar] [CrossRef]
- Wu, J.; Zheng, A.; Liu, Y.; Jiao, D.; Zeng, D.; Wang, X.; Cao, L.; Jiang, X. Enhanced bone regeneration of the silk fibroin electrospun scaffolds through the modification of the graphene oxide functionalized by BMP-2 peptide. Int. J. Nanomed. 2019, 14, 733–751. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, P.; Kundu, B.; Naskar, D.; Kim, H.W.; Maiti, T.K.; Bhattacharya, D.; Kundu, S.C. Silk scaffolds in bone tissue engineering: An overview. Acta Biomater. 2017, 63, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, D.; Ye, Z.; Wang, L.; Ge, L.; Pathak, J.L. Rare earth smart nanomaterials for bone tissue engineering and implantology: Advances, challenges, and prospects. Bioeng. Transl. Med. 2021, 7, e10262. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Luo, D.; Liu, Y. Effect of the nano/microscale structure of biomaterial scaffolds on bone regeneration. Int. J. Oral Sci. 2020, 12, 6. [Google Scholar] [CrossRef] [PubMed]
- Sarikaya, B.; Aydin, H.M. Collagen/Beta-Tricalcium Phosphate Based Synthetic Bone Grafts via Dehydrothermal Processing. Biomed. Res. Int. 2015, 2015, 576532. [Google Scholar] [CrossRef] [PubMed]
- Satija, N.K.; Singh, V.K.; Verma, Y.K.; Gupta, P.; Sharma, S.; Afrin, F.; Sharma, M.; Sharma, P.; Tripathi, R.P.; Gurudutta, G.U. Mesenchymal stem cell-based therapy: A new paradigm in regenerative medicine. J. Cell. Mol. Med. 2009, 13, 4385–4402. [Google Scholar] [CrossRef] [PubMed]
- Moradi, S.L.; Golchin, A.; Hajishafieeha, Z.; Khani, M.M.; Ardeshirylajimi, A. Bone tissue engineering: Adult stem cells in combination with electrospun nanofibrous scaffolds. J. Cell. Physiol. 2018, 233, 6509–6522. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Y.; Gou, W.; Lu, Q.; Peng, J.; Lu, S. Role of mesenchymal stem cells in bone regeneration and fracture repair: A review. Int. Orthop. 2013, 37, 2491–2498. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.L.B.K.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Liu, G.; Halim, A.; Ju, Y.; Luo, Q.; Song, A.G. Mesenchymal Stem Cell Migration and Tissue Repair. Cells 2019, 8, 784. [Google Scholar] [CrossRef] [PubMed]
- Yong, K.W.; Choi, J.R.; Choi, J.Y.; Cowie, A.C. Recent Advances in Mechanically Loaded Human Mesenchymal Stem Cells for Bone Tissue Engineering. Int. J. Mol. Sci. 2020, 21, 5816. [Google Scholar] [CrossRef]
- Hirano, Y.; Aziz, M.; Yang, W.L.; Wang, Z.; Zhou, M.; Ochani, M.; Khader, A.; Wang, P. Neutralization of osteopontin attenuates neutrophil migration in sepsis-induced acute lung injury. Crit. Care 2015, 19, 53. [Google Scholar] [CrossRef]
- Liu, X.; Duan, B.; Cheng, Z.; Jia, X.; Mao, L.; Fu, H.; Kong, D. SDF-1/CXCR4 axis modulates bone marrow mesenchymal stem cell apoptosis, migration and cytokine secretion. Protein Cell 2011, 2, 845–854. [Google Scholar] [CrossRef]
- Khatiwala, C.B.; Kim, P.D.; Peyton, S.R.; Putnam, A.J. ECM compliance regulates osteogenesis by influencing MAPK signaling downstream of RhoA and ROCK. J. Bone Miner. Res. 2009, 24, 886–898. [Google Scholar] [CrossRef] [PubMed]
- Lazennec, G.; Jorgensen, C. Concise review: Adult multipotent stromal cells and cancer: Risk or benefit? Stem Cells 2008, 26, 1387–1394. [Google Scholar] [CrossRef]
- Gunn, W.G.; Conley, A.; Deininger, L.; Olson, S.D.; Prockop, D.J.; Gregory, C.A. A crosstalk between myeloma cells and marrow stromal cells stimulates production of DKK1 and interleukin-6: A potential role in the development of lytic bone disease and tumor progression in multiple myeloma. Stem Cells 2006, 24, 986–991. [Google Scholar] [CrossRef]
- Oliveira, É.R.; Nie, L.; Podstawczyk, D.; Allahbakhsh, A.; Ratnayake, J.; Brasil, D.L.; Shavandi, A. Advances in Growth Factor Delivery for Bone Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 903. [Google Scholar] [CrossRef] [PubMed]
- James, A.W.; LaChaud, G.; Shen, J.; Asatrian, G.; Nguyen, V.; Zhang, X.; Ting, K.; Soo, C. A Review of the Clinical Side Effects of Bone Morphogenetic Protein-2. Tissue Eng. B Rev. 2016, 22, 284–297. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.N.; Green, J.; Wang, Z.; Deng, Y.; Qiao, M.; Peabody, M.; Zhang, Q.; Ye, J.; Yan, Z.; Denduluri, S.; et al. Bone Morphogenetic Protein (BMP) signaling in development and human diseases. Genes Dis. 2014, 1, 87–105. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.N.; Kasper, F.K.; Mikos, A.G. Strategies for controlled delivery of growth factors and cells for bone regeneration. Adv. Drug Deliv. Rev. 2012, 64, 1292–1309. [Google Scholar] [CrossRef]
- Jacinto-Tinajero, J.C.; Ascencio, D.; Marquina, B.; Barrios-Payán, J.; Gutierrez, M.C.; Lim, M.G.; Pando, R.H. Induction of bone formation in abdominal implants constituted by collagen sponges embedded with plant-based human transforming growth factor family proteins in ectopic dog model. J. Exp. Orthop. 2014, 1, 11. [Google Scholar] [CrossRef]
- Mohan, S.P.; Jaishangar, N.; Devy, S.; Narayanan, A.; Cherian, D.; Madhavan, S.S. Platelet-Rich Plasma and Platelet-Rich Fibrin in Periodontal Regeneration: A Review. J. Pharm. Bioallied Sci. 2019, 11 (Suppl. S2), S126–S130. [Google Scholar] [CrossRef]
- Mussano, F.; Genova, T.; Munaron, L.; Petrillo, S.; Erovigni, F.; Carossa, S. Cytokine, chemokine, and growth factor profile of platelet-rich plasma. Platelets 2016, 27, 467–471. [Google Scholar] [CrossRef]
- Tannoury, C.A.; An, H.S. Complications with the use of bone morphogenetic protein 2 (BMP-2) in spine surgery. Spine J. 2014, 14, 552–559. [Google Scholar] [CrossRef]
- Laschke, M.W.; Menger, M.D. Prevascularization in tissue engineering: Current concepts and future directions. Biotechnol. Adv. 2016, 34, 112–121. [Google Scholar] [CrossRef]
- Rücker, C.; Kirch, H.; Pullig, O.; Walles, H. Strategies and First Advances in the Development of Prevascularized Bone Implants. Curr. Mol. Biol. Rep. 2016, 2, 149–157. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Utzinger, U.; Baggett, B.; Weiss, J.A.; Hoying, J.B.; Edgar, L.T. Large-scale time series microscopy of neovessel growth during angiogenesis. Angiogenesis 2015, 18, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Jafarkhani, M.; Salehi, Z.; Aidun, A.; Shokrgozar, M.A. Bioprinting in Vascularization Strategies. Iran Biomed. J. 2019, 23, 9–20. [Google Scholar] [CrossRef]
- Masson-Meyers, D.S.; Tayebi, L. Vascularization strategies in tissue engineering approaches for soft tissue repair. J. Tissue Eng. Regen. Med. 2021, 15, 747–762. [Google Scholar] [CrossRef] [PubMed]
- Heller, M.; Frerick-Ochs, E.V.; Bauer, H.K.; Schiegnitz, E.; Flesch, D.; Brieger, J.; Stein, R.; Al-Nawas, B.; Brochhausen, C.; Thüroff, J.W.; et al. Tissue engineered pre-vascularized buccal mucosa equivalents utilizing a primary triculture of epithelial cells, endothelial cells and fibroblasts. Biomaterials 2016, 77, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Ross, D.; Wang, G.; Jia, W.; Kirkpatrick, S.J.; Zhao, F. Upgrading prevascularization in tissue engineering: A review of strategies for promoting highly organized microvascular network formation. Acta Biomater. 2019, 95, 112–130. [Google Scholar] [CrossRef] [PubMed]
- Kazimierczak, P.; Przekora, A. Bioengineered Living Bone Grafts-A Concise Review on Bioreactors and Production Techniques In Vitro. Int. J. Mol. Sci. 2022, 23, 1765. [Google Scholar] [CrossRef]
- Jia, W.; Gungor-Ozkerim, P.S.; Zhang, Y.S.; Yue, K.; Zhu, K.; Liu, W.; Pi, Q.; Byambaa, B.; Dokmeci, M.R.; Shin, S.R.; et al. Direct 3D bioprinting of perfusable vascular constructs using a blend bioink. Biomaterials 2016, 106, 58–68. [Google Scholar] [CrossRef]
- Chen, H.C.; Hu, Y.C. Bioreactors for tissue engineering. Biotechnol. Lett. 2006, 28, 1415–1423. [Google Scholar] [CrossRef]
- Hansmann, J.; Groeber, F.; Kahlig, A.; Kleinhans, C.; Walles, H. Bioreactors in tissue engineering—Principles, applications and commercial constraints. Biotechnol. J. 2013, 8, 298–307. [Google Scholar] [CrossRef]
- Gaspar, D.A.; Gomide, V.; Monteiro, F.J. The role of perfusion bioreactors in bone tissue engineering. Biomatter 2012, 2, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jakus, A.E.; Kural, M.; Qian, H.; Engler, A.; Ghaedi, M.; Shah, R.; Steinbacher, D.M.; Niklason, L.E. Vascularization of Natural and Synthetic Bone Scaffolds. Cell Transplant. 2018, 27, 1269–1280. [Google Scholar] [CrossRef] [PubMed]
- Ressler, A.; Antunović, M.; Teruel-Biosca, L.; Ferrer, G.G.; Babić, S.; Urlić, I.; Ivanković, M.; Ivanković, H. Osteogenic differentiation of human mesenchymal stem cells on substituted calcium phosphate/chitosan composite scaffold. Carbohydr. Polym. 2022, 277, 118883. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.R.; Lipphaus, A.; Ergin, M.; Salehi, S.; Gehweiler, D.; Rudert, M.; Hansmann, J.; Herrmann, M. Modeling of the Human Bone Environment: Mechanical Stimuli Guide Mesenchymal Stem Cell-Extracellular Matrix Interactions. Materials 2021, 14, 4431. [Google Scholar] [CrossRef] [PubMed]
- Datta, N.; Pham, Q.P.; Sharma, U.; Sikavitsas, V.I.; Jansen, J.A.; Mikos, A.G. In vitro generated extracellular matrix and fluid shear stress synergistically enhance 3D osteoblastic differentiation. Proc. Natl. Acad. Sci. USA 2006, 103, 2488–2493. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, V.; Bechtel, G.; Tchantchaleishvili, V. Artificial tissue creation under microgravity conditions: Considerations and future applications. Artif. Organs. 2021, 45, 1446–1455. [Google Scholar] [CrossRef] [PubMed]
- Cazzaniga, A.; Ille, F.; Wuest, S.; Haack, C.; Koller, A.; Giger-Lange, C.; Zocchi, M.; Egli, M.; Castiglioni, S.; Maier, J.A. Scalable Microgravity Simulator Used for Long-Term Musculoskeletal Cells and Tissue Engineering. Int. J. Mol. Sci. 2020, 21, 8908. [Google Scholar] [CrossRef] [PubMed]
- Sakai, S.; Mishima, H.; Ishii, T.; Akaogi, H.; Yoshioka, T.; Ohyabu, Y.; Chang, F.; Ochiai, N.; Uemura, T. Rotating three-dimensional dynamic culture of adult human bone marrow-derived cells for tissue engineering of hyaline cartilage. J. Orthop. Res. 2009, 27, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Sheyn, D.; Pelled, G.; Netanely, D.; Domany, E.; Gazit, D. The effect of simulated microgravity on human mesenchymal stem cells cultured in an osteogenic differentiation system: A bioinformatics study. Tissue Eng. A 2010, 16, 3403–3412. [Google Scholar] [CrossRef] [PubMed]
- Caliogna, L.; Medetti, M.; Bina, V.; Brancato, A.; Castelli, A.; Jannelli, E.; Ivone, A.; Gastaldi, G.; Annunziata, S.; Mosconi, M.; et al. Pulsed Electromagnetic Fields in Bone Healing: Molecular Pathways and Clinical Applications. Int. J. Mol. Sci. 2021, 22, 7403. [Google Scholar] [CrossRef]
- Wang, T.; Yang, L.; Jiang, J.; Liu, Y.; Fan, Z.; Zhong, C.; He, C. Pulsed Electromagnetic Fields: Promising Treatment for Osteoporosis. Osteoporos. Int. 2019, 30, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.T.; Chang, W.H.; Chang, K.; Hou, R.J.; Wu, T.W. Pulsed electromagnetic fields affect osteoblast proliferation and differentiation in bone tissue engineering. Bioelectromagnetics 2007, 28, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Ross, C.L.; Syed, I.; Smith, T.L.; Harrison, B.S. The regenerative effects of electromagnetic field on spinal cord injury. Electromagn. Biol. Med. 2017, 36, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Krakowczyk, L.; Maciejewski, A.; Szymczyk, C.; Wierzgoń, J.; Szumniak, R.; Jędrzejewski, P.; Grajek, M.; Dobrut, M.; Ulczok, R.; Półtorak, S. The use of prefabrication technique in microvascular reconstructive surgery. Contemp. Oncol. 2012, 16, 546–550. [Google Scholar] [CrossRef]
- Wei, X.; Li, Q. Prefabrication as a term in flap surgery: Do we need a broader definition? J. Reconstr. Microsurg. 2013, 29, 559–560. [Google Scholar] [CrossRef]
- Shen, T.Y. Vascular implantation into skin flap: Experimental study and clinical application: A preliminary report. Plast. Reconstr. Surg. 1981, 68, 404–410. [Google Scholar]
- Pribaz, J.J.; Fine, N. Prelamination: Defining the prefabricated flap—A case report and review. Microsurgery 1994, 15, 618–623. [Google Scholar] [CrossRef]
- Tan, B.K.; Chen, H.C.; He, T.M.; Song, I.C. Flap prefabrication—The bridge between conventional flaps and tissue-engineered flaps. Ann. Acad. Med. Singap. 2004, 33, 662–666. [Google Scholar]
- Trung, V.T.; Van Long, P.; Van, H.T. Restoration of Ear Defects by Prefabricated Radial Forearm Flap. Plast. Reconstr. Surg. Glob. Open 2020, 8, e2616. [Google Scholar] [CrossRef]
- Flaherty, F.; Militelli, F.; Vizcay, M. Partial Ear Reconstruction with a Prelaminated Induced Expanded Radial Artery Flap. Plast. Reconstr. Surg. Glob. Open 2021, 9, e3344. [Google Scholar] [CrossRef]
- Stevens, M.M.; Marini, R.P.; Schaefer, D.; Aronson, J.; Langer, R.; Shastri, V.P. In vivo engineering of organs: The bone bioreactor. Proc. Natl. Acad. Sci. USA 2005, 102, 11450–11455. [Google Scholar] [CrossRef] [PubMed]
- Holt, G.E.; Halpern, J.L.; Dovan, T.T.; Hamming, D.; Schwartz, H.S. Evolution of an in vivo bioreactor. J. Orthop. Res. 2005, 23, 916–923. [Google Scholar] [CrossRef]
- Buehrer, G.; Balzer, A.; Arnold, I.; Beier, J.P.; Körner, C.; Bleiziffer, O.; Brandl, A.; Weis, C.; Horch, R.E.; Kneser, U.; et al. Combination of BMP2 and MSCs significantly increases bone formation in the rat arterio-venous loop model. Tissue Eng. A 2015, 21, 96–105. [Google Scholar] [CrossRef]
- Han, D.; Guan, X.; Wang, J.; Wei, J.; Li, Q. Rabbit tibial periosteum and saphenous arteriovenous vascular bundle as an in vivo bioreactor to construct vascularized tissue-engineered bone: A feasibility study. Artif. Organs. 2014, 38, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Huang, X.; Shen, J.; Wang, C.; Dang, X.; Mankin, H.; Duan, Z.; Wang, K. Development of a new pre-vascularized tissue-engineered construct using predifferentiated rADSCs, arteriovenous vascular bundle and porous nano-hydroxyapatide-polyamide 66 scaffold. BMC Musculoskelet. Disord. 2013, 14, 318. [Google Scholar] [CrossRef] [PubMed]
- Sever, C.; Uygur, F.; Kose, G.T.; Urhan, M.; Haholu, A.; Kulahci, Y.; Sinan, O.; Cihan, S.; Omer, O. Prefabrication of vascularized bone graft using an interconnected porous calcium hydroxyapatite ceramic in presence of vascular endothelial growth factor and bone marrow mesenchymal stem cells: Experimental study in rats. Indian J. Plast. Surg. 2012, 45, 444–452. [Google Scholar] [CrossRef]
- Boos, A.M.; Loew, J.S.; Weigand, A.; Deschler, G.; Klumpp, D.; Arkudas, A.; Bleiziffer, O.; Gulle, H.; Kneser, U.; Horch, R.E.; et al. Engineering axially vascularized bone in the sheep arteriovenous-loop model. J. Tissue Eng. Regen. Med. 2013, 7, 654–664. [Google Scholar] [CrossRef]
- Runyan, C.M.; Vu, A.T.; Rumburg, A.; Bove, K.; Racadio, J.; Billmire, D.A.; Taylor, J.A. Repair of a Critical Porcine Tibial Defect by Means of Allograft Revitalization. Plast. Reconstr. Surg. 2015, 136, 461e–473e. [Google Scholar] [CrossRef]
- Tatara, A.M.; Shah, S.R.; Demian, N.; Ho, T.; Shum, J.; van den Beucken, J.J.J.P.; Jansen, J.A.; Wong, M.E.; Mikos, A.G. Reconstruction of large mandibular defects using autologous tissues generated from in vivo bioreactors. Acta Biomater. 2016, 45, 72–84. [Google Scholar] [CrossRef]
- Akar, B.; Tatara, A.M.; Sutradhar, A.; Hsiao, H.Y.; Miller, M.; Cheng, M.H.; Mikos, A.G.; Brey, E.M. Large Animal Models of an In Vivo Bioreactor for Engineering Vascularized Bone. Tissue Eng. B Rev. 2018, 24, 317–325. [Google Scholar] [CrossRef]
- Top, H.; Aygit, C.; Sarikaya, A.; Cakir, B.; Cakir, B.; Unlu, E. Bone flap prefabrication: An experimental study in rabbits. Ann. Plast. Surg. 2005, 54, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.-L.; Tremp, M.; Ho, C.-K.; Sun, Y.; Liu, K.; Li, Q. Prefabrication of a functional bone graft with a pedicled periosteal flap as an in vivo bioreactor. Sci. Rep. 2017, 7, 18038. [Google Scholar] [CrossRef] [PubMed]
- Watson, E.; Tatara, A.M.; van den Beucken, J.J.J.P.; Jansen, J.A.; Wong, M.E.; Mikos, A.G. An Ovine Model of In Vivo Bioreactor-Based Bone Generation. Tissue Eng. C Methods 2020, 26, 384–396. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Kaji, Y.; Nakamura, O.; Tobiume, S.; Yamamoto, T. Prefabrication of Vascularized Allogenic Bone Graft in a Rat by Implanting a Flow-Through Vascular Pedicle and Basic Fibroblast Growth Factor Containing Hydroxyapatite/Collagen Composite. J. Reconstr. Microsurg. 2017, 33, 367–376. [Google Scholar] [CrossRef]
- Zhang, H.; Han, D. Research progress of in vivo bioreactor as vascularization strategies in bone tissue engineering. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2014, 28, 1173–1176. [Google Scholar]
- Aliyev, A.; Ekin, Ö.; Bitik, O.; Korkusuz, P.; Yersal, N.C.; Çelik, H.H.; Tunçbilek, G. A Novel Method of Neo-osseous Flap Prefabrication: Induction of Free Calvarial Periosteum with Bioactive Glass. J. Reconstr. Microsurg. 2018, 34, 307–314, Erratum in J. Reconstr. Microsurg. 2018, 34, e1. [Google Scholar] [CrossRef]
- Ersoy, B.; Bayramiçli, M.; Ercan, F.; Şirinoğlu, H.; Turan, P.; Numanoğlu, A. Comparison of bone prefabrication with vascularized periosteal flaps, hydroxyapatite, and bioactive glass in rats. J. Reconstr. Microsurg. 2015, 31, 291–299. [Google Scholar] [CrossRef]
- Dong, Z.; Li, B.; Zhao, J.; Ma, Q.; Bai, S.; Yang, W.; Li, G.; Ma, G.; Liu, Y. Prefabrication of vascularized bone grafts using a combination of bone marrow mesenchymal stem cells and vascular bundles with β-tricalcium phosphate ceramics. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114 (Suppl. S5), S153–S159. [Google Scholar] [CrossRef]
- Omar, O.; Engstrand, T.; Linder, L.K.B.; Åberg, J.; Shah, F.A.; Palmquist, A.; Birgersson, U.; Elgali, I.; Pujari-Palmer, M.; Engqvist, H.; et al. In situ bone regeneration of large cranial defects using synthetic ceramic implants with a tailored composition and design. Proc. Natl. Acad. Sci. USA 2020, 117, 26660–26671. [Google Scholar] [CrossRef]
- Ma, D.; Ren, L.; Cao, Z.; Li, J.; Cao, J.; Tian, W.; Yao, H. Prefabrication of axially vascularized bone by combining β-tricalciumphosphate, arteriovenous loop, and cell sheet technique. Tissue Eng. Regen. Med. 2016, 13, 579–584. [Google Scholar] [CrossRef]
- Wiltfang, J.; Rohnen, M.; Egberts, J.H.; Lützen, U.; Wieker, H.; Açil, Y.; Naujokat, H. Man as a Living Bioreactor: Prefabrication of a Custom Vascularized Bone Graft in the Gastrocolic Omentum. Tissue Eng. C Methods 2016, 22, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, O.; Kaji, Y.; Imaizumi, Y.; Yamagami, Y.; Yamamoto, T. Prefabrication of vascularized bone allograft in a recipient rat using a flow-through vascular pedicle, bone morphogenetic protein, and bisphosphonate. J. Reconstr. Microsurg. 2013, 29, 241–248. [Google Scholar] [CrossRef]
- Abu-Shahba, A.G.; Wilkman, T.; Kornilov, R.; Adam, M.; Salla, K.M.; Lindén, J.; Lappalainen, A.K.; Björkstrand, R.; Seppänen-Kaijansinkko, R.; Mannerström, B. Periosteal Flaps Enhance Prefabricated Engineered Bone Reparative Potential. J. Dent. Res. 2021, 101, 220345211037247. [Google Scholar] [CrossRef]
- Hokugo, A.; Sawada, Y.; Sugimoto, K.; Fukuda, A.; Mushimoto, K.; Morita, S.; Tabata, Y. Preparation of prefabricated vascularized bone graft with neoangiogenesis by combination of autologous tissue and biodegradable materials. Int. J. Oral Maxillofac. Surg. 2006, 35, 1034–1040. [Google Scholar] [CrossRef]
- Kuzmenka, D.; Sewohl, C.; König, A.; Flath, T.; Hahnel, S.; Schulze, F.P.; Hacker, M.C.; Schulz-Siegmund, M. Sustained Calcium(II)-Release to Impart Bioactivity in Hybrid Glass Scaffolds for Bone Tissue Engineering. Pharmaceutics 2020, 12, 1192. [Google Scholar] [CrossRef] [PubMed]
- Sparks, D.S.; Savi, F.M.; Saifzadeh, S.; Schuetz, M.A.; Wagels, M.; Hutmacher, D.W. Convergence of Scaffold-Guided Bone Reconstruction and Surgical Vascularization Strategies-A Quest for Regenerative Matching Axial Vascularization. Front. Bioeng. Biotechnol. 2020, 7, 448. [Google Scholar] [CrossRef] [PubMed]
- Weigand, A.; Beier, J.P.; Arkudas, A.; Al-Abboodi, M.; Polykandriotis, E.; Horch, R.E.; Boos, A.M. The Arteriovenous (AV) Loop in a Small Animal Model to Study Angiogenesis and Vascularized Tissue Engineering. J. Vis. Exp. 2016, 117, e54676. [Google Scholar] [CrossRef]
- Tian, T.; Zhang, T.; Lin, Y.; Cai, X. Vascularization in Craniofacial Bone Tissue Engineering. J. Dent. Res. 2018, 97, 969–976. [Google Scholar] [CrossRef]
- Wang, J.H.; Chen, J.; Kuo, S.M.; Mitchell, G.M.; Lim, S.Y.; Liu, G.S. Methods for Assessing Scaffold Vascularization In Vivo. Methods Mol. Biol. 2019, 1993, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Stephens, C.J.; Spector, J.A.; Butcher, J.T. Biofabrication of thick vascularized neo-pedicle flaps for reconstructive surgery. Transl. Res. 2019, 211, 84–122. [Google Scholar] [CrossRef] [PubMed]
- Später, T.; Ampofo, E.; Menger, M.D.; Laschke, M.W. Combining Vascularization Strategies in Tissue Engineering: The Faster Road to Success? Front. Bioeng. Biotechnol. 2020, 8, 592095. [Google Scholar] [CrossRef] [PubMed]
- McGregor, I.A.; Morgan, G. Axial and random pattern flaps. Br. J. Plast. Surg. 1973, 26, 202–213. [Google Scholar] [CrossRef]
- Cassell, O.C.; Hofer, S.O.; Morrison, W.A.; Knight, K.R. Vascularisation of tissue-engineered grafts: The regulation of angiogenesis in reconstructive surgery and in disease states. Br. J. Plast. Surg. 2002, 55, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Marion, N.W.; Hollister, S.; Mao, J.J. Tissue formation and vascularization in anatomically shaped human joint condyle ectopically in vivo. Tissue Eng. A 2009, 15, 3923–3930. [Google Scholar] [CrossRef] [PubMed]
- Horch, R.E.; Kneser, U.; Polykandriotis, E.; Schmidt, V.J.; Sun, J.; Arkudas, A. Tissue engineering and regenerative medicine -where do we stand? J. Cell. Mol. Med. 2012, 16, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Kneser, U.; Polykandriotis, E.; Ohnolz, J.; Heidner, K.; Grabinger, L.; Euler, S.; Amann, K.U.; Hess, A.; Brune, K.; Greil, P.; et al. Engineering of vascularized transplantable bone tissues: Induction of axial vascularization in an osteoconductive matrix using an arteriovenous loop. Tissue Eng. 2006, 12, 1721–1731. [Google Scholar] [CrossRef] [PubMed]
- Warnke, P.; Springer, I.; Wiltfang, J.; Acil, Y.; Eufinger, H.; Wehmöller, M.; Russo, P.; Bolte, H.; Sherry, E.; Behrens, E.; et al. Growth and transplantation of a custom vascularised bone graft in a man. Lancet 2004, 364, 766–770. [Google Scholar] [CrossRef]
- Kokemueller, H.; Spalthoff, S.; Nolff, M.; Tavassol, F.; Essig, H.; Stuehmer, C.; Bormann, K.H.; Rücker, M.; Gellrich, N.C. Prefabrication of vascularized bioartificial bone grafts in vivo for segmental mandibular reconstruction: Experimental pilot study in sheep and first clinical application. Int. J. Oral Maxillofac. Surg. 2010, 39, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Horch, R.E.; Beier, J.P.; Kneser, U.; Arkudas, A. Successful human long-term application of in situ bone tissue engineering. J. Cell. Mol. Med. 2014, 18, 1478–1485. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Kang, N.; Wang, Q.; Dong, P.; Lv, X.; Cao, Y.; Xiao, R. The dose-effect relationship between the seeding quantity of human marrow mesenchymal stem cells and in vivo tissue-engineered bone yield. Cell Transplant. 2015, 24, 1957–1968. [Google Scholar] [CrossRef]
- Okuda, T.; Uysal, A.C.; Tobita, M.; Hyakusoku, H.; Mizuno, H. Prefabrication of tissue engineered bone grafts: An experimental study. Ann. Plast. Surg. 2010, 64, 98–104. [Google Scholar] [CrossRef]
- Liu, Y.; Möller, B.; Wiltfang, J.; Warnke, P.H.; Terheyden, H. Tissue engineering of a vascularized bone graft of critical size with an osteogenic and angiogenic factor-based in vivo bioreactor. Tissue Eng. A 2014, 20, 3189–3197. [Google Scholar] [CrossRef] [PubMed]
- Heliotis, M.; Lavery, K.M.; Ripamonti, U.; Tsiridis, E.; di Silvio, L. Transformation of a prefabricated hydroxyapatite/osteogenic protein-1 implant into a vascularised pedicled bone flap in the human chest. Int J. Oral Maxillofac. Surg. 2006, 35, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Bahney, C.S.; Zondervan, R.L.; Allison, P.; Theologis, A.; Ashley, J.W.; Ahn, J.; Miclau, T.; Marcucio, R.S.; Hankenson, K.D. Cellular biology of fracture healing. J. Orthop. Res. 2019, 37, 35–50. [Google Scholar] [CrossRef]
- Chang, H.; Knothe Tate, M.L. Concise review: The periosteum: Tapping into a reservoir of clinically useful progenitor cells. Stem Cells Transl. Med. 2012, 1, 480–491. [Google Scholar] [CrossRef] [PubMed]
- Squier, C.A.; Ghoneim, S.; Kremenak, C.R. Ultrastructure of the periosteum from membrane bone. J. Anat. 1990, 171, 233–239. [Google Scholar] [PubMed]
- Nau, C.; Henrich, D.; Seebach, C.; Schröder, K.; Fitzsimmons, S.-J.; Hankel, S.; Barker, J.H.; Marzi, I.; Frank, J. Treatment of large bone defects with a vascularized periosteal flap in combination with biodegradable scaffold seeded with bone marrow-derived mononuclear cells: An experimental study in rats. Tissue Eng. A 2016, 22, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.-H.; Brey, E.M.; Ulusal, B.G.; Wei, F.-C. Mandible augmentation for osseointegrated implants using tissue engineering strategies. Plast. Reconstr. Surg. 2006, 118, 1e–4e. [Google Scholar] [CrossRef] [PubMed]
- Vranckx, J.J.; Den Hondt, M.; Delaere, P. Prefabrication and prelamination strategies for the reconstruction of complex defects of trachea and larynx. J. Reconstr. Microsurg. 2014, 30, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Jaquet, Y.; Higgins, K.M.; Enepekides, D.J. The temporoparietal fascia flap: A versatile tool in head and neck reconstruction. Curr. Opin. Otolaryngol. Head Neck Surg. 2011, 19, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Zeng, X.; Wang, X.; Zhu, R.; Pei, G. Efficacy of prevascularization for segmental bone defect repair using β-tricalcium phosphate scaffold in rhesus monkey. Biomaterials 2014, 35, 7407–7415. [Google Scholar] [CrossRef]
- Brey, E.M.; Cheng, M.H.; Allori, A.; Satterfield, W.; Chang, D.W.; Patrick, C.W., Jr.; Miller, M.J. Comparison of guided bone formation from periosteum and muscle fascia. Plast. Reconstr. Surg. 2007, 119, 1216–1222. [Google Scholar] [CrossRef] [PubMed]
- Spalthoff, S.; Jehn, P.; Zimmerer, R.; Möllmann, U.; Gellrich, N.C.; Kokemueller, H. Heterotopic bone formation in the musculus latissimus dorsi of sheep using β-tricalcium phosphate scaffolds: Evaluation of an extended prefabrication time on bone formation and matrix degeneration. Int. J. Oral Maxillofac. Surg. 2015, 44, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, N.; Kiyono, T.; Wada, M.R.; Umeda, R.; Goto, Y.; Nonaka, I.; Shimizu, S.; Yasumoto, S.; Inagawa-Ogashiwa, M. Osteogenic properties of human myogenic progenitor cells. Mech. Dev. 2008, 125, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Peng, X.; Mao, C.; Tian, J.H.; Zhang, S.W.; Xu, F.; Tu, J.J.; Liu, S.; Hu, M.; Yu, G.Y. The value of SPECT/CT in monitoring prefabricated tissue-engineered bone and orthotopic rhBMP-2 implants for mandibular reconstruction. PLoS ONE 2015, 10, e0137167. [Google Scholar] [CrossRef][Green Version]
- Chan, J.K.; Harry, L.; Williams, G.; Nanchahal, J. Soft tissue reconstruction of open fractures of the lower limb: Muscle versus fasciocutaneous flaps. Plast. Reconstr. Surg. 2012, 130, 284e–295e. [Google Scholar] [CrossRef]
- Kamei, Y.; Toriyama, K.; Takada, T.; Yagi, S. Tissue-engineering bone from omentum. Nagoya J. Med. Sci. 2010, 72, 111–117. [Google Scholar] [PubMed]
- Sadegh, A.B.; Basiri, E.; Oryan, A.; Mirshokraei, P. Wrapped omentum with periosteum concurrent with adipose derived adult stem cells for bone tissue engineering in dog model. Cell Tissue Bank. 2014, 15, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Tokashiki, K.; Okamoto, I.; Okada, T.; Sato, H.; Yamashita, T.; Matsuki, T.; Kondo, T.; Fushimi, C.; Masubuchi, T.; Miura, K.; et al. Postoperative Complications and Swallowing Function after Jejunal and Skin Flap Reconstruction for Hypopharyngeal Carcinoma-A Multicenter Retrospective Study. J. Clin. Med. 2022, 11, 1464. [Google Scholar] [CrossRef]
- Weigand, A.; Beier, J.P.; Hess, A.; Gerber, T.; Arkudas, A.; Horch, R.E.; Boos, A.M. Acceleration of vascularized bone tissue-engineered constructs in a large animal model combining intrinsic and extrinsic vascularization. Tissue Eng. A 2015, 21, 1680–1694. [Google Scholar] [CrossRef]
- Polykandriotis, E.; Arkudas, A.; Beier, J.P.; Hess, A.; Greil, P.; Papadopoulos, T.; Kopp, J.; Bach, A.D.; Horch, R.E.; Kneser, U. Intrinsic axial vascularization of an osteoconductive bone matrix by means of an arteriovenous vascular bundle. Plast. Reconstr. Surg. 2007, 120, 855–868. [Google Scholar] [CrossRef] [PubMed]
- Eweida, A.M.; Nabawi, A.S.; Abouarab, M.; Kayed, M.; Elhammady, H.; Etaby, A.; Khalil, M.R.; Shawky, M.S.; Kneser, U.; Horch, R.E.; et al. Enhancing mandibular bone regeneration and perfusion via axial vascularization of scaffolds. Clin. Oral Investig. 2014, 18, 1671–1678. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Ruan, C.; Ma, Y.; Huang, Z.; Huang, Z.; Zhou, G.; Zhang, J.; Wang, H.; Wu, Z.; Qiu, G. Fabrication of Vascularized Bone Flaps with Sustained Release of Recombinant Human Bone Morphogenetic Protein-2 and Arteriovenous Bundle. Tissue Eng. A 2018, 24, 1413–1422. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Hua, C.; Tang, X.; Wang, Y.; Zhang, F.; Xiang, Z. Angiogenesis and osteogenesis of non-vascularised autogenous bone graft with arterial pedicle implantation. J. Plast. Reconstr. Aesthet. Surg. 2010, 63, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Sung, K.C.; Tsutsumi, A.; Ohba, S.; Ueda, K.; Morrison, W.A. Tissue engineering skin flaps: Which vascular carrier, arteriovenous shunt loop or arteriovenous bundle, has more potential for angiogenesis and tissue generation? Plast. Reconstr. Surg. 2003, 112, 1636–1644. [Google Scholar] [CrossRef] [PubMed]
- Mian, R.; Morrison, W.A.; Hurley, J.V.; Penington, A.J.; Romeo, R.; Tanaka, Y.; Knight, K.R. Formation of new tissue from an arteriovenous loop in the absence of added extracellular matrix. Tissue Eng. 2000, 6, 595–603. [Google Scholar] [CrossRef]
- Beier, J.P.; Horch, R.E.; Hess, A.; Arkudas, A.; Heinrich, J.; Loew, J.; Gulle, H.; Polykandriotis, E.; Bleiziffer, O.; Kneser, U. Axial vascularization of a large volume calcium phosphate ceramic bone substitute in the sheep AV loop model. J. Tissue Eng. Regen. Med. 2010, 4, 216–223. [Google Scholar] [CrossRef]
- Eweida, A.M.; Nabawi, A.S.; Marei, M.K.; Khalil, M.R.; Elhammady, H.A. Mandibular reconstruction using an axially vascularized tissue-engineered construct. Ann. Surg. Innov. Res. 2011, 5, 2, Erratum in Ann. Surg. Innov. Res. 2012, 6, 4. [Google Scholar] [CrossRef]
- Huang, R.-L.; Kobayashi, E.; Liu, K.; Li, Q. Bone Graft Prefabrication Following the In Vivo Bioreactor Principle. EBioMedicine 2016, 12, 43–54. [Google Scholar] [CrossRef]
- Orringer, J.S.; Shaw, W.W.; Borud, L.J.; Freymiller, E.G.; Wang, S.A.; Markowitz, B.L. Total mandibular and lower lip reconstruction with a prefabricated osteocutaneous free flap. Plast. Reconstr. Surg. 1999, 104, 793–797. [Google Scholar] [CrossRef]
- Mesimäki, K.; Lindroos, B.; Törnwall, J.; Mauno, J.; Lindqvist, C.; Kontio, R.; Miettinen, S.; Suuronen, R. Novel maxillary reconstruction with ectopic bone formation by GMP adipose stem cells. Int J. Oral Maxillofac. Surg. 2009, 38, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Safak, T.; Akyürek, M.; Ozcan, G.; Keçik, A.; Aydin, M. Osteocutaneous flap prefabrication based on the principle of vascular induction: An experimental and clinical study. Plast. Reconstr. Surg. 2000, 105, 1304–1313. [Google Scholar] [CrossRef] [PubMed]
- Vacanti, C.A.; Bonassar, L.J.; Vacanti, M.P.; Shufflebarger, J. Replacement of an avulsed phalanx with tissue-engineered bone. N. Engl. J. Med. 2001, 344, 1511–1514, Erratum in N. Engl. J. Med. 2001, 345, 704. [Google Scholar] [CrossRef] [PubMed]
- Gronthos, S. Reconstruction of human mandible by tissue engineering. Lancet 2004, 364, 735–736. [Google Scholar] [CrossRef] [PubMed]
- Cancedda, R.; Giannoni, P.; Mastrogiacomo, M. A tissue engineering approach to bone repair in large animal models and in clinical practice. Biomaterials 2007, 28, 4240–4250. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Ueda, M.; Hibi, H.; Nagasaka, T. Translational research for injectable tissue-engineered bone regeneration using mesenchymal stem cells and platelet-rich plasma: From basic research to clinical case study. Cell Transplant. 2004, 13, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Marcacci, M.; Kon, E.; Moukhachev, V.; Lavroukov, A.; Kutepov, S.; Quarto, R.; Mastrogiacomo, M.; Cancedda, R. Stem cells associated with macroporous bioceramics for long bone repair: 6- to 7-year outcome of a pilot clinical study. Tissue Eng. 2007, 13, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Iino, M.; Mori, Y.; Chikazu, D.; Saijyo, H.; Ohkubo, K.; Takato, T. Clinical application of bone regeneration by in vivo tissue engineering. Clin. Calcium 2008, 18, 1757–1766. (In Japanese) [Google Scholar] [PubMed]
- Leonhardt, H.; Pradel, W.; Mai, R.; Markwardt, J.; Lauer, G. Prefabricated bony radial forearm flap for secondary mandible reconstruction after radiochemotherapy. Head Neck 2009, 31, 1579–1587. [Google Scholar] [CrossRef]
- Sadigh, P.L.; Jeng, S.F. Prelamination of the anterolateral thigh flap with a fibula graft to successfully reconstruct a mandibular defect. Plast. Reconstr. Surg. Glob. Open 2015, 3, e497. [Google Scholar] [CrossRef][Green Version]
- Rüegg, E.M.; Gniadek, P.; Modarressi, A.; Baratti-Mayer, D.; Pittet-Cuénod, B. Facial bone reconstruction with prefabricated vascularized calvarium flaps in children and young adults: Advantages and long-term results. J. Craniomaxillofac. Surg. 2016, 44, 1880–1888. [Google Scholar] [CrossRef] [PubMed]
- Banaszewski, J.; Gaggl, A.; Buerger, H.; Wierzbicka, M.; Pabiszczak, M.; Andruszko, A. One-Step Laryngotracheal Reconstruction with Prefabricated Corticoperiosteal Flap. Ann. Thorac. Surg. 2019, 107, e333–e335. [Google Scholar] [CrossRef] [PubMed]
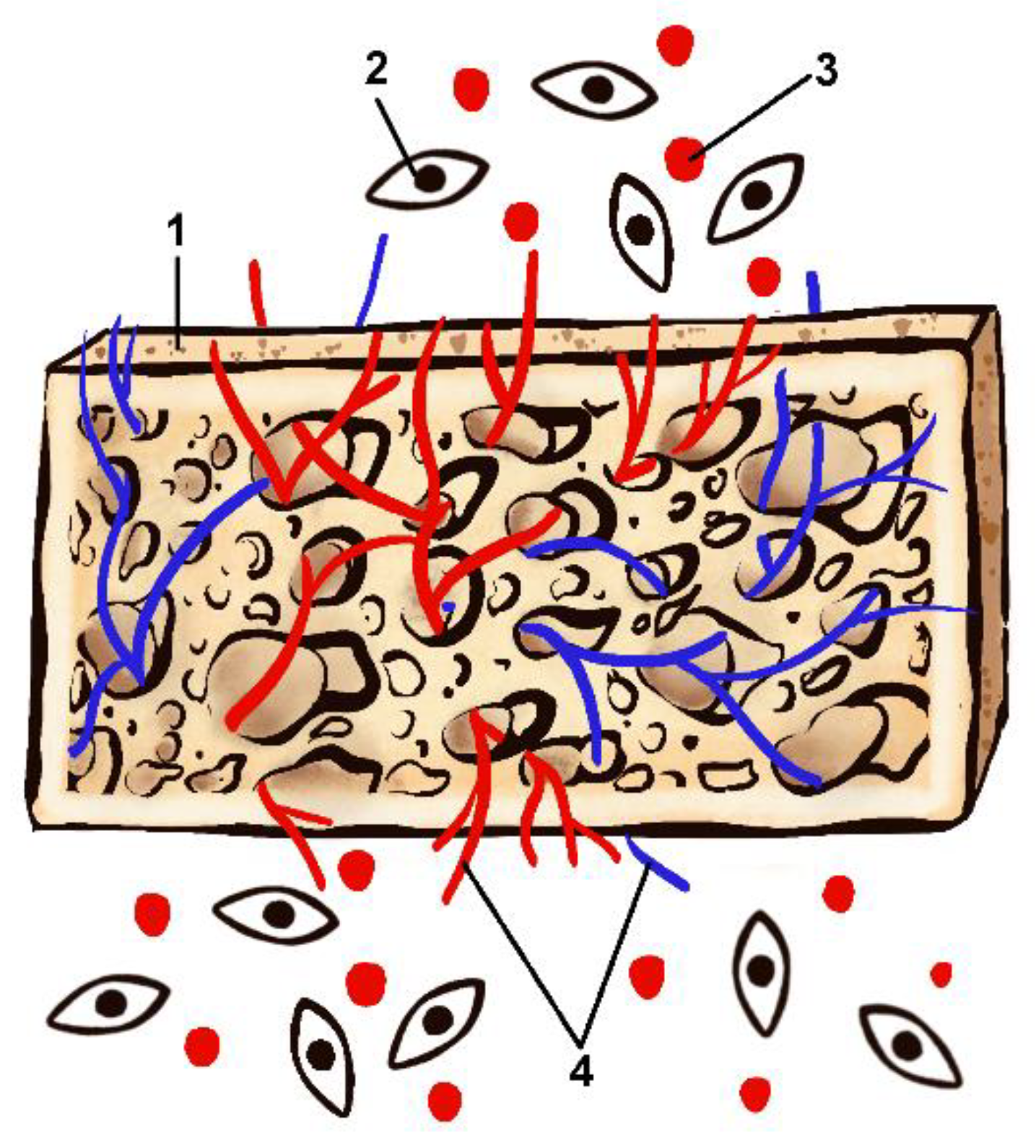
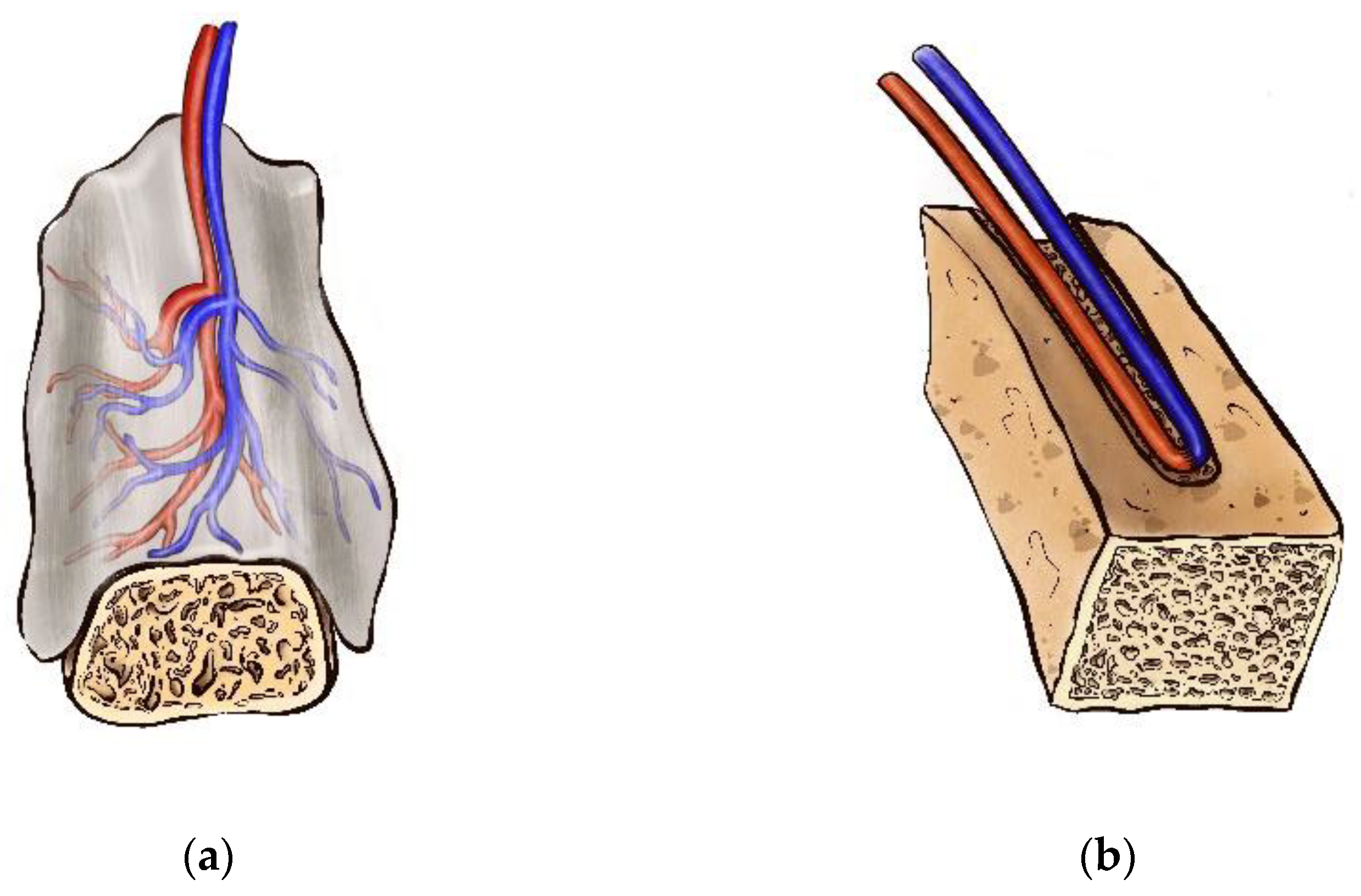
| Scaffold Type | Advantages | Disadvantages | Fabrication Technique | Sterilization Method |
|---|---|---|---|---|
| Natural polymers | Biocompatibility Cell adhesion, proliferation, Angiogenesis Low immunogenicity Antimicrobial properties | Poor mechanical strength High biodegradability rate | Electrospinning Lyophilization Salt-leaching 3D printing (fused deposition modeling) | Supercritical carbon dioxide Lyophilization combined with gas plasma Peracetic acid Ethanol UV irradiation |
| Synthetic polymers | Biocompatibility Appropriate mechanical stability Controlled biodegradation rate | Lack of degradation (in the group of non-biodegradable polymers) | Salt-leaching 3D printing (fused deposition modeling) Fused deposition modeling Stereolithography | Plasma sterilization (Hydrogen peroxide) Supercritical carbon dioxide Ethanol Antibiotics Dry heat Electron beam irradiation Gamma irradiation UV irradiation Ethylene oxide |
| Hydrogels | Biocompatibility Osteoconductivity, Cell adhesion, proliferation Hydrophilic characteristics Porosity | Poor mechanical strength | Electrospinning 3D printing | Ethanol Ethylene oxide Autoclaving Supercritical carbon dioxide Lyophilization Electron beam irradiation Gamma irradiation UV irradiation |
| Bioceramic scaffolds | Biocompatibility Porosity Osteoconductive and osteoinductive properties High mechanical strength Individualized scaffolds Easily sterilized and visualized Controlled pore size | Brittle structure (HA) | Particle/salt leaching Gas foaming Phase separation Selective laser sintering Fused deposition modeling Electron beam melting Stereolithography | Steam Dry heat Ethylene oxide Electron beam irradiation Gamma irradiation UV irradiation |
| Metallic scaffolds | Biocompatibility High mechanical strength and stiffness Osteoconductive properties Ability to promote osteointegration Individualized scaffolds Easily sterilized and visualized Controlled pore size | Corrosion | Stereolithography Electron beam melting Selective laser melting | Steam Dry heat Ethylene oxide (ETO) Electron beam irradiation Gamma irradiation UV irradiation |
| Biological scaffolds | Biomimetic properties Identical microstructure and porosity | Foreign body and inflammatory response | Lyophilization | Supercritical carbon dioxide Gamma irradiation |
| Composite scaffolds | Combination of different scaffolds advantages and compensating disadvantages | Combination of different scaffolds advantages and compensating disadvantages Limited new bone formation | Electrospinning Lyophilization Particle/salt leaching Gas foaming Phase separation Additive manufacturing techniques (selective laser sintering, fused deposition modeling and electron beam melting) Stereolithography | Electron beam irradiation Gamma irradiation UV irradiation |
| Source of MMSCs | Differentiation Potential |
|---|---|
| Bone marrow MMSCs | Osteoblasts, Adipocytes, Myocytes, Neurons, Astrocytes, Hepatocytes, Cardiomyocytes, Chondrocytes, Mesangial cells |
| Adipose tissue MMSCs | Chondrocytes, Osteoblasts, Adipocytes, Myocytes |
| Tooth pulp MMSCs | Odontoblasts, Chondrocytes, Osteoblasts, Adipocytes |
| Muscle tissue MMSCs | Osteoblasts, Adipocytes, Chondrocytes, Neurons, Endothelial cells |
| Growth Factors | Sources | Functions |
|---|---|---|
| Bone Morphogenic Protein (BMP) |
MMSCs Osteoblasts Endothelial cells Chondrocytes | Induction of the bone growth Cells migration, proliferation, differentiation |
| Vascular Endothelial Growth Factor (VEGF) | Platelets Osteoblasts Chondrocytes Endothelial cells | Angiogenesis (regulation of migration and proliferation of endothelial cells) |
| Platelet-Derived Growth Factor (PDGF) | Platelets Osteoblasts Endothelial cells | Bone cells proliferation Angiogenesis |
| Transforming Growth Factor-Beta (TGF-β) | Platelets Osteoblasts Chondrocytes Endothelial cells Fibroblasts | Induction of the bone growth Osteoprogenitor cells migration, proliferation, differentiation |
| Fibroblast Growth Factor (FGF) | MMSCs Osteoblasts Chondrocytes Endothelial cells | Induction of the bone growth Angiogenesis Osteoblasts proliferation |
| Insulin-Like Growth Factor (IGF) | Osteoblasts Chondrocytes Endothelial cells | Osteoblasts proliferation ECM synthesis stimulate Osteoclasts proliferation |
| Vascularization Strategy | Vascularization Technique |
|---|---|
| Use of the angiogenic GF (VEGF, PDGF, FGF) | Direct incorporation of the GF in the scaffold |
| Use of the angiogenic cell cultures | Direct delivery of endothelial cells into the implantation site (scaffold-based or scaffold free techniques) |
| Hypoxia-induced vascularization | Promoting the proliferation and sprouting of endothelial cells Recruitment of pericytes Inducing the expression of proangiogenic factors |
| Use of microvascular adipose tissue fragments | Seeding of the microvascular isolates onto the scaffolds |
| 3D-bioprinting | Combining angiogenic GF and angiogenic cells with 3D-printing techniques (Laser-based methods, Extrusion printing) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsiklin, I.L.; Shabunin, A.V.; Kolsanov, A.V.; Volova, L.T. In Vivo Bone Tissue Engineering Strategies: Advances and Prospects. Polymers 2022, 14, 3222. https://doi.org/10.3390/polym14153222
Tsiklin IL, Shabunin AV, Kolsanov AV, Volova LT. In Vivo Bone Tissue Engineering Strategies: Advances and Prospects. Polymers. 2022; 14(15):3222. https://doi.org/10.3390/polym14153222
Chicago/Turabian StyleTsiklin, Ilya L., Aleksey V. Shabunin, Alexandr V. Kolsanov, and Larisa T. Volova. 2022. "In Vivo Bone Tissue Engineering Strategies: Advances and Prospects" Polymers 14, no. 15: 3222. https://doi.org/10.3390/polym14153222
APA StyleTsiklin, I. L., Shabunin, A. V., Kolsanov, A. V., & Volova, L. T. (2022). In Vivo Bone Tissue Engineering Strategies: Advances and Prospects. Polymers, 14(15), 3222. https://doi.org/10.3390/polym14153222








