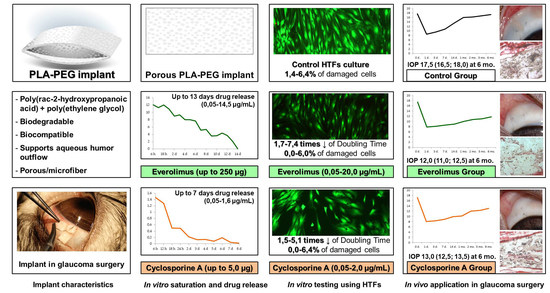
PLA-PEG Implant as a Drug Delivery System in Glaucoma Surgery: Experimental Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Saturation of Glaucoma PLA-PEG Implants with CsA or Everolimus
2.2. In Vitro Drug Release Examination
2.3. In Vitro Cell Experiments
2.3.1. Human Tenon’s Fibroblasts Primary Culture
2.3.2. Examination of HTFs Inhibition
2.4. In Vivo Study
2.4.1. Animals
2.4.2. Experiment Design
2.4.3. Anesthesia
2.4.4. Implant Preparation
2.4.5. Surgical Technique
2.4.6. Pre- and Postoperative Examinations
2.4.7. Histologic Examination
2.5. Statistical Analyses
3. Results
3.1. In Vitro Saturation and Desorption
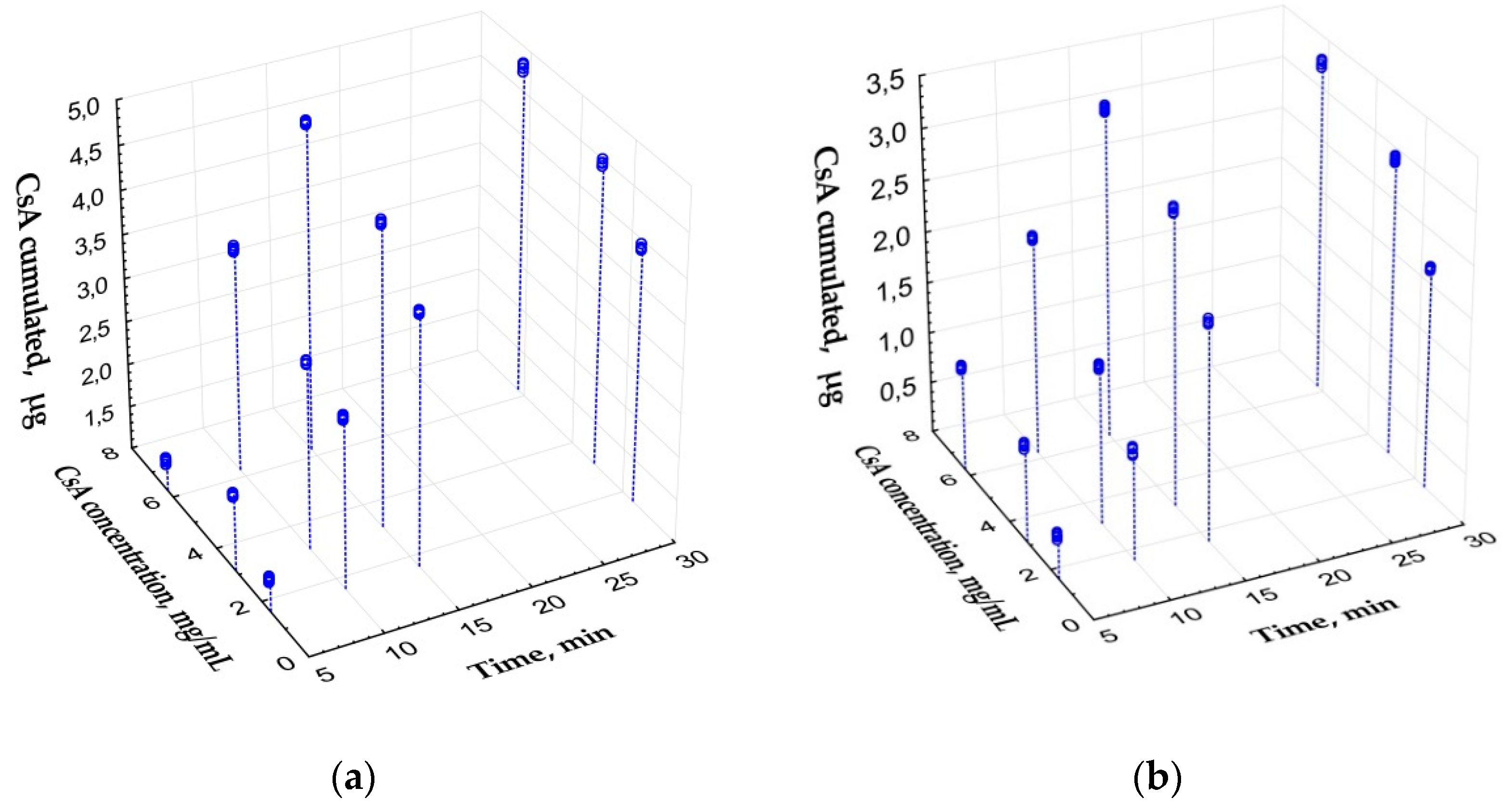
3.1.1. Saturation of PLA-PEG Implants with CsA
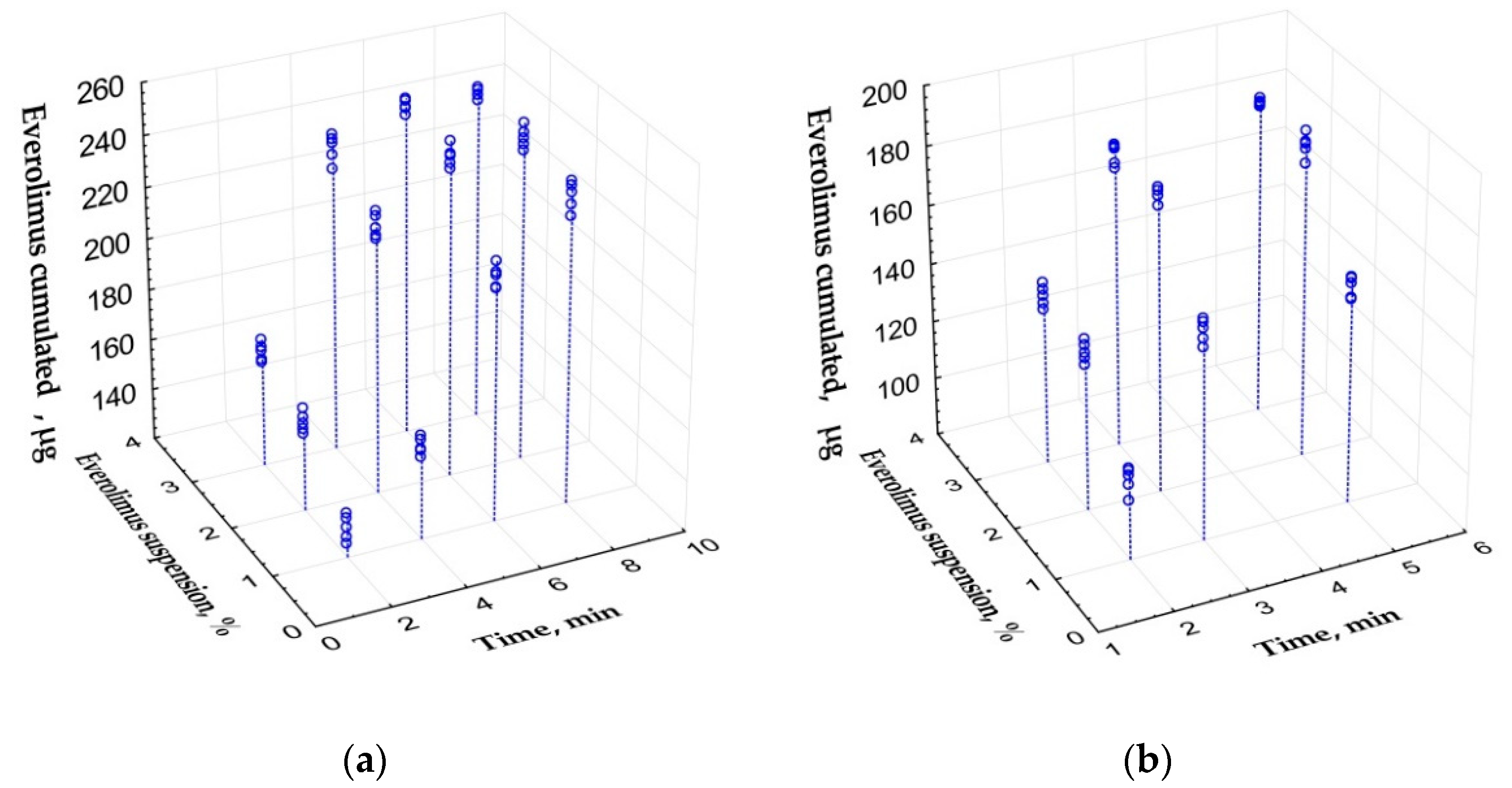
3.1.2. Saturation of PLA-PEG Implants with Everolimus
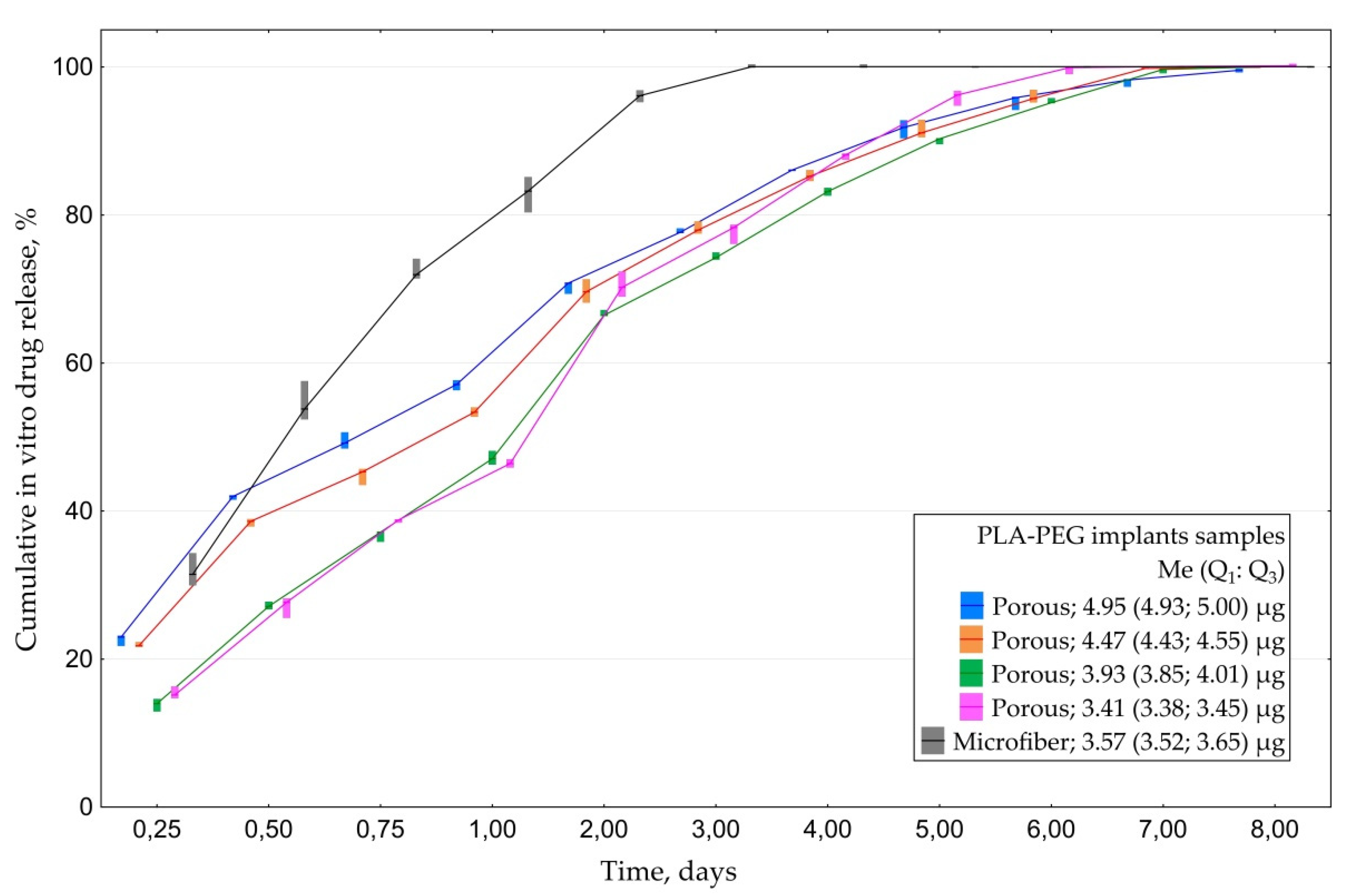
3.1.3. In Vitro CsA Release
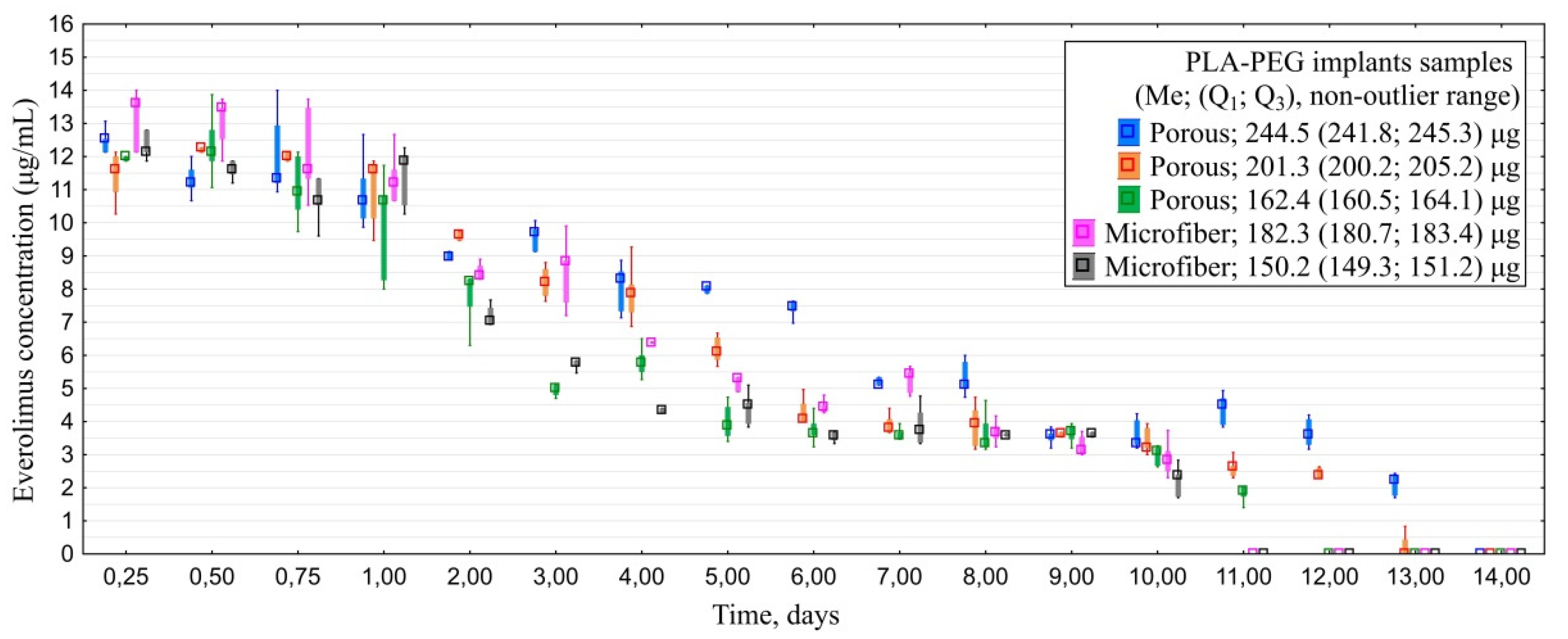
3.1.4. In Vitro Everolimus Release
3.2. In Vitro Cell Culture Study
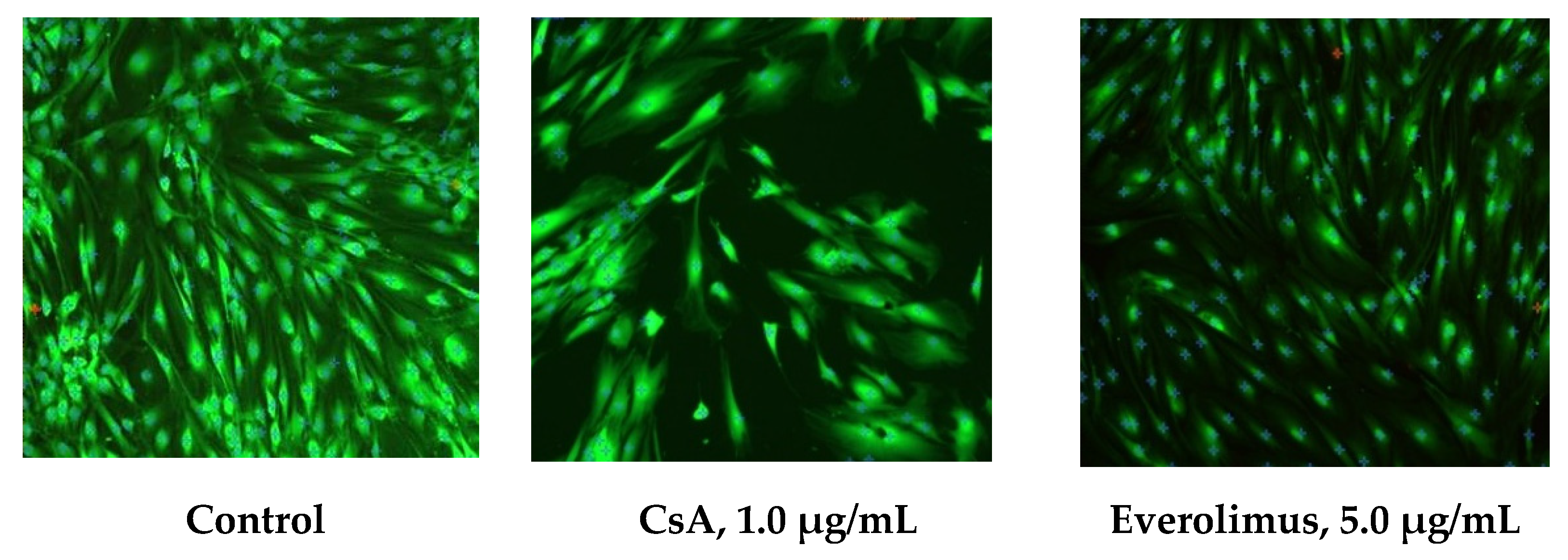
3.2.1. In Vitro HTFs Inhibition
3.2.2. In Vitro Cytotoxicity Evaluation
3.3. In Vivo Surgery Results
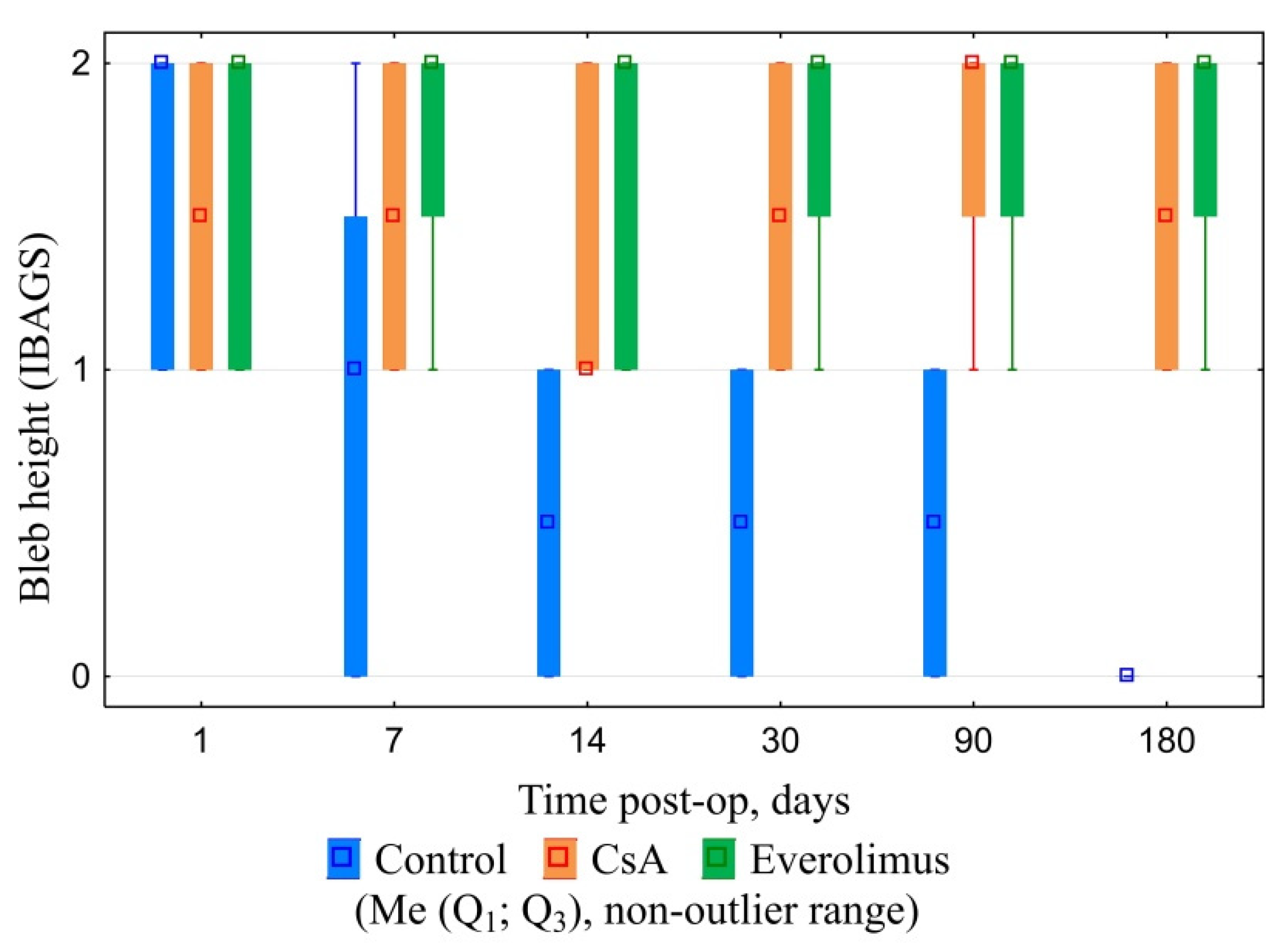
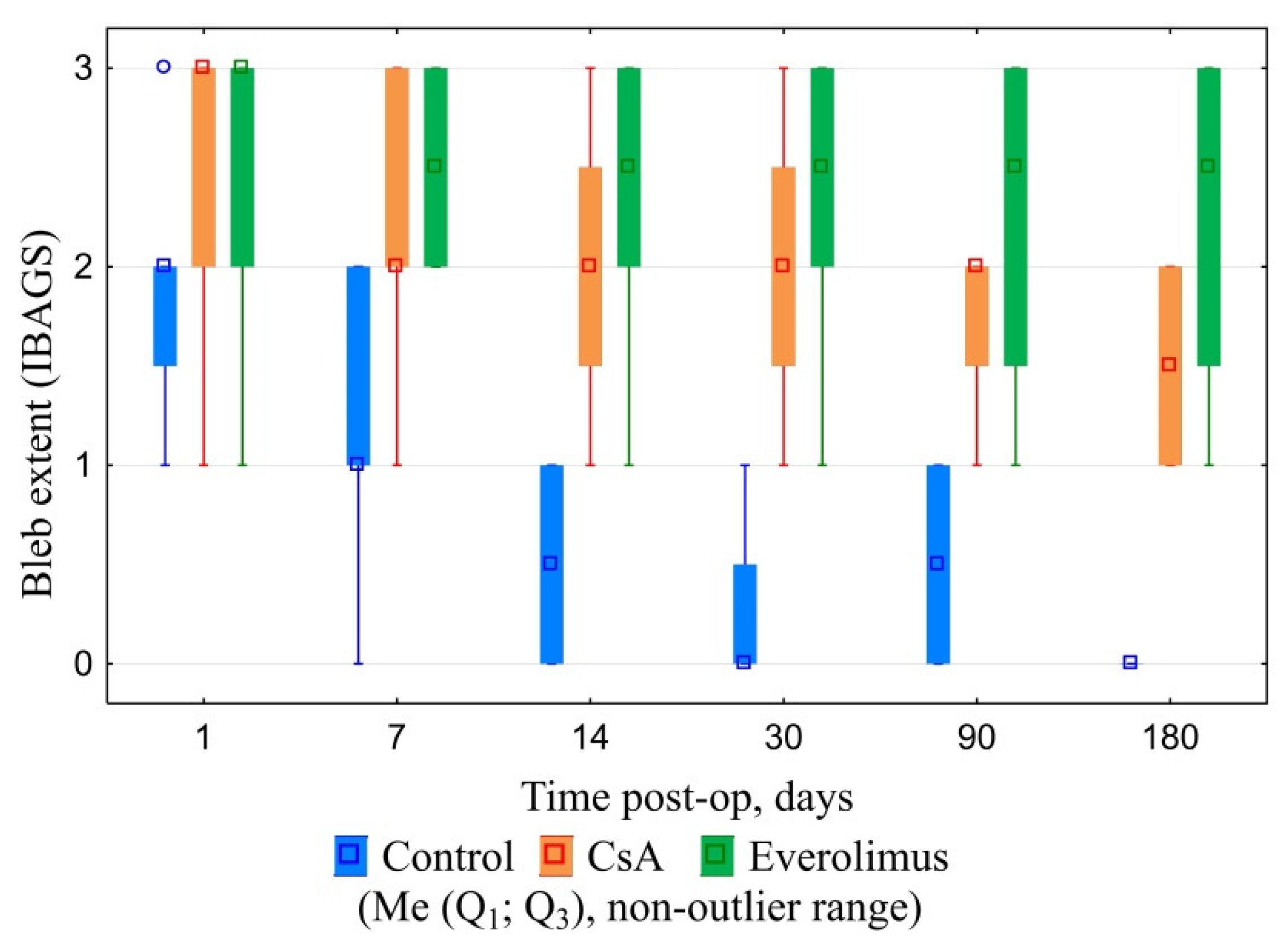
3.3.1. Ophthalmic Examination
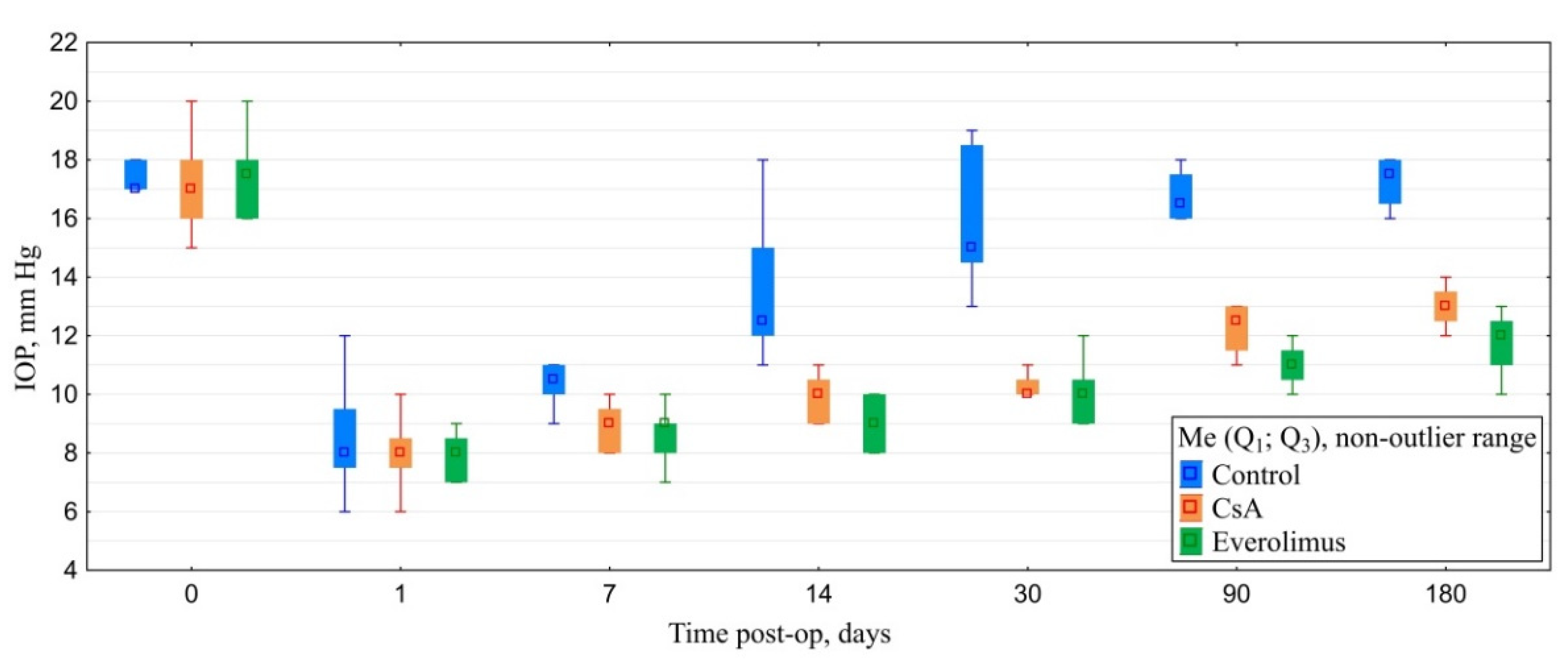
3.3.2. Postoperative IOP Dynamics
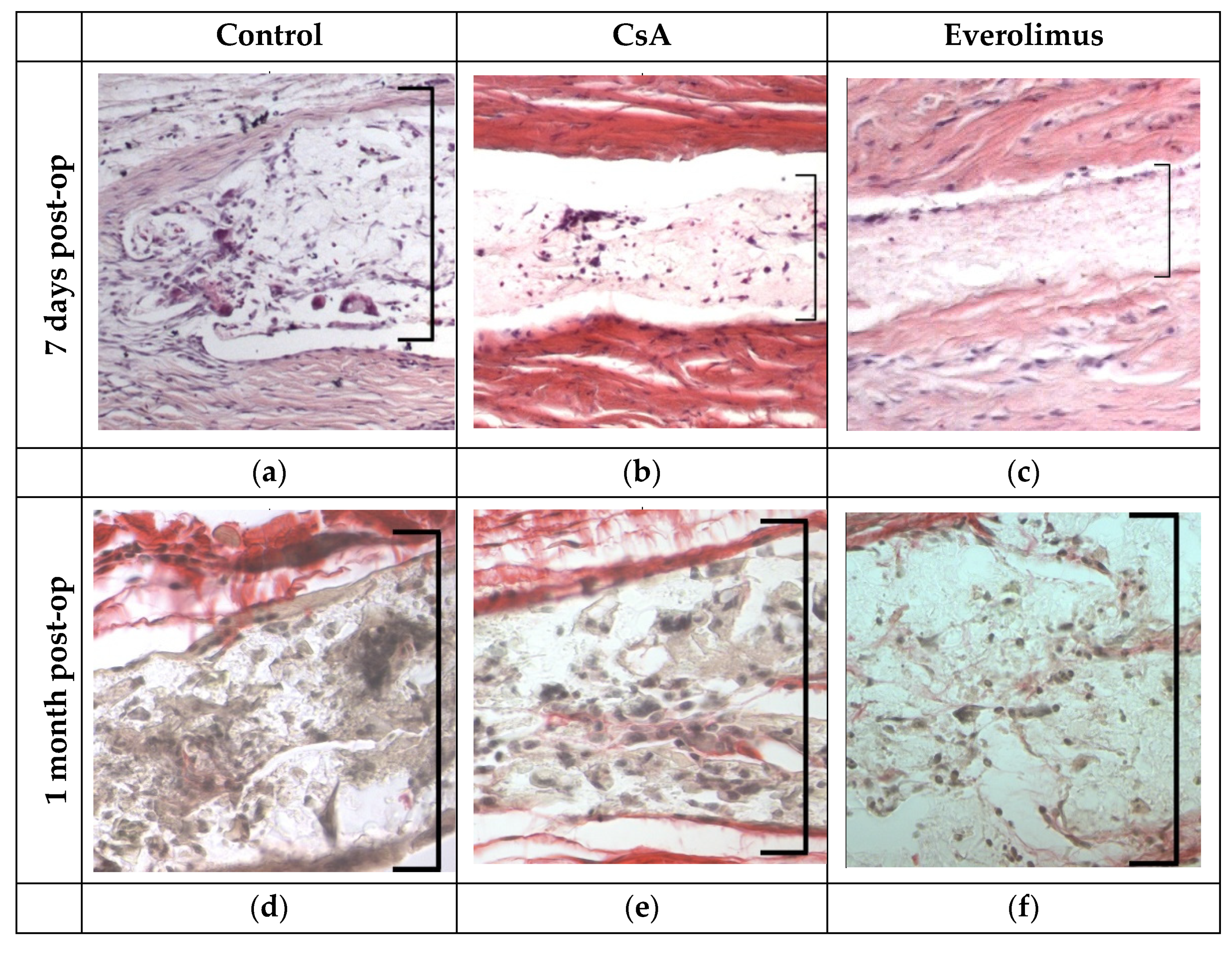
3.3.3. Histological Examination
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zada, M.; Pattamatta, U.; White, A. Modulation of Fibroblasts in Conjunctival Wound Healing. Ophthalmology 2018, 125, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Masoumpour, M.B.; Nowroozzadeh, M.H.; Razeghinejad, M.R. Current and Future Techniques in Wound Healing Modulation after Glaucoma Filtering Surgeries. Open Ophthalmol. J. 2016, 10, 68–85. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Holló, G. Wound Healing and Glaucoma Surgery: Modulating the Scarring Process with Conventional Antimetabolites and New Molecules. Dev. Ophthalmol. 2017, 59, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef]
- Jayaram, H. Intraocular pressure reduction in glaucoma: Does every mmHg count? Taiwan J. Ophthalmol. 2020, 10, 255–258. [Google Scholar] [CrossRef]
- Burr, J.; Azuara-Blanco, A.; Avenell, A.; Tuulonen, A. Medical versus surgical interventions for open angle glaucoma. Cochrane Database Syst. Rev. 2012, 9, CD004399. [Google Scholar] [CrossRef]
- Chong, R.S.; Crowston, J.G.; Wong, T.T. Experimental models of glaucoma filtration surgery. Acta Ophthalmol. 2021, 99, 9–15. [Google Scholar] [CrossRef]
- Van Bergen, T.; Van de Velde, S.; Vandewalle, E.; Moons, L.; Stalmans, I. Improving patient outcomes following glaucoma surgery: State of the art and future perspectives. Clin. Ophthalmol. 2014, 8, 857–867. [Google Scholar] [CrossRef]
- Yamanaka, O.; Kitano-Izutani, A.; Tomoyose, K.; Reinach, P.S. Pathobiology of wound healing after glaucoma filtration surgery. BMC Ophthalmol. 2015, 15 (Suppl. S1), 157. [Google Scholar] [CrossRef]
- Trelford, C.B.; Denstedt, J.T.; Armstrong, J.J.; Hutnik, C.M.L. The Pro-Fibrotic Behavior of Human Tenon’s Capsule Fibroblasts in Medically Treated Glaucoma Patients. Clin. Ophthalmol. 2020, 14, 1391–1402. [Google Scholar] [CrossRef]
- Boimer, C.; Birt, C.M. Preservative exposure and surgical outcomes in glaucoma patients: The PESO study. J. Glaucoma 2013, 22, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, M.; Indar, A.; Wormald, R. Intra-operative mitomycin C for glaucoma surgery. Cochrane Database Syst. Rev. 2001, 1, CD002897. [Google Scholar] [CrossRef]
- Green, E.; Wilkins, M.; Bunce, C.; Wormald, R. 5-Fluorouracil for glaucoma surgery. Cochrane Database Syst. Rev. 2014, 2, CD001132. [Google Scholar] [CrossRef] [PubMed]
- Fan Gaskin, J.C.; Nguyen, D.Q.; Soon Ang, G.; O’Connor, J.; Crowston, J.G. Wound Healing Modulation in Glaucoma Filtration Surgery-Conventional Practices and New Perspectives: The Role of Antifibrotic Agents (Part I). J. Curr. Glaucoma Pract. 2014, 8, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Andrés-Guerrero, V.; Perucho-González, L.; García-Feijoo, J.; Morales-Fernández, L.; Saenz-Frances, F.; Herrero-Vanrell, R.; Júlvez, L.P.; Llorens, V.P.; Martinez-De-La-Casa, J.M.; Konstas, A.-G.P. Current Perspectives on the Use of Anti-VEGF Drugs as Adjuvant Therapy in Glaucoma. Adv. Ther. 2017, 34, 378–395. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.W.; Cheng, S.W.; Wei, R.L.; Lu, G.C. Anti-vascular endothelial growth factor for control of wound healing in glaucoma surgery. Cochrane Database Syst. Rev. 2016, 2016, CD009782. [Google Scholar] [CrossRef]
- Fakhraie, G.; Lopes, J.F.; Spaeth, G.L.; Almodin, J.; Ichhpujai, P.; Moster, M.R. Effects of postoperative cyclosporine ophthalmic emulsion 0.05% (Restasis) following glaucoma surgery. Clin. Exp. Ophthalmol. 2009, 37, 842–848. [Google Scholar] [CrossRef]
- Dai, Z.X.; Song, X.L.; Yu, X.B.; Sun, J.G.; Sun, X.H. Cyclosporine A-loaded drug delivery systems inhibit scar formation after glaucoma surgery in rabbits. Chin. Med. J. 2019, 132, 1381–1384. [Google Scholar] [CrossRef]
- Sun, J.; Liu, X.; Lei, Y.; Tang, M.; Dai, Z.; Yang, X.; Yu, X.; Yu, L.; Sun, X.; Ding, J. Sustained subconjunctival delivery of cyclosporine A using thermogelling polymers for glaucoma filtration surgery. J. Mater. Chem. B 2017, 31, 6400–6411. [Google Scholar] [CrossRef]
- Cinik, R.; Yüksel, N.; Pirhan, D.; Aslan, M.Ş.; Subaşı, C.; Karaöz, E. The Effect of Everolimus on Scar Formation in Glaucoma Filtering Surgery in a Rabbit Model. Curr. Eye Res. 2016, 41, 1438–1446. [Google Scholar] [CrossRef]
- Faulds, D.; Goa, K.L.; Benfield, P. Erratum to: Cyclosporin: A Review of its Pharmacodynamic and Pharmacokinetic Properties, and Therapeutic Use in Immunoregulatory Disorders. Drugs 1993, 46, 377. [Google Scholar] [CrossRef]
- Matsuda, S.; Koyasu, S. Mechanisms of action of cyclosporine. Immunopharmacology 2000, 47, 119–125. [Google Scholar] [CrossRef]
- Houghton, P.J. Everolimus. Clin. Cancer Res. 2010, 16, 1368–1372. [Google Scholar] [CrossRef] [PubMed]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Mukhopadhyay, S.; Tung, K.; Patel, D.; Foster, D.A. Rapamycin-induced G1 cell cycle arrest employs both TGF-β and Rb pathways. Cancer Lett. 2015, 360, 134–140. [Google Scholar] [CrossRef]
- Stepanov, A.V.; Gamzaeva, U.S. Drainage surgery of glaucoma. Russ. Pediatric Ophthalmol. 2016, 11, 158–164. [Google Scholar] [CrossRef]
- Khodzhaev, N.S.; Sidorova, A.V.; Kolomeytsev, M.N. Basic characteristics of antiglaucomatous drainages. Fyodorov J. Ophthalmic Surg. 2017, 4, 80–86. [Google Scholar] [CrossRef]
- García-Estrada, P.; García-Bon, M.A.; López-Naranjo, E.J.; Basaldúa-Pérez, D.N.; Santos, A.; Navarro-Partida, J. Polymeric Implants for the Treatment of Intraocular Eye Diseases: Trends in Biodegradable and Non-Biodegradable Materials. Pharmaceutics 2021, 13, 701. [Google Scholar] [CrossRef]
- Pawar Rajendra, P.; Tekale Sunil, U.; Shisodia Suresh, U.; Totre Jalinder, T.; Domb Abraham, J. Biomedical Applications of Poly(Lactic Acid). Recent Pat. Regen. Med. 2014, 4, 40–51. [Google Scholar] [CrossRef]
- Slonimskij, A.Y.; Alekseev, I.B.; Dolgij, S.S.; Korigodskij, A.R. New biodegradable drainage “Glautex” in the surgical treatment of glaucoma. Glaucoma 2012, 4, 55–59. [Google Scholar]
- Khodjaev, N.S.; Kolomeytsev, M.N.; Sidorova, A.V.; Molchanov, V.V.; Sedush, N.G.; Tenchurin, T.H. Experimental-Morphological Study of Several Types of Fibrillar Structured Drainages for Glaucoma Surgery. Ophthalmol. Russ. 2018, 15, 211–219. [Google Scholar] [CrossRef]
- Kolomeytsev, M.N.; Khodzhaev, N.S.; Sidorova, A.V. Poverhnostnyj mehanizm rezorbcii drenazhej kak faktor, sposobstvujushhij povysheniju gipotenzivnoj jeffektivnosti antiglaukomatoznyh operacij. Sovrem. Tehnol. V Oftalmol. 2018, 4, 119–122. [Google Scholar]
- Lattanzio, F.; Crouch, E.R.; Mitrev, P.V.; Williams, P.B.; Allen, R.C. Cyclosporin as an adjunct to glaucoma filtration surgery. J. Glaucoma 2005, 14, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Crowston, J.G.; Cordeiro, M.F.; Akbar, A.N.; Khaw, P.T. The role of the immune system in conjunctival wound healing after glaucoma surgery. Surv. Ophthalmol. 2000, 45, 49–68. [Google Scholar] [CrossRef]
- Clark, R.A.F.; Henson, P.M. Overview and General Considerations of Wound Repair. In The Molecular and Cellular Biology of Wound Repair, 2nd ed.; Plenum Press: New York, NY, USA, 1996; pp. 3–33. [Google Scholar]
- Yan, Z.C.; Bai, Y.J.; Tian, Z.; Hu, H.Y.; You, X.H.; Lin, J.X.; Liu, S.R.; Zhuo, Y.H.; Luo, R.J. Anti-proliferation effects of Sirolimus sustained delivery film in rabbit glaucoma filtration surgery. Mol. Vis. 2011, 17, 2495–2506. [Google Scholar]
- Eren, K.; Turgut, B.; Akin, M.M.; Demir, T. The Suppression of Wound Healing Response with Sirolimus and Sunitinib Following Experimental Trabeculectomy in a Rabbit Model. Curr. Eye Res. 2016, 41, 367–376. [Google Scholar] [CrossRef]
- Lallemand, F.; Schmitt, M.; Bourges, J.L.; Gurny, R.; Benita, S.; Garrigue, J.S. Cyclosporine A delivery to the eye: A comprehensive review of academic and industrial efforts. Eur. J. Pharm. Biopharm. 2017, 117, 14–28. [Google Scholar] [CrossRef] [PubMed]
- Garweg, J.G.; Wegmann-Burns, M.; Goldblum, D. Effects of daunorubicin, mitomycin C, azathioprine and cyclosporin A on human retinal pigmented epithelial, corneal endothelial and conjunctival cell lines. Graefes Arch. Clin. Exp. Ophthalmol. 2006, 244, 382–389. [Google Scholar] [CrossRef][Green Version]
- Pérez-Rico, C.; Germain, F.; Castro-Rebollo, M.; Moreno-Salgueiro, A.; Teus, M.Á. Effect of topical 0.05% cyclosporine A on corneal endothelium in patients with dry eye disease. Int. J. Ophthalmol. 2013, 6, 471–474. [Google Scholar] [CrossRef][Green Version]
- Tang-Liu, D.D.; Acheampong, A. Ocular pharmacokinetics and safety of ciclosporin, a novel topical treatment for dry eye. Clin. Pharmacokinet. 2005, 44, 247–261. [Google Scholar] [CrossRef]
- Aono, J.; Ruiz-Rodriguez, E.; Qing, H.; Findeisen, H.M.; Jones, K.L.; Heywood, E.B.; Bruemmer, D. Telomerase Inhibition by Everolimus Suppresses Smooth Muscle Cell Proliferation and Neointima Formation Through Epigenetic Gene Silencing. JACC Basic Transl. Sci. 2016, 1, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Böhm, M.; Stegemann, A.; Metze, D.; Scharffetter-Kochanek, K.; Sindrilaru, A. The mammalian target of rapamycin inhibitor everolimus suppresses proliferation, metabolic activity and collagen synthesis of human fibroblasts in vitro and exerts antifibrogenic effects in vivo. Br. J. Dermatol. 2017, 177, e130–e132. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.K.; Chen, Y.H.; Kuo, Y.H.; Ke, M.C.; Tseng, Y.C.; Wu, P.C. Evaluation of the Effect of Everolimus on Retinal Pigment Epithelial Cells and Experimental Proliferative Vitreoretinopathy. Curr. Eye Res. 2018, 43, 333–339. [Google Scholar] [CrossRef]
- Leonardi, A.; DeFranchis, G.; Fregona, I.A.; Violato, D.; Plebani, M.; Secchi, A.G. Effects of cyclosporin A on human conjunctival fibroblasts. Arch. Ophthalmol. 2001, 119, 1512–1517. [Google Scholar] [CrossRef] [PubMed]
- Viveiros, M.M.; Kakizaki, F.Y.; Hércules, L.A.; Padovani, C.R.; Candeias, J.M.; Schellini, S.A. In vitro study of cyclosporine A 0.05% on primary and recurrent pterygium fibroblasts. Int. Ophthalmol. 2016, 36, 237–242. [Google Scholar] [CrossRef] [PubMed]





| CsA Conc, μg/mL | PI, rel. un. | DT, h | PDL, PI, rel. un. |
|---|---|---|---|
| 0.00 | 2.35 (1.92; 2.36) | 39.0 (38.7; 50.9) | 1.55 (1.53; 1.56) |
| 0.05 | 1.70 (1.63; 1.75) * | 62.9 (59.5; 67.8) * | 0.96 (0.91; 0.97) * |
| 0.20 | 1.52 (1.41; 1.56) * | 79.2 (75.3; 96.6) * | 0.75 (0.73; 0.76) * |
| 0.50 | 1.46 (1.41; 1.48) * | 88.1 (84.9; 97.8) * | 0.62 (0.54; 0.62) * |
| 1.00 | 1.38 (1.37: 1.41) * | 102.4 (97.4; 107.0) * | - |
| 2.00 | 1.22 (1.17; 1.29) * | 182.5 (155.0; 208.7) * | 0.70 (0.55; 0.75) * |
| Everolimus Conc, μg/mL | PI, rel. un. | DT, h | PDL, rel. un. |
|---|---|---|---|
| 0.0 | 1.96 (1.94; 1.99) | 49.6 (48.2; 50.0) | 1.32 (1.31; 1.49) |
| 0.5 | 1.43 (1.21; 1.46) * | 93.1 (87.7; 175.0) * | 0.63 (0.40; 0.67) * |
| 1.0 | 1.23 (1.22; 1.23) * | 162.1 (161.3; 168.8) * | 0.41 (0.41; 0.42) * |
| 5.0 | 1.17 (1.07; 1.22) * | 188.3 (145.5; 342.3) * | 0.51 (0.51; 0.60) * |
| 10.0 | 1.13 (1.12; 1.14) * | 279.7 (262.3; 289.1) * | 0.41 (0.32; 0.54) * |
| 15.0 | 1.29 (1.28; 1.31) * | 129.3 (124.7; 133.8) * | 0.73 (0.65; 0.79) * |
| 20.0 | 1.54 (1.37; 1.59) * | 77.1 (72.2; 104.6) * | 0.85 (0.68; 0.87) * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Germanova, V.N.; Karlova, E.V.; Volova, L.T.; Zolotarev, A.V.; Rossinskaya, V.V.; Zakharov, I.D.; Korigodskiy, A.R.; Boltovskaya, V.V.; Nefedova, I.F.; Radaykina, M.V. PLA-PEG Implant as a Drug Delivery System in Glaucoma Surgery: Experimental Study. Polymers 2022, 14, 3419. https://doi.org/10.3390/polym14163419
Germanova VN, Karlova EV, Volova LT, Zolotarev AV, Rossinskaya VV, Zakharov ID, Korigodskiy AR, Boltovskaya VV, Nefedova IF, Radaykina MV. PLA-PEG Implant as a Drug Delivery System in Glaucoma Surgery: Experimental Study. Polymers. 2022; 14(16):3419. https://doi.org/10.3390/polym14163419
Chicago/Turabian StyleGermanova, Viktoriya N., Elena V. Karlova, Larisa T. Volova, Andrey V. Zolotarev, Viktoriya V. Rossinskaya, Ivan D. Zakharov, Aleksandr R. Korigodskiy, Violetta V. Boltovskaya, Irina F. Nefedova, and Mariya V. Radaykina. 2022. "PLA-PEG Implant as a Drug Delivery System in Glaucoma Surgery: Experimental Study" Polymers 14, no. 16: 3419. https://doi.org/10.3390/polym14163419
APA StyleGermanova, V. N., Karlova, E. V., Volova, L. T., Zolotarev, A. V., Rossinskaya, V. V., Zakharov, I. D., Korigodskiy, A. R., Boltovskaya, V. V., Nefedova, I. F., & Radaykina, M. V. (2022). PLA-PEG Implant as a Drug Delivery System in Glaucoma Surgery: Experimental Study. Polymers, 14(16), 3419. https://doi.org/10.3390/polym14163419