Synthesis of Covalent Organic Frameworks (COFs)-Nanocellulose Composite and Its Thermal Degradation Studied by TGA/FTIR
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
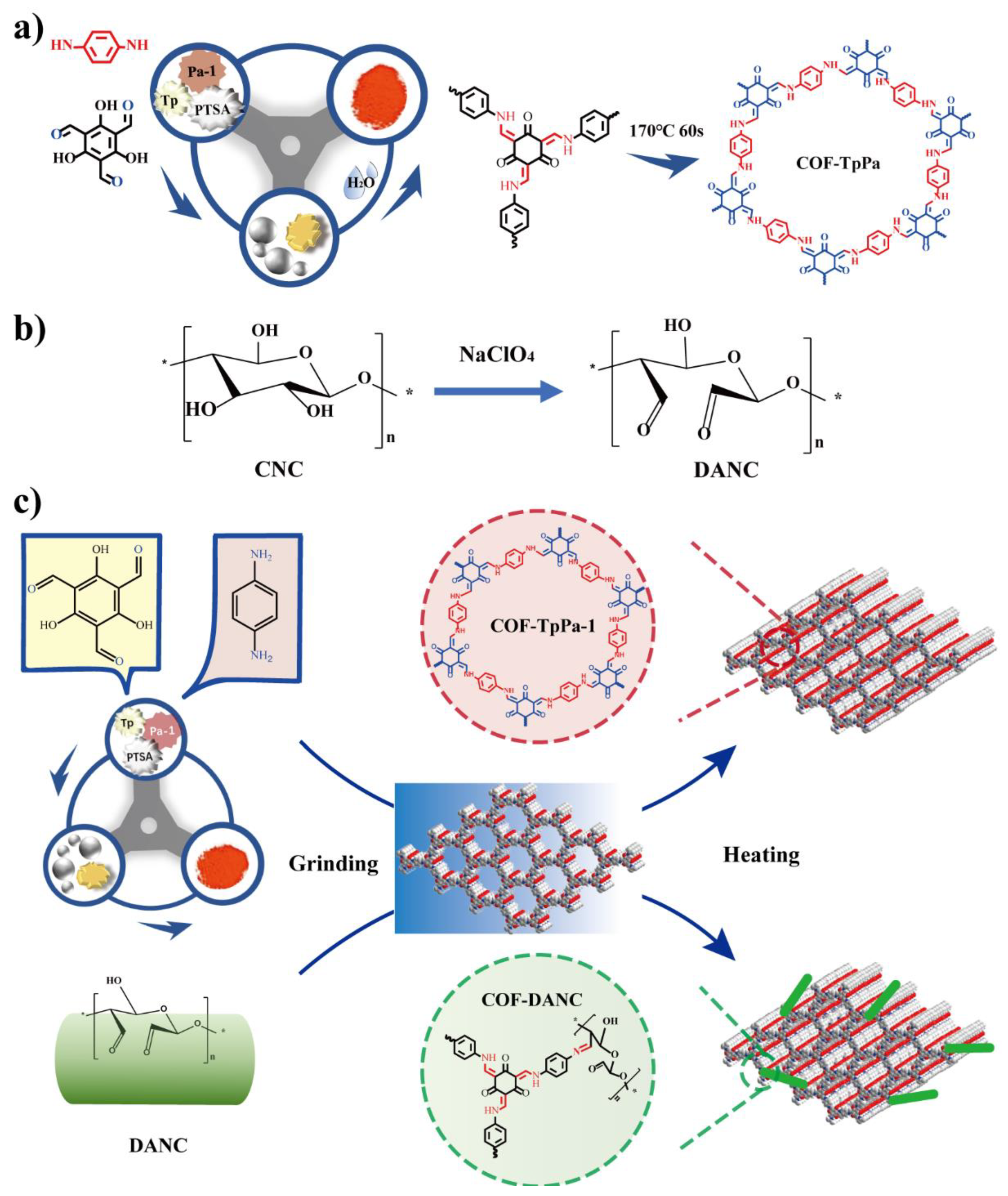
2.2. Preparation of Dialdehyde Cellulose Nanocrystals (DANC) by Periodic Acid Oxidation
2.3. Preparation of TpPa-1-DANC
2.4. Characterization
3. Results and Discussion
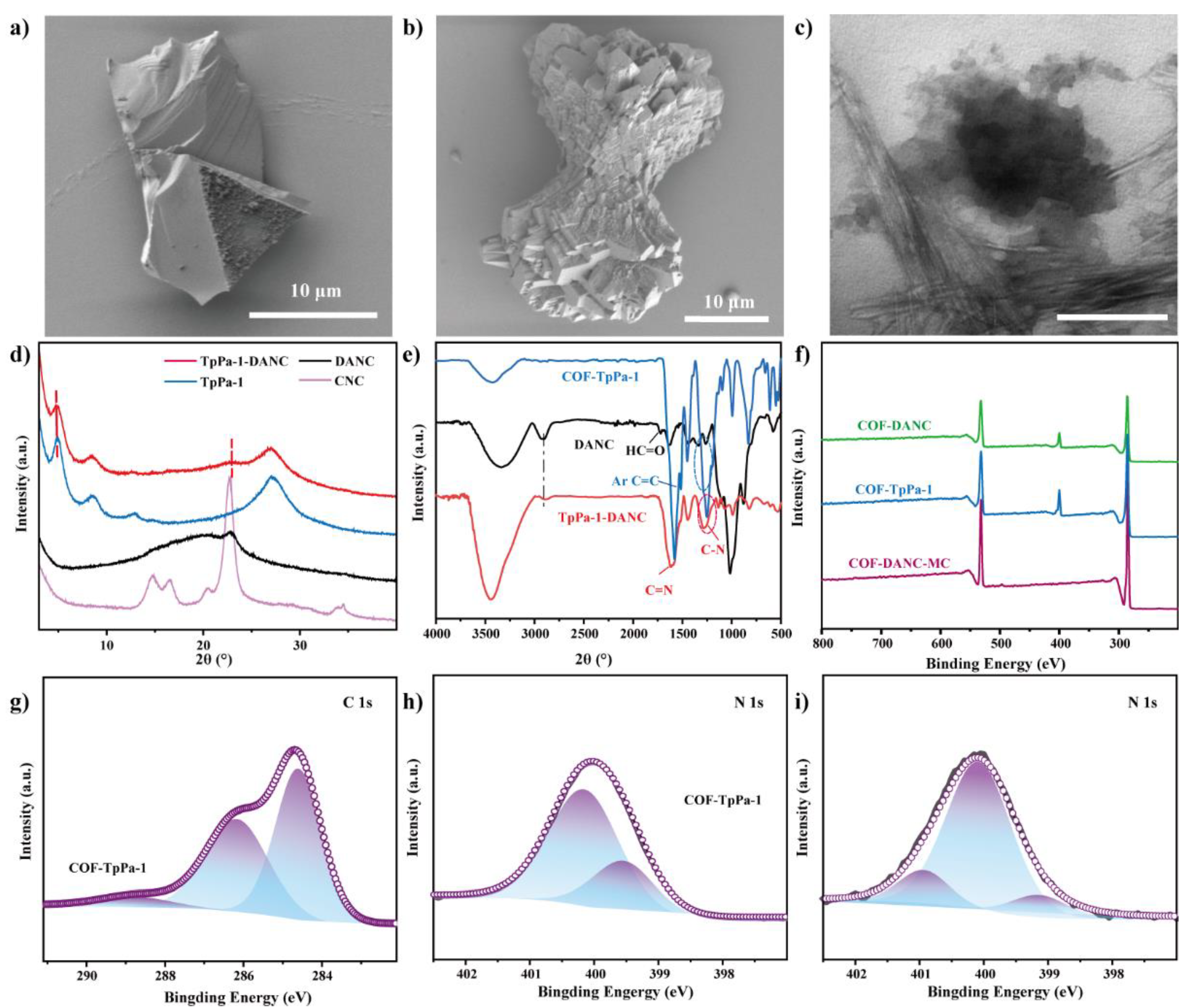
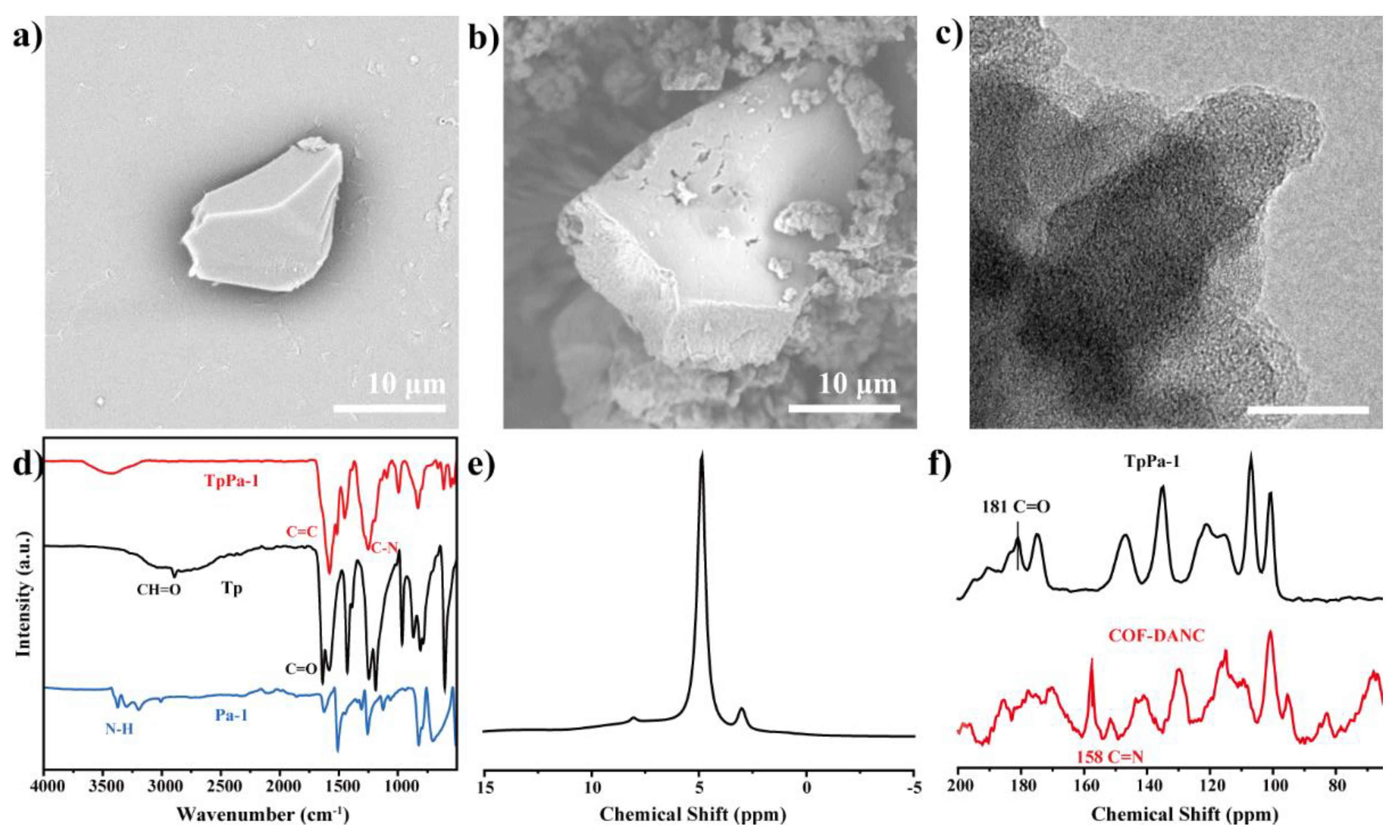
3.1. Morphology Analysis of CNCs and DANC
3.2. Characterization of COF-TpPa-1 by Mechanochemical Method
3.3. Characterization of TpPa-1-DANC Composites by Mechanochemical Method
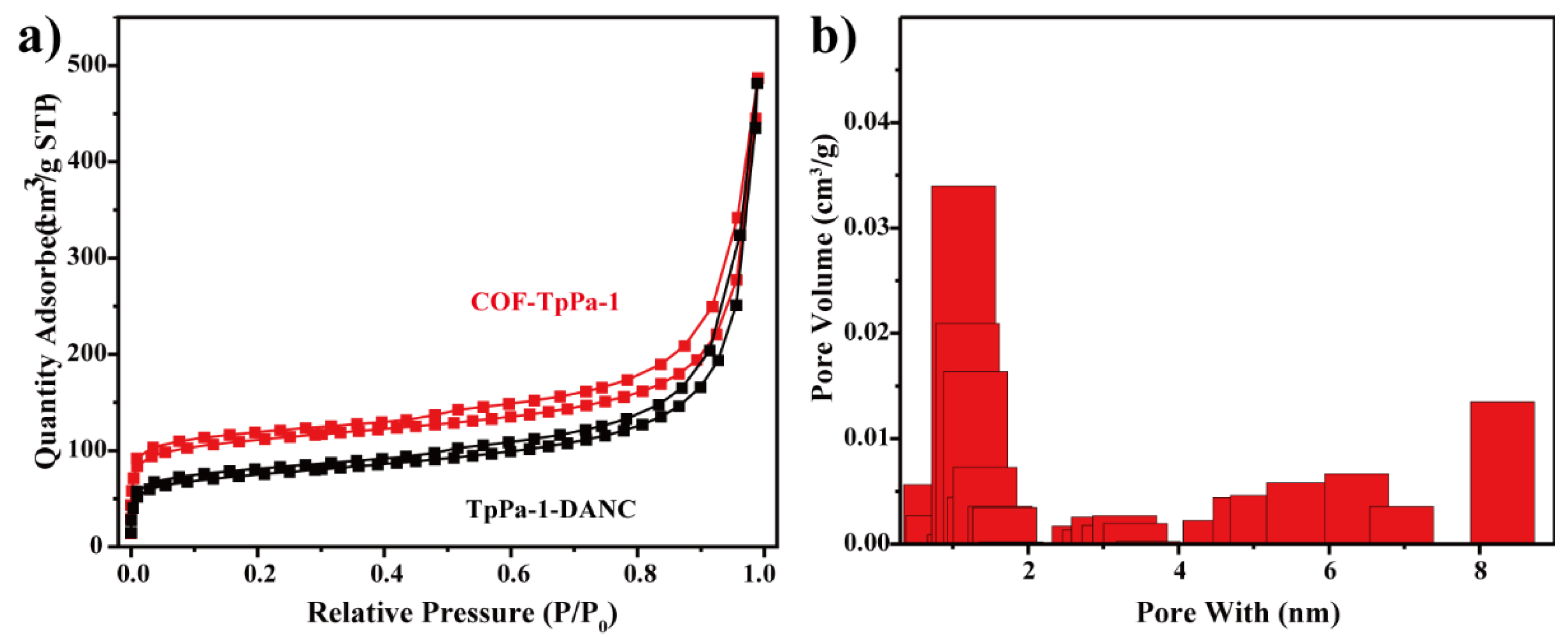
3.3.1. Physical Properties
3.3.2. Chemical Properties
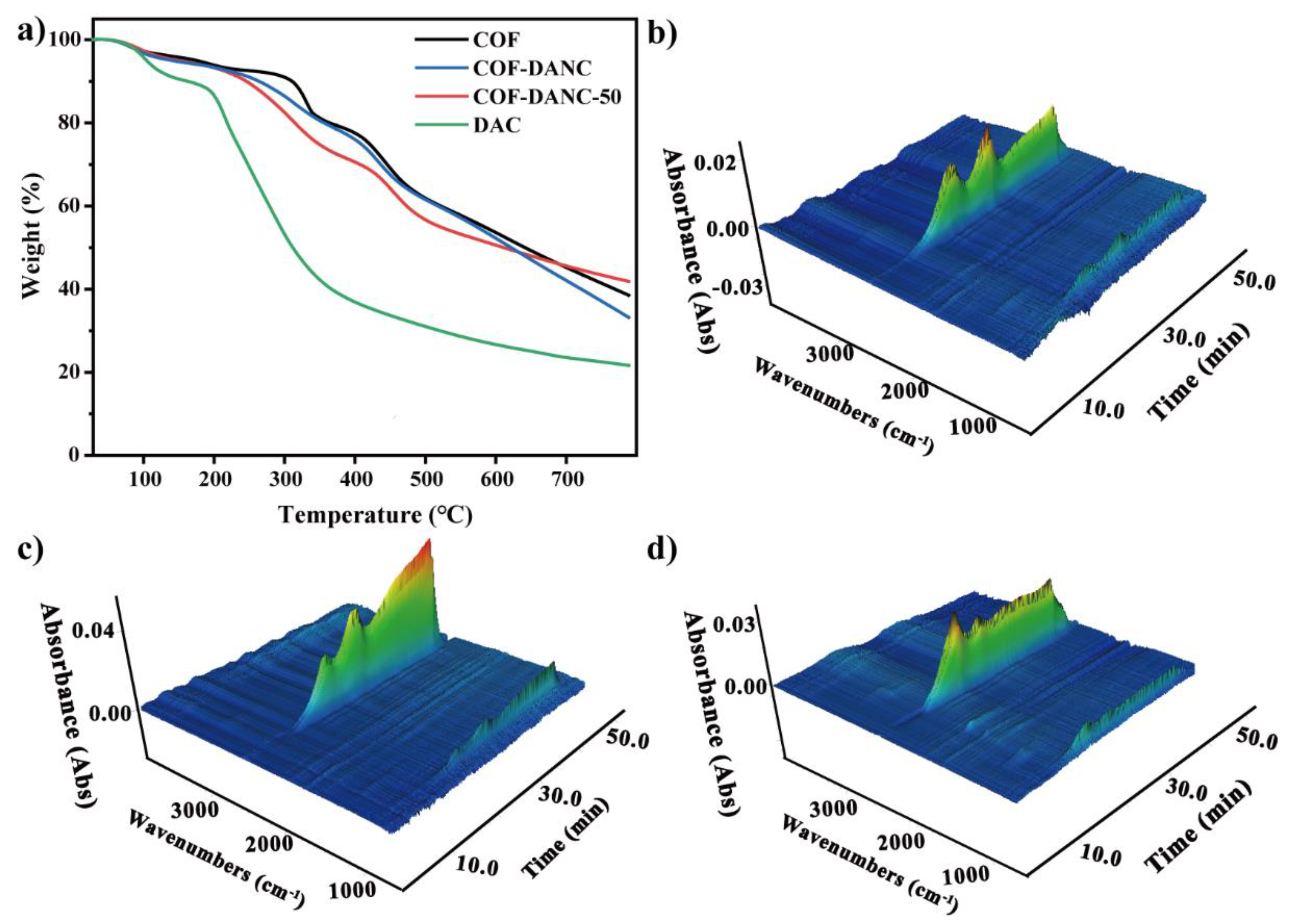
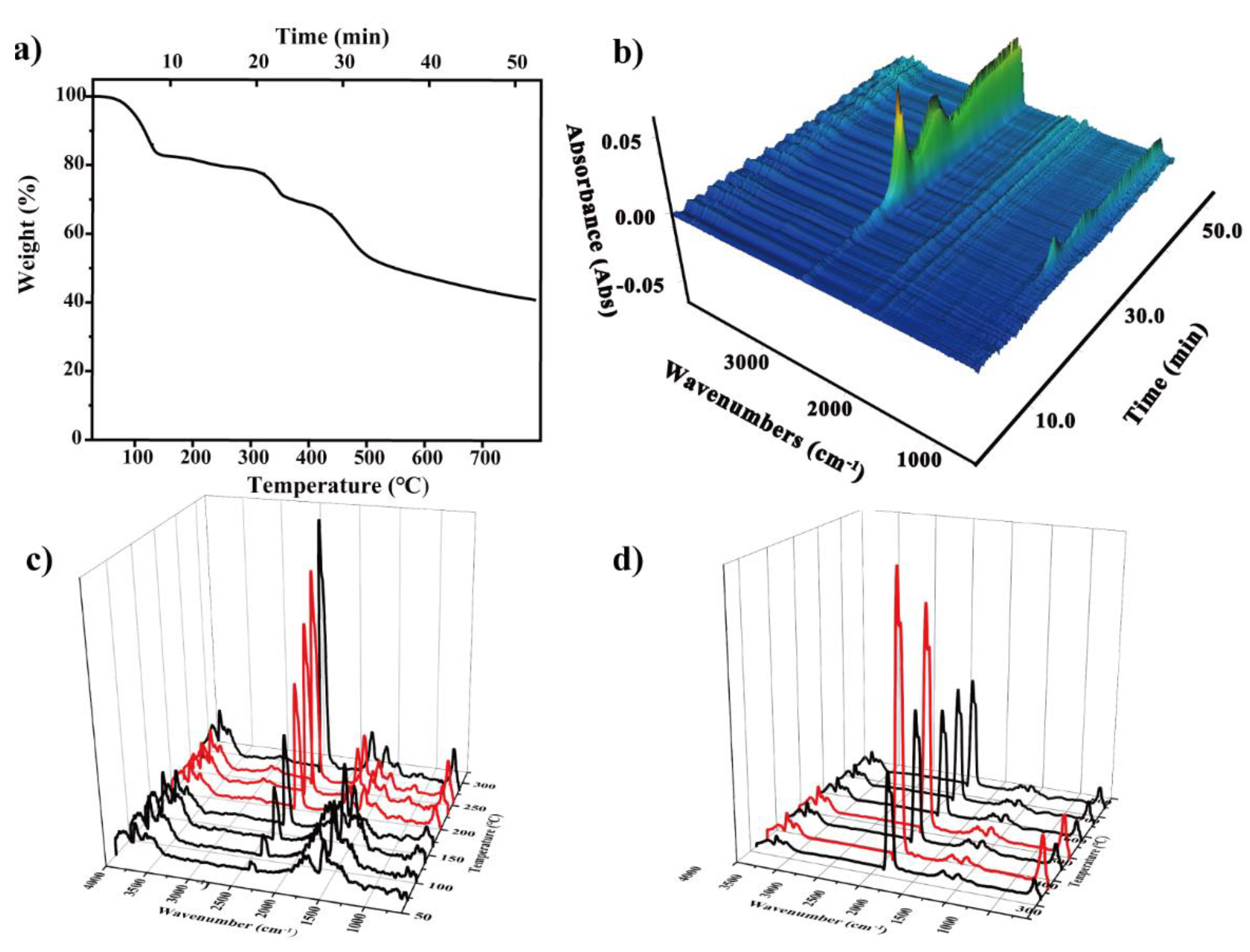
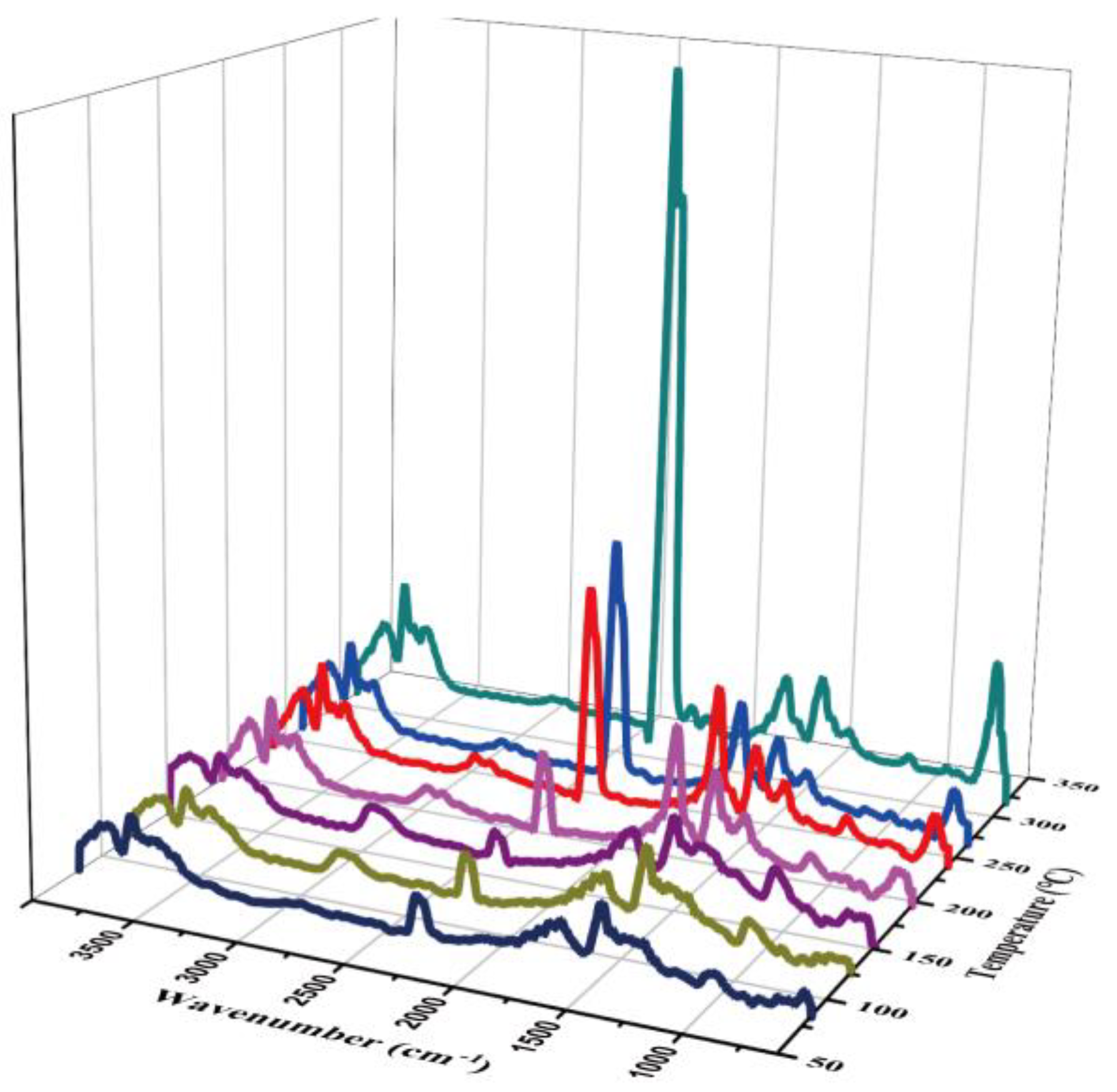
3.3.3. Thermal Analysis
- At about 100 °C, it can be seen in Figure 6 that at 100 (5 min) and 137 °C (7 min), the characteristic peak of water increased at 3400–3700 and 1500–1700 cm−1, which may come from the water on the material surface. In addition, there was a certain time error in the delay of gas transmission.
- At about 19 min (and 300 °C), the absorbance of CO increased slightly. The appearance at 1380 cm−1 indicated the vibration of the C–C skeleton. By 30.6 min, a large amount of CO2 was produced.
- The third stage was about 400 °C, which shows the large-scale cracking of the frame. With the increase in temperature, the peak value of 2300 cm− increased. The maximum weight loss peak was about 40.2 min. The absorption peak at 3500–3700 cm− showed that the gas still contains water. This means that when the temperature reached 400 °C, the composite began to decompose.
3.3.4. Structure Analysis of Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hasell, T.; Miklitz, M.; Stephenson, A.; Little, M.A.; Chong, S.Y.; Clowes, R.; Chen, L.; Holden, D.; Tribello, G.A.; Jelfs, K.E.; et al. Porous Organic Cages for Sulfur Hexafluoride Separation. J. Am. Chem. Soc. 2016, 138, 1653–1659. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-S.M.; Wu, T.-H.; Alston, B.M.; Briggs, M.E.; Hasell, T.; Hu, C.-C.; Cooper, A.I. Porosity-engineered carbons for supercapacitive energy storage using conjugated microporous polymer precursors. J. Mater. Chem. A 2016, 4, 7665–7673. [Google Scholar] [CrossRef] [Green Version]
- Benyettou, F.; Kaddour, N.; Prakasam, T.; Das, G.; Sharma, S.K.; Thomas, S.A.; Bekhti-Sari, F.; Whelan, J.; Alkhalifah, M.A.; Khair, M.; et al. In vivo oral insulin delivery via covalent organic frameworks. Chem. Sci. 2021, 12, 6037–6047. [Google Scholar] [CrossRef] [PubMed]
- Diercks, C.S.; Yaghi, O.M. The atom, the molecule, and the covalent organic framework. Science 2017, 355, eaal1585. [Google Scholar] [CrossRef] [PubMed]
- Côté, A.P.; Benin, A.I.; Ockwig, N.W.; O’Keeffe, M.; Matzger, A.J.; Yaghi, O.M. Porous, Crystalline, Covalent Organic Frameworks. Science 2005, 310, 1166–1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geng, K.; He, T.; Liu, R.; Dalapati, S.; Tan, K.T.; Li, Z.; Tao, S.; Gong, Y.; Jiang, Q.; Jiang, D. Covalent Organic Frameworks: Design, Synthesis, and Functions. Chem. Rev. 2020, 120, 8814–8933. [Google Scholar] [CrossRef]
- Huang, N.; Chen, X.; Krishna, R.; Jiang, D. Two-Dimensional Covalent Organic Frameworks for Carbon Dioxide Capture through Channel-Wall Functionalization. Angew. Chem. Int. Ed. 2015, 54, 2986–2990. [Google Scholar] [CrossRef] [Green Version]
- Chen, R.; Wang, Y.; Ma, Y.; Mal, A.; Gao, X.-Y.; Gao, L.; Qiao, L.; Li, X.-B.; Wu, L.-Z.; Wang, C. Rational design of isostructural 2D porphyrin-based covalent organic frameworks for tunable photocatalytic hydrogen evolution. Nat. Commun. 2021, 12, 1354. [Google Scholar] [CrossRef]
- Shinde, D.B.; Aiyappa, H.B.; Bhadra, M.; Biswal, B.P.; Wadge, P.; Kandambeth, S.; Garai, B.; Kundu, T.; Kurungot, S.; Banerjee, R. A mechanochemically synthesized covalent organic framework as a proton-conducting solid electrolyte. J. Mater. Chem. A 2016, 4, 2682–2690. [Google Scholar] [CrossRef]
- Wang, H.; Zeng, Z.; Xu, P.; Li, L.; Zeng, G.; Xiao, R.; Tang, Z.; Huang, D.; Tang, L.; Lai, C.; et al. Recent progress in covalent organic framework thin films: Fabrications, applications and perspectives. Chem. Soc. Rev. 2019, 48, 488–516. [Google Scholar] [CrossRef]
- Bondancia, T.J.; Mattoso, L.H.C.; Marconcini, J.M.; Farinas, C.S. A new approach to obtain cellulose nanocrystals and ethanol from eucalyptus cellulose pulp via the biochemical pathway. Biotechnol. Prog. 2017, 33, 1085–1095. [Google Scholar] [CrossRef] [PubMed]
- Bondancia, T.J.; Florencio, C.; Baccarin, G.S.; Farinas, C.S. Cellulose nanostructures obtained using enzymatic cocktails with different compositions. Int. J. Biol. Macromol. 2022, 207, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Kamperidou, V.; Terzopoulou, P. Anaerobic Digestion of Lignocellulosic Waste Materials. Sustainability 2021, 13, 12810. [Google Scholar] [CrossRef]
- Lagerwall, J.P.F.; Schütz, C.; Salajkova, M.; Noh, J.; Hyun Park, J.; Scalia, G.; Bergström, L. Cellulose nanocrystal-based materials: From liquid crystal self-assembly and glass formation to multifunctional thin films. NPG Asia Mater. 2014, 6, e80. [Google Scholar] [CrossRef] [Green Version]
- Joly, F.-X.; Coulis, M. Comparison of cellulose vs. plastic cigarette filter decomposition under distinct disposal environments. Waste Manag. 2018, 72, 349–353. [Google Scholar] [CrossRef] [Green Version]
- Schiavi, D.; Francesconi, S.; Taddei, A.R.; Fortunati, E.; Balestra, G.M. Exploring cellulose nanocrystals obtained from olive tree wastes as sustainable crop protection tool against bacterial diseases. Sci. Rep. 2022, 12, 6149. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, G.; Yao, X.; Gao, S.; Xie, J.; Xu, H.; Lin, N. Electrochemical chiral sensor based on cellulose nanocrystals and multiwall carbon nanotubes for discrimination of tryptophan enantiomers. Cellulose 2018, 25, 3861–3871. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, X.; Cranston, E.D.; Zhu, S. Flexible and Porous Nanocellulose Aerogels with High Loadings of Metal-Organic-Framework Particles for Separations Applications. Adv. Mater. 2016, 28, 7652–7657. [Google Scholar] [CrossRef]
- Qian, L.; Lei, D.; Duan, X.; Zhang, S.; Song, W.; Hou, C.; Tang, R. Design and preparation of metal-organic framework papers with enhanced mechanical properties and good antibacterial capacity. Carbohydr. Polym. 2018, 192, 44–51. [Google Scholar] [CrossRef]
- Wan, X.; Wang, X.; Chen, G.; Guo, C.; Zhang, B. Covalent organic framework/nanofibrillated cellulose composite membrane loaded with Pd nanoparticles for dechlorination of dichlorobenzene. Mater. Chem. Phys. 2020, 246, 122574. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, J.; Han, G.; Bai, Y.; Ge, Q.; Ma, J.; Lau, C.H.; Shao, L. Molecularly soldered covalent organic frameworks for ultrafast precision sieving. Sci. Adv. 2021, 7, eabe8706. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.K.; Vijayakumar, V.; Halder, A.; Ghosh, M.; Addicoat, M.; Bansode, U.; Kurungot, S.; Banerjee, R. Weak Intermolecular Interactions in Covalent Organic Framework-Carbon Nanofiber Based Crystalline yet Flexible Devices. ACS Appl Mater Interfaces 2019, 11, 30828–30837. [Google Scholar] [CrossRef] [PubMed]
- Maschita, J.; Banerjee, T.; Lotsch, B.V. Direct and Linker-Exchange Alcohol-Assisted Hydrothermal Synthesis of Imide-Linked Covalent Organic Frameworks. Chem. Mater. 2022, 34, 2249–2258. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Yaghi, O.M. Storage of Hydrogen, Methane, and Carbon Dioxide in Highly Porous Covalent Organic Frameworks for Clean Energy Applications. J. Am. Chem. Soc. 2009, 131, 8875–8883. [Google Scholar] [CrossRef]
- Díaz de Greñu, B.; Torres, J.; García-González, J.; Muñoz-Pina, S.; de los Reyes, R.; Costero, A.M.; Amorós, P.; Ros-Lis, J.V. Microwave-Assisted Synthesis of Covalent Organic Frameworks: A Review. ChemSusChem 2021, 14, 208–233. [Google Scholar] [CrossRef]
- Głowniak, S.; Szczęśniak, B.; Choma, J.; Jaroniec, M. Advances in Microwave Synthesis of Nanoporous Materials. Adv. Mater. 2021, 33, 2103477. [Google Scholar] [CrossRef]
- Li, Y.; Chen, W.; Xing, G.; Jiang, D.; Chen, L. New synthetic strategies toward covalent organic frameworks. Chem. Soc. Rev. 2020, 49, 2852–2868. [Google Scholar] [CrossRef]
- Das, G.; Balaji Shinde, D.; Kandambeth, S.; Biswal, B.P.; Banerjee, R. Mechanosynthesis of imine, beta-ketoenamine, and hydrogen-bonded imine-linked covalent organic frameworks using liquid-assisted grinding. Chem. Commun. (Camb) 2014, 50, 12615–12618. [Google Scholar] [CrossRef]
- Do, J.L.; Friscic, T. Mechanochemistry: A Force of Synthesis. ACS Cent. Sci. 2017, 3, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Szczęśniak, B.; Borysiuk, S.; Choma, J.; Jaroniec, M. Mechanochemical synthesis of highly porous materials. Mater. Horiz. 2020, 7, 1457–1473. [Google Scholar] [CrossRef]
- Casco, M.E.; Kirchhoff, S.; Leistenschneider, D.; Rauche, M.; Brunner, E.; Borchardt, L. Mechanochemical synthesis of N-doped porous carbon at room temperature. Nanoscale 2019, 11, 4712–4718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, H.; Tan, J.; Ren, Y.; Zhao, M.; Liu, J.; Wang, H.; Liu, J.; Zhao, Z. Mechanochemical Synthesis of Highly Porous CeMnOx Catalyst for the Removal of NOx. Ind. Eng. Chem. Res. 2019, 58, 16472–16478. [Google Scholar] [CrossRef]
- Khayum, M.A.; Vijayakumar, V.; Karak, S.; Kandambeth, S.; Bhadra, M.; Suresh, K.; Acharambath, N.; Kurungot, S.; Banerjee, R. Convergent Covalent Organic Framework Thin Sheets as Flexible Supercapacitor Electrodes. ACS Appl. Mater. Interfaces 2018, 10, 28139–28146. [Google Scholar] [CrossRef] [PubMed]
- Bondeson, D.; Mathew, A.; Oksman, K. Optimization of the isolation of nanocrystals from microcrystalline celluloseby acid hydrolysis. Cellulose 2006, 13, 171. [Google Scholar] [CrossRef]
- Liimatainen, H.; Suopajärvi, T.; Sirviö, J.; Hormi, O.; Niinimäki, J. Fabrication of cationic cellulosic nanofibrils through aqueous quaternization pretreatment and their use in colloid aggregation. Carbohydr. Polym. 2014, 103, 187–192. [Google Scholar] [CrossRef]
- Ahvenainen, P.; Kontro, I.; Svedström, K. Comparison of sample crystallinity determination methods by X-ray diffraction for challenging cellulose I materials. Cellulose 2016, 23, 1073–1086. [Google Scholar] [CrossRef] [Green Version]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Karak, S.; Kandambeth, S.; Biswal, B.P.; Sasmal, H.S.; Kumar, S.; Pachfule, P.; Banerjee, R. Constructing Ultraporous Covalent Organic Frameworks in Seconds via an Organic Terracotta Process. J. Am. Chem. Soc. 2017, 139, 1856–1862. [Google Scholar] [CrossRef]
- Ansari, F.; Salajková, M.; Zhou, Q.; Berglund, L.A. Strong Surface Treatment Effects on Reinforcement Efficiency in Biocomposites Based on Cellulose Nanocrystals in Poly(vinyl acetate) Matrix. Biomacromolecules 2015, 16, 3916–3924. [Google Scholar] [CrossRef]
- Zheng, T.; Clemons, C.M.; Pilla, S. Comparative Study of Direct Compounding, Coupling Agent-Aided and Initiator-Aided Reactive Extrusion to Prepare Cellulose Nanocrystal/PHBV (CNC/PHBV) Nanocomposite. ACS Sustain. Chem. Eng. 2020, 8, 814–822. [Google Scholar] [CrossRef]
- Li, H.; Wu, B.; Mu, C.; Lin, W. Concomitant degradation in periodate oxidation of carboxymethyl cellulose. Carbohydr. Polym. 2011, 84, 881–886. [Google Scholar] [CrossRef]
- Kandambeth, S.; Mallick, A.; Lukose, B.; Mane, M.V.; Heine, T.; Banerjee, R. Construction of crystalline 2D covalent organic frameworks with remarkable chemical (acid/base) stability via a combined reversible and irreversible route. J. Am. Chem. Soc. 2012, 134, 19524–19527. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Fu, X.-B.; Li, Y.; Xia, T.; Pan, L.; Yao, Y.-F. Solid-state NMR study of adsorbed water molecules in covalent organic framework materials. Microporous Mesoporous Mater. 2020, 305, 110287. [Google Scholar] [CrossRef]
- Halder, A.; Ghosh, M.; Khayum, M.A.; Bera, S.; Addicoat, M.; Sasmal, H.S.; Karak, S.; Kurungot, S.; Banerjee, R. Interlayer Hydrogen-Bonded Covalent Organic Frameworks as High-Performance Supercapacitors. J. Am. Chem. Soc. 2018, 140, 10941–10945. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Wu, Q.; Han, L.; Wang, S.; Fang, S.; Zhang, Z.; Sun, S. Synthesis of conjugated covalent organic frameworks/graphene composite for supercapacitor electrodes. RSC Adv. 2015, 5, 27290–27294. [Google Scholar] [CrossRef]
- Çalışkan, M.; Baran, T. Design of a palladium nanocatalyst produced from Schiff base modified dialdehyde cellulose and its application in aryl halide cyanation and reduction of nitroarenes. Cellulose 2022, 29, 4475–4493. [Google Scholar] [CrossRef]
- Gao, R.; Xiao, S.; Gan, W.; Liu, Q.; Amer, H.; Rosenau, T.; Li, J.; Lu, Y. Mussel Adhesive-Inspired Design of Superhydrophobic Nanofibrillated Cellulose Aerogels for Oil/Water Separation. ACS Sustain. Chem. Eng. 2018, 6, 9047–9055. [Google Scholar] [CrossRef]
- Zhong, X.; Liang, W.; Lu, Z.; Qiu, M.; Hu, B. Ultra-high capacity of graphene oxide conjugated covalent organic framework nanohybrid for U(VI) and Eu(III) adsorption removal. J. Mol. Liq. 2021, 323, 114603. [Google Scholar] [CrossRef]
- Cui, S. Thermal Degradation of As-Synthesized MOFs Studied by TG-FTIR. Sepectroscopy Spectr. Anal. 2012, 32, 131–132. [Google Scholar] [CrossRef]
- Pérez-Carvajal, J.; Boix, G.; Imaz, I.; Maspoch, D. The Imine-Based COF TpPa-1 as an Efficient Cooling Adsorbent That Can Be Regenerated by Heat or Light. Adv. Energy Mater. 2019, 9, 1901535. [Google Scholar] [CrossRef]
- Gopalakrishnan, V.N.; Nguyen, D.-T.; Becerra, J.; Sakar, M.; Ahad, J.M.E.; Jautzy, J.J.; Mindorff, L.M.; Béland, F.; Do, T.-O. Manifestation of an Enhanced Photoreduction of CO2 to CO over the In Situ Synthesized rGO–Covalent Organic Framework under Visible Light Irradiation. ACS Appl. Energy Mater. 2021, 4, 6005–6014. [Google Scholar] [CrossRef]

| Element | Atomic % | |
|---|---|---|
| COF | COF-DANC | |
| C 1s | 73.15 | 72.18 |
| O 1s | 18.40 | 20.25 |
| N 1s | 8.45 | 7.57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, C.; Pang, S.; Chen, Z.; Bi, L.; Wang, S.; Liang, C.; Qin, C. Synthesis of Covalent Organic Frameworks (COFs)-Nanocellulose Composite and Its Thermal Degradation Studied by TGA/FTIR. Polymers 2022, 14, 3158. https://doi.org/10.3390/polym14153158
Zhu C, Pang S, Chen Z, Bi L, Wang S, Liang C, Qin C. Synthesis of Covalent Organic Frameworks (COFs)-Nanocellulose Composite and Its Thermal Degradation Studied by TGA/FTIR. Polymers. 2022; 14(15):3158. https://doi.org/10.3390/polym14153158
Chicago/Turabian StyleZhu, Chunxia, Shuyu Pang, Zhaoxia Chen, Lehua Bi, Shuangfei Wang, Chen Liang, and Chengrong Qin. 2022. "Synthesis of Covalent Organic Frameworks (COFs)-Nanocellulose Composite and Its Thermal Degradation Studied by TGA/FTIR" Polymers 14, no. 15: 3158. https://doi.org/10.3390/polym14153158
APA StyleZhu, C., Pang, S., Chen, Z., Bi, L., Wang, S., Liang, C., & Qin, C. (2022). Synthesis of Covalent Organic Frameworks (COFs)-Nanocellulose Composite and Its Thermal Degradation Studied by TGA/FTIR. Polymers, 14(15), 3158. https://doi.org/10.3390/polym14153158







