Experimental Study on Phenol-Formaldehyde Resin Aggregates as In-Depth Conformance Control Agents Stabilized by Polymer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methodology
2.2.1. Measurement of PFR Solution Turbidity
2.2.2. Cellulose Membrane Filtration
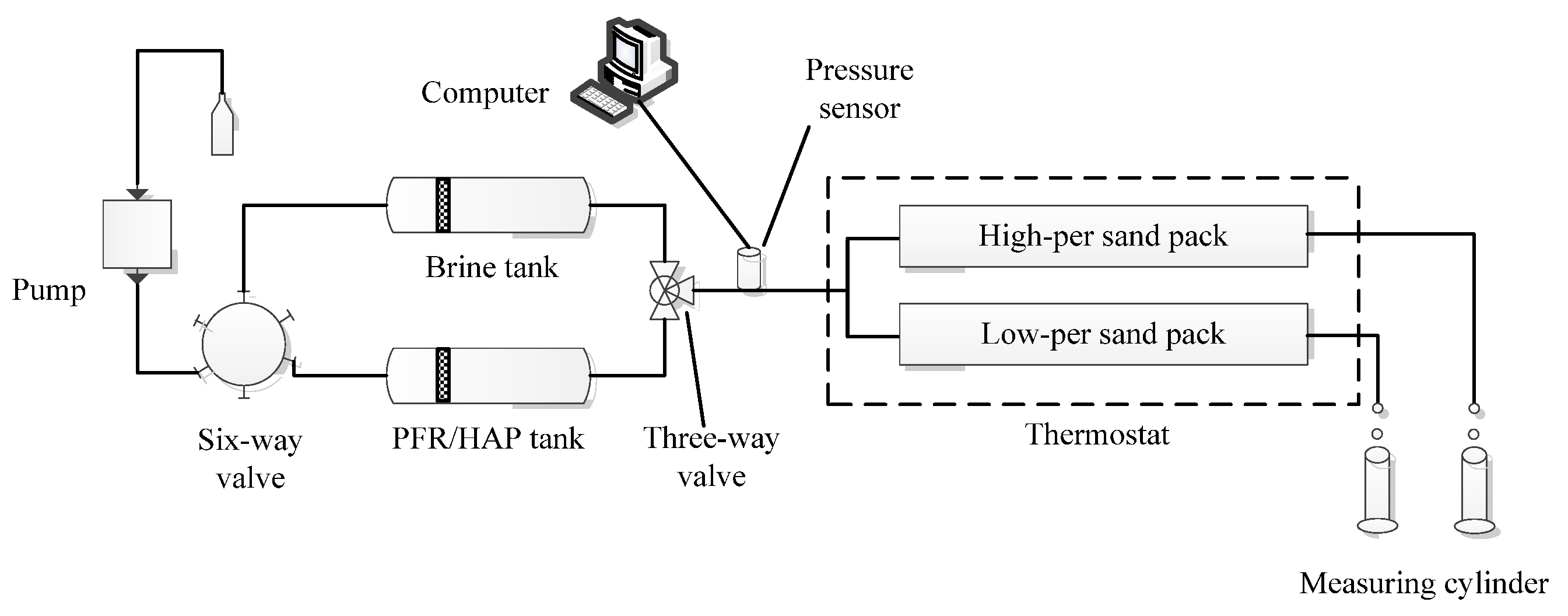
2.2.3. PFR/HAP Migration Behavior in the Sand Pack
2.2.4. Oil Displacement in Parallel Sand Packs
3. Results
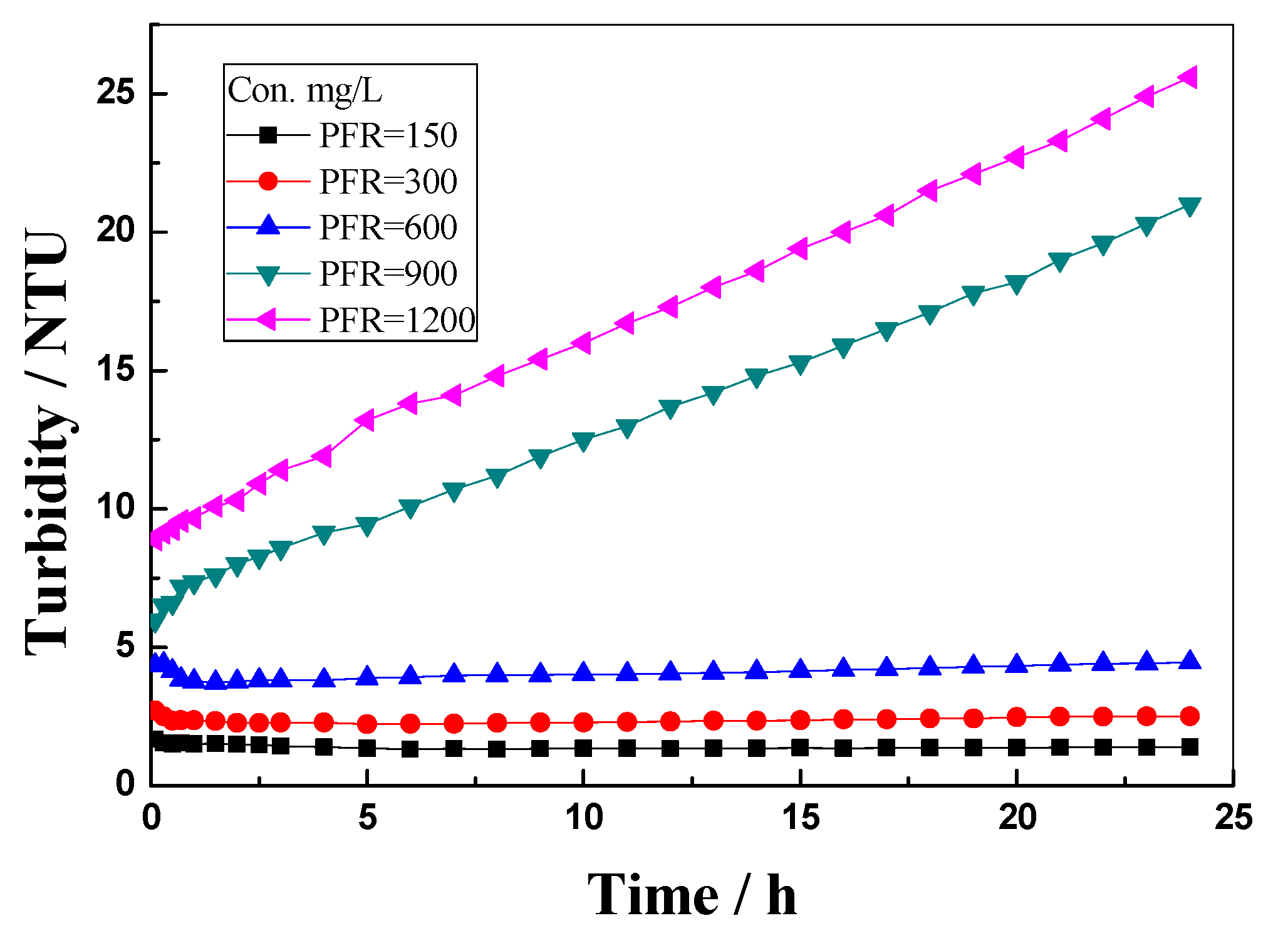
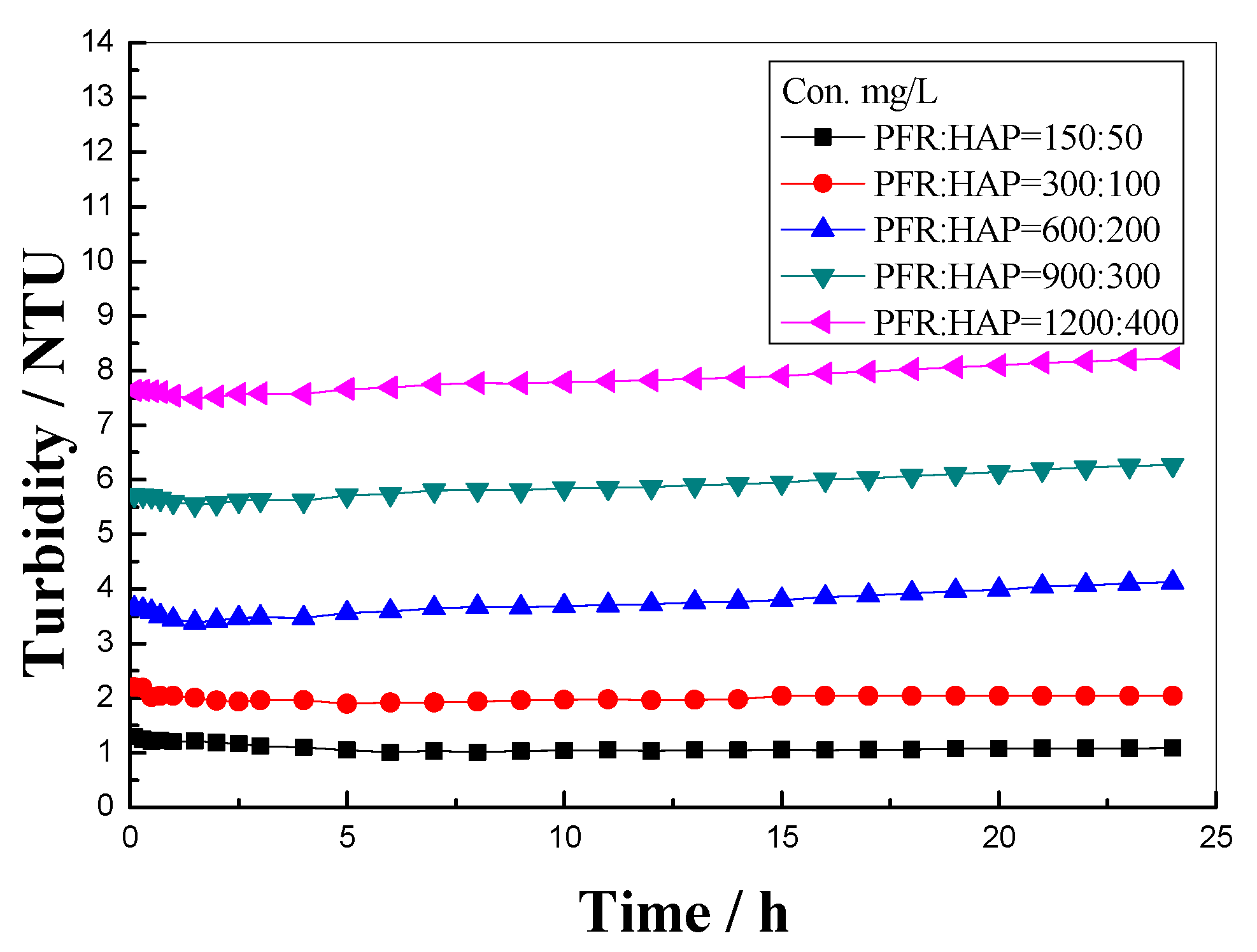
3.1. PFR Dispersion Stability
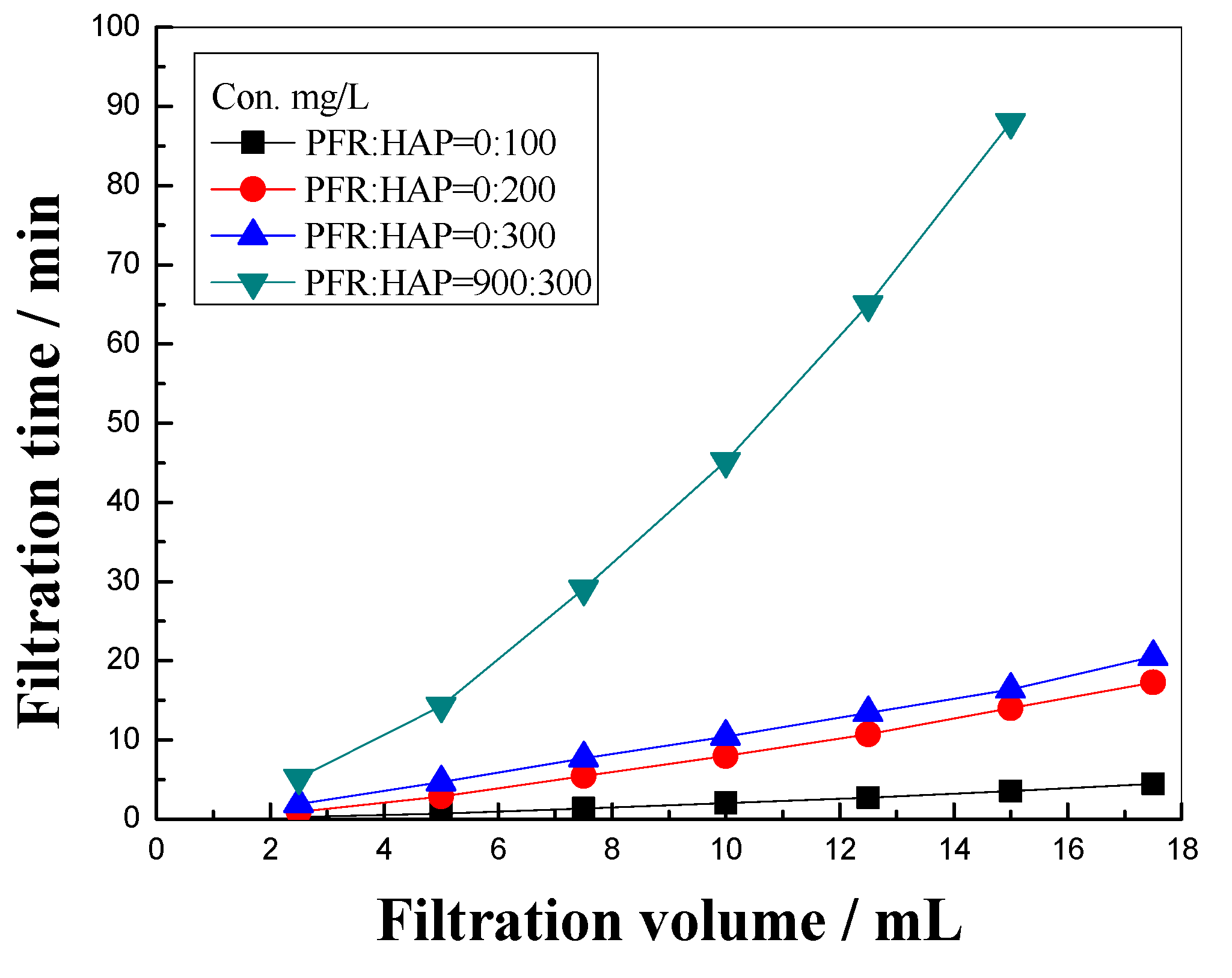
3.2. Blocking Property of PFR/HAP and HAP Solution in Cellulose Membrane
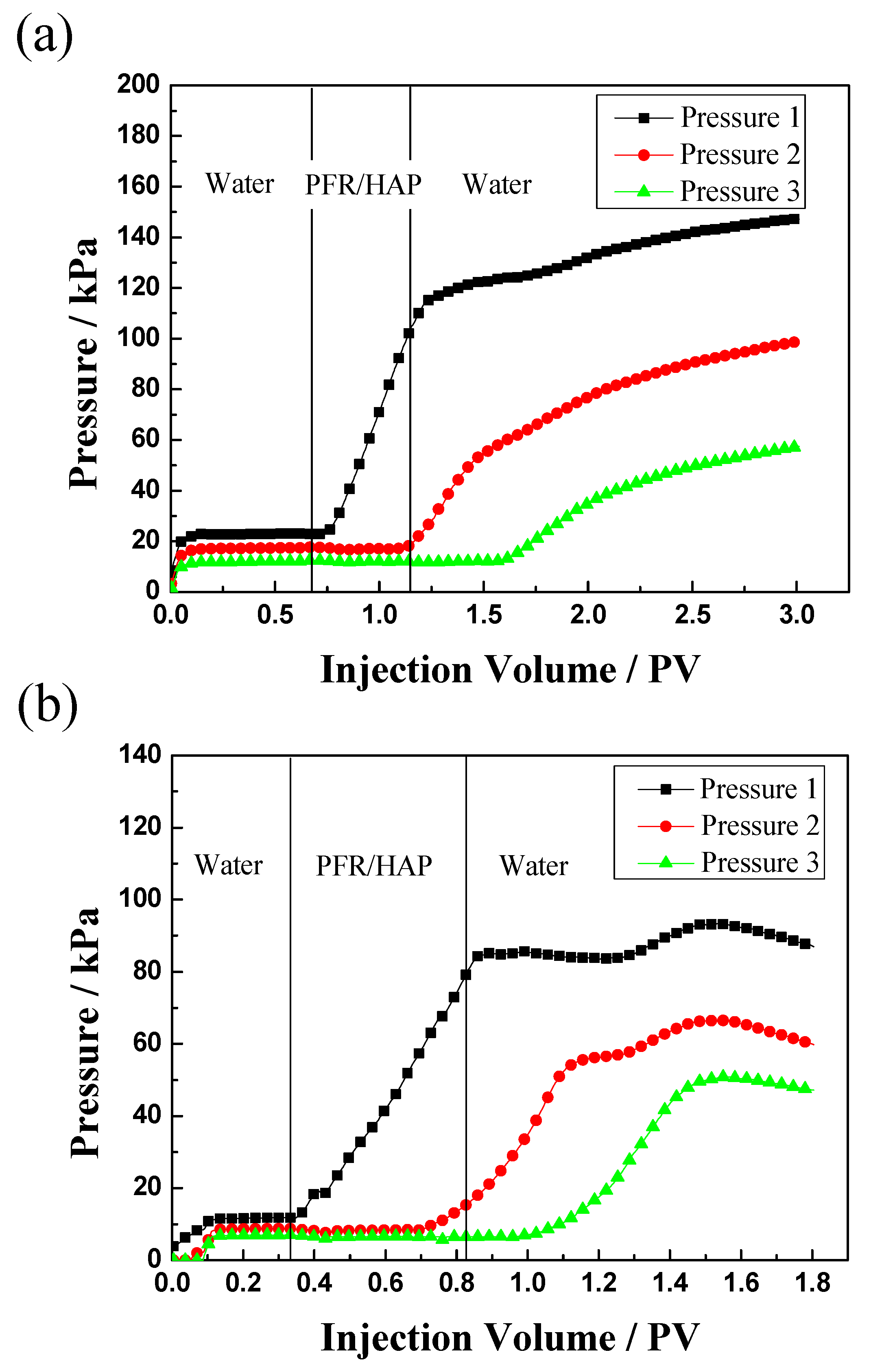
3.3. Flow Behavior in Sand Packs
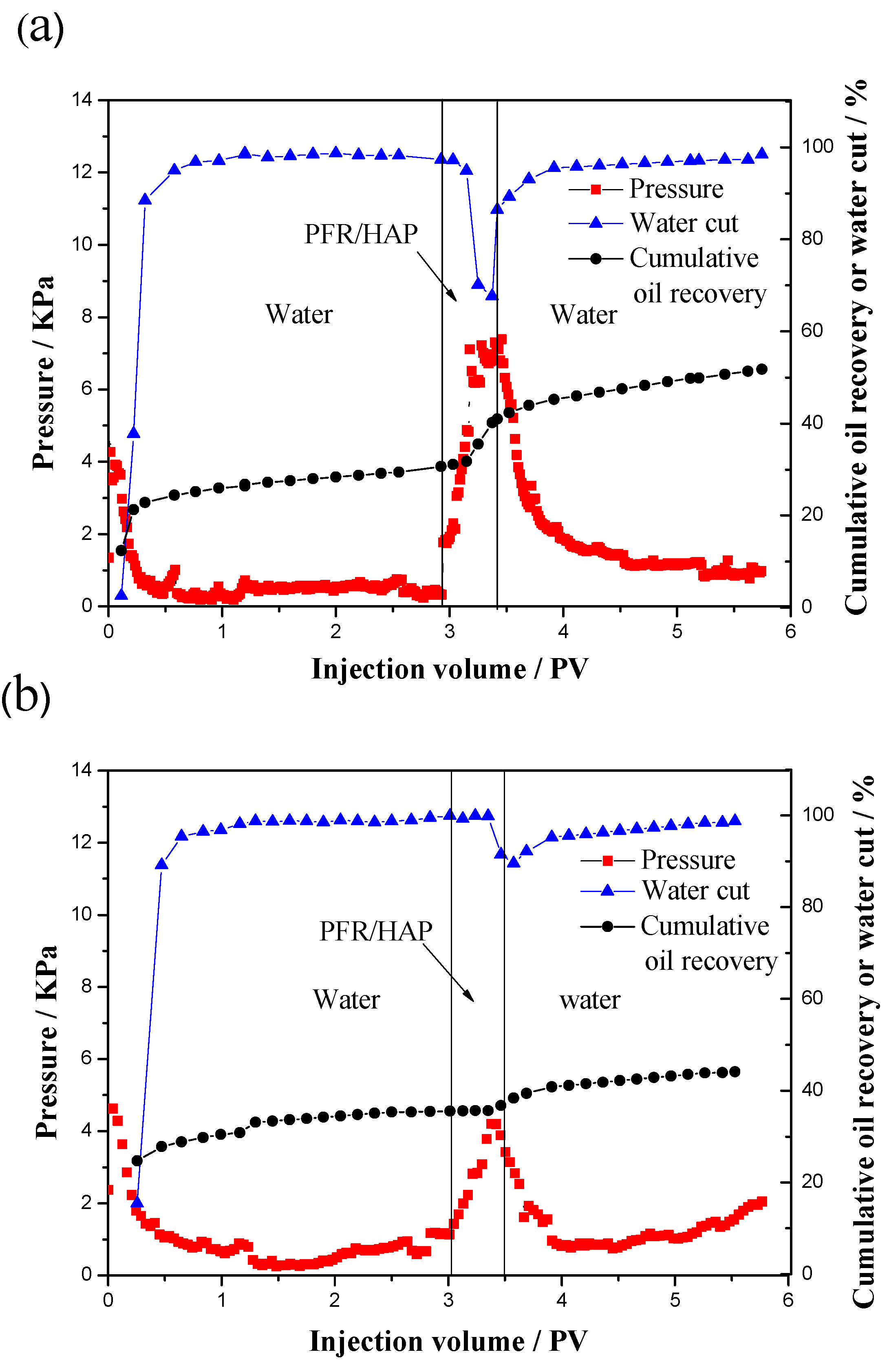
3.4. Oil Displacement in Parallel Sand Packs
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Setiati, R.; Malinda, M.T.; Sabrina, J. The potential of polymer for enhanced oil recovery process on oil refinery: A literature research. IOP Conf. Ser. Earth Environ. Sci. 2021, 737, 012046. [Google Scholar] [CrossRef]
- Deng, Z.; Liu, M.; Qin, J.; Sun, H.; Zhang, H.; Zhi, K.; Zhu, D. Mechanism study of water control and oil recovery improvement by polymer gels based on nuclear magnetic resonance. J. Pet. Sci. Eng. 2021, 209, 109881. [Google Scholar] [CrossRef]
- Zhu, D.; Bai, B.; Hou, J. Polymer Gel Systems for Water Management in High-Temperature Petroleum Reservoirs: A Chemical Review. Energy Fuels 2017, 31, 13063–13087. [Google Scholar] [CrossRef]
- Hamouma, M.; Delbos, A.; Dalmazzone, C.; Colin, A. Polymer Surfactant Interactions in Oil Enhanced Recovery Processes. Energy Fuels 2021, 35, 9312–9321. [Google Scholar] [CrossRef]
- Zhu, D.-Y.; Luo, R.-T.; Liu, Y.; Qin, J.-H.; Zhao, Q.; Zhang, H.-J.; Wang, W.-S.; Wang, Z.-Y.; Zhu, M.-E.; Wang, Y.-P.; et al. Development of re-crosslinkable dispersed particle gels for conformance improvement in extremely high-temperature reservoirs. Pet. Sci. 2022; in press. [Google Scholar] [CrossRef]
- Sharma, H.; Dufour, S.; Arachchilage, G.W.P.P.; Weerasooriya, U.; Pope, G.A.; Mohanty, K. Alternative alkalis for ASP flooding in anhydrite containing oil reservoirs. Fuel 2021, 140, 407–420. [Google Scholar] [CrossRef]
- Yusuf, M.; Wathon, M.H.; Thanasaksukthawee, V.; Saul, A.; Tangparitkul, S. Adsorption of Saponin Natural Surfactant on Carbonate Rock and Comparison to Synthetic Surfactants: An Enhanced Oil Recovery Prospective. Energy Fuels 2021, 35, 11193–11202. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, H.; Zheng, W.; Li, X.; Wang, F.; Li, X.; Zhang, D.; Turtabayev, S.; Kang, W. Research on synthesis and salt thickening behavior of a binary copolymer amphiphilic polymer. J. Pet. Sci. Eng. 2021, 204, 108713. [Google Scholar] [CrossRef]
- Zhu, D.; Peng, S.; Zhao, S.; Wei, M.; Bai, B. Comprehensive Review of Sealant Materials for Leakage Remediation Technology in Geological CO2 Capture and Storage Process. Energy Fuels 2021, 35, 4711–4742. [Google Scholar] [CrossRef]
- Nowrouzi, I.; Mohammadi, A.H.; Manshad, A.K. Chemical Enhanced Oil Recovery by Different Scenarios of Slug Injection into Carbonate/Sandstone Composite Oil Reservoirs Using an Anionic Surfactant Derived from Rapeseed Oil. Energy Fuels 2021, 35, 1248–1258. [Google Scholar] [CrossRef]
- Jouenne, S. Polymer flooding in high temperature, high salinity conditions: Selection of polymer type and polymer chemistry, thermal stability. J. Pet. Sci. Eng. 2020, 195, 107545. [Google Scholar] [CrossRef]
- Gao, Q.; Zhong, C.; Han, P.; Cao, R. Characteristics of Preferential Flow Paths in Reservoirs After Polymer Flooding and an Adaptive Compound Flooding Method. Chem. Technol. Fuels Oils 2021, 57, 368–375. [Google Scholar] [CrossRef]
- Zhu, D.-Y.; Fang, X.-Y.; Sun, R.-X.; Xu, Z.-H.; Liu, Y.; Liu, J.-Y. Development of degradable pre-formed particle gel (DPPG) as temporary plugging agent for petroleum drilling and production. Pet. Sci. 2021, 18, 479–494. [Google Scholar] [CrossRef]
- Tajik, S.; Shahrabadi, A.; Rashidi, A.; Jalilian, M.; Yadegari, A. Application of functionalized silica-graphene nanohybrid for the enhanced oil recovery performance. Colloids Surf. A Physicochem. Eng. Asp. 2018, 556, 253–265. [Google Scholar] [CrossRef]
- Sircar, A.; Rayavarapu, K.; Bist, N.; Yadav, K.; Singh, S. Applications of nanoparticles in enhanced oil recovery. Pet. Res. 2021, 7, 77–90. [Google Scholar] [CrossRef]
- Haruna, M.A.; Nourafkan, E.; Hu, Z.; Wen, D. Improved Polymer Flooding in Harsh Environments by Free-Radical Polymerization and the Use of Nanomaterials. Energy Fuels 2019, 33, 1637–1648. [Google Scholar] [CrossRef]
- Rueda, E.; Akarri, S.; Torsæter, O.; Moreno, R.B.Z.L. Experimental Investigation of the Effect of Adding Nanoparticles to Polymer Flooding in Water-Wet Micromodels. Nanomaterials 2020, 10, 1489. [Google Scholar] [CrossRef]
- Shi, G.; Tang, K.; Wang, F.; Luo, Q.; Bai, L.; Sun, P.; Zhu, D. Visualized Study of a Nanoparticle-Assisted Foam System to Enhance Oil Recovery by Nuclear Magnetic Resonance Online Flooding Experiment. Energy Fuels 2020, 35, 465–472. [Google Scholar] [CrossRef]
- Meng, X.; Li, M.; Lin, M.; Yang, Z.; Zhang, J.; Wang, B.; Feng, L. Dispersion and Blocking Properties of Sulfonated Phenol Formaldehyde Resin. J. Dispers. Sci. Technol. 2016, 37, 1607–1612. [Google Scholar] [CrossRef]
- Meng, X.; Li, M.; Lin, M.; Yang, Z.; Zhang, J.; Fang, Z. Synthesis and molecular structure characterization of water-soluble sulfonated methyl phenolic resin. Chin. Adhes. 2017, 26, 21–24. [Google Scholar]
- Zhu, D.Y.; Deng, Z.H.; Chen, S.W. A review of nuclear magnetic resonance(NMR) technology applied in the characterization of polymer gels for petroleum reservoir conformance control. Pet. Res. 2021, 18, 16. [Google Scholar] [CrossRef]
- Xu, L.; Liu, S.; Qiu, Z.; Gong, H.; Fan, H.; Zhu, T.; Zhang, H.; Dong, M. Hydrophobic effect further improves the rheological behaviors and oil recovery of polyacrylamide/nanosilica hybrids at high salinity. Chem. Eng. Sci. 2020, 232, 116369. [Google Scholar] [CrossRef]
- Hua, Z.; Lin, M.; Dong, Z.; Li, M.; Zhang, G.; Yang, J. Study of deep profile control and oil displacement technologies with nanoscale polymer microspheres. J. Colloid Interface Sci. 2014, 424, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Wang, Q. New progress and prospect of oilfields development technologies in China. Pet. Explor. Dev. 2018, 45, 698–711. [Google Scholar] [CrossRef]
- Meng, X.; Li, M.; Lin, M.; Zhang, J.; Yang, Z.; Feng, L. Aggregation behavior and reversibility of sulfonated phenol formaldehyde resin. J. Dispers. Sci. Technol. 2016, 38, 328–333. [Google Scholar] [CrossRef]



| Ion | Na+ | Ca2+ | Mg2+ | Cl− | CO32− | HCO3− | SO42− | Total |
|---|---|---|---|---|---|---|---|---|
| Concentration/mg/L | 1686.8 | 0.7 | 0.8 | 1214.3 | 96.8 | 2196.0 | 0.7 | 5196.3 |
| Test | Sand Pack | K/mD | Perm. Ratio | PFR: HAP/ mg/L | RFw/% | Incremental Oil Recovery/% | ||
|---|---|---|---|---|---|---|---|---|
| Single | Total | Single | Total | |||||
| 1 | High-perm | 480 | 2.1 | 510:170 | 64.3 | 58.7 | 8.4 | 10.9 |
| Low-perm | 230 | 52.6 | 13.8 | |||||
| 2 | High-perm | 1250 | 5.0 | 600:200 | 63.6 | 52.1 | 6.2 | 18.3 |
| Low-perm | 250 | 41.0 | 30.0 | |||||
| 3 | High-perm | 1630 | 8.1 | 1200:400 | 63.1 | 48.2 | 7.3 | 19.2 |
| Low-perm | 200 | 34.3 | 30.2 | |||||
| 4 | High-perm | 2220 | 11.1 | 1800:600 | 58.2 | 30.7 | 11.1 | 21.1 |
| Low-perm | 200 | 1.7 | 31.3 | |||||
| 5 | High-perm | 2030 | 11.3 | 900:300 | 63.4 | 35.5 | 8.7 | 8.4 |
| Low-perm | 180 | 0.0 | 8.0 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, X.; Zhang, G.; Wu, J.; Zhao, X.; Wang, L.; Zhang, F. Experimental Study on Phenol-Formaldehyde Resin Aggregates as In-Depth Conformance Control Agents Stabilized by Polymer. Polymers 2022, 14, 3159. https://doi.org/10.3390/polym14153159
Meng X, Zhang G, Wu J, Zhao X, Wang L, Zhang F. Experimental Study on Phenol-Formaldehyde Resin Aggregates as In-Depth Conformance Control Agents Stabilized by Polymer. Polymers. 2022; 14(15):3159. https://doi.org/10.3390/polym14153159
Chicago/Turabian StyleMeng, Xianxing, Guiqing Zhang, Jian Wu, Xiong Zhao, Lin Wang, and Fang Zhang. 2022. "Experimental Study on Phenol-Formaldehyde Resin Aggregates as In-Depth Conformance Control Agents Stabilized by Polymer" Polymers 14, no. 15: 3159. https://doi.org/10.3390/polym14153159
APA StyleMeng, X., Zhang, G., Wu, J., Zhao, X., Wang, L., & Zhang, F. (2022). Experimental Study on Phenol-Formaldehyde Resin Aggregates as In-Depth Conformance Control Agents Stabilized by Polymer. Polymers, 14(15), 3159. https://doi.org/10.3390/polym14153159






