Co/ZnO/Nitrogen-Doped Carbon Composite Anode Derived from Metal Organic Frameworks for Lithium Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of ZIF-8
2.2. Synthesis of the ZIF-8@ZIF-67 Composite
2.3. Synthesis of the ZIF-8/ZIF-67 Composite
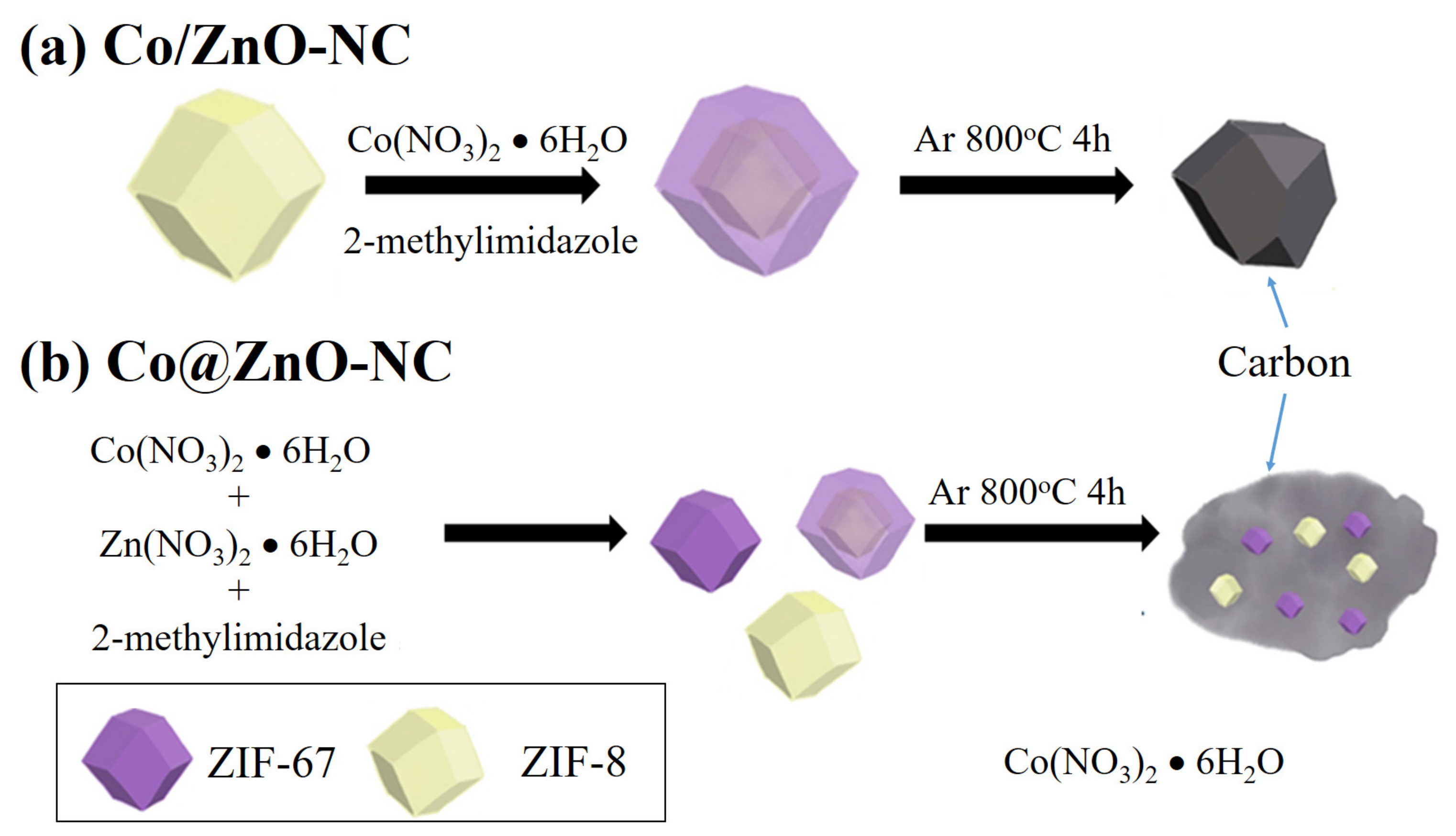
2.4. Synthesis of Co/ZnO-NC and Co@ZnO-NC Composites
2.5. Characterizations
2.6. Electrochemical Measurements
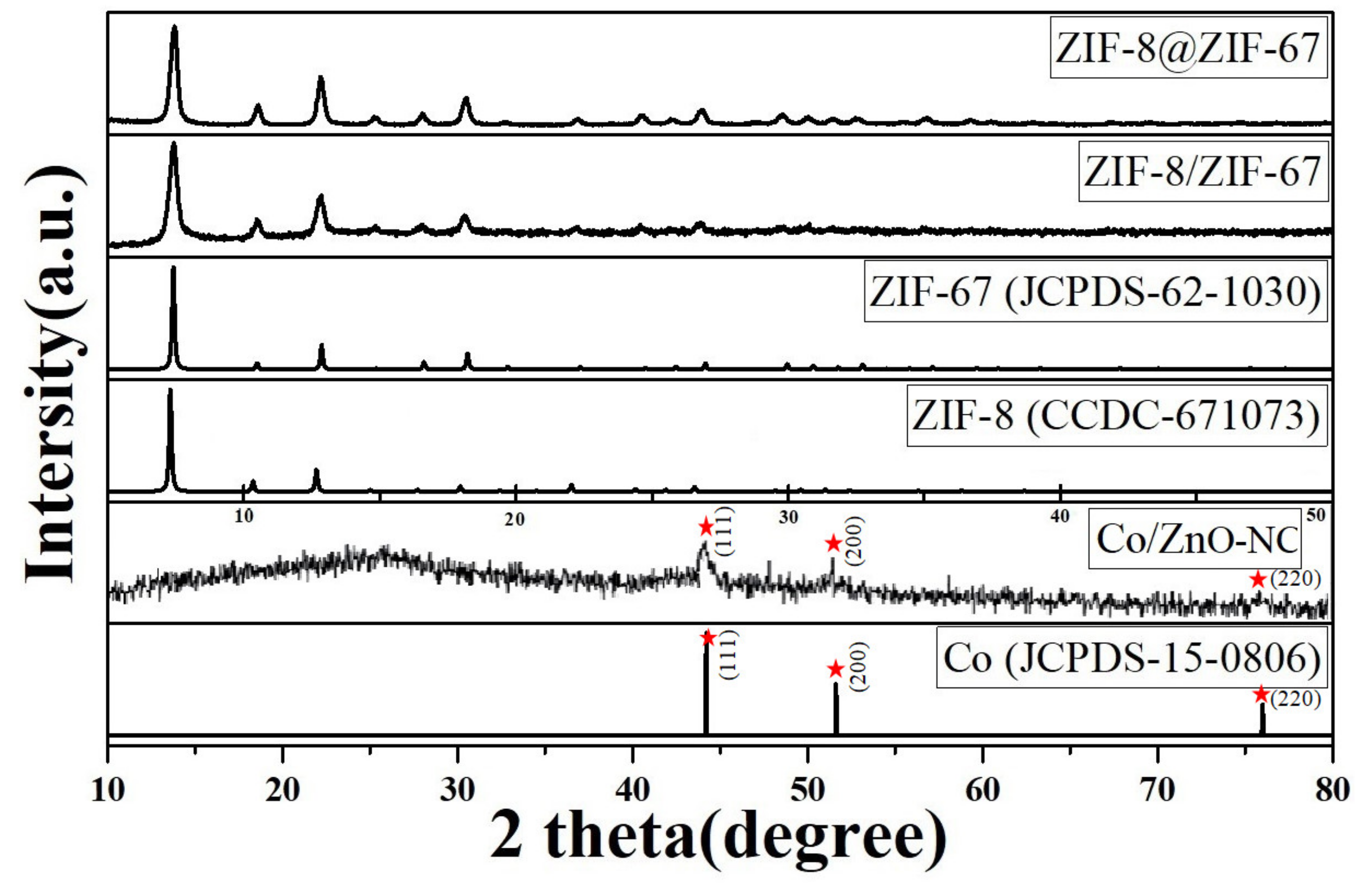
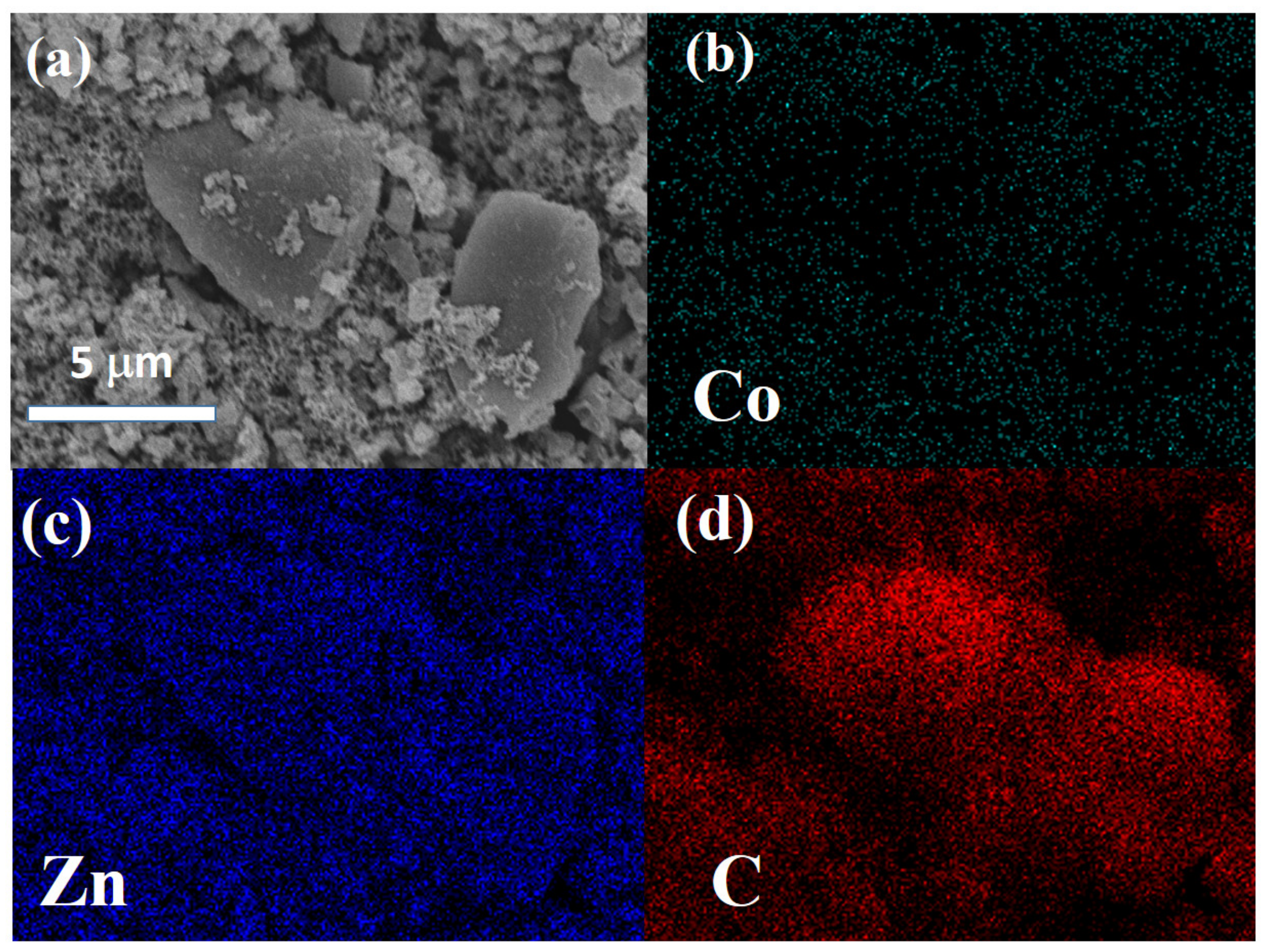
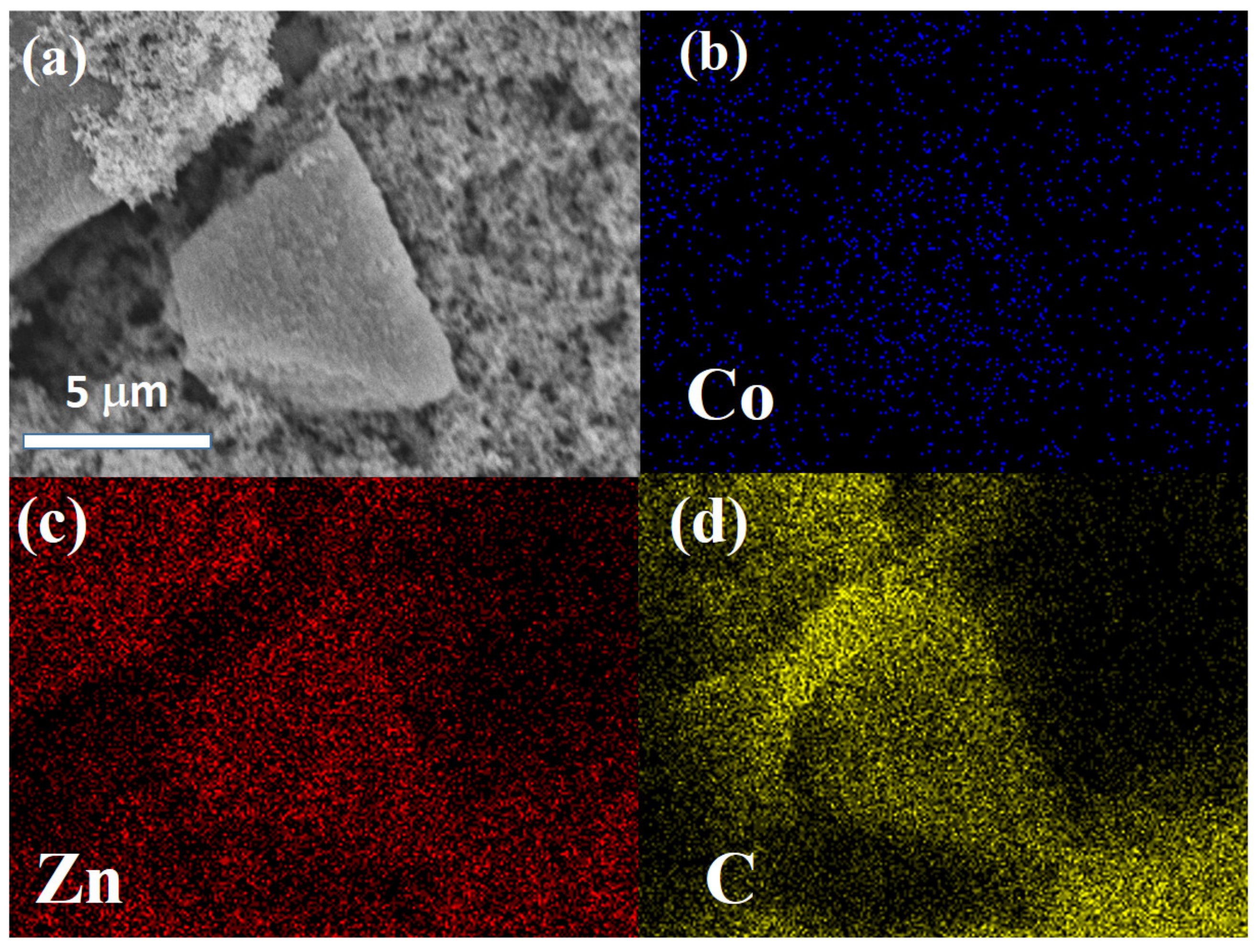
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hassoun, J.; Panero, S.; Reale, P.; Scrosati, B. A new, safe, high-rate and high-energy polymer lithium-ion battery. Adv. Mater. 2009, 21, 4807–4810. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, A. The birth of the lithium-ion battery. Angew. Chem. Int. Ed. 2012, 51, 5798–5800. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.K.; Wang, G.X.; Guo, Z.; Wang, J.; Konstantinov, K. Nanomaterials for lithium-ion rechargeable batteries. J. Nanosci. Nanotechnol. 2006, 6, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Manthiram, A. An outlook on lithium ion battery technology. ACS Cent. Sci. 2017, 3, 1063–1069. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, L.; Yu, C. Highly crystallized Fe2O3 nanocrystals on graphene: A lithium ion battery anode material with enhanced cycling. RSC Adv. 2013, 4, 495–499. [Google Scholar] [CrossRef][Green Version]
- Lian, P.; Zhu, X.; Xiang, H.; Li, Z.; Yang, W.; Wang, H. Enhanced cycling performance of Fe3O4–graphene nanocomposite as an anode material for lithium-ion batteries. Electrochim. Acta 2010, 56, 834–840. [Google Scholar] [CrossRef]
- Wang, B.; Côté, A.P.; Furukawa, H.; O’Keeffe, M.; Yaghi, O.M. Colossal cages in zeolitic imidazolate frameworks as selective carbon dioxide reservoirs. Nature 2008, 453, 207–211. [Google Scholar] [CrossRef]
- Fairen-Jimenez, D.; Moggach, S.; Wharmby, M.; Wright, P.; Parsons, S.; Duren, T. Opening the gate: Framework flexibility in ZIF-8 explored by experiments and simulations. J. Am. Chem. Soc. 2011, 133, 8900–8902. [Google Scholar] [CrossRef]
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 1999, 402, 276–279. [Google Scholar] [CrossRef]
- Mu, L.; Liu, B.; Liu, H.; Yang, Y.; Sun, C.; Chen, G. A novel method to improve the gas storage capacity of ZIF-8. J. Mater. Chem. 2012, 22, 12246–12252. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, Z.; Xu, J.; Huang, Y.; Song, Y. Evidence of pressure enhanced CO2 storage in ZIF-8 probed by FTIR spectroscopy. J. Am. Chem. Soc. 2013, 135, 9287–9290. [Google Scholar] [CrossRef]
- Liu, D.; Ma, X.; Xi, H.; Lin, Y. Gas transport properties and propylene/propane separation characteristics of ZIF-8 membranes. J. Membr. Sci. 2014, 451, 85–93. [Google Scholar] [CrossRef]
- Kumar, R.; Jayaramulu, K.; Maji, T.K.; Rao, C. Hybrid nanocomposites of ZIF-8 with graphene oxide exhibiting tunable morphology, significant CO 2 uptake and other novel properties. Chem. Commun. 2013, 49, 4947–4949. [Google Scholar] [CrossRef]
- McEwen, J.; Hayman, J.-D.; Yazaydin, A.O. A comparative study of CO2, CH4 and N2 adsorption in ZIF-8, Zeolite-13X and BPL activated carbon. Chem. Phys. 2013, 412, 72–76. [Google Scholar] [CrossRef]
- Zhang, Z.; Xian, S.; Xi, H.; Wang, H.; Li, Z. Improvement of CO2 adsorption on ZIF-8 crystals modified by enhancing basicity of surface. Chem. Eng. Sci. 2011, 66, 4878–4888. [Google Scholar] [CrossRef]
- Thomas, A.; Ahamed, R.; Prakash, M. Selection of a suitable ZIF-8/ionic liquid (IL) based composite for selective CO 2 capture: The role of anions at the interface. RSC Adv. 2020, 10, 39160–39170. [Google Scholar] [CrossRef]
- Feng, S.; Zhang, X.; Shi, D.; Wang, Z. Zeolitic imidazolate framework-8 (ZIF-8) for drug delivery: A critical review. Front. Chem. Sci. Eng. 2021, 15, 221–237. [Google Scholar] [CrossRef]
- Zheng, C.; Wang, Y.; Phua, S.Z.F.; Lim, W.Q.; Zhao, Y. ZnO–DOX@ ZIF-8 core–shell nanoparticles for pH-responsive drug delivery. ACS Biomater. Sci. Eng. 2017, 3, 2223–2229. [Google Scholar] [CrossRef]
- Sun, C.-Y.; Qin, C.; Wang, X.-L.; Yang, G.-S.; Shao, K.-Z.; Lan, Y.-Q.; Su, Z.-M.; Huang, P.; Wang, C.-G.; Wang, E.-B. Zeolitic imidazolate framework-8 as efficient pH-sensitive drug delivery vehicle. Dalton Trans. 2012, 41, 6906–6909. [Google Scholar] [CrossRef]
- Kaur, H.; Mohanta, G.C.; Gupta, V.; Kukkar, D.; Tyagi, S. Synthesis and characterization of ZIF-8 nanoparticles for controlled release of 6-mercaptopurine drug. J. Drug Deliv. Sci. Technol. 2017, 41, 106–112. [Google Scholar] [CrossRef]
- Ren, H.; Zhang, L.; An, J.; Wang, T.; Li, L.; Si, X.; He, L.; Wu, X.; Wang, C.; Su, Z. Polyacrylic acid@ zeolitic imidazolate framework-8 nanoparticles with ultrahigh drug loading capability for pH-sensitive drug release. Chem. Commun. 2014, 50, 1000–1002. [Google Scholar] [CrossRef] [PubMed]
- Bux, H.; Chmelik, C.; Krishna, R.; Caro, J. Ethene/ethane separation by the MOF membrane ZIF-8: Molecular correlation of permeation, adsorption, diffusion. J. Membr. Sci. 2011, 369, 284–289. [Google Scholar] [CrossRef]
- Hara, N.; Yoshimune, M.; Negishi, H.; Haraya, K.; Hara, S.; Yamaguchi, T. Diffusive separation of propylene/propane with ZIF-8 membranes. J. Membr. Sci. 2014, 450, 215–223. [Google Scholar] [CrossRef]
- Lai, Z. Development of ZIF-8 membranes: Opportunities and challenges for commercial applications. Curr. Opin. Chem. Eng. 2018, 20, 78–85. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, W.; Liu, D.; Liu, B.; Chen, G.; Zhong, C. Effect of temperature on gas adsorption and separation in ZIF-8: A combined experimental and molecular simulation study. Chem. Eng. Sci. 2011, 66, 6297–6305. [Google Scholar] [CrossRef]
- Dai, H.; Xia, B.; Wen, L.; Du, C.; Su, J.; Luo, W.; Cheng, G. Synergistic catalysis of AgPd@ ZIF-8 on dehydrogenation of formic acid. Appl. Catal. B Environ. 2015, 165, 57–62. [Google Scholar] [CrossRef]
- Kuo, C.-H.; Tang, Y.; Chou, L.-Y.; Sneed, B.T.; Brodsky, C.N.; Zhao, Z.; Tsung, C.-K. Yolk–shell nanocrystal@ ZIF-8 nanostructures for gas-phase heterogeneous catalysis with selectivity control. J. Am. Chem. Soc. 2012, 134, 14345–14348. [Google Scholar] [CrossRef]
- Chizallet, C.; Lazare, S.; Bazer-Bachi, D.; Bonnier, F.; Lecocq, V.; Soyer, E.; Quoineaud, A.-A.; Bats, N. Catalysis of transesterification by a nonfunctionalized metal−organic framework: Acido-basicity at the external surface of ZIF-8 probed by FTIR and ab initio calculations. J. Am. Chem. Soc. 2010, 132, 12365–12377. [Google Scholar] [CrossRef]
- Sisi, A.J.; Fathinia, M.; Khataee, A.; Orooji, Y. Systematic activation of potassium peroxydisulfate with ZIF-8 via sono-assisted catalytic process: Mechanism and ecotoxicological analysis. J. Mol. Liq. 2020, 308, 113018. [Google Scholar] [CrossRef]
- Zhu, M.; Srinivas, D.; Bhogeswararao, S.; Ratnasamy, P.; Carreon, M.A. Catalytic activity of ZIF-8 in the synthesis of styrene carbonate from CO2 and styrene oxide. Catal. Commun. 2013, 32, 36–40. [Google Scholar] [CrossRef]
- Dang, T.T.; Zhu, Y.; Ngiam, J.S.; Ghosh, S.C.; Chen, A.; Seayad, A.M. Palladium nanoparticles supported on ZIF-8 as an efficient heterogeneous catalyst for aminocarbonylation. ACS Catal. 2013, 3, 1406–1410. [Google Scholar] [CrossRef]
- Li, B.; Wen, H.M.; Cui, Y.; Zhou, W.; Qian, G.; Chen, B. Emerging multifunctional metal–organic framework materials. Adv. Mater. 2016, 28, 8819–8860. [Google Scholar] [CrossRef]
- Phan, A.; Doonan, C.J.; Uribe-Romo, F.J.; Knobler, C.B.; O’Keeffe, M.; Yaghi, O.M. Synthesis, structure, and carbon dioxide capture properties of zeolitic imidazolate frameworks. Acc. Chem. Res. 2009, 43, 58–67. [Google Scholar] [CrossRef]
- Lee, Y.-R.; Jang, M.-S.; Cho, H.-Y.; Kwon, H.-J.; Kim, S.; Ahn, W.-S. ZIF-8: A comparison of synthesis methods. Chem. Eng. J. 2015, 271, 276–280. [Google Scholar] [CrossRef]
- Tai, Z.; Shi, M.; Chong, S.; Chen, Y.; Shu, C.; Dai, X.; Tan, Q.; Liu, Y. N-doped ZIF-8-derived carbon (NC-ZIF) as an anodic material for lithium-ion batteries. J. Alloys Compd. 2019, 800, 1–7. [Google Scholar] [CrossRef]
- Son, S.; Lim, D.; Nam, D.; Kim, J.; Shim, S.E.; Baeck, S.-H. N, S-doped nanocarbon derived from ZIF-8 as a highly efficient and durable electro-catalyst for oxygen reduction reaction. J. Solid State Chem. 2019, 274, 237–242. [Google Scholar] [CrossRef]
- Tang, J.; Salunkhe, R.R.; Liu, J.; Torad, N.L.; Imura, M.; Furukawa, S.; Yamauchi, Y. Thermal conversion of core–shell metal–organic frameworks: A new method for selectively functionalized nanoporous hybrid carbon. J. Am. Chem. Soc. 2015, 137, 1572–1580. [Google Scholar] [CrossRef]
- Cheng, E.; Huang, S.; Chen, D.; Huang, R.; Wang, Q.; Hu, Z.; Jiang, Y.; Li, Z.; Zhao, B.; Chen, Z. Porous ZnO/Co3O4/N-doped carbon nanocages synthesized via pyrolysis of complex metal–organic framework (MOF) hybrids as an advanced lithium-ion battery anode. Acta Crystallogr. Sect. C Struct. Chem. 2019, 75, 969–978. [Google Scholar] [CrossRef]
- Huang, M.; Mi, K.; Zhang, J.; Liu, H.; Yu, T.; Yuan, A.; Kong, Q.; Xiong, S. MOF-derived bi-metal embedded N-doped carbon polyhedral nanocages with enhanced lithium storage. J. Mater. Chem. A 2017, 5, 266–274. [Google Scholar] [CrossRef]
- Zhao, J.; Yao, S.; Hu, C.; Li, Z.; Wang, J.; Feng, X. Porous ZnO/Co3O4/CoO/Co composite derived from Zn-Co-ZIF as improved performance anodes for lithium-ion batteries. Mater. Lett. 2019, 250, 75–78. [Google Scholar] [CrossRef]
- Gao, C.; Jiang, Z.; Wang, P.; Jensen, L.R.; Zhang, Y.; Yue, Y. Optimized assembling of MOF/SnO2/Graphene leads to superior anode for lithium ion batteries. Nano Energy 2020, 74, 104868. [Google Scholar] [CrossRef]
- Gao, C.; Wang, P.; Wang, Z.; Kær, S.K.; Zhang, Y.; Yue, Y. The disordering-enhanced performances of the Al-MOF/graphene composite anodes for lithium ion batteries. Nano Energy 2019, 65, 104032. [Google Scholar] [CrossRef]
- Gao, C.; Jiang, Z.; Qi, S.; Wang, P.; Jensen, L.R.; Johansen, M.; Christensen, C.K.; Zhang, Y.; Ravnsbæk, D.B.; Yue, Y. Metal-organic framework glass anode with an exceptional cycling-induced capacity enhancement for Lithium-ion batteries. Adv. Mater. 2022, 34, 2110048. [Google Scholar] [CrossRef]
- Muruganantham, R.; Gu, Y.-J.; Song, Y.-D.; Kung, C.-W.; Liu, W.-R. Ce-MOF derived ceria: Insights into the Na-ion storage mechanism as a high-rate performance anode material. Appl. Mater. Today 2021, 22, 100935. [Google Scholar] [CrossRef]
- Wang, G.; Yang, J.; Park, J.; Gou, X.; Wang, B.; Liu, H.; Yao, J. Facile synthesis and characterization of graphene nanosheets. J. Phys. Chem. C 2008, 112, 8192–8195. [Google Scholar] [CrossRef]
- Tuccitto, N.; Riela, L.; Zammataro, A.; Spitaleri, L.; Li-Destri, G.; Sfuncia, G.; Nicotra, G.; Pappalardo, A.; Capizzi, G.; Trusso Sfrazzetto, G. Functionalized carbon nanoparticle-based sensors for chemical warfare agents. ACS Appl. Nano Mater. 2020, 3, 8182–8191. [Google Scholar] [CrossRef]
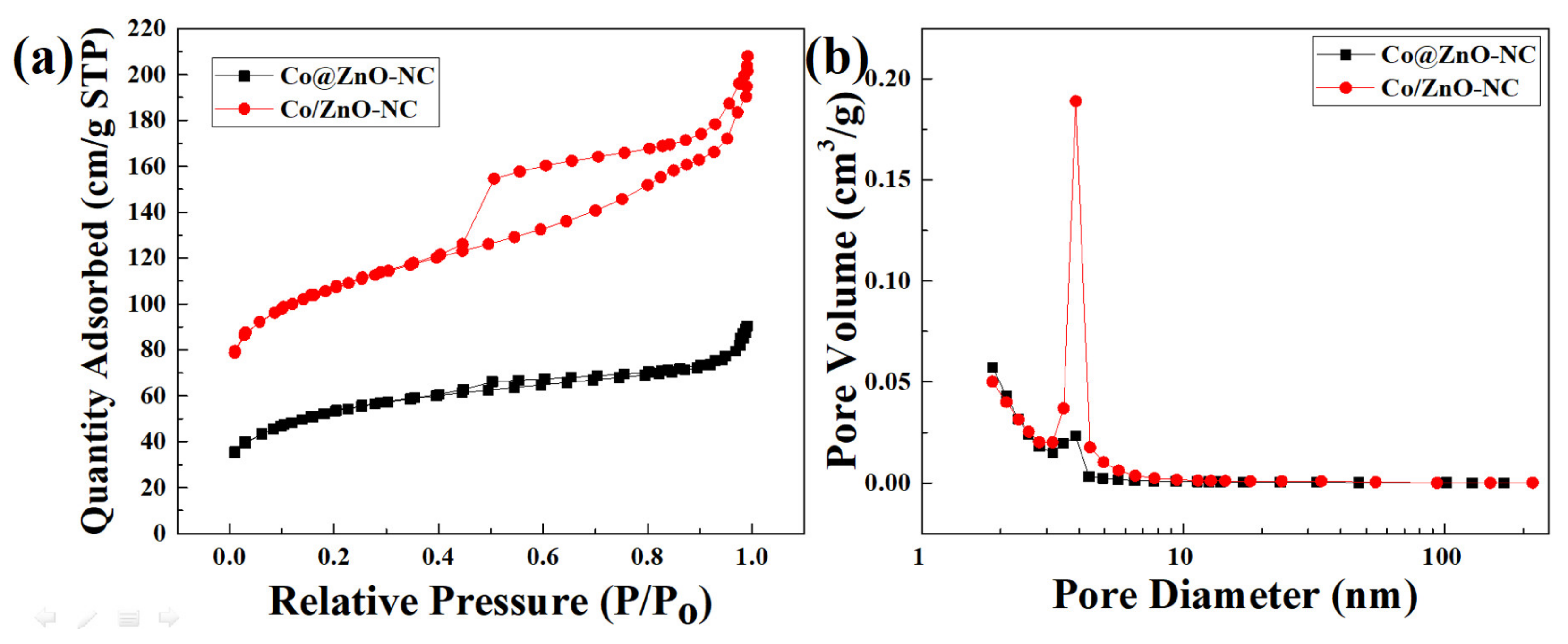
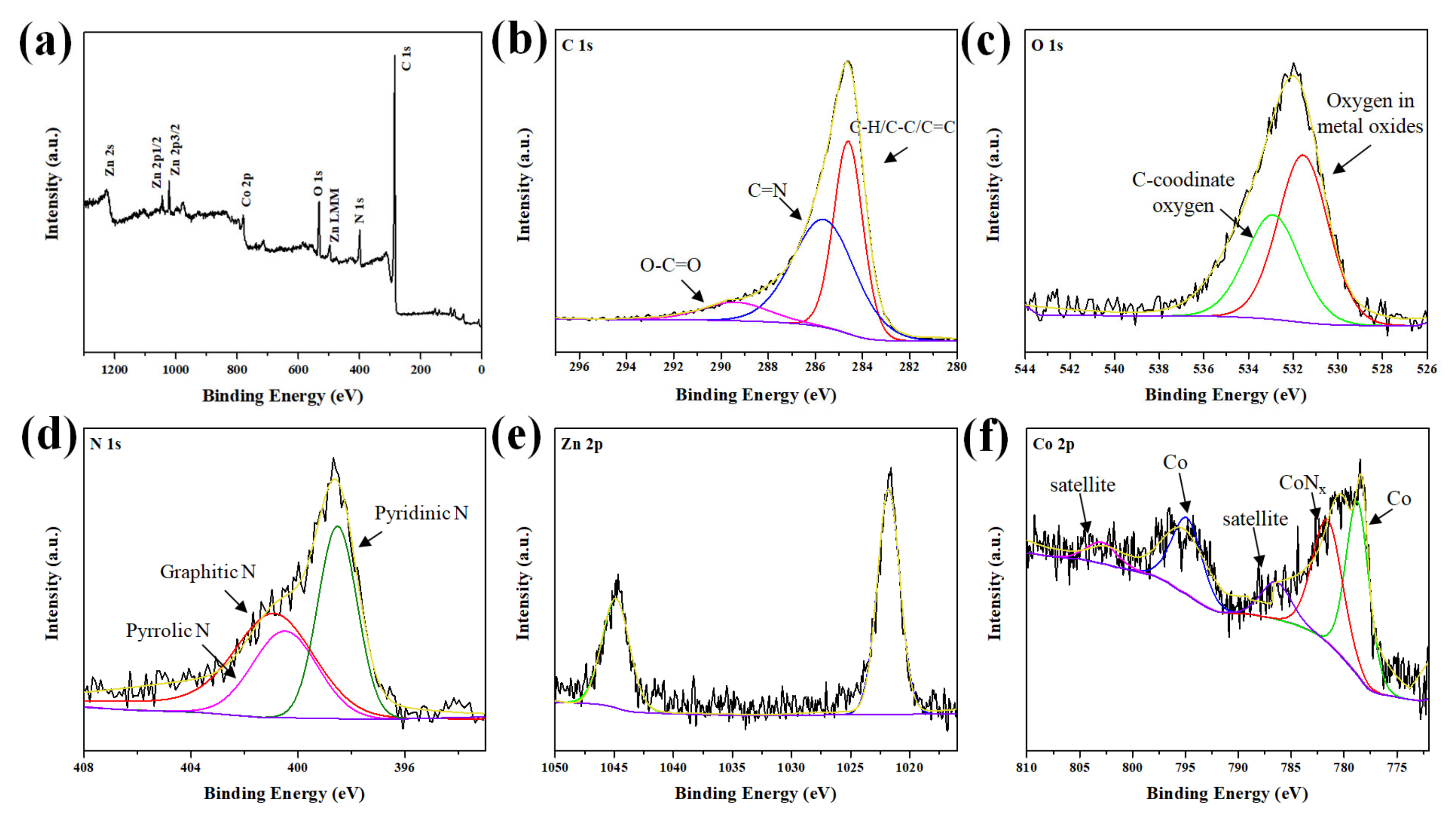
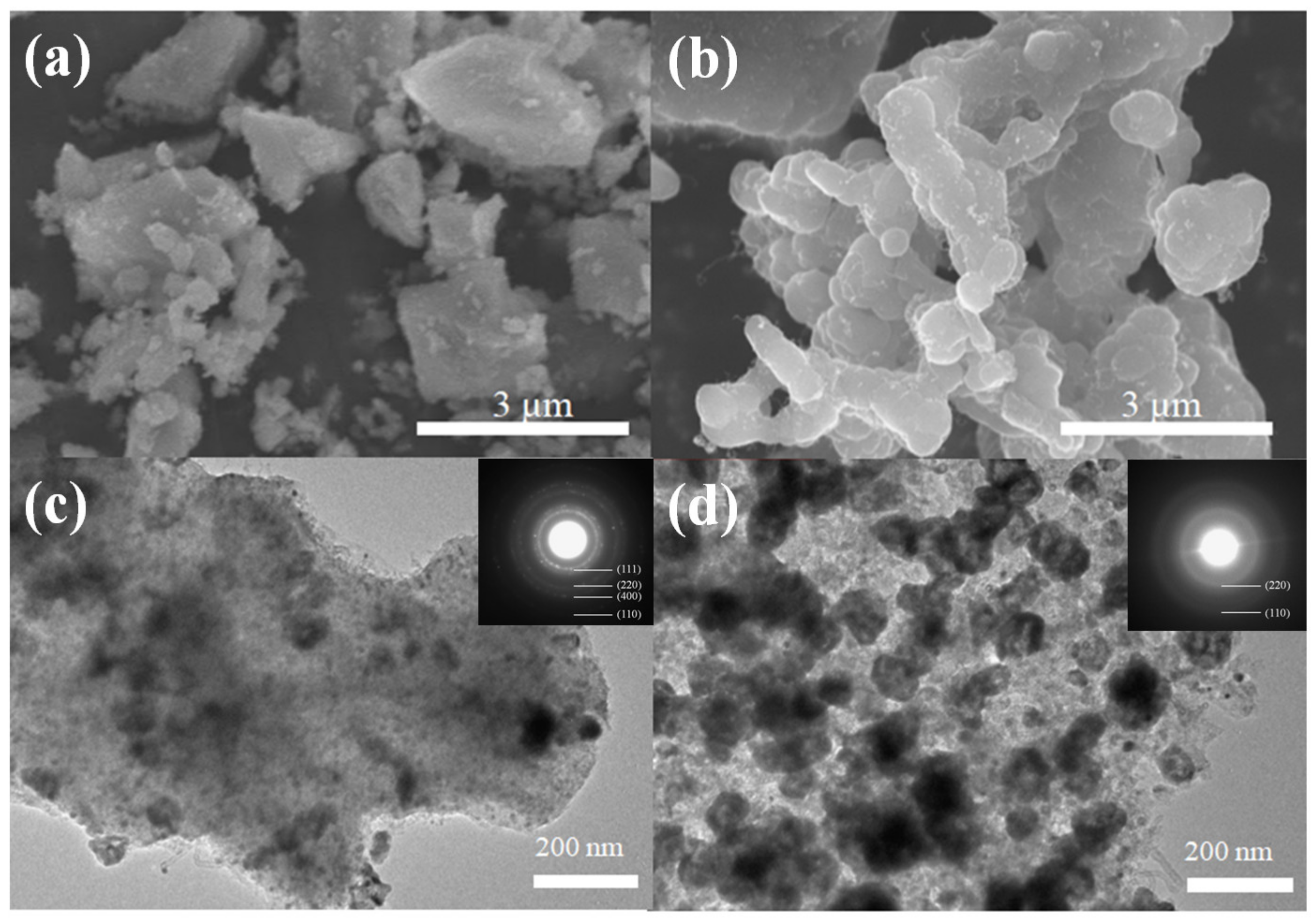
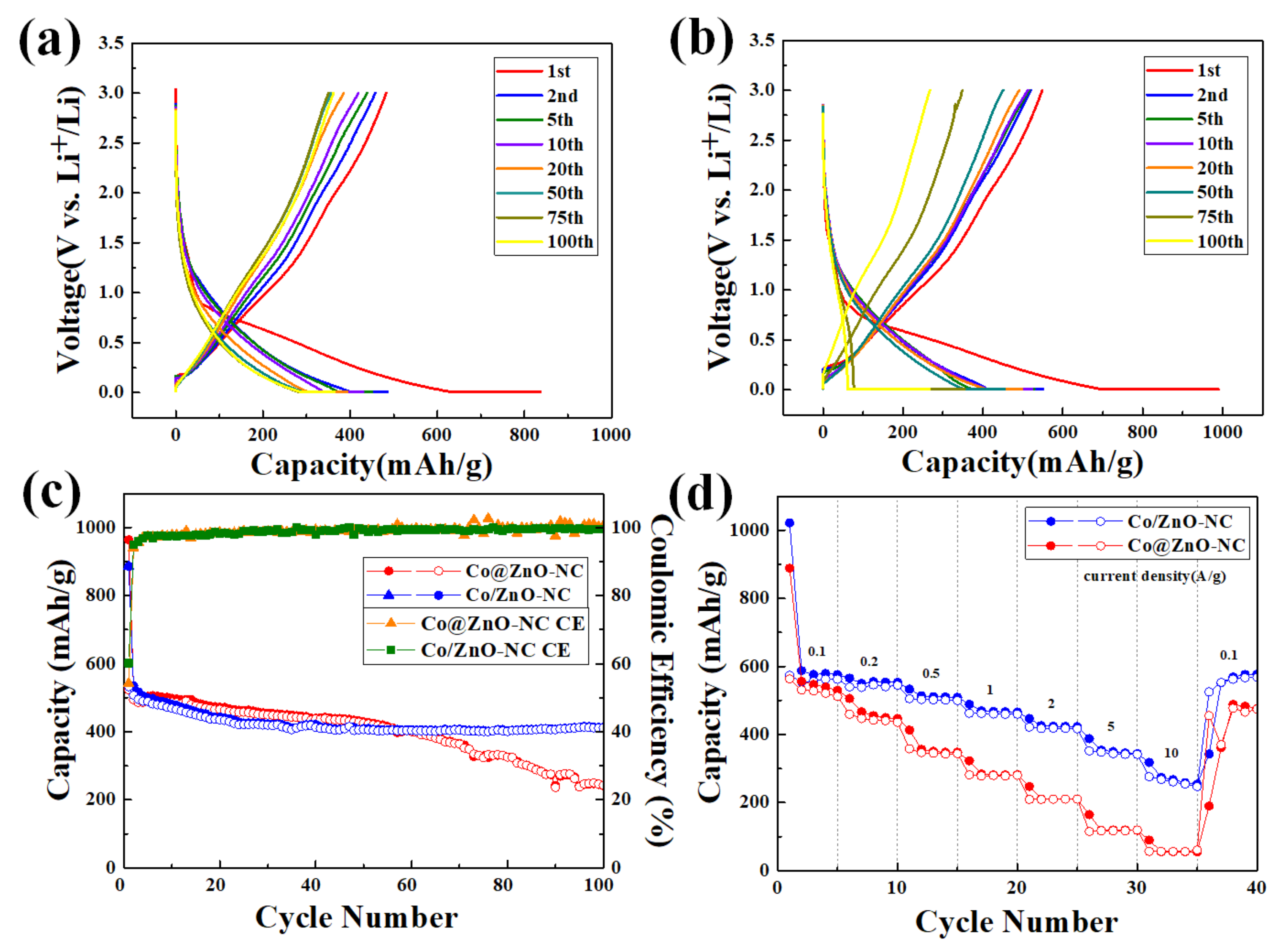
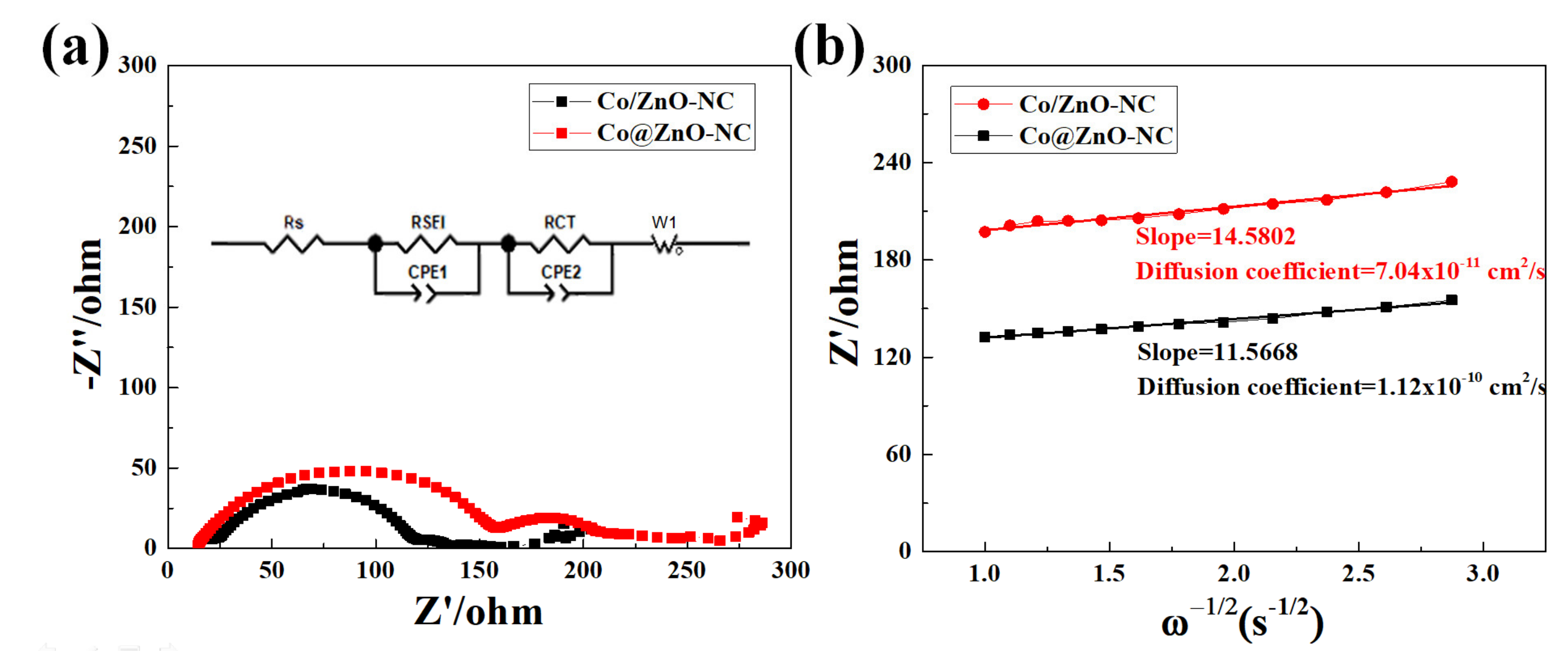
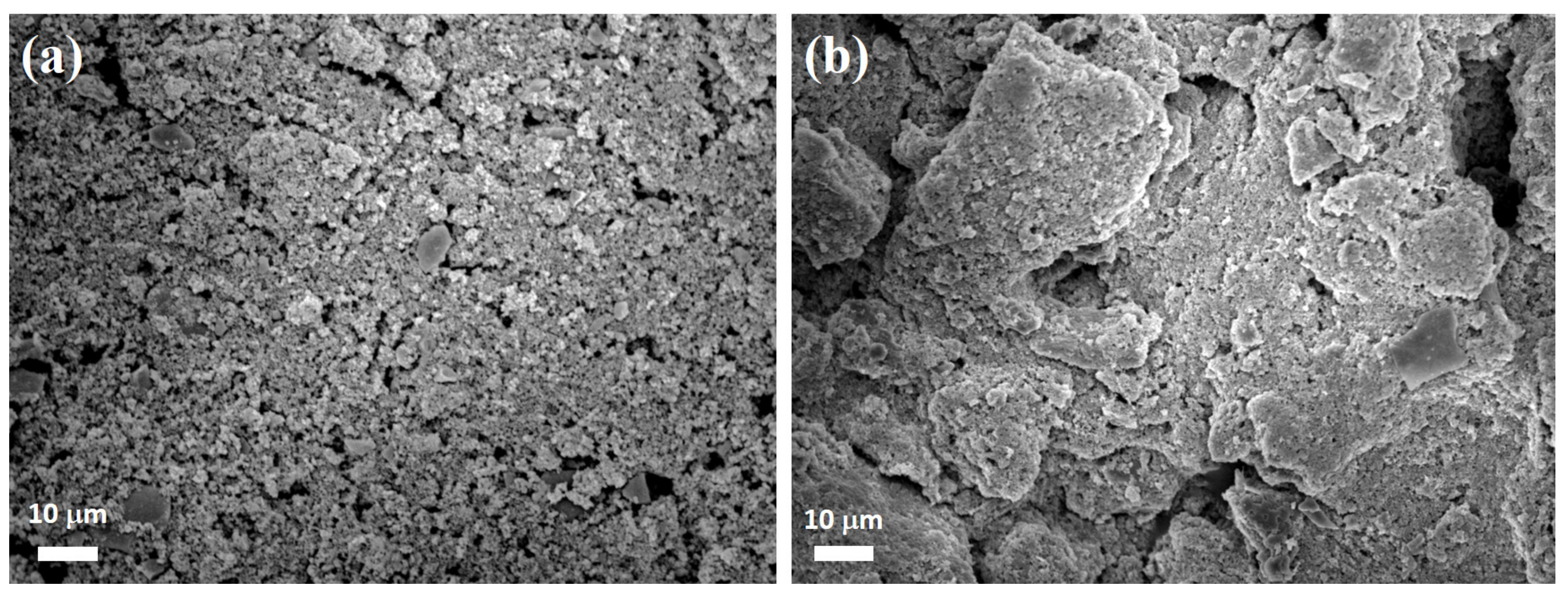
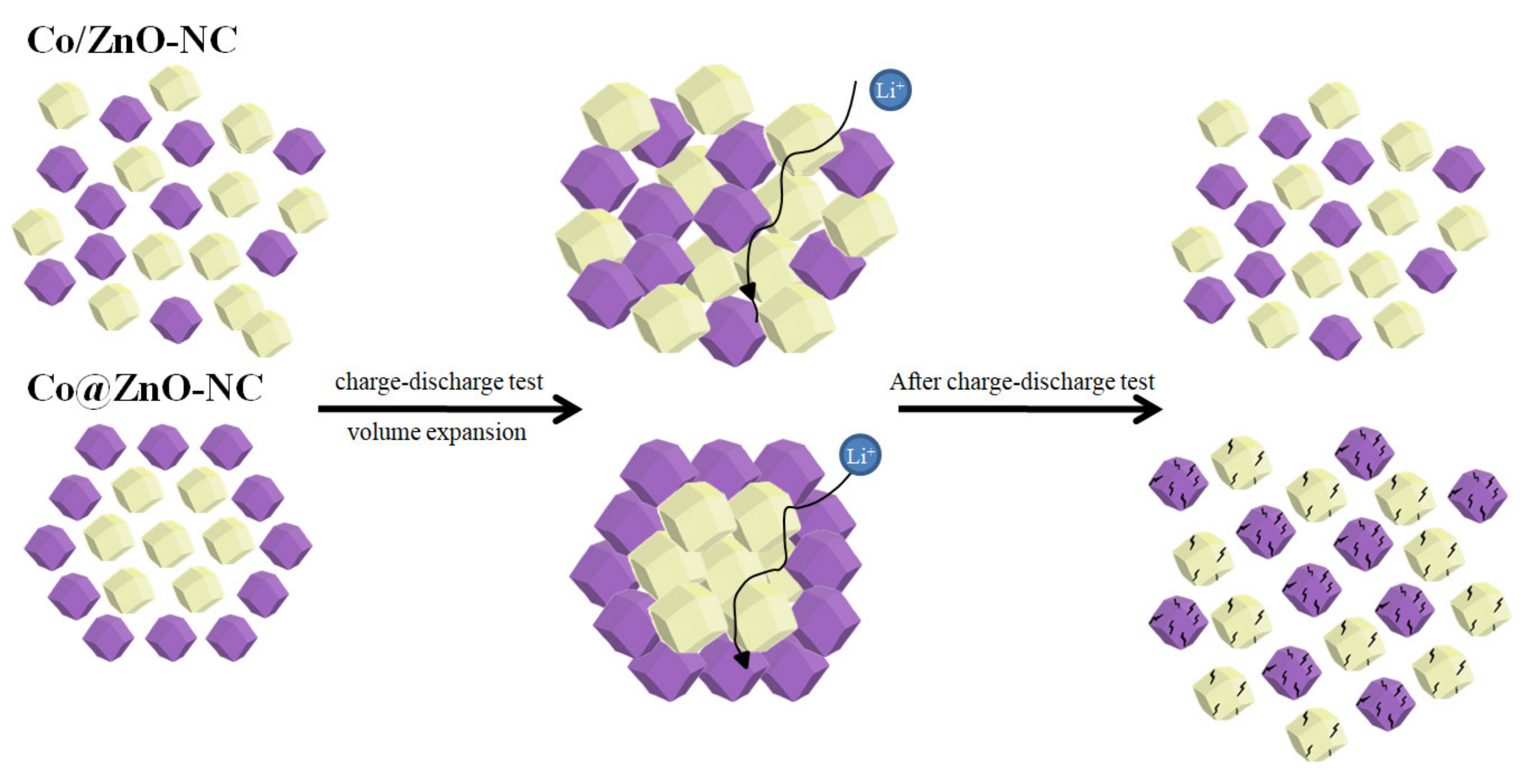
| Components | Co/ZnO-NC | Co@ZnO-NC |
|---|---|---|
| Rs (Ω) | 23 | 18 |
| RSEI (Ω) | 97 | 144 |
| RCT (Ω) | 15 | 49 |
| σ (Ω/s0.5) | 14.58 | 11.56 |
| D (cm2/s) | 7.04 × 10−11 | 1.12 × 10−10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.-C.; Huang, C.-H.; Liu, W.-R. Co/ZnO/Nitrogen-Doped Carbon Composite Anode Derived from Metal Organic Frameworks for Lithium Ion Batteries. Polymers 2022, 14, 3085. https://doi.org/10.3390/polym14153085
Chang Y-C, Huang C-H, Liu W-R. Co/ZnO/Nitrogen-Doped Carbon Composite Anode Derived from Metal Organic Frameworks for Lithium Ion Batteries. Polymers. 2022; 14(15):3085. https://doi.org/10.3390/polym14153085
Chicago/Turabian StyleChang, Ya-Chun, Chia-Hung Huang, and Wei-Ren Liu. 2022. "Co/ZnO/Nitrogen-Doped Carbon Composite Anode Derived from Metal Organic Frameworks for Lithium Ion Batteries" Polymers 14, no. 15: 3085. https://doi.org/10.3390/polym14153085
APA StyleChang, Y.-C., Huang, C.-H., & Liu, W.-R. (2022). Co/ZnO/Nitrogen-Doped Carbon Composite Anode Derived from Metal Organic Frameworks for Lithium Ion Batteries. Polymers, 14(15), 3085. https://doi.org/10.3390/polym14153085








