Wearable Natural Rubber Latex Gloves with Curcumin for Torn Glove Detection in Clinical Settings
Abstract
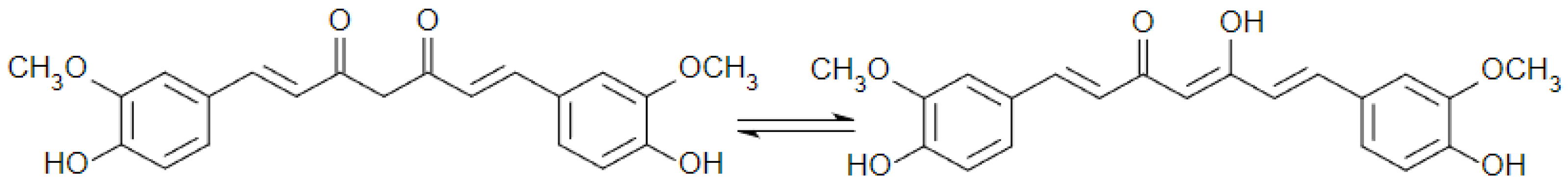
1. Introduction
2. Materials and Methods
2.1. Materials and Instrumentation
2.2. Preparation of NRL Filled with Curcumin (NRL-Cur Mixture)
2.3. Preparation of Double Coating Films (LC)
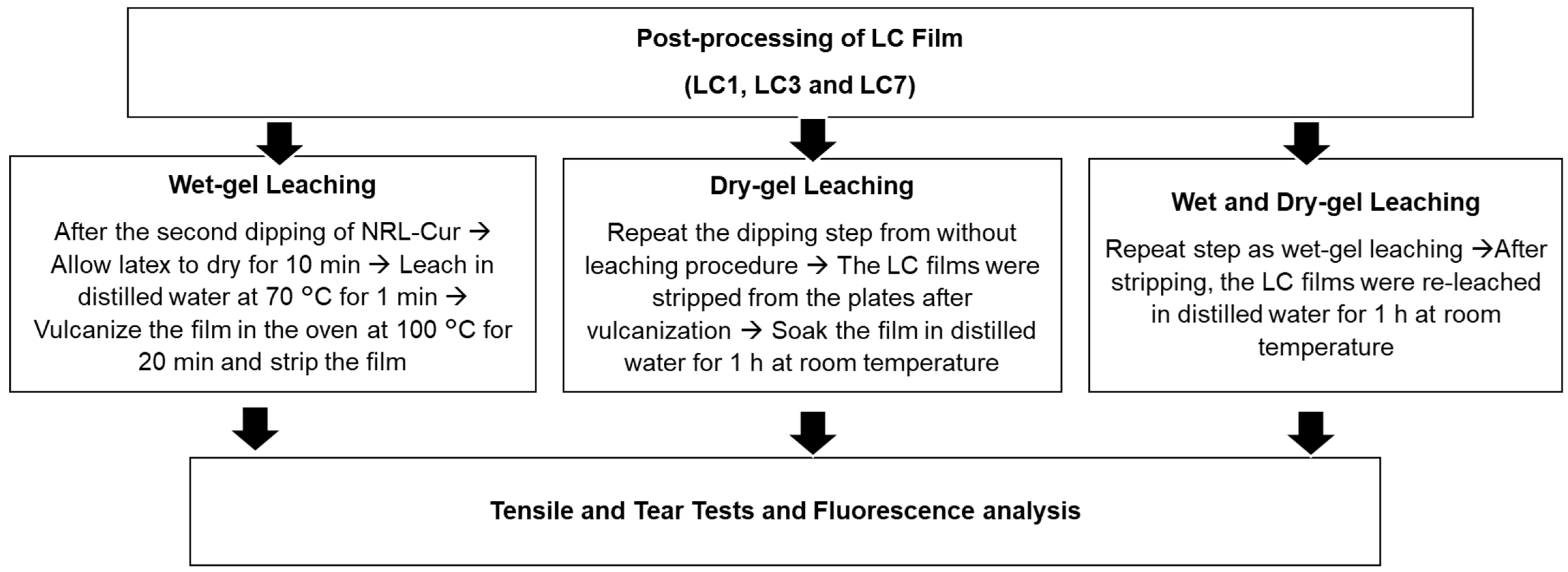
2.4. Post-Processing of LC Film
2.5. Characterization of Mechanical Properties
2.6. Fluorescence Spectroscopy of LC Films
2.7. Characterization of LC Films
2.8. Thermogravimetric Analysis
3. Results and Discussions
3.1. Mechanical Properties of the LC Films
3.2. Fluorescence Effect of the Torn/Cut LC Films Based on Dwell Time of Dipping
3.3. Effect of Leaching Process on Mechanical Properties of the LC Films
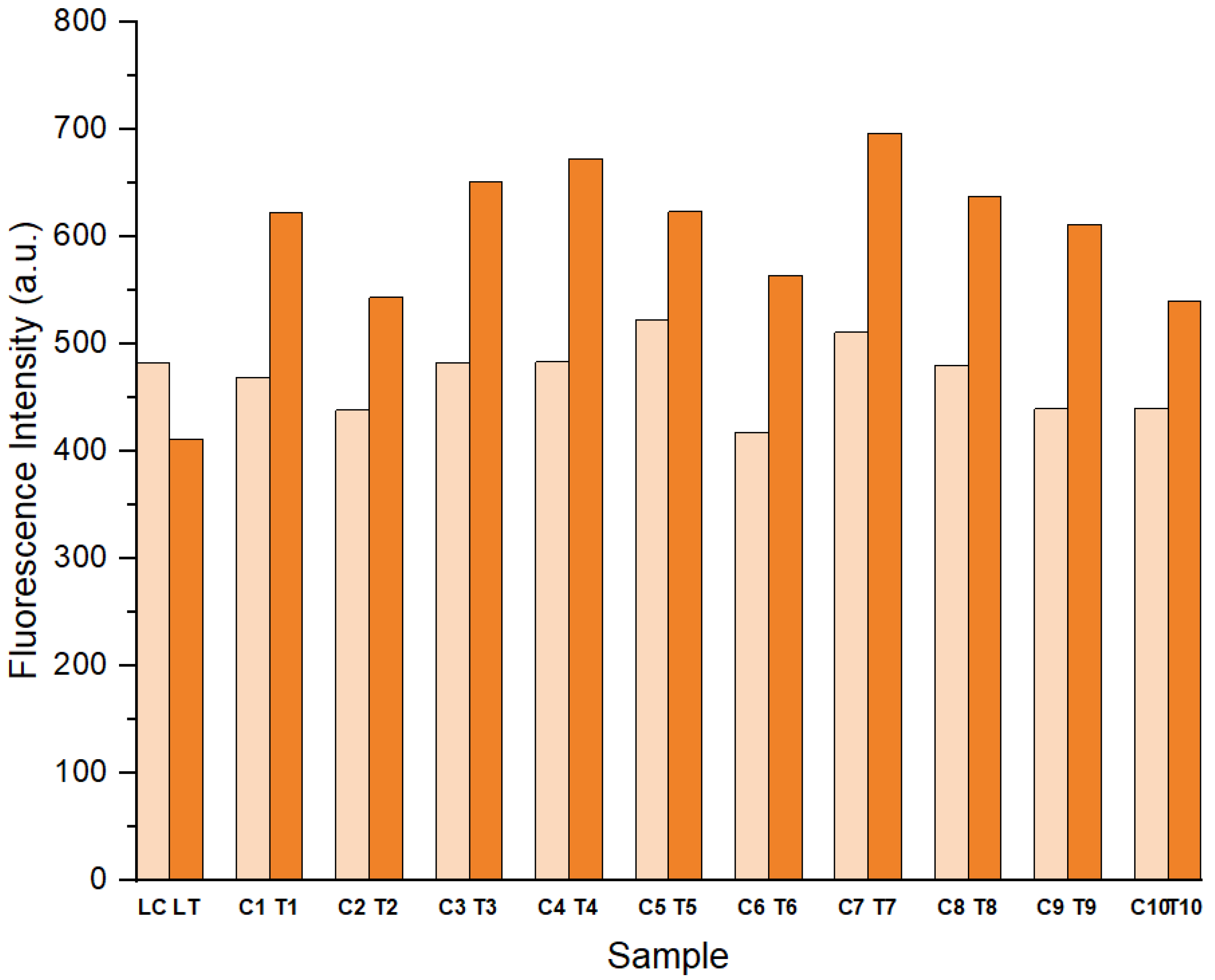
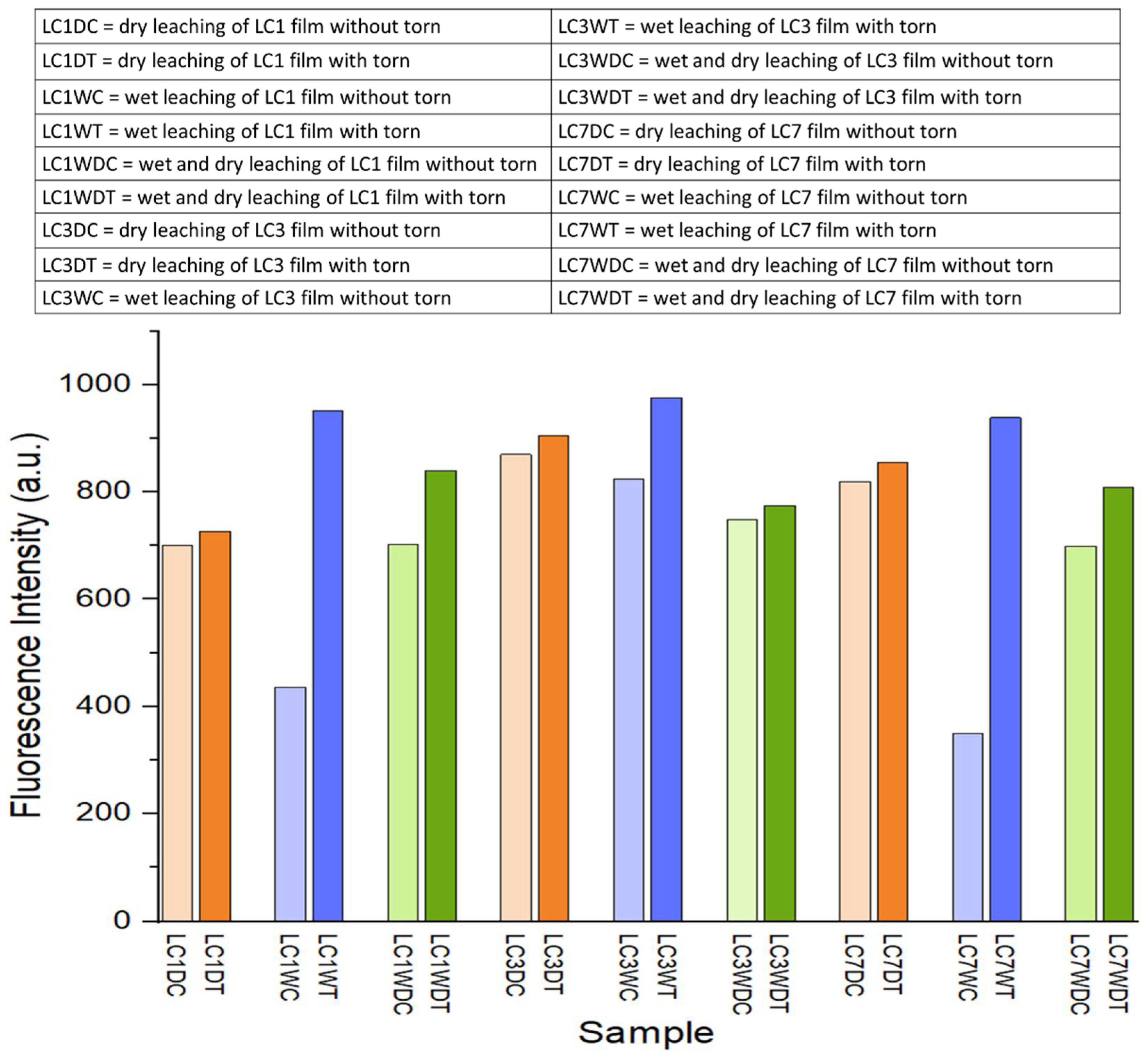
3.4. Effect of Leaching on Fluorescence Analysis of LC Films
3.5. FTIR Study
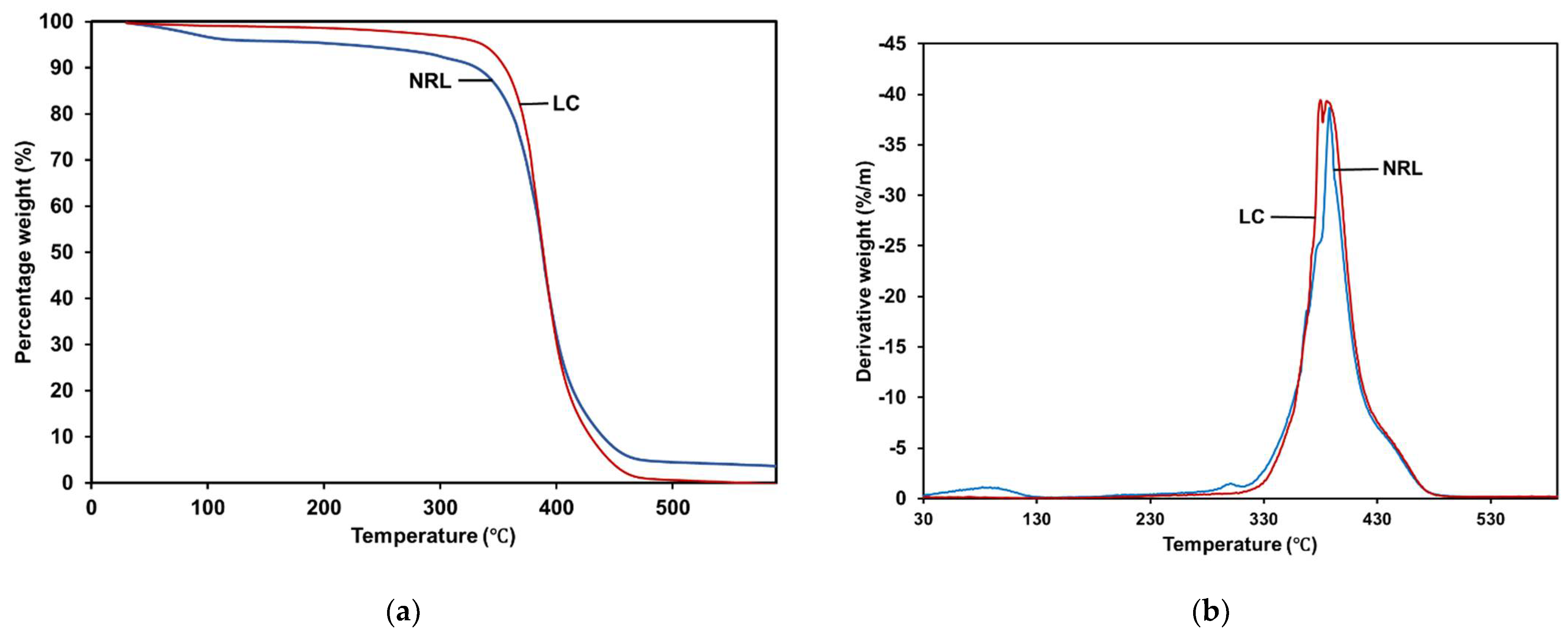
3.6. Thermal Properties by Thermogravimetric Analyzer (TGA)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chan, K.Y.; Singh, V.A.; Oun, B.H.; To, B.H.S. The rate of glove perforations in orthopaedic procedures: Single versus double gloving. A prospective study. Med. J. Malays. 2006, 61 (Suppl. B), 3–7. [Google Scholar]
- Osman, M.O.; Jensen, S.L. Surgical Gloves: Current Problems. World J. Surg. 1999, 23, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Dodds, R.D.A.; Guy, P.J.; Peacock, A.M.; Duffy, S.R.; Barker, S.G.E.; Thomas, M.H. Surgical glove perforation. Br. J. Surg. 2005, 75, 966–968. [Google Scholar] [CrossRef] [PubMed]
- Han, C.D.; Kim, J.; Moon, S.H.; Lee, B.H.; Kwon, H.M.; Park, K.K. A Randomized Prospective Study of Glove Perforation in Orthopaedic Surgery: Is a Thick Glove More Effective? J. Arthroplast. 2013, 28, 1878–1881. [Google Scholar] [CrossRef]
- Tanner, J. Surgical Gloves: Perforation and Protection. J. Perioper. Pract. 2006, 16, 148–152. [Google Scholar] [CrossRef]
- Yinusa, W.; Li, Y.H.; Ho, W.Y.; Leong, J.C.Y.; Chow, W. Glove punctures in orthopaedic surgery. Int. Orthop. 2004, 28, 36–39. [Google Scholar] [CrossRef]
- Makama, J.G.; Okeme, I.M.; Makama, E.J.; Ameh, E.A. Glove Perforation Rate in Surgery: A Randomized, Controlled Study To Evaluate the Efficacy of Double Gloving. Surg. Infect. 2016, 17, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Gordon, A.M.; Hudson, P.W.; Bowman, J.R.; Watson, S.L.; Leddy, L.R.; Khoury, J.G.; Patt, J.C.; Tubb, C.C.; Ames, S.E.; McGwin, G.; et al. Workplace Hazards in Orthopaedic Surgery Training: A Nationwide Resident Survey Involving Sharps-related Injuries. J. Am. Acad. Orthop. Surg. 2022, 30, 428–436. [Google Scholar] [CrossRef]
- Germaine, R.L.S.; Hanson, J.; de Gara, C.J. Double gloving and practice attitudes among surgeons. Am. J. Surg. 2003, 185, 141–145. [Google Scholar] [CrossRef]
- Goldman, A.H.; Haug, E.; Owen, J.R.; Wayne, J.S.; Golladay, G.J. High Risk of Surgical Glove Perforation From Surgical Rotatory Instruments. Clin. Orthop. Relat. Res. 2016, 474, 2513–2517. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, C.; Pan, H.; Li, L.; Yu, Y.; Liu, B. An insight into the in vivo imaging potential of curcumin analogues as fluorescence probes. Asian J. Pharm. Sci. 2021, 16, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Khorasani, M.Y.; Langari, H.; Sany, S.B.T.; Rezayi, M.; Sahebkar, A. The role of curcumin and its derivatives in sensory applications. Mater. Sci. Eng. C 2019, 103, 109792. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, H.; El Kurdi, R.; Patra, D. A Novel Study on the Self-Assembly Behavior of Poly(lactic-co-glycolic acid) Polymer Probed by Curcumin Fluorescence. ACS Omega 2022, 7, 9551–9558. [Google Scholar] [CrossRef]
- Sirawatcharin, S.; Saithongdee, A.; Chaicham, A.; Tomapatanaget, B.; Imyim, A.; Praphairaksit, N. Naked-eye and Colorimetric Detection of Arsenic(III) Using Difluoroboron-curcumin in Aqueous and Resin Bead Support Systems. Anal. Sci. 2014, 30, 1129–1134. [Google Scholar] [CrossRef]
- Bhopate, D.P.; Mahajan, P.G.; Garadkar, K.M.; Kolekar, G.B.; Patil, S.R. A highly selective and sensitive single click novel fluorescent off–on sensor for copper and sulfide ions detection directly in aqueous solution using curcumin nanoparticles. New J. Chem. 2015, 39, 7086–7096. [Google Scholar] [CrossRef]
- Xu, G.; Wang, J.; Si, G.; Wang, M.; Xue, X.; Wu, B.; Zhou, S. A novel highly selective chemosensor based on curcumin for detection of Cu2+ and its application for bioimaging. Sens. Actuators B Chem. 2016, 230, 684–689. [Google Scholar] [CrossRef]
- Ponnuvel, K.; Santhiya, K.; Padmini, V. Curcumin based chemosensor for selective detection of fluoride and cyanide anions in aqueous media. Photochem. Photobiol. Sci. 2016, 15, 1536–1543. [Google Scholar] [CrossRef]
- Liu, Y.; Ouyang, Q.; Li, H.; Zhang, Z.; Chen, Q. Development of an Inner Filter Effects-Based Upconversion Nanoparticles–Curcumin Nanosystem for the Sensitive Sensing of Fluoride Ion. ACS Appl. Mater. Interfaces 2017, 9, 18314–18321. [Google Scholar] [CrossRef]
- Park, S.; Lee, S.-Y. Significant enhancement of curcumin photoluminescence by a photosensitizing organogel: An optical sensor for pyrrole detection. Sens. Actuators B Chem. 2015, 220, 318–325. [Google Scholar] [CrossRef]
- Ashraf, P.M.; Lalitha, K.; Edwin, L. Synthesis of polyaniline hybrid composite: A new and efficient sensor for the detection of total volatile basic nitrogen molecules. Sens. Actuators B Chem. 2015, 208, 369–378. [Google Scholar] [CrossRef]
- Ramamoorthy, J.; Sathya, V.; Lavanya, R.; Padmini, V. Highly selective and sensitive response of curcumin thioether derivative for the detection of hypochlorous acid by fluorimetric method. J. Iran. Chem. Soc. 2022, 19, 3327–3335. [Google Scholar] [CrossRef]
- Devasena, T.; Balasubramanian, N.; Muninathan, N.; Baskaran, K.; John, S.T. Curcumin Is an Iconic Ligand for Detecting Environmental Pollutants. Bioinorg. Chem. Appl. 2022, 2022, 9248988. [Google Scholar] [CrossRef]
- Chandran, N.; Janardhanan, P.; Bayal, M.; Pilankatta, R.; Nair, S.S. Development of a paper printed colorimetric sensor based on Cu-Curcumin nanoparticles for evolving point-of-care clinical diagnosis of sodium. Sci. Rep. 2022, 12, 1–15. [Google Scholar] [CrossRef]
- Geng, F.; Wang, Y.; Qu, P.; Zhang, Y.; Dong, H.; Xu, M. Naked-eye detection of Cys using simple molecular systems of curcumin and Hg2+. Anal. Methods 2013, 5, 3965–3969. [Google Scholar] [CrossRef]
- Patil, R.; Gangalum, P.R.; Wagner, S.; Portilla-Arias, J.; Ding, H.; Rekechenetskiy, A.; Konda, B.; Inoue, S.; Black, K.L.; Ljubimova, J.Y.; et al. Curcumin Targeted, Polymalic Acid-Based MRI Contrast Agent for the Detection of Aβ Plaques in Alzheimer’s Disease. Macromol. Biosci. 2015, 15, 1212–1217. [Google Scholar] [CrossRef]
- Alipour, E.; Shahabi, H.; Mahmoudi-Badiki, T. Introducing curcumin as an electrochemical DNA hybridization indicator and its application for detection of human interleukin-2 gene. J. Solid State Electrochem. 2016, 20, 1645–1653. [Google Scholar] [CrossRef]
- Ojani, R.; Raoof, J.-B.; Zamani, S. A novel voltammetric sensor for amoxicillin based on nickel–curcumin complex modified carbon paste electrode. Bioelectrochemistry 2012, 85, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Pourreza, N.; Golmohammadi, H. Hemoglobin detection using curcumin nanoparticles as a colorimetric chemosensor. RSC Adv. 2015, 5, 1712–1717. [Google Scholar] [CrossRef]
- Estephan, M.; El Kurdi, R.; Patra, D. Curcumin-embedded DBPC liposomes coated with chitosan layer as a fluorescence nanosensor for the selective detection of ribonucleic acid. Luminescence 2022, 37, 422–430. [Google Scholar] [CrossRef]
- Erna, K.H.; Felicia, W.X.L.; Rovina, K.; Vonnie, J.M.; Huda, N. Development of curcumin/rice starch films for sensitive detection of hypoxanthine in chicken and fish meat. Carbohydr. Polym. Technol. Appl. 2022, 3, 100189. [Google Scholar] [CrossRef]
- Dai, H.; Huang, Z.; Liu, X.; Bi, J.; Shu, Z.; Xiao, A.; Wang, J. Colorimetric ELISA based on urease catalysis curcumin as a ratiometric indicator for the sensitive determination of aflatoxin B1 in grain products. Talanta 2022, 246, 123495. [Google Scholar] [CrossRef] [PubMed]
- Sarih, N.M.; Gwee, K.; Maher, S.; Rashid, A.A. Natural Rubber (NR) Latex Films with Antimicrobial Properties for Stethoscope Diaphragm Covers. Materials 2022, 15, 3433. [Google Scholar] [CrossRef] [PubMed]
- Ritter, M.A.; Olberding, E.M.; Malinzak, R.A. Ultraviolet Lighting During Orthopaedic Surgery and the Rate of Infection. JBJS 2007, 89, 1935–1940. [Google Scholar] [CrossRef]
- Ritter, M.A. Operating Room Environment. Clin. Orthop. Relat. Res. 1999, 369, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Yew, G.Y.; Tham, T.C.; Law, C.L.; Chu, D.-T.; Ogino, C.; Show, P.L. Emerging crosslinking techniques for glove manufacturers with improved nitrile glove properties and reduced allergic risks. Mater. Today Commun. 2019, 19, 39–50. [Google Scholar] [CrossRef]
- Huda, N.; Mohd, A.-H. Leaching A Critical Factor in processing of rubber examination gloves. Malays. Rubber Technol. Dev. 2014, 1, 16–21. [Google Scholar]
- Ab Rahman, M.F.; Rusli, A.; Kuwn, M.T.; Azura, A.R. Effect of Latex Compound Dwell Time for the Production of Prototyped Biodegradable Natural Rubber Latex Gloves. IOP Conf. Ser. Mater. Sci. Eng. 2019, 548, 012017. [Google Scholar] [CrossRef]
- Myant, C.; Reddyhoff, T.; Spikes, H. Laser-induced fluorescence for film thickness mapping in pure sliding lubricated, compliant, contacts. Tribol. Int. 2010, 43, 1960–1969. [Google Scholar] [CrossRef][Green Version]
- Goldys, E.M.; Drozdowicz-Tomsia, K.; Xie, F.; Shtoyko, T.; Matveeva, E.; Gryczynski, I.; Gryczynski, Z. Fluorescence Amplification by Electrochemically Deposited Silver Nanowires with Fractal Architecture. J. Am. Chem. Soc. 2007, 129, 12117–12122. [Google Scholar] [CrossRef]
- Chirinos, H.D.; Guedes, S.M.L. The manufacute of gloves using RVNRL: Parameters of the coagulant dipping process. Braz. J. Chem. Eng. 1998, 15, 334–342. [Google Scholar] [CrossRef]
- Ramli, R.; Jaapar, J.; Singh, M.S.J.; Yatim, A.H.M. Physical Properties and Fatigue Lifecycles of Natural Rubber Latex Gloves. Adv. Environ. Biol. 2014, 8, 2714–2722. [Google Scholar]
- Mathew, S.; Varghese, S. Extractable Proteins in Latex Products: Effect of Vulcanization Methods and Leaching. Rubber Sci. 2019, 31, 249–258. [Google Scholar]
- Wei, F.; Yu, H.; Zeng, Z.; Liu, H.; Wang, Q.; Wang, J.; Li, S. Preparation and structural characterization of hydroxylethyl methacrylate grafted natural rubber latex. Polímeros 2014, 24, 283–290. [Google Scholar] [CrossRef][Green Version]
- Chen, X.; Zou, L.-Q.; Niu, J.; Liu, W.; Peng, S.-F.; Liu, C.-M. The Stability, Sustained Release and Cellular Antioxidant Activity of Curcumin Nanoliposomes. Molecules 2015, 20, 14293–14311. [Google Scholar] [CrossRef] [PubMed]
- Nabil, H.; Ismail, H.; Azura, A. Recycled Polyethylene Terephthalate Filled Natural Rubber Compounds: Effects of Filler Loading and Types of Matrix. J. Elastomers Plast. 2011, 43, 429–449. [Google Scholar] [CrossRef]
- Chaichua, B.; Prasassarakich, P.; Poompradub, S. In situ silica reinforcement of natural rubber by sol–gel process via rubber solution. J. Sol-Gel Sci. Technol. 2009, 52, 219–227. [Google Scholar] [CrossRef]
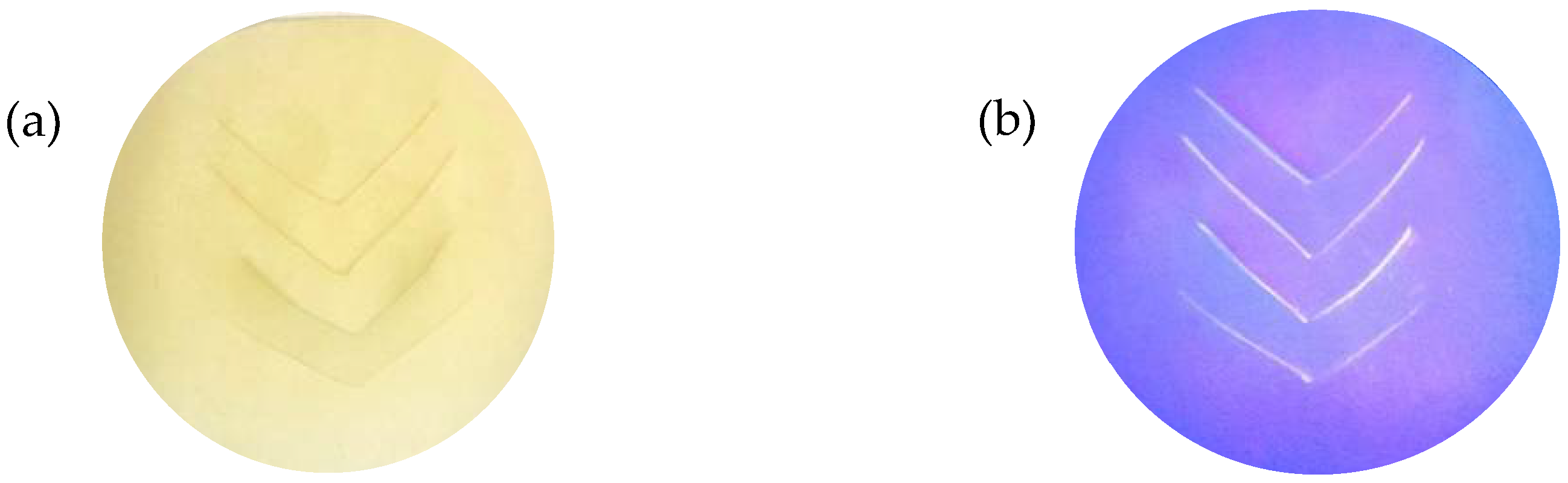

| Samples | Dwell Time | Thickness (mm) | |
|---|---|---|---|
| NRL (44%) | NRL-Cur (26%) | MEAN (±SD) | |
| LC1 | 10_10 | 5_5 | 0.22 (2.78 × 10−17) |
| LC2 | 10_10 | 5 | 0.21 (2.78 × 10−17) |
| LC3 | 10_5_10 | 5_5 | 0.24 (4.90 × 10−3) |
| LC4 | 10_5_10 | 5 | 0.23 (4.00 × 10−3) |
| LC5 | 15_15 | 5_5 | 0.24 (8.94 × 10−3) |
| LC6 | 15_15 | 5 | 0.23 (4.90 × 10−3) |
| LC7 | 15_5_15 | 5_5 | 0.26 (4.90 × 103) |
| LC8 | 15_5_15 | 5 | 0.25 (4.30 × 103) |
| LC9 | 15_15 | 10_5_10 | 0.26 (0.00) |
| LC10 | 15_15 | 10_10 | 0.25 (4.71 × 10−3) |
| L | 10_10 | 0.15 (0.00) | |
| Samples | Tensile Strength (MPa) | Tear Strength (N/mm) |
|---|---|---|
| Mean (±SD) | Mean (±SD) | |
| LC1 | 19.10 (0.96) | 40.55 (10.15) |
| LC2 | 18.09 (0.69) | 45.78 (9.49) |
| LC3 | 18.82 (1.76) | 40.13 (7.87) |
| LC4 | 18.96 (1.76) | 41.25 (8.60) |
| LC5 | 19.14 (0.73) | 41.72 (1.66) |
| LC6 | 19.13 (1.47) | 44.23 (9.51) |
| LC7 | 18.67 (0.81) | 39.84 (4.23) |
| LC8 | 18.31 (0.77) | 43.05 (14.41) |
| LC9 | 20.10 (0.45) | 39.97 (13.08) |
| LC10 | 18.35 (0.55) | 40.20 (3.19) |
| L | 17.92 (0.17) | 37.73 (6.14) |
| Leaching Conditions | LC1 | LC3 | LC7 | |||
|---|---|---|---|---|---|---|
| Tensile (MPa) | Tear (N/mm) | Tensile (MPa) | Tear (N/mm) | Tensile (MPa) | Tear (N/mm) | |
| Unleached | 19.10 | 40.55 | 18.82 | 41.72 | 18.67 | 39.84 |
| Wet | 21.23 | 52.02 | 23.36 | 54.15 | 21.98 | 57.91 |
| Dry | 21.60 | 53.86 | 23.04 | 54.30 | 21.09 | 55.49 |
| Wet + Dry | 21.90 | 58.60 | 23.81 | 65.47 | 22.74 | 63.85 |
| LC Films | Without Leaching | Wet Leaching | Dry Leaching | Wet-Dry Leaching |
|---|---|---|---|---|
| LC1 |  |  |  |  |
| LC3 |  |  |  |  |
| LC7 |  |  |  |  |
| Type of Film | T5% (°C) | T50% (°C) | T75% (°C) | Total Weight Loss (%) |
|---|---|---|---|---|
| NRL | 211.92 | 387.92 | 405.92 | 96.329 |
| LC | 336.92 | 388.92 | 403.92 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarih, N.M.; Dzulkafly, N.S.; Maher, S.; Rashid, A.A. Wearable Natural Rubber Latex Gloves with Curcumin for Torn Glove Detection in Clinical Settings. Polymers 2022, 14, 3048. https://doi.org/10.3390/polym14153048
Sarih NM, Dzulkafly NS, Maher S, Rashid AA. Wearable Natural Rubber Latex Gloves with Curcumin for Torn Glove Detection in Clinical Settings. Polymers. 2022; 14(15):3048. https://doi.org/10.3390/polym14153048
Chicago/Turabian StyleSarih, Norfatirah Muhamad, Nuur Syuhada Dzulkafly, Simon Maher, and Azura A. Rashid. 2022. "Wearable Natural Rubber Latex Gloves with Curcumin for Torn Glove Detection in Clinical Settings" Polymers 14, no. 15: 3048. https://doi.org/10.3390/polym14153048
APA StyleSarih, N. M., Dzulkafly, N. S., Maher, S., & Rashid, A. A. (2022). Wearable Natural Rubber Latex Gloves with Curcumin for Torn Glove Detection in Clinical Settings. Polymers, 14(15), 3048. https://doi.org/10.3390/polym14153048







