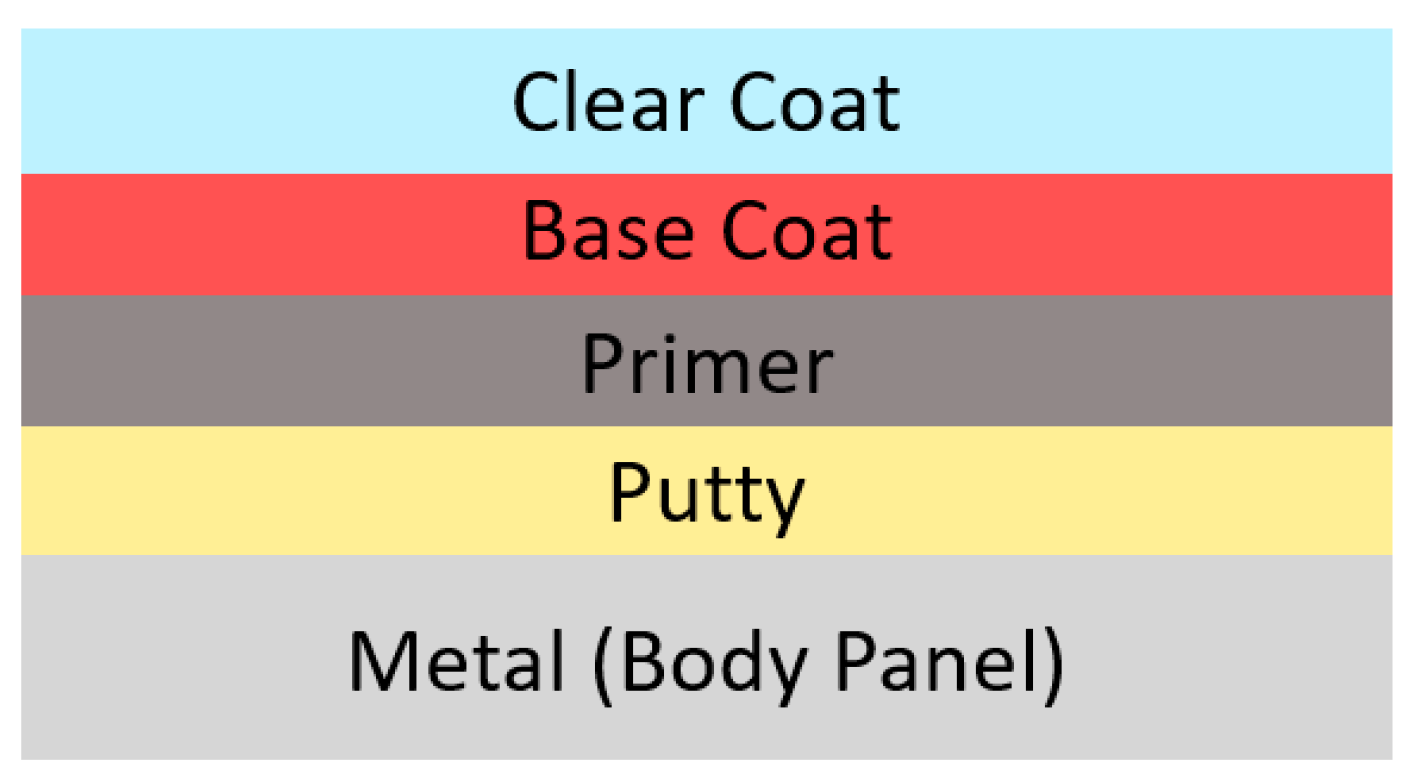
Free-Radical Photopolymerization for Curing Products for Refinish Coatings Market
Abstract
:1. Introduction
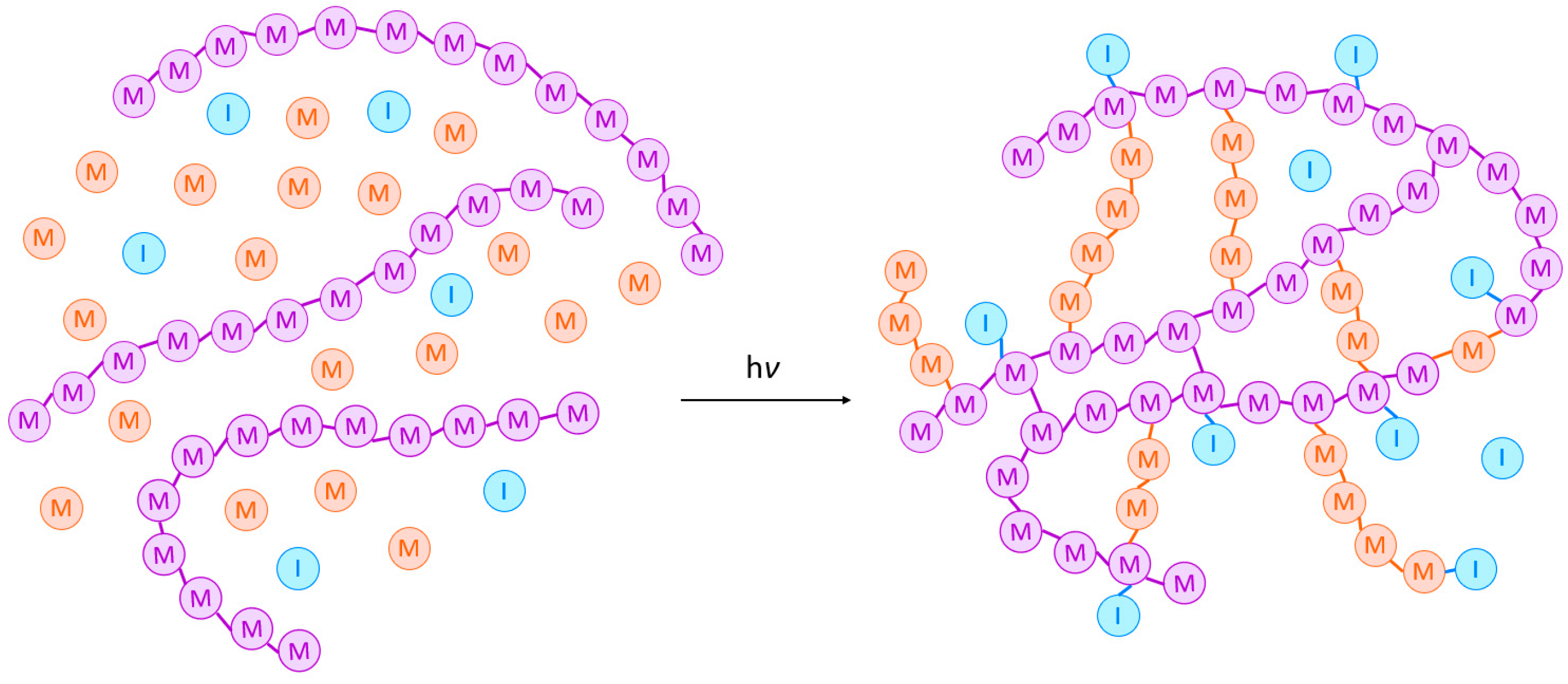
2. Free Radical Polymerization
2.1. Mechanism
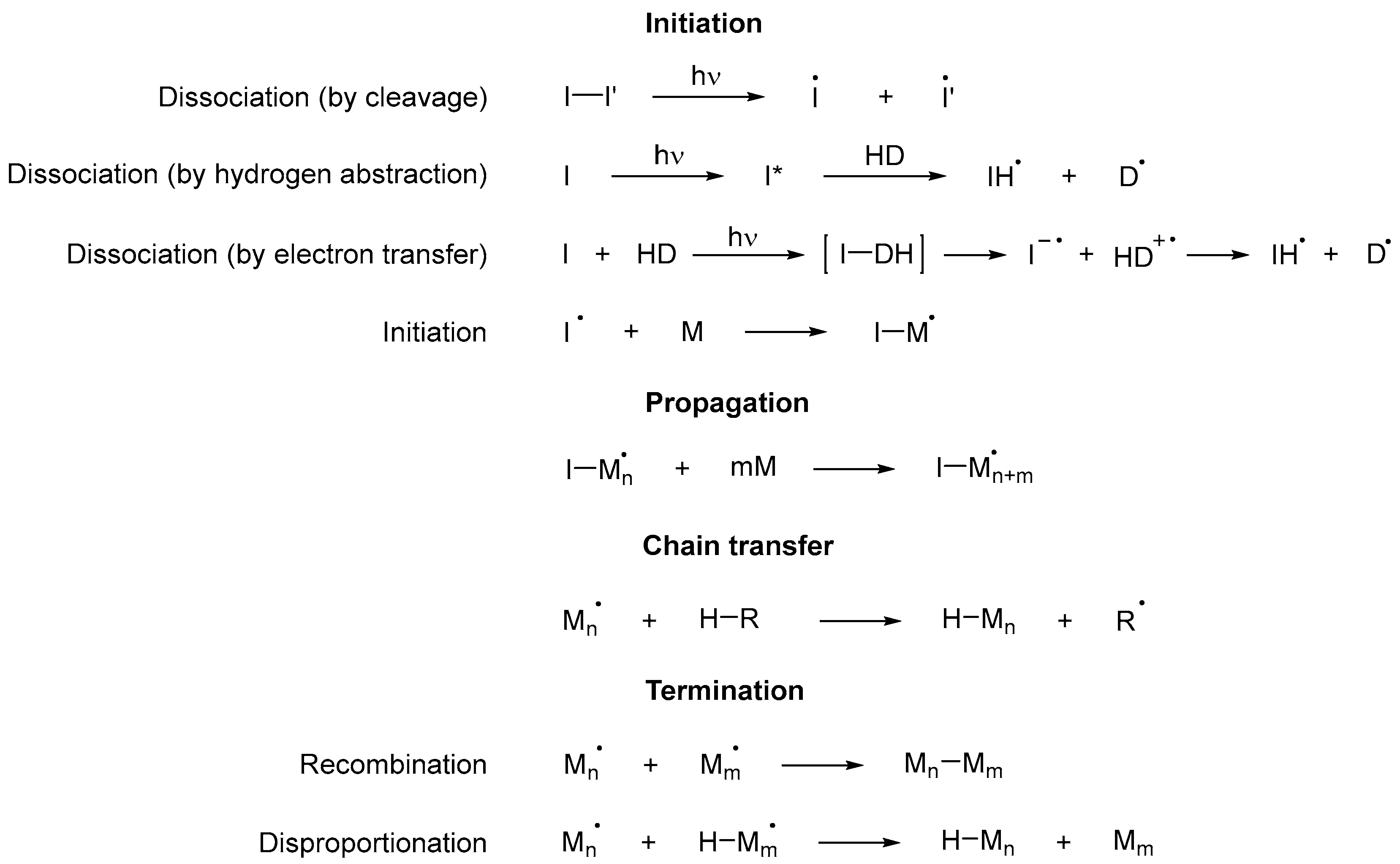
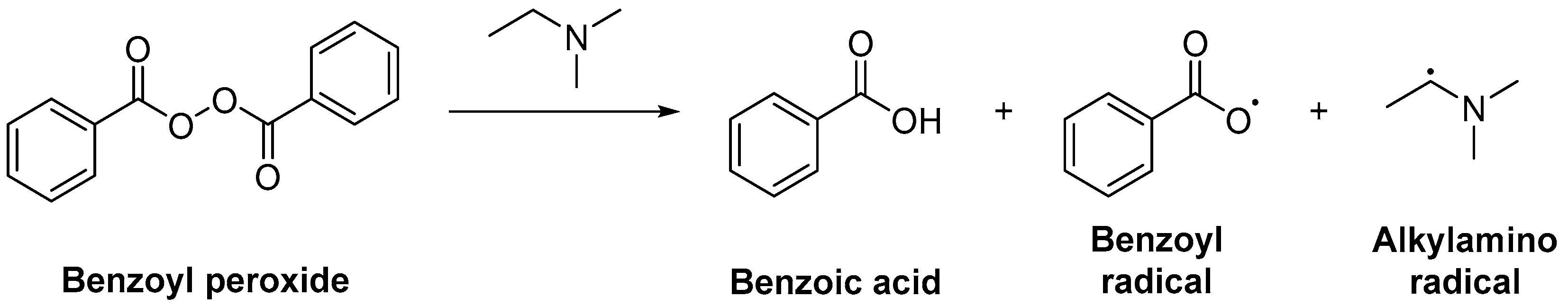
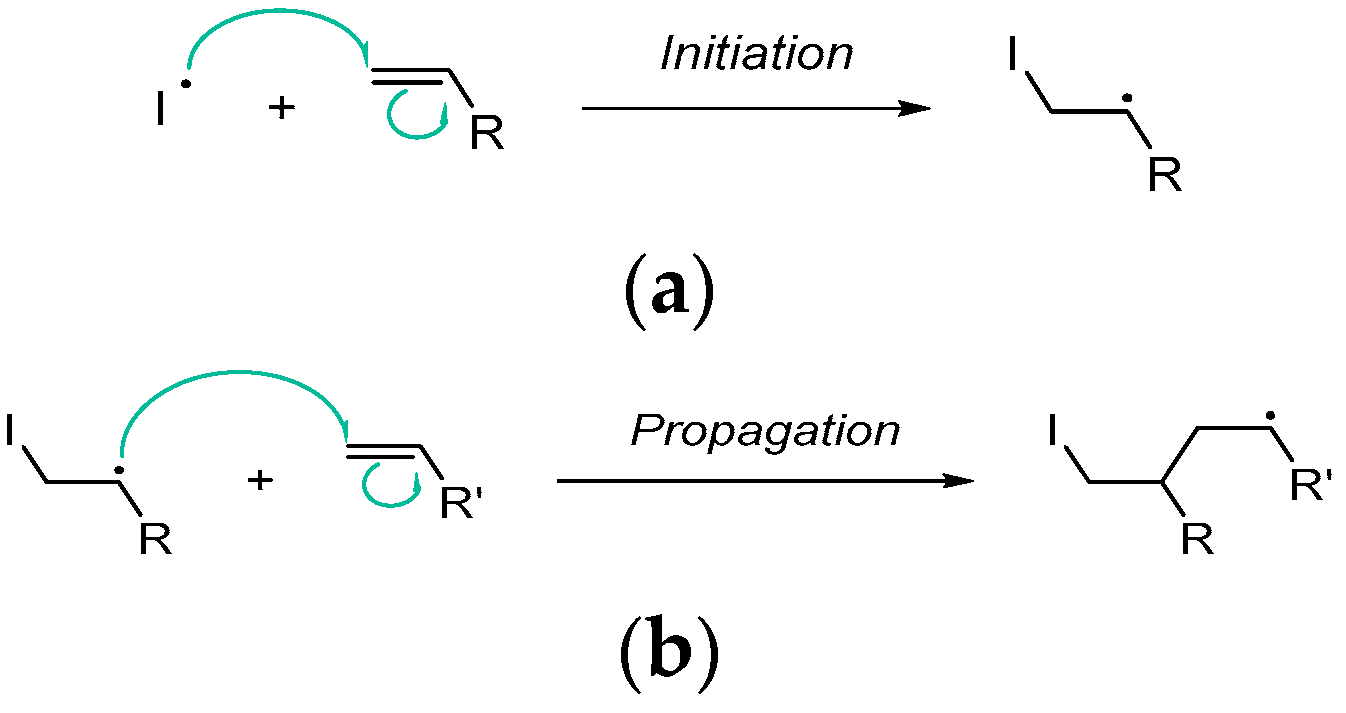
2.1.1. Initiation
2.1.2. Propagation
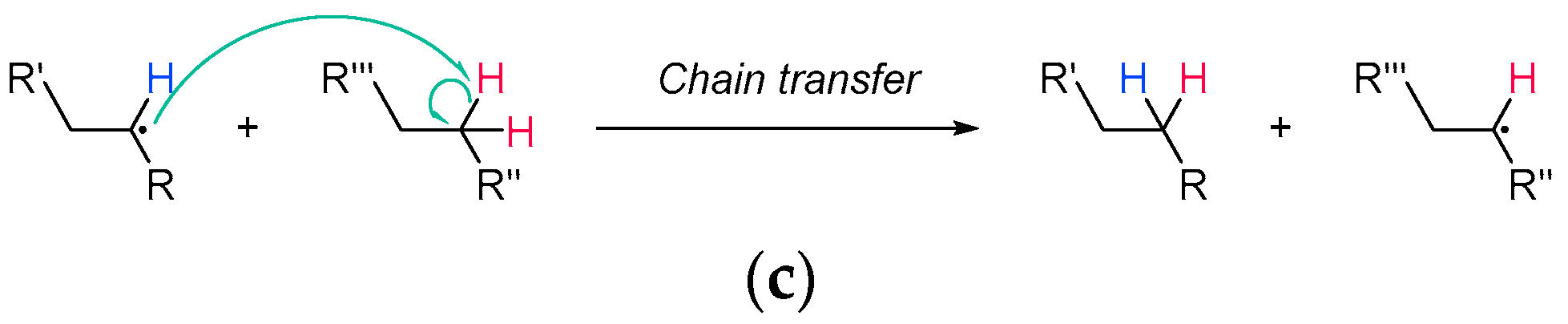
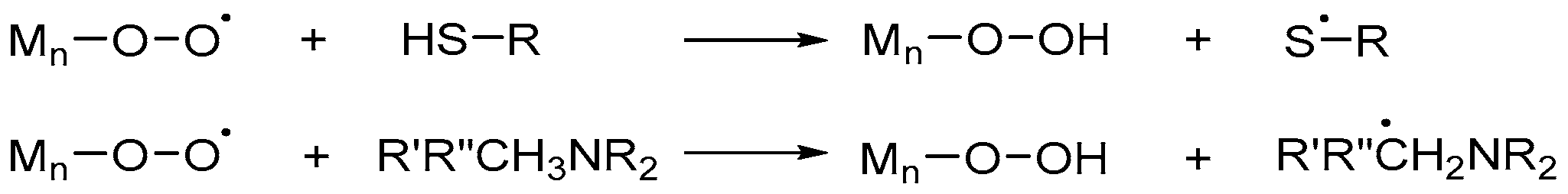
2.1.3. Chain Transfer
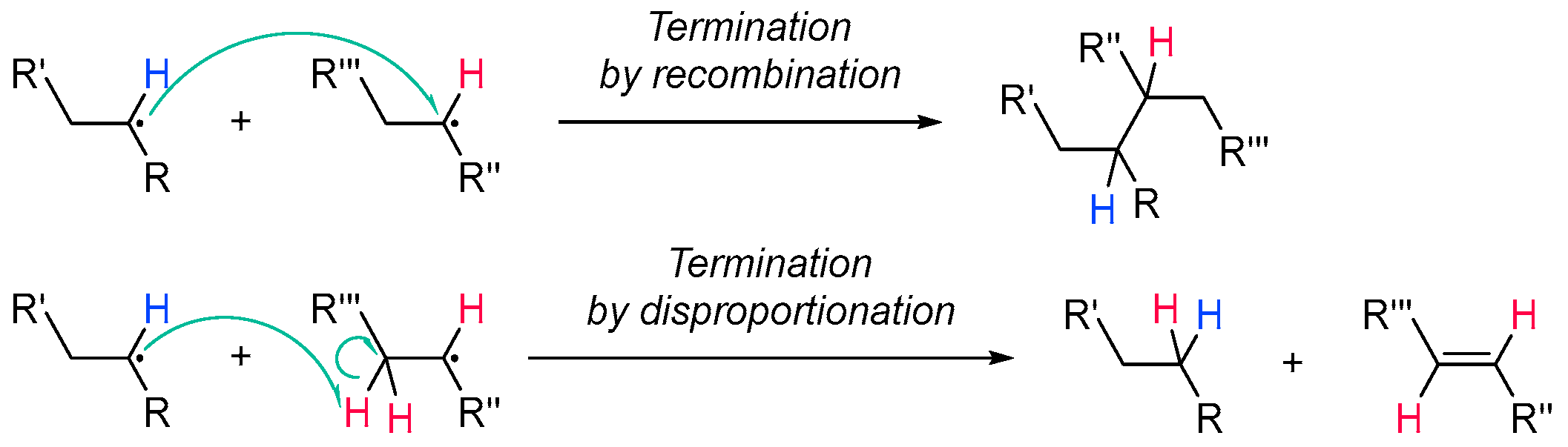
2.1.4. Termination
2.2. Inhibition by Oxygen
3. Constituents of a Free Radical UV Curing System
3.1. Resins
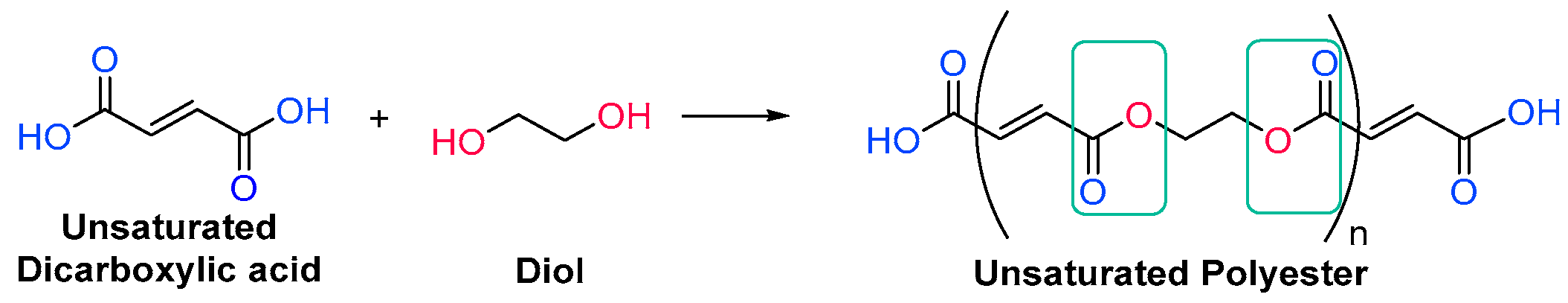
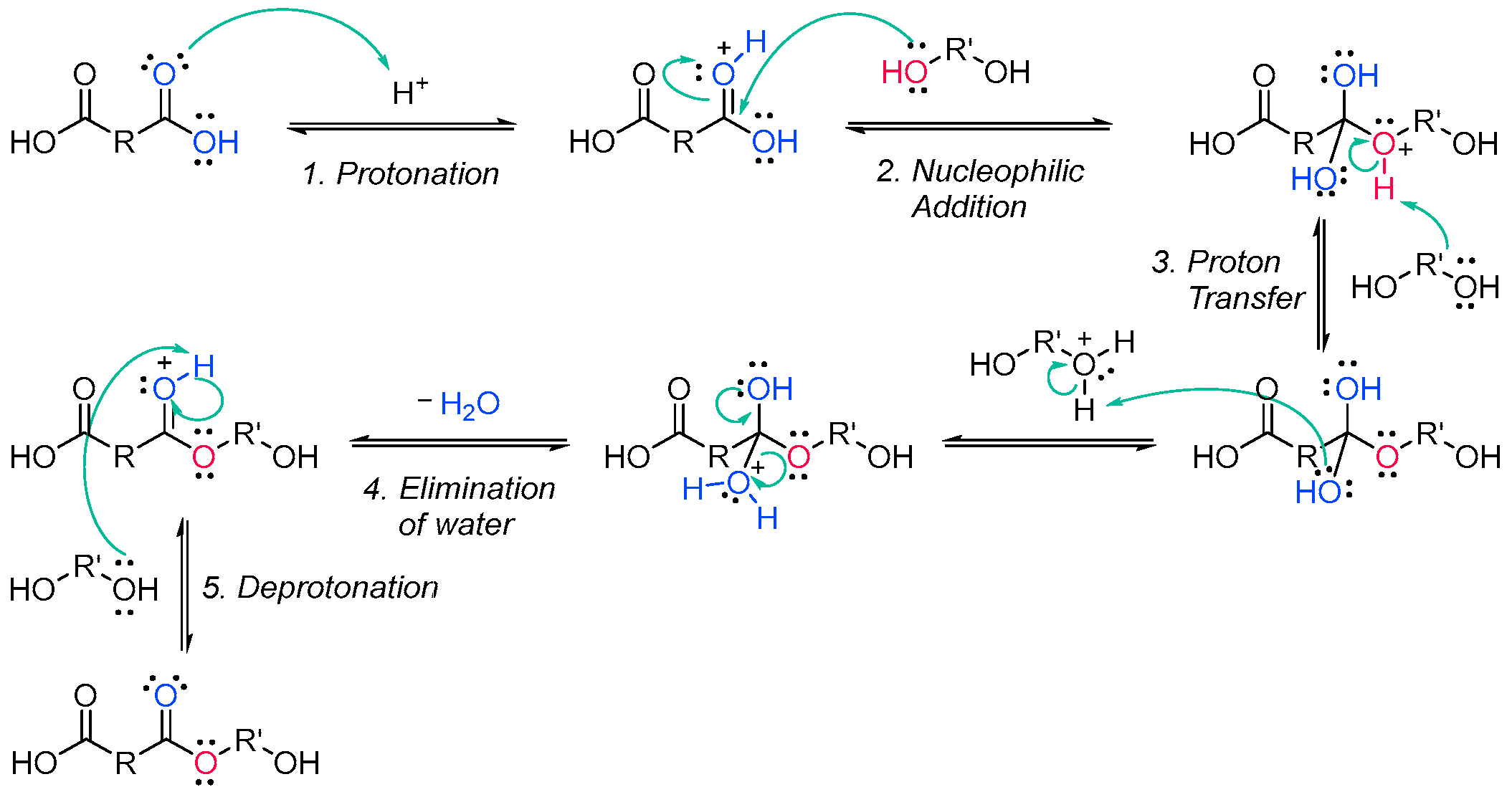
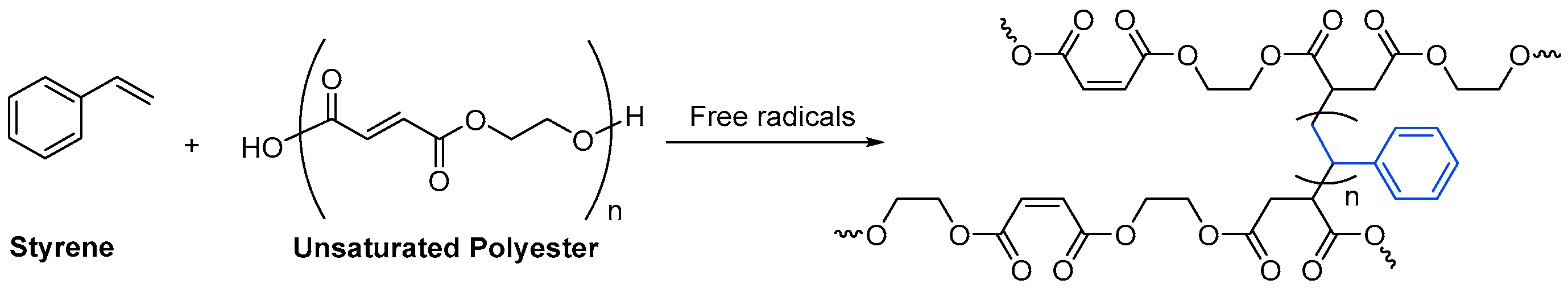
3.1.1. Unsaturated Resins
3.1.2. Acrylate Resins
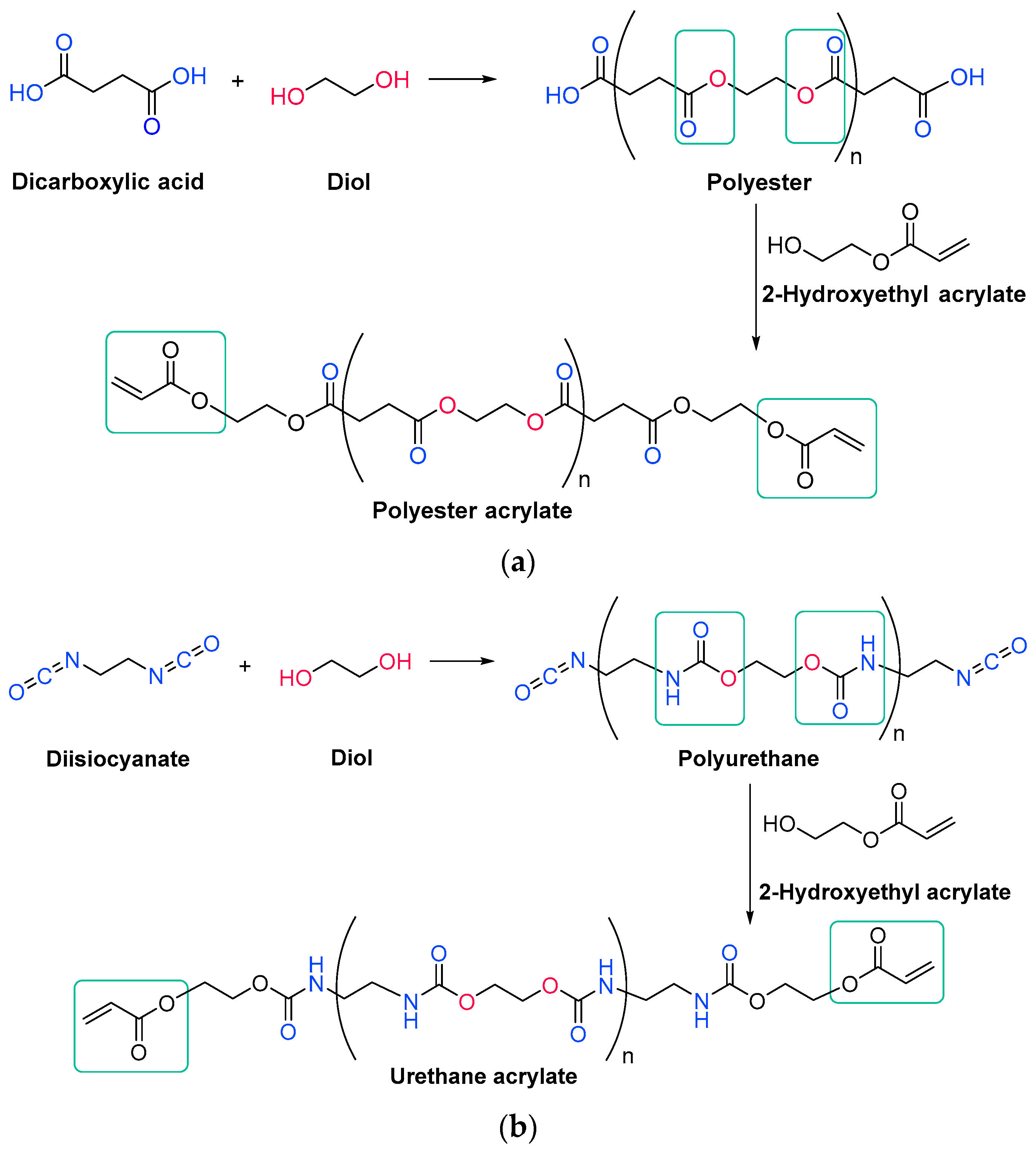

3.2. Monomers
3.2.1. Unsaturated Monomers
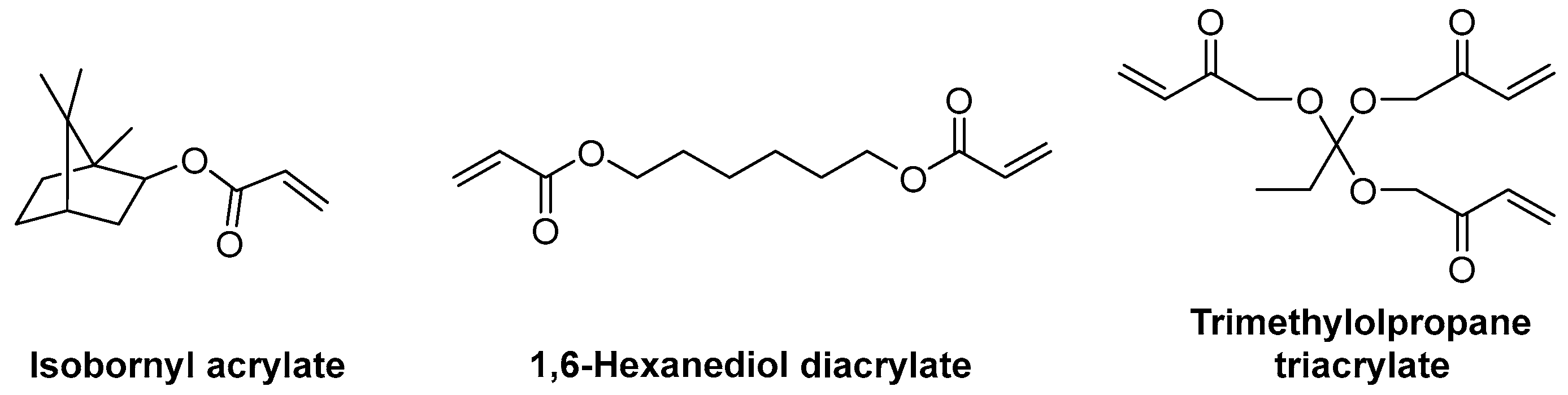
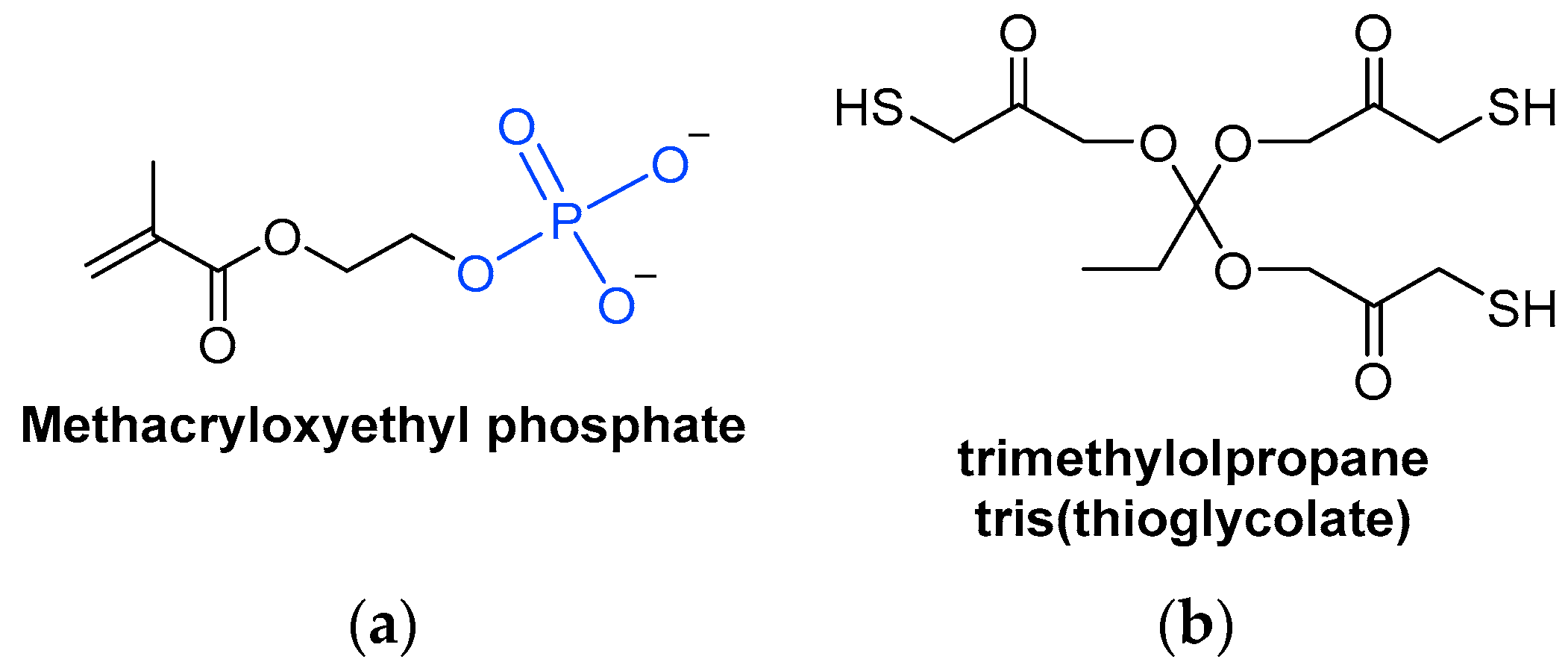
3.2.2. Acrylate Monomers
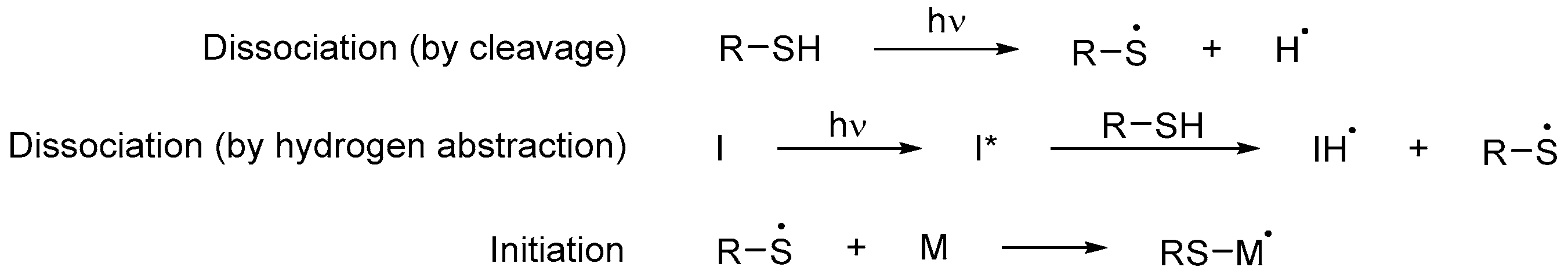
3.2.3. Thiol Monomers
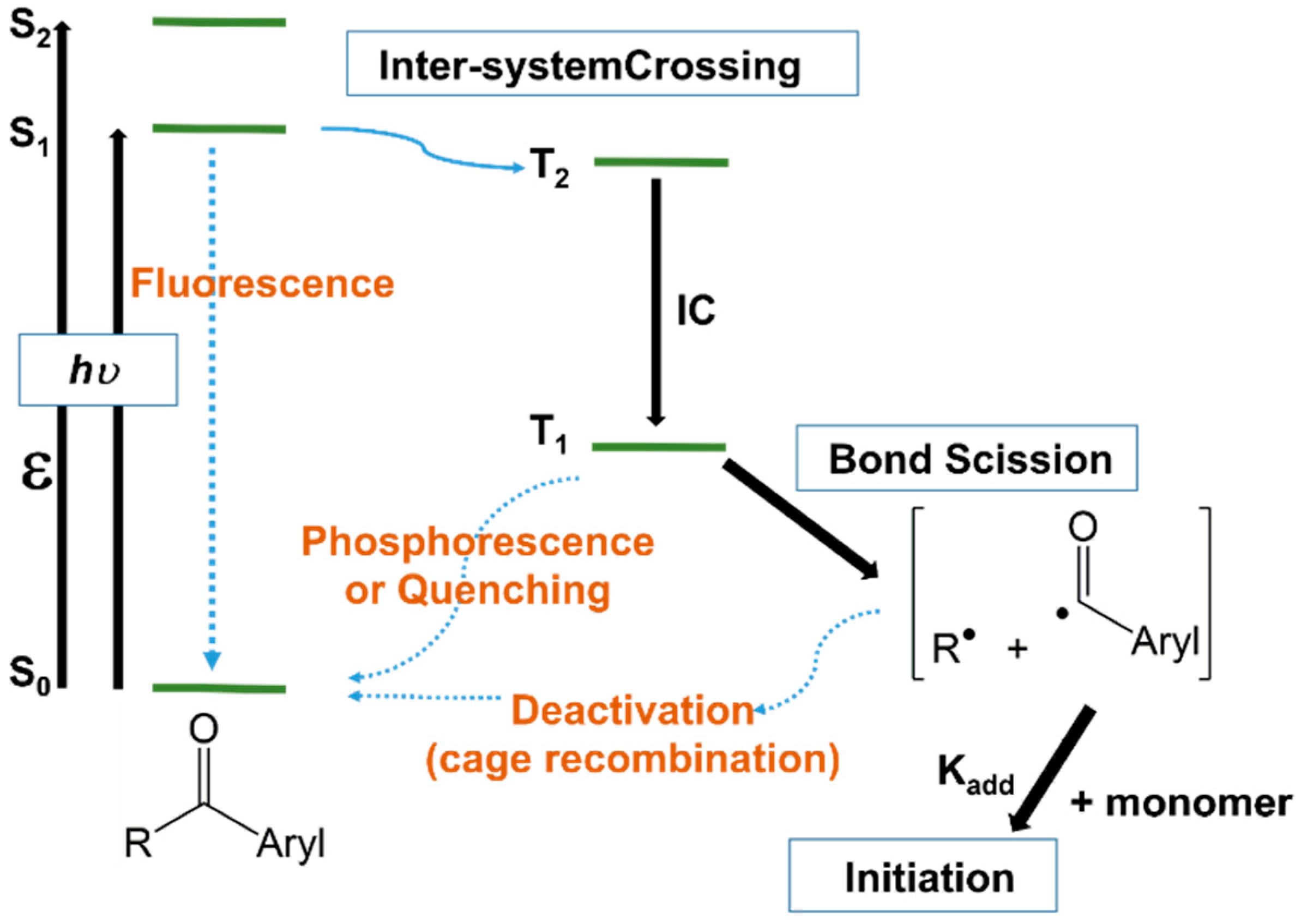
3.3. Photoinitiators
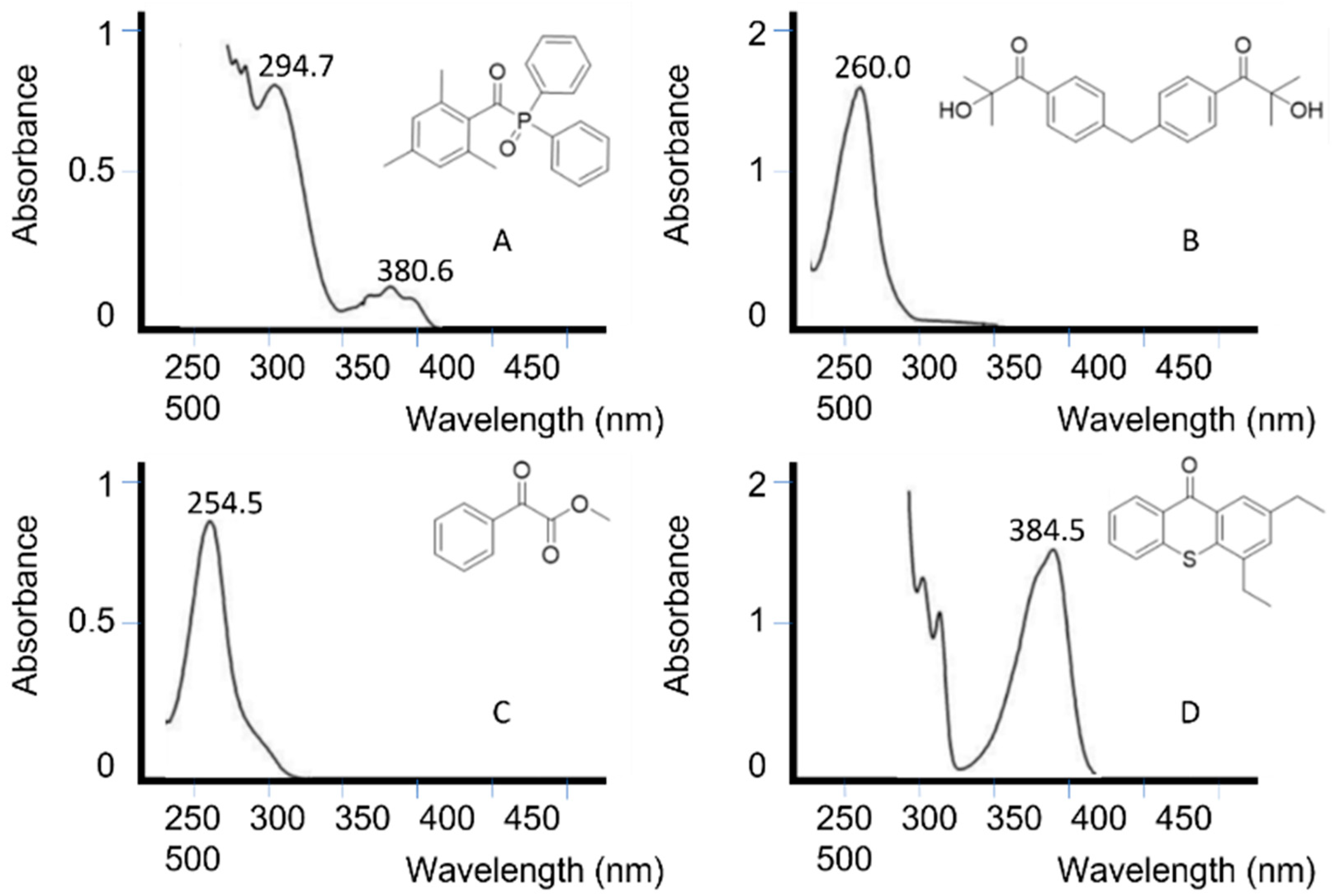
3.3.1. Unimolecular Photoinitiators
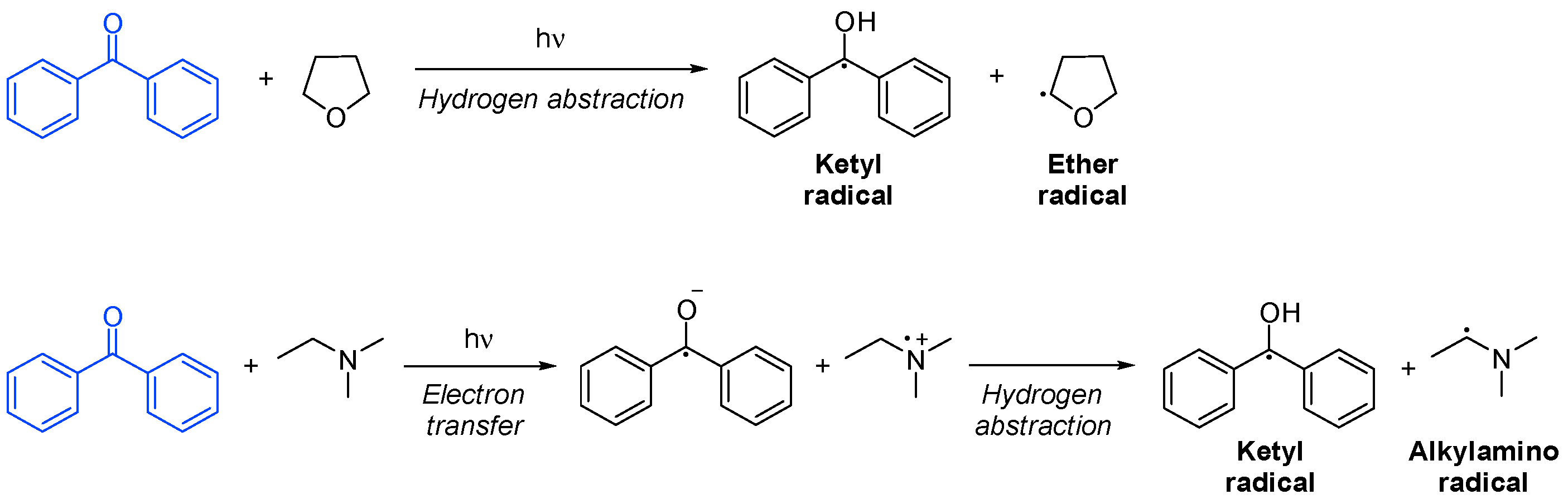
3.3.2. Bimolecular Photoinitiators
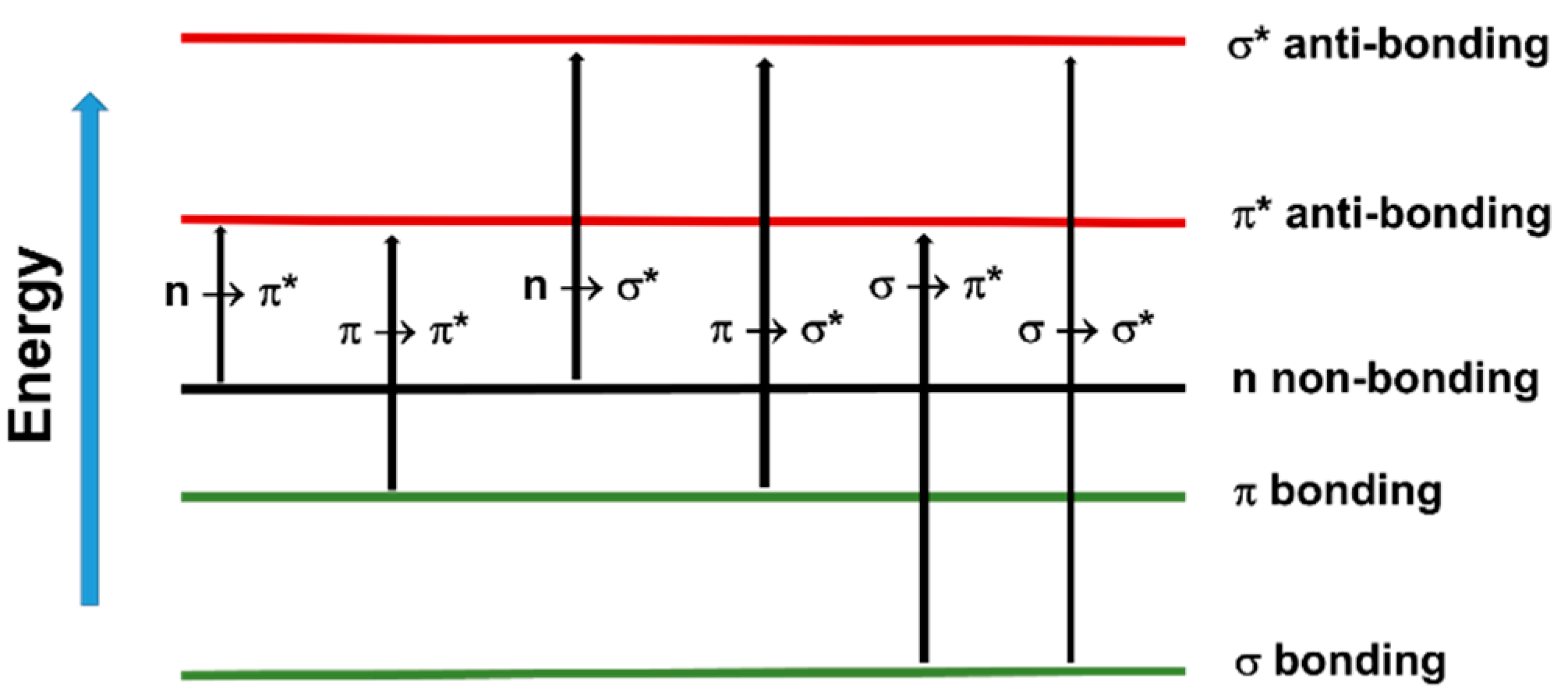
3.4. UV Light
4. Additives
4.1. Mineral Fillers
4.2. Thinners/Thickeners
4.3. Microspheres
4.4. Pigments
4.5. Solvents
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, M.H.; Lee, J.-B.; Choi, K.-Y. Discoloration mechanism of UV-curable polymer/metal hybrid coating. J. Ind. Eng. Chem. 2013, 19, 292–298. [Google Scholar] [CrossRef]
- Melchiors, M.; Sonntag, M.; Kobusch, C.; Jürgens, E. Recent developments in aqueous two-component polyurethane (2K-PUR) coatings. Prog. Org. Coat. 2000, 40, 99–109. [Google Scholar] [CrossRef]
- Jang, S.-C.; Yi, S.-C.; Hong, J.-W. Synthesis and performance of reactive light stabilizers for weather-resistant UV-curable coatings. J. Ind. Eng. Chem. 2005, 11, 964–970. [Google Scholar]
- Akafuah, N.K.; Poozesh, S.; Salaimeh, A.; Patrick, G.; Lawler, K.; Saito, K. Evolution of the Automotive Body Coating Process—A Review. Coatings 2016, 6, 24. [Google Scholar] [CrossRef] [Green Version]
- Decker, C.; Masson, F.; Schwalm, R. Weathering resistance of waterbased UV-cured polyurethane-acrylate coatings. Polym. Degrad. Stab. 2004, 83, 309–320. [Google Scholar] [CrossRef]
- Nkongho, E.; Kolla, T.E.; Akaho, A.; Ngando, M.T. Optimizing catalytic drying of paints and varnishes: Case study at Smalto. J. Chem. Pharm. Res. 2014, 6, 138–147. [Google Scholar]
- Oyama, T. Simultaneous improvement of mechanical properties and curing temperature of cyanate ester resin by in situ generated modifier polymer having phenolic OH group. Polymer 2020, 202, 122611. [Google Scholar]
- Javadi, A.; Mehr, H.S.; Sobani, M.; Soucek, M.D. Cure-on-command technology: A review of the current state of the art. Prog. Org. Coat. 2016, 100, 2–31. [Google Scholar] [CrossRef]
- Ostovar, S.; Rezvani, A.; Luque, R.; Carrillo-Carrión, C. Core-shell iron oxide@cathecol-polymer@palladium/copper nanocomposites as efficient and sustainable catalysts in cross-coupling reactions. Mol. Catal. 2020, 493, 111042. [Google Scholar] [CrossRef]
- Xu, C.; Fang, R.; Luque, R.; Chen, L.; Li, Y. Functional metal–organic frameworks for catalytic applications. Coord. Chem. Rev. 2019, 388, 268–292. [Google Scholar] [CrossRef]
- Amiens, C.; Ciuculescu-Pradines, D.; Philippot, K. Controlled metal nanostructures: Fertile ground for coordination chemists. Coord. Chem. Rev. 2016, 308, 409–432. [Google Scholar] [CrossRef]
- Sharmin, E.; Zafar, F.; Akram, D.; Alam, M.; Ahmad, S. Recent advances in vegetable oils based environment friendly coatings: A review. Ind. Crops Prod. 2015, 76, 215–229. [Google Scholar] [CrossRef]
- Fertier, L.; Koleilat, H.; Stemmelen, M.; Giani, O.; Joly-Duhamel, C.; Lapinte, V.; Robin, J.-J. The use of renewable feedstock in UV-curable materials-A new age for polymers and green chemistry. Prog. Polym. Sci. 2013, 38, 932–962. [Google Scholar] [CrossRef]
- Barkane, A.; Kampe, E.; Platnieks, O.; Gaidukovs, S. Cellulose nanofibers: A comparative study of reinforcing effects in UV-cured vegetable oil nanocomposites. Nanomaterials 2021, 11, 1791. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Hu, Y.; Tang, J.; Zhang, J.; Wang, C.; Shang, Q.; Feng, G.; Liu, G.; Zhou, Y.; Lei, W. High-Performance Soybean-Oil-Based Epoxy Acrylate Resins: “green” Synthesis and Application in UV-Curable Coatings. ACS Sustain. Chem. Eng. 2018, 6, 8340–8349. [Google Scholar] [CrossRef]
- Alsulami, Q.A.; Albukhari, S.M.; Hussein, M.A.; Tay, G.S.; Rozman, H.D. Biodegradable lignin as a reactive raw material in UV curable systems. Polym. Plast. Technol. Mater. 2020, 59, 1387–1406. [Google Scholar] [CrossRef]
- Wang, W.; Mattoussi, H. Engineering the Bio-Nano Interface Using a Multifunctional Coordinating Polymer Coating. Acc. Chem. Res. 2020, 53, 1124–1138. [Google Scholar] [CrossRef]
- Corrigan, N.; Jung, K.; Moad, G.; Hawker, C.J.; Matyjaszewski, K.; Boyer, C. Reversible-deactivation radical polymerization (Controlled/living radical polymerization): From discovery to materials design and applications. Prog. Polym. Sci. 2020, 111, 101311. [Google Scholar] [CrossRef]
- Shim, G.-S.; Kim, J.-S.; Kim, H.-J. Behavior and Adhesion Performance of Acrylic PSAs using Semi-IPN Structure and UV/UV Stepwise Curing. J. Ind. Eng. Chem. 2020, 89, 139–146. [Google Scholar] [CrossRef]
- Sangermano, M. Advances in cationic photopolymerization. Pure Appl. Chem. 2012, 84, 2089–2101. [Google Scholar] [CrossRef]
- Lee, B.-H.; Choi, J.-H.; Kim, H.-J.; Kim, J.-I.; Park, J.-Y. Properties of UV-curable coatings for wood floorings as a function of UV dose. J. Ind. Eng. Chem. 2004, 10, 608–613. [Google Scholar]
- Mousavi, S.M.; Hashemi, S.A.; Bahrani, S.; Yousefi, K.; Behbudi, G.; Babapoor, A.; Omidifar, N.; Lai, C.W.; Gholami, A.; Chiang, W.-H. Recent Advancements in Polythiophene-Based Materials and their Biomedical, Geno Sensor and DNA Detection. Int. J. Mol. Sci. 2021, 22, 6850. [Google Scholar] [CrossRef] [PubMed]
- Barszczewska-Rybarek, I.; Chladek, G. Studies on the Curing Efficiency and Mechanical Properties of Bis-GMA and TEGDMA Nanocomposites Containing Silver Nanoparticles. Int. J. Mol. Sci. 2018, 19, 3937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mangal, U.; Kwon, J.S.; Choi, S.H. Bio-Interactive Zwitterionic Dental Biomaterials for Improving Biofilm Resistance: Characteristics and Applications. Int. J. Mol. Sci. 2020, 21, 9087. [Google Scholar] [CrossRef]
- Park, Y.; Byun, H.; An, J.; Kim, S.; Lee, J.H. Large-scale fabrication of a highly flexible and transparent adhesive film embedded with elastic hybrid nanoparticles. J. Ind. Eng. Chem. 2021, 97, 299–306. [Google Scholar] [CrossRef]
- Bouzrati-Zerelli, M.; Maier, M.; Dietlin, C.; Fabrice, M.-S.; Fouassier, J.P.; Klee, J.E.; Lalevée, J. A novel photoinitiating system producing germyl radicals for the polymerization of representative methacrylate resins: Camphorquinone/R3GeH/iodonium salt. Dent. Mater. 2016, 32, 1226–1234. [Google Scholar] [CrossRef]
- Rudawska, A.; Frigione, M. Effect of Diluents on Mechanical Characteristics of Epoxy Compounds. Polymers 2020, 14, 2277. [Google Scholar] [CrossRef]
- Atif, M.; Bongiovanni, R.; Yang, J. Cationically UV-Cured Epoxy Composites. Polym. Rev. 2015, 55, 90–106. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, D.G.; Lee, J.W.; Oh, D.G.; Son, I.; Lee, J.H.; Kim, H.; Jung, H.W. Relationship between crosslinking and surface mechanical properties of UV curable coatings for automotive interior plastic parts. Polymer 2020, 44, 38–48. [Google Scholar] [CrossRef]
- Cavallaro, G.; Milioto, S.; Konnova, S.; Fakhrullina, G.; Akhatova, F.; Lazzara, G.; Fakhrullin, R.; Lvov, Y. Halloysite/Keratin Nanocomposite for Human Hair Photoprotection Coating. ACS Appl. Mater. Interfaces 2020, 12, 24348–24362. [Google Scholar] [CrossRef]
- Sadjadi, S.; Koohestani, F.; Pareras, G.; Nekoomanesh-Haghighi, M.; Bahri-Laleh, N.; Poater, A. Combined experimental and computational study on the role of ionic liquid containing ligand in the catalytic performance of halloysite-based hydrogenation catalyst. J. Mol. Liq. 2021, 331, 115740. [Google Scholar] [CrossRef]
- Karimi, S.; Bahri-Laleh, N.; Sadjadi, S.; Pareras, G.; Nekoomanesh-Haghighi, M.; Poater, A. Pd on nitrogen rich polymer-halloysite nanocomposite as an environmentally benign and sustainable catalyst for hydrogenation of polyalfaolefin based lubricants. J. Ind. Eng. Chem. 2021, 97, 441–451. [Google Scholar] [CrossRef]
- Mehdizadeh, M.; Sadjadi, S.; Poater, A.A. Mansouri, N. Bahri-Laleh, Molecular modelling aided catalyst design for PAO oils hydrofinishing. J. Mol. Liq. 2022, 352, 118675. [Google Scholar] [CrossRef]
- Tabrizi, M.; Sadjadi, S.; Pareras, G.; Nekoomanesh-Haghighi, M.; Bahri-Laleh, N.; Poater, A. Efficient hydro-finishing of polyalfaolefin based lubricants under mild reaction condition using Pd on ligands decorated halloysite. J. Colloid Interface Sci. 2021, 581, 939–953. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, S.; Sadjadi, S.; Bahri-Laleh, N.; Nekoomanesh-Haghighi, M.; Poater, A. Study of the effect of the ligand structure on the catalytic activity of Pd@ ligand decorated halloysite: Combination of experimental and computational studies. Appl. Organomet. Chem. 2019, 33, e4891. [Google Scholar] [CrossRef]
- Shams, A.; Sadjadi, S.; Duran, J.; Simon, S.; Poater, A.; Bahri-Laleh, N. Effect of support hydrophobicity of halloysite based catalysts on the PAO hydrofinishing performance. Appl. Organomet. Chem. 2022, 36, e6719. [Google Scholar] [CrossRef]
- Sangermano, M.; Roppolo, I.; Chiappone, A. New Horizons in Cationic Photopolymerization. Polymers 2018, 10, 136. [Google Scholar] [CrossRef] [Green Version]
- Decker, C. Kinetic Study and New Applications of UV Radiation Curing. Macromol. Rapid Commun. 2002, 23, 1067–1093. [Google Scholar] [CrossRef]
- Xiao, P.; Zhang, J.; Dumur, F.; Tehfe, M.A.; Morlet-Savarya, F.; Graffa, B.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Visible light sensitive photoinitiating systems: Recent progress in cationic and radical photopolymerization reactions under soft conditions. Prog. Polym. Sci. 2015, 41, 32–66. [Google Scholar] [CrossRef]
- Crivello, J.V. Cationic polymerization: Iodonium and sulfonium photoinitiators. Adv. Polym. Sci. 1984, 62, 2–23. [Google Scholar]
- Decker, C. Photoinitiated curing of multifunctional monomers. Acta Polym. 1994, 45, 333–347. [Google Scholar] [CrossRef]
- Fouassier, J.-P.; Lalevée, J. Photoinitiators and Light Sources: Novel Developments. Photoinitiators 2021, 1, 537–557. [Google Scholar]
- Cai, Y.; Jessop, J.L.P. Decreased oxygen inhibition in photopolymerized acrylate/epoxide hybrid polymer coatings as demonstrated by Raman spectroscopy. Polymer 2006, 47, 6560–6566. [Google Scholar] [CrossRef]
- Bahri-Laleh, N.; Hanifpour, A.; Mirmohammadi, S.A.; Poater, A.; Nekoomanesh-Haghighi, M.; Talarico, G.; Cavallo, L. Computational modeling of heterogeneous Ziegler-Natta catalysts for olefins polymerization. Prog. Polym. Sci. 2018, 84, 89–114. [Google Scholar] [CrossRef]
- Ryu, H.; Choi, H.; Shin, J.H.; Hong, H.; Park, B.; Lee, E.G.; Kim, T.-I. Non-yellowish and heat-resistant adhesive for a transparent heat sinking film. J. Ind. Eng. Chem. 2021, 103, 275–282. [Google Scholar] [CrossRef]
- Myers, T.N. Initiators, Free-Radical. In Encyclopedia of Polymer Science and Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2002. [Google Scholar]
- Kabatc, J.; Paczkowski, J. Acceleration of the free radical polymerization by using N-alkoxypyridinium salt as co-initiator in hemicyanine dye/borate salt photoinitiating system. J. Photochem. Photobiol. A Chem. 2006, 184, 184–192. [Google Scholar] [CrossRef]
- Sautrot-Ba, P.; Jockusch, S.; Malval, J.-P.; Brezová, V.; Rivard, M.; Abbad-Andaloussi, S.; Blacha-Grzechnik, A.; Versace, D.-L. Quinizarin Derivatives as Photoinitiators for Free-Radical and Cationic Photopolymerizations in the Visible Spectral Range. Macromolecules 2020, 53, 1129–1141. [Google Scholar] [CrossRef]
- Tomal, W.; Pilch, M.; Chachaj-Brekiesz, A.; Galek, M.; Morlet-Savary, F.; Graff, B.; Dietlin, C.; Lalevée, J.; Ortyl, J. Photoinitiator-catalyst systems based on: Meta -terphenyl derivatives as photosensitisers of iodonium and thianthrenium salts for visible photopolymerization in 3D printing processes. Polym. Chem. 2020, 11, 4604–4621. [Google Scholar] [CrossRef]
- Breloy, L.; Losantos, R.; Sampedro, D.; Marazzi, M.; Malval, J.-P.; Heo, Y.; Akimoto, J.; Ito, Y.; Brezová, V.; Versace, D.-L. Allyl amino-thioxanthone derivatives as highly efficient visible light H-donors and co-polymerizable photoinitiators. Polym. Chem. 2020, 11, 4297–4312. [Google Scholar] [CrossRef]
- Balcerak, A.; Kabatc, J. The photooxidative sensitization of bis(: P-substituted diphenyl)iodonium salts in the radical polymerization of acrylates. RSC Adv. 2019, 9, 28490–28499. [Google Scholar] [CrossRef] [Green Version]
- Kabatc, J.; Zadruyńska, A.; Czech, Z.; Kowalczyk, A. The synthesis, spectroscopic and electrochemical properties, and application of new dyeing photoinitiator systems for acrylate monomers polymerization. Dyes Pigm. 2012, 92, 724–731. [Google Scholar] [CrossRef]
- Zoller, A.; Gigmes, D.; Guillaneuf, Y. Simulation of radical polymerization of methyl methacrylate at room temperature using a tertiary amine/BPO initiating system. Polym. Chem. 2015, 6, 5719–5727. [Google Scholar] [CrossRef]
- Topa, M.; Ortyl, J. Moving Towards a Finer Way of Light-Cured Resin-Based Restorative Dental Materials: Recent Advances in Photoinitiating Systems Based on Iodonium Salts. Materials 2020, 13, 4093. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Panahi, F.; Bahri-Laleh, N.; Sabzi, M.; Pareras, G.; Falcone, B.N.; Poater, A. pH-Responsive Gelation in Metallo-Supramolecular Polymers Based on the Protic Pyridinedicarboxamide Ligand. Chem. Mater. 2022, 34, 6155–6169. [Google Scholar] [CrossRef]
- Tullier, M. Thiol-Acrylate Polymerization Kinetics and Applications in Microfluidics. Ph.D. Thesis, Louisiana State University, Baton Rouge, LA, USA, 2016. [Google Scholar]
- Bongiovanni, R.; Vacche, S.D.; Vitale, A. Photoinduced processes as a way to sustainable polymers and innovation in polymeric materials. Polymers 2021, 13, 2293. [Google Scholar] [CrossRef]
- Trusiano, G.; Vitale, A.; Bonneaud, C.; Pugliese, D.; Vacche, S.D.; Joly-Duhamel, C.; Friesen, C.M.; Bongiovanni, R. Vinyl ethers and epoxides photoinduced copolymerization with perfluoropolyalkylether monomers. Colloid Polym. Sci. 2021, 299, 509–521. [Google Scholar] [CrossRef]
- Ay, E.; Raad, Z.; Dautel, O.; Dumur, F.; Wantz, G.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Oligomeric Photocatalysts in Photoredox Catalysis: Toward High Performance and Low Migration Polymerization Photoinitiating Systems. Macromolecules 2016, 49, 2124–2134. [Google Scholar] [CrossRef]
- Takashima, M.; Shichiri, M.; Hagihara, Y.; Yoshida, Y.; Niki, E. Reactivity toward oxygen radicals and antioxidant action of thiol compounds. BioFactors 2012, 38, 240–248. [Google Scholar] [CrossRef]
- Hoyle, C.E.; Cole, M.; Bachemin, M.; Kuang, W.; Jönsson, S. Photoinitiated Polymerization of Selected Thiol-ene Systems. ACS Symp. Ser. 2003, 847, 52–64. [Google Scholar]
- Chen, Z.; Wang, L.; Lin, J.; Du, L. A theoretical insight into the curing mechanism of phthalonitrile resins promoted by aromatic amines. Phys. Chem. Chem. Phys. 2021, 23, 17300–17309. [Google Scholar] [CrossRef]
- Xia, Y.-Z.; Zhang, D.; Li, Z.; Lin, H.; Chen, X.-N.; Oliver, S.; Shi, S.-X.; Lei, L. Toughness modification of cationic UV-cured cycloaliphatic epoxy resin by hydroxyl polymers with different structures. Eur. Polym. J. 2020, 127, 109594. [Google Scholar] [CrossRef]
- Zhou, L.-P.; Liu, H.-T.; Liu, A.; Zhou, L.; Du, C.; Li, Y. Easily fabricated icephobic surface with external and self-replenishing properties. Appl. Surf. Sci. 2022, 579, 152069. [Google Scholar] [CrossRef]
- Das, T.K.; Poater, A. Review on the Use of Heavy Metal Deposits from Water Treatment Waste towards Catalytic Chemical Syntheses. Int. J. Mol. Sci. 2021, 22, 13383. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, A.D.; Kratochvíl, P.; Stepto, R.F.T.; Suter, U.W. Glossary of basic terms in polymer science (IUPAC Recommendations 1996). Pure Appl. Chem. 1996, 68, 2287–2311. [Google Scholar] [CrossRef]
- Obi, B.E. Polymer Chemistry and Synthesis. In Polymeric Foams Structure-Property-Performance; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Zhao, D.; Liu, S.; Wu, Y.; Guan, T.; Sun, N.; Ren, B. Self-healing UV light-curable resins containing disulfide group: Synthesis and application in UV coatings. Prog. Org. Coat. 2019, 133, 289–298. [Google Scholar] [CrossRef]
- Gao, Y.; Romero, P.; Zhang, H.; Huang, M.; Lai, F. Unsaturated polyester resin concrete: A review. Constr. Build. Mater. 2019, 228, 116709. [Google Scholar] [CrossRef]
- Maurya, S.D.; Kurmvanshi, S.K.; Mohanty, S.; Nayak, S.K. A Review on Acrylate-Terminated Urethane Oligomers and Polymers: Synthesis and Applications. Polym. Plast. Technol. Eng. 2018, 57, 625–656. [Google Scholar] [CrossRef]
- Nava, H. Polyesters, Unsaturated. In Encyclopedia of Polymer Science and Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015. [Google Scholar]
- Fischer, E.; Speier, A. Darstellung der Ester. Chem. Ber. 1895, 28, 3252–3258. [Google Scholar] [CrossRef]
- Zimmermann, H.; Rudolph, J. Protonic States and the Mechanism of Acid-Catalysed Esterification. Angew. Chem. Int. Ed. 1965, 4, 40–49. [Google Scholar] [CrossRef]
- Slark, A.T.; Sherrington, D.C.; Titterton, A.; Martin, I.K. Branched methacrylate copolymers from multifunctional comonomers: The effect of multifunctional monomer functionality on polymer architecture and properties. J. Mater. Chem. 2003, 13, 2711–2720. [Google Scholar] [CrossRef]
- Xie, F.W.; Halley, P.J.; Avérous, L. Rheology to understand and optimize processibility, structures and properties of starch polymeric materials. Prog. Polym. Sci. 2012, 37, 595–623. [Google Scholar] [CrossRef]
- Park, M.N.; Oh, S.W.; Ahn, B.H.; Moon, M.J.; Kang, Y.S. Synthesis of poly(methyl urethane) acrylate oligomer using 2-Isocyanatoethyl methacrylate for UV curable coating. J. Nanosci. Nanotechnol. 2009, 9, 1277–1280. [Google Scholar] [CrossRef] [PubMed]
- Pretula, J.; Slomkowski, S.; Penczek, S. Polylactides-methods of synthesis and characterization. Adv. Drug Deliv. Rev. 2016, 107, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Kale, G.; Auras, R.; Singh, S.P.; Narayan, R. Biodegradability of polylactide bottles in real and simulated composting conditions. Polym. Test. 2007, 26, 1049–1061. [Google Scholar] [CrossRef]
- Garlotta, D. A literature review of poly(lactic acid). J. Polym. Environ. 2001, 9, 63–84. [Google Scholar] [CrossRef]
- Paraskar, P.M.; Hatkar, V.M.; Kulkarni, R.D. Facile synthesis and characterization of renewable dimer acid-based urethane acrylate oligomer and its utilization in UV-curable coatings. Prog. Org. Coat. 2020, 149, 105946. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, S.H.; Han, Y.K.; Kim, Y.H. Synthesis and degradation of end-group functionalized polylactide. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 973–985. [Google Scholar] [CrossRef]
- Ren, J.M.; Mckenzie, T.G.; Fu, Q.; Wong, E.H.H.; Xu, J.T.; An, Z.; Shanmugam, S.; Davis, T.P.; Boyer, C.; Qiao, G.G. Star polymers. Chem. Rev. 2016, 116, 6743–6836. [Google Scholar] [CrossRef]
- Cameron, D.J.A.; Shaver, M.P. Aliphatic polyester polymer stars: Synthesis, properties and applications in biomedicine and nanotechnology. Chem. Soc. Rev. 2011, 40, 1761–1776. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Z.; Liu, Q.; Pan, X.; Wang, J.; Jin, M.; Li, J. Preparation and properties of star-shaped UV-curable polyester methacrylate resins with Low viscosity derived from renewable resources. Prog. Org. Coat. 2021, 157, 106324. [Google Scholar] [CrossRef]
- Bonneaud, C.; Burgess, J.; Vitale, A.; Trusiano, G.; Joly-Duhamel, C.; Friesen, C.M.; Bongiovanni, R. Perfluoropolyalkylether Maleimides for Protection From Oxygen Inhibition and Surface Modification of Photoinitiator-Free UV-Cured Polymers. Front. Mater. 2020, 6, 346. [Google Scholar] [CrossRef] [Green Version]
- Khudyakov, I.V. Fast photopolymerization of acrylate coatings: Achievements and problems. Prog. Org. Coat. 2018, 121, 151–159. [Google Scholar] [CrossRef]
- Chattopadhyay, D.K.; Raju, K.V.S.N. Structural engineering of polyurethane coatings for high performance applications. Prog. Polym. Sci. 2007, 32, 352–418. [Google Scholar] [CrossRef]
- Chattopadhyay, D.K.; Panda, S.S.; Raju, K.V.S.N. Thermal and mechanical properties of epoxy acrylate/methacrylates UV cured coatings. Prog. Org. Coat. 2005, 54, 10–19. [Google Scholar] [CrossRef]
- Akindoyo, J.O.; Beg, M.D.H.; Ghazali, S.; Islam, M.R.; Jeyaratnam, N.; Yuvaraj, A.R. Polyurethane types, synthesis and applications—A review. RSC Adv. 2016, 6, 114453–114482. [Google Scholar] [CrossRef] [Green Version]
- Dworakowska, S.; Bogdał, D.; Zaccheria, F.; Ravasio, N. The role of catalysis in the synthesis of polyurethane foams based on renewable raw materials. Catal. Today 2014, 223, 148–156. [Google Scholar] [CrossRef]
- Heath, D.; Cooper, S.A. Polyurethanes. In Encyclopedia of Polymer Science and Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013. [Google Scholar]
- Çanak, T.C.; Kiraylar, E.; Serhatli, E. Preparation and Application of Urethane Acrylate Coatings for Enhanching Mechanical Properties of Coagulated Surfaces. Karaelmas Fen Ve Mühendislik Derg. 2016, 6, 359–368. [Google Scholar]
- Madhi, A.; Hadavand, B.S.; Amoozadeh, A. UV-curable urethane acrylate zirconium oxide nanocomposites: Synthesis, study on viscoelastic properties and thermal behavior. J. Compos. Mater. 2018, 52, 691–701. [Google Scholar] [CrossRef]
- Raheem, K.; Cassidy, J.; Betts, A.; Ryan, B. Use of confocal Raman microscopy to characterise ethyl cyanoacrylate adhesive depth curing. Phys. Chem. Chem. Phys. 2020, 22, 23899–23907. [Google Scholar] [CrossRef]
- Mailhot-Jensen, B.; Komvopoulos, K.; Ward, B.; Tian, Y.; Somorjai, G. Mechanical and friction properties of thermoplastic polyurethanes determined by scanning force microscopy. J. Appl. Phys. 2001, 89, 5712–5719. [Google Scholar] [CrossRef]
- Parvate, S.; Mahanwar, P.; Dispers, J. Advances in self-crosslinking of acrylic emulsion: What we know and what we would like to know. Sci. Technol. 2019, 40, 519–536. [Google Scholar] [CrossRef]
- Bae, C.J.; Ramachandran, A.; Chung, K.; Park, S. Ceramic Stereolithography: Additive Manufacturing for 3D Complex Ceramic Structures. J. Korean Ceram. Soc. 2017, 54, 470–477. [Google Scholar] [CrossRef] [Green Version]
- Roper, T.M.; Kwee, T.; Lee, T.Y.; Guymon, C.A.; Hoyle, C.E. Photopolymerization of pigmented thiol–ene systems. Polym. J. 2004, 45, 2921–2929. [Google Scholar] [CrossRef]
- Ahmed, M.M.M.; Chi, Y.-T.; Hung, Y.-H.; Reyes, L.M.C.; Yeh, J.-M. UV-cured electroactive polyurethane acrylate coatings with superhydrophobic surface structure of biomimetic peacock feather for anticorrosion application. Prog. Org. Coat. 2022, 165, 106679. [Google Scholar] [CrossRef]
- Truc, T.A.; Pébère, N.; Hang, T.T.X.; Hervaud, Y.; Boutevin, B. Evaluation of corrosion performance of a UV-cured polyurethane coating in the presence of organic phosphorous compounds. Prog. Org. Coat. 2004, 49, 130–136. [Google Scholar] [CrossRef]
- Findik, V.; Findik, B.K.; Aviyente, V.; Monari, A. Origins of the photoinitiation capacity of aromatic thiols as photoinitiatiors: A computational study. Phys. Chem. Chem. Phys. 2021, 23, 24377–24385. [Google Scholar] [CrossRef]
- Chawla, M.; Poater, A.; Besalú-Sala, P.; Kalra, K.; Oliva, R.; Cavallo, L. Theoretical characterization of sulfur-to-selenium substitution in an emissive RNA alphabet: Impact on H-bonding potential and photophysical properties. Phys. Chem. Chem. Phys. 2018, 20, 7676–7685. [Google Scholar] [CrossRef] [Green Version]
- Chawla, M.; Poater, A.; Oliva, R.; Cavallo, L. Structural and energetic characterization of the emissive RNA alphabet based on the isothiazolo [4,3-d]pyrimidine heterocycle core. Phys. Chem. Chem. Phys. 2016, 18, 18045–18053. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Mondal, S.; Nechab, M.; Dumur, F.; Bertrand, M.P.; Graff, B.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. New Thiols for Photoinitiator-Free Thiol-Acrylate Polymerization. Macromol. Chem. Phys. 2013, 214, 1302–1308. [Google Scholar] [CrossRef]
- Lago, M.A.; de Quirós, A.R.; Sendón, R.; Bustos, J.; Nieto, M.T.; Paseiro, P. Photoinitiators: A food safety review. Food Addit. Contam. Chem. Anal. Control Expo. Risk Assess. 2015, 32, 779–798. [Google Scholar] [CrossRef]
- Rist, G.; Borer, A.; Dietliker, K.; Desobry, V.; Fouassier, J.P.; Ruhlmann, D. Sensitization of α-aminoketone photoinitiators: A time-resolved CIDNP and laser spectroscopy investigation. Macromolecules 1992, 25, 4182–4190. [Google Scholar] [CrossRef]
- Noble, B.B.; Mater, A.C.; Smith, L.M.; Coote, M.L. The effects of Lewis acid complexation on type I radical photoinitiators and implications for pulsed laser polymerization. Polym. Chem. 2016, 7, 6400–6412. [Google Scholar] [CrossRef]
- Tomal, W.; Ortyl, J. Water-Soluble Photoinitiators in Biomedical Applications. Polymers 2020, 12, 1073. [Google Scholar] [CrossRef] [PubMed]
- Kabatc, J.; Jurek, K. New two- and three-cationic polymethine dyes. Synthesis, properties and application. Dyes Pigm. 2015, 112, 24–33. [Google Scholar] [CrossRef]
- Giacoletto, N.; Ibrahim-Ouali, M.; Dumur, F. Recent Advances on Squaraine-based Photoinitiators of Polymerization. Eur. Polym. J. 2021, 150, 110427. [Google Scholar] [CrossRef]
- Kabatc, J.; Kostrzewska, K.; Jurek, K. Squaric acid derivative effects on the kinetics of photopolymerization of different monomers. RSC Adv. 2016, 6, 74715–74725. [Google Scholar] [CrossRef]
- Balcerak, A.; Kwiatkowska, D.; Kabatc, J. Novel photoinitiators based on difluoroborate complexes of squaraine dyes for radical polymerization of acrylates upon visible light. Polym. Chem. 2022, 13, 220–234. [Google Scholar] [CrossRef]
- Skotnicka, A.; Kabatc, J. New BODIPY Dyes Based on Benzoxazole as Photosensitizers in Radical Polymerization of Acrylate Monomers. Materials 2022, 15, 662. [Google Scholar] [CrossRef]
- Germer, T.A.; Zwinkels, J.C.; Tsai, B.K. Theoretical Concepts in Spectrophotometric Measurements. Exp. Methods Phys. Sci. 2014, 46, 11–66. [Google Scholar]
- Maverakis, E.; Miyamura, Y.; Bowen, M.P.; Correa, G.; Ono, Y.; Goodarzi, H. Light, including ultraviolet. J. Autoimmun. 2010, 34, J247–J257. [Google Scholar] [CrossRef] [Green Version]
- Baroncini, M.; Canton, M.; Casimiro, L.; Corrà, S.; Groppi, J.; la Rosa, M.; Silvi, S.; Credi, A. Photoactive Molecular-Based Devices, Machines and Materials: Recent Advances. Eur. J. Inorg. Chem. 2018, 2018, 4589–4603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, X.; Gao, Y.; Nie, J.; Sun, F. Methyl Benzoylformate Derivative Norrish Type I Photoinitiators for Deep-Layer Photocuring under Near-UV or Visible LED. Macromolecules 2021, 54, 3854–3864. [Google Scholar] [CrossRef]
- Robert, J.D.; Caseiro, M.C. Basic Principles of Organic Chemistry, 2nd ed.; W.A. Benjamin, Inc.: Menlo Park, CA, USA, 1977; Photochemistry. [Google Scholar]
- Sangermano, M.; Razza, N.; Crivello, J.V. Cationic UV-curing: Technology and applications. Macromol. Mater. Eng. 2014, 299, 775–793. [Google Scholar] [CrossRef]
- Dietlin, C.; Schweizer, S.; Xiao, P.; Zhang, J.; Morlet-Savary, F.; Graff, B.; Fouassiera, J.-P.; Lalevée, J. Photopolymerization upon LEDs: New photoinitiating systems and strategies. Polym. Chem. 2015, 6, 3895–3912. [Google Scholar] [CrossRef]
- Rongxia, B.; Shiyong, L.; Wencai, X.; Ruiqiang, M.; Di, H.; Kelin, M.; Jiangwei, H.; Yong, X.; Caichang, L. Characterization of the UV-visible absorption spectra of commonly used photoinitiators. J. Syst. Control Eng. 2017, 1, 18–20. [Google Scholar]
- Ashfaq, A.; Clochard, M.-C.; Coqueret, X.; Dispenza, C.; Driscoll, M.S.; Ulański, P.; Al-Sheikhly, M. Polymerization Reactions and Modifications of Polymers by Ionizing Radiation. Polymers 2020, 12, 2877. [Google Scholar] [CrossRef]
- Zhang, W.-X.; Lub, J.; Schenning, A.P.H.J.; Zhou, G.F.; de Haan, L.T. Polymer Stabilized Cholesteric Liquid Crystal Siloxane for Temperature-Responsive Photonic Coatings. Int. J. Mol. Sci. 2020, 21, 1803. [Google Scholar] [CrossRef] [Green Version]
- Trusiano, G.; Vitale, A.; Rizzello, M.; Bonneaud, C.; Joly-Duhamel, C.; Friesen, C.M.; Bongiovanni, R. Controlling perfluoropolyalkylether rearrangements at the surface of photocured networks. Eur. Polym. J. 2019, 121, 109285. [Google Scholar] [CrossRef]
- Bonneaud, C.; Howell, J.; Bongiovanni, R.; Joly-Duhamel, C.; Friesen, C.M. Diversity of Synthetic Approaches to Functionalized Perfluoropolyalkylether. Macromolecules 2021, 54, 521–550. [Google Scholar] [CrossRef]
- Bongiovanni, R.; di Gianni, A.; Priola, A.; Pollicino, A. Surface modification of polyethylene for improving the adhesion of a highly fluorinated UV-cured coating. Eur. Polym. J. 2007, 43, 3787–3794. [Google Scholar] [CrossRef]
- Jiang, B.; Shi, X.; Zhang, T.; Huang, Y. Recent advances in UV/thermal curing silicone polymers. Chem. Eng. J. 2022, 435, 134843. [Google Scholar] [CrossRef]
- Friedman, G.M. Occurrence of Talc as a Clay Mineral in Sedimentary Rocks. Nature 1965, 207, 283–284. [Google Scholar] [CrossRef]
- Mulderig, A.; Beaucage, G.; Vogtt, K.; Jiang, H.; Kuppa, V. Quantification of branching in fumed silica. J. Aerosol Sci. 2017, 109, 28–37. [Google Scholar] [CrossRef]
- Arnaud, S.P.; Malitowski, N.M.; Casamayor, K.M.; Robert, T. Itaconic Acid-Based Reactive Diluents for Renewable and Acrylate-Free UV-Curing Additive Manufacturing Materials. ACS Sustain. Chem. Eng. 2022, 9, 17142–17151. [Google Scholar] [CrossRef]
- Plummer, J.F. Microspheres. In Encyclopedia of Polymer Science and Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011. [Google Scholar]
- Macarie, L.; Ilia, G. Influence of pigment properties on UV-curing efficiency. J. Appl. Polym. Sci. 2007, 104, 247–252. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribas-Massonis, A.; Cicujano, M.; Duran, J.; Besalú, E.; Poater, A. Free-Radical Photopolymerization for Curing Products for Refinish Coatings Market. Polymers 2022, 14, 2856. https://doi.org/10.3390/polym14142856
Ribas-Massonis A, Cicujano M, Duran J, Besalú E, Poater A. Free-Radical Photopolymerization for Curing Products for Refinish Coatings Market. Polymers. 2022; 14(14):2856. https://doi.org/10.3390/polym14142856
Chicago/Turabian StyleRibas-Massonis, Aina, Magalí Cicujano, Josep Duran, Emili Besalú, and Albert Poater. 2022. "Free-Radical Photopolymerization for Curing Products for Refinish Coatings Market" Polymers 14, no. 14: 2856. https://doi.org/10.3390/polym14142856
APA StyleRibas-Massonis, A., Cicujano, M., Duran, J., Besalú, E., & Poater, A. (2022). Free-Radical Photopolymerization for Curing Products for Refinish Coatings Market. Polymers, 14(14), 2856. https://doi.org/10.3390/polym14142856