Biosorption Potential of Microbial and Residual Biomass of Saccharomyces pastorianus Immobilized in Calcium Alginate Matrix for Pharmaceuticals Removal from Aqueous Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Analytical Procedure
2.2. Biosorbent Preparation and Characterization
2.2.1. Synthesis of Biosorbent Containing Saccharomyces pastorianus Dried Biomass
2.2.2. Synthesis of Biosorbent Containing Residual Biomass of Saccharomyces pastorianus
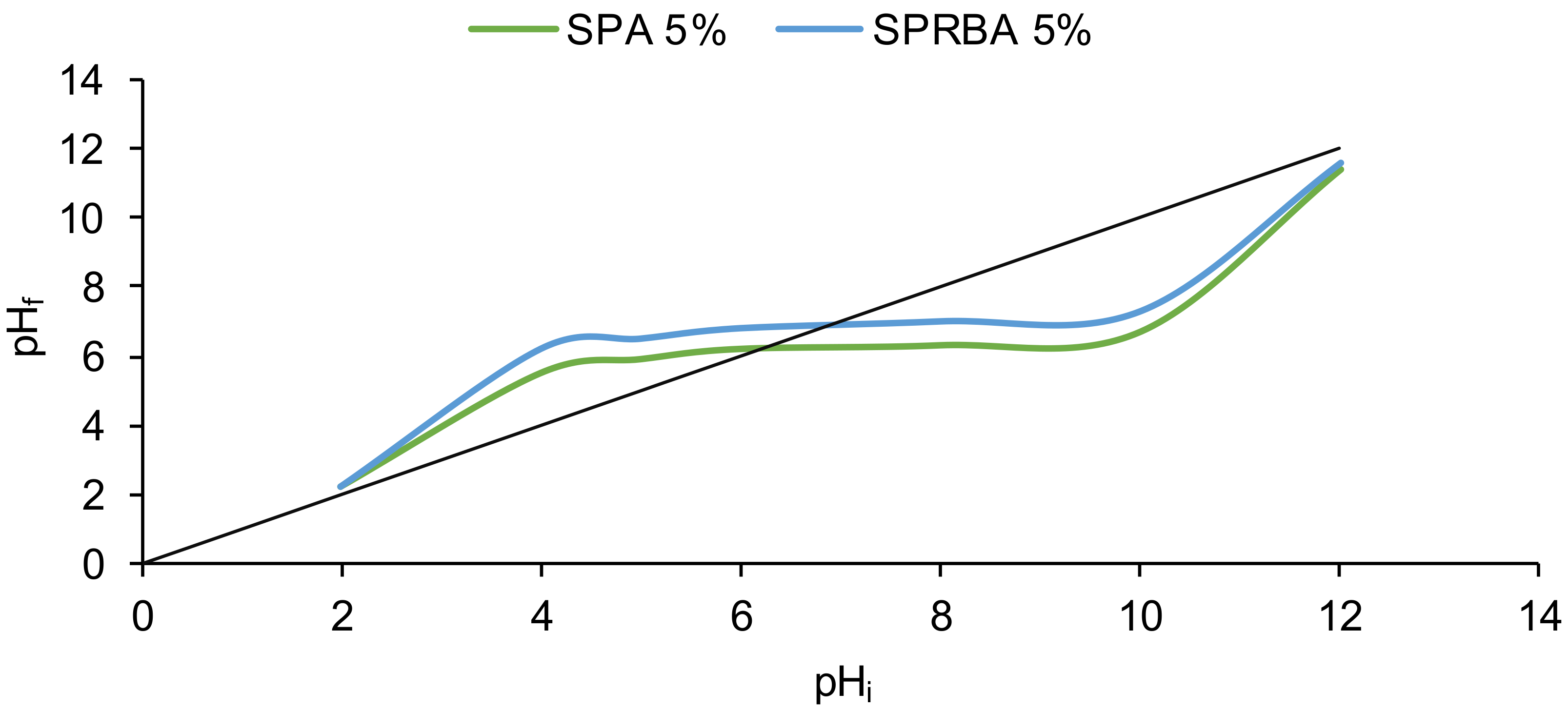
2.2.3. Biosorbents Characterization (SEM, FTIR, Point of Zero Charge)
2.3. Biosorption Process (pH, Biosorbent Dose, Initial Contaminant Concentration)
2.4. Kinetics and Equilibrium Isotherms
2.5. Statiscal Analysis
3. Results and Discussion
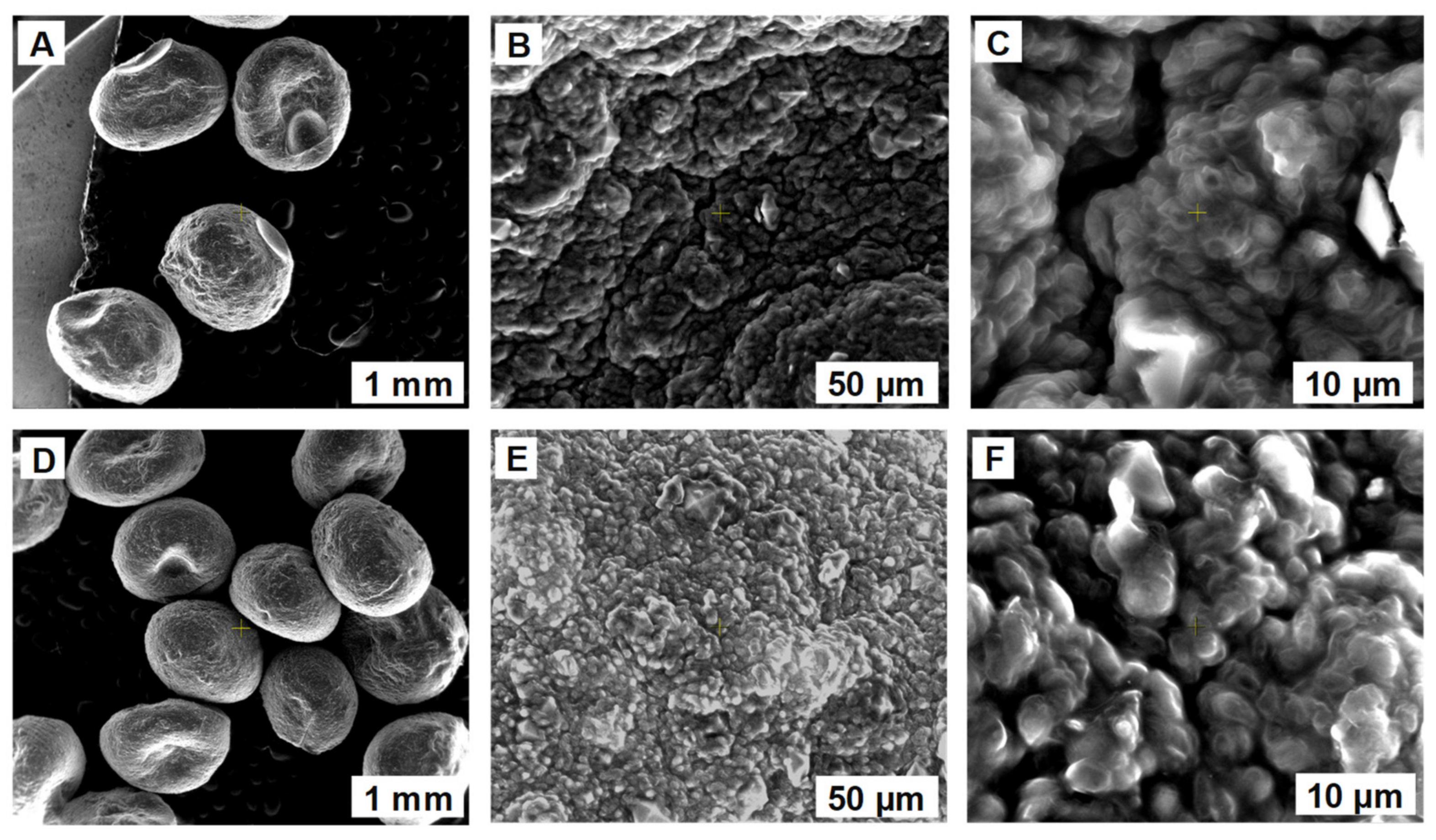
3.1. Biosorbents Preparation and Characterization
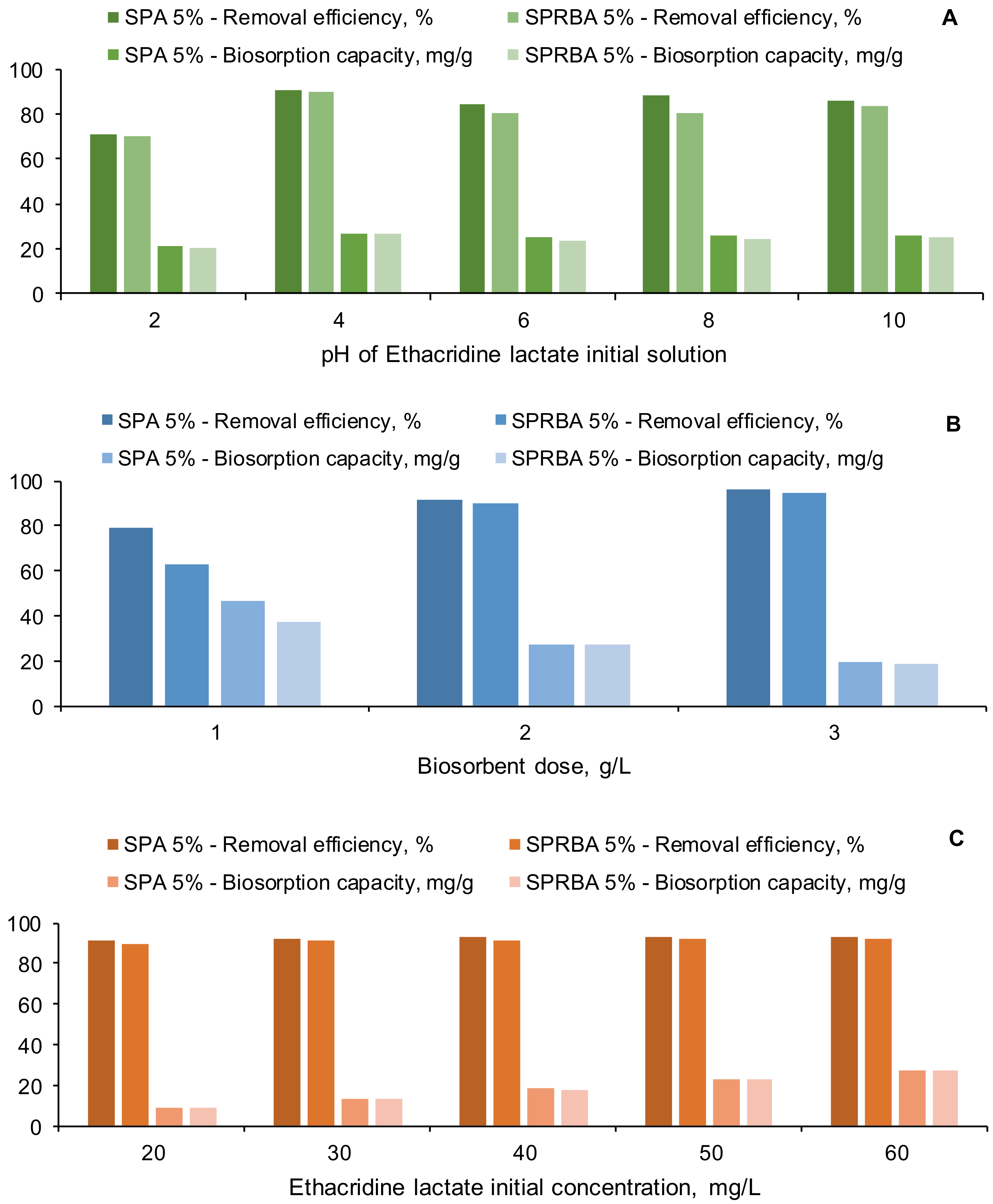
3.2. Impact of pH, Biosorbent Dose and EL Initial Concentration on the Biosorption Process
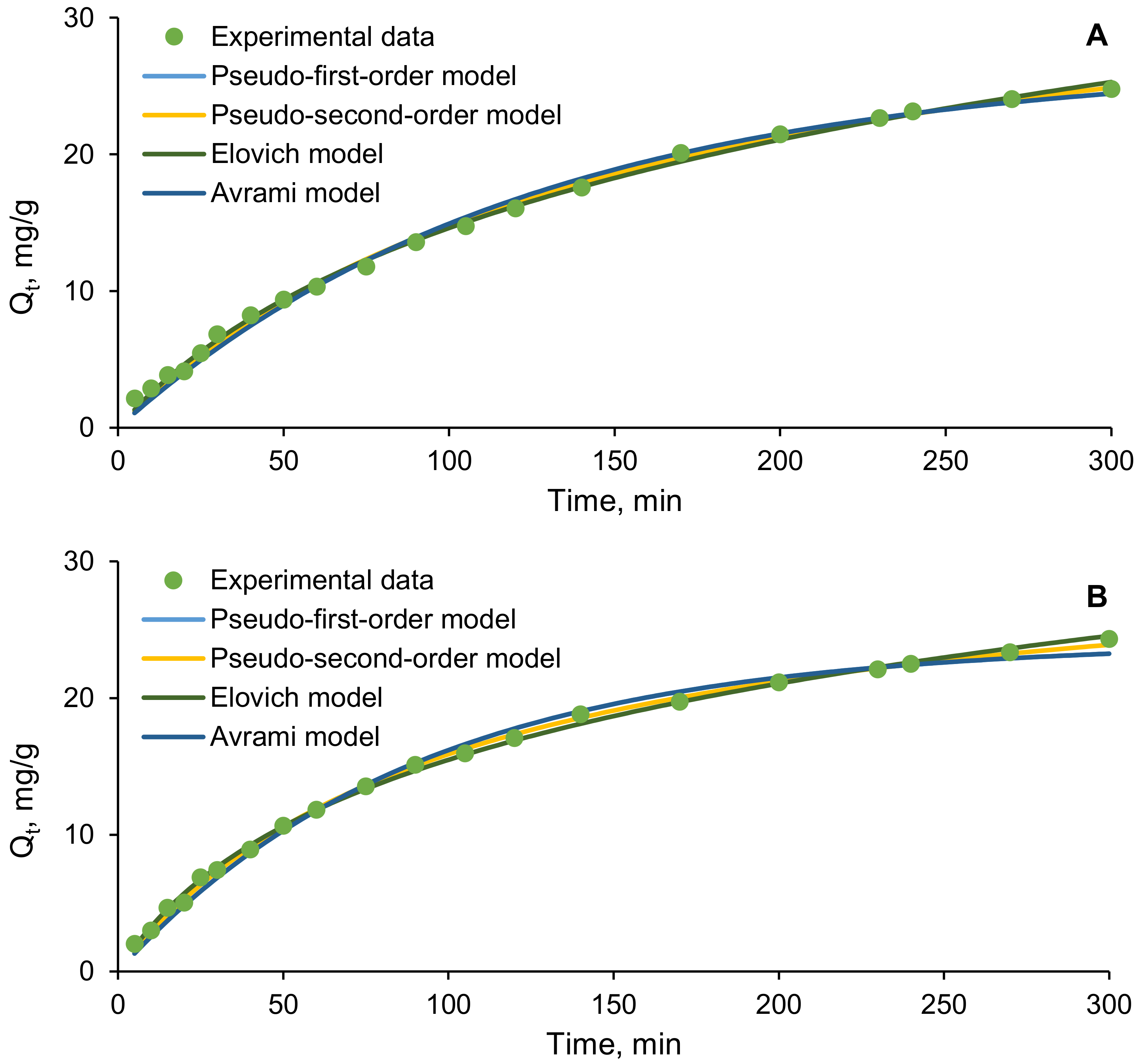
3.3. Biosorption Kinetics
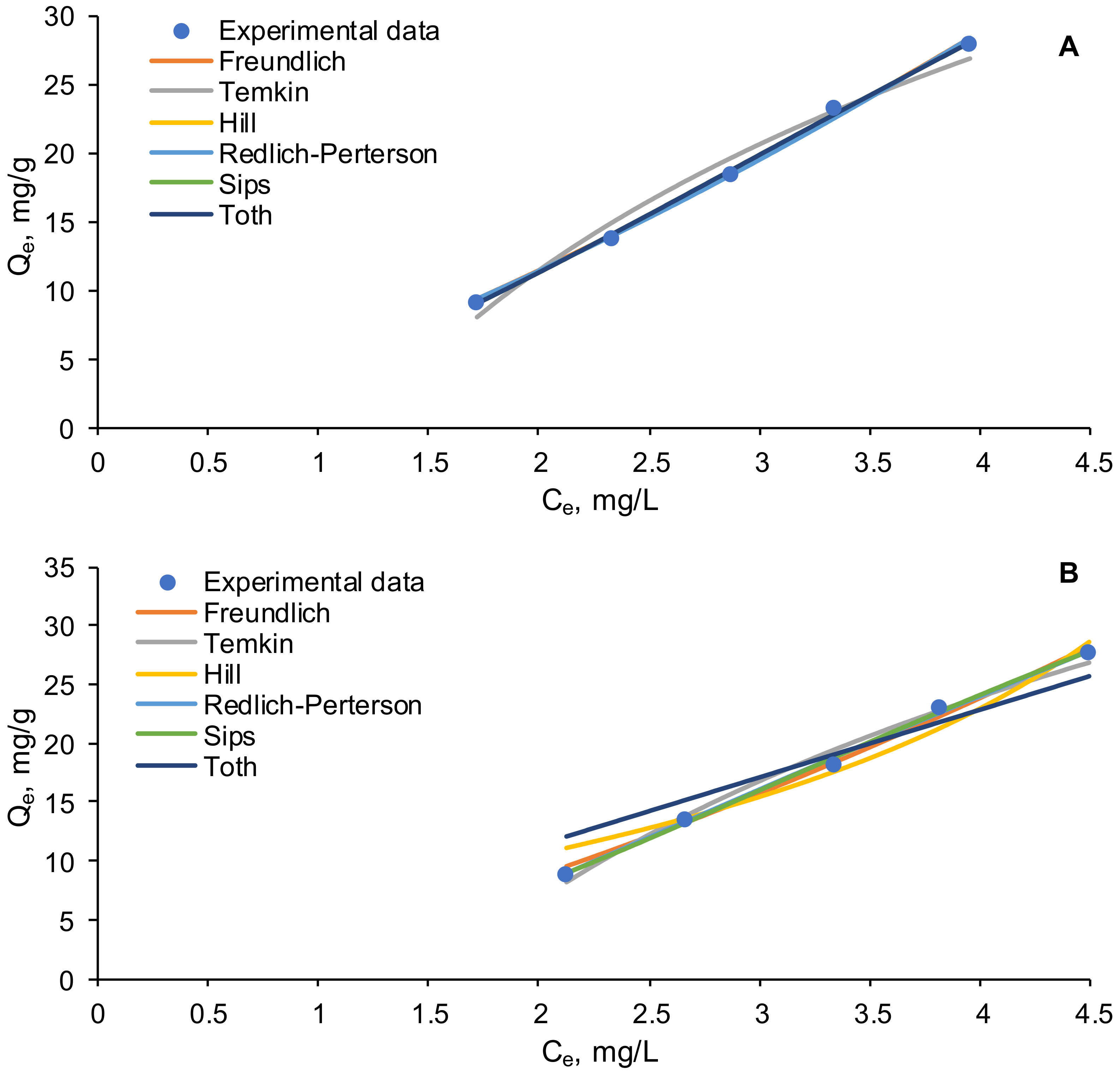
3.4. Equilibrium Isotherms
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Felis, E.; Kalka, J.; Sochacki, A.; Kowalska, K.; Bajkacz, S.; Harnisz, M.; Korzeniewska, E. Antimicrobial Pharmaceuticals in the Aquatic Environment—Occurrence and Environmental Implications. Eur. J. Pharmacol. 2020, 866, 172813. [Google Scholar] [CrossRef]
- Carvalho, I.T.; Santos, L. Antibiotics in the Aquatic Environments: A review of the European Scenario. Environ. Int. 2016, 94, 736–757. [Google Scholar] [CrossRef] [PubMed]
- Berkner, S.; Konradi, S.; Schönfeld, J. Antibiotic Resistance and the Environment—There and Back Again. EMBO Rep. 2014, 15, 740–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molnar, E.; Maasz, G.; Pirger, Z. Environmental Risk Assessment of Pharmaceuticals at a Seasonal Holiday Destination in the Largest Freshwater Shallow Lake in Central Europe. Environ. Sci. Pollut. Res. 2021, 28, 59233–59243. [Google Scholar] [CrossRef]
- Ginebreda, A.; Muñoz, I.; de Alda, M.L.; Brix, R.; López-Doval, J.; Barceló, D. Environmental Risk Assessment of Pharmaceuticals in Rivers: Relationships between Hazard Indexes and Aquatic Macroinvertebrate Diversity Indexes in the Llobregat River (NE Spain). Environ. Int. 2010, 36, 153–162. [Google Scholar] [CrossRef]
- Guzel, E.Y.; Cevik, F.; Daglioglu, N. Determination of Pharmaceutical Active Compounds in Ceyhan River, Turkey: Seasonal, Spatial Variations and Environmental Risk Assessment. Hum. Ecol. Risk Assess. Int. J. 2019, 25, 1980–1995. [Google Scholar] [CrossRef]
- Hernando, M.D.; Mezcua, M.; Fernández-Alba, A.R.; Barceló, D. Environmental Risk Assessment of Pharmaceutical Residues in Wastewater Effluents, Surface Waters and Sediments. Talanta 2006, 69, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Adewuyi, A. Chemically Modified Biosorbents and their role in the Removal of Emerging Pharmaceutical Waste in the Water System. Water 2020, 12, 1551. [Google Scholar] [CrossRef]
- Silva, A.; Delerue-Matos, C.; Figueiredo, S.A.; Freitas, O.M. The Use of Algae and Fungi for Removal of Pharmaceuticals by Bioremediation and Biosorption Processes: A Review. Water 2019, 11, 1555. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, M.O.; Moreira, N.F.F.; Ribeiro, A.R.; Pereira, M.F.R.; Silva, A.M.T. Occurrence and Removal of Organic Micropollutants: An Overview of the Watch List of EU Decision 2015/495. Water Res. 2016, 94, 257–279. [Google Scholar] [CrossRef]
- Loos, R.; Carvalho, R.; António, D.C.; Comero, S.; Locoro, G.; Tavazzi, S.; Paracchini, B.; Ghiani, M.; Lettieri, T.; Blaha, L.; et al. EU-Wide Monitoring Survey on Emerging Polar Organic Contaminants in Wastewater Treatment Plant Effluents. Water Res. 2013, 47, 6475–6487. [Google Scholar] [CrossRef] [PubMed]
- European, C.; Joint Research, C.; Napierska, D.; Sanseverino, I.; Loos, R.; Marinov, D.; Lettieri, T. Review of the 1st Watch List under the Water Framework Directive and Recommendations for the 2nd Watch List; Publications Office of the European Union: Luxembourg, 2018. [Google Scholar]
- Akhtar, J.; Amin, N.A.S.; Shahzad, K. A Review on Removal of Pharmaceuticals from Water by Adsorption. Desalination Water Treat. 2016, 57, 12842–12860. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, T.; Zhang, Y.; Ge, F.; Steel, R.M.; Sun, L. Advances in Technologies for Pharmaceuticals and Personal Care Products Removal. J. Mater. Chem. A 2017, 5, 12001–12014. [Google Scholar] [CrossRef]
- Silva, C.P.; Jaria, G.; Otero, M.; Esteves, V.I.; Calisto, V. Waste-Based Alternative Adsorbents for the Remediation of Pharmaceutical Contaminated Waters: Has a Step Forward Already Been Taken? Bioresour. Technol. 2018, 250, 888–901. [Google Scholar] [CrossRef] [Green Version]
- Adewuyi, A.; Oderinde, R.A. Chemically Modified Vermiculite Clay: A Means to Remove Emerging Contaminant from Polluted Water System in Developing Nation. Polym. Bull. 2019, 76, 4967–4989. [Google Scholar] [CrossRef]
- Lach, J. Adsorption of Chloramphenicol on Commercial and Modified Activated Carbons. Water 2019, 11, 1141. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Pan, C.; Zheng, X.; Liu, S.; Hu, F.; Xu, L.; Xu, G.; Peng, X. Removal of Ciprofloxacin with Aluminum-Pillared Kaolin Sodium Alginate Beads (CA-Al-KABs): Kinetics, Isotherms, and BBD Model. Water 2020, 12, 905. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, A.Z.; Wang, H.; Hu, L.; Zhang, Y.; Xu, P. Treatment of Produced Water in the Permian Basin for Hydraulic Fracturing: Comparison of Different Coagulation Processes and Innovative Filter Media. Water 2020, 12, 770. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Yang, Y.; Lu, Y.; Chen, Y.; Li, W.; Wang, S. Effect of Heavy Metal Ions on Steroid Estrogen Removal and Transport in SAT Using DLLME as a Detection Method of Steroid Estrogen. Water 2020, 12, 589. [Google Scholar] [CrossRef] [Green Version]
- Vrinceanu, N.; Hlihor, R.M.; Simion, A.I.; Rusu, L.; Fekete-Kertész, I.; Barka, N.; Favier, L. New Evidence of the Enhanced Elimination of a Persistent Drug used as a Lipid Absorption Inhibitor by Advanced Oxidation with UV-A and Nanosized Catalysts. Catalysts 2019, 9, 761. [Google Scholar] [CrossRef] [Green Version]
- Favier, L.; Rusu, L.; Simion, A.I.; Hlihor, R.M.; Păcală, M.L.; Augustyniak, A. Efficient Degradation of Clofibric Acid by Heterogeneous Photocatalytic Oxidation Process. Environ. Eng. Manag. J. 2019, 18, 1683–1692. [Google Scholar] [CrossRef]
- Favier, L.; Harja, M.; Simion, A.I.; Rusu, L.; Kadmi, Y.; Pacala, M.L.; Bouzaza, A. Advanced Oxidation Process for the Removal of Chlorinated Phenols in Aqueous Suspensions. J. Environ. Prot. Ecol. 2016, 17, 1132–1141. [Google Scholar]
- Torres, E. Biosorption: A Review of the Latest Advances. Processes 2020, 8, 1584. [Google Scholar] [CrossRef]
- Reddy, S.; Osborne, J.W. Biodegradation and biosorption of Reactive Red 120 Dye by Immobilized Pseudomonas guariconensis: Kinetic and Toxicity Study. Water Environ. Res. 2020, 92, 1230–1241. [Google Scholar] [CrossRef]
- Wang, L.; Xiao, H.; He, N.; Sun, D.; Duan, S. Biosorption and Biodegradation of the Environmental Hormone Nonylphenol by Four Marine Microalgae. Sci. Rep. 2019, 9, 5277. [Google Scholar] [CrossRef]
- Santaeufemia, S.; Abalde, J.; Torres, E. Eco-Friendly Rapid Removal of Triclosan from Seawater using Biomass of a Microalgal Species: Kinetic and Equilibrium Studies. J. Hazard. Mater. 2019, 369, 674–683. [Google Scholar] [CrossRef]
- Contreras-Cortés, A.G.; Almendariz-Tapia, F.J.; Cortez-Rocha, M.O.; Burgos-Hernández, A.; Rosas-Burgos, E.C.; Rodríguez-Félix, F.; Gómez-Álvarez, A.; Quevedo-López, M.Á.; Plascencia-Jatomea, M. Biosorption of Copper by Immobilized Biomass of Aspergillus australensis. Effect of Metal on the Viability, Cellular Components, Polyhydroxyalkanoates Production, and oxidative stress. Environ. Sci. Pollut. Res. 2020, 27, 28545–28560. [Google Scholar] [CrossRef]
- Moghazy, R.M.; Labena, A.; Husien, S. Eco-Friendly Complementary Biosorption Process of Methylene Blue using Micro-Sized Dried Biosorbents of two Macro-Algal Species (Ulva fasciata and Sargassum dentifolium): Full Factorial Design, Equilibrium, and Kinetic Studies. Int. J. Biol. Macromol. 2019, 134, 330–343. [Google Scholar] [CrossRef]
- Rusu, L.; Grigoraș, C.-G.; Suceveanu, E.M.; Simion, A.-I.; Dediu Botezatu, A.V.; Istrate, B.; Doroftei, I. Eco-Friendly Biosorbents Based on Microbial Biomass and Natural Polymers: Synthesis, Characterization and Application for the Removal of Drugs and Dyes from Aqueous Solutions. Materials 2021, 14, 4810. [Google Scholar] [CrossRef]
- Rusu, L.; Grigoraș, C.-G.; Simion, A.-I.; Suceveanu, E.-M.; Blaga, A.-C.; Harja, M. Encapsulation of Saccharomyces pastorianus Residual Biomass in Calcium Alginate Matrix with Insights in Ethacridine Lactate Biosorption. Polymers 2022, 14, 170. [Google Scholar] [CrossRef]
- Ahmad, A.; Bhat, A.H.; Buang, A. Enhanced biosorption of transition metals by living Chlorella vulgaris Immobilized in Ca-Alginate Beads. Environ. Technol. 2019, 40, 1793–1809. [Google Scholar] [CrossRef] [PubMed]
- Páez-Vélez, C.; Castro-Mayorga, J.L.; Dussán, J. Effective Gold Biosorption by Electrospun and Electrosprayed Bio-Composites with Immobilized Lysinibacillus sphaericus CBAM5. Nanomaterials 2020, 10, 408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, G.G. Saccharomyces species in the Production of Beer. Beverages 2016, 2, 34. [Google Scholar] [CrossRef]
- Flores-Copa, V.; Romero-Soto, L.; Romero-Calle, D.; Alvarez-Aliaga, M.T.; Orozco-Gutierrez, F.; Vega-Baudrit, J.; Martín, C.; Carrasco, C. Residual Brewing Yeast as Substrate for Co-Production of Cell Biomass and Biofilm Using Candida maltosa SM4. Fermentation 2021, 7, 84. [Google Scholar] [CrossRef]
- Sütterlin, H.; Alexy, R.; Coker, A.; Kümmerer, K. Mixtures of Quaternary Ammonium Compounds and Anionic Organic Compounds in the Aquatic Environment: Elimination and Biodegradability in the Closed Bottle Test Monitored by LC–MS/MS. Chemosphere 2008, 72, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Aronson, J.K. (Ed.) Ethacridine. In Meyler’s Side Effects of Drugs, 16th ed.; Elsevier: Oxford, UK, 2016; p. 171. [Google Scholar]
- Wainwright, M. Acridine—A Neglected Antibacterial Chromophore. J. Antimicrob. Chemother. 2001, 47, 1–13. [Google Scholar] [CrossRef]
- Lehtola, C.J.; Brown, C.M.; Becker, W.J. Hazard Communications—OSHA Standard 1910.1200. Available online: https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.1200 (accessed on 18 March 2022).
- Talman, R.Y.; Salihi, E.C.; Gokturk, S.; Bastug, A.S. Removal of Ethacridine Lactate from Aqueous Solutions onto Bentonite and Activated Carbon. Fresenius Environ. Bull. 2015, 24, 3603–3608. [Google Scholar]
- Talman, R.Y.; Gokturk, S.; Bastug, A.S.; Caliskan, E. Adsorption of Rivanol on Activated Carbon from Aqueous Solutions. EMChiE 2010 Conf. Proc. 2010, 2, 815–821. [Google Scholar]
- Jing, H.; Huang, X.; Du, X.; Mo, L.; Ma, C.; Wang, H. Facile Synthesis of pH-Responsive Sodium Alginate/Carboxymethyl Chitosan Hydrogel Beads Promoted by Hydrogen Bond. Carbohydr. Polym. 2022, 278, 118993. [Google Scholar] [CrossRef]
- Daemi, H.; Barikani, M. Synthesis and Characterization of Calcium Alginate Nanoparticles, Sodium Homopolymannuronate Salt and its Calcium Nanoparticles. Sci. Iran. 2012, 19, 2023–2028. [Google Scholar] [CrossRef] [Green Version]
- Manuja, A.; Kumar, S.; Dilbaghi, N.; Bhanjana, G.; Chopra, M.; Kaur, H.; Kumar, R.; Manuja, B.; Singh, S.; Yadav, S. Quinapyramine sulfate-loaded sodium alginate nanoparticles show enhanced trypanocidal activity. Nanomedicine 2014, 9, 1625–1634. [Google Scholar] [CrossRef] [PubMed]
- Cardenas-Jiron, G.; Leal, D.; Matsuhiro, B.; Osorio-Roman, I.O. Vibrational spectroscopy and density functional theory calculations of poly-D-mannuronate and heteropolymeric fractions from sodium alginate. J. Raman Spectrosc. 2011, 42, 870–878. [Google Scholar] [CrossRef]
- Larosa, C.; Salerno, M.; de Lima, J.S.; Merijs Meri, R.; da Silva, M.F.; de Carvalho, L.B.; Converti, A. Characterisation of bare and tannase-loaded calcium alginate beads by microscopic, thermogravimetric, FTIR and XRD analyses. Int. J. Biol. Macromol. 2018, 115, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Moreno Rivas, S.C.; Armenta Corral, R.I.; Frasquillo Félix, M.d.C.; Islas Rubio, A.R.; Vázquez Moreno, L.; Ramos-Clamont Montfort, G. Removal of cadmium from aqueous solutions by Saccharomyces cerevisiae–alginate system. Materials 2019, 12, 4128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowalczuk, D.; Pitucha, M. Application of FTIR method for the assessment of immobilization of active substances in the matrix of biomedical materials. Materials 2019, 12, 2972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, M.-H.; Kim, M.-S.; Han, M.; Kwak, D.-H. Experimental application of a zero-point charge based on pH as a simple indicator of microplastic particle aggregation. Chemosphere 2022, 299, 134388. [Google Scholar] [CrossRef] [PubMed]
- Okada, S.; Nakahzara, H.; Isaka, H. Adsorption of drugs on microcrystalline cellulose suspended in aqueous solutions. Chem. Pharm. Bull. 1987, 35, 761–768. [Google Scholar] [CrossRef] [Green Version]
- Plazinski, W.; Rudzinski, W.; Plazinska, A. Theoretical models of sorption kinetics including a surface reaction mechanism: A review. Adv. Colloid Interface Sci. 2009, 152, 2–13. [Google Scholar] [CrossRef]
- Chatterjee, A.; Schiewer, S. Multi-resistance kinetic models for biosorption of Cd by raw and immobilized citrus peels in batch and packed-bed columns. Chem. Eng. J. 2014, 244, 105–116. [Google Scholar] [CrossRef]
- Marco-Brown, J.L.; Areco, M.M.; Torres Sánchez, R.M.; dos Santos Afonso, M. Adsorption of picloram herbicide on montmorillonite: Kinetic and equilibrium studies. Colloids Surf. A Physicochem. Eng. Asp. 2014, 449, 121–128. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption kinetic models: Physical meanings, applications, and solving methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef] [PubMed]
- Piorkowska, E.; Galeski, A.; Haudin, J.-M. Critical assessment of overall crystallization kinetics theories and predictions. Prog. Polym. Sci. 2006, 31, 549–575. [Google Scholar] [CrossRef]
- George, R.; Sugunan, S. Kinetics of adsorption of lipase onto different mesoporous materials: Evaluation of Avrami model and leaching studies. J. Mol. Catal. B Enzym. 2014, 105, 26–32. [Google Scholar] [CrossRef]
- Adeola, A.O.; de Lange, J.; Forbes, P.B.C. Adsorption of antiretroviral drugs, efavirenz and nevirapine from aqueous solution by graphene wool: Kinetic, equilibrium, thermodynamic and computational studies. Appl. Surf. Sci. Adv. 2021, 6, 100157. [Google Scholar] [CrossRef]
- Altalhi, T.A.; Ibrahim, M.M.; Mersal, G.A.M.; Mahmoud, M.H.H.; Kumeria, T.; El-Desouky, M.G.; El-Bindary, A.A.; El-Bindary, M.A. Adsorption of doxorubicin hydrochloride onto thermally treated green adsorbent: Equilibrium, kinetic and thermodynamic studies. J. Mol. Struct. 2022, 1263, 133160. [Google Scholar] [CrossRef]
- Rudzinski, W.; Plazinski, W. On the applicability of the pseudo-second order equation to represent the kinetics of adsorption at solid/solution interfaces: A theoretical analysis based on the statistical rate theory. Adsorption 2009, 15, 181. [Google Scholar] [CrossRef]
- Benedini, L.; Placente, D.; Ruso, J.; Messina, P. Adsorption/desorption study of antibiotic and anti-inflammatory drugs onto bioactive hydroxyapatite nano-rods. Mater. Sci. Eng. C 2019, 99, 180–190. [Google Scholar] [CrossRef]
- Zaheer, Z.; Al-Asfar, A.; Aazam, E.S. Adsorption of methyl red on biogenic Ag@Fe nanocomposite adsorbent: Isotherms, kinetics and mechanisms. J. Mol. Liq. 2019, 283, 287–298. [Google Scholar] [CrossRef]
- Kiran, B.; Kaushik, A. Chromium binding capacity of Lyngbya putealis exopolysaccharides. Biochem. Eng. J. 2008, 38, 47–54. [Google Scholar] [CrossRef]
- Rangabhashiyam, S.; Anu, N.; Giri Nandagopal, M.S.; Selvaraju, N. Relevance of isotherm models in biosorption of pollutants by agricultural byproducts. J. Environ. Chem. Eng. 2014, 2, 398–414. [Google Scholar] [CrossRef]
- Obradović, M.; Daković, A.; Smiljanić, D.; Ožegović, M.; Marković, M.; Rottinghaus, G.E.; Krstić, J. Ibuprofen and diclofenac sodium adsorption onto functionalized minerals: Equilibrium, kinetic and thermodynamic studies. Microporous Mesoporous Mater. 2022, 335, 111795. [Google Scholar] [CrossRef]
- Daikh, S.; Ouis, D.; Benyoucef, A.; Mouffok, B. Equilibrium, kinetic and thermodynamic studies for evaluation of adsorption capacity of a new potential hybrid adsorbent based on polyaniline and chitosan for Acetaminophen. Chem. Phys. Lett. 2022, 798, 139565. [Google Scholar] [CrossRef]
- Yadav, S.; Asthana, A.; Singh, A.K.; Chakraborty, R.; Vidya, S.S.; Susan, M.A.B.H.; Carabineiro, S.A.C. Adsorption of cationic dyes, drugs and metal from aqueous solutions using a polymer composite of magnetic/β-cyclodextrin/activated charcoal/Na alginate: Isotherm, kinetics and regeneration studies. J. Hazard. Mater. 2021, 409, 124840. [Google Scholar] [CrossRef] [PubMed]



| Kinetic Model | Equation | Parameters Significance 1 |
|---|---|---|
| Pseudo-first-order | k1 is the pseudo-first-order constant rate, 1/min t is the contact time, min | |
| Pseudo-second-order | k2 is the pseudo-second-order constant rate, g/(mg·min) t is the contact time, min | |
| Elovich | β is the extent of surface coverage and activation energy for chemisorption, g/mg α is the initial adsorption rate, mg/(g·min) t is the contact time, min | |
| Avrami | kAv is the overall rate constant, 1/min nAv is parameter related to the adsorption, dimensionless t is the contact time, min |
| Equilibrium Isotherm | Equation | Constants Significance 1 |
|---|---|---|
| Freundlich | KF is Freundlich constant, (mg/g)(L/mg)1/n n is Freundlich constant, dimensionless | |
| Temkin | R is gas constant, R = 8.314 J/(mol K) T is temperature, K KT is Temkin constant, L/mg b is Temkin constant, J/mg | |
| Hill | QH is Hill maximum uptake, mg/g KD is Hill constant, L/mg nH is the cooperativity coefficient of the binding interaction, dimensionless | |
| Redlich–Peterson | KR is Redlich–Peterson constant, L/g aR is Redlich–Peterson constant, L/mg bR is Redlich–Peterson exponent, dimensionless | |
| Sips | QS is Sips maximum uptake, mg/g KS is Sips constant, L/mg BS is Sips exponent, dimensionless | |
| Toth | QT is Toth maximum uptake, mg/g aT is Toth constant, L/mg nT is Toth constant, dimensionless |
| Statistical Parameter | Mathematical Expression |
|---|---|
| RMSE | |
| MPSD | |
| HYBRID | |
| χ2 | |
| R2 |
| Kinetic Model | EL Initial Concentration, mg/L | Kinetic Parameters | ||||||
|---|---|---|---|---|---|---|---|---|
| Qe | k1 | k2 | α | β | kAv | nAv | ||
| Pseudo-first-order | 20 | 8.7441 | 0.0072 | - | - | - | - | - |
| 30 | 12.9952 | 0.0080 | - | - | - | - | - | |
| 40 | 17.8438 | 0.0082 | - | - | - | - | - | |
| 50 | 22.7148 | 0.0079 | - | - | - | - | - | |
| 60 | 26.7650 | 0.0081 | - | - | - | - | - | |
| Pseudo-second-order | 20 | 12.5990 | - | 0.0004 | - | - | - | - |
| 30 | 18.4352 | - | 0.0003 | - | - | - | - | |
| 40 | 25.2392 | - | 0.0002 | - | - | - | - | |
| 50 | 32.2967 | - | 0.0001 | - | - | - | - | |
| 60 | 37.6285 | - | 0.0001 | - | - | - | - | |
| Elovich | 20 | - | - | - | 0.0781 | 0.2349 | - | - |
| 30 | - | - | - | 0.1321 | 0.1636 | - | - | |
| 40 | - | - | - | 0.1854 | 0.1201 | - | - | |
| 50 | - | - | - | 0.2773 | 0.0932 | - | - | |
| 60 | - | - | - | 0.2822 | 0.0818 | - | - | |
| Avrami | 20 | 8.7441 | - | - | - | - | 0.0998 | 0.0724 |
| 30 | 12.9952 | - | - | - | - | 0.0788 | 0.1026 | |
| 40 | 17.8438 | - | - | - | - | 0.0813 | 0.1010 | |
| 50 | 22.7148 | - | - | - | - | 0.0911 | 0.0876 | |
| 60 | 26.6750 | - | - | - | - | 0.0818 | 0.0996 | |
| Kinetic Model | EL Initial Concentration, mg/L | Kinetic Parameters | ||||||
|---|---|---|---|---|---|---|---|---|
| Qe | k1 | k2 | α | β | kAv | nAv | ||
| Pseudo-first-order | 20 | 7.8596 | 0.0108 | - | - | - | - | - |
| 30 | 12.2111 | 0.1034 | - | - | - | - | - | |
| 40 | 16.1200 | 0.0108 | - | - | - | - | - | |
| 50 | 20.4265 | 0.0107 | - | - | - | - | - | |
| 60 | 20.0882 | 0.0111 | - | - | - | - | - | |
| Pseudo-second-order | 20 | 10.5802 | - | 0.0008 | - | - | - | - |
| 30 | 16.5292 | - | 0.0005 | - | - | - | - | |
| 40 | 21.5583 | - | 0.0004 | - | - | - | - | |
| 50 | 27.2783 | - | 0.0003 | - | - | - | - | |
| 60 | 31.9313 | - | 0.0003 | - | - | - | - | |
| Elovich | 20 | - | - | - | 0.1171 | 0.3088 | - | - |
| 30 | - | - | - | 0.1728 | 0.1961 | - | - | |
| 40 | - | - | - | 0.2474 | 0.1538 | - | - | |
| 50 | - | - | - | 0.3147 | 0.1221 | - | - | |
| 60 | - | - | - | 0.3919 | 0.1059 | - | - | |
| Avrami | 20 | 7.8596 | - | - | - | - | 0.0892 | 0.1212 |
| 30 | 12.2111 | - | - | - | - | 0.0721 | 0.1434 | |
| 40 | 16.1200 | - | - | - | - | 0.1027 | 0.1055 | |
| 50 | 20.4265 | - | - | - | - | 0.1238 | 0.0871 | |
| 60 | 24.0882 | - | - | - | - | 0.1307 | 0.0851 | |
| Kinetic Model | EL Initial Concentration, mg/L | Statistical Error Function | ||||
|---|---|---|---|---|---|---|
| RMSE | MPSD | HYBRID | Χ2 | R2 | ||
| Pseudo-first-order | 20 | 0.1841 | 17.2244 | 1.8257 | 0.3317 | 0.9942 |
| 30 | 0.1932 | 6.7392 | 1.0871 | 0.2092 | 0.9973 | |
| 40 | 0.2364 | 5.1621 | 1.0964 | 0.2139 | 0.9979 | |
| 50 | 0.3155 | 6.0400 | 1.7042 | 0.3290 | 0.9976 | |
| 60 | 0.5595 | 15.2104 | 7.3399 | 2.0171 | 0.9944 | |
| Pseudo-second-order | 20 | 0.1510 | 19.7638 | 1.5343 | 0.2355 | 0.9961 |
| 30 | 0.1549 | 9.2838 | 1.1011 | 0.1836 | 0.9983 | |
| 40 | 0.1871 | 4.8811 | 0.8149 | 0.1437 | 0.9986 | |
| 50 | 0.2474 | 5.8828 | 1.2903 | 0.2207 | 0.9985 | |
| 60 | 0.4223 | 12.5293 | 4.5434 | 1.2118 | 0.9968 | |
| Elovich | 20 | 0.1364 | 23.9650 | 1.7086 | 0.2186 | 0.9968 |
| 30 | 0.1894 | 13.6630 | 1.9533 | 0.2889 | 0.9974 | |
| 40 | 0.2487 | 7.9983 | 1.5949 | 0.2557 | 0.9976 | |
| 50 | 0.3035 | 8.6210 | 2.1413 | 0.3315 | 0.9978 | |
| 60 | 0.3633 | 10.1217 | 2.9546 | 1.2118 | 0.9976 | |
| Avrami | 20 | 0.1841 | 17.7237 | 1.9332 | 0.3317 | 0.9942 |
| 30 | 0.1932 | 6.9345 | 1.1511 | 0.2093 | 0.9973 | |
| 40 | 0.2364 | 5.3118 | 1.1610 | 0.2139 | 0.9979 | |
| 50 | 0.3155 | 6.2151 | 1.8044 | 0.3290 | 0.9976 | |
| 60 | 0.5595 | 15.6514 | 7.7718 | 2.0171 | 0.9944 | |
| Kinetic Model | EL Initial Concentration, mg/L | Statistical Error Function | ||||
|---|---|---|---|---|---|---|
| RMSE | MPSD | HYBRID | Χ2 | R2 | ||
| Pseudo-first-order | 20 | 0.1294 | 29.7826 | 2.3720 | 0.2648 | 0.9971 |
| 30 | 0.2273 | 27.5194 | 3.2055 | 0.3819 | 0.9962 | |
| 40 | 0.3170 | 5.8542 | 1.5394 | 0.2926 | 0.9957 | |
| 50 | 0.4459 | 6.5103 | 2.5212 | 0.5034 | 0.9946 | |
| 60 | 0.5538 | 11.1238 | 4.7950 | 1.0813 | 0.9939 | |
| Pseudo-second-order | 20 | 0.1393 | 37.4927 | 3.6818 | 0.3536 | 0.9966 |
| 30 | 0.1722 | 34.5163 | 4.3600 | 0.4102 | 0.9978 | |
| 40 | 0.1734 | 8.0616 | 1.0131 | 0.1594 | 0.9987 | |
| 50 | 0.2201 | 4.6546 | 0.7909 | 0.1439 | 0.9987 | |
| 60 | 0.2696 | 6.9319 | 1.5108 | 0.3230 | 0.9985 | |
| Elovich | 20 | 0.2163 | 47.4033 | 6.3809 | 0.5866 | 0.9920 |
| 30 | 0.2526 | 43.7950 | 7.5420 | 0.6712 | 0.9953 | |
| 40 | 0.2654 | 14.2927 | 2.8698 | 0.4012 | 0.9970 | |
| 50 | 0.2665 | 9.4590 | 1.9043 | 0.2899 | 0.9980 | |
| 60 | 0.2921 | 5.1609 | 1.2527 | 0.2144 | 0.9983 | |
| Avrami | 20 | 0.3774 | 148.7241 | 44.2646 | −0.9447 | 0.9971 |
| 30 | 0.2273 | 28.3171 | 3.3940 | 0.3819 | 0.9962 | |
| 40 | 0.3170 | 6.0240 | 1.6299 | 0.2926 | 0.9957 | |
| 50 | 0.4459 | 6.6991 | 2.6695 | 0.5034 | 0.9946 | |
| 60 | 0.5538 | 11.4463 | 5.0771 | 1.0813 | 0.9939 | |
| Parameter | Freundlich | Temkin | Hill | Redlich- Peterson | Sips | Toth |
|---|---|---|---|---|---|---|
| KF | 4.5154 | - | - | - | - | - |
| n | 0.7476 | - | - | - | - | - |
| KT | - | 0.8281 | - | - | - | - |
| b | - | 108.2646 | - | - | - | - |
| QH | - | - | 96.2335 | - | - | - |
| KD | - | - | 24.0381 | - | - | - |
| nH | - | - | 1.6712 | - | - | - |
| KR | - | - | - | 0.0070 | - | - |
| aR | - | - | - | −0.9985 | - | - |
| bR | - | - | - | 0.8198 | - | - |
| QS | - | - | - | - | 96.2335 | - |
| KS | - | - | - | - | 0.1490 | - |
| BS | - | - | - | - | 1.6702 | - |
| QT | - | - | - | - | - | 6.6691 |
| aT | - | - | - | - | - | −0.9415 |
| nT | - | - | - | - | - | −0.000003 |
| Parameter | Freundlich | Temkin | Hill | Redlich- Peterson | Sips | Toth |
|---|---|---|---|---|---|---|
| KF | 3.2340 | - | - | - | - | - |
| n | 0.6937 | - | - | - | - | - |
| KT | - | 0.6558 | - | - | - | - |
| b | - | 98.6681 | - | - | - | - |
| QH | - | - | 0.0008 | - | - | - |
| KD | - | - | −0.9998 | - | - | - |
| nH | - | - | −0.00006 | - | - | - |
| KR | - | - | - | 6.8807 | - | - |
| aR | - | - | - | 3.5347 | - | - |
| bR | - | - | - | −2.3021 | - | - |
| QS | - | - | - | - | 64.2878 | - |
| KS | - | - | - | - | 0.1948 | - |
| BS | - | - | - | - | 2.0508 | - |
| QT | - | - | - | - | - | 5.7159 |
| aT | - | - | - | - | - | −0.9286 |
| nT | - | - | - | - | - | 0.000006 |
| Equilibrium Isotherm Model | Statistical Error Function | ||||
|---|---|---|---|---|---|
| RMSE | MPSD | HYBRID | Χ2 | R2 | |
| Freundlich | 0.5895 | 2.2028 | 0.9141 | 0.027 | 0.9973 |
| Temkin | 0.9787 | 9.3994 | 11.0666 | 0.3417 | 0.9784 |
| Hill | 0.2396 | 2.2021 | 0.7805 | 0.0157 | 0.9987 |
| Redlich–Peterson | 0.3907 | 3.4869 | 1.9578 | 0.0392 | 0.9965 |
| Sips | 0.2396 | 2.2021 | 0.7805 | 0.0157 | 0.9987 |
| Toth | 1.5706 | 20.8824 | 48.2691 | 0.8271 | 0.9444 |
| Equilibrium Isotherm Model | Statistical Error Function | ||||
|---|---|---|---|---|---|
| RMSE | MPSD | HYBRID | Χ2 | R2 | |
| Freundlich | 0.5285 | 5.0164 | 3.0804 | 0.0901 | 0.9936 |
| Temkin | 0.7386 | 6.2446 | 5.5365 | 0.1646 | 0.9876 |
| Hill | 1.3477 | 18.2624 | 35.5931 | 0.6222 | 0.9587 |
| Redlich–Peterson | 0.3462 | 2.9609 | 1.5882 | 0.0315 | 0.9972 |
| Sips | 0.3447 | 3.1836 | 1.6884 | 0.0336 | 0.9972 |
| Toth | 1.9571 | 27.7742 | 79.9008 | 1.2840 | 0.9129 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusu, L.; Grigoraș, C.-G.; Simion, A.-I.; Suceveanu, E.-M.; Istrate, B.; Harja, M. Biosorption Potential of Microbial and Residual Biomass of Saccharomyces pastorianus Immobilized in Calcium Alginate Matrix for Pharmaceuticals Removal from Aqueous Solutions. Polymers 2022, 14, 2855. https://doi.org/10.3390/polym14142855
Rusu L, Grigoraș C-G, Simion A-I, Suceveanu E-M, Istrate B, Harja M. Biosorption Potential of Microbial and Residual Biomass of Saccharomyces pastorianus Immobilized in Calcium Alginate Matrix for Pharmaceuticals Removal from Aqueous Solutions. Polymers. 2022; 14(14):2855. https://doi.org/10.3390/polym14142855
Chicago/Turabian StyleRusu, Lăcrămioara, Cristina-Gabriela Grigoraș, Andrei-Ionuț Simion, Elena-Mirela Suceveanu, Bogdan Istrate, and Maria Harja. 2022. "Biosorption Potential of Microbial and Residual Biomass of Saccharomyces pastorianus Immobilized in Calcium Alginate Matrix for Pharmaceuticals Removal from Aqueous Solutions" Polymers 14, no. 14: 2855. https://doi.org/10.3390/polym14142855
APA StyleRusu, L., Grigoraș, C.-G., Simion, A.-I., Suceveanu, E.-M., Istrate, B., & Harja, M. (2022). Biosorption Potential of Microbial and Residual Biomass of Saccharomyces pastorianus Immobilized in Calcium Alginate Matrix for Pharmaceuticals Removal from Aqueous Solutions. Polymers, 14(14), 2855. https://doi.org/10.3390/polym14142855












