Flame Retardant Coatings: Additives, Binders, and Fillers
Abstract
:1. Introduction
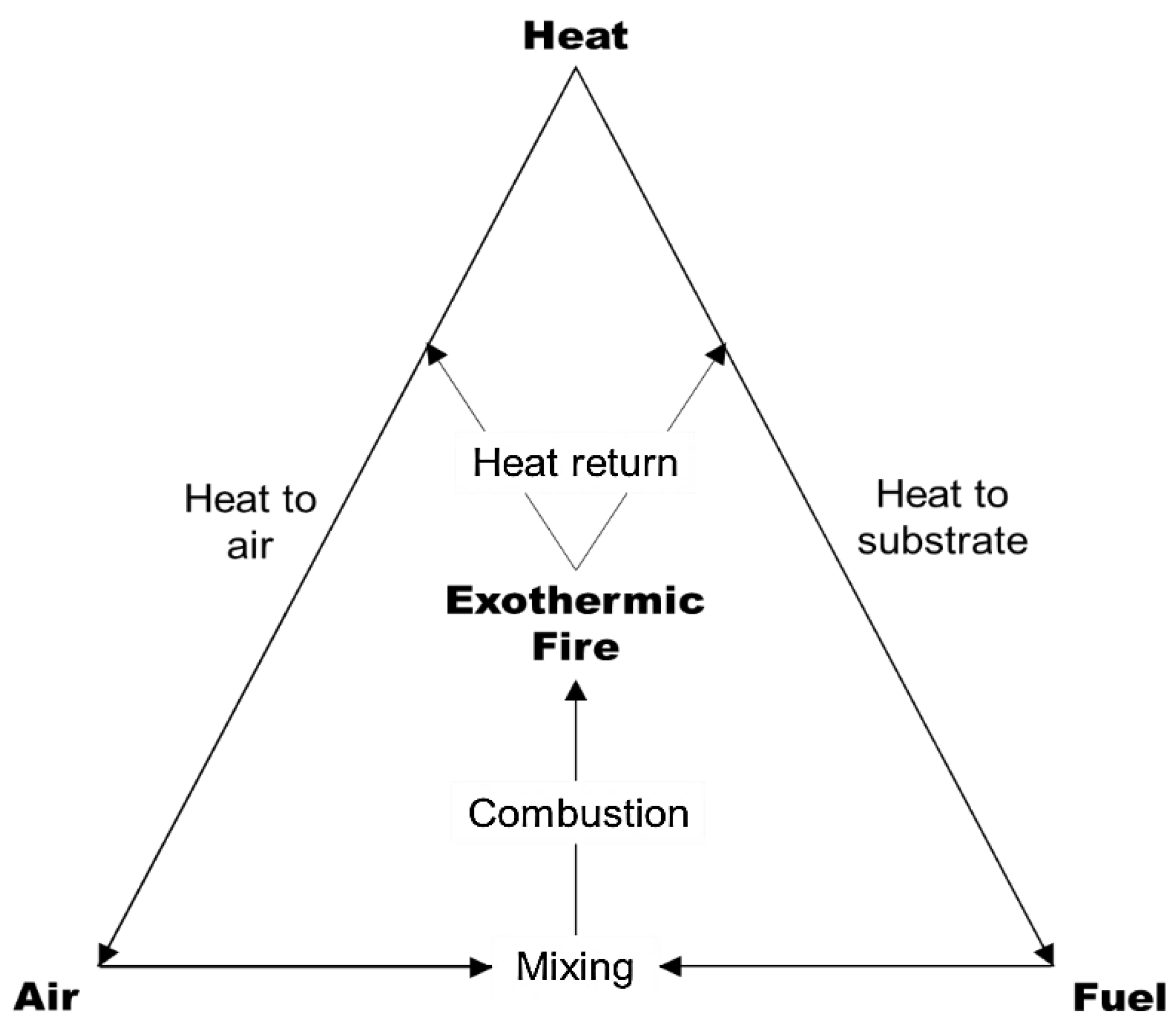
2. Thermal Degradation and Flammability
3. Flame Retardant
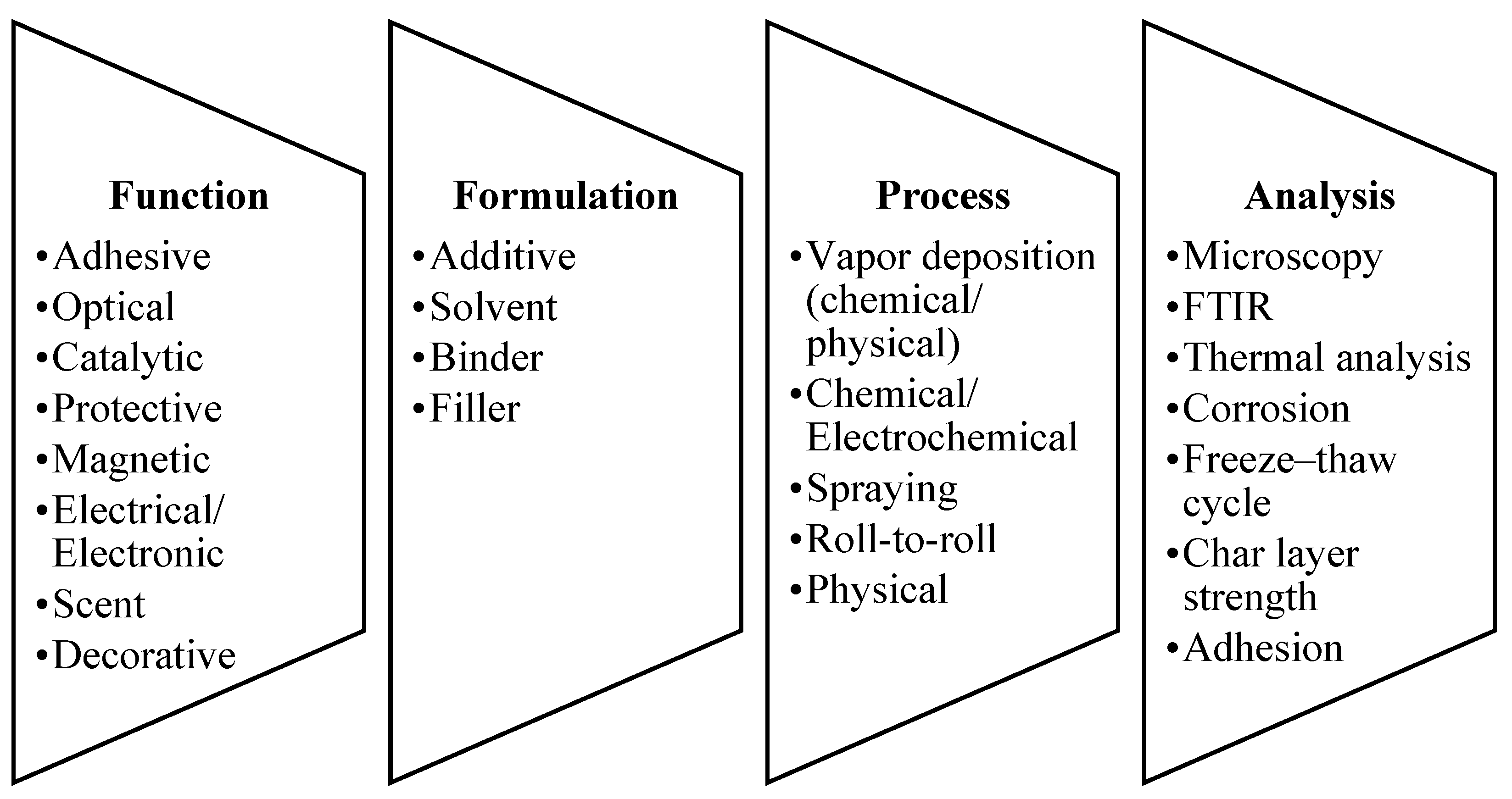
4. Flame Retardant Coatings
Types of Flame Retardant Coatings
5. Intumescent Coatings
6. Intumescent Flame Retardant Coatings
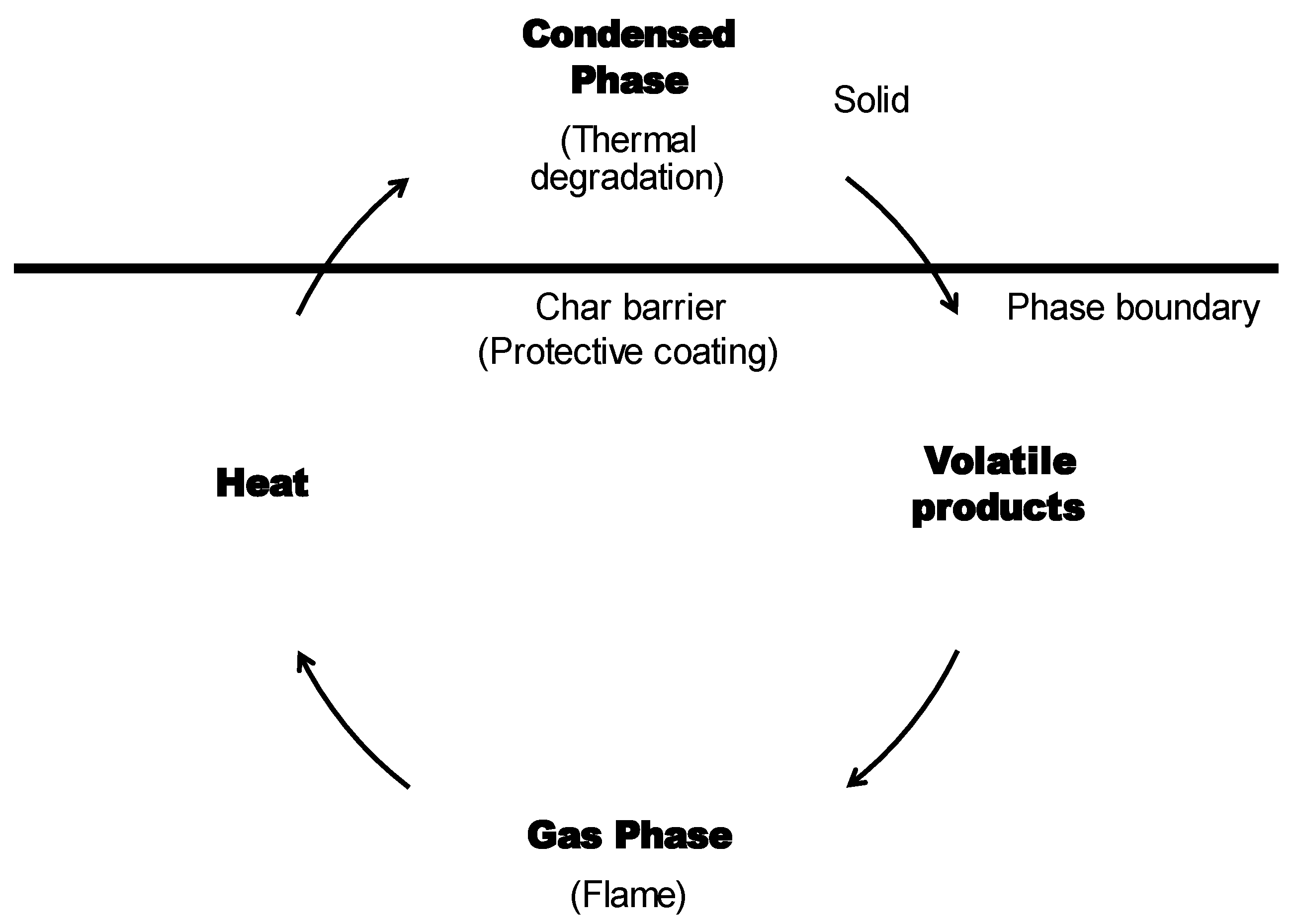
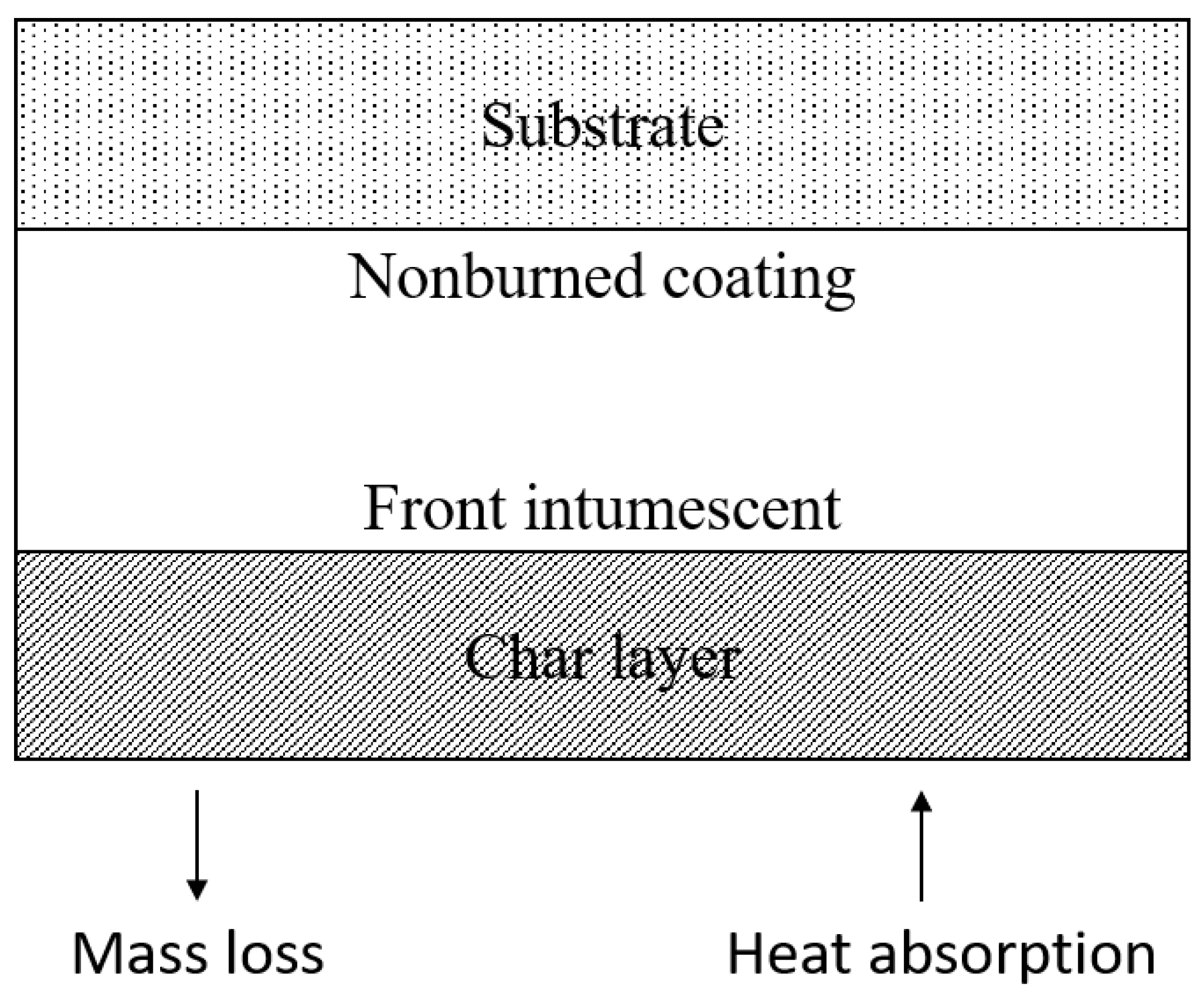
6.1. Mechanisms of Intumescent Flame Retardant Coatings
6.1.1. Physical Mechanisms
6.1.2. Chemical Mechanisms
7. Flame Retardant Coating Formulations and Designs: The Implementation of Polymer Materials
7.1. Additives
7.1.1. Ammonium Polyphosphate
7.1.2. Pentaerythritol Phosphate Alcohol
7.1.3. Melamine
7.1.4. Boric Acid/Borate
7.2. Binders
7.2.1. Ethylene Vinyl Acetate
7.2.2. Epoxy Resin
7.2.3. Polyamide
7.2.4. Cellulose
7.3. Fillers
7.3.1. Aluminum Trihydroxide (Al(OH)3)
7.3.2. Magnesium Carbonate (MgCO3)
7.3.3. Magnesium Hydroxide (Mg(OH)2)
7.3.4. Titanium Dioxide (TiO2)
7.3.5. Expandable Graphite
7.3.6. Fly Ash
7.3.7. Cenospheres
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Howarth, G.A.; Manock, H.L. Water-Borne Polyurethane Dispersions and Their Use in Functional Coatings. Surf. Coatings Int. 1997, 80, 324–328. [Google Scholar] [CrossRef]
- Tietema, R. Large-Scale Industrial Coating Applications and Systems. In Comprehensive Materials Processing; Hashmi, S., Batalha, G.F., Van Tyne, C.J., Yilbas, B.B.T.-C.M.P., Eds.; Elsevier: Oxford, UK, 2014; pp. 519–561. ISBN 978-0-08-096533-8. [Google Scholar]
- Mozetič, M. Surface Modification to Improve Properties of Materials. Materials 2019, 12, 441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chason, M.; Brazis, P.W.; Zhang, J.; Kalyanasundaram, K.; Gamota, D.R. Printed Organic Semiconducting Devices. Proc. IEEE 2005, 93, 1348–1356. [Google Scholar] [CrossRef]
- Zaghloul, M.M.Y.; Zaghloul, M.Y.M.; Zaghloul, M.M.Y. Experimental and Modeling Analysis of Mechanical-Electrical Behaviors of Polypropylene Composites Filled with Graphite and MWCNT Fillers. Polym. Test. 2017, 63, 467–474. [Google Scholar] [CrossRef]
- Jilani, A. Advance Deposition Techniques for Thin Film and Coating. In Modern Technologies for Creating the THin-Film Systems and Coatings; Abdel-wahab, M.S., Ed.; IntechOpen: Rijeka, Croatia, 2017; ISBN 978-953-51-3004-8. [Google Scholar]
- Akafuah, N.K.; Poozesh, S.; Salaimeh, A.; Patrick, G.; Lawler, K.; Saito, K. Evolution of the Automotive Body Coating Process—A Review. Coatings 2016, 6, 24. [Google Scholar] [CrossRef] [Green Version]
- Machiels, J.; Appeltans, R.; Bauer, D.K.; Segers, E.; Henckens, Z.; Van Rompaey, W.; Adons, D.; Peeters, R.; Geiβler, M.; Kuehnoel, K.; et al. Screen Printed Antennas on Fiber-Based Substrates for Sustainable HF RFID Assisted E-Fulfilment Smart Packaging. Materials 2021, 14, 5500. [Google Scholar] [CrossRef]
- Rasouli, R.; Barhoum, A.; Uludag, H. A Review of Nanostructured Surfaces and Materials for Dental Implants: Surface Coating, Patterning and Functionalization for Improved Performance. Biomater. Sci. 2018, 6, 1312–1338. [Google Scholar] [CrossRef]
- Allahdini, A.; Jafari, R.; Momen, G. Transparent Non-Fluorinated Superhydrophobic Coating with Enhanced Anti-Icing Performance. Prog. Org. Coat. 2022, 165, 106758. [Google Scholar] [CrossRef]
- Mehta, A.; Vasudev, H.; Singh, S. Recent Developments in the Designing of Deposition of Thermal Barrier Coatings—A Review. Mater. Today Proc. 2020, 26, 1336–1342. [Google Scholar] [CrossRef]
- Thakare, J.G.; Pandey, C.; Mahapatra, M.M.; Mulik, R.S. Thermal Barrier Coatings—A State of the Art Review. Met. Mater. Int. 2021, 27, 1947–1968. [Google Scholar] [CrossRef]
- Klemm, D.; Petzold-Welcke, K.; Kramer, F.; Richter, T.; Raddatz, V.; Fried, W.; Nietzsche, S.; Bellmann, T.; Fischer, D. Biotech Nanocellulose: A Review on Progress in Product Design and Today’s State of Technical and Medical Applications. Carbohydr. Polym. 2021, 254, 117313. [Google Scholar] [CrossRef] [PubMed]
- Laguna, O.H.; Lietor, P.F.; Godino, F.J.I.; Corpas-Iglesias, F.A. A Review on Additive Manufacturing and Materials for Catalytic Applications: Milestones, Key Concepts, Advances and Perspectives. Mater. Des. 2021, 208, 109927. [Google Scholar] [CrossRef]
- Yang, Q.; Yuan, F.; Xu, L.; Yan, Q.; Yang, Y.; Wu, D.; Guo, F.; Yang, G. An Update of Moisture Barrier Coating for Drug Delivery. Pharmaceutics 2019, 11, 436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caputo, P.; Abe, A.A.; Loise, V.; Porto, M.; Calandra, P.; Angelico, R.; Oliviero Rossi, C. The Role of Additives in Warm Mix Asphalt Technology: An Insight into Their Mechanisms of Improving an Emerging Technology. Nanomaterials 2020, 10, 1202. [Google Scholar] [CrossRef]
- Enekvist, M.; Liang, X.; Zhang, X.; Dam-Johansen, K.; Kontogeorgis, G.M. Computer-Aided Design and Solvent Selection for Organic Paint and Coating Formulations. Prog. Org. Coat. 2022, 162, 106568. [Google Scholar] [CrossRef]
- Xie, L.; Wang, J.; Lu, Q.; Hu, W.; Yang, D.; Qiao, C.; Peng, X.; Peng, Q.; Wang, T.; Sun, W.; et al. Surface Interaction Mechanisms in Mineral Flotation: Fundamentals, Measurements, and Perspectives. Adv. Colloid Interface Sci. 2021, 295, 102491. [Google Scholar] [CrossRef]
- Zhang, J.; Zeng, H. Intermolecular and Surface Interactions in Engineering Processes. Engineering 2021, 7, 63–83. [Google Scholar] [CrossRef]
- Wolfe, D.; Eden, T. Cold Spray Particle Deposition for Improved Wear Resistance. In Woodhead Publishing Series in Metals and Surface Engineering; Champagne, V., Ed.; Woodhead Publishing: Sawston, UK, 2007; pp. 264–301. ISBN 978-1-84569-181-3. [Google Scholar]
- Boentoro, T.W.; Szyszka, B. Protective Coatings for Optical Surfaces. In Woodhead Publishing Series in Electronic and Optical Materials; Piegari, A., Flory, F., Eds.; Woodhead Publishing: Sawston, UK, 2013; pp. 540–563. ISBN 978-0-85709-594-7. [Google Scholar]
- Vargel, C. Protection of Aluminium. In Corrosion of Aluminium; Elsevier: Amsterdam, The Netherlands, 2020; pp. 383–443. ISBN 978-0-08-099925-8. [Google Scholar]
- Maurel, V.; Bartsch, M.; Vidal-Sétif, M.-H.; Vaßen, R.; Guipont, V. Coated Single Crystal Superalloys: Processing, Characterization, and Modeling of Protective Coatings. In Nickel Base Single Crystals across Length Scales; Cailletaud, G., Cormier, J., Eggeler, G., Maurel, V., Nazé, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 283–338. ISBN 978-0-12-819357-0. [Google Scholar]
- Eduok, U.; Ohaeri, E.; Szpunar, J. Self-Healing Composite Coatings with Protective and Anticorrosion Potentials: Classification by Healing Mechanism. In Woodhead Publishing Series in Composites Science and Engineering; Khan, A., Jawaid, M., Raveendran, S.N., Ahmed Asiri, A.M.B.T.-S.-H.C.M., Eds.; Woodhead Publishing: Sawston, UK, 2020; pp. 123–162. ISBN 978-0-12-817354-1. [Google Scholar]
- Pahade, V.S.; Chavan, P.S.; Baisane, V.P. A Review Paper on Vapour Deposition Coating. Int. J. Eng. Appl. Sci. 2016, 3, 75–78. [Google Scholar]
- Fuseini, M.; Zaghloul, M.M.Y.; Elkady, M.F.; El-Shazly, A.H. Evaluation of Synthesized Polyaniline Nanofibres as Corrosion Protection Film Coating on Copper Substrate by Electrophoretic Deposition. J. Mater. Sci. 2022, 57, 6085–6101. [Google Scholar] [CrossRef]
- Møller, V.B.; Dam-Johansen, K.; Frankær, S.M.; Kiil, S. Acid-Resistant Organic Coatings for the Chemical Industry: A Review. J. Coatings Technol. Res. 2017, 14, 279–306. [Google Scholar] [CrossRef]
- Du, Z.; Wen, S.; Wang, J.; Yin, C.; Yu, D.; Luo, J. The Review of Powder Coatings. J. Mater. Sci. Chem. Eng. 2016, 4, 54–59. [Google Scholar] [CrossRef] [Green Version]
- Łatka, L.; Pawłowski, L.; Winnicki, M.; Sokołowski, P.; Małachowska, A.; Kozerski, S. Review of Functionally Graded Thermal Sprayed Coatings. Appl. Sci. 2020, 10, 5153. [Google Scholar] [CrossRef]
- Srikanth, A.; Mohammed Thalib Basha, G.; Venkateshwarlu, B. A Brief Review on Cold Spray Coating Process. Mater. Today Proc. 2020, 22, 1390–1397. [Google Scholar] [CrossRef]
- Park, J.; Shin, K.; Lee, C. Roll-to-Roll Coating Technology and Its Applications: A Review. Int. J. Precis. Eng. Manuf. 2016, 17, 537–550. [Google Scholar] [CrossRef]
- Puetz, J.; Aegerter, M.A. Dip Coating Technique BT-Sol-Gel Technologies for Glass Producers and Users. In Sol-Gel Technologies for Glass Producers and Users; Aegerter, M.A., Mennig, M., Eds.; Springer: Boston, MA, USA, 2004; pp. 37–48. ISBN 978-0-387-88953-5. [Google Scholar]
- Sahu, N.; Parija, B.; Panigrahi, S. Fundamental Understanding and Modeling of Spin Coating Process: A Review. Indian J. Phys. 2009, 83, 493–502. [Google Scholar] [CrossRef] [Green Version]
- Velázquez, M.M. Langmuir-Blodgett Methodology: A Versatile Technique to Build 2D Material Films. In Two-Dimensional Materials; Alejo, T., Ed.; IntechOpen: Rijeka, Croatia, 2016; ISBN 978-953-51-2555-6. [Google Scholar]
- Ogabi, R.; Manescau, B.; Chetehouna, K.; Gascoin, N. A Study of Thermal Degradation and Fire Behaviour of Polymer Composites and Their Gaseous Emission Assessment. Energies 2021, 14, 7070. [Google Scholar] [CrossRef]
- Kohse-Höinghaus, K. Combustion in the Future: The Importance of Chemistry. Proc. Combust. Inst. 2020, 38, 1–56. [Google Scholar] [CrossRef]
- Varma, A.; Mukasyan, A.S.; Rogachev, A.S.; Manukyan, K. V Solution Combustion Synthesis of Nanoscale Materials. Chem. Rev. 2016, 116, 14493–14586. [Google Scholar] [CrossRef]
- Chandraratne, M.R. Recent Advances in Thermochemical Conversion of Biomass. In Recent Perspectives in Pyrolysis Research; Giorcelli, M., Bartoli, M., Eds.; IntechOpen: Rijeka, Croatia, 2021; ISBN 978-1-83969-915-3. [Google Scholar]
- Kordi, M.; Seyyedi, S.M. Biomass Gasification Systems and Different Types of Gasifiers, Effective Parameters on Gasification Process Efficiency: An Overview. J. Appl. Dyn. Syst. Control 2021, 4, 1–17. [Google Scholar]
- Lowden, L.; Hull, T. Flammability Behaviour of Wood and a Review of the Methods for Its Reduction. Fire Sci. Rev. 2013, 2, 4. [Google Scholar] [CrossRef] [Green Version]
- Zaman, C.Z. Pyrolysis: A Sustainable Way to Generate Energy from Waste. In Pyrolysis; Pal, K., Ed.; IntechOpen: Rijeka, Croatia, 2017; ISBN 978-953-51-3312-4. [Google Scholar]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Abu-Omar, M.; Scott, S.L.; Suh, S. Degradation Rates of Plastics in the Environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef] [Green Version]
- Shen, J.; Liang, J.; Lin, X.; Lin, H.; Yu, J.; Wang, S. The Flame-Retardant Mechanisms and Preparation of Polymer Composites and Their Potential Application in Construction Engineering. Polymers 2022, 14, 82. [Google Scholar] [CrossRef]
- Maqsood, M.; Seide, G. Biodegradable Flame Retardants for Biodegradable Polymer. Biomolecules 2020, 10, 1038. [Google Scholar] [CrossRef]
- Madyaratri, E.W.; Ridho, M.R.; Aristri, M.A.; Lubis, M.A.; Iswanto, A.H.; Nawawi, D.S.; Antov, P.; Kristak, L.; Majlingová, A.; Fatriasari, W. Recent Advances in the Development of Fire-Resistant Biocomposites—A Review. Polymers 2022, 14, 362. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, S.; Yoon, H. Fire-Safe Polymer Composites: Flame-Retardant Effect of Nanofillers. Polymers 2021, 13, 540. [Google Scholar] [CrossRef]
- Xu, Y. Introductory Chapter: Flame Retardant and Thermally Insulating Polymers. In Flame Retardant and Thermally Insulating Polymers; IntechOpen: Rijeka, Croatia, 2021; ISBN 978-1-83968-715-0. [Google Scholar]
- Salmeia, K.A.; Fage, J.; Liang, S.; Gaan, S. An Overview of Mode of Action and Analytical Methods for Evaluation of Gas Phase Activities of Flame Retardants. Polymers 2015, 7, 504–526. [Google Scholar] [CrossRef] [Green Version]
- Wilén, C.E.; Pfaendner, R. Improving Weathering Resistance of Flame-Retarded Polymers. J. Appl. Polym. Sci. 2013, 129, 925–944. [Google Scholar] [CrossRef]
- Venier, M.; Salamova, A.; Hites, R.A. Halogenated Flame Retardants in the Great Lakes Environment. Acc. Chem. Res. 2015, 48, 1853–1861. [Google Scholar] [CrossRef] [Green Version]
- Daley, R. Flame Retardant Troubles Attributable to Weak Chemical Regulations. Public Health Rep. 2011, 126, 458–459. [Google Scholar] [CrossRef] [Green Version]
- Vahidi, G.; Bajwa, D.S.; Shojaeiarani, J.; Stark, N.; Darabi, A. Advancements in Traditional and Nanosized Flame Retardants for Polymers—A Review. J. Appl. Polym. Sci. 2021, 138, e50050. [Google Scholar] [CrossRef]
- Baby, A.; Tretsiakova-McNally, S.; Arun, M.; Joseph, P.; Zhang, J. Reactive and Additive Modifications of Styrenic Polymers with Phosphorus-Containing Compounds and Their Effects on Fire Retardance. Molecules 2020, 25, 3779. [Google Scholar] [CrossRef]
- Babushok, V.I.; Deglmann, P.; Krämer, R.; Linteris, G.T. Influence of Antimony-Halogen Additives on Flame Propagation. Combust. Sci. Technol. 2017, 189, 290–311. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Hao, J.; Gaan, S. Recent Studies on the Decomposition and Strategies of Smoke and Toxicity Suppression for Polyurethane Based Materials. RSC Adv. 2016, 6, 74742–74756. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Kumar Kundu, C.; Li, Z.; Li, X.; Zhang, Z. Flame Retardant Treatments for Polypropylene: Strategies and Recent Advances. Compos. Part A Appl. Sci. Manuf. 2021, 145, 106382. [Google Scholar] [CrossRef]
- Shaw, S.D.; Blum, A.; Weber, R.; Kannan, K.; Rich, D.; Lucas, D.; Koshland, C.P.; Dobraca, D.; Hanson, S.; Birnbaum, L.S. Halogenated Flame Retardants: Do the Fire Safety Benefits Justify the Risks? Rev. Environ. Health 2010, 25, 261–305. [Google Scholar] [CrossRef]
- Mensah, R.A.; Shanmugam, V.; Narayanan, S.; Renner, J.S.; Babu, K.; Neisiany, R.E.; Försth, M.; Sas, G.; Das, O. A Review of Sustainable and Environment-Friendly Flame Retardants Used in Plastics. Polym. Test. 2022, 108, 107511. [Google Scholar] [CrossRef]
- Salasinska, K.; Borucka, M.; Leszczyńska, M.; Zatorski, W.; Celiński, M.; Gajek, A.; Ryszkowska, J. Analysis of Flammability and Smoke Emission of Rigid Polyurethane Foams Modified with Nanoparticles and Halogen-Free Fire Retardants. J. Therm. Anal. Calorim. 2017, 130, 131–141. [Google Scholar] [CrossRef] [Green Version]
- Levinṭa, N.; Vuluga, Z.; Teodorescu, M.; Corobea, M.C. Halogen-Free Flame Retardants for Application in Thermoplastics Based on Condensation Polymers. SN Appl. Sci. 2019, 1, 422. [Google Scholar] [CrossRef] [Green Version]
- Lai, X.; Qiu, J.; Li, H.; Zeng, X.; Tang, S.; Chen, Y.; Chen, Z. Flame-Retardant and Thermal Degradation Mechanism of Caged Phosphate Charring Agent with Melamine Pyrophosphate for Polypropylene. Int. J. Polym. Sci. 2015, 2015, 360274. [Google Scholar] [CrossRef]
- Prabhakar, M.N.; Shah, A.U.R.; Song, J.-I. A Review on the Flammability and Flame Retardant Properties of Natural Fibers and Polymer Matrix Based Composites. Compos. Res. 2015, 28, 29–39. [Google Scholar] [CrossRef] [Green Version]
- Salmeia, K.A.; Gaan, S.; Malucelli, G. Recent Advances for Flame Retardancy of Textiles Based on Phosphorus Chemistry. Polymers 2016, 8, 319. [Google Scholar] [CrossRef] [Green Version]
- Nousiainen, P.; Heidari, S. Flame Retardant Chemical Mechanisms of Flame Retardant Viscose Fibres and Blends with Polyester. Makromol. Chem. Macromol. Symp. 1993, 74, 41–57. [Google Scholar] [CrossRef]
- Wang, F.; Liu, H.; Yan, L. Comparative Study of Fire Resistance and Char Formation of Intumescent Fire-Retardant Coatings Reinforced with Three Types of Shell Bio-Fillers. Polymers 2021, 13, 4333. [Google Scholar] [CrossRef]
- Cao, X.; Lu, K.; Li, Y. Isolated Protective Char Layers by Nanoclay Network: Significantly Improved Flame Retardancy and Mechanical Performance of TPV/MH Composites by Small Amount of Nanoclay. Ind. Eng. Chem. Res. 2015, 54, 6912–6921. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, D.; Li, Z.; Li, Z.; Peng, X.; Liu, C.; Zhang, Y.; Zheng, P. Recent Developments in the Flame-Retardant System of Epoxy Resin. Materials 2020, 13, 2145. [Google Scholar] [CrossRef]
- Shi, X.-H.; Li, X.-L.; Li, Y.-M.; Li, Z.; Wang, D.-Y. Flame-Retardant Strategy and Mechanism of Fiber Reinforced Polymeric Composite: A Review. Compos. Part B Eng. 2022, 233, 109663. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, L.; Semple, K.; Zhang, M.; Zhang, W.; Dai, C. Development of Biodegradable Flame-Retardant Bamboo Charcoal Composites, Part I: Thermal and Elemental Analyses. Polymers 2020, 12, 2217. [Google Scholar] [CrossRef]
- Lazar, S.T.; Kolibaba, T.J.; Grunlan, J.C. Flame-Retardant Surface Treatments. Nat. Rev. Mater. 2020, 5, 259–275. [Google Scholar] [CrossRef]
- Horrocks, A.R. The Potential for Bio-Sustainable Organobromine-Containing Flame Retardant Formulations for Textile Applications-a Review. Polymers 2020, 12, 2160. [Google Scholar] [CrossRef]
- Weil, E. Fire-Protective and Flame-Retardant Coatings—A State-of-the-Art Review. J. Fire Sci. 2011, 29, 259–296. [Google Scholar] [CrossRef]
- Mariappan, T. Fire Retardant Coatings. In New Technologies in Protective Coatings; IntechOpen: Rijeka, Croatia, 2017; ISBN 978-953-51-3492-3. [Google Scholar]
- Czupryński, A. Flame Spraying of Aluminum Coatings Reinforced with Particles of Carbonaceous Materials as an Alternative for Laser Cladding Technologies. Materials 2019, 12, 3467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopera-Valle, A.; McDonald, A. Application of Flame-Sprayed Coatings as Heating Elements for Polymer-Based Composite Structures. J. Therm. Spray Technol. 2015, 24, 1289–1301. [Google Scholar] [CrossRef]
- Chang, S.; Slopek, R.P.; Condon, B.; Grunlan, J.C. Surface Coating for Flame-Retardant Behavior of Cotton Fabric Using a Continuous Layer-by-Layer Process. Ind. Eng. Chem. Res. 2014, 53, 3805–3812. [Google Scholar] [CrossRef]
- Nontasak, W.; Thongnuanchan, B.; Ninjan, R.; Lopattananon, N.; Wannavilai, P.; Nakason, C. Fire-Retardant Wood Coating Based on Natural Rubber Bearing Methacrylic Functionality. J. Polym. Eng. 2021, 41, 44–53. [Google Scholar] [CrossRef]
- Zheng, C.; Li, D.; Ek, M. Improving Fire Retardancy of Cellulosic Thermal Insulating Materials by Coating with Bio-Based Fire Retardants. Nord. Pulp Pap. Res. J. 2019, 34, 96–106. [Google Scholar] [CrossRef] [Green Version]
- Abdeen, D.H.; El Hachach, M.; Koc, M.; Atieh, M.A. A Review on the Corrosion Behaviour of Nanocoatings on Metallic Substrates. Materials 2019, 12, 210. [Google Scholar] [CrossRef] [Green Version]
- Awang, M.; Khalili, A.A.; Pedapati, S.R. A Review: Thin Protective Coating for Wear Protection in High-Temperature Application. Metals 2020, 10, 42. [Google Scholar] [CrossRef] [Green Version]
- Ye, X. Thermal Insulation Coatings in Energy Saving. In Energy-Efficient Approaches in Industrial Applications; IntechOpen: Rijeka, Croatia, 2019; ISBN 978-1-78985-520-3. [Google Scholar]
- Beh, J.H.; Yew, M.C.; Saw, L.H.; Yew, M.K. Fire Resistance and Mechanical Properties of Intumescent Coating Using Novel Bioash for Steel. Coatings 2020, 10, 1117. [Google Scholar] [CrossRef]
- Kim, K.; Kim, W. Effect of Heat Treatment on Microstructure and Thermal Conductivity of Thermal Barrier Coating. Materials 2021, 14, 7801. [Google Scholar] [CrossRef]
- Yin, J.J.K.; Yew, M.C.; Yew, M.K.; Saw, L.H. Preparation of Intumescent Fire Protective Coating for Fire Rated Timber Door. Coatings 2019, 9, 738. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Yu, B.; Song, P.; Maluk, C.; Wang, H. Surface-Coating Engineering for Flame Retardant Flexible Polyurethane Foams: A Critical Review. Compos. Part B Eng. 2019, 176, 107185. [Google Scholar] [CrossRef]
- Huang, D.; Chen, C.; Xu, Z.; Li, D.; Shi, L.; Liang, G. Fire Behaviors of Two-Layer Coated Latex Foam with an Extremely Thin Surface Layer under Bottom Ventilation Conditions. Process Saf. Environ. Prot. 2021, 148, 1164–1178. [Google Scholar] [CrossRef]
- Popescu, C.M.; Pfriem, A. Treatments and Modification to Improve the Reaction to Fire of Wood and Wood Based Products—An Overview. Fire Mater. 2020, 44, 100–111. [Google Scholar] [CrossRef] [Green Version]
- Zverev, V.G.; Zinchenko, V.I.; Tsimbalyuk, A.F. Physical and Mechanical Properties and Thermal Protection Efficiency of Intumescent Coatings. IOP Conf. Ser. Mater. Sci. Eng. 2016, 124, 012109. [Google Scholar] [CrossRef] [Green Version]
- Rossi, S.; Lindmark, H.; Fedel, M. Colored Paints Containing NIR-Reflective Pigments Exposed to Accelerated Ultraviolet Radiation Aging with Possible Application as Roof Coatings. Coatings 2020, 10, 1135. [Google Scholar] [CrossRef]
- Yew, M.C.; Ramli Sulong, N.H.; Yew, M.K.; Amalina, M.A.; Johan, M.R. Fire Propagation Performance of Intumescent Fire Protective Coatings Using Eggshells as a Novel Biofiller. Sci. World J. 2014, 2014, 805094. [Google Scholar] [CrossRef]
- Zielecka, M.; Rabajczyk, A.; Cygańczuk, K.; Pastuszka, Ł.; Jurecki, L. Silicone Resin-Based Intumescent Paints. Materials 2020, 13, 4785. [Google Scholar] [CrossRef]
- Kandola, B.K.; Luangtriratana, P.; Duquesne, S.; Bourbigot, S. The Effects of Thermophysical Properties and Environmental Conditions on Fire Performance of Intumescent Coatings on Glass Fibre-Reinforced Epoxy Composites. Materials 2015, 8, 5216–5237. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, R.B.R.S.; Moreno, A.L., Jr.; Vieira, L.C.M. Intumescent Paint as Fire Protection Coating. Rev. IBRACON Estrut. Mater. 2017, 10, 220–231. [Google Scholar] [CrossRef] [Green Version]
- Mouritz, A.P.; Feih, S.; Kandare, E.; Gibson, A.G. Thermal–Mechanical Modelling of Laminates with Fire Protection Coating. Compos. Part B Eng. 2013, 48, 68–78. [Google Scholar] [CrossRef] [Green Version]
- Larsen, M.E.; Reinholz, E.L.; Coker, E.N.; Jameson, K.J.; Hoffmeister, K.N.G.; Adee, S.M. Thermochemical Model of Intumescing Fire Protection Coating. In Proceedings of the 2nd Thermal and Fluid Engineering Conference and 4th International Workshop on Heat Transfer (TFEC-IWHT), Las Vegas, NV, USA, 2–5 April 2017; pp. 843–857. [Google Scholar]
- Griffin, G.J. The Modeling of Heat Transfer across Intumescent Polymer Coatings. J. Fire Sci. 2010, 28, 249–277. [Google Scholar] [CrossRef]
- Geoffroy, L.; Samyn, F.; Jimenez, M.; Bourbigot, S. Intumescent Polymer Metal Laminates for Fire Protection. Polymers 2018, 10, 995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, F. Fire Blanket and Intumescent Coating Materials for Failure Resistance. MRS Bull. 2021, 46, 429–434. [Google Scholar] [CrossRef]
- Korolchenko, D.A.; Eremina, T.Y. Method of Mathematical Modeling for the Experimental Evaluation of Flame-Retardant Materials’ Parameters. Materials 2021, 15, 11. [Google Scholar] [CrossRef]
- Kandola, B.K.; Williams, K.V.; Ebdon, J.R. Organo-Inorganic Hybrid Intumescent Fire Retardant. Molecules 2020, 25, 688. [Google Scholar] [CrossRef] [Green Version]
- Yasir, M.; Ahmad, F.; Yusoff, P.S.M.M.; Ullah, S.; Jimenez, M. Latest Trends for Structural Steel Protection by Using Intumescent Fire Protective Coatings: A Review. Surf. Eng. 2020, 36, 334–363. [Google Scholar] [CrossRef]
- Jin, F.L.; Zhao, M.; Park, M.; Park, S.J. Recent Trends of Foaming in Polymer Processing: A Review. Polymers 2019, 11, 953. [Google Scholar] [CrossRef] [Green Version]
- Garbacz, T.; Tor-świątek, A.; Jachowicz, T. Effect of Chemical Blowing Agent on the Pvc Cellular Coating Extrusion. Materials 2020, 13, 5752. [Google Scholar] [CrossRef]
- Oliwa, R.; Ryszkowska, J.; Oleksy, M.; Auguścik-Królikowska, M.; Gzik, M.; Bartoń, J.; Budzik, G. Effects of Various Types of Expandable Graphite and Blackcurrant Pomace on the Properties of Viscoelastic Polyurethane Foams. Materials 2021, 14, 1801. [Google Scholar] [CrossRef]
- Mazela, B.; Batista, A.; Grześkowiak, W. Expandable Graphite as a Fire Retardant for Cellulosic Materials—A Review. Forests 2020, 11, 755. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on Nanoparticles and Nanostructured Materials: History, Sources, Toxicity and Regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [Green Version]
- Pomázi, Á.; Toldy, A. Multifunctional Gelcoats for Fiber Reinforced Composites. Coatings 2019, 9, 173. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Jiang, S.; Liang, R.; Liao, Z.; You, G. A Green Highly-Effective Surface Flame-Retardant Strategy for Rigid Polyurethane Foam: Transforming UV-Cured Coating into Intumescent Self-Extinguishing Layer. Compos. Part A Appl. Sci. Manuf. 2019, 125, 105534. [Google Scholar] [CrossRef]
- Winandy, J.E.; Morrell, J.J. Improving the Utility, Performance, and Durability of Wood- and Bio-Based Composites. Ann. For. Sci. 2017, 74, 25. [Google Scholar] [CrossRef] [Green Version]
- Azwa, Z.N.; Yousif, B.F.; Manalo, A.C.; Karunasena, W. A Review on the Degradability of Polymeric Composites Based on Natural Fibres. Mater. Des. 2013, 47, 424–442. [Google Scholar] [CrossRef] [Green Version]
- Frigione, M.; Lettieri, M. Novel Attribute of Organic-Inorganic Hybrid Coatings for Protection and Preservation of Materials (Stone and Wood) Belonging to Cultural Heritage. Coatings 2018, 8, 319. [Google Scholar] [CrossRef] [Green Version]
- Watson, D.A.V.; Schiraldi, D.A. Biomolecules as Flame Retardant Additives for Polymers: A Review. Polymers 2020, 12, 849. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Rong, X.; Zhao, L.; Xing, X.; Ma, H. Effects of Substrate Surface Characteristics on the Adhesion Properties of Geopolymer Coatings. ACS Omega 2022, 7, 11988–11994. [Google Scholar] [CrossRef]
- Dogan, M.; Dogan, S.D.; Savas, L.A.; Ozcelik, G.; Tayfun, U. Flame Retardant Effect of Boron Compounds in Polymeric Materials. Compos. Part B Eng. 2021, 222, 109088. [Google Scholar] [CrossRef]
- Yan, L.; Tang, X.; Xie, X.; Xu, Z. Fire Resistance, Thermal and Anti-Ageing Properties of Transparent Fire-Retardant Coatings Modified with Different Molecular Weights of Polyethylene Glycol Borate. Polymers 2021, 13, 4206. [Google Scholar] [CrossRef]
- Nazaré, S.; Davis, R.D. A Review of Fire Blocking Technologies for Soft Furnishings. Fire Sci. Rev. 2012, 1, 1. [Google Scholar] [CrossRef] [Green Version]
- Lim, K.-S.; Bee, S.-T.; Sin, L.T.; Tee, T.-T.; Ratnam, C.T.; Hui, D.; Rahmat, A.R. A Review of Application of Ammonium Polyphosphate as Intumescent Flame Retardant in Thermoplastic Composites. Compos. Part B Eng. 2016, 84, 155–174. [Google Scholar] [CrossRef]
- Bourbigot, S.; Sarazin, J.; Bensabath, T.; Samyn, F.; Jimenez, M. Intumescent Polypropylene: Reaction to Fire and Mechanistic Aspects. Fire Saf. J. 2019, 105, 261–269. [Google Scholar] [CrossRef]
- Gérard, C.; Fontaine, G.; Bourbigot, S. New Trends in Reaction and Resistance to Fire of Fire-Retardant Epoxies. Materials 2010, 3, 4476–4499. [Google Scholar] [CrossRef]
- Xu, B.; Shao, L.; Wang, J.; Liu, Y.; Qian, L. Enhancement of the Intumescent Flame Retardant Efficiency in Polypropylene by Synergistic Charring Effect of a Hypophosphite/Cyclotetrasiloxane Bi-Group Compound. Polym. Degrad. Stab. 2020, 181, 109281. [Google Scholar] [CrossRef]
- Lu, W.; Ye, J.; Zhu, L.; Jin, Z.; Matsumoto, Y. Intumescent Flame Retardant Mechanism of Lignosulfonate as a Char Forming Agent in Rigid Polyurethane Foam. Polymers 2021, 13, 1585. [Google Scholar] [CrossRef]
- Jimenez, M.; Duquesne, S.; Bourbigot, S. Characterization of the Performance of an Intumescent Fire Protective Coating. Surf. Coat. Technol. 2006, 201, 979–987. [Google Scholar] [CrossRef]
- Aqlibous, A.; Tretsiakova-McNally, S.; Fateh, T. Waterborne Intumescent Coatings Containing Industrial and Bio-Fillers for Fire Protection of Timber Materials. Polymers 2020, 12, 757. [Google Scholar] [CrossRef] [Green Version]
- Amir, N.; Majid, A.A.A.; Ahmad, F. Effects of Hybrid Fibre Reinforcement on Fire Resistance Performance and Char Morphology of Intumescent Coating. MATEC Web Conf. 2016, 38, 03001. [Google Scholar] [CrossRef] [Green Version]
- Mariappan, T. Recent Developments of Intumescent Fire Protection Coatings for Structural Steel: A Review. J. Fire Sci. 2016, 34, 120–163. [Google Scholar] [CrossRef]
- Maqsood, M.; Seide, G. Investigation of the Flammability and Thermal Stability of Halogen-Free Intumescent System in Biopolymer Composites Containing Biobased Carbonization Agent and Mechanism of Their Char Formation. Polymers 2019, 11, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jadhav, S.D.; Jhabarmal, J. A Review of Non-Halogenated Flame Retardant. Pharma Innov. J. 2018, 380, 380–386. [Google Scholar]
- Mougel, C.; Garnier, T.; Cassagnau, P.; Sintes-Zydowicz, N. Phenolic Foams: A Review of Mechanical Properties, Fire Resistance and New Trends in Phenol Substitution. Polymer 2019, 164, 86–117. [Google Scholar] [CrossRef]
- Luda, M.P.; Zanetti, M. Cyclodextrins and Cyclodextrin Derivatives as Green Char Promoters in Flame Retardants Formulations for Polymeric Materials. A Review. Polymers 2019, 11, 664. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Chen, H.; Zhang, Y.; Zhang, Y.; Kan, W.; Pan, M. Flame Retardancy of High-Density Polyethylene Composites with P,N-Doped Cellulose Fibrils. Polymers 2020, 12, 336. [Google Scholar] [CrossRef] [Green Version]
- Rosace, G.; Castellano, A.; Trovato, V.; Iacono, G.; Malucelli, G. Thermal and Flame Retardant Behaviour of Cotton Fabrics Treated with a Novel Nitrogen-Containing Carboxyl-Functionalized Organophosphorus System. Carbohydr. Polym. 2018, 196, 348–358. [Google Scholar] [CrossRef]
- Pallmann, J.; Ren, Y.; Mahltig, B.; Huo, T. Phosphorylated Sodium Alginate/APP/DPER Intumescent Flame Retardant Used for Polypropylene. J. Appl. Polym. Sci. 2019, 136, 47794. [Google Scholar] [CrossRef]
- Xu, Z.; Chu, Z.; Yan, L.; Chen, H.; Jia, H.; Tang, W. Effect of Chicken Eggshell on the Flame-Retardant and Smoke Suppression Properties of an Epoxy-Based Traditional APP-PER-MEL System. Polym. Compos. 2019, 40, 2712–2723. [Google Scholar] [CrossRef]
- Liang, C.; Du, Y.; Wang, Y.; Ma, A.; Huang, S.; Ma, Z. Intumescent Fire-Retardant Coatings for Ancient Wooden Architectures with Ideal Electromagnetic Interference Shielding. Adv. Compos. Hybrid Mater. 2021, 4, 979–988. [Google Scholar] [CrossRef]
- Kang, S.; Choi, J.Y.; Choi, S. Mechanism of Heat Transfer through Porous Media of Inorganic Intumescent Coating in Cone Calorimeter Testing. Polymers 2019, 11, 221. [Google Scholar] [CrossRef] [Green Version]
- Nyazika, T.; Jimenez, M.; Samyn, F.; Bourbigot, S. Modeling Heat Transfers across a Silicone-Based Intumescent Coating. J. Phys. Conf. Ser. 2018, 1107, 32012. [Google Scholar] [CrossRef]
- Zybina, O.; Gravit, M. Basic Ingredients of Intumescent Compositions. In Intumescent Coatings for Fire Protection of Building Structures and Materials; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–51. ISBN 978-3-030-59422-0. [Google Scholar]
- Hobbs, C.E. Recent Advances in Bio-Based Flame Retardant Additives for Synthetic Polymeric Materials. Polymers 2019, 11, 224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, M.H.M.; Rahman, H.A.; Amirnordin, S.H.; Khan, N.A. Eco-Friendly Flame-Retardant Additives for Polyurethane Foams: A Short Review. Key Eng. Mater. 2018, 791, 19–28. [Google Scholar]
- Ahmed, L.; Zhang, B.; Shen, R.; Agnew, R.J.; Park, H.; Cheng, Z.; Mannan, M.S.; Wang, Q. Fire Reaction Properties of Polystyrene-Based Nanocomposites Using Nanosilica and Nanoclay as Additives in Cone Calorimeter Test. J. Therm. Anal. Calorim. 2018, 132, 1853–1865. [Google Scholar] [CrossRef]
- Vahabi, H.; Laoutid, F.; Mehrpouya, M.; Saeb, M.R.; Dubois, P. Flame Retardant Polymer Materials: An Update and the Future for 3D Printing Developments. Mater. Sci. Eng. R Rep. 2021, 144, 100604. [Google Scholar] [CrossRef]
- Blum, A.; Behl, M.; Birnbaum, L.S.; Diamond, M.L.; Phillips, A.; Singla, V.; Sipes, N.S.; Stapleton, H.M.; Venier, M. Organophosphate Ester Flame Retardants: Are They a Regrettable Substitution for Polybrominated Diphenyl Ethers? Environ. Sci. Technol. Lett. 2019, 6, 638–649. [Google Scholar] [CrossRef]
- Beach, M.W.; Kearns, K.L.; Davis, J.W.; Stutzman, J.R.; Lee, D.; Lai, Y.; Monaenkova, D.; Kram, S.; Hu, J.; Lukas, C. Stability Assessment of a Polymeric Brominated Flame Retardant in Polystyrene Foams under Application-Relevant Conditions. Environ. Sci. Technol. 2021, 55, 3050–3058. [Google Scholar] [CrossRef]
- Bika, S.H.; Adeniji, A.O.; Okoh, A.I.; Okoh, O.O. Spatiotemporal Distribution and Analysis of Organophosphate Flame Retardants in the Environmental Systems: A Review. Molecules 2022, 27, 573. [Google Scholar] [CrossRef]
- Alias, A.H.; Norizan, M.N.; Sabaruddin, F.A.; Asyraf, M.R.; Norrrahim, M.N.; Ilyas, A.R.; Kuzmin, A.M.; Rayung, M.; Shazleen, S.S.; Nazrin, A.; et al. Hybridization of MMT/Lignocellulosic Fiber Reinforced Polymer Nanocomposites for Structural Applications: A Review. Coatings 2021, 11, 1355. [Google Scholar] [CrossRef]
- Schirp, A.; Hellmann, A. Fire Retardancy Improvement of High-Density Polyethylene Composites Based on Thermomechanical Pulp Treated with Ammonium Polyphosphate. Polym. Compos. 2019, 40, 2410–2423. [Google Scholar] [CrossRef]
- Zybina, O.; Gravit, M. Technology Basis of the Thermolytic Synthesis of Char Formation Polymeric System. In Intumescent Coatings for Fire Protection of Building Structures and Materials; Springer International Publishing: Cham, Switzerland, 2020; pp. 53–90. ISBN 978-3-030-59422-0. [Google Scholar]
- He, W.; Song, P.; Yu, B.; Fang, Z.; Wang, H. Flame Retardant Polymeric Nanocomposites through the Combination of Nanomaterials and Conventional Flame Retardants. Prog. Mater. Sci. 2020, 114, 100687. [Google Scholar] [CrossRef]
- Mahmoud Zaghloul, M.Y.; Yousry Zaghloul, M.M.; Yousry Zaghloul, M.M. Developments in Polyester Composite Materials—An in-Depth Review on Natural Fibres and Nano Fillers. Compos. Struct. 2021, 278, 114698. [Google Scholar] [CrossRef]
- Wang, D.; Zhong, L.; Zhang, C.; Zhang, F.; Zhang, G. A Novel Reactive Phosphorous Flame Retardant for Cotton Fabrics with Durable Flame Retardancy and High Whiteness Due to Self-Buffering. Cellulose 2018, 25, 5479–5497. [Google Scholar] [CrossRef]
- Vasiljević, J.; Čolović, M.; Čelan Korošin, N.; Šobak, M.; Štirn, Ž.; Jerman, I. Effect of Different Flame-Retardant Bridged DOPO Derivatives on Properties of in Situ Produced Fiber-Forming Polyamide 6. Polymers 2020, 12, 657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourbigot, S.; Sarazin, J.; Bensabath, T. Intumescent Polypropylene in Extreme Fire Conditions. Fire Saf. J. 2021, 120, 103082. [Google Scholar] [CrossRef]
- Morgan, A.B. The Future of Flame Retardant Polymers—Unmet Needs and Likely New Approaches. Polym. Rev. 2019, 59, 25–54. [Google Scholar] [CrossRef]
- Chen, R.; Huang, X.; Zheng, R.; Xie, D.; Mei, Y.; Zou, R. Flame-Retardancy and Thermal Properties of a Novel Phosphorus-Modified PCM for Thermal Energy Storage. Chem. Eng. J. 2020, 380, 122500. [Google Scholar] [CrossRef]
- Zhang, K.; Wu, H.; Wang, T.; Yao, M.; Xie, J.; Jiao, Y. Flame-Retardant Effect of Cross-Linked Phosphazene Derivatives and Pentaerythritol Derivatives on Polypropylene. J. Therm. Anal. Calorim. 2021, 145, 3067–3075. [Google Scholar] [CrossRef]
- Ma, J.; He, L.; Huang, L.; Peng, Q.; Cai, X. Synthesis of Aliphatic–Aromatic Polyamide Carbonized System with Phosphoramide Structure and Study on Its Thermal Degradation Mechanism and Flame Retardancy in Polypropylene System. J. Therm. Anal. Calorim. 2021, 145, 3041–3051. [Google Scholar] [CrossRef]
- Balabanovich, A.I. Thermal Decomposition Study of Intumescent Additives: Pentaerythritol Phosphate and Its Blend with Melamine Phosphate. Thermochim. Acta 2005, 435, 188–196. [Google Scholar] [CrossRef]
- Huang, S.; Xu, J.; Deng, H.; Liu, J.; Xiao, Y. Comparison of Pentaerythrotol and Its Derivatives as Intumescent Flame Retardants for Polypropylene. Adv. Mater. Sci. Eng. 2018, 2018, 6153252. [Google Scholar] [CrossRef] [Green Version]
- Schartel, B. Phosphorus-Based Flame Retardancy Mechanisms-Old Hat or a Starting Point for Future Development? Materials 2010, 3, 4710–4745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malucelli, G. Flame-Retardant Systems Based on Chitosan and Its Derivatives: State of the Art and Perspectives. Molecules 2020, 25, 4046. [Google Scholar] [CrossRef]
- Araby, S.; Philips, B.; Meng, Q.; Ma, J.; Laoui, T.; Wang, C.H. Recent Advances in Carbon-Based Nanomaterials for Flame Retardant Polymers and Composites. Compos. Part B Eng. 2021, 212, 108675. [Google Scholar] [CrossRef]
- Rezvani Ghomi, E.; Khosravi, F.; Mossayebi, Z.; Saedi Ardahaei, A.; Morshedi Dehaghi, F.; Khorasani, M.; Neisiany, R.E.; Das, O.; Marani, A.; Mensah, R.A.; et al. The Flame Retardancy of Polyethylene Composites: From Fundamental Concepts to Nanocomposites. Molecules 2020, 25, 5157. [Google Scholar] [CrossRef] [PubMed]
- Zaghloul, M.M.Y.M. Mechanical Properties of Linear Low-Density Polyethylene Fire-Retarded with Melamine Polyphosphate. J. Appl. Polym. Sci. 2018, 135, 46770. [Google Scholar] [CrossRef]
- Rong, Z.; Li, Y.; Lim, R.Z.; Wang, H.; Dong, Z.; Li, K.; Wang, X. Fire-Retardant Effect of Titania-Polyurea Coating and Additional Enhancement via Aromatic Diamine and Modified Melamine Polyphosphate. npj Mater. Degrad. 2022, 6, 38. [Google Scholar] [CrossRef]
- Arastehnejad, N.; Sulaiman, M.R.; Gupta, R.K. Nitrogen-Based Ecofriendly Flame Retardants for Polyurethane Foams. In Polyurethane Chemistry: Renewable Polyols and Isocyanates; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2021; Volume 1380, pp. 167–185. ISBN 9780841298408. [Google Scholar]
- Palve, A.M.; Suroshe, J.S.; Gupta, R.K. Recent Developments in Nitrogen- and Phosphorous-Based Flame Retardants for Polyurethanes. In Materials and Chemistry of Flame-Retardant Polyurethanes Volume 2: Green Flame Retardants; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2021; Volume 1400, pp. 11–189. ISBN 9780841298002. [Google Scholar]
- Jia, D.; Yang, J.; He, J.; Li, X.; Yang, R. Melamine-Based Polyol Containing Phosphonate and Alkynyl Groups and Its Application in Rigid Polyurethane Foam. J. Mater. Sci. 2021, 56, 870–885. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, L.; Zhao, H. Recyclable Flame Retardant Paper Made from Layer-by-Layer Assembly of Zinc Coordinated Multi-Layered Coatings. Cellulose 2018, 25, 5309–5321. [Google Scholar] [CrossRef]
- Bartoli, M.; Malucelli, G.; Tagliaferro, A. Overview on Classification of Flame-Retardant Additives for Polymeric Matrix. In Materials and Chemistry of Flame-Retardant Polyurethanes Volume 1: A Fundamental Approach; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2021; Volume 1399, pp. 3–59. ISBN 9780841298026. [Google Scholar]
- Jordanov, I.; Kolibaba, T.J.; Lazar, S.; Magovac, E.; Bischof, S.; Grunlan, J.C. Flame Suppression of Polyamide through Combined Enzymatic Modification and Addition of Urea to Multilayer Nanocoating. J. Mater. Sci. 2020, 55, 15056–15067. [Google Scholar] [CrossRef]
- Islam, M.N.; Rahman, F.; Das, A.K.; Hiziroglu, S. An Overview of Different Types and Potential of Bio-Based Adhesives Used for Wood Products. Int. J. Adhes. Adhes. 2022, 112, 102992. [Google Scholar] [CrossRef]
- Priya; Sharma, A.K.; Kaith, B.S.; Simran; Bhagyashree; Arora, S. Synthesis of Dextrin-Polyacrylamide and Boric Acid Based Tough and Transparent, Self-Healing, Superabsorbent Film. Int. J. Biol. Macromol. 2021, 182, 712–721. [Google Scholar] [CrossRef] [PubMed]
- Kundu, D. Oron Nutrition of Crops in Relation to Yield and Quality- A Review. Agric. Res. Technol. Access J. 2016, 2, 430–433. [Google Scholar] [CrossRef]
- Vani, C.N.; Prajwal, S.; Sundararaj, R.; Dhamodaran, T.K. Chemical Preservatives in Wood Protection. In Science of Wood Degradation and its Protection; Sundararaj, R., Ed.; Springer: Singapore, 2022; pp. 559–587. ISBN 978-981-16-8797-6. [Google Scholar]
- Davesne, A.-L.; Jimenez, M.; Samyn, F.; Bourbigot, S. Thin Coatings for Fire Protection: An Overview of the Existing Strategies, with an Emphasis on Layer-by-Layer Surface Treatments and Promising New Solutions. Prog. Org. Coat. 2021, 154, 106217. [Google Scholar] [CrossRef]
- Jamadar, P.D.; Sharma, R.; Wagh, H.K. Review on Development of False Ceiling Material from Coconut Shell Powder Reinforced PLA with Increase Fire Retardancy. AIP Conf. Proc. 2022, 2393, 020029. [Google Scholar]
- Ji, J.; Huang, S.; Liu, S.; Yuan, Y.; Zhao, J.; Zhang, S. A Novel Biomass-Derived Schiff Base Waterborne Epoxy Coating for Flame Retardation and Anti-Bacteria. Polym. Degrad. Stab. 2022, 199, 109910. [Google Scholar] [CrossRef]
- Quan, Y.-Y.; Chen, Z.; Lai, Y.; Huang, Z.-S.; Li, H. Recent Advances in Fabricating Durable Superhydrophobic Surfaces: A Review in the Aspects of Structures and Materials. Mater. Chem. Front. 2021, 5, 1655–1682. [Google Scholar] [CrossRef]
- Sykam, K.; Försth, M.; Sas, G.; Restás, Á.; Das, O. Phytic Acid: A Bio-Based Flame Retardant for Cotton and Wool Fabrics. Ind. Crops Prod. 2021, 164, 113349. [Google Scholar] [CrossRef]
- Xu, Z.; Chu, Z.; Yan, L. Enhancing the Flame-Retardant and Smoke Suppression Properties of Transparent Intumescent Fire-Retardant Coatings by Introducing Boric Acid as Synergistic Agent. J. Therm. Anal. Calorim. 2018, 133, 1241–1252. [Google Scholar] [CrossRef]
- Kumar, R.; Gunjal, J.; Chauhan, S. Effect of Borax-Boric Acid and Ammonium Polyphosphate on Flame Retardancy of Natural Fiber Polyethylene Composites. Maderas-Cienc. Tecnol. 2022, 24, 1–21. [Google Scholar]
- Hansen-Bruhn, I.; Poulsen, A.V.; Abildgaard, U.; Ravnsbæk, J.B.; Hinge, M. Effect of Titania, Barite, and Kaolinite Fillers on Char Layer Formation in Water-Based Intumescent Fire-Retardant Coatings. J. Coat. Technol. Res. 2022, 1–9. [Google Scholar] [CrossRef]
- Yang, X.; Shen, A.; Jiang, Y.; Meng, Y.; Wu, H. Properties and Mechanism of Flame Retardance and Smoke Suppression in Asphalt Binder Containing Organic Montmorillonite. Constr. Build. Mater. 2021, 302, 124148. [Google Scholar] [CrossRef]
- Yew, M.C.; Yew, M.K.; Saw, L.H.; Ng, T.C.; Durairaj, R.; Beh, J.H. Influences of Nano Bio-Filler on the Fire-Resistive and Mechanical Properties of Water-Based Intumescent Coatings. Prog. Org. Coat. 2018, 124, 33–40. [Google Scholar] [CrossRef]
- Mun, G.A.; Bekbassov, T.; Beksultanov, Z.; Yermukhambetova, B.B.; Azhgaliyev, B.; Azhgaliyev, N.; Dergunov, S.A. Modified Graft Copolymers Based on Ethylene Vinyl Acetate as Depressants for Waxy Crude Oil and Their Effect on the Rheological Properties of Oil. J. Pet. Sci. Eng. 2022, 213, 110298. [Google Scholar] [CrossRef]
- Macko, T.; Arndt, J.-H.; Brüll, R. HPLC Separation of Ethylene–Vinyl Acetate Copolymers According to Chemical Composition. Chromatographia 2019, 82, 725–732. [Google Scholar] [CrossRef]
- Velencoso, M.M.; Battig, A.; Markwart, J.C.; Schartel, B.; Wurm, F.R. Molecular Firefighting—How Modern Phosphorus Chemistry Can Help Solve the Challenge of Flame Retardancy. Angew. Chem. Int. Ed. 2018, 57, 10450–10467. [Google Scholar] [CrossRef] [Green Version]
- Karaer Özmen, F.; Üreyen, M.E.; Koparal, A.S. Cleaner Production of Flame-Retardant-Glass Reinforced Epoxy Resin Composite for Aviation and Reducing Smoke Toxicity. J. Clean. Prod. 2020, 276, 124065. [Google Scholar] [CrossRef]
- Yang, G.; Wu, W.; Dong, H.; Wang, Y.; Qu, H.; Xu, J. Synergistic Flame-Retardant Effects of Aluminum Phosphate and Trimer in Ethylene–Vinyl Acetate Composites. J. Therm. Anal. Calorim. 2018, 132, 919–926. [Google Scholar] [CrossRef]
- Yao, M.; Wu, H.; Liu, H.; Zhou, Z.; Wang, T.; Jiao, Y.; Qu, H. In-Situ Growth of Boron Nitride for the Effect of Layer-by-Layer Assembly Modified Magnesium Hydroxide on Flame Retardancy, Smoke Suppression, Toxicity and Char Formation in EVA. Polym. Degrad. Stab. 2021, 183, 109417. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Sapuan, S.M.; Asyraf, M.R.M.; Dayana, D.A.Z.N.; Amelia, J.J.N.; Rani, M.S.A.; Norrrahim, M.N.; Nurazzi, N.M.; Aisyah, H.A.; Sharma, S.; et al. Polymer Composites Filled with Metal Derivatives: A Review of Flame Retardants. Polymers 2021, 13, 1701. [Google Scholar] [CrossRef]
- Hou, Y.; Xu, Z.; Chu, F.; Gui, Z.; Song, L.; Hu, Y.; Hu, W. A Review on Metal-Organic Hybrids as Flame Retardants for Enhancing Fire Safety of Polymer Composites. Compos. Part B Eng. 2021, 221, 109014. [Google Scholar] [CrossRef]
- Liu, J.; He, Y.; Chang, H.; Guo, Y.; Li, H.; Pan, B. Simultaneously Improving Flame Retardancy, Water and Acid Resistance of Ethylene Vinyl Acetate Copolymer by Introducing Magnesium Hydroxide/Red Phosphorus Co-Microcapsule and Carbon Nanotube. Polym. Degrad. Stab. 2020, 171, 109051. [Google Scholar] [CrossRef]
- de Keer, L.; Kilic, K.; van Steenberge, P.; Daelemans, L.; Kodura, D.; Frisch, H.; de Clerck, K.; Reyniers, M.-F.; Barner-Kowollik, C.; Dauskardt, R.; et al. From Time Dependent Incorporation of Molecular Building Blocks to Application Properties for Inorganic and Organic Three-Dimensional Network Polymers. Res. Sq. 2020. [Google Scholar] [CrossRef]
- Kandola, B.K.; Magnoni, F.; Ebdon, J.R. Flame Retardants for Epoxy Resins: Application-Related Challenges and Solutions. J. Vinyl Addit. Technol. 2022, 28, 17–49. [Google Scholar] [CrossRef]
- Ruiz de Luzuriaga, A.; Solera, G.; Azcarate-Ascasua, I.; Boucher, V.; Grande, H.-J.; Rekondo, A. Chemical Control of the Aromatic Disulfide Exchange Kinetics for Tailor-Made Epoxy Vitrimers. Polymer 2022, 239, 124457. [Google Scholar] [CrossRef]
- Aziz, T.; Zheng, J.; Jamil, M.I.; Fan, H.; Ullah, R.; Iqbal, M.; Ali, A.; Khan, F.U.; Ullah, A. Enhancement in Adhesive and Thermal Properties of Bio-based Epoxy Resin by Using Eugenol Grafted Cellulose Nanocrystals. J. Inorg. Organomet. Polym. Mater. 2021, 31, 3290–3300. [Google Scholar] [CrossRef]
- Yan, L.; Xu, Z.; Deng, N.; Chu, Z. Synergistic Effects of Mono-Component Intumescent Flame Retardant Grafted with Carbon Black on Flame Retardancy and Smoke Suppression Properties of Epoxy Resins. J. Therm. Anal. Calorim. 2019, 138, 915–927. [Google Scholar] [CrossRef]
- Fang, F.; Huo, S.; Shen, H.; Ran, S.; Wang, H.; Song, P.; Fang, Z. A Bio-Based Ionic Complex with Different Oxidation States of Phosphorus for Reducing Flammability and Smoke Release of Epoxy Resins. Compos. Commun. 2020, 17, 104–108. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Z.; Wang, D.-Y. Polydopamine-Assisted Strategies for Preparation of Fire-Safe Polymeric Materials: A Review. Eur. Polym. J. 2020, 138, 109973. [Google Scholar] [CrossRef]
- Zhi, M.; Yang, X.; Fan, R.; Yue, S.; Zheng, L.; Liu, Q.; He, Y. A Comprehensive Review of Reactive Flame-Retardant Epoxy Resin: Fundamentals, Recent Developments, and Perspectives. Polym. Degrad. Stab. 2022, 201, 109976. [Google Scholar] [CrossRef]
- Suriani, M.J.; Radzi, F.S.; Ilyas, R.A.; Petrů, M.; Sapuan, S.M.; Ruzaidi, C.M. Flammability, Tensile, and Morphological Properties of Oil Palm Empty Fruit Bunches Fiber/Pet Yarn-Reinforced Epoxy Fire Retardant Hybrid Polymer Composites. Polymers 2021, 13, 1282. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, L.; Shi, D.; Song, F. Synthesis and Application of Low-Cost Layered Double Hydroxides Intercalated by Gluconic Acid Anion for Flame Retardancy and Tensile Strength Conservation of High Filling Epoxy Resin. J. Colloid Interface Sci. 2021, 594, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Aziz, T.; Mehmood, S.; Haq, F.; Ullah, R.; Khan, F.U.; Ullah, B.; Raheel, M.; Iqbal, M.; Ullah, A. Synthesis and Modification of Silica-Based Epoxy Nanocomposites with Different Sol–Gel Process Enhanced Thermal and Mechanical Properties. J. Appl. Polym. Sci. 2021, 138, 51191. [Google Scholar] [CrossRef]
- Niu, H.; Nabipour, H.; Wang, X.; Song, L.; Hu, Y. Phosphorus-Free Vanillin-Derived Intrinsically Flame-Retardant Epoxy Thermoset with Extremely Low Heat Release Rate and Smoke Emission. ACS Sustain. Chem. Eng. 2021, 9, 5268–5277. [Google Scholar] [CrossRef]
- Gao, T.-Y.; Wang, F.-D.; Xu, Y.; Wei, C.-X.; Zhu, S.-E.; Yang, W.; Lu, H.-D. Luteolin-Based Epoxy Resin with Exceptional Heat Resistance, Mechanical and Flame Retardant Properties. Chem. Eng. J. 2022, 428, 131173. [Google Scholar] [CrossRef]
- Vahabi, H.; Saeb, M.R.; Formela, K.; Cuesta, J.-M.L. Flame Retardant Epoxy/Halloysite Nanotubes Nanocomposite Coatings: Exploring Low-Concentration Threshold for Flammability Compared to Expandable Graphite as Superior Fire Retardant. Prog. Org. Coat. 2018, 119, 8–14. [Google Scholar] [CrossRef]
- Huang, J.; Guo, W.; Wang, X.; Song, L.; Hu, Y. Intrinsically Flame Retardant Cardanol-Based Epoxy Monomer for High-Performance Thermosets. Polym. Degrad. Stab. 2021, 186, 109519. [Google Scholar] [CrossRef]
- Picard, M.; Mohanty, A.K.; Misra, M. Recent Advances in Additive Manufacturing of Engineering Thermoplastics: Challenges and Opportunities. RSC Adv. 2020, 10, 36058–36089. [Google Scholar] [CrossRef]
- Murray, J.J.; Robert, C.; Gleich, K.; McCarthy, E.D.; Ó Brádaigh, C.M. Manufacturing of Unidirectional Stitched Glass Fabric Reinforced Polyamide 6 by Thermoplastic Resin Transfer Moulding. Mater. Des. 2020, 189, 108512. [Google Scholar] [CrossRef]
- Drobny, J.G. Automotive Applications of Thermoplastic Elastomers. Chem. List. 2009, 103, 1–19. [Google Scholar] [CrossRef]
- Marset, D.; Dolza, C.; Fages, E.; Gonga, E.; Gutiérrez, O.; Gomez-Caturla, J.; Ivorra-Martinez, J.; Sanchez-Nacher, L.; Quiles-Carrillo, L. The Effect of Halloysite Nanotubes on the Fire Retardancy Properties of Partially Biobased Polyamide 610. Polymers 2020, 12, 3050. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yang, M.; Cai, K.; Chen, Y.; Liu, S.; Liu, W.; Liu, J. Effect of the Flame Retardants and Glass Fiber on the Polyamide 66/Polyphenylene Oxide Composites. Materials 2022, 15, 813. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Yang, H.; Yu, B.; Shi, Y.; Wang, W.; Song, L.; Hu, Y.; Zhang, Y. Phosphorus and Nitrogen-Containing Polyols: Synergistic Effect on the Thermal Property and Flame Retardancy of Rigid Polyurethane Foam Composites. Ind. Eng. Chem. Res. 2016, 55, 10813–10822. [Google Scholar] [CrossRef]
- Horrocks, A.R.; Sitpalan, A.; Kandola, B.K. Design and Characterisation of Bicomponent Polyamide 6 Fibres with Specific Locations of Each Flame Retardant Component for Enhanced Flame Retardancy. Polym. Test. 2019, 79, 106041. [Google Scholar] [CrossRef]
- Quan, Y.; Zhang, Z.; Tanchak, R.N.; Wang, Q. A Review on Cone Calorimeter for Assessment of Flame-Retarded Polymer Composites. J. Therm. Anal. Calorim. 2022, 1–26. [Google Scholar] [CrossRef]
- Ahmed, L.; Zhang, B.; Hatanaka, L.C.; Mannan, M.S. Application of Polymer Nanocomposites in the Flame Retardancy Study. J. Loss Prev. Process Ind. 2018, 55, 381–391. [Google Scholar] [CrossRef]
- Vahabi, H.; Sonnier, R.; Taguet, A.; Otazaghine, B.; Saeb, M.R.; Beyer, G. Halloysite Nanotubes (HNTs)/Polymer Nanocomposites: Thermal Degradation and Flame Retardancy. In Micro and Nano Technologies; Cavallaro, G., Fakhrullin, R., Pasbakhsh, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 67–93. ISBN 978-0-12-816783-0. [Google Scholar]
- Zhu, C.; Li, S.; Li, J.; Clement, M.; Rudd, C.; Yi, X.; Liu, X. Fire Performance of Sandwich Composites with Intumescent Mat Protection: Evolving Thermal Insulation, Post-Fire Performance and Rail Industry Testing. Fire Saf. J. 2020, 116, 103205. [Google Scholar] [CrossRef]
- Moustafa, H.; Youssef, A.M.; Darwish, N.A.; Abou-Kandil, A.I. Eco-Friendly Polymer Composites for Green Packaging: Future Vision and Challenges. Compos. Part B Eng. 2019, 172, 16–25. [Google Scholar] [CrossRef]
- Kusmono; Listyanda, R.F.; Wildan, M.W.; Ilman, M.N. Preparation and Characterization of Cellulose Nanocrystal Extracted from Ramie Fibers by Sulfuric Acid Hydrolysis. Heliyon 2020, 6, e05486. [Google Scholar] [CrossRef]
- Kandhola, G.; Djioleu, A.; Rajan, K.; Labbé, N.; Sakon, J.; Carrier, D.J.; Kim, J.-W. Maximizing Production of Cellulose Nanocrystals and Nanofibers from Pre-Extracted Loblolly Pine Kraft Pulp: A Response Surface Approach. Bioresour. Bioprocess. 2020, 7, 19. [Google Scholar] [CrossRef]
- Volpe, R.; Zabaniotou, A.A.; Skoulou, V. Synergistic Effects between Lignin and Cellulose during Pyrolysis of Agricultural Waste. Energy Fuels 2018, 32, 8420–8430. [Google Scholar] [CrossRef]
- Solar, J.; Caballero, B.M.; López-Urionabarrenechea, A.; Acha, E.; Arias, P.L. Pyrolysis of Forestry Waste in a Screw Reactor with Four Sequential Heating Zones: Influence of Isothermal and Nonisothermal Profiles. Ind. Eng. Chem. Res. 2021, 60, 18627–18639. [Google Scholar] [CrossRef]
- Horrocks, A.R. Fundamentals: Flammability, Ignition, and Fire Spread in Polymers. In Analysis of Flame Retardancy in Polymer Science; Vahabi, H., Saeb, M.R., Malucelli, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–72. ISBN 978-0-12-824045-8. [Google Scholar]
- Boran Torun, S. Investigation of the Properties of Fiberboards Made from Microcrystalline Cellulose and Antimony Trioxide Added Melamine Formaldehyde Adhesive. Int. J. Adhes. Adhes. 2022, 113, 103084. [Google Scholar] [CrossRef]
- Ali, S.; Hussain, S.A.; Tohir, M.Z.M. Fire Test and Effects of Fire Retardant on the Natural Ability of Timber: A Review. Pertanika J. Sci. Technol. 2019, 27, 867–895. [Google Scholar]
- Karslioğlu, A.; Onur, M.İ.; Balaban, E. Investigation of Boron Waste Usage in Civil Engineering Applications. Nigde Omer Halisdemir Univ. J. Eng. Sci. 2022, 1, 1. [Google Scholar] [CrossRef]
- Cetiner, I.; Shea, A.D. Wood Waste as an Alternative Thermal Insulation for Buildings. Energy Build. 2018, 168, 374–384. [Google Scholar] [CrossRef]
- Theodosiou, T.; Tsikaloudaki, K.; Tsoka, S.; Chastas, P. Thermal Bridging Problems on Advanced Cladding Systems and Smart Building Facades. J. Clean. Prod. 2019, 214, 62–69. [Google Scholar] [CrossRef]
- Gupta, P.; Verma, C.; Maji, P.K. Flame Retardant and Thermally Insulating Clay Based Aerogel Facilitated by Cellulose Nanofibers. J. Supercrit. Fluids 2019, 152, 104537. [Google Scholar] [CrossRef]
- Khalili, P.; Blinzler, B.; Kádár, R.; Blomqvist, P.; Sandinge, A.; Bisschop, R.; Liu, X. Ramie Fabric Elium® Composites with Flame Retardant Coating: Flammability, Smoke, Viscoelastic and Mechanical Properties. Compos. Part A Appl. Sci. Manuf. 2020, 137, 105986. [Google Scholar] [CrossRef]
- Jianjun, L.; Yuxiang, O. Theory of Flame Retardation of Polymeric Materials; De Gruyter: Berlin, Germany, 2019; ISBN 9783110349351. [Google Scholar]
- Idumah, C.I. Emerging Advancements in Flame Retardancy of Polypropylene Nanocomposites. J. Thermoplast. Compos. Mater. 2020, 0892705720930782. [Google Scholar] [CrossRef]
- Sim, M.-J.; Cha, S.-H.; Lee, J.-C. Enhancement of Flame Retardancy and Physical Property for Poly(Vinyl Chloride) Having Renewable Cardanol-Based Self-Polymerizable Phosphonate under Heat Treatment Process. Polym. Test. 2021, 100, 107266. [Google Scholar] [CrossRef]
- Zhao, L.; Duan, G.; Zhang, G.; Yang, H.; He, S.; Jiang, S. Electrospun Functional Materials toward Food Packaging Applications: A Review. Nanomaterials 2020, 10, 150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, D.; Nunes, S.P.; Vankelecom, I.F.J.; Figoli, A.; Lee, Y.M. Recent Advances in Polymer Membranes Employing Non-Toxic Solvents and Materials. Green Chem. 2021, 23, 9815–9843. [Google Scholar] [CrossRef]
- Shen, R.; Fan, T.; Quan, Y.; Ma, R.; Zhang, Z.; Li, Y.; Wang, Q. Thermal Stability and Flammability of Cotton Fabric with TiO2 Coatings Based on Biomineralization. Mater. Chem. Phys. 2022, 282, 125986. [Google Scholar] [CrossRef]
- Shen, R.; Quan, Y.; Zhang, Z.; Ma, R.; Wang, Q. Metal–Organic Framework as an Efficient Synergist for Intumescent Flame Retardants against Highly Flammable Polypropylene. Ind. Eng. Chem. Res. 2022, 61, 7292–7302. [Google Scholar] [CrossRef]
- Kairytė, A.; Kremensas, A.; Vaitkus, S.; Członka, S.; Strąkowska, A. Fire Suppression and Thermal Behavior of Biobased Rigid Polyurethane Foam Filled with Biomass Incineration Waste Ash. Polymers 2020, 12, 683. [Google Scholar] [CrossRef] [Green Version]
- Yu, K.; Liu, Y.; Yang, Y. Review on Form-Stable Inorganic Hydrated Salt Phase Change Materials: Preparation, Characterization and Effect on the Thermophysical Properties. Appl. Energy 2021, 292, 116845. [Google Scholar] [CrossRef]
- Kant, K.; Biwole, P.H.; Shamseddine, I.; Tlaiji, G.; Pennec, F.; Fardoun, F. Recent Advances in Thermophysical Properties Enhancement of Phase Change Materials for Thermal Energy Storage. Sol. Energy Mater. Sol. Cells 2021, 231, 111309. [Google Scholar] [CrossRef]
- Soliman, S.; Casel, C.; Krug, R.; Krastl, G.; Hahn, B. Influence of Filler Geometry and Viscosity of Composite Luting Materials on Marginal Adhesive Gap Width and Occlusal Surface Height of All-Ceramic Partial Crowns. Dent. Mater. 2022, 38, 601–612. [Google Scholar] [CrossRef]
- Liang, L.; Xi, F.; Tan, W.; Meng, X.; Hu, B.; Wang, X. Review of Organic and Inorganic Pollutants Removal by Biochar and Biochar-Based Composites. Biochar 2021, 3, 255–281. [Google Scholar] [CrossRef]
- Qiu, M.; Liu, L.; Ling, Q.; Cai, Y.; Yu, S.; Wang, S.; Fu, D.; Hu, B.; Wang, X. Biochar for the Removal of Contaminants from Soil and Water: A Review. Biochar 2022, 4, 19. [Google Scholar] [CrossRef]
- Ding, S.; Zhu, G.; Zhao, S.; Wu, W.; Jin, P.; Jiao, Y.; Zhai, W.; Zhou, L.; Luo, Y. Simultaneously Optimized Healing Efficiency and Mechanical Strength in Polymer Composites Reinforced by Ultrahigh Loading Fillers Based on Interfacial Energy and Dynamic Disulfide Bonds. Polymer 2022, 251, 124711. [Google Scholar] [CrossRef]
- Mazurek, P.; Vudayagiri, S.; Skov, A. How to Tailor Flexible Silicone Elastomers with Mechanical Integrity_ a Tutorial Review. Chem. Soc. Rev. 2019, 48, 1448–1464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tshai, K.Y.; Kong, I. Advancement in Flame Retardancy of Natural Fibre Reinforced Composites with Macro to Nanoscale Particulates Additives. In Woodhead Publishing Series in Composites Science and Engineering; Goh, K.L., Aswathi, M.K., De Silva, R.T., Thomas, S., Eds.; Woodhead Publishing: Sawston, UK, 2020; pp. 311–342. ISBN 978-0-08-102665-6. [Google Scholar]
- Ielo, I.; Giacobello, F.; Sfameni, S.; Rando, G.; Galletta, M.; Trovato, V.; Rosace, G.; Plutino, M.R. Nanostructured Surface Finishing and Coatings: Functional Properties and Applications. Materials 2021, 14, 2733. [Google Scholar] [CrossRef] [PubMed]
- Chu, R.; Wu, J.; Meng, X.; Wan, Y.; Miao, Z.; Yu, S.; Bai, L. Inhibition Mechanism of Spontaneous Combustion by Nano-Sized Complex Inhibitor for Chinese Lignite in Low-Temperature Oxidation. Chem. Eng. Commun. 2021, 208, 29–40. [Google Scholar] [CrossRef]
- Guan, Y.; Zhao, W.; Liu, K.; Guo, T.; Wang, D.; Cui, M.; Fu, S.; Fan, X.; Wei, X. Depolymerization of Alkaline Lignin over Mesoporous KF/γ-Al2O3. New J. Chem. 2020, 44, 14411–14420. [Google Scholar] [CrossRef]
- Zaghloul, M.M.Y.; Mohamed, Y.S.; El-Gamal, H. Fatigue and Tensile Behaviors of Fiber-Reinforced Thermosetting Composites Embedded with Nanoparticles. J. Compos. Mater. 2018, 53, 709–718. [Google Scholar] [CrossRef]
- Zhu, J.; Wei, Z.; Luo, Z.; Yu, L.; Yin, K. Phase Changes during Various Treatment Processes for Incineration Bottom Ash from Municipal Solid Wastes: A Review in the Application-Environment Nexus. Environ. Pollut. 2021, 287, 117618. [Google Scholar] [CrossRef]
- Pachta, V.; Tsardaka, E.-C.; Stefanidou, M. The Role of Flame Retardants in Cement Mortars Exposed at Elevated Temperatures. Constr. Build. Mater. 2021, 273, 122029. [Google Scholar] [CrossRef]
- Barroso, G.; Li, Q.; Bordia, R.K.; Motz, G. Polymeric and Ceramic Silicon-Based Coatings—A Review. J. Mater. Chem. A 2019, 7, 1936–1963. [Google Scholar] [CrossRef]
- Hsissou, R.; Seghiri, R.; Benzekri, Z.; Hilali, M.; Rafik, M.; Elharfi, A. Polymer Composite Materials: A Comprehensive Review. Compos. Struct. 2021, 262, 113640. [Google Scholar] [CrossRef]
- Bergeret, A. Surface Treatments in Fiber-Reinforced Composites. In Woodhead Publishing Series in Composites Science and Engineering; Joseph, K., Oksman, K., George, G., Wilson, R., Appukuttan, S., Eds.; Woodhead Publishing: Sawston, UK, 2021; pp. 47–81. ISBN 978-0-12-821090-1. [Google Scholar]
- Palve, A.M.; Vani, O.V.; Gupta, R.K. Metal Oxide-Based Compounds as Flame Retardants for Polyurethanes. In Materials and Chemistry of Flame-Retardant Polyurethanes Volume 2: Green Flame Retardants; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2021; Volume 1400, pp. 121–136. ISBN 9780841298002. [Google Scholar]
- Zhang, T.; Wang, C.; Wang, Y.; Qian, L.; Han, Z. Enhanced Flame Retardancy in Ethylene–Vinyl Acetate Copolymer/Magnesium Hydroxide/Polycarbosilane Blends. Polymers 2022, 14, 36. [Google Scholar] [CrossRef] [PubMed]
- Bokka, S.; Achary, S.N.; Chowdhury, A. Structure, Property, Processing and Applications of Fire Retardant Materials: A Brief Review. Adv. Mater. Res. 2022, 1170, 87–116. [Google Scholar] [CrossRef]
- Wu, Y.; Yao, C.; Hu, Y.; Zhu, X.; Qing, Y.; Wu, Q. Comparative Performance of Three Magnesium Compounds on Thermal Degradation Behavior of Red Gum Wood. Materials 2014, 7, 637–652. [Google Scholar] [CrossRef] [Green Version]
- Yashwanth, B.L.; Shotorban, B.; Mahalingam, S.; Weise, D.R. An Investigation of the Influence of Heating Modes on Ignition and Pyrolysis of Woody Wildland Fuel. Combust. Sci. Technol. 2015, 187, 780–796. [Google Scholar] [CrossRef] [Green Version]
- Satdive, A.; Tayde, S.; Chatterjee, A. Flammability Properties of the Bionanocomposites Reinforced with Fire Retardant Filler. In Polymer Based Bio-nanocomposites; Muthukumar, C., Thiagamani, S.M.K., Krishnasamy, S., Nagarajan, R., Siengchin, S., Eds.; Springer Singapore: Singapore, 2022; pp. 69–86. ISBN 978-981-16-8578-1. [Google Scholar]
- Merachtsaki, D.; Tsardaka, E.-C.; Anastasiou, E.; Zouboulis, A. Evaluation of the Protection Ability of a Magnesium Hydroxide Coating against the Bio-Corrosion of Concrete Sewer Pipes, by Using Short and Long Duration Accelerated Acid Spraying Tests. Materials 2021, 14, 4897. [Google Scholar] [CrossRef]
- Ding, C.; Tai, Y.; Wang, D.; Tan, L.; Fu, J. Superhydrophobic Composite Coating with Active Corrosion Resistance for AZ31B Magnesium Alloy Protection. Chem. Eng. J. 2019, 357, 518–532. [Google Scholar] [CrossRef]
- Wan, L.; Deng, C.; Zhao, Z.-Y.; Chen, H.; Wang, Y.-Z. Flame Retardation of Natural Rubber: Strategy and Recent Progress. Polymers 2020, 12, 429. [Google Scholar] [CrossRef] [Green Version]
- Zaghloul, M.M.Y.; Zaghloul, M.M.Y. Influence of Flame Retardant Magnesium Hydroxide on the Mechanical Properties of High Density Polyethylene Composites. J. Reinf. Plast. Compos. 2017, 36, 1802–1816. [Google Scholar] [CrossRef]
- Bartczak, P.; Grząbka-Zasadzińska, A. Minerals as Flame-Retardant Fillers in Polyurethanes. In Materials and Chemistry of Flame-Retardant Polyurethanes Volume 2: Green Flame Retardants; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2021; Volume 1400, pp. 6–87. ISBN 9780841298002. [Google Scholar]
- Luong, V.-T.; Amal, R.; Scott, J.A.; Ehrenberger, S.; Tran, T. A Comparison of Carbon Footprints of Magnesium Oxide and Magnesium Hydroxide Produced from Conventional Processes. J. Clean. Prod. 2018, 202, 1035–1044. [Google Scholar] [CrossRef]
- Xu, S.; Li, S.-Y.; Zhang, M.; Zeng, H.-Y.; Du, J.-Z.; Chen, C.-R. Effect of P3O105− Intercalated Hydrotalcite on the Flame Retardant Properties and the Degradation Mechanism of a Novel Polypropylene/Hydrotalcite System. Appl. Clay Sci. 2018, 163, 196–203. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, M.; Li, S.-Y.; Zeng, H.-Y.; Du, J.-Z.; Chen, C.-R.; Pan, Y. Surface Modification of Phosphorus-Containing Hydrotalcite Using Rare-Earth Coupling Agent and Its Application in Polypropylene. Powder Technol. 2019, 342, 555–561. [Google Scholar] [CrossRef]
- Shen, H.; Liu, Y. One-Step Synthesis of Hydrophobic Magnesium Hydroxide Nanoparticles and Their Application in Flame-Retardant Polypropylene Composites. Chin. J. Chem. Eng. 2018, 26, 2199–2205. [Google Scholar] [CrossRef]
- Meucci, M.; Haveriku, S.; Badalassi, M.; Cardelli, C.; Ruggeri, G.; Pucci, A. Effect of Polyolefin Elastomers’ Characteristics and Natural Magnesium Hydroxide Content on the Properties of Halogen-Free Flame-Retardant Polyolefin Composites. Micro 2022, 2, 164–182. [Google Scholar] [CrossRef]
- Seidi, F.; Movahedifar, E.; Naderi, G.; Akbari, V.; Ducos, F.; Shamsi, R.; Vahabi, H.; Saeb, M.R. Flame Retardant Polypropylenes: A Review. Polymers 2020, 12, 1701. [Google Scholar] [CrossRef]
- Łopiński, J.; Schmidt, B.; Bai, Y.; Kowalczyk, K. Effect of the B:Zn:H2O Molar Ratio on the Properties of Poly(Vinyl Acetate) and Zinc Borate-Based Intumescent Coating Materials Exposed to a Quasi-Real Cellulosic Fire. Polymers 2020, 12, 2542. [Google Scholar] [CrossRef]
- Arogundade, A.I.; Megat-Yusoff, P.S.M.; Ahmad, F.; Bhat, A.H.; Afolabi, L.O. Modification of Bauxite Residue with Oxalic Acid for Improved Performance in Intumescent Coatings. J. Mater. Res. Technol. 2021, 12, 679–687. [Google Scholar] [CrossRef]
- Chaisaenrith, P.; Taksakulvith, P.; Pavasupree, S. Effect of Nano Titanium Dioxide in Intumescent Fireproof Coating on Thermal Performance and Char Morphology. Mater. Today Proc. 2021, 47, 3462–3467. [Google Scholar] [CrossRef]
- Yang, W.-J.; Wei, C.-X.; Lu, H.-D.; Yang, W.; Yuen, R.K.K. Flame Retardant Polyurethane Nanocomposites. In Materials and Chemistry of Flame-Retardant Polyurethanes Volume 1: A Fundamental Approach; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2021; Volume 1399, pp. 10–221. ISBN 9780841298026. [Google Scholar]
- Mahović Poljaček, S.; Tomašegović, T.; Leskovšek, M.; Stanković Elesini, U. Effect of SiO2 and TiO2 Nanoparticles on the Performance of UV Visible Fluorescent Coatings. Coatings 2021, 11, 928. [Google Scholar] [CrossRef]
- Ramesh, M.; Rajeshkumar, L.; Bhoopathi, R. Carbon Substrates: A Review on Fabrication, Properties and Applications. Carbon Lett. 2021, 31, 557–580. [Google Scholar] [CrossRef]
- Lee, S.; Kim, H.M.; Seong, D.G.; Lee, D. Synergistic Improvement of Flame Retardant Properties of Expandable Graphite and Multi-Walled Carbon Nanotube Reinforced Intumescent Polyketone Nanocomposites. Carbon 2019, 143, 650–659. [Google Scholar] [CrossRef]
- Zheng, Z.; Liu, Y.; Dai, B.; Meng, C.; Guo, Z. Fabrication of Cellulose-Based Halogen-Free Flame Retardant and Its Synergistic Effect with Expandable Graphite in Polypropylene. Carbohydr. Polym. 2019, 213, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Tang, B.; Chen, J.; Shan, L.; Gao, Y.; Yang, K.; Wang, Y.; Sun, K.; Fan, R.; Yu, J. Epoxy Composites with High Cross-Plane Thermal Conductivity by Constructing All-Carbon Multidimensional Carbon Fiber/Graphite Networks. Compos. Sci. Technol. 2021, 203, 108610. [Google Scholar] [CrossRef]
- Li, F.; Zhen, H.; Li, L.; Li, Y.; Wang, Q.; Cheng, X. A Template-Method Synthesis of Mesoporous-MgO/Expanded Graphite for Enhancing Thermal Properties of Methyl Palmitate-Lauric Acid Phase Change Materials. Mater. Today Energy 2022, 26, 100999. [Google Scholar] [CrossRef]
- Mahto, A.; Khandelwal, M. Expandable Graphite for Flame-Retardant Polyurethane Foams. In Materials and Chemistry of Flame-Retardant Polyurethanes Volume 2: Green Flame Retardants; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2021; Volume 1400, pp. 5–65. ISBN 9780841298002. [Google Scholar]
- Atanes, E.; Cuesta-García, B.; Nieto-Márquez, A.; Fernández-Martínez, F. A Mixed Separation-Immobilization Method for Soluble Salts Removal and Stabilization of Heavy Metals in Municipal Solid Waste Incineration Fly Ash. J. Environ. Manag. 2019, 240, 359–367. [Google Scholar] [CrossRef]
- Du, B.; Li, J.; Fang, W.; Liu, Y.; Yu, S.; Li, Y.; Liu, J. Characterization of Naturally Aged Cement-Solidified MSWI Fly Ash. Waste Manag. 2018, 80, 101–111. [Google Scholar] [CrossRef]
- Yakubu, Y.; Zhou, J.; Shu, Z.; Zhang, Y.; Wang, W.; Mbululo, Y. Potential Application of Pre-Treated Municipal Solid Waste Incineration Fly Ash as Cement Supplement. Environ. Sci. Pollut. Res. 2018, 25, 16167–16176. [Google Scholar] [CrossRef]
- Pivák, A.; Pavlíková, M.; Záleská, M.; Lojka, M.; Jankovský, O.; Pavlík, Z. Magnesium Oxychloride Cement Composites with Silica Filler and Coal Fly Ash Admixture. Materials 2020, 13, 2537. [Google Scholar] [CrossRef]
- Hossain, N.; Bhuiyan, M.A.; Pramanik, B.K.; Nizamuddin, S.; Griffin, G. Waste Materials for Wastewater Treatment and Waste Adsorbents for Biofuel and Cement Supplement Applications: A Critical Review. J. Clean. Prod. 2020, 255, 120261. [Google Scholar] [CrossRef]
- Quina, M.J.; Bontempi, E.; Bogush, A.; Schlumberger, S.; Weibel, G.; Braga, R.; Funari, V.; Hyks, J.; Rasmussen, E.; Lederer, J. Technologies for the Management of MSW Incineration Ashes from Gas Cleaning: New Perspectives on Recovery of Secondary Raw Materials and Circular Economy. Sci. Total Environ. 2018, 635, 526–542. [Google Scholar] [CrossRef]
- Marinina, O.; Nevskaya, M.; Jonek-Kowalska, I.; Wolniak, R.; Marinin, M. Recycling of Coal Fly Ash as an Example of an Efficient Circular Economy: A Stakeholder Approach. Energies 2021, 14, 3597. [Google Scholar] [CrossRef]
- Zanoletti, A.; Ciacci, L. The Reuse of Municipal Solid Waste Fly Ash as Flame Retardant Filler: A Preliminary Study. Sustainability 2022, 14, 2038. [Google Scholar] [CrossRef]
- Jiao, C.; Wang, H.; Chen, X. Preparation of Modified Fly Ash Hollow Glass Microspheres Using Ionic Liquids and Its Flame Retardancy in Thermoplastic Polyurethane. J. Therm. Anal. Calorim. 2018, 133, 1471–1480. [Google Scholar] [CrossRef]
- Nakonieczny, D.S.; Antonowicz, M.; Paszenda, Z. Surface Modification Methods of Ceramic Filler in Ceramic-Carbon Fibre Composites for Bioengineering Applications—A Systematic Review. Rev. Adv. Mater. Sci. 2020, 59, 586–605. [Google Scholar] [CrossRef]
- Kim, Y.; Hwang, S.; Choi, J.; Lee, J.; Yu, K.; Baeck, S.-H.; Shim, S.E.; Qian, Y. Valorization of Fly Ash as a Harmless Flame Retardant via Carbonation Treatment for Enhanced Fire-Proofing Performance and Mechanical Properties of Silicone Composites. J. Hazard. Mater. 2021, 404, 124202. [Google Scholar] [CrossRef] [PubMed]
- Murmu, A.L.; Dhole, N.; Patel, A. Stabilisation of Black Cotton Soil for Subgrade Application Using Fly Ash Geopolymer. Road Mater. Pavement Des. 2020, 21, 867–885. [Google Scholar] [CrossRef]
- Paciorek-Sadowska, J.; Czupryński, B.; Borowicz, M.; Liszkowska, J. Rigid Polyurethane–Polyisocyanurate Foams Modified with Grain Fraction of Fly Ashes. J. Cell. Plast. 2019, 56, 53–72. [Google Scholar] [CrossRef]
- Vigneshwaran, S.; Uthayakumar, M.; Arumugaprabu, V.; Deepak Joel Johnson, R. Influence of Filler on Erosion Behavior of Polymer Composites: A Comprehensive Review. J. Reinf. Plast. Compos. 2018, 37, 1011–1019. [Google Scholar] [CrossRef]
- Blanco, I.; Catauro, M. Geopolymers-Design, Preparation, and Applications. Polymers 2022, 14, 853. [Google Scholar] [CrossRef]
- Mohd Basri, M.S.; Yek, T.H.; Talib, R.A.; Mohamed Amin Tawakkal, I.S.; Kamarudin, S.H.; Mazlan, N.; Maidin, N.A.; Ab Rahman, M.H. Rice Husk Ash/Silicone Rubber-Based Binary Blended Geopolymer Coating Composite: Fire Retardant, Moisture Absorption, Optimize Composition, and Microstructural Analysis. Polymers 2021, 13, 985. [Google Scholar] [CrossRef]
- Danish, A.; Mosaberpanah, M.A. Formation Mechanism and Applications of Cenospheres: A Review. J. Mater. Sci. 2020, 55, 4539–4557. [Google Scholar] [CrossRef]
- Hanif, A.; Usman, M. Fly Ash Cenosphere: Characterization, Processing, and Properties. In Handbook of Fly Ash; Butterworth-Heinemann: Oxford, UK, 2021; p. 57. [Google Scholar]
- Yadav, V.K.; Yadav, K.K.; Tirth, V.; Jangid, A.; Gnanamoorthy, G.; Choudhary, N.; Islam, S.; Gupta, N.; Son, C.T.; Jeon, B.-H. Recent Advances in Methods for Recovery of Cenospheres from Fly Ash and Their Emerging Applications in Ceramics, Composites, Polymers and Environmental Cleanup. Crystals 2021, 11, 1067. [Google Scholar] [CrossRef]
- Zanjad, N.; Pawar, S.; Nayak, C. Use of Fly Ash Cenosphere in the Construction Industry: A Review. Mater. Today Proc. 2022, 62, 2185–2190. [Google Scholar] [CrossRef]
- Huang, Z.; Padmaja, K.; Li, S.; Liew, J.Y.R. Mechanical Properties and Microstructure of Ultra-Lightweight Cement Composites with Fly Ash Cenospheres after Exposure to High Temperatures. Constr. Build. Mater. 2018, 164, 760–774. [Google Scholar] [CrossRef]
- Perumal Arulselvan, S.; Vellalapalayam Gurusamy, S. Characterization of Fly Ash Cenosphere—Capric Acid Composite as Phase Change Material for Thermal Energy Storage in Buildings. Energy Sources Part A Recover. Util. Environ. Eff. 2022, 44, 2088–2102. [Google Scholar] [CrossRef]
- Liu, S.; Niu, Y.; Wen, L.; Liang, Y.; Yan, B.; Wang, D.; Hui, S. Experimental Studies of Ash Film Fractions Based on Measurement of Cenospheres Geometry in Pulverized Coal Combustion. Front. Energy 2021, 15, 91–98. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Liu, B.; Liang, P.; Zhang, Y. Effectiveness and Mechanism of Carbamide/Fly Ash Cenosphere with Bilayer Spherical Shell Structure as Explosion Suppressant of Coal Dust. J. Hazard. Mater. 2019, 365, 555–564. [Google Scholar] [CrossRef]
- Rybiński, P.; Syrek, B.; Bradło, D.; Żukowski, W.; Anyszka, R.; Imiela, M. Influence of Cenospheric Fillers on the Thermal Properties, Ceramisation and Flammability of Nitrile Rubber Composites. J. Compos. Mater. 2018, 52, 2815–2827. [Google Scholar] [CrossRef]




| Inert Gas Dilution | Thermal Quenching | Physical Dilution | Chemical Interaction | Protective Char |
|---|---|---|---|---|
|
|
|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohd Sabee, M.M.S.; Itam, Z.; Beddu, S.; Zahari, N.M.; Mohd Kamal, N.L.; Mohamad, D.; Zulkepli, N.A.; Shafiq, M.D.; Abdul Hamid, Z.A. Flame Retardant Coatings: Additives, Binders, and Fillers. Polymers 2022, 14, 2911. https://doi.org/10.3390/polym14142911
Mohd Sabee MMS, Itam Z, Beddu S, Zahari NM, Mohd Kamal NL, Mohamad D, Zulkepli NA, Shafiq MD, Abdul Hamid ZA. Flame Retardant Coatings: Additives, Binders, and Fillers. Polymers. 2022; 14(14):2911. https://doi.org/10.3390/polym14142911
Chicago/Turabian StyleMohd Sabee, Mohd Meer Saddiq, Zarina Itam, Salmia Beddu, Nazirul Mubin Zahari, Nur Liyana Mohd Kamal, Daud Mohamad, Norzeity Amalin Zulkepli, Mohamad Danial Shafiq, and Zuratul Ain Abdul Hamid. 2022. "Flame Retardant Coatings: Additives, Binders, and Fillers" Polymers 14, no. 14: 2911. https://doi.org/10.3390/polym14142911
APA StyleMohd Sabee, M. M. S., Itam, Z., Beddu, S., Zahari, N. M., Mohd Kamal, N. L., Mohamad, D., Zulkepli, N. A., Shafiq, M. D., & Abdul Hamid, Z. A. (2022). Flame Retardant Coatings: Additives, Binders, and Fillers. Polymers, 14(14), 2911. https://doi.org/10.3390/polym14142911








