Relation between Droplet Size Distributions and Physical Stability for Zein Microfluidized Emulsions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
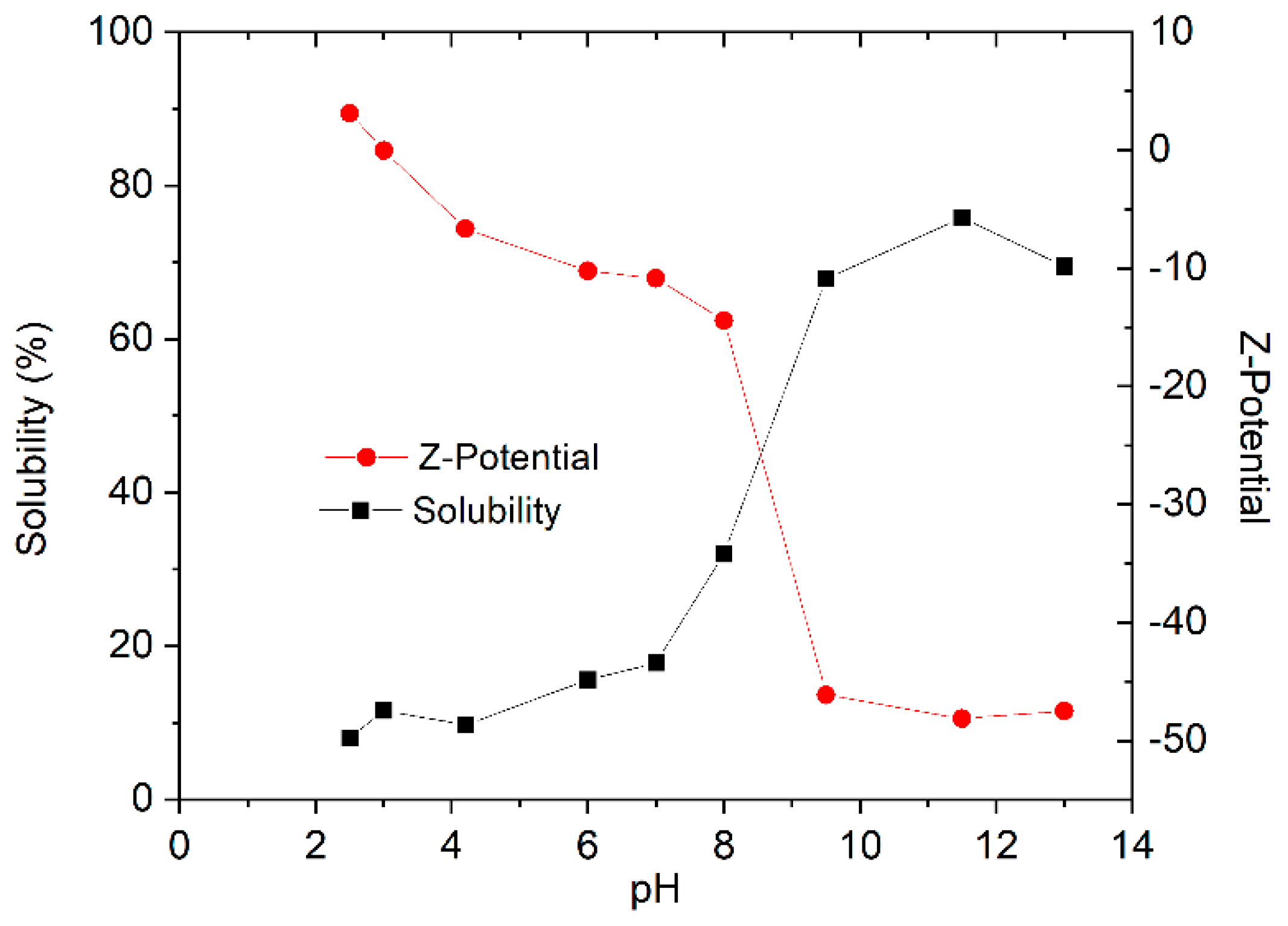
2.2. Functional Properties Determination: Solubility and Zeta Potential
2.3. Microfluidization of Food Emulsions Formulated with Zein Protein
2.4. Design of Experiments
2.5. Laser Diffraction Measurements
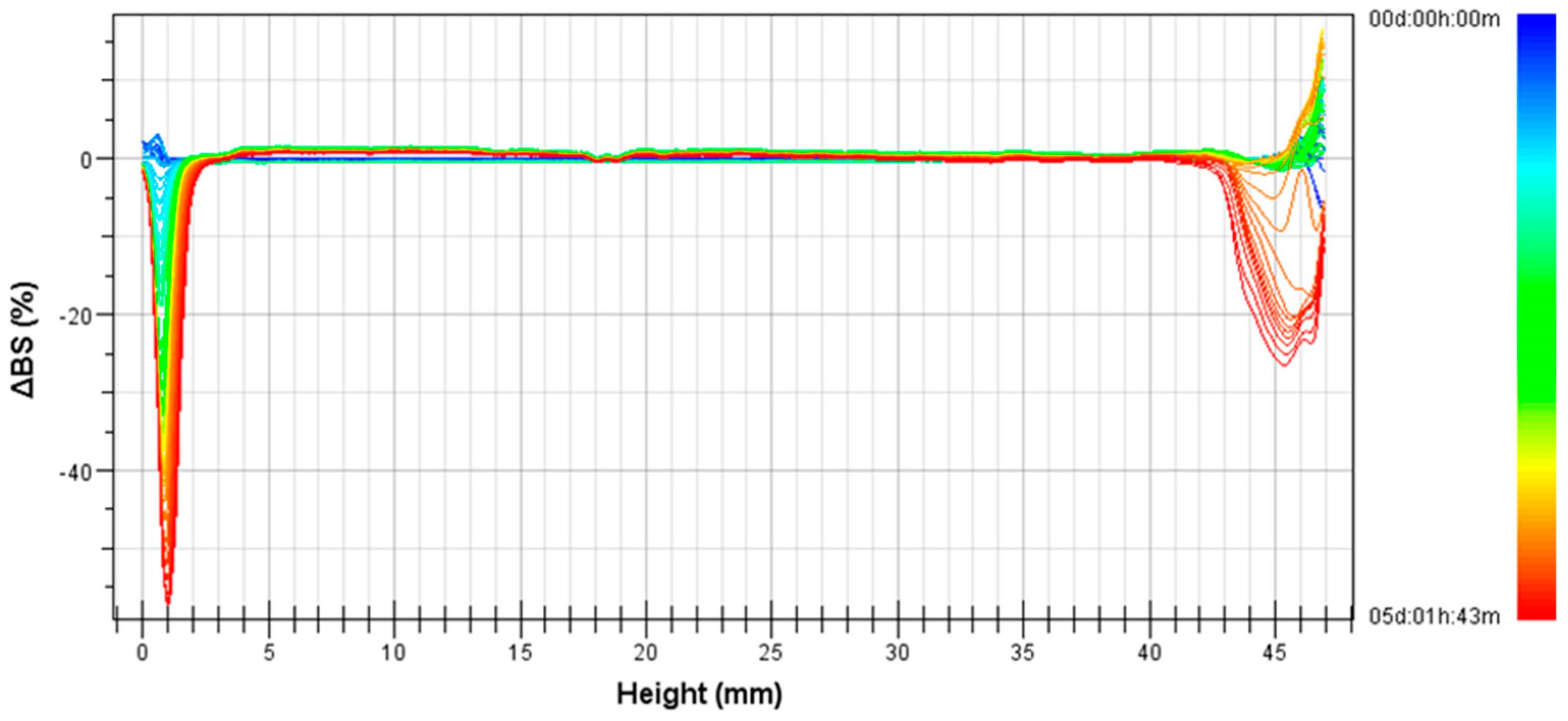
2.6. Multiple Light Scattering Technique
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chanamai, R.; McClements, D.J. Dependence of Creaming and Rheology of Monodisperse Oil-in-Water Emulsions on Droplet Size and Concentration. Colloids Surf. A Physicochem. Eng. Asp. 2000, 172, 79–86. [Google Scholar] [CrossRef]
- Gallegos, C.; Valencia, C.; Partal, P.; Franco, J.M.; Maglio, O.; Abrahamsson, M.; Brito-de la Fuente, E. Droplet-Size Distribution and Stability of Commercial Injectable Lipid Emulsions Containing Fish Oil. Am. J. Health-Syst. Pharm. 2012, 69, 1332–1335. [Google Scholar] [CrossRef] [PubMed]
- Martin-Piñero, M.J.; García, M.C.; Muñoz, J.; Alfaro-Rodriguez, M.-C. Influence of the Welan Gum Biopolymer Concentration on the Rheological Properties, Droplet Size Distribution and Physical Stability of Thyme Oil/W Emulsions. Int. J. Biol. Macromol. 2019, 133, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Giteru, S.G.; Ali, M.A.; Oey, I. Recent progress in understanding fundamental interactions and applications of zein. Food Hydrocoll. 2021, 120, 106948. [Google Scholar] [CrossRef]
- Li, M.; Yu, M. Development of a nanoparticle delivery system based on zein/polysaccharide complexes. J. Food Sci. 2021, 85, 4108–4117. [Google Scholar] [CrossRef]
- Champrasert, O.; Orfila, C.; Suwannaporn, P. Acrylamide mitigation using zein–polysaccharide complex particles. Food Hydrocoll. 2022, 124, 107317. [Google Scholar] [CrossRef]
- Altan, A.; Aytac, Z.; Uyar, T. Carvacrol Loaded Electrospun Fibrous Films from Zein and Poly (Lactic Acid) for Active Food Packaging. Food Hydrocoll. 2018, 81, 48–59. [Google Scholar] [CrossRef] [Green Version]
- Zou, Y.; van Baalen, C.; Yang, X.; Scholten, E. Tuning Hydrophobicity of Zein Nanoparticles to Control Rheological Behavior of Pickering Emulsions. Food Hydrocoll. 2018, 80, 130–140. [Google Scholar] [CrossRef]
- Kasaai, M.R. Zein and Zein-Based Nano-Materials for Food and Nutrition Applications: A Review. Trends Food Technol. 2018, 79, 184–197. [Google Scholar] [CrossRef]
- Li, M.; He, S. Utilization of Zein-Based Particles in Pickering Emulsions: A Review. Food Rev. Int. 2021, 1–21. [Google Scholar] [CrossRef]
- Santos, J.; Alcaide-González, M.A.; Trujillo-Cayado, L.A.; Carrillo, F.; Alfaro-Rodríguez, M.C. Development of Food-Grade Pickering Emulsions Stabilized by a Biological Macromolecule (Xanthan Gum) and Zein. Int. J. Biol. Macromol. 2020, 153, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Argos, P.; Pedersen, K.; Marks, M.D.; Larkins, B.A. A Structural Model for Maize Zein Proteins. J. Biol. Chem. 1982, 257, 9984–9990. [Google Scholar] [CrossRef]
- Cabra, V.; Arreguin, R.; Vazquez-Duhalt, R.; Farres, A. Effect of Alkaline Deamidation on the Structure, Surface Hydrophobicity, and Emulsifying Properties of the Z19 α-Zein. J. Agric. Food Chem. 2007, 55, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Rayner, M.; Dejmek, P. Engineering Aspects of Food Emulsification and Homogenization; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Ramos, O.L.; Pereira, R.N.; Cerqueria, M.A.; Teixeira, J.A.; Vicente, A.A. Advances in Processing Technologies for Bio-Based Nanosystems in Food; CRC Press: Boca Raton, FL, USA, 2019; Volume 1, ISBN 1351710052. [Google Scholar]
- Iordache, M.; Jelen, P. High Pressure Microfluidization Treatment of Heat Denatured Whey Proteins for Improved Functionality. Innov. Food Sci. Emerg. Technol. 2003, 4, 367–376. [Google Scholar] [CrossRef]
- Cikrikci, S.; Demirkesen, I.; Mert, B. Production of Hazelnut Skin Fibres and Utilisation in a Model Bakery Product. Qual. Assur. Saf. Crop. Foods 2016, 8, 195–206. [Google Scholar] [CrossRef]
- Chen, J.; Liang, R.-H.; Liu, W.; Liu, C.-M.; Li, T.; Tu, Z.-C.; Wan, J. Degradation of High-Methoxyl Pectin by Dynamic High Pressure Microfluidization and Its Mechanism. Food Hydrocoll. 2012, 28, 121–129. [Google Scholar] [CrossRef]
- Box, G.E.P.; Wilson, K.B. On the Experimental Attainment of Optimum Conditions. J. R. Stat. Soc. Ser. B Methodol. 1951, 13, 1–45. [Google Scholar] [CrossRef]
- Santos, J.; Trujillo-Cayado, L.A.; Alcaide, M.D.Á.; Alfaro, M.D.C. Impact of Microfluidization on the Emulsifying Properties of Zein-Based Emulsions: Influence of Diutan Gum Concentration. Materials 2021, 14, 3695. [Google Scholar] [CrossRef]
- Santos, J.; Trujillo-Cayado, L.A.; Carrello, H.; Cidade, M.T.; Alfaro, M.C. Optimization of sonication parameters to obtain food emulsions stabilized by zein: Formation of zein–diutan gum/zein–guar gum complexes. J. Sci. Food Agric. 2022, 102, 2127–2134. [Google Scholar] [CrossRef]
- Shi, L.-E.; Ying, G.-Q.; Zhang, X.-Y.; Tang, Z.-X.; Chen, J.-S.; Xiong, W.-Y.; Liu, H.-Z. Medium Optimization for 5′-Phosphodiesterase Production from Penicillium Citrinum Using Response Surface Methodology. Food Technol. Biotechnol. 2007, 45, 126–133. [Google Scholar]
- Margeta, D.; Faraguna, F.; Sertić Bionda, K.; Jukić, A. Modeling of Dibenzothiophene Oxidative Desulfurization Using Response Surface Methodology. Pet. Sci. Technol. 2018, 36, 68–74. [Google Scholar] [CrossRef]
- Mochi, V.T.; Pacheco, J.R.; Cremasco, M.A. Response Surface Methodology Applied to Ozone Generation. Ozone Sci. Eng. 2010, 32, 372–378. [Google Scholar] [CrossRef]
- Peterson, G.L. A Simplification of the Protein Assay Method of Lowry et al. Which Is More Generally Applicable. Anal. Biochem. 1977, 83, 346–356. [Google Scholar] [CrossRef]
- Tan, W.F.; Koopal, L.K.; Weng, L.P.; van Riemsdijk, W.H.; Norde, W. Humic Acid Protein Complexation. Geochim. Et Cosmochim. Acta 2008, 72, 2090–2099. [Google Scholar] [CrossRef]
- Shukla, R.; Cheryan, M. Zein: The Industrial Protein from Corn. Ind. Crop. Prod. 2001, 13, 171–192. [Google Scholar] [CrossRef]
- Wu, Y.V. Emulsifying activity and emulsion stability of corn gluten meal. J. Sci. Food Agric. 2001, 81, 1223–1227. [Google Scholar] [CrossRef]
- Jafari, S.M.; He, Y.; Bhandari, B. Production of Sub-Micron Emulsions by Ultrasound and Microfluidization Techniques. J. Food Eng. 2007, 82, 478–488. [Google Scholar] [CrossRef]
- Jafari, S.M.; He, Y.; Bhandari, B. Optimization of Nano-Emulsions Production by Microfluidization. Eur. Food Res. Technol. 2007, 225, 733–741. [Google Scholar] [CrossRef]
- Santos, J.; Trujillo-Cayado, L.A.; Calero, N.; Muñoz, J. Physical Characterization of Eco-Friendly O/W Emulsions Developed through a Strategy Based on Product Engineering Principles. AIChE J. 2014, 60, 2644–2653. [Google Scholar] [CrossRef]

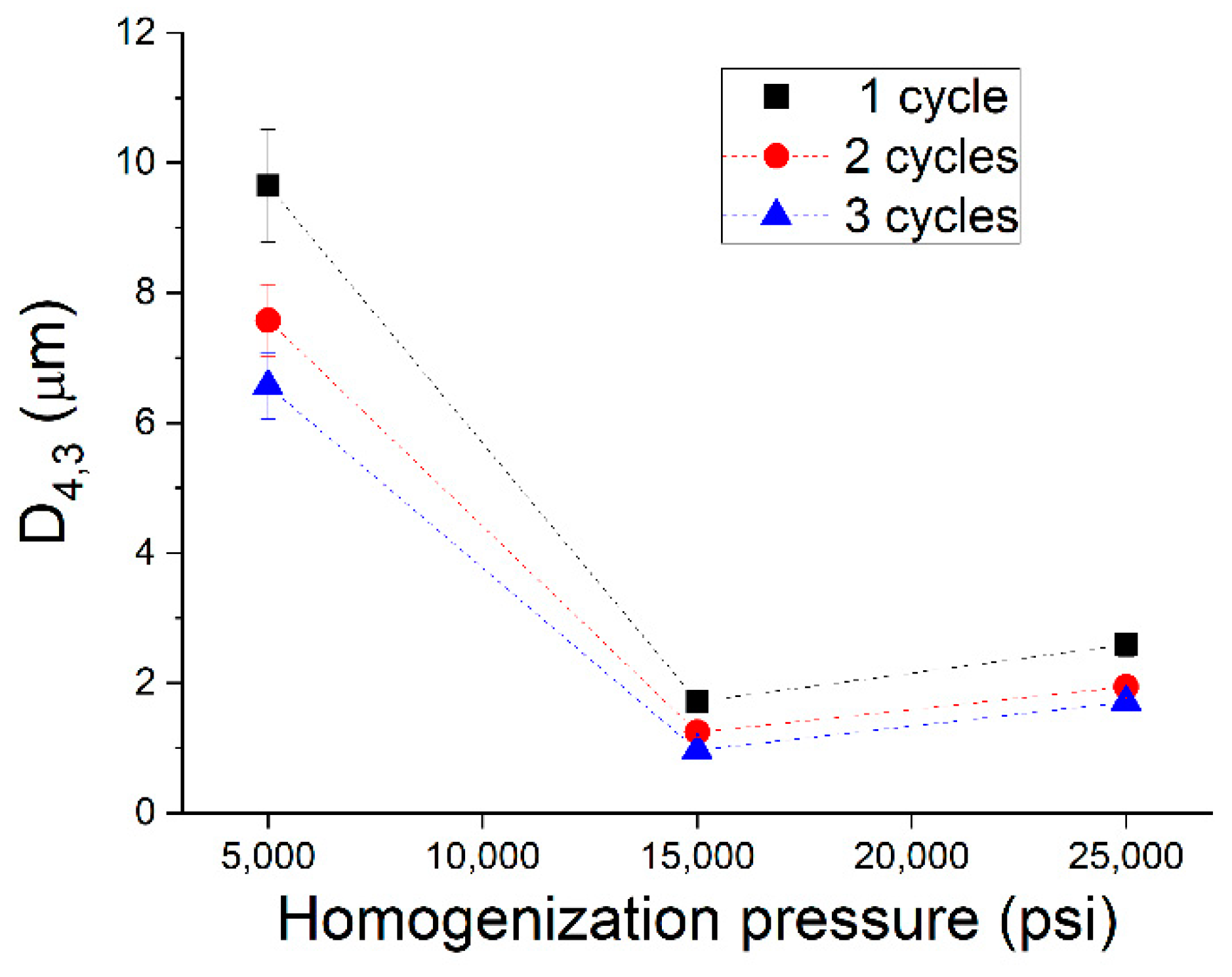
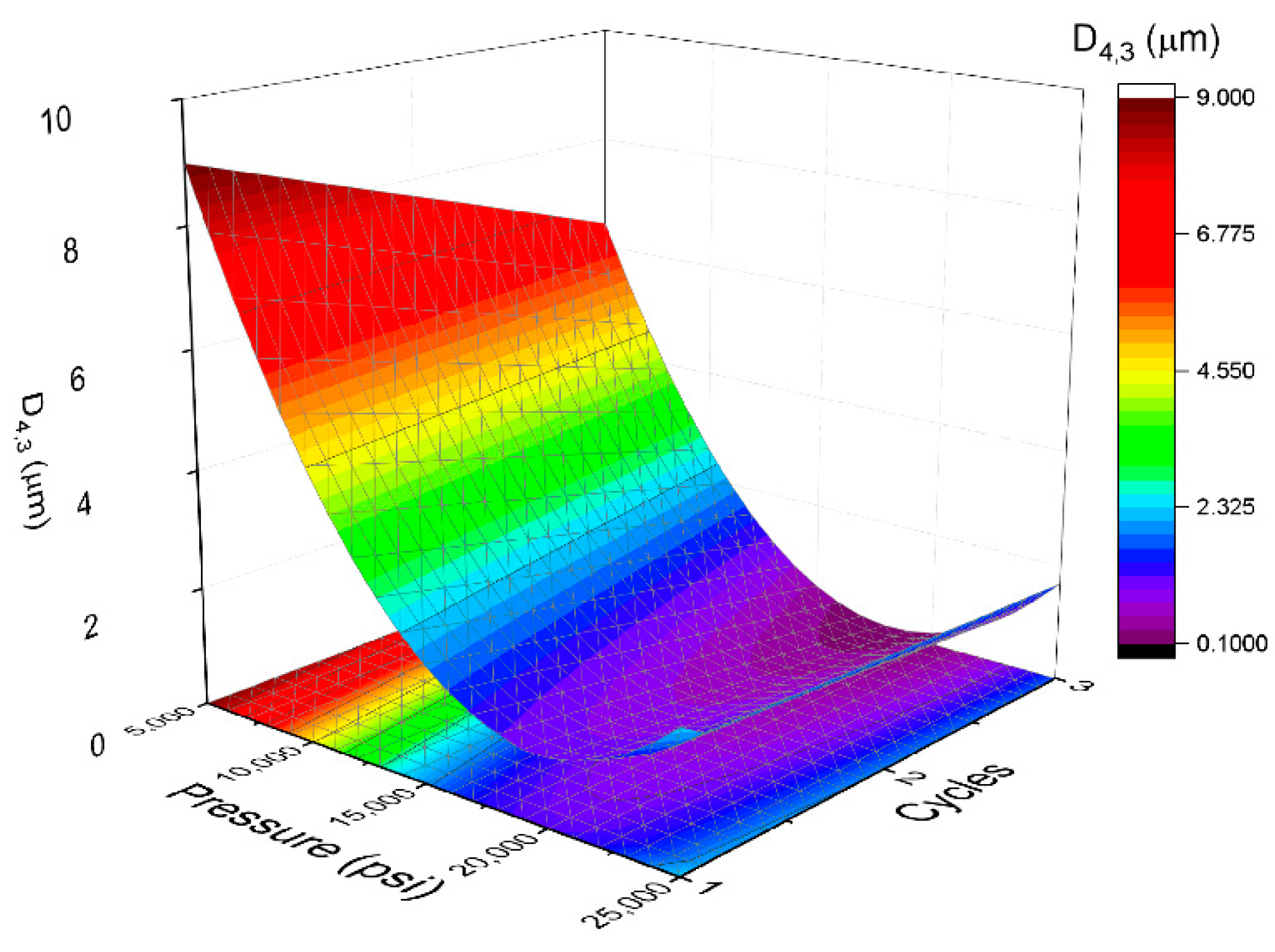



| Sample | X1 | X2 | Pressure | Cycles | D4,3 (μm) | span | TSI |
|---|---|---|---|---|---|---|---|
| 1 | −1 | −1 | 5000 | 1 | 9.65 ± 0.87 | 1.980 | 16.35 |
| 2 | −1 | 0 | 5000 | 2 | 7.58 ± 0.55 | 1.925 | 13.51 |
| 3 | −1 | 1 | 5000 | 3 | 6.57 ± 0.51 | 1.718 | 13.97 |
| 4 | 0 | −1 | 15,000 | 1 | 1.72 ± 0.11 | 1.015 | 6.22 |
| 5 | 0 | 0 | 15,000 | 2 | 1.22 ± 0.07 | 1.051 | 6.02 |
| 6 | 0 | 0 | 15,000 | 2 | 1.25 ± 0.09 | 1.071 | 6.09 |
| 7 | 0 | 0 | 15,000 | 2 | 1.27 ± 0.08 | 1.06 | 6.05 |
| 8 | 0 | 1 | 15,000 | 3 | 0.97 ± 0.06 | 1.629 | 6.10 |
| 9 | 1 | −1 | 25,000 | 1 | 2.59 ± 0.18 | 1.228 | 6.75 |
| 10 | 1 | 0 | 25,000 | 2 | 1.94 ± 0.16 | 1.455 | 6.58 |
| 11 | 1 | 1 | 25,000 | 3 | 1.71 ± 0.13 | 1.702 | 6.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, J.; Trujillo-Cayado, L.A.; Carrillo, F.; López-Castejón, M.L.; Alfaro-Rodríguez, M.C. Relation between Droplet Size Distributions and Physical Stability for Zein Microfluidized Emulsions. Polymers 2022, 14, 2195. https://doi.org/10.3390/polym14112195
Santos J, Trujillo-Cayado LA, Carrillo F, López-Castejón ML, Alfaro-Rodríguez MC. Relation between Droplet Size Distributions and Physical Stability for Zein Microfluidized Emulsions. Polymers. 2022; 14(11):2195. https://doi.org/10.3390/polym14112195
Chicago/Turabian StyleSantos, Jenifer, Luis Alfonso Trujillo-Cayado, Francisco Carrillo, María Luisa López-Castejón, and María Carmen Alfaro-Rodríguez. 2022. "Relation between Droplet Size Distributions and Physical Stability for Zein Microfluidized Emulsions" Polymers 14, no. 11: 2195. https://doi.org/10.3390/polym14112195
APA StyleSantos, J., Trujillo-Cayado, L. A., Carrillo, F., López-Castejón, M. L., & Alfaro-Rodríguez, M. C. (2022). Relation between Droplet Size Distributions and Physical Stability for Zein Microfluidized Emulsions. Polymers, 14(11), 2195. https://doi.org/10.3390/polym14112195








