Cryo-Structuring of Polymeric Systems. Poly(Vinyl Alcohol)-Based Cryogels Loaded with the Poly(3-hydroxybutyrate) Microbeads and the Evaluation of Such Composites as the Delivery Vehicles for Simvastatin †
Abstract
:1. Introduction
2. Results and Discussion
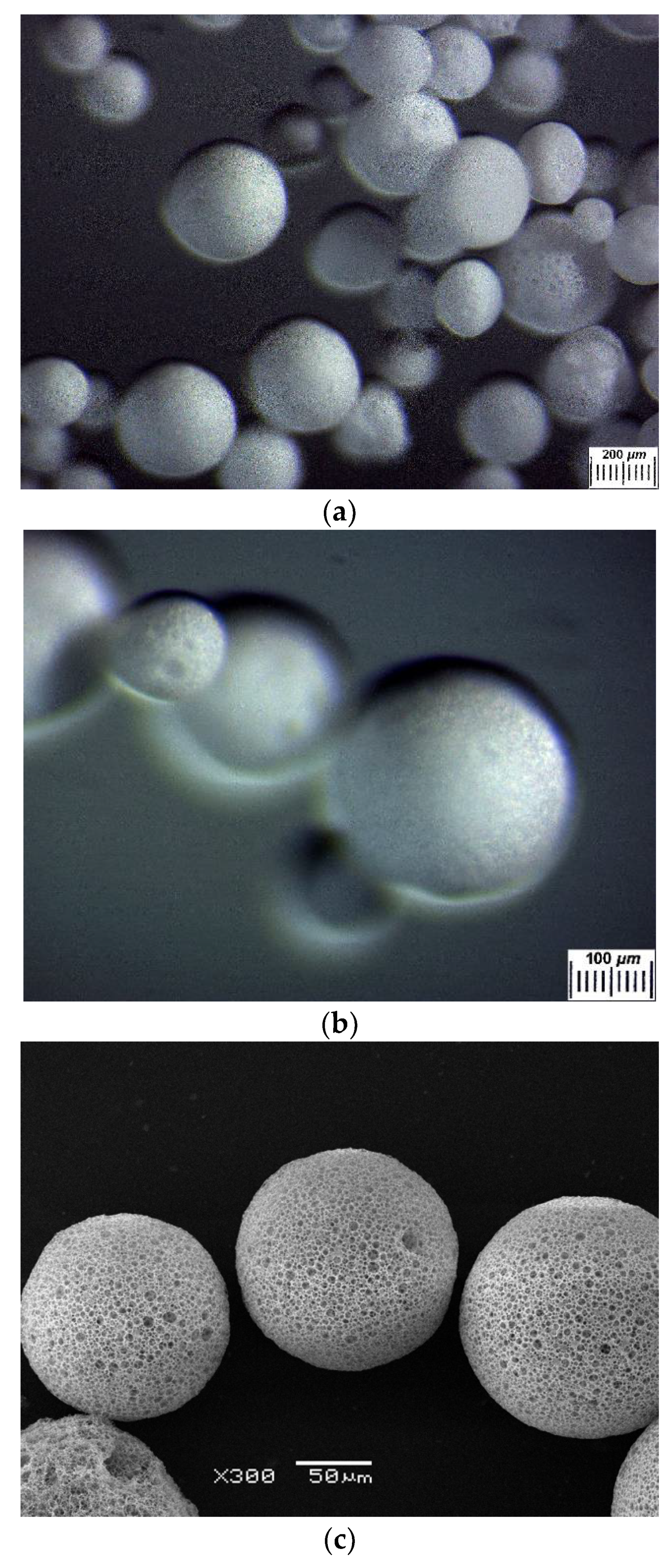
2.1. Preparation of Composite PVA Cryogels Filled with the PHB-Based Microbeads
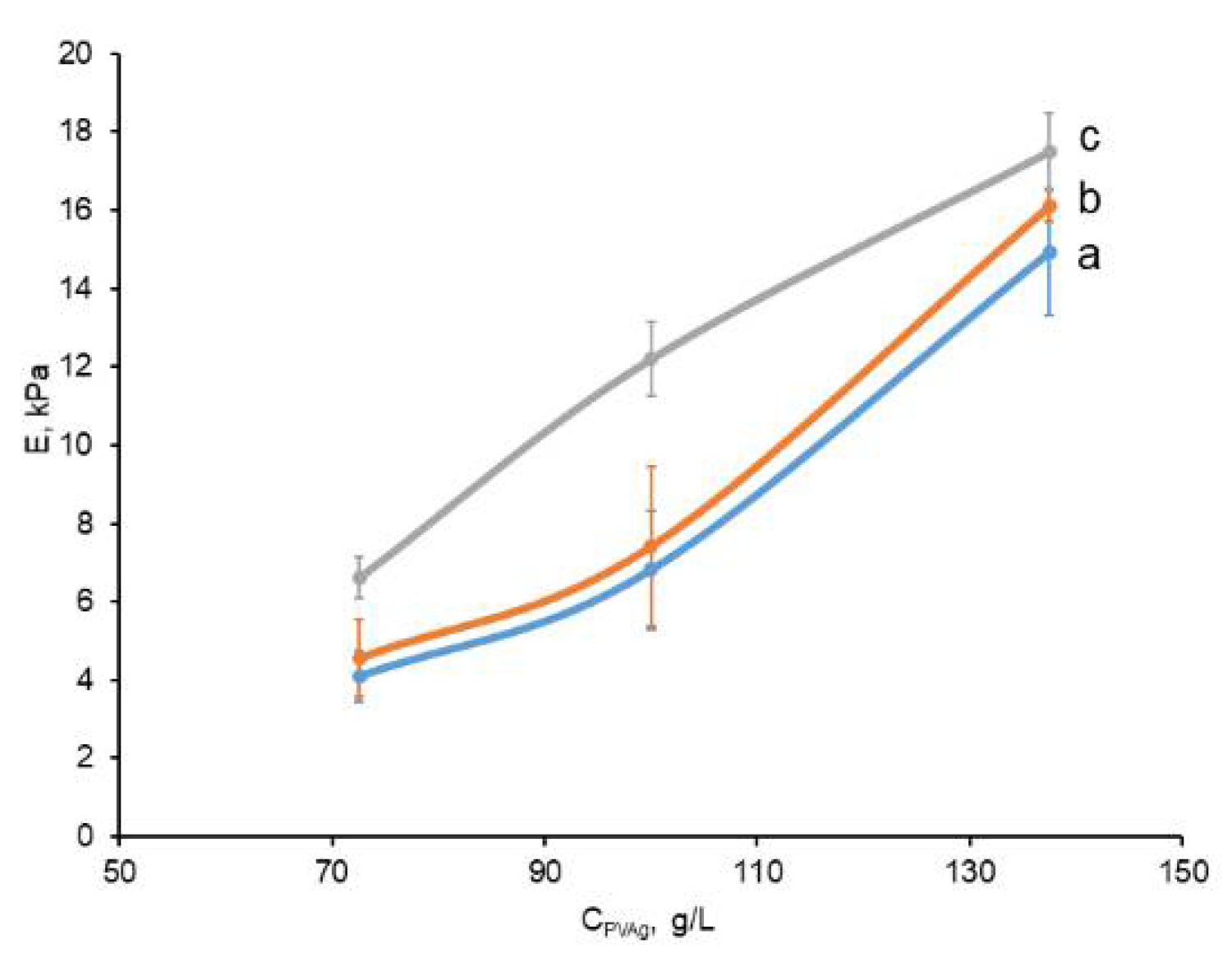
2.2. Physico-Mechanical Characteristics of the Prepared Non-Filled and Composite PVA Cryogels
2.3. Structural Features of the Composite PVA Cryogels
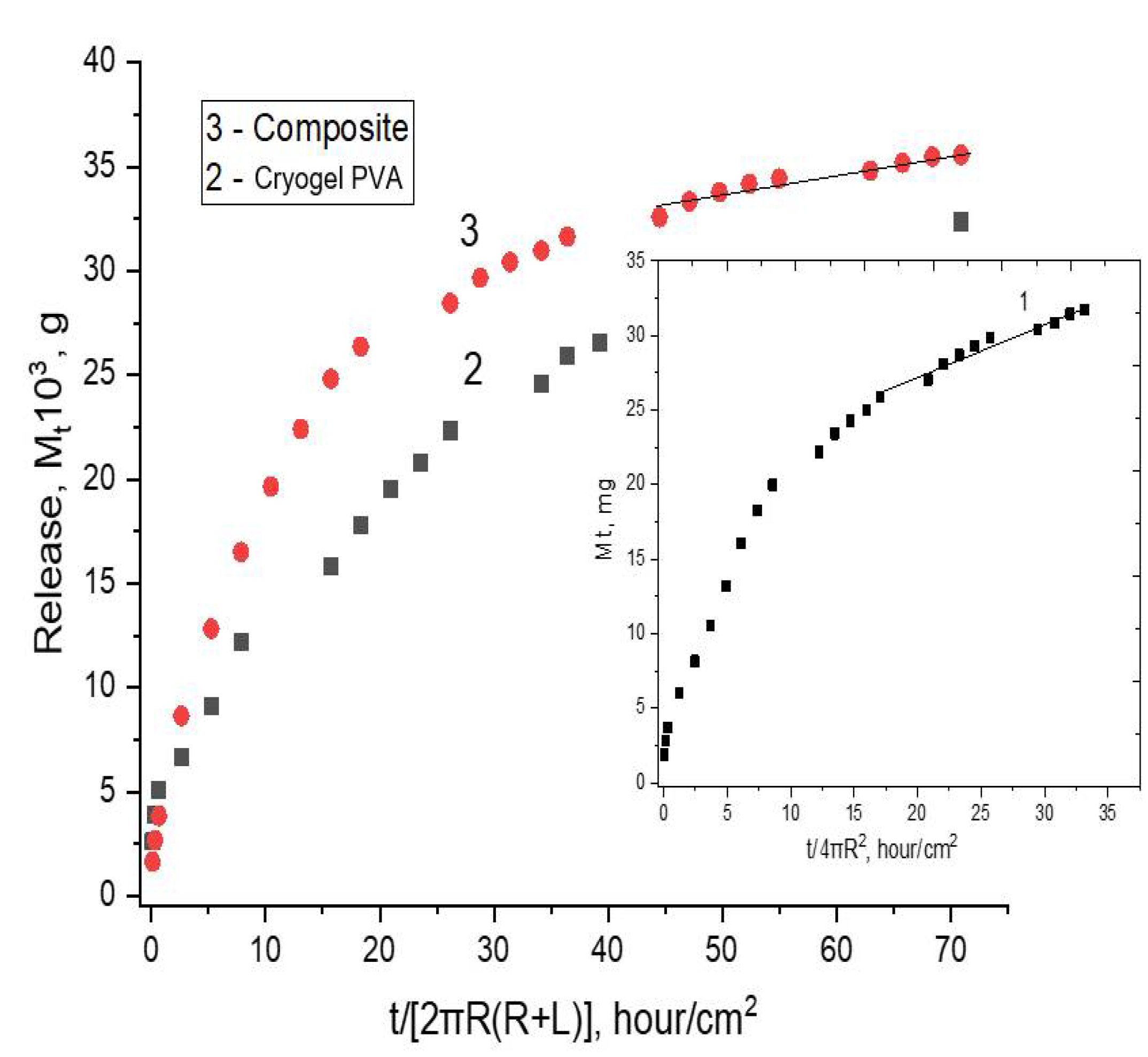
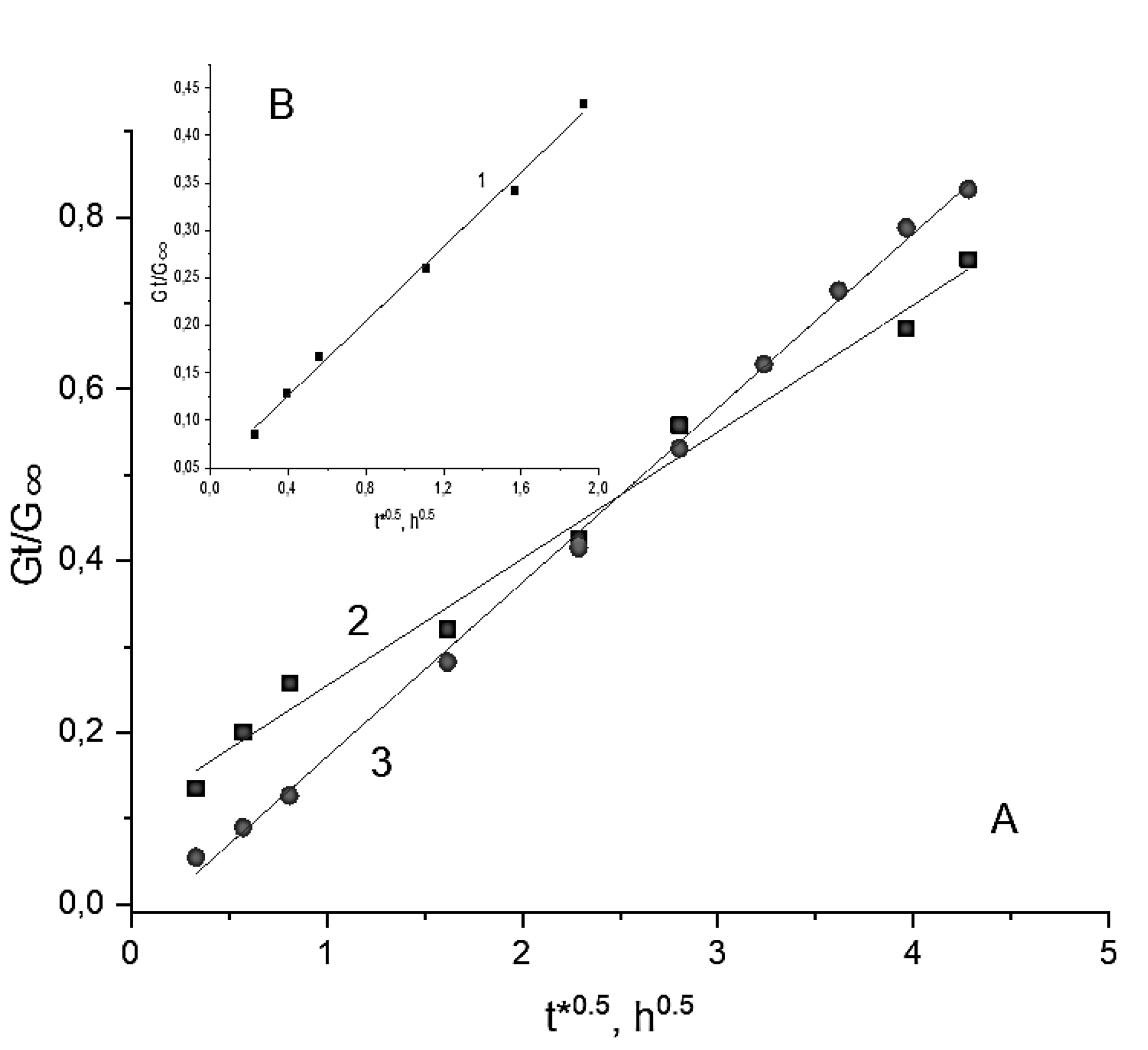
2.4. Loading and Release of the Simvastatin in, and from, the cPVACGs
3. Experimental
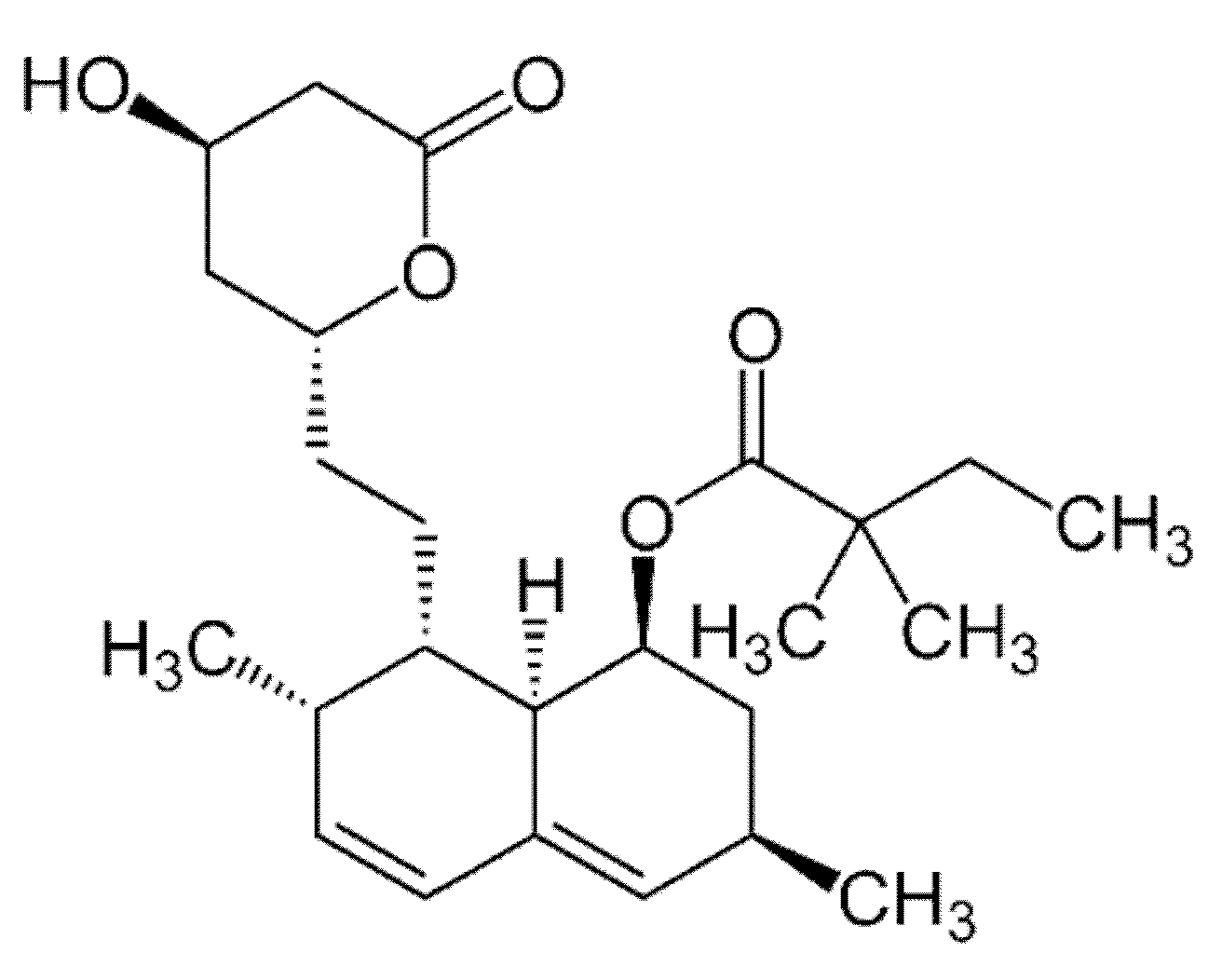
3.1. Materials
3.2. Methods
3.2.1. “Empty” and Loaded with Simvastatin Poly(3-hydroxybutyrate) Microbeads
3.2.2. Composite PVA Cryogels Filled with PHB-Based Microbeads
3.2.3. Physico-Mechanical Characteristics of Composite and Filler-Free PVA Cryogels
3.2.4. Optical Microscopy and SEM Studies
3.2.5. Simvastatin Release
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nambu, M. Rubber-like poly(vinyl alcohol) gel. Kobunshi Ronbunshu 1990, 47, 695–703. (In Japanese) [Google Scholar] [CrossRef]
- Peppas, N.A.; Stauffer, S.R. Reinforced uncrosslinked poly (vinyl alcohol) gels produced by cyclic freezing-thawing processes: A short review. J. Control. Release 1991, 16, 305–310. [Google Scholar] [CrossRef]
- Lozinsky, V.I. Cryotropic gelation of poly(vinyl alcohol) solutions. Russ. Chem. Rev. 1998, 67, 573–586. [Google Scholar] [CrossRef]
- Hassan, C.M.; Peppas, N.A. Structure and applications of poly(vinyl alcohol) hydrogels produced by conventional crosslinking or by freezing/thawing methods. Adv. Polym. Sci. 2000, 153, 37–65. [Google Scholar] [CrossRef]
- Lozinsky, V.I. Cryogels on the basis of natural and synthetic polymers: Preparation, properties and areas of implementation. Russ. Chem. Rev. 2002, 71, 489–511. [Google Scholar] [CrossRef]
- Gutiérrez, M.C.; Aranaz, I.; Ferrer, M.L.; del Monto, F. Production and properties of poly(vinyl alcohol) cryogels: Recent developments. In Macroporous Polymers: Production, Properties and Biological/Biomedical Applications; Mattiasson, B., Kumar, A., Galaev, I., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 83–115. ISBN 978-1-4200-8461-0. [Google Scholar]
- Alves, M.H.; Jensen, B.E.B.; Smith, A.A.A.; Zelikin, A.N. Poly(vinyl alcohol) physical hydrogels: New vista on a long serving biomaterial. Macromol. Biosci. 2011, 11, 1293–1313. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Okay, O. Basic principles of cryotropic gelation. Adv. Polym. Sci. 2014, 263, 49–102. [Google Scholar] [CrossRef]
- De Rosa, C.; Auriemma, F.; Girolamo, R.D. Kinetic analysis of cryotropic gelation of poly(vinyl alcohol)/water solutions by small-angle neutron scattering. Adv. Polym. Sci. 2014, 263, 159–197. [Google Scholar] [CrossRef]
- Chu, K.C.; Rutt, B.K. Poly(vinyl alcohol) cryogel: An ideal phantom material for MR studies of arterial flow and elasticity. Magn. Reson. Med. 1997, 37, 314–319. [Google Scholar] [CrossRef]
- Hoskins, P.R. Simulation and validation of arterial ultrasound imagining and blood flow. Ultrasound Med. Biol. 2008, 34, 693–717. [Google Scholar] [CrossRef]
- Ghanbari, H.; Viatage, H.; Kidane, A.G.; Burriesci, G.; Tavakoli, M.; Seifalian, A.M. Polymeric heart valves: New materials, emerging hopes. Trends Biotechnol. 2009, 27, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.I.; Walsh, S.P.; Schwatz, Z.; Boyan, B.D. A review of polyvinyl alcohol and its uses in cartilage and orthopedic applications. J. Biomed. Mater. Res. B 2012, 100, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Gajra, B.; Pandya, S.S.; Vidyasagar, G.; Rabari, H.; Dedania, R.R.; Rao, S. Poly(vinyl alcohol) hydrogel and its pharmaceutical and biomedical applications: A review. Int. J. Pharm. Res. 2012, 4, 20–26. [Google Scholar]
- Wan, W.; Bannerman, A.D.; Yang, L.; Mak, H. Poly(vinyl alcohol) cryogels for biomedical applications. Adv. Polym. Sci. 2014, 263, 283–321. [Google Scholar] [CrossRef]
- Teodorescu, M.; Bercea, M.; Morariu, S. Biomaterials of poly(vinyl alcohol) and natural polymers. Polym. Rev. 2018, 58, 247–287. [Google Scholar] [CrossRef]
- Lozinsky, V.I. Cryostructuring of polymeric systems as a tool for the creation of innovative materials of biomedical purposes. In Synthesis and Functional Properties of Hydrid Nanoforms of Bioactive and Drug Substances; Melnikov, M.Y., Trakhtenberg, L.I., Eds.; Tekhnosfera Publishing House: Moscow, Russia, 2019; Chapter 3; pp. 68–100. ISBN 978-5-94836-561_3. (In Russian) [Google Scholar]
- Memic, A.; Colombani, T.; Eggermont, L.J.; Rezaeeyazdi, M.; Steingold, J.; Rogers, Z.J.; Navare, K.J.; Mohammed, H.S.; Bencherif, S.A. Latest advances in cryogel technology for biomedical applications. Adv. Ther. 2019, 2, 1800114. [Google Scholar] [CrossRef] [Green Version]
- Lozinsky, V.I. Cryostructuring of polymeric systems. 55. Retrospective view on the more than 40-years studies performed in the A.N.Nesmeyanov Institute of Organoelement Compounds with respect of the cryostructuring processes in polymeric systems. Gels 2020, 6, 29. [Google Scholar] [CrossRef]
- Eigel, D.; Werner, C.; Newland, B. Cryogel biomaterials for neuroscience applications. Neurochem. Int. 2021, 147, 105012. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Vakula, A.V.; Zubov, A.L. Application of poly(vinyl alcohol) cryogels in biotechnology. IV. Literature data overview. Sov. Biotechnol. 1992, 4, 4–17. [Google Scholar]
- Varfolomeev, S.D.; Rainina, E.I.; Lozinsky, V.I. Cryoimmobilized enzymes and cells in organic synthesis. Pure Appl. Chem. 1992, 64, 1193–1196. [Google Scholar] [CrossRef] [Green Version]
- Lozinsky, V.I.; Plieva, F.M. Poly(vinyl alcohol) cryogels employed as matrices for cell immobilization. 3. Overview of recent research and developments. Enzym. Microb. Technol. 1998, 23, 227–242. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Plieva, F.M.; Galaev, I.Y.; Mattiasson, B. The potential of polymeric cryogels in bioseparation. Bioseparation 2001, 10, 163–188. [Google Scholar] [CrossRef] [PubMed]
- Bacheva, A.V.; Plieva, F.M.; Lysogorskaya, E.N.; Filippova, I.Y.; Lozinsky, V.I. Peptide synthesis in organic media with subtilisin 72 immobilized on poly(vinyl alcohol)-cryogel carrier. Bioorg. Med. Chem. Lett. 2001, 11, 1005–1008. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Galaev, I.Y.; Plieva, F.M.; Savina, I.N.; Jungvid, H.; Mattiasson, B. Polymeric cryogels as promising materials of biotechnological interest. Trends Biotechnol. 2003, 21, 445–451. [Google Scholar] [CrossRef]
- Plieva, F.M.; Galaev, I.Y.; Noppe, W.; Mattiasson, B. Cryogel applications in microbiology. Trends Microbiol. 2008, 16, 543–551. [Google Scholar] [CrossRef]
- Mattiasson, B. Cryogels for biotechnological applications. Adv. Polym. Sci. 2014, 263, 245–282. [Google Scholar] [CrossRef]
- Berillo, D.; Al-Jwaid, A.; Caplin, J. Polymeric materials used for immobilization of bacteria for the bioremediation of contaminants in water. Polymers 2021, 13, 1073. [Google Scholar] [CrossRef]
- Inoue, T. Gelled Vinyl Alcohol Polymers and Articles Therefrom. US Patent 3,875,302A, 1 April 1975. [Google Scholar]
- Ikoma, S.; Nomoto, E.; Yokoi, H. Preparation and some properties of poly(vinyl alcohol)-silica composite hydrogels. Kobunshi Ronbunshu 1990, 47, 1001–1004. [Google Scholar] [CrossRef] [Green Version]
- Lozinsky, V.I.; Zubov, A.I.; Kulakova, V.K.; Titova, E.F.; Rogozhin, S.V. Study of cryostructurization of polymer systems. IX. Poly(vinyl alcohol) cryogels filled with particles of cross-linked dextran gel. J. Appl. Polym. Sci. 1992, 44, 1423–1435. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Zubov, A.L.; Titova, E.I. Poly(vinyl alcohol) cryogels which are used as matrices for cell immobilization. 2. Entrapped cells resemble porous fillers in their effects on the properties of PVA-cryogel carrier. Enzym. Microb. Technol. 1997, 20, 182–190. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Savina, I.N. Study of cryostructuration of polymer systems. 22. Poly (vinyl alcohol) composite cryogels filled with dispersed particles of various hydrophilicity/hydrophobicity. Colloid J. 2002, 64, 336–343. [Google Scholar] [CrossRef]
- Savina, I.N.; Lozinsky, V.I. Study of cryostructuration of polymer systems. 23. Poly(vinyl alcohol) composite cryogels filled with dispersed particles that contain ionogenic groups. Colloid J. 2004, 66, 343–349. [Google Scholar] [CrossRef]
- Savina, E.N.; Hanora, A.; Plieva, F.M.; Galaev, I.Y.; Mattiasson, B.; Lozinsky, V.I. Study of cryostructuration of polymer systems. 24. Poly(vinyl alcohol) cryogels filled with particles of strong anion-exchanger: Properties of the composite materials and potential application. J. Appl. Polym. Sci. 2005, 95, 529–538. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Bakeeva, I.V.; Presnyak, E.P.; Damshkaln, L.G.; Zubov, V.P. Study of cryostructuring of polymer systems. 26. Heterophase organic-inorganic cryogels prepared via freezing-thawing of aqueous solutions of poly(vinyl alcohol) with added tetramethoxysilane. J. Appl. Polym. Sci. 2007, 105, 2689–2702. [Google Scholar] [CrossRef]
- Kokabi, M.; Sirousazar, M.; Hassan, Z.M. PVA-clay nanocomposite hydrogels for wound dressing. Eur. Polym. J. 2007, 43, 773–781. [Google Scholar] [CrossRef]
- Pan, Y.; Xiong, D.; Chen, X. Mechanical properties of nanohydroxyapatite reinforced poly(vinyl alcohol) gel composites as biomaterial. J. Mater. Sci. 2007, 42, 5129–5134. [Google Scholar] [CrossRef]
- Millon, L.E.; Oates, C.J.; Wan, W. Compression properties of polyvinyl alcohol–bacterial cellulose nanocomposite. J. Biomed. Mater. Res. 2009, 90, 922–929. [Google Scholar] [CrossRef]
- Podorozhko, E.A.; Korlyukov, A.A.; Lozinsky, V.I. Cryostructuring of polymer systems. 30. Poly(vinyl alcohol)-based composite cryogels filled with small disperse oil droplets: A gel system capable of mechanically-induced releasing of the lipophilic constituents. J. Appl. Polym. Sci. 2010, 117, 1332–1349. [Google Scholar] [CrossRef]
- Maiolo, A.S.; Amado, M.N.; Gonzalez, J.S.; Alvarez, V.A. Development and characterization of poly(vinyl alcohol) hydrogels for potential use as an articular cartilage replacement. Mater. Sci. Eng. C 2012, 32, 1490–1495. [Google Scholar] [CrossRef]
- Podorozhko, E.A.; D’yakonova, E.A.; Kolosova, O.Y.; Klabukova, L.F.; Lozinsky, V.I. Study of cryostructuring of polymer systems. 34. Composite poly(vinyl alcohol) cryogels filled with microparticles of polymeric dispersion. Colloid J. 2012, 74, 711–719. [Google Scholar] [CrossRef]
- Podorozhko, E.A.; D’yakonova, E.A.; Lozinsky, V.I. Cryostructuring of polymeric systems. 37. Composite cryogels from dispersions of poly(butadiene-co-styrene) latex in aqueous poly(vinyl alcohol) solution. Colloid J. 2015, 77, 46–57. [Google Scholar] [CrossRef]
- Timofejeva, A.; D’Este, M.; Loca, D. Calcium phosphate/polyvinyl alcohol composite hydrogels: A review on the freeze-thaw synthesis approach and applications in regenerative medicine. Eur. Polym. J. 2017, 95, 547–565. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Podorozhko, E.A.; Nikitina, Y.B.; Klabukova, L.F.; Vasil’iev, V.G.; Burmistrov, A.A.; Kondrashov, Y.G.; Vasiliev, N.K. A study of cryostructuring of polymeric systems. 45. Effect of porosity of dispersed filler on physicochemical characteristics of composite poly(vinyl alcohol) cryogels. Colloid J. 2017, 79, 497–507. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Cui, J.; Li, S.; Yuan, M.; Wang, T.; Hu, Q. Fabrication and characterization of metal organic frameworks/poly(vinyl alcohol) cryogel and their application in extraction of non-steroidal anti-inflammatory drugs in water samples. Anal. Chim. Acta 2018, 1022, 45–52. [Google Scholar] [CrossRef]
- Altunina, L.K.; Fufaeva, M.S.; Manzhai, V.N.; Bondaletov, V.G.; Fisenko, D.V. Effect of an oxidized polymeric petroleum resin on the properties of cryogels. Chem. Sustain. Dev. 2019, 27, 120–125. [Google Scholar] [CrossRef]
- Reguieg, F.; Ricci, L.; Bouyacoub, N.; Belbachir, M.; Bertoldo, M. Thermal characterization by DSC and TGA analyses of PVA hydrogels with organic and sodium MMT. Polym. Bull. 2020, 77, 929–948. [Google Scholar] [CrossRef]
- Bakeeva, I.V.; Doktorova, A.V.; Damshkaln, L.G.; Lozinsky, V.I. A study of cryostructuring of polymer systems. 54. Hybrid organo-inorganic poly(vinyl alcohol) cryogels filled with in situ formed silica. Colloid J. 2021, 83, 49–63. [Google Scholar] [CrossRef]
- Tamer, T.M.; Sabet, M.M.; Omer, A.M.; Abbas, E.; Eid, A.I.; Mohy-Eldin, M.S.; Hassan, M.A. Hemostatic and antibacterial PVA/kaolin composite sponges loaded with penicillin-streptomycin for wound dressing applications. Sci. Rep. 2021, 11, 3428. [Google Scholar] [CrossRef]
- Cascone, M.G.; Zhu, Z.H.; Borselli, F. Poly(vinyl alcohol) hydrogels as hydrophilic matrices for the release of lipophilic drugs loaded in PLGA nanoparticles. J. Mater. Sci. Mater. Med. 2002, 13, 29–32. [Google Scholar] [CrossRef]
- Mandal, T.K.; Bostanian, L.A.; Graves, R.A.; Chapman, S.R. Poly(d,l-lactide-co-glycolide) encapsulated poly(vinyl alcohol) hydrogel as a drug delivery system. Pharm. Res. 2002, 19, 1713–1719. [Google Scholar] [CrossRef]
- Galeska, I.; Kim, T.K.; Patil, S.D.; Bhardwaj, U.; Chattopadhyay; Papadimitrakopoulos, F.; Burgess, D.J. Controlled release of dexamethasone from PLGA microspheres embedded within polyacid-containing PVA hydrogels. AAPS J. 2006, 7, E231–E240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Zhang, S.M.; Chen, P.P.; Cheng, L.; Zhou, W.; Tang, W.X.; Chen, Z.W.; Ke, C.M. Controlled release of insulin from PLGA nanoparticles embedded within PVA hydrogels. J. Mater. Sci. Mater. Med. 2007, 18, 2205–2210. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Bae, H.; Chung, N.; Lee, H.; Choi, S.; Hwang, S.; Lee, J. Freezing/thawing processing of PVA in the preparation of structured microspheres for protein drug delivery. Macromol. Res. 2011, 19, 130–136. [Google Scholar] [CrossRef]
- Gomes, C.; Moreira, R.G.; Castell-Perez, E. Poly(DL-lactide-co-glycolide) (PLGA) nanoparticles with entrapped trans-cinnamaldehyde and eugenol for antimicrobial delivery applications. J. Food Sci. 2011, 76, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Spiller, K.L.; Liu, Y.; Holloway, J.L.; Maher, S.A.; Cao, Y.; Liu, W.; Zhou, G.; Lowman, A.M. A novel method for the direct fabrication of growth factor-loaded microspheres within porous nondegradable hydrogels: Controlled release for cartilage tissue engineering. J. Control. Release 2012, 157, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Mokhtarzadeh, A.; Alibakhshi, A.; Hejazi, M.; Omidi, Y.; Dolatabadi, J.E.N. Bacterial-derived biopolymers: Advanced natural nanomaterials for drug delivery and tissue engineering. TrAC-Trend Anal. Chem. 2016, 82, 367–384. [Google Scholar] [CrossRef]
- Bonartsev, A.P.; Bonartseva, G.A.; Shaitan, K.V.; Kirpichnikov, M.P. Poly(3-hydroxybutyrate) and poly(3-hydroxybutyrate)-based biopolymer systems. Biochem. Suppl. Ser. B Biomed. Chem. 2011, 5, 10–21. [Google Scholar] [CrossRef]
- Bonartsev, A.P.; Bonartseva, G.A.; Reshetov, I.V.; Kirpichnikov, M.P.; Shaitan, K.V. Application of polyhydroxyalkanoates in medicine and the biological activity of natural poly(3-hydroxybutyrate). Acta Nat. 2019, 11, 4–16. [Google Scholar] [CrossRef]
- Shishatskaya, E.I.; Volova, T.G.; Gordeev, S.A.; Puzyr, A.P. Degradation of p(3HB) and p(3HB-co-3HV) in biological media. J. Biomater. Sci. Polym. Ed. 2005, 16, 643–657. [Google Scholar] [CrossRef]
- Voinova, V.; Bonartseva, G.; Bonartsev, A. Effect of poly(3-hydroxyalkanoates) as natural polymers on mesenchymal stem cells. World J. Stem Cells 2019, 11, 764–786. [Google Scholar] [CrossRef]
- Bonartsev, A.P.; Zharkova, I.I.; Yakovlev, S.G.; Myshkina, V.L.; Makhina, T.K.; Zernov, A.L.; Kudryashova, K.S.; Feofanov, A.V.; Akulina, E.A.; Ivanova, E.V.; et al. 3D-Scaffolds from poly(3-hydroxybutyrate)-poly(ethylene glycol) copolymer for Tissue Engineering. J. Biomater. Tissue Eng. 2016, 6, 42–52. [Google Scholar] [CrossRef]
- Giavaresi, G.; Tschon, M.; Daly, J.H.; Liggat, J.J.; Sutherland, D.S.; Agheli, H.; Fini, M.; Torricelli, P.; Giardino, R. In vitro and in vivo response to nanotopographically-modified surfaces of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and polycaprolactone. J. Biomater. Sci. Polym. Ed. 2006, 17, 1405–1423. [Google Scholar] [CrossRef] [PubMed]
- Solheim, E.; Sudmann, B.; Bang, G.; Sudmann, E. Biocompatibility and effect on osteogenesis of poly(ortho ester) compared to poly(DL-lactic acid). J. Biomed. Mater. Res. 2000, 49, 257–263. [Google Scholar] [CrossRef]
- Ceonzo, K.; Gaynor, A.; Shaffer, L.; Kojima, K.; Vacanti, C.A.; Stahl, G.L. Polyglycolic acid-induced inflammation: Role of hydrolysis and resulting complement activation. Tissue Eng. 2006, 12, 301–308. [Google Scholar] [CrossRef]
- Kim, M.S.; Ahn, H.H.; Shin, Y.N.; Cho, M.H.; Khang, G.; Lee, H.B. An in vivo study of the host tissue response to subcutaneous implantation of PLGA- and/or porcine small intestinal submucosa-based scaffolds. Biomaterials 2008, 28, 5137–5143. [Google Scholar] [CrossRef] [PubMed]
- Simvastatin. Available online: https://www.drugs.com/monograph/simvastatin.html (accessed on 22 May 2022).
- Du, Z.; Chen, J.; Yan, F.; Xiao, Y. Effects of simvastatin on bone healing around titanium implants in osteoporotic rats. Clin. Oral Impl. Res. 2009, 20, 145–150. [Google Scholar] [CrossRef] [Green Version]
- Chou, J.; Ito, T.; Bishop, D.; Otsuka, M.; Ben-Nissan, B.; Milthorpe, B. Controlled release of simvastatin from biomimetic b-TCP drug delivery system. PLoS ONE 2013, 8, e54676. [Google Scholar] [CrossRef] [Green Version]
- Bonartsev, A.P.; Zernov, A.L.; Yakovlev, S.G.; Zharkova, I.I.; Myshkina, V.L.; Mahina, T.K.; Bonartseva, G.A.; Andronova, N.V.; Smirnova, G.B.; Borisova, J.A.; et al. New poly (3-hydroxybutyrate) microparticles with paclitaxel sustained release for intraperitoneal administration. Anti-Cancer Agents Med. Chem. 2017, 17, 434–441. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Damshkaln, L.G.; Kurochkin, I.N.; Kurochkin, I.I. Study of cryostructuring of polymer systems. 28. Physicochemical and morphological properties of poly(vinyl alcohol) cryogels formed via multiple freezing-thawing technique. Colloid J. 2008, 70, 189–198. [Google Scholar] [CrossRef]
- Masri, C.; Chagnon, G.; Favier, D. Influence of processing parameters on the macroscopic mechanical behavior of PVA hydrogels. Mater. Sci. Eng. C 2017, 75, 769–776. [Google Scholar] [CrossRef] [Green Version]
- Joshi, N.; Suman, K.; Joshi, Y.M. Rheological behavior of aqueous poly(vinyl alcohol) solution during freeze-thaw gelation process. Macromolecules 2020, 53, 3452–3463. [Google Scholar] [CrossRef]
- Mori, Y.; Tokura, H.; Yoshikawa, M. Properties of hydrogels synthesized by freezing and thawing aqueous poly(vinyl alcohol) solutions and their applications. J. Mater. Sci. 1997, 32, 491–496. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Damshkaln, L.G.; Shaskol’skii, B.L.; Babushkina, T.A.; Kurochkin, I.N.; Kurochkin, I.I. Study of cryostructuring of polymer systems. 27. Physicochemical properties of poly(vinyl alcohol) cryogels and features of their macroporous morphology. Colloid J. 2007, 69, 747–764. [Google Scholar] [CrossRef]
- Gun’ko, V.M.; Savina, I.N.; Mikhalovsky, S.V. Cryogels: Morphological, structural and adsorption characterization. Adv. Coll. Interface Sci 2013, 187–188, 1–46. [Google Scholar] [CrossRef] [Green Version]
- Karimi, A.; Daud, W.M.A.W. Materials, preparation, and characterization of PVA/MMT nanocomposite hydrogels: A review. Polym. Compos. 2017, 38, 1086–1102. [Google Scholar] [CrossRef]
- Lipatov, Y.S. Relaxation and viscoelastic properties of heterogeneous polymeric compositions. Adv. Polym. Sci. 1977, 22, 1–59. [Google Scholar] [CrossRef]
- Kudaibergenov, S.E.; Ibraeva, Z.E.; Yashkarova, M.G.; Bekturov, E.A. Hydrogel Composite Materials; Shakarim State University Publisher: Semei, Kazakhstan, 2011; 148p, ISSN 978-601-280-085-2. (In Russian) [Google Scholar]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Clarendon Press: Oxford, UK, 1975; 414p, ISBN 0198533446 9780198533443. [Google Scholar]
- Cao, K.; Liu, Y.; Olkhov, A.A.; Siracusa, V.; Iordanskii, A.L. PLLA-PHB fiber membranes obtained by solvent-free electrospinning for short-time drug delivery. Drug Deliv. Transl. Res. 2018, 8, 291–302. [Google Scholar] [CrossRef]
- Zhuikov, V.A.; Zhuikova, Y.V.; Makhina, T.K.; Myshkina, V.L.; Rusakov, A.; Useinov, A.; Voinova, V.V.; Bonartseva, G.A.; Berlin, A.A.; Bonartsev, A.P.; et al. Comparative structure-property characterization of poly(3-hydroxybutyrate-co-3-hydroxyvalerate)s films under hydrolytic and enzymatic degradation: Finding a transition point in 3-hydroxyvalerate content. Polymers 2020, 12, 728. [Google Scholar] [CrossRef] [Green Version]
- Peppas, N.A.; Sahlin, J.J. A simple equation for the description of solute release. III. Coupling of diffusion and relaxation. Internat. J. Pharm. 1989, 57, 169–172. [Google Scholar] [CrossRef]
- Bruschi, M.L. Strategies to Modify the Drug Release from Pharmaceutical Systems; Elsevier-Woodhead Publishing: Amsterdam, The Netherlands, 2015; 208p, ISBN 9780081000922. [Google Scholar]
- Bonartsev, A.P.; Livshits, V.A.; Makhina, T.A.; Myshkina, V.L.; Bonartseva, G.A.; Iordanskii, A.L. Controlled release profiles of dipyridamole from biodegradable microspheres on the base of poly(3-hydroxybutyrate). Express Polym. Lett. 2007, 1, 797–803. [Google Scholar] [CrossRef]
- Olkhov, A.A.; Vlasov, S.V.; Iordanskii, A.L.; Zaikov, G.E.; Lobo, V.M. Water transport, structure features and mechanical behavior of biodegradable PHB/PVA blends. J. Appl. Polym. Sci. 2003, 90, 1471–1476. [Google Scholar] [CrossRef]

| Series | Sample | PVA Concentration (g/L) | PHB Concentration a (g/L) |
|---|---|---|---|
| 1 | a | 72.6 | - |
| b | 11.4 | ||
| c | 22.8 | ||
| 2 | a | 100.0 | - |
| b | 11.4 | ||
| c | 22.8 | ||
| 3 | a | 137.5 | - |
| b | 11.4 | ||
| c | 22.8 |
| Polymeric | Diffusional Impact | Kinetic Impact | ||||
|---|---|---|---|---|---|---|
| Carriers Loaded with SVN | Radius of Carrier, [cm] | ∆(Gt/G∞)/∆t0.5 [s−0.5] a | Drug Diffusivity (D’), [cm2/s] | R-Square, Origin® | kG × 106 [s−1] | R-Square, Origin® |
| PHB-micro-beads | 0.0125 | 0.196 | 8.72 × 10−10 | 0.994 | 3.75 | 0.964 |
| PVA cryogel | 0.563 | 0.148 | 2.69 × 10−6 | 0.985 | 5.63 | 0.998 |
| PVA/PHB composite | 0.563 | 0.203 | 5.05 × 10−6 | 0.997 | 2.29 | 0.968 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michurov, D.A.; Makhina, T.K.; Siracusa, V.; Bonartsev, A.P.; Lozinsky, V.I.; Iordanskii, A.L. Cryo-Structuring of Polymeric Systems. Poly(Vinyl Alcohol)-Based Cryogels Loaded with the Poly(3-hydroxybutyrate) Microbeads and the Evaluation of Such Composites as the Delivery Vehicles for Simvastatin. Polymers 2022, 14, 2196. https://doi.org/10.3390/polym14112196
Michurov DA, Makhina TK, Siracusa V, Bonartsev AP, Lozinsky VI, Iordanskii AL. Cryo-Structuring of Polymeric Systems. Poly(Vinyl Alcohol)-Based Cryogels Loaded with the Poly(3-hydroxybutyrate) Microbeads and the Evaluation of Such Composites as the Delivery Vehicles for Simvastatin. Polymers. 2022; 14(11):2196. https://doi.org/10.3390/polym14112196
Chicago/Turabian StyleMichurov, Dmitrii A., Tatiana K. Makhina, Valentina Siracusa, Anton P. Bonartsev, Vladimir I. Lozinsky, and Alexey L. Iordanskii. 2022. "Cryo-Structuring of Polymeric Systems. Poly(Vinyl Alcohol)-Based Cryogels Loaded with the Poly(3-hydroxybutyrate) Microbeads and the Evaluation of Such Composites as the Delivery Vehicles for Simvastatin" Polymers 14, no. 11: 2196. https://doi.org/10.3390/polym14112196
APA StyleMichurov, D. A., Makhina, T. K., Siracusa, V., Bonartsev, A. P., Lozinsky, V. I., & Iordanskii, A. L. (2022). Cryo-Structuring of Polymeric Systems. Poly(Vinyl Alcohol)-Based Cryogels Loaded with the Poly(3-hydroxybutyrate) Microbeads and the Evaluation of Such Composites as the Delivery Vehicles for Simvastatin. Polymers, 14(11), 2196. https://doi.org/10.3390/polym14112196









