Recent Advances in 3D Bioprinting: A Review of Cellulose-Based Biomaterials Ink
Abstract
:1. Introduction
2. 3D Bioprinting Techniques
2.1. Extrusion/Liquid Deposition Modeling
2.2. Stereolithography
2.3. Inkjet
2.4. Laser-Assisted Bioprinting
3. Potential and Limitation of Hydrogel as Biomaterial Ink
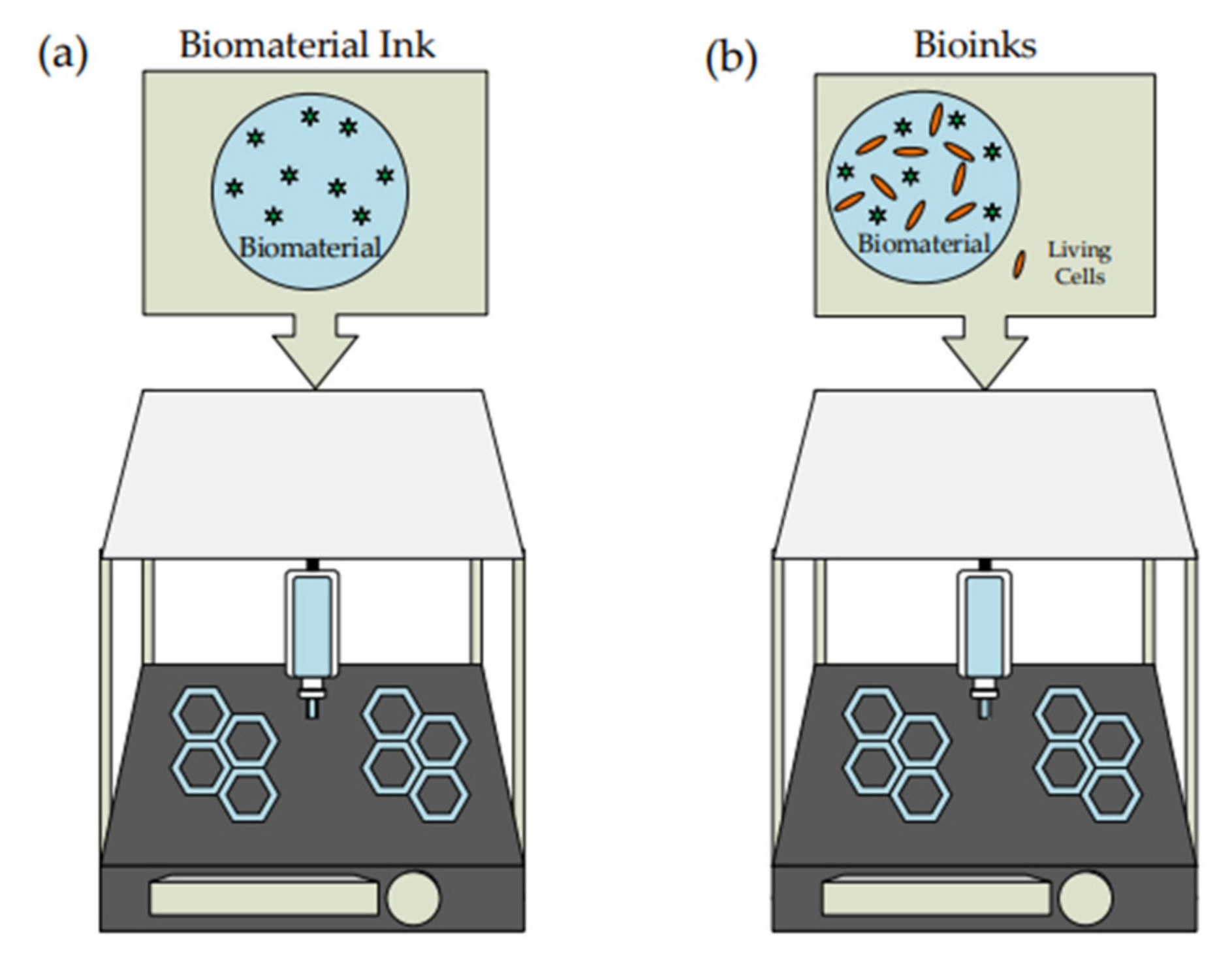
3.1. Biomaterial Ink
3.2. Potential of Biopolymer Hydrogel
4. 3D Bioprinting Application
4.1. Tissue Engineering
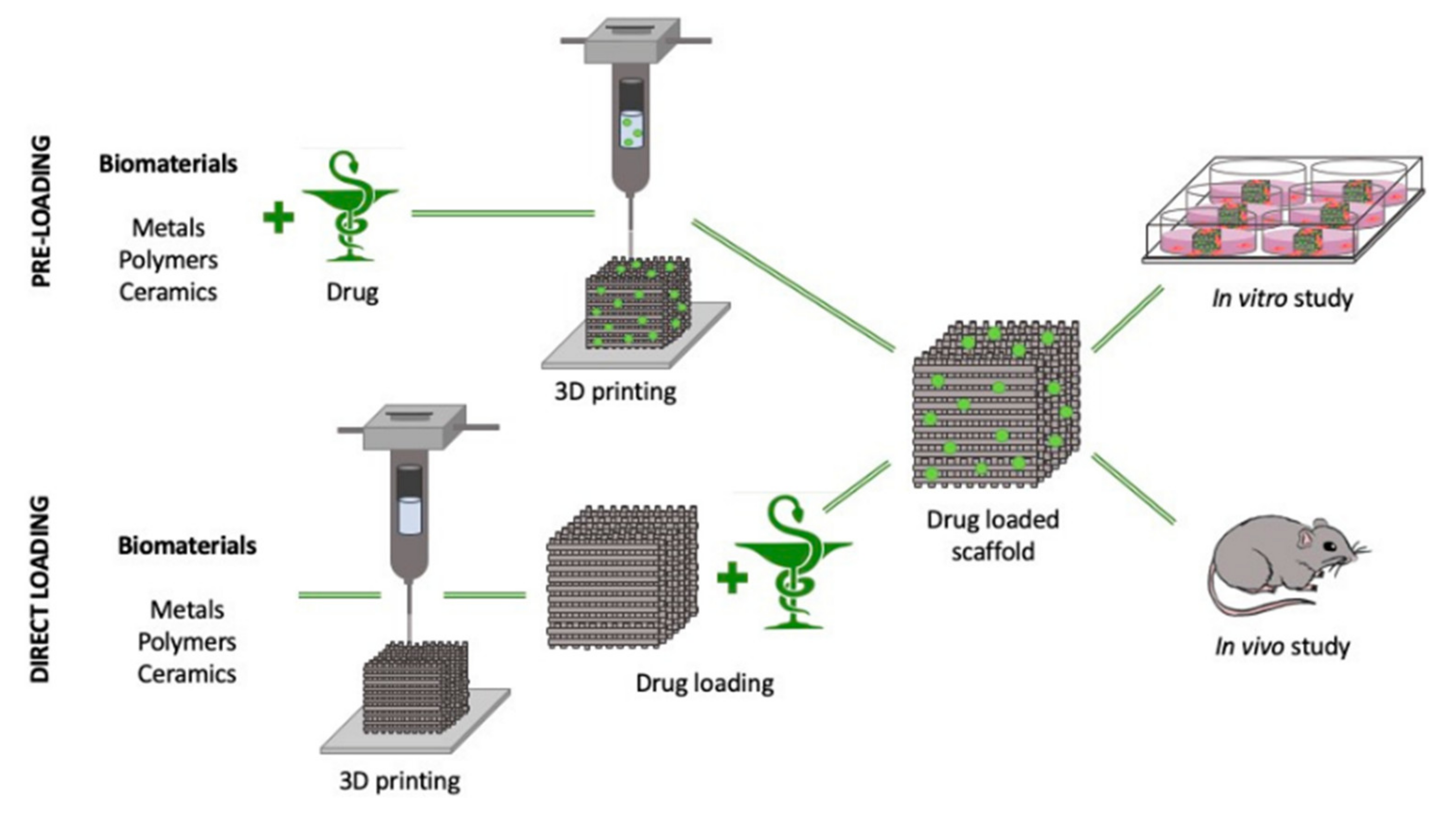
4.2. Drug Delivery
4.3. Protein Study
4.4. Immobilization of Microalgae
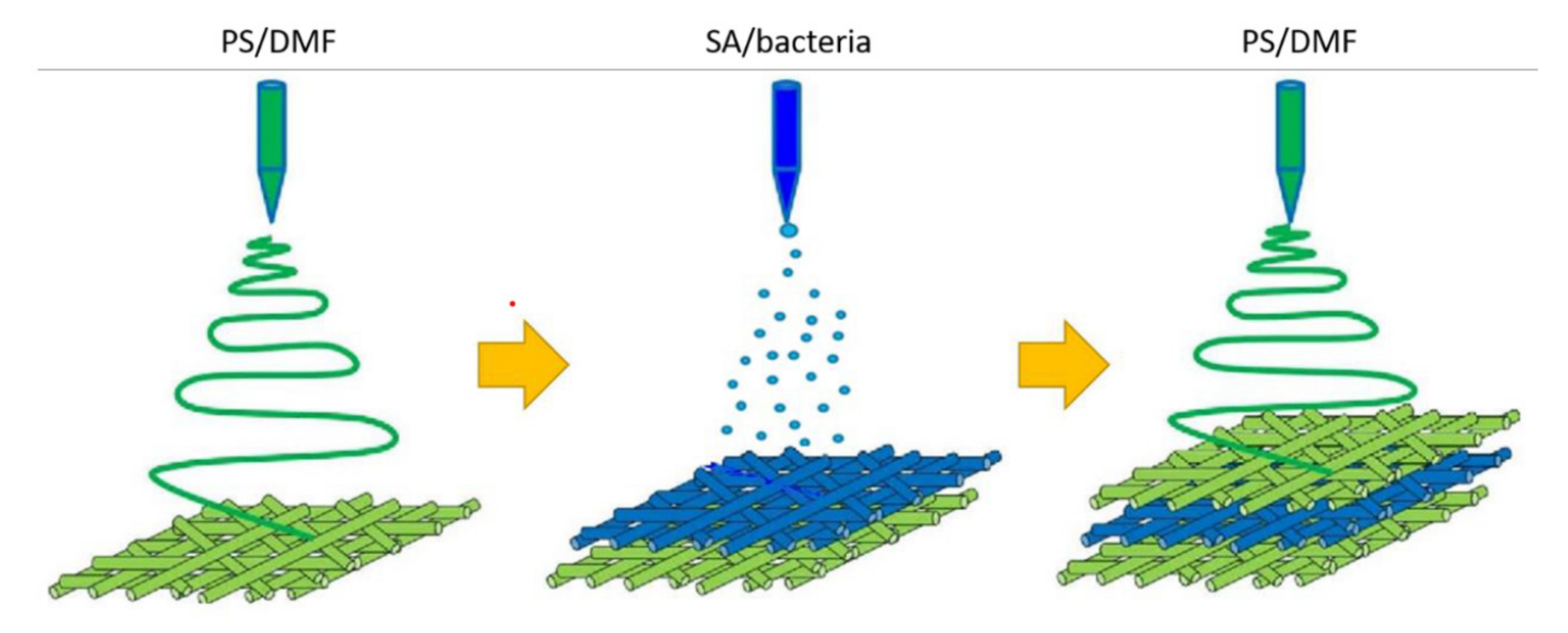
4.5. Immobilization of Bacteria
4.6. Immobilization of Human and Animal Cells
5. Cellulose-Based as Biomaterial Ink
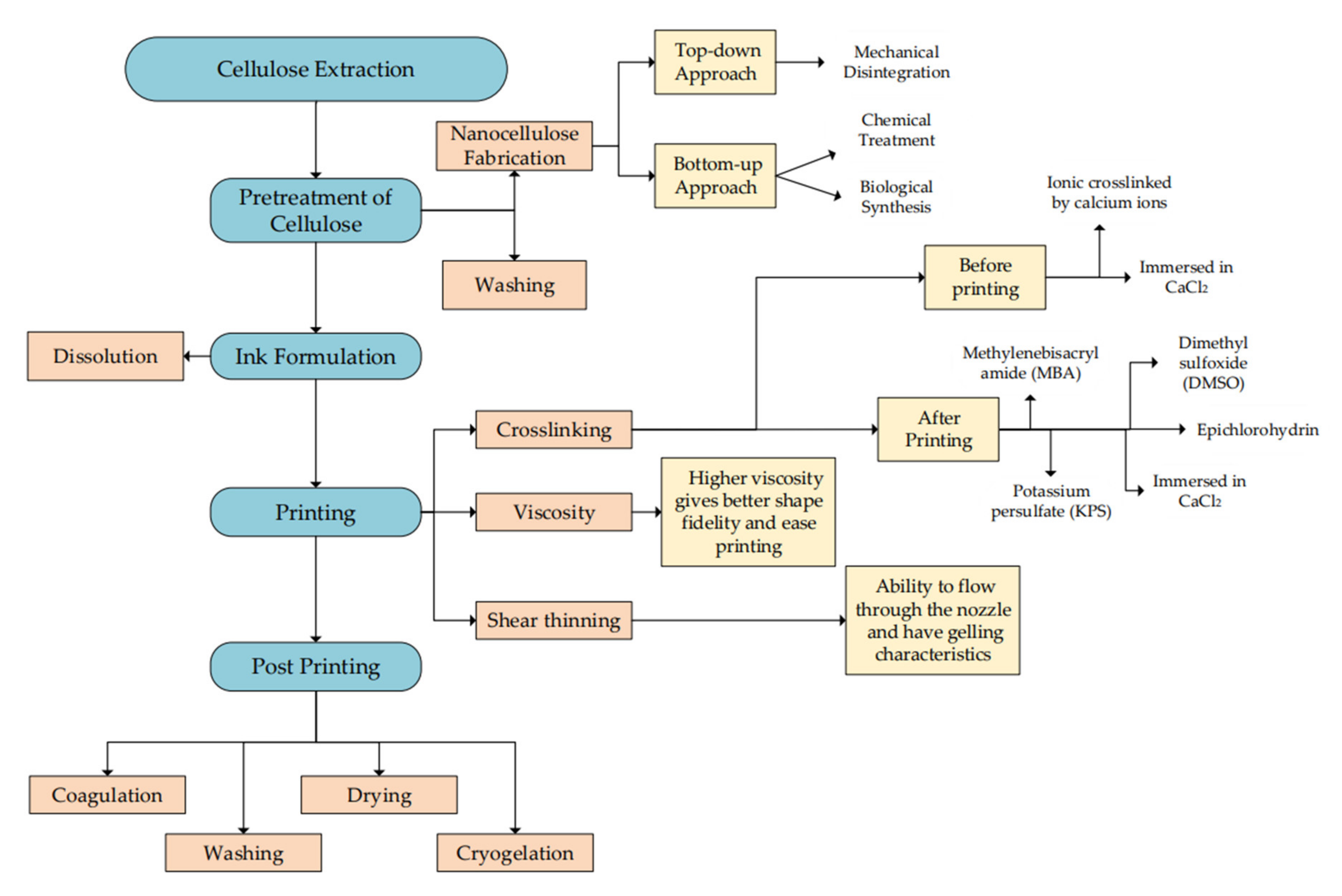
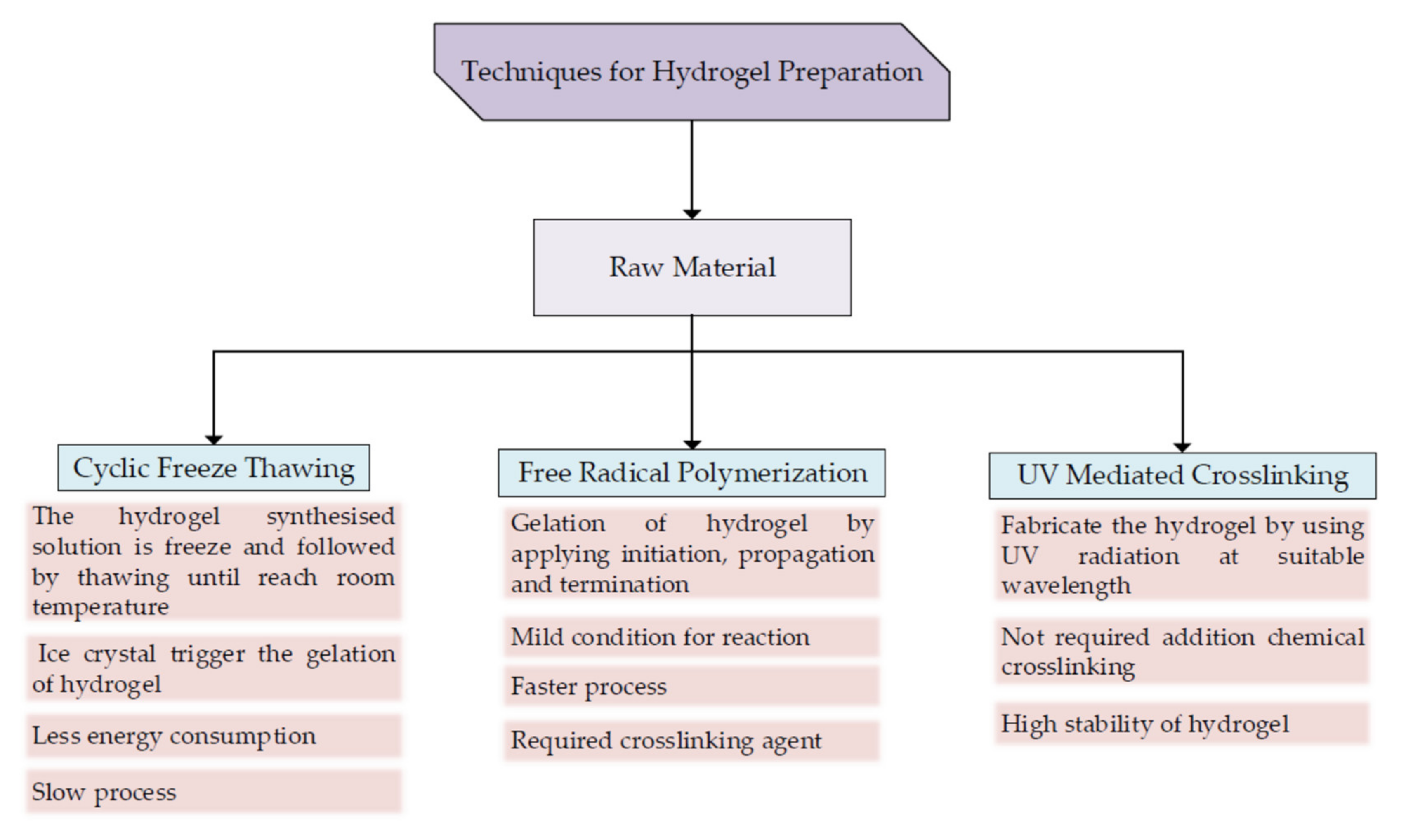
5.1. Preparation for Biomaterial Ink
5.2. Curing of 3D-Printed Cellulose Hydrogel
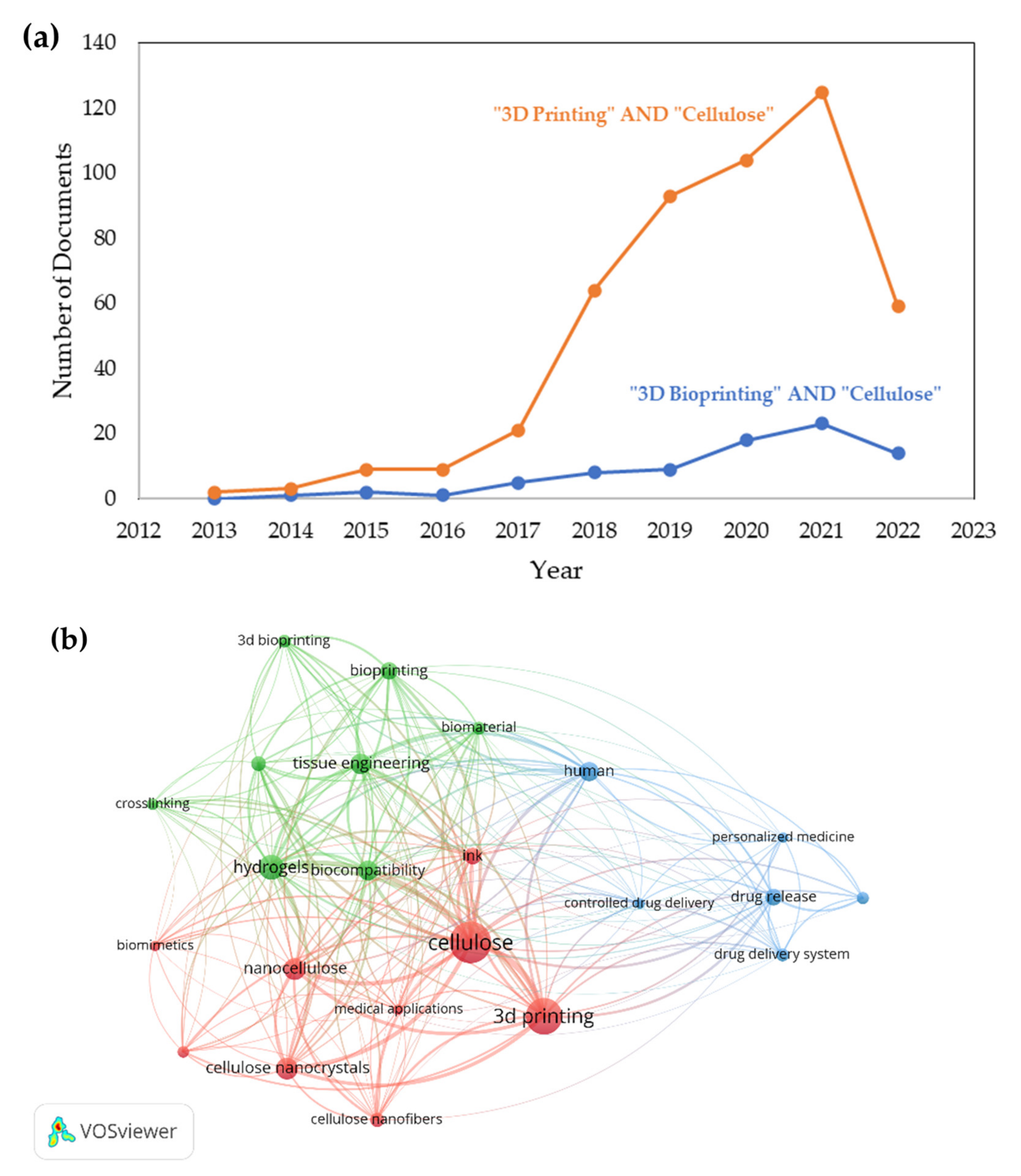
5.3. Recent Trends of 3D Bioprinting Cellulose
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mukherjee, P.; Rani, A.; Saravanan, P. Polymeric materials for 3D bioprinting. In 3D Printing Technology in Nanomedicine; Elsevier: Amsterdam, The Netherlands, 2019; pp. 63–81. [Google Scholar] [CrossRef]
- Goel, A.; Meher, M.K.; Gulati, K.; Poluri, K.M. Fabrication of biopolymer-based organs and tissues using 3D bioprinting. In 3D Printing Technology in Nanomedicine; Elsevier: Amsterdam, The Netherlands, 2019; pp. 43–62. [Google Scholar] [CrossRef]
- Mehanny, S.; Abu-El Magd, E.E.; Ibrahim, M.; Farag, M.; Gil-San-Millan, R.; Navarro, J.; El Habbak, A.E.H.; El-Kashif, E. Extraction and Characterization of Nanocellulose from Three Types of Palm Residues. J. Mater. Res. Technol. 2021, 10, 526–537. [Google Scholar] [CrossRef]
- Thayer, P.; Martinez, H.; Gatenholm, E. History and trends of 3D bioprinting. In 3D Bioprinting Principles and Protocols; Springer: Berlin/Heidelberg, Germany, 2020; Volume 2140, pp. 3–18. [Google Scholar]
- Kačarević, Ž.P.; Rider, P.M.; Alkildani, S.; Retnasingh, S.; Smeets, R.; Jung, O.; Ivanišević, Z.; Barbeck, M. An Introduction to 3D Bioprinting: Possibilities, Challenges and Future Aspects. Materials 2018, 11, 2199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marija, Đ.; Obeid, S.; Mad, M.; Cviji, S.; Ibri, S. Paracetamol Extended Release FDM 3D Printlets: Evaluation of Formulation Variables on Printability and Drug Release. Int. J. Pharm. 2020, 592, 120053. [Google Scholar] [CrossRef]
- Ioannidis, K.; Danalatos, R.I.; Tsaniras, S.C.; Kaplani, K.; Lokka, G.; Kanellou, A.; Papachristou, D.J.; Bokias, G.; Lygerou, Z.; Taraviras, S. A Custom Ultra-Low-Cost 3D Bioprinter Supports Cell Growth and Differentiation. Front. Bioeng. Biotechnol. 2020, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Lu, C.; Jian, Z.; Zhang, T.; Chen, Z.; Zhu, Q.; Tai, Z.; Liu, Y. 3D Bioprinting for Fabricating Artificial Skin Tissue. Colloids Surf. B: Biointerfaces 2021, 208, 112041. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D Bioprinting of Tissues and Organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, J.; Yao, Q.; Ji, C. 3D Printing with Cellulose Materials. Cellulose 2018, 25, 4275–4301. [Google Scholar] [CrossRef]
- Ayyoubi, S.; Cerda, J.R.; Fernandez-Garcia, R.; Knief, P.; Lalatsa, A.; Healy, A.M.; Serrano, D.R. 3D Printed Spherical Mini-Tablets: Geometry versus Composition Effects in Controlling Dissolution from Personalised Solid Dosage Forms. Int. J. Pharm. 2021, 597, 120336. [Google Scholar] [CrossRef]
- Markstedt, K.; Sundberg, J.; Gatenholm, P. 3D Bioprinting of Cellulose Structures from an Ionic Liquid. 3d Print. Addit. Manuf. 2014, 1, 115–121. [Google Scholar] [CrossRef]
- Mohan, D.; Khairullah, N.F.; How, Y.P.; Sajab, M.S. 3D Printed Laminated CaCO 3—Nanocellulose Films as Controlled-Release 5-Fluorouracil. Polymers 2020, 12, 986. [Google Scholar] [CrossRef] [Green Version]
- Monfared, M.; Mawad, D.; Rnjak-Kovacina, J.; Stenzel, M.H. 3D Bioprinting of Dual-Crosslinked Nanocellulose Hydrogels for Tissue Engineering Applications. J. Mater. Chem. B 2021, 9, 6163–6175. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, F.; Mohan, D.; Sajab, M.S.; Bakarudin, S.B.; Kaco, H. Evaluation of the Compatibility of Organosolv Lignin-Graphene Nanoplatelets with Photo-Curable Polyurethane in Stereolithography 3D Printing. Polymers 2019, 11, 1544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anandakrishnan, N.; Ye, H.; Guo, Z.; Chen, Z.; Mentkowski, K.I.; Lang, J.K.; Rajabian, N.; Andreadis, S.T.; Ma, Z.; Spernyak, J.A.; et al. Fast Stereolithography Printing of Large-Scale Biocompatible Hydrogel Models. Adv. Healthc. Mater. 2021, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Carnero, B.; Bao-Varela, C.; Gómez-Varela, A.I.; Álvarez, E.; Flores-Arias, M.T. Microfluidic Devices Manufacturing with a Stereolithographic Printer for Biological Applications. Mater. Sci. Eng. C 2021, 129, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Bi, Z.; Bian, X.; Chen, G.; Zhang, X. Study on Linear Bio-Structure Print Process Based on Alginate Bio-Ink in 3D Bio-Fabrication. Bio-Des. Manuf. 2020, 3, 109–121. [Google Scholar] [CrossRef]
- Li, J.; Chen, M.; Fan, X.; Zhou, H. Recent Advances in Bioprinting Techniques: Approaches, Applications and Future Prospects. J. Transl. Med. 2016, 14, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Cui, X.; Dean, D.; Ruggeri, Z.M.; Boland, T. Cell Damage Evaluation of Thermal Inkjet Printed Chinese Hamster Ovary Cells. Biotechnol. Bioeng. 2010, 106, 963–969. [Google Scholar] [CrossRef]
- Tan, C.T.; Liang, K.; Ngo, Z.H.; Dube, C.T.; Lim, C.Y. Application of 3d Bioprinting Technologies to the Management and Treatment of Diabetic Foot Ulcers. Biomedicines 2020, 8, 441. [Google Scholar] [CrossRef]
- Markstedt, K.; Mantas, A.; Tournier, I.; Martínez Ávila, H.; Hägg, D.; Gatenholm, P. 3D Bioprinting Human Chondrocytes with Nanocellulose-Alginate Bioink for Cartilage Tissue Engineering Applications. Biomacromolecules 2015, 16, 1489–1496. [Google Scholar] [CrossRef]
- Keriquel, V.; Oliveira, H.; Rémy, M.; Ziane, S.; Delmond, S.; Rousseau, B.; Rey, S.; Catros, S.; Amédée, J.; Guillemot, F.; et al. In Situ Printing of Mesenchymal Stromal Cells, by Laser-Assisted Bioprinting, for in Vivo Bone Regeneration Applications. Sci. Rep. 2017, 7, 1778. [Google Scholar] [CrossRef]
- Devillard, R.; Pages, E.; Correa, M.M.; Keriquel, V.; Remy, M.; Kalisky, J.; Ali, M.; Guillotin, B.; Guillemot, F. Cell Patterning by Laser-Assisted Bioprinting. Methods Cell Biol. 2014, 119, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Guillotin, B.; Ali, M.; Ducom, A.; Catros, S.; Keriquel, V.; Souquet, A.; Remy, M.; Fricain, J.; Guillemot, F. Laser-assisted bioprinting for tissue engineering. In Biofabrication; Elsevier: Amsterdam, The Netherlands, 2013; pp. 95–118. [Google Scholar] [CrossRef]
- Hölzl, K.; Lin, S.; Tytgat, L.; Van Vlierberghe, S.; Gu, L.; Ovsianikov, A. Bioink Properties before, during and after 3D Bioprinting. Biofabrication 2016, 8, 032002. [Google Scholar] [CrossRef] [PubMed]
- Smallman, R.E.; Bishop, R.J. Chapter 13—Biomaterials. In Modern Physical Metallurgy and Materials Engineering, 6th ed.; Butterworth-Heinemann: Oxford, UK, 1999; pp. 394–405. [Google Scholar] [CrossRef]
- Ng, I.C.; Pawijit, P.; Tan, J.; Yu, H. Anatomy and physiology for biomaterials research and development. In Encyclopedia of Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; Volume 1, pp. 225–236. [Google Scholar] [CrossRef]
- Kuhn, L.T. Biomaterials. In Introduction to Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2005; pp. 255–312. [Google Scholar] [CrossRef]
- Raghavendra, G.M.; Varaprasad, K.; Jayaramudu, T. Biomaterials: Design, development and biomedical applications. In Nanotechnology Applications for Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2015; pp. 21–44. [Google Scholar] [CrossRef]
- Gopinathan, J.; Noh, I. Recent Trends in Bioinks for 3D Printing. Biomater. Res. 2018, 22, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gungor-Ozkerim, P.S.; Inci, I.; Zhang, Y.S.; Khademhosseini, A.; Dokmeci, M.R. Bioinks for 3D Bioprinting: An Overview. Biomater. Sci. 2018, 6, 915–946. [Google Scholar] [CrossRef] [Green Version]
- Mohan, S.; Oluwafemi, O.S.; Kalarikkal, N.; Thomas, S.; Songca, S.P. Biopolymers—Application in Nanoscience and Nanotechnology. Recent Adv. Biopolym. 2016, 1, 47–66. [Google Scholar] [CrossRef] [Green Version]
- Maraveas, C. Production of Sustainable and Biodegradable Polymers from Agricultural Waste. Polymers 2020, 12, 1127. [Google Scholar] [CrossRef]
- Jämsä, M.; Kosourov, S.; Rissanen, V.; Hakalahti, M.; Pere, J.; Ketoja, J.A.; Tammelin, T.; Allahverdiyeva, Y. Versatile Templates from Cellulose Nanofibrils for Photosynthetic Microbial Biofuel Production. J. Mater. Chem. A 2018, 6, 5825–5835. [Google Scholar] [CrossRef] [Green Version]
- Krujatz, F.; Lode, A.; Brüggemeier, S.; Schütz, K.; Kramer, J.; Bley, T.; Gelinsky, M.; Weber, J. Green Bioprinting: Viability and Growth Analysis of Microalgae Immobilized in 3D-Plotted Hydrogels versus Suspension Cultures. Eng. Life Sci. 2015, 15, 1–28. [Google Scholar] [CrossRef]
- Müller, A.; Wesarg, F.; Hessler, N.; Müller, F.A.; Kralisch, D.; Fischer, D. Loading of Bacterial Nanocellulose Hydrogels with Proteins Using a High-Speed Technique. Carbohydr. Polym. 2014, 106, 410–413. [Google Scholar] [CrossRef]
- Chang, C.; Duan, B.; Cai, J.; Zhang, L. Superabsorbent Hydrogels Based on Cellulose for Smart Swelling and Controllable Delivery. Eur. Polym. J. 2010, 46, 92–100. [Google Scholar] [CrossRef]
- Zhang, X.; Morits, M.; Jonkergouw, C.; Ora, A.; Valle-Delgado, J.J.; Farooq, M.; Ajdary, R.; Huan, S.; Linder, M.; Rojas, O.; et al. Three-Dimensional Printed Cell Culture Model Based on Spherical Colloidal Lignin Particles and Cellulose Nanofibril-Alginate Hydrogel. Biomacromolecules 2020, 21, 1875–1885. [Google Scholar] [CrossRef]
- Dai, L.; Lu, J.; Kong, F.; Liu, K.; Wei, H.; Si, C. Reversible Photo-Controlled Release of Bovine Serum Albumin by Azobenzene-Containing Cellulose Nanofibrils-Based Hydrogel. Adv. Compos. Hybrid Mater. 2019, 2, 462–470. [Google Scholar] [CrossRef]
- Ji, L.; Zhang, F.; Zhu, L.; Jiang, J. An In-Situ Fabrication of Bamboo Bacterial Cellulose/Sodium Alginate Nanocomposite Hydrogels as Carrier Materials for Controlled Protein Drug Delivery. Int. J. Biol. Macromol. 2021, 170, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.S.; Yang, K.; Kim, Y.M.; Nam, K.; Roh, Y.H. Cellulose Nanocrystals as Support Nanomaterials for Dual Droplet-Based Freeform 3D Printing. Carbohydr. Polym. 2021, 272, 118469. [Google Scholar] [CrossRef]
- Müller, A.; Ni, Z.; Hessler, N.; Wesarg, F.; Müller, F.A.; Kralisch, D.; Fischer, D. The Biopolymer Bacterial Nanocellulose as Drug Delivery System: Investigation of Drug Loading and Release Using the Model Protein Albumin. J. Pharm. Sci. 2012, 101, 2271–2280. [Google Scholar] [CrossRef] [PubMed]
- Gawande, N.; Mungray, A.A. Superabsorbent Polymer (SAP) Hydrogels for Protein Enrichment. Sep. Purif. Technol. 2015, 150, 86–94. [Google Scholar] [CrossRef]
- Moreno-Garrido, I. Microalgae Immobilization: Current Techniques and Uses. Bioresour. Technol. 2008, 99, 3949–3964. [Google Scholar] [CrossRef] [PubMed]
- González-Delgado, Á.D.; Barajas-Solano, A.F.; Peralta-Ruiz, Y.Y. Microalgae Immobilization Using Hydrogels for Environmental Applications: Study of Transient Photopolymerization. Chem. Eng. Trans. 2016, 47, 457–462. [Google Scholar] [CrossRef]
- Vasilieva, S.; Shibzukhova, K.; Morozov, A.; Solovchenko, A.; Bessonov, I.; Kopitsyna, M.; Lukyanov, A.; Chekanov, K.; Lobakova, E. Immobilization of Microalgae on the Surface of New Cross-Linked Polyethylenimine-Based Sorbents. J. Biotechnol. 2018, 281, 31–38. [Google Scholar] [CrossRef]
- Deng, J.; Jia, M.; Zeng, Y.Q.; Li, W.; He, J.; Ren, J.; Bai, J.; Zhang, L.; Li, J.; Yang, S. Enhanced Treatment of Organic Matter in Slaughter Wastewater through Live Bacillus Velezensis Strain Using Nano Zinc Oxide Microsphere. Environ. Pollut. 2022, 292, 1–7. [Google Scholar] [CrossRef]
- Shemer, B.; Shpigel, E.; Hazan, C.; Kabessa, Y.; Agranat, A.J.; Belkin, S. Detection of Buried Explosives with Immobilized Bacterial Bioreporters. Microb. Biotechnol. 2020, 14, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, H.; Zhang, M.; Shen, X.; Zhang, X.; Wu, F.; Hu, J.; Wang, B.; Wang, X. Removal of PAHs at High Concentrations in a Soil Washing Solution Containing TX-100 via Simultaneous Sorption and Biodegradation Processes by Immobilized Degrading Bacteria in PVA-SA Hydrogel Beads. J. Hazard. Mater. 2021, 410, 124533. [Google Scholar] [CrossRef]
- Güngörmüşler, M.; Tamayol, A.; Levin, D.B. Hydrogen Production by Immobilized Cells of Clostridium Intestinale Strain URNW Using Alginate Beads. Appl. Biochem. Biotechnol. 2021, 193, 1558–1573. [Google Scholar] [CrossRef] [PubMed]
- Grzywaczyk, A.; Zdarta, A.; Jankowska, K.; Biadasz, A.; Zdarta, J.; Jesionowski, T.; Kaczorek, E.; Smułek, W. New Biocomposite Electrospun Fiber/Alginate Hydrogel for Probiotic Bacteria Immobilization. Materials 2021, 14, 3861. [Google Scholar] [CrossRef] [PubMed]
- Schaffner, M.; Rühs, P.A.; Coulter, F.; Kilcher, S.; Studart, A.R. 3D Printing of Bacteria into Functional Complex Materials. Sci. Adv. 2017, 3, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Zhang, F.; Zhai, W.; Cheng, S.; Li, J.; Wang, Y. Unraveling of Advances in 3D-Printed Polymer-Based Bone Scaffolds. Polymers 2022, 14, 566. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Nishiyama, Y.; Henmi, C. 3D Micro-fabrication by inkjet 3D biofabrication for 3D tissue engineering. In Proceedings of the 2008 International Symposium on Micro-NanoMechatronics and Human Science, Nagoya, Japan, 6–9 November 2008; pp. 451–456. [Google Scholar] [CrossRef]
- Zhu, W.; Ma, X.; Gou, M.; Mei, D.; Zhang, K.; Chen, S. 3D Printing of Functional Biomaterials for Tissue Engineering. Curr. Opin. Biotechnol. 2016, 40, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Vijayavenkataraman, S. 3D-Printable Conductive Materials for Tissue Engineering and Biomedical Applications. Bioprinting 2021, 24, e00166. [Google Scholar] [CrossRef]
- Limongi, T.; Susa, F.; Allione, M.; Fabrizio, E.D. Drug Delivery Applications of Three-Dimensional Printed (3DP) Mesoporous Scaffolds. Pharmaceutics 2020, 12, 851. [Google Scholar] [CrossRef]
- Bhatt, P.; Trehan, S.; Inamdar, N.; Mourya, V.K.; Misra, A. Polymers in drug delivery: An update. In Applications of Polymers in Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar] [CrossRef]
- Hermann, J.R. Protein and the Body. Agric. Sci. Nat. Resour. 2005, 3163, 1–4. [Google Scholar]
- Schiffman, J.D.; Schauer, C.L. A Review: Electrospinning of Biopolymer Nanofibers and Their Applications. Polym. Rev. 2008, 48, 317–352. [Google Scholar] [CrossRef]
- Aryal, S. Proteins—Definition, Properties, Structure, Classification, Functions. Available online: https://microbenotes.com/proteins-properties-structure-classification-and-functions (accessed on 31 December 2021).
- Proteins—Physical & Chemical Properties|A-Level Biology Revision Notes. Available online: https://alevelbiology.co.uk/notes/proteins-physical-chemical-properties (accessed on 31 December 2021).
- Beetul, K.; Gopeechund, A.; Kaullysing, D.; Mattan-Moorgawa, S.; Puchooa, D.; Bhagooli, R. Challenges and opportunities in the present era of marine algal applications. In Algae-Organisms for Imminent Biotechnology; Thajuddin, N., Dhanasekaran, D., Eds.; IntechOpen: London, UK, 2016; Volume 40. [Google Scholar] [CrossRef] [Green Version]
- Barkia, I.; Saari, N.; Manning, S.R. Microalgae for High-Value Products towards Human Health and Nutrition. Mar. Drugs 2019, 17, 304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The Promising Future of Microalgae: Current Status, Challenges, and Optimization of a Sustainable and Renewable Industry for Biofuels, Feed, and Other Products. Microb. Cell Factories 2018, 17, 1–21. [Google Scholar] [CrossRef]
- Satpati, G.G.; Pal, R. Microalgae—Biomass to Biodiesel: A Review. J. Algal Biomass Util. 2018, 9, 11–37. [Google Scholar]
- Hossain, S.M.Z. Biochemical Conversion of Microalgae Biomass into Biofuel. Chem. Eng. Technol. 2019, 42, 2594–2607. [Google Scholar] [CrossRef]
- Raja, R.; Shanmugam, H.; Ganesan, V.; Carvalho, I.S. Biomass from Microalgae: An Overview. Oceanogr. Open Access 2014, 2, 1–7. [Google Scholar] [CrossRef]
- Poirier, D.R.; Michaux, E.; Sumiko Fulleringer, M. Design of a small scale algae cultivation system to produce biodiesel. In Design Proposal; McGill University: Montreal, QC, Canada, 2008. [Google Scholar]
- Singh, S.P.; Singh, P. Effect of Temperature and Light on the Growth of Algae Species: A Review. Renew. Sustain. Energy Rev. 2015, 50, 431–444. [Google Scholar] [CrossRef]
- Chew, K.W.; Yap, J.Y.; Show, P.L.; Suan, N.H.; Juan, J.C.; Ling, T.C.; Lee, D.J.; Chang, J.S. Microalgae Biorefinery: High Value Products Perspectives. Bioresour. Technol. 2017, 229, 1–43. [Google Scholar] [CrossRef]
- Lauritano, C.; Andersen, J.H.; Hansen, E.; Albrigtsen, M.; Escalera, L.; Esposito, F.; Helland, K.; Hanssen, K.O.; Romano, G.; Ianora, A. Bioactivity Screening of Microalgae for Antioxidant, Anti-Inflammatory, Anticancer, Anti-Diabetes, and Antibacterial Activities. Front. Mar. Sci. 2016, 3, 68. [Google Scholar] [CrossRef] [Green Version]
- Caliari, S.R.; Burdick, J.A. A Practical Guide to Hydrogels for Cell Culture. Nat. Methods 2016, 13, 405–414. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Nanda, M.; Verma, M. Application of Agar Liquid-Gel Transition in Cultivation and Harvesting of Microalgae for Biodiesel Production. Bioresour. Technol. 2017, 243, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Maidak, B.L.; Larsen, N.; McCaughey, M.J.; Overbeek, R.; Olsen, G.J.; Fogel, K.; Blandy, J.; Woese, C.R. The ribosomal database project. Nucleic Acids Res. 1994, 22, 3485–3487. [Google Scholar] [CrossRef] [Green Version]
- Maier, R.M. Bacterial growth. In Environmental Microbiology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar] [CrossRef]
- Wang, T.; Wusigale; Kuttappan, D.; Amalaradjou, M.A.; Luo, Y.; Luo, Y. Polydopamine-Coated Chitosan Hydrogel Beads for Synthesis and Immobilization of Silver Nanoparticles to Simultaneously Enhance Antimicrobial Activity and Adsorption Kinetics. Adv. Compos. Hybrid Mater. 2021, 4, 696–706. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial Biofilms: A Common Cause of Persistent Infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deliorman, M.; Wolfenden, M.L.; Suo, Z.; Beech, I.B.; Yang, X.; Avci, R. Immobilization and Trapping of Living Bacteria and Applications in Corrosion Studies. In Understanding Biocorrosion: Fundamentals and Applications; Woodhead Publishing Limited: Sawston, UK, 2014. [Google Scholar] [CrossRef]
- Connell, J.L.; Ritschdorff, E.T.; Whiteley, M.; Shear, J.B. 3D Printing of Microscopic Bacterial Communities. Appl. Phys. Sci. 2013, 110, 18380–18385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.; Wang, J.; Li, Z.; Yue, M.; Qing, X.; Zhang, P.; Liao, X.; Fan, Z.; Yang, S. PVA/SA/MXene Dual-Network Conductive Hydrogel for Wearable Sensor to Monitor Human Motions. J. Appl. Polym. Sci. 2022, 139, 51627. [Google Scholar] [CrossRef]
- Sakai, S.; Kotani, T.; Harada, R.; Goto, R.; Morita, T.; Bouissil, S.; Dubessay, P.; Pierre, G.; Michaud, P.; Boutachfaiti, R.E.; et al. Development of Phenol-Grafted Polyglucuronic Acid and Its Application to Extrusion-Based Bioprinting Inks. Carbohydr. Polym. 2022, 277, 118820. [Google Scholar] [CrossRef]
- Bin, Y.; Dongzhen, Z.; Xiaoli, C.; Jirigala, E.; Wei, S.; Zhao, L.; Tian, H.; Ping, Z.; Jianjun, L.; Yuzhen, W.; et al. Modeling Human Hypertrophic Scars with 3D Preformed Cellular Aggregates Bioprinting. Bioact. Mater. 2022, 10, 247–254. [Google Scholar] [CrossRef]
- Chen, L.; Li, Z.; Zheng, Y.; Zhou, F.; Zhao, J.; Zhai, Q.; Zhang, Z.; Liu, T.; Chen, Y.; Qi, S. 3D-Printed Dermis-Specific Extracellular Matrix Mitigates Scar Contraction via Inducing Early Angiogenesis and Macrophage M2 Polarization. Bioact. Mater. 2022, 10, 236–246. [Google Scholar] [CrossRef]
- Trinh, K.T.L.; Le, N.X.T.; Lee, N.Y. Microfluidic-Based Fabrication of Alginate Microparticles for Protein Delivery and Its Application in the in Vitro Chondrogenesis of Mesenchymal Stem Cells. J. Drug Deliv. Sci. Technol. 2021, 66, 102735. [Google Scholar] [CrossRef]
- Hsu, C.-C.; George, J.H.; Waller, S.; Besnard, C.; Nagel, D.A.; Hill, E.J.; Coleman, M.D.; Korsunsky, A.M.; Cui, Z.; Ye, H. Increased Connectivity of HiPSC-Derived Neural Networks in Multiphase Granular Hydrogel Scaffolds. Bioact. Mater. 2022, 9, 358–372. [Google Scholar] [CrossRef] [PubMed]
- Zennifer, A.; Senthilvelan, P.; Sethuraman, S.; Sundaramurthi, D. Key Advances of Carboxymethyl Cellulose in Tissue Engineering & 3D Bioprinting Applications. Carbohydr. Polym. 2021, 256, 117561. [Google Scholar] [CrossRef] [PubMed]
- Estelle, K.T.; Gozen, B.A. Humidity-Controlled Direct Ink Writing for Micro-Additive Manufacturing with Water-Based Inks. J. Manuf. Processes 2021, 69, 583–592. [Google Scholar] [CrossRef]
- Phanthong, P.; Reubroycharoen, P.; Hao, X.; Xu, G.; Abudula, A.; Guan, G. Nanocellulose: Extraction and Application. Carbon Resour. Convers. 2018, 1, 32–43. [Google Scholar] [CrossRef]
- Shak, K.P.Y.; Pang, Y.L.; Mah, S.K. Nanocellulose: Recent Advances and Its Prospects in Environmental Remediation. Beilstein J. Nanotechnol. 2018, 9, 2479–2498. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Davoudpour, Y.; Islam, M.N.; Mustapha, A.; Sudesh, K.; Dungani, R.; Jawaid, M. Production and Modification of Nanofibrillated Cellulose Using Various Mechanical Processes: A Review. Carbohydr. Polym. 2014, 99, 649–665. [Google Scholar] [CrossRef]
- Cheng, Q.; Wang, S.; Rials, T.G. Composites: Part A Poly (Vinyl Alcohol) Nanocomposites Reinforced with Cellulose Fibrils Isolated by High Intensity Ultrasonication. Compos. Part A 2009, 40, 218–224. [Google Scholar] [CrossRef]
- Siro, I.; Plackett, D. Microfibrillated Cellulose and New Nanocomposite Materials: A Review. Cellulose 2010, 17, 459–494. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Ono, T. Separation and Characterization of Cellulose Fibers from Cypress Wood Treated with Ionic Liquid Prior to Laccase Treatment. Bioresour. Technol. 2013, 127, 132–137. [Google Scholar] [CrossRef]
- Peppas, N.A.; Slaughter, B.V.; Kanzelberger, M.A. Hydrogels. Polym. Sci. A Compr. Ref. 2012, 9, 385–395. [Google Scholar] [CrossRef]
- Sudhakar, C.K.; Upadhyay, N.; Jain, A.; Verma, A.; Charyulu, R.N.; Jain, S. Hydrogels-Promising Candidates for Tissue Engineering. In Nanotechnology Applications for Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar] [CrossRef]
- Korah, L.V.; Anilkumar, G.; Thomas, S. Hydrogels, DNA, and RNA Polypeptides for the Preparation of Biomaterials. In Fundamental Biomaterials: Polymers; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Gonzalez, J.S.; Ludueña, L.N.; Ponce, A.; Alvarez, V.A. Poly (Vinyl Alcohol)/Cellulose Nanowhiskers Nanocomposite Hydrogels for Potential Wound Dressings. Mater. Sci. Eng. 2014, 34, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Mallakpour, S.; Tukhani, M.; Hussain, C.M. Recent Advancements in 3D Bioprinting Technology of Carboxymethyl Cellulose-Based Hydrogels: Utilization in Tissue Engineering. Adv. Colloid Interface Sci. 2021, 292, 102415. [Google Scholar] [CrossRef] [PubMed]
- Kayra, N.; Aytekin, A.O. Synthesis of Cellulose-Based Hydrogels: Preparation, Formation, Mixture, and Modification. Polym. Polym. Compos. A Ref. Ser. 2019, 14, 407–434. [Google Scholar]
- Lan, W.; He, L.; Liu, Y. Preparation and Properties of Sodium Carboxymethyl Cellulose/Sodium Alginate/Chitosan Composite Film. Coatings 2018, 8, 291. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Meng, Y.; Shen, W.; Dou, J.; Liu, R.; Jin, Q.; Fang, S. PH-Responsive Injectable Polysaccharide Hydrogels with Self-Healing, Enhanced Mechanical Properties Based on POSS. React. Funct. Polym. 2020, 70, 282–287. [Google Scholar] [CrossRef]
- Deng, S.; Li, X.; Yang, W.; He, K.; Ye, X. Injectable in Situ Cross-Linking Hyaluronic Acid/Carboxymethyl Cellulose Based Hydrogels for Drug Release. J. Biomater. Sci. Polym. Ed. 2018, 29, 1643–1655. [Google Scholar] [CrossRef]
- Hu, X.; Yang, Z.; Kang, S.; Jiang, M.; Zhou, Z.; Gou, J.; Hui, D.; He, J. Cellulose Hydrogel Skeleton by Extrusion 3D Printing of Solution. Nanotechnol. Rev. 2020, 9, 345–353. [Google Scholar] [CrossRef]
- Yang, J.; Han, C.; Zhang, X.; Xu, F.; Sun, R. Cellulose Nanocrystals Mechanical Reinforcement in Composite Hydrogels with Multiple Cross-Links: Correlations between Dissipation Properties and Deformation Mechanisms. Macromolecules 2014, 47, 4077–4086. [Google Scholar] [CrossRef]
- Du, H.; Liu, W.; Zhang, M.; Si, C.; Zhang, X.; Li, B. Cellulose Nanocrystals and Cellulose Nanofibrils Based Hydrogels for Biomedical Applications. Carbohydr. Polym. 2019, 209, 130–144. [Google Scholar] [CrossRef]
- Wang, H.; Zuo, M.; Ding, N.; Yan, G.; Zeng, X.; Tang, X.; Sun, Y.; Lei, T.; Lin, L. Preparation of Nanocellulose with High-Pressure Homogenization from Pretreated Biomass with Cooking with Active Oxygen and Solid Alkali. ACS Sustain. Chem. Eng. 2019, 7, 9378–9386. [Google Scholar] [CrossRef]
- Butylina, S.; Geng, S.; Laatikainen, K.; Oksman, K. Cellulose Nanocomposite Hydrogels: From Formulation to Material Properties. Fronteirs 2020, 8, 655. [Google Scholar] [CrossRef] [PubMed]
- Lewis, L.; Hatzikiriakos, S.G.; Hamad, W.Y.; Maclachlan, M.J. Freeze—Thaw Gelation of Cellulose Nanocrystals. ACS Macro Lett. 2019, 8, 48–491. [Google Scholar] [CrossRef]
- Yang, J.; Han, C.; Xu, F.; Sun, R. Simple Approach to Reinforce Hydrogels with Cellulose Nanocrystals. Nanoscale 2014, 6, 5934–5943. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, X.; Xu, F. Design of Cellulose Nanocrystals Template-Assisted Composite Hydrogels: Insights from Static to Dynamic Alignment. Macromolecules 2015, 48, 1231–1239. [Google Scholar] [CrossRef]
- Beck, R.C.R.; Chaves, P.S.; Goyanes, A.; Vukosavljevic, B.; Buanz, A.; Windbergs, M.; Basit, A.W.; Gaisford, S. 3D Printed Tablets Loaded with Polymeric Nanocapsules: An Innovative Approach to Produce Customized Drug Delivery Systems. Int. J. Pharm. 2017, 528, 268–279. [Google Scholar] [CrossRef]
- Mohan, D.; Teong, Z.K.; Sajab, M.S.; Hidayatul, N.; Kamarudin, N.; Kaco, H. Intact Fibrillated 3D-Printed Cellulose Macrofibrils/CaCO 3 for Controlled Drug Delivery. Polymers 2021, 13, 1912. [Google Scholar] [CrossRef]
- Wilson, W.C.; Boland, T. Cell and Organ Printing 1: Protein and Cell Printers. Anat. Rec. 2003, 272, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, S.; Yu, K.; Meyer, A.S.; Karana, E.; Aubin-Tam, M.-E. Bioprinting of Regenerative Photosynthetic Living Materials. Adv. Funct. Mater. 2021, 31, 2170222. [Google Scholar] [CrossRef]
- Lode, A.; Krujatz, F.; Brüggemeier, S.; Quade, M.; Schütz, K.; Knaack, S.; Weber, J.; Bley, T.; Gelinsky, M. Green Bioprinting: Fabrication of Photosynthetic Algae-Laden Hydrogel Scaffolds for Biotechnological and Medical Applications. Eng. Life Sci. 2015, 15, 177–183. [Google Scholar] [CrossRef]
- Milojevic, M.; Harih, G.; Vihar, B.; Vajda, J.; Gradisnik, L.; Zidaric, T.; Kleinschek, K.S.; Maver, U.; Maver, T. Hybrid 3D Printing of Advanced Hydrogel-Based Wound Dressings with Tailorable Properties. Pharmaceutics 2021, 13, 564. [Google Scholar] [CrossRef]
- Aarstad, O.; Heggset, E.B.; Pedersen, I.S.; Bjørnøy, S.H.; Syverud, K.; Strand, B.L. Mechanical Properties of Composite Hydrogels of Alginate and Cellulose Nanofibrils. Polymers 2017, 9, 378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habib, A.; Sathish, V.; Mallik, S.; Khoda, B. 3D Printability of Alginate-Carboxymethyl Cellulose Hydrogel. Materials 2018, 11, 454. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Type of Protein | Hydrogel | Release and Uptake | Refs. |
|---|---|---|---|
| Protein albumin | Bacterial nanocellulose, BNC |
| [37,43] |
| BSA | Superabsorbent polymer hydrogel: Carboxymethyl cellulose, CMC, and cellulose (Crosslinker: NaOH/urea aqueous with epichlorohydrin (ECH)) |
| [38] |
| BSA | Injectable polysaccharides-based hydrogel: cation octa (-chloroammoniumpropyl) silsesquioxane (OCAPS), chitosan, and oxidized hydroxypropyl cellulose | The encapsulation efficiency of hydrogel is approximately 100%. | [39] |
| BSA | Photo-response hydrogel (PR-gel): 4arm-PEG and azobenzene into CNF | Photo-controlled released BSA using UV and visible light
| [40] |
| BSA | Multi-layered sphere using alginate and CNC | Sustained release of the protein into the gastric environment. | [42] |
| BSA | Sodium alginate-bamboo-bacterial cellulose hydrogel | Lower released rate due to stronger hydrophobic adsorption generated by lignin. | [41] |
| Lysozyme | Sodium alginate-bamboo-bacterial cellulose hydrogel | Faster released at the early phase (conducted by electrostatic adsorption) and suitable pH-dependent released. | [41] |
| Crosslinker Material | Hydrogel Composition | Processing Technique | Benefit | Refs. |
|---|---|---|---|---|
| CaCl2 | CNF and alginates | Adding CaCl2 to the bioink solution. |
| [22] |
| CMC/SA/chitosan | Soak the film in the CaCl2 for 2 min. |
| [102] | |
| Alginic acid sodium salt and methylcellulose | Immersion in crosslinking solution for 10 min. | [36] | ||
| Ca2+ | CNF | Ionic crosslinking before printing. |
| [14] |
| CNF, alginates, and colloidal lignin particle nanocomposites scaffold | Stored in Dulbecco’s phosphate buffer solution (DPBS) for 7 days with Ca2+ and Mg2+ ions. |
| [103] | |
| PVA and SA | 1st: Crosslinked PVA by freeze-thawing method 2nd: Crosslinked sodium alginates by immersed in CaCl2. |
| [82] | |
| ECH | CMC and cellulose | Addition of crosslinker into hydrogel solution at 30 °C and stir for 2 h. Kept for 12 h at 60 °C for the formation of the gel. Hydroxyl group of cellulose and CMC crosslinked by nucleophilic attack. |
| [38] |
| Disulfide bonds | Hyaluronic acid/carboxymethyl cellulose-based hydrogels (HA/CMC) | Oxidation reaction of dissolved oxygen in solution between the thiol groups a 37 °C. |
| [104] |
| Thiol–ene photoreaction of norbornene groups | PEG and CNF | Under visible light by exposing the blue light for 3 min at 460 nm and 25 mW cm−1. |
| [14] |
| Dimethyl sulfoxide (DMSO) and N, N’-Methylenebisacrylamide (MBA) | Cotton cellulose | Immersion in the crosslinked solution after printing. | Provides better ability for reswelling and compression properties. | [105] |
| N,N′-methylenebisacrylamide (MBA) and potassium persulfate (KPS) | CNC−poly(acrylamide) | Stir hydrogel and crosslinker in a nitrogen bubble for deoxygenation in 10 min. |
| [106] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan Jusoh, W.N.L.; Sajab, M.S.; Mohamed Abdul, P.; Kaco, H. Recent Advances in 3D Bioprinting: A Review of Cellulose-Based Biomaterials Ink. Polymers 2022, 14, 2260. https://doi.org/10.3390/polym14112260
Wan Jusoh WNL, Sajab MS, Mohamed Abdul P, Kaco H. Recent Advances in 3D Bioprinting: A Review of Cellulose-Based Biomaterials Ink. Polymers. 2022; 14(11):2260. https://doi.org/10.3390/polym14112260
Chicago/Turabian StyleWan Jusoh, Wan Nazihah Liyana, Mohd Shaiful Sajab, Peer Mohamed Abdul, and Hatika Kaco. 2022. "Recent Advances in 3D Bioprinting: A Review of Cellulose-Based Biomaterials Ink" Polymers 14, no. 11: 2260. https://doi.org/10.3390/polym14112260
APA StyleWan Jusoh, W. N. L., Sajab, M. S., Mohamed Abdul, P., & Kaco, H. (2022). Recent Advances in 3D Bioprinting: A Review of Cellulose-Based Biomaterials Ink. Polymers, 14(11), 2260. https://doi.org/10.3390/polym14112260







