Modified Starch-Based Adhesives: A Review
Abstract
1. Introduction
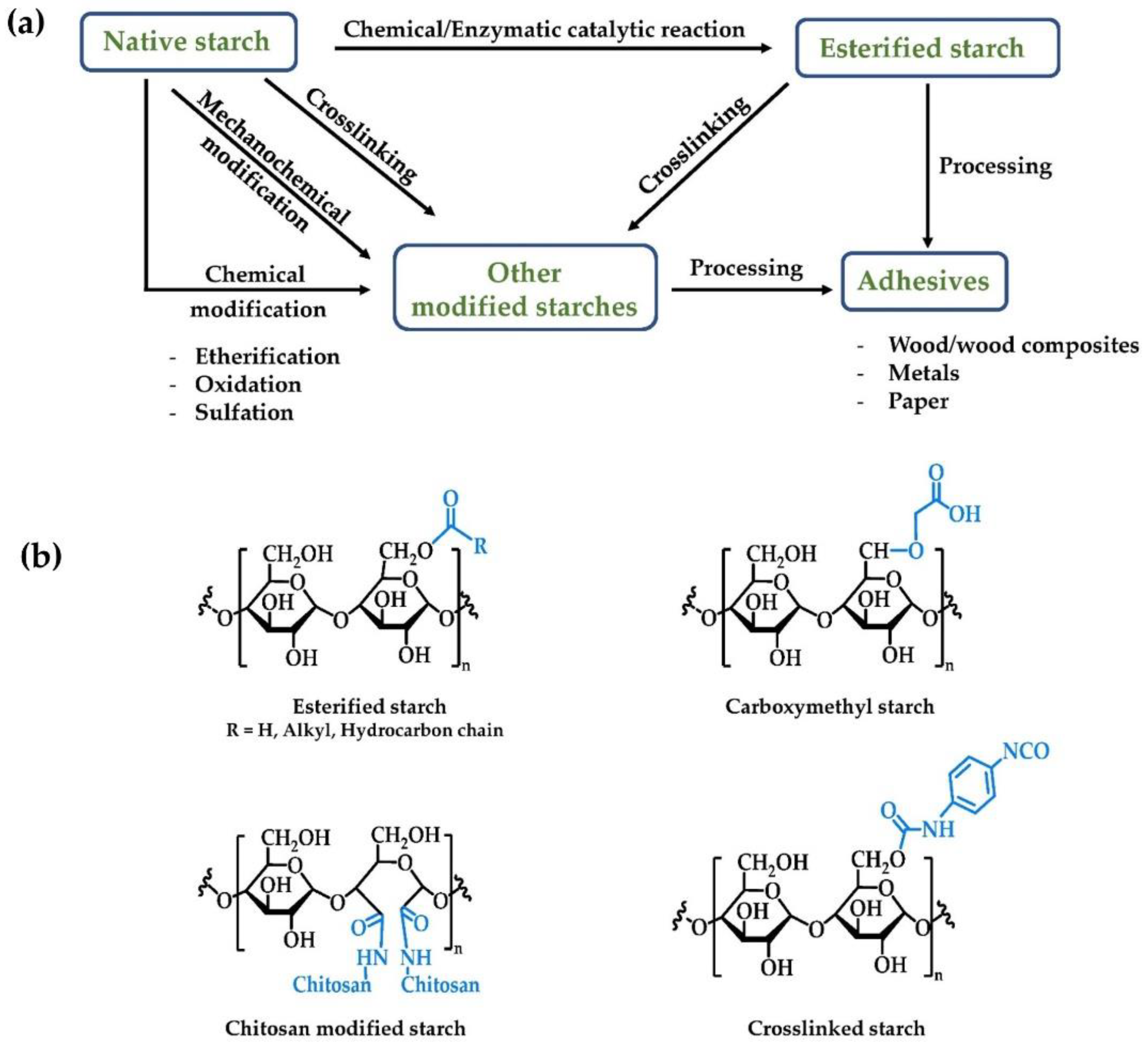
2. Esterified Starch
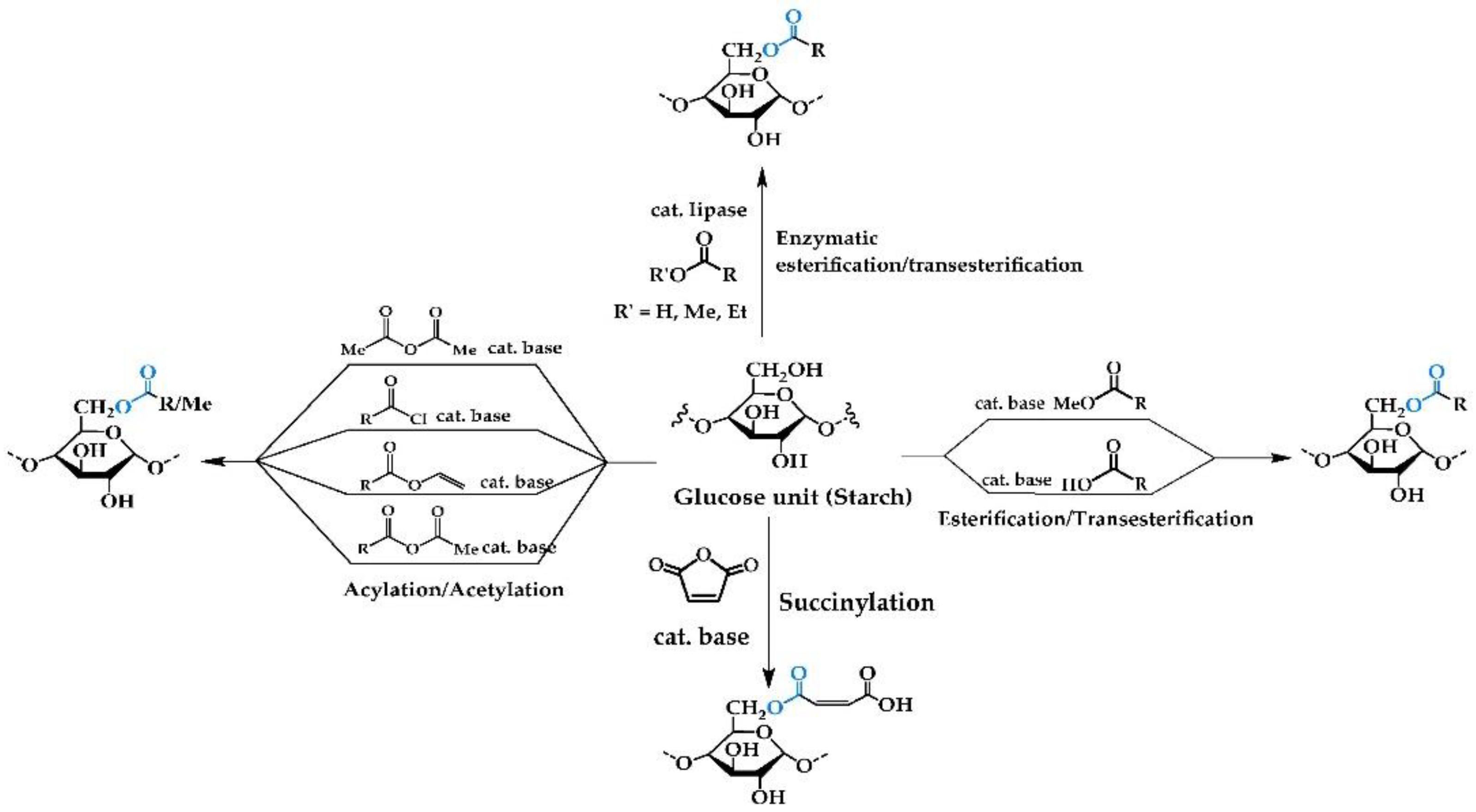
2.1. Chemical Reactions
2.1.1. Esterification/Transesterification
2.1.2. Acylation/Acetylation/Alkylation
2.1.3. Succinylation
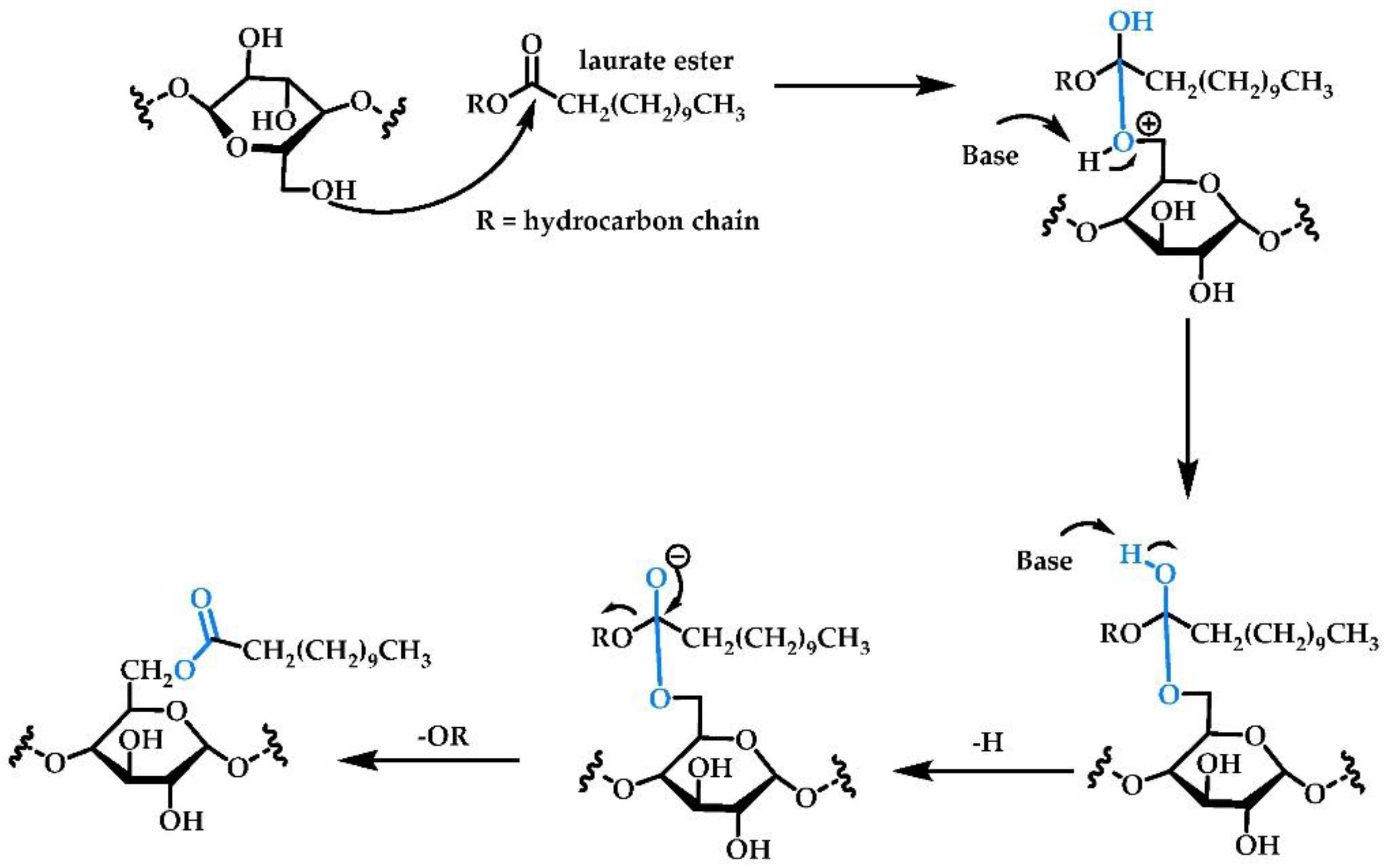
2.1.4. Enzymatic Reactions with Free/Immobilized Lipase
2.1.5. Mechanochemical Processes
2.2. Properties of Starches
2.2.1. Degree of Substitution
Determination of DS via Titration
Determination of DS via 1H-NMR Spectroscopy
2.2.2. Morphology of Native and Modified Starches
2.2.3. Viscosity of Starch Pastes
2.2.4. Solubility/Hydrophobicity
2.2.5. Thermal Properties of Starches
2.3. Adhesives Derived from Esterified Starch
3. Other Modified Starches for Adhesive Applications
4. Conclusions and Perspectives
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Adhesives & Sealants Market by Adhesive Formulating Technology (Water-based, Solvent-based, Hot-melt, Reactive), Sealant Resin Type (Silicone, Polyurethane, Plastisol, Emulsion, Polysulfide, Butyl), Application, Region—Global Forecast to 2026. Available online: https://www.marketsandmarkets.com/Market-Reports/adhesive-sealants-market-421.html?gclid=CjwKCAjwloCSBhAeEiwA3hVo_a_Futuj6Pu555meM1rkldUxwvGxozpuIlUvFIfXlGf0ePpsb70gjhoC8C8QAvD_BwE (accessed on 30 March 2022).
- Heinrich, L.A. Future opportunities for bio-based adhesives—Advantages beyond renewability. Green Chem. 2019, 21, 1866–1888. [Google Scholar] [CrossRef]
- Alinejad, M.; Henry, C.; Nikafshar, S.; Gondaliya, A.; Bagheri, S.; Chen, N.; Singh, S.K.; Hodge, D.B.; Nejad, M. Lignin-Based Polyurethanes: Opportunities for Bio-Based Foams, Elastomers, Coatings and Adhesives. Polymers 2019, 11, 1202. [Google Scholar] [CrossRef] [PubMed]
- Tenorio-Alfonso, A.; Sánchez, M.C.; Franco, J.M. Preparation, Characterization and Mechanical Properties of Bio-Based Polyurethane Adhesives from Isocyanate-Functionalized Cellulose Acetate and Castor Oil for Bonding Wood. Polymers 2017, 9, 132. [Google Scholar] [CrossRef]
- Maaßen, W.; Oelmann, S.; Peter, D.; Oswald, W.; Willenbacher, N.; Meier, M.A. Novel Insights into Pressure-Sensitive Adhesives Based on Plant Oils. Macromol. Chem. Phys. 2015, 216, 1609–1618. [Google Scholar] [CrossRef]
- Pizzi, A. Recent developments in eco-efficient bio-based adhesives for wood bonding: Opportunities and issues. J. Adhes. Sci. Technol. 2006, 20, 829–846. [Google Scholar] [CrossRef]
- Junistia, L.; Sugih, A.K.; Manurung, R.; Picchioni, F.; Janssen, L.P.B.M.; Heeres, H.J. Synthesis of Higher Fatty Acid Starch Esters using Vinyl Laurate and Stearate as Reactants. Starch-Starke 2008, 60, 667–675. [Google Scholar] [CrossRef]
- Oikawa, H.; Ichihara, A.; Sakamura, S. Biosynthetic Study of Betaenone B: Origin of the Oxygen Atoms and Accumulation of Deoxygenated Intermediate using P-450 Inhibitor. J. Chem. Soc. Chem. Commun. 1988, 600–602. [Google Scholar] [CrossRef]
- Kumoro, A.C.; Amalia, R.; Budiyati, C.S.; Retnowati, D.S.; Ratnawati, R. Preparation and characterization of physicochemical properties of glacial acetic acid modified Gadung (Diocorea hispida Dennst) flours. J. Food Sci. Technol. 2015, 52, 6615–6622. [Google Scholar] [CrossRef]
- Rosida, D.F.; Yuliani, R.; Djajati, S. Modification of Colocasia esculenta Starch with Acetylation Process. In Proceedings of the 4th International Seminar of Research Month, NST Proceedings, Surabaya, Indonesia, 9 October 2009. [Google Scholar]
- Golachowski, A.; Drożdż, W.; Golachowska, M.; Kapelko-Żeberska, M.; Raszewski, B. Production and Properties of Starch Citrates—Current Research. Foods 2020, 9, 1311. [Google Scholar] [CrossRef]
- Kapelko-Zeberska, M.; Buksa, K.; Szumny, A.; Zieba, T.; Gryszkin, A. Analysis of molecular structure of starch citrate obtained by a well-stablished method. LWT 2016, 69, 334–341. [Google Scholar] [CrossRef]
- Amini, H.W.; Masruri; Ulfa, S.M. Study on Esterification Reaction of Starch Isolated from Cassava (Manihot esculeta) with Acetic Acid and Isopropyl Myrtistate Using Ultrasonicator. In IOP Conference Series: Materials Science and Engineering, Proceedings of the International Conference on Chemistry and Material Science (IC2MS) 2017, Malang, Indonesia, 4–5 November 2017; IOP Publishing: Bristol, UK, 2017. [Google Scholar]
- Xie, W.; Wang, Y. Synthesis of high fatty acid starch esters with 1-butyl-3-methylimidazolium chloride as a reaction medium. Starch-Starke 2011, 63, 190–197. [Google Scholar] [CrossRef]
- Lukasiewicz, M.; Kowalski, S. Low power microwave-assisted enzymatic esterification of starch. Starch-Starke 2012, 64, 188–197. [Google Scholar] [CrossRef]
- Amaraweera, S.M.; Gunathilake, C.; Gunawardene, O.H.P.; Fernando, N.M.L.; Wanninayaka, D.B.; Dassanayake, R.S.; Rajapaksha, S.M.; Manamperi, A.; Fernando, C.A.N.; Kulatunga, A.K.; et al. Development of Starch-Based Materials Using Current Modification Techniques and Their Applications: A Review. Molecules 2021, 26, 6880. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.; Shogren, R.L.; Selling, G.; Salch, J.; Willett, J.L.; Buchanan, C.M. Rapid and environmentally friendly preparation of starch esters. Carbohydr. Polym. 2008, 74, 137–141. [Google Scholar] [CrossRef]
- Tupa, M.; Maldonado, L.; Vázquez, A.; Foresti, M.L. Simple organocatalytic route for the synthesis of starch esters. Carbohydr. Polym. 2013, 98, 349–357. [Google Scholar] [CrossRef]
- Thiebaud, S.; Aburto, J.; Alric, I.; Borredon, E.; Bikiaris, D.; Prinos, J.; Panayiotou, C. Properties of fatty-acid esters of starch and theirblends with LDPE. J. Appl. Polym. Sci. 1997, 65, 705–721. [Google Scholar] [CrossRef]
- Aburto, J.; Alric, I.; Thiebaud, S.; Borredon, E.; Bikiaris, D.; Prinos, J.; Panayiotou, C. Panayiotou Synthesis, Characterization, and Biodegradability of Fatty-Acid Esters of Amylose and Starch. J. Appl. Polym. Sci. 1999, 74, 1440–1451. [Google Scholar] [CrossRef]
- Tupa, M.V.; Altuna, L.; Herrera, M.L.; Foresti, M.L. Preparation and Characterization of Modified Starches Obtained in Acetic Anhydride/Tartaric Acid Medium. Starch-Starke 2020, 72, 1900300. [Google Scholar] [CrossRef]
- Amos, R.C.; Mesnager, J.; Kuska, M.; Gauthier, M. Production of Cyclic Anhydride-Modified Starches. Polymers 2021, 13, 1504. [Google Scholar] [CrossRef]
- Kim, H.S.; Choi, H.S.; Kim, B.Y.; Baik, M.Y. Characterization of Acetylated Corn Starch Prepared under Ultrahigh Pressure (UHP). J. Agric. Food Chem. 2010, 58, 3573–3579. [Google Scholar] [CrossRef]
- Bhuniya, S.P.; Rahman, S.; Satyanand, A.J.; Gharia, M.M.; Dave, A.M. Novel route to synthesis of allyl starch and biodegradable hydrogel by copolymerizing allyl-modified starch with methacrylic acid and acrylamide. J. Polym. Sci. A Polym. Chem. 2003, 41, 1650–1658. [Google Scholar] [CrossRef]
- Rosu, A.M.; Rafin, C.; Surpateanu, G.; Brabie, G.; Miron, D.N.; Veignie, E. Synthesis of alkylated potato starch derivatives and their potential in the aqueous solubilization of benzo[a]pyrene. Carbohydr. Polym. 2013, 93, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Franssen, M.C.R.; Boeriu, C.G. Chemically Modified Starch; Allyl- and Epoxy-Starch Derivatives. In Starch Polymers; Elsevier: Amsterdam, The Netherlands, 2014; pp. 145–184. [Google Scholar]
- Fang, J.M.; Fowler, P.A.; Sayers, C.; Williams, P.A. The chemical modification of a range of starches under aqueous reaction conditions. Carbohydr. Polym. 2004, 55, 283–289. [Google Scholar] [CrossRef]
- Hong, J.; Zeng, X.A.; Brennan, C.S.; Brennan, M.; Han, Z. Recent Advances in Techniques for Starch Esters and the Applications: A Review. Foods 2016, 5, 50. [Google Scholar] [CrossRef] [PubMed]
- Ačkar, Đ.; Babić, J.; Jozinović, A.; Miličević, B.; Jokić, S.; Miličević, R.; Rajič, M.; Šubarić, D. Starch Modification by Organic Acids and Their Derivatives: A Review. Molecules 2015, 20, 19554–19570. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Gu, J.; Tan, H.; Zhang, Y.; Huo, P. Physicochemical properties of starch adhesives enhanced by esterification modification with dodecenyl succinic anhydride. Int. J. Biol. Macromol. 2018, 112, 1257–1263. [Google Scholar] [CrossRef]
- Huang, Q.; Fu, X.; He, X.; Luo, F.; Yu, S.; Li, L. The effect of enzymatic pretreatments on subsequent octenyl succinic anhydride modifications of cornstarch. Food Hydrocoll. 2010, 24, 60–65. [Google Scholar] [CrossRef]
- Hu, H.; Liu, W.; Shi, J.; Huang, Z.; Zhang, Y.; Huang, A.; Yang, M.; Qin, X.; Shen, F. Structure and functional properties of octenyl succinic anhydride modified starch prepared by a non-conventional technology. Starch-Starke 2016, 68, 151–159. [Google Scholar] [CrossRef]
- Lu, X.; Luo, Z.; Fu, X.; Xiaos, Z. Two-Step Method of Enzymatic Synthesis of Starch Laurate in Ionic Liquids. J. Agric. Food Chem. 2013, 61, 9882–9891. [Google Scholar] [CrossRef]
- Prasertpornsakun, N.; Raita, M.; Laosiripojana, N.; Champreda, V. Biocatalytic synthesis of starch esters by immobilized lipase on magnetic microparticles. Biosci. Biotechnol. BioChem. 2015, 79, 1750–1758. [Google Scholar] [CrossRef]
- Horchani, H.; Chaâbounib, M.; Gargouria, Y.; Sayaria, A. Solvent-free lipase-catalyzed synthesis of long-chain starch esters using microwave heating: Optimization by response surface methodology. Carbohydr. Polym. 2010, 79, 466–474. [Google Scholar] [CrossRef]
- Luna, C.; Luna, D.; Calero, J.; Bautista, F.M.; Romero, A.A.; Posadillo, A.; Verdugo-Escamilla, C. 7—Biochemical catalytic production of biodiesel. In Handbook of Biofuels Production, 2nd ed.; Woodhead Publishing: Sawston, UK, 2016; pp. 165–199. [Google Scholar]
- Ilesanmi, O.I.; Adekunle, A.E.; Omolaiye, J.A.; Olorode, E.M.; Ogunkanmi, A.L. Isolation, optimization, and molecular characterization of lipase producing bacteria from contaminated soil. Sci. Afr. 2020, 8, e00279. [Google Scholar] [CrossRef]
- Osbon, Y.; Kumar, M. Biocatalysis and Strategies for Enzyme Improvement. In Biophysical Chemistry: Advance Applications, 1st ed.; Khalid, M.A.A., Ed.; IntechOpen: London, UK, 2020; Volume 7. [Google Scholar]
- Zhang, Y.; Gan, T.; Hu, H.; Huang, Z.; Huang, A.; Zhu, Y.; Feng, Y.; Yang, M. A Green Technology for the Preparation of High Fatty Acid Starch Esters: Solid-Phase Synthesis of Starch Laurate Assisted by Mechanical Activation with Stirring Ball Mill as Reactor. Ind. Eng. Chem. Res. 2014, 53, 2114–2120. [Google Scholar] [CrossRef]
- Huang, Z.Q.; Xie, X.L.; Chen, Y.; Lu, J.P.; Tong, J.P. Ball-milling treatment effect on physicochemical properties and features for cassava and maize starches. Comptes Rendus Chim. 2008, 11, 73–79. [Google Scholar] [CrossRef]
- Huang, Z.Q.; Lu, J.P.; Li, X.H.; Tong, Z.F. Effect of mechanical activation on physicochemical properties and structure of cassava starch. Carbohydr. Polym. 2007, 68, 128–135. [Google Scholar] [CrossRef]
- Singh, N.; Singh, J.; Kaur, L.; Sodhi, N.S.; Gill, B.S. Morphological, thermal, and rheological properties of starches from different botanical sources. Food Chem. 2003, 81, 219–231. [Google Scholar] [CrossRef]
- Marichelvam, M.K.; Jawaid, M.; Asim, M. Corn and Rice Starch-Based Bioplastics as Alternative Packaging Materials. Fibers 2019, 7, 32. [Google Scholar] [CrossRef]
- Ogunsona, E.; Ojogbo, E.; Mekonnen, T. Advanced material applications of starch and its derivatives. Eur. Polym. J. 2018, 108, 570–581. [Google Scholar] [CrossRef]
- Stojanović, Ž.; Jeremić, K.; Jovanović, S.; Lechner, M.D. A Comparison of Some Methods for the Determination of the Degree of Substitution of Carboxymethyl Starch. Starch-Starke 2005, 57, 79–83. [Google Scholar] [CrossRef]
- Wang, S.Y.; Zhang, C.; Liu, Q.Q.; Wang, Z.J.; Wan, K.X.; Qian, J.Y.; Zhang, L.; Wu, C.; Li, Q. Modification of potato starch by critical melting pretreatment combined with freeze-thawing: Preparation, morphology, structure, and functionality. LWT 2022, 158, 113109. [Google Scholar] [CrossRef]
- Ojogbo, E.; Blanchard, R.; Mekonnen, T. Hydrophobic and Melt Processable Starch-Laurate Esters: Synthesis, Structure–Property Correlations. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 2611–2622. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, H.; Huang, J.; Ge, Z.; Guo, J.; Feng, X.; Xu, Q. Improvement of the bonding properties of cassava starch-based wood adhesives by using different types of acrylic ester. Int. J. Biol. Macromol. 2019, 126, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Hui, R.; Qi-he, C.; Liang, F.M.; Qiong, X.; Guo-qing, H. Preparation and properties of octenyl succinic anhydride modified potato starch. Food Chem. 2009, 114, 81–86. [Google Scholar] [CrossRef]
- Muljana, H.; Knoop, S.V.D.; Keijzer, D.; Picchioni, F.; Janssen, L.P.B.M.; Heeres, H.J. Synthesis of fatty acid starch esters in supercritical carbon dioxide. Carbohydr. Polym. 2010, 82, 346–354. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, S.; Zhang, B.; Qiao, D.; Pu, H.; Liu, S.; Li, L. Structural features, and thermal property of propionylated starches with different amylose/amylopectin ratio. Int. J. Biol. Macromol. 2017, 97, 123–130. [Google Scholar] [CrossRef]
- Vamadevan, V.; Bertoft, E.; Seetharaman, K. On the importance of organization of glucan chains on thermal properties of starch. Carbohydr. Polym. 2013, 92, 1653–1659. [Google Scholar] [CrossRef]
- Silaket, P.; Chatakanonda, P.; Tran, T.; Wansuksri, R.; Piyachomkwan, K.; Sriroth, K. Thermal properties of esterified cassava starches and their maltodextrins in various water systems. Starch-Starke 2014, 66, 1022–1032. [Google Scholar] [CrossRef]
- Yang, B.Y.; Montgomery, R. Preparation and Physical Properties of Starch Mixed Esters. Starch-Starke 2008, 60, 146–158. [Google Scholar] [CrossRef]
- Zhao, K.; Li, B.; Xu, M.; Jing, L.; Gou, M.; Yu, Z.; Zheng, J.; Li, W. Microwave pretreated esterification improved the substitution degree, structural and physicochemical properties of potato starch esters. LWT 2018, 90, 116–123. [Google Scholar] [CrossRef]
- Sindhu, R.; Devi, A.; Khatkar, B.S. Morphology, structure and functionality of acetylated, oxidized and heat moisture treated amaranth starches. Food Hydrocoll. 2021, 118, 106800. [Google Scholar] [CrossRef]
- Neumann, U.; Wiege, B.; Warwel, S. Synthesis of Hydrophobic Starch Esters by Reaction of Starch with Various Carboxylic Acid Imidazolides. Starch-Starke 2002, 54, 449–453. [Google Scholar] [CrossRef]
- Gao, J.; Luo, Z.G.; Luo, F.X. Ionic liquids as solvents for dissolution of corn starch and homogeneous synthesis of fatty-acid starch esters without catalysts. Carbohydr. Polym. 2012, 89, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Hu, Q.; Qian, J.; Li, T.; Ma, P.; Shi, D.; Dong, W.; Chen, M. Preparation and properties of thermoplastic poly(caprolactone) composites containing high amount of esterified starch without plasticizer. Carbohydr. Polym. 2016, 139, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; He, X.; Zuo, Y.; Li, X.; Wu, Y. Synthesis and characterization of lactic acid esterified starch by an in-situ solid phase method. Int. J. Biol. Macromol. 2020, 156, 1316–1322. [Google Scholar] [CrossRef]
- Zhong, C.; Xiong, Y.; Lu, H.; Luo, S.; Wu, J.; Ye, J.; Liu, C. Preparation and characterization of rice starch citrates by superheated steam: A new strategy of producing resistant starch. LWT 2022, 154, 112890. [Google Scholar] [CrossRef]
- Rajan, A.; Sudha, J.D.; Abraham, T.E. Enzymatic modification of cassava starch by fungal lipase. Ind. Crops Prod. 2008, 27, 50–59. [Google Scholar] [CrossRef]
- Rajan, A.; Prasad, V.S.; Abraham, T.E. Enzymatic esterification of starch using recovered coconut oil. Int. J. Biol. Macromol. 2006, 39, 265–272. [Google Scholar] [CrossRef]
- Zarski, A.; Bajer, K.; Zarska, S.; Kapusniak, J. From high oleic vegetable oils to hydrophobic starch derivatives: I. Development and structural studies. Carbohydr. Polym. 2019, 214, 124–130. [Google Scholar] [CrossRef]
- Deng, X.; Han, X.; Hu, X.; Zheng, S.; Liu, K. Enzyme-Catalyzed Starch Esterification in Deep Eutectic Solvent. ChemistrySelect 2019, 4, 565–569. [Google Scholar] [CrossRef]
- Söyler, Z.; Meier, M.A.R. Catalytic Transesterification of Starch with Plant Oils: A Sustainable and Efficient Route to Fatty Acid Starch Esters. ChemSusChem 2017, 10, 182–188. [Google Scholar] [CrossRef]
- Winkler, H.; Vorwerg, W.; Rihm, R. Thermal and mechanical properties of fatty acid starch esters. Carbohydr. Polym. 2014, 102, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Winkler, H.; Vorwerg, W.; Wetzel, H. Synthesis, and properties of fatty acid starch esters. Carbohydr. Polym. 2013, 98, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Aburto, J.; Alric, I.; Borredon, E. Organic Solvent-free Transesterification of Various Starches with Lauric Acid Methyl Ester and Triacyl Glycerides. Starch-Starke 2005, 57, 145–152. [Google Scholar] [CrossRef]
- Geng, F.; Chang, P.R.; Yu, J.; Ma, X. The fabrication and the properties of pretreated corn starch laurate. Carbohydr. Polym. 2010, 80, 360–365. [Google Scholar] [CrossRef]
- Hermawan, E.; Rosyanti, L.; Megasari, L.; Sugih, A.K.; Muljana, H. Transesterification of Sago Starch Using Various Fatty Acid Methyl Esters in Densified CO2. Int. J. Chem. Eng. Appl. 2015, 6, 152. [Google Scholar] [CrossRef][Green Version]
- Aburto, J.; Hamaili, H.; Mouysset-Baziard, G.; Senocq, F.; Alric, I.; Borredon, E.; Toulouse. Free-solvent Synthesis and Properties of Higher Fatty Esters of Starch—Part 2. Starch-Starke 1999, 51, 302–307. [Google Scholar] [CrossRef]
- Xu, Q.; Wen, J.; Wang, Z. Preparation and Properties of Cassava Starch-based Wood Adhesives. BioResources 2016, 11, 6756–6767. [Google Scholar] [CrossRef]
- Owodunni, A.A.; Lamaming, J.; Hashim, R.; Abdulwahab Taiwo, O.F.; Hussin, M.H.; Mohamad Kassim, M.H.; Bustami, Y.; Sulaiman, O.; Mohamad Amini, M.H.; Hiziroglu, S. Properties of green particleboard manufactured from coconut fiber using a potato starch-based adhesive. BioResources 2020, 15, 2279–2292. [Google Scholar] [CrossRef]
- Lamaming, J.; Heng, N.B.; Owodunni, A.A.; Lamaming, S.Z.; Khadir, N.K.A.; Hashim, R.; Sulaiman, O.; Kassim, M.H.M.; Hussin, M.H.; Bustami, Y.; et al. Characterization of rubberwood particleboard made using carboxymethyl starch mixed with polyvinyl alcohol as adhesive. Compos. Part B Eng. 2020, 183, 107731. [Google Scholar] [CrossRef]
- Zhang, Z.; Macquarrie, D.; Clark, J.H.; Matharu, A.S. Chemical modification of starch and the application of expanded starch and its esters in hot melt adhesive. RSC Adv. 2014, 4, 41947–41955. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Z.; Gu, Z.; Hong, Y.; Cheng, L. Preparation, characterization, and properties of starch-based wood adhesive. Carbohydr. Polym. 2012, 88, 699–706. [Google Scholar] [CrossRef]
- Xing, J.; Li, T.; Yu, Y.; Chen, C.; Chang, J. Development and characterization of a new bio-adhesive for wood using cassava starch and bio-oil. Int. J. Adhes. Adhes. 2018, 87, 91–97. [Google Scholar] [CrossRef]
- Qiao, Z.; Gu, J.; Lv, S.; Cao, J.; Tan, H.; Zhang, Y. Preparation and Properties of Normal Temperature Cured Starch-Based Wood Adhesive. Bioresources 2016, 11, 4839–4849. [Google Scholar] [CrossRef]
- Gu, Y.; Cheng, L.; Gu, Z.; Hong, Y.; Li, Z.; Li, C. Preparation, characterization and properties of starch-based adhesive for wood-based panels. Int. J. Biol. Macromol. 2019, 134, 247–254. [Google Scholar] [CrossRef]
- Zia-ud-Din; Xiong, H.; Wang, Z.; Chen, L.; Ullah, I. Effects of sucrose fatty acid ester addition on the structural, rheological and retrogradation behavior of high amylose starch-based wood adhesive. Int. J. Adhes. Adhes. 2019, 89, 51–58. [Google Scholar] [CrossRef]
- Yang, C.; Lin, Y.; Cheng, F.; Zhou, M.; Tan, L.; Zhu, P. Synthesis and Characterization of Corn Starch Phthalate by a Semidry Method. Starch-Starke 2019, 71, 1800315. [Google Scholar] [CrossRef]
- Desai, S.D.; Patel, J.V.; Sinha, V.K. Polyurethane adhesive system from biomaterial-based polyol for bonding wood. Int. J. Adhes. Adhes. 2003, 23, 393–399. [Google Scholar] [CrossRef]
- Huicochea, E.F. Introductory Chapter: Starch Modifications. Applications of Modified Starches; Huicochea, E.F., Villalobos, R.R., Eds.; InTechOpen: London, UK, 2018; p. 78. [Google Scholar]
- Egharevba, H.O. Chemical Properties of Starch and Its Application in the Food Industry. Chemical Properties of Starch; InTechOpen: London, UK, 2019; Available online: https://www.intechopen.com/chapters/68437 (accessed on 14 April 2022).
- Ganorkar, P.M.; Kulkarni, A.S. Studies on preparation and functional properties of carboxymethyl starch from sorghum. Int. Food Res. J. 2013, 20, 2205–2210. [Google Scholar]
- Li, S.; Mujyambere, J.M.V.; Liu, M. Synthesis of Carboxymethyl Starch with High Degree of Substitution by a Modified Dry Process. In Advanced Materials Research; Trans Tech Publications Ltd.: Bach, Switzerland, 2011; Volume 233–235, pp. 306–310. [Google Scholar]
- Detduangchan, N.; Sridach, W.; Wittaya, T. Enhancement of the properties of biodegradable rice starch films by using chemical crosslinking agents. Int. Food Res. J. 2014, 21, 1189–1199. [Google Scholar]
- Kim, M.; Lee, S.J. Characteristics of crosslinked potato starch and starch-filled linear low-density polyethylene films. Carbohydr. Polym. 2002, 50, 331–337. [Google Scholar] [CrossRef]
- Seligra, P.G.; Jaramillo, C.M.; Fama, L.; Goyanes, S. Biodegradable and non-retrogradable eco-films based on starch–glycerol with citric acid as crosslinking agent. Carbohydr. Polym. 2016, 138, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Mewada, R.; Mehta, T. Crosslinking of starch and its effect on viscosity behaviour. Rev. Chem. Eng. 2016, 32, 265–270. [Google Scholar] [CrossRef]
- Praveen, T.K.; Bhushanam, M.V.N.; Prasad, Y.R. Preparation, Evaluation and Characterization of Starch and Grafted Starch. IIJPSR 2021, 12, 4001–4010. [Google Scholar]
- Zia-ud-Din; Chen, L.; Ullah, I.; Wang, P.K.; Javaid, A.B.; Hu, C.; Zhang, M.; Ahamd, I.; Xiong, H.; Wang, Z. Synthesis, and characterization of starch-g-poly (vinyl acetate-co-butyl acrylate) bio-based adhesive for wood application. Int. J. Biol. Macromol. 2018, 114, 1186–1193. [Google Scholar] [CrossRef] [PubMed]
- Kramer, M.E. Structure and function of starch-based edible films and coatings. In Edible Films and Coatings for Food Applications; Springer: New York, NY, USA, 2009; pp. 113–134. [Google Scholar]
- Zhang, Y.; Ding, L.; Gu, J.; Tan, H.; Zhu, L. Preparation, and properties of a starch-based wood adhesive with high bonding strength and water resistance. Carbohydr. Polym. 2015, 115, 32–37. [Google Scholar] [CrossRef]
- Li, Z.; Wang, J.; Li, C.; Gu, Z.; Cheng, L.; Hong, Y. Effects of montmorillonite addition on the performance of starch-based wood adhesive. Carbohydr. Polym. 2015, 115, 394–400. [Google Scholar] [CrossRef]
- Chen, L.; Xiong, Z.; Xiong, H.; Wang, Z.; Din, Z.U.; Nawaz, A.; Wang, P.; Hu, C. Effects of nano-TiO2 on bonding performance, structure stability and film-forming properties of starch-g-VAc based wood adhesive. Carbohydr. Polym. 2018, 200, 477–486. [Google Scholar] [CrossRef]
- Zhao, X.F.; Peng, L.Q.; Wang, H.L.; Wang, Y.B.; Zhang, H. Environment-friendly urea-oxidized starch adhesive with zero formaldehyde-emission. Carbohydr. Polym. 2018, 181, 1112–1118. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, M.; Zhang, J.; Li, J.; Shi, S.Q.; Gao, Q. A High-Performance Bio-Adhesive Using Hyperbranched Aminated Soybean Polysaccharide and Bio-Based Epoxide. Adv. Mater. Interfaces 2020, 7, 2000148. [Google Scholar] [CrossRef]
- Yin, H.; Zheng, P.; Zhang, E.; Rao, J.; Lin, Q.; Fan, M.; Zhu, Z.; Zeng, Q.; Chen, N. Improved wet shear strength in eco-friendly starch-cellulosic adhesives for woody composites. Carbohydr. Polym. 2020, 250, 116884. [Google Scholar] [CrossRef]
- Wu, Q.; Shao, W.; Xia, N.; Wang, P.; Kong, F. A separable paper adhesive based on the starch―lignin composite. Carbohydr. Polym. 2020, 229, 115488. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhuang, B.; Wang, X.; Wu, Z.; Wei, W.; Aladejana, J.Y.; Hou, X.; Yves, K.G.; Xie, Y.; Liu, J. Chitosan used as a specific coupling agent to modify starch in preparation of adhesive film. J. Clean. Prod. 2020, 277, 123210. [Google Scholar] [CrossRef]
- Moreno, O.; Cárdenas, J.; Atarés, L.; Chiralt, A. Influence of starch oxidation on the functionality of starch-gelatin based active films. Carbohydr. Polym. 2017, 178, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Woggum, T.; Sirivongpaisal, P.; Wittaya, T. Properties and characteristics of dual-modified rice starch based biodegradable films. Int. J. Biol. Macromol. 2014, 67, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Akmana, F.; Kazachenko, A.S.; Vasilyeva, N.Y.; Malyar, Y.N. Synthesis and characterization of starch sulfates obtained by the sulfamic acid-urea complex. J. Mol. Struct. 2020, 1208, 127899. [Google Scholar] [CrossRef]
- Kazachenko, A.S.; Vasilyeva, N.Y.; Malyar, Y.N.; Kazachenko, A.S.; Slyusareva, E.A. Synthesis of sulfated starch-casein complex. IOP Conf. Ser. Mater. Sci. Eng. 2020, 862, 062013. [Google Scholar] [CrossRef]
- Lubis, M.A.R.; Park, B.D.; Hong, M.K. Tuning of Adhesion and Disintegration of Oxidized Starch Adhesives for the Recycling of Medium Density Fiberboard. BioResources 2020, 15, 5156–5178. [Google Scholar] [CrossRef]
- Su, M.; Wu, J.; Pan, P.; Wang, H. Preparation, and characterization of a water-resistant polyamide-oxidized starch-methyl methacrylate eco-friendly wood adhesive. Int. J. Biol. Macromol. 2022, 194, 763–769. [Google Scholar] [CrossRef]
- Sulaiman, N.S.; Hahim, R.; Amini, M.H.M.; Sulaiman, O.; Hiziroglu, S. Evaluation of the Properties of Particleboard Made Using Oil Palm Starch Modified with Epichlorohydrin. BioResources 2013, 8, 283–301. [Google Scholar] [CrossRef]
- Chen, X.; Sun, C.; Wang, Q.; Tan, H.; Zhang, Y. Preparation of glycidyl methacrylate grafted starch adhesive to apply in high-performance and environment-friendly plywood. Int. J. Biol. Macromol. 2022, 194, 954–961. [Google Scholar] [CrossRef]
- Chen, L.; Xiong, Z.; Li, Q.; Din, Z.U.; Xiong, H. Sodium dodecyl sulfate improves the properties of bio-based wood adhesive derived from micronized starch: Microstructure and rheological behaviors. Int. J. Biol. Macromol. 2019, 140, 1026–1036. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Chen, Q.; Tan, H.; Gu, J.; Zhang, Y. A Low-Cost, Formaldehyde-Free, and High-Performance Starch-based Wood Adhesive. BioResources 2019, 14, 1405–1418. [Google Scholar]
- Nasiri, A.; Wearing, J.; Dubé, M.A. Using Lignin to Modify Starch-Based Adhesive Performance. ChemEngineering 2020, 4, 3. [Google Scholar] [CrossRef]
- Gadhave, R.V.; Mahanwar, P.; Gadekar, P. Starch-Based Adhesives for Wood/Wood Composite Bonding: Review. OJP Chem. 2017, 7, 19–32. [Google Scholar] [CrossRef]
- Eisen, A.; Bussa, M.; Röder, H. A review of environmental assessments of biobased against petrochemical adhesives. J. Clean. Prod. 2020, 277, 124277. [Google Scholar] [CrossRef]
- Magalhães, S.; Alves, L.; Medronho, B.; Fonseca, A.C.; Romano, A.; Coelho, J.F.J.; Norgren, M. Brief Overview on Bio-Based Adhesives and Sealants. Polymers 2019, 11, 1685. [Google Scholar] [CrossRef]
| Type of Starch | Ratio of Amylose to Amylopectin | Gelatinization Temperature (°C) a | % Solubility | % Crystallinity b |
|---|---|---|---|---|
| Rice | 30:70 | 68–77 | 11–18, 95 °C | 38 |
| Potato | 18:82 | 58–68 | 82, 95 °C | 23–53 |
| Cassava | 18:82 | 60–80 | - | 31–59 |
| Wheat | 20:80 | 58–64 | 1.55, 100 °C | 43–48 |
| Corn | 28:72 | 62–72 | 22, 95 °C | 22–28 |
| Modification of Starch | Type of Starch | Degree of Substitution (DS) | Ref. | |
|---|---|---|---|---|
| Reaction | Reagent–Catalyst | |||
| Acylation | Carboxylic acid (butanoic, hexanoic, octanoic, palmitic acid) | Potato | 2.52–3.00 | [54] |
| Acetylation | Acetic anhydride-iodine | Corn | 0.12–2.97 | [17] |
| Acetic anhydride-tartaric acid | Corn | 0.06–1.54 | [21] | |
| Acetic anhydride and glacial acetic acid | Potato | 0.015–0.054 | [55] | |
| Vinyl acetate | Amaranth grain | 0.22 | [56] | |
| Esterification | Carboxylic acid imidazolides (C8, C12, C16)—methanolic KOCH3 | Potato | 1.76 | [57] |
| Lauric acid—K2CO3 | Cassava | 0.0148–0.0412 | [39] | |
| Lauroyl chloride | Corn | 0.45–2.92 | [47] | |
| Lauric acid, palmitic acid, and stearic acid | Corn | 0.053–0.100 | [58] | |
| Stearyl chloride | Corn | 0.25–1.58 | [59] | |
| Lactic acid—stannous octoate | Corn | 0.015–0.12 | [60] | |
| Citric acid | Rice | 0.015–0.064 | [61] | |
| Enzymatic esterification | Oleic acid—immobilized lipase | Maize | 2.86 | [35] |
| Hydrolyzed recovered coconut oil (lauric acid) and palmitic acid—fungal lipase | Cassava | 1.1 and 1.04 | [62] | |
| Lauric acid-lipase in ionic liquid | Maize | 0.048–0.171 | [33] | |
| Carboxylic acid (acetic, lauric, and stearic acid)-lipase | Maize | 0.016–0.513 | [15] | |
| Recovered coconut oil-lipase | Maize | 1.55 | [63] | |
| Hydrolyzed rapeseed oil—immobilized fungal lipase | Potato | 0.15–1.36 | [64] | |
| Palmitic, lauric, decanoic acids—lipase in deep eutectic solvent | Native | 0.07–0.19 | [65] | |
| Transesterification Transesterification | Olive oil or high oleic sunflower oil-TBD | Maize | 1.29–1.33 | [66] |
| Fatty acid vinyl ester | Maize | 1.40–1.73 and 2.20–2.63 | [67] | |
| Vinyl laurate—Cs2CO3 | Maize | 1.75–2.44 | [68] | |
| Methyl laurate—potassium laurate | Maize | 0.08–0.62 | [69] | |
| Vinyl laurate and vinyl stearate—Na2HPO4, K2CO3 and Na acetate | Corn | 0.24–2.96 | [7] | |
| Methyl laurate | Corn | 0.2673–0.7034 | [70] | |
| Fatty acid methyl ester | Sago | 0.45 | [71] | |
| Starch | Modification | Properties | Utilization | Ref. |
|---|---|---|---|---|
| Cassava | Esterification using dodecenyl succinic anhydride (DDSA) as reactant and polymethylene polyphenyl polyisocyanate (PAPI) | Improved bonding strength and water resistance of esterified starch adhesive | Adhesive film for plywood | [30] |
| Cassava | Grafted with olein monomer (vinyl acetate; VAc and butyl acrylate; BA as co-monomer) | Enhanced the storage stability of starch wood adhesive and glass transition temperature | Wood adhesive | [73] |
| Cassava | Grafted with different typrs of acrylic ester | Improved performance and shear strength under both dry and wet conditions | Wood adhesive | [48] |
| Cassava | Esterification with acid in bio-oil and grafted copolymerization with vinyl acetate and butyl acrylate | Lower viscosities, improved shelf life and mildew resistance of bio-adhesive | Wood adhesive | [78] |
| High amylose corn starch (HACS) | Esterification using propionic anhydride as agent and catalytic 4-dimethyl aminopyridine (DMAP) | Surface area of HACS was increased with improved stability, hydrophobicity, and adhesion strength | Hot melt adhesive bonded Al plate | [76] |
| Corn | Esterification using maleic anhydride | Improved shear strength and thermal stability | Wood adhesive | [79] |
| Corn | Grafted with vinyl acetate and crosslinked with N-methylol acrylamide | Improved water resistance and can be used in hot pressimg processes | Wood-based panel adhesive | [80] |
| Corn | Addition of sucrose fatty acid ester | Enhanced shear-thinning, solid-like behavior, and anti-retrodegradation of starch | Wood adhesive | [81] |
| Corn | Esterification with phthalic anhydride in DMF | Reduced viscosity and thermal decomposition | Adhesive | [82] |
| Waxy corn | Grafted with vinyl acetate | Increased shear strength under both dry and wet conditions | Wood adhesive | [77] |
| Potato | Transesterification with natural oil and combined with toluene 2,4-diisocyanate | Good resistance to cold and hot water, moderate resistance to acid and weak resistance to akali | Polyurethane (PU) adhesive for wood | [83] |
| Reaction of Modified Starch | Starch | Properties | Utilization | Ref. |
|---|---|---|---|---|
| Oxidation and modification with chitosan | Corn | Improved dry and wet shear strength of plywood | An adhesive film for plywood | [102] |
| Oxidation using H2O2 and then crosslinking with B-pMDI and citric acid | Corn | Improved physical properties, mechanical properties, and water resistance | Medium density fiberboard | [107] |
| Oxidation using KMnO4, then crosslinking and copolymerization with polyamide and methyl methacrylate | Corn | Improved wet shear strength and water resistance | An adhesive for plywood | [108] |
| Oxidation using KMnO4, polycondensation reaction with urea and addition of nano-TiO2 | Corn | The nano-TiO2 effectively improves dry shear strength and viscosity of the nano-TiO2-U-OSt adhesive. | An adhesive | [98] |
| Oxidation using H2O2 and crosslinking with polyamidoamine-epichlorohydrin (PAH) | Rice | Enhanced thermal stability, hydrophobicity, wet-cohesion, and adhesiveness | An adhesive for wood composites | [100] |
| Etherification with carboxymethyl and use of POCl3 as crosslinking agent | Wheat | The modified starch mixed with PVA improves solid content, heat and water resistance but decrease viscosity. | Adhesive for particleboard | [75] |
| Etherification with epichlorohydrin | Oil palm | Improved mechanical strength (modulus, elasticity, and internal bond), solid content and viscosity | Adhesive for particleboard | [109] |
| Graft copolymerization with glycidyl methacrylate (GMA) and crosslinking with sodium trimetaphosphate (STMP). | Cassava | Improved water resistance and bonding strength | An adhesive for plywood | [110] |
| Graft copolymerization with sodium dodecyl sulfate (SDS) | Micronized (MS) | Improved shear strength and decreased viscosity of micronized starch with increasing SDS contents | Wood adhesive | [111] |
| Graft copolymerization with lignin | Corn | Improved adhesive bond strength and moisture resistance, including extended shelf-life. | An adhesive for paper | [101] |
| Crosslinking with polyphenylene isocyanate (PAPI) with poly vinyl alcohol (PVOH) as a protective colloid | Cassava | Improved water resistance, shear strength, mobility and storage stability of starch adhesive | Wood adhesive | [112] |
| Crosslinking with lignin | Corn | Lignin improved the strength and water resistance of adhesive | An adhesive for cardboard application | [113] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watcharakitti, J.; Win, E.E.; Nimnuan, J.; Smith, S.M. Modified Starch-Based Adhesives: A Review. Polymers 2022, 14, 2023. https://doi.org/10.3390/polym14102023
Watcharakitti J, Win EE, Nimnuan J, Smith SM. Modified Starch-Based Adhesives: A Review. Polymers. 2022; 14(10):2023. https://doi.org/10.3390/polym14102023
Chicago/Turabian StyleWatcharakitti, Jidapa, Ei Ei Win, Jaturavit Nimnuan, and Siwaporn Meejoo Smith. 2022. "Modified Starch-Based Adhesives: A Review" Polymers 14, no. 10: 2023. https://doi.org/10.3390/polym14102023
APA StyleWatcharakitti, J., Win, E. E., Nimnuan, J., & Smith, S. M. (2022). Modified Starch-Based Adhesives: A Review. Polymers, 14(10), 2023. https://doi.org/10.3390/polym14102023







