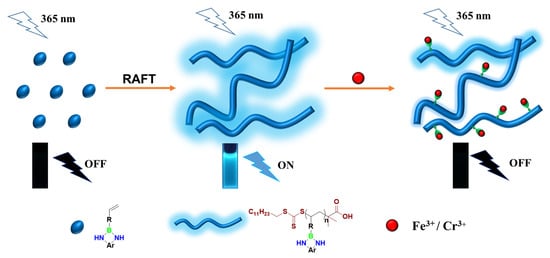
Novel NBN-Embedded Polymers and Their Application as Fluorescent Probes in Fe3+ and Cr3+ Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
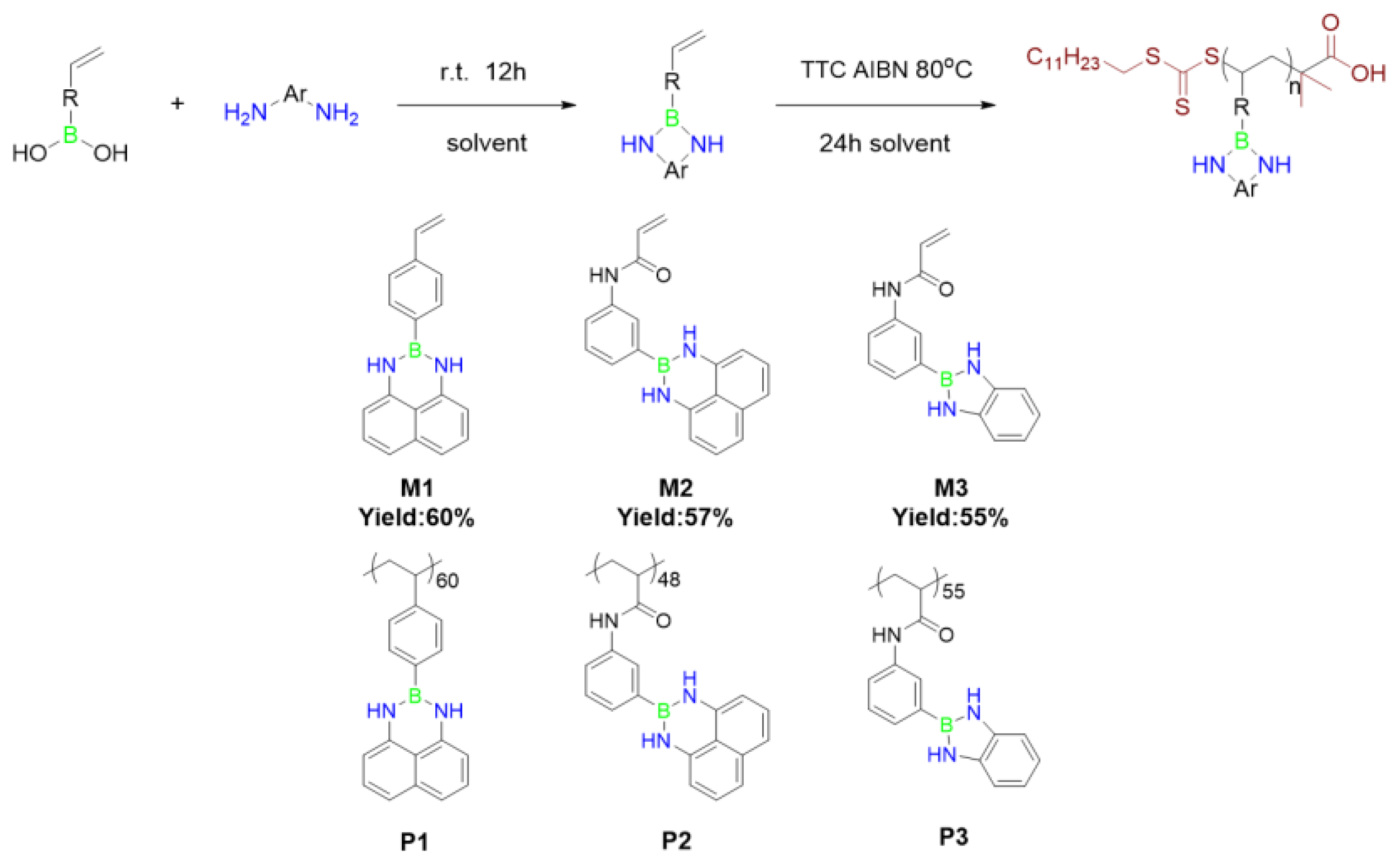
2.2. Synthesis of the Monomer
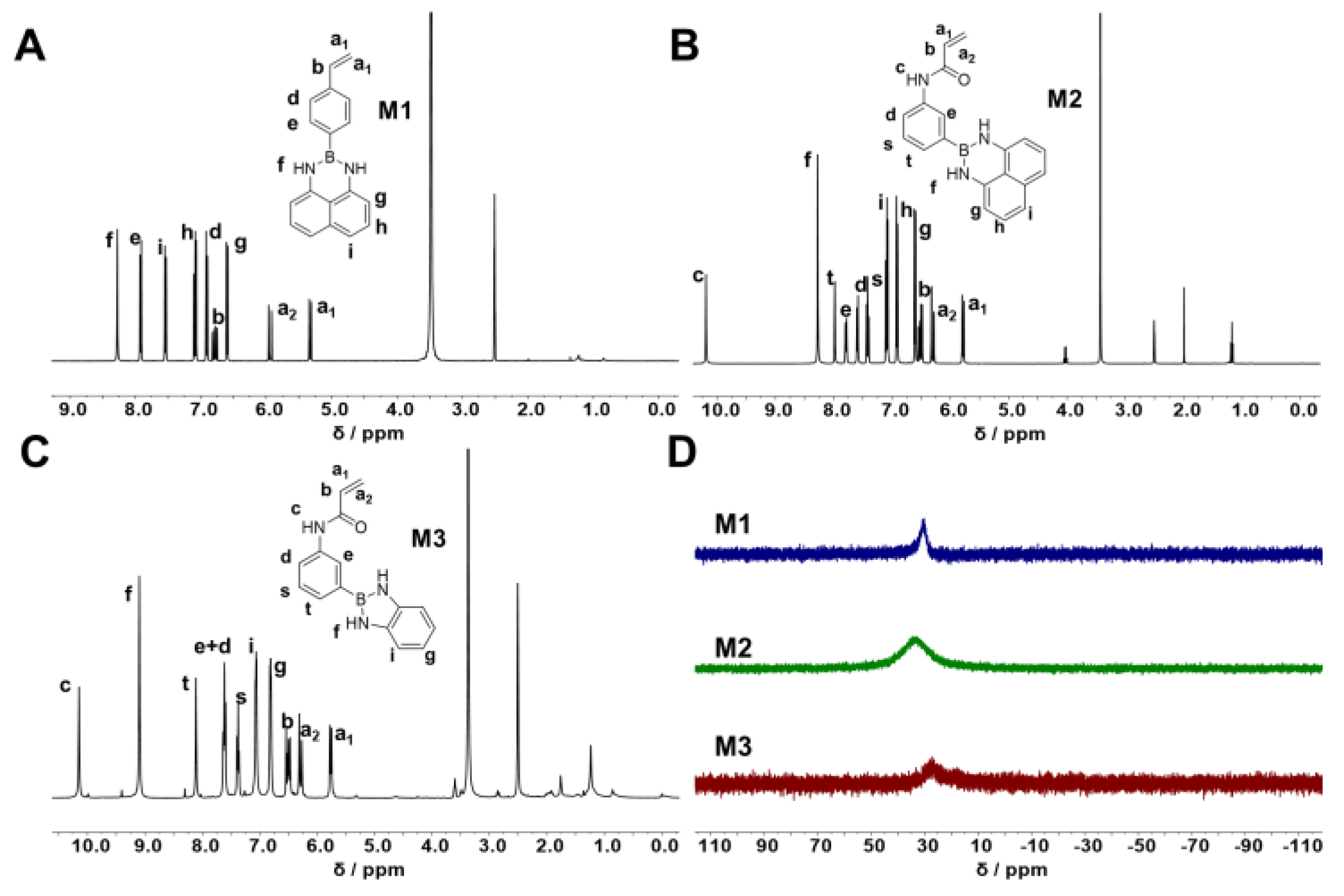
2.2.1. Synthesis of 2-(4-Vinylphenyl)-2,3-dihydro-1H-naphtho[1,8-de][1,3,2]diazaborinine (M1)
2.2.2. Synthesis of N-(4-(1H-naphtho[1,8-de][1,3,2]diazaborinin-2(3H)-yl)phenyl)acrylamide (M2)
2.2.3. Synthesis of N-(4-(1H-benzo[d][1,3,2]diazaborol-2(3H)-yl)phenyl)acrylamide (M3)
2.3. Synthesis of the Polymer
2.3.1. Synthesis of P1
2.3.2. Synthesis of P2
2.4. The Fluorescence Detection of Metal Ion
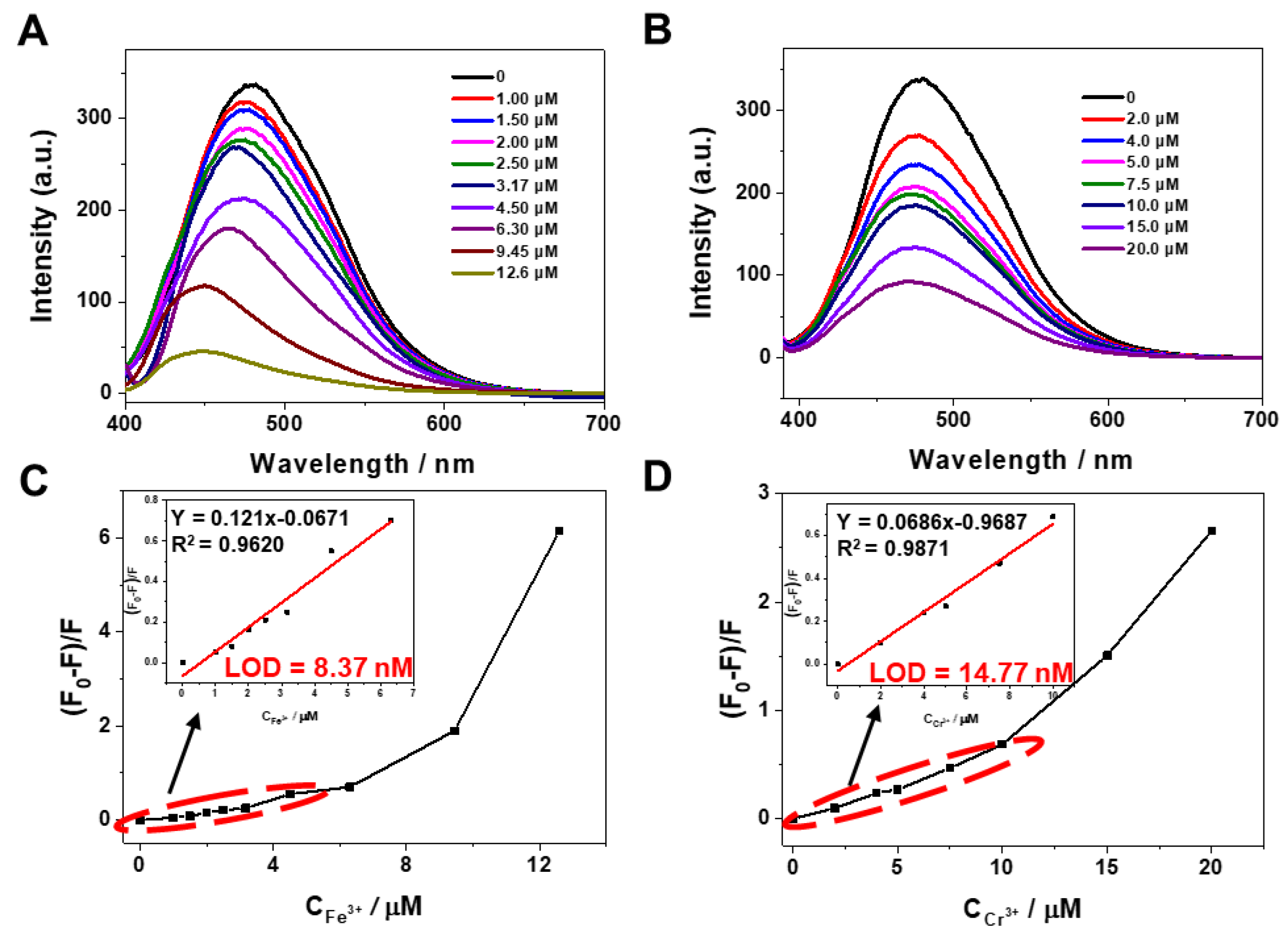
2.4.1. The Limit of Detection (LOD)
2.4.2. The Time Sensitivity Test
2.5. Characterizations
2.6. Theoretical Calculations
3. Results and Discussion
3.1. Fabrication of NBN-Embedded Polymer
3.1.1. Synthesis of NBN-Containing Monomer
3.1.2. Synthesis of NBN-Embedded Polymers
3.2. Photophysical Properties
3.3. Metal Ion Fluorescence Detection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, M.; Gou, H.; Al-Ogaidi, I.; Wu, N. Nanostructured Sensors for Detection of Heavy Metals: A Review. ACS Sustain. Chem. Eng. 2013, 1, 713–723. [Google Scholar] [CrossRef]
- Dwivedi, S.; Mishra, S.; Tripathi, R.D. Ganga Water Pollution: A Potential Health Threat to Inhabitants of Ganga Basin. Environ. Int. 2018, 117, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Say, R.; Birlik, E.; Denizli, A.; Ersöz, A. Removal of Heavy Metal ions by Dithiocarbamate-Anchored Polymer/Organosmectite Composites. Appl. Clay Sci. 2006, 31, 298–305. [Google Scholar] [CrossRef]
- Zhang, L.; Peng, D.; Liang, R.-P.; Qiu, J.-D. Graphene-Based Optical Nanosensors for Detection of Heavy metal Ions. TrAC Trends Anal. Chem. 2018, 102, 280–289. [Google Scholar] [CrossRef]
- Christodoulou, K.; Leontidis, E.; Achilleos, M.; Polydorou, C.; Krasia-Christoforou, T. Semi-Interpenetrating Polymer Networks with Predefined Architecture for Metal Ion Fluorescence Monitoring. Polymers 2016, 8, 411. [Google Scholar] [CrossRef] [Green Version]
- Xiu, W.; Dai, L.Z. Well-Dispersed Zero-Valent Iron Supported on Fe3O4/g-C3N4 Composites via A Facile Approach with Versatile Photoredox Catalysis. J. Nanopart. Res. 2018, 20, 317. [Google Scholar]
- Liu, X.F.; Theil, E.C. Ferritins: Dynamic Management of Biological Iron and Oxygen Chemistry. Acc. Chem. Res. 2005, 3, 167–175. [Google Scholar] [CrossRef]
- Li, S.Z.; Zhang, X.Y. Iron in Cardiovascular Disease: Challenges and Potentials. Front. Cardiovasc. Med. 2021, 8, 707138. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, H.; Zhang, Y.; Zhao, Y.; Yuan, H.; He, X.; Wu, Y.; Zhang, S. Copper Nanoclusters-Based Fluorescent Sensor Array to Identify Metal Ions and Dissolved Organic Matter. J. Hazard. Mater. 2022, 428, 128158. [Google Scholar] [CrossRef]
- Hou, J.; Jia, P.; Yang, K.; Bu, T.; Zhao, S.; Li, L.; Wang, L. Fluorescence and Colorimetric Dual-Mode Ratiometric Sensor Based on Zr–Tetraphenylporphyrin Tetrasulfonic Acid Hydrate Metal–Organic Frameworks for Visual Detection of Copper Ions. ACS Appl. Mater. Interfaces 2022, 14, 13848–13857. [Google Scholar] [CrossRef]
- Naksen, P.; Boonruang, S.; Yuenyong, N.; Lee, H.L.; Ramachandran, P.; Anutrasakda, W.; Amatatongchai, M.; Pencharee, S.; Jarujamrus, P. Sensitive Detection of Trace Level Cd(II) Triggered by Chelation Enhanced Fluorescence (CHEF) “Turn On”: Nitrogen-Doped Graphene Quantum Dots (N-GQDs) as Fluorometric Paper-based Sensor. Talanta 2022, 242, 123305. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, H.; Huang, H.; Cao, X.; Chen, X.; Cao, D. Porous Organic Polymers as a Platform for Sensing Applications. Chem. Soc. Rev. 2022. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jiang, M.; Yan, L.; Peng, R.; Huangfu, M.; Guo, X.; Li, Y.; Wu, P. Multifunctional Luminescent Eu(III)-Based Metal–Organic Framework for Sensing Methanol and Detection and Adsorption of Fe(III) Ions in Aqueous Solution. Inorg. Chem. 2016, 55, 12660–12668. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gao, X.; Shi, W.; Ma, H. Design Strategies for Water-Soluble Small Molecular Chromogenic and Fluorogenic Probes. Chem. Rev. 2013, 114, 590–659. [Google Scholar] [CrossRef] [PubMed]
- She, M.; Wang, Z.; Chen, J.; Li, Q.; Liu, P.; Chen, F.; Zhang, S.; Li, J. Design Strategy and Recent Progress of Fluorescent Probe for Noble Metal Ions (Ag, Au, Pd, and Pt). Coord. Chem. Rev. 2021, 432, 213712. [Google Scholar] [CrossRef]
- Dai, Y.; Zhao, P.; Wang, L.; Ding, Y.; Hu, A. Controlled Synthesis of Soluble Conjugated Polymeric Nanoparticles for Fluorescence Detection. RSC Adv. 2017, 7, 25740–25745. [Google Scholar] [CrossRef] [Green Version]
- Farahani, Y.D.; Safarifard, V. Highly Selective Detection of Fe3+, Cd2+ and CH2Cl2 Based on a Fluorescent Zn-MOF with Azine-Decorated Pores. J. Solid State Chem. 2019, 275, 131–140. [Google Scholar] [CrossRef]
- Deng, Y.; Niu, W.; Wang, Z.; Feng, L. Synthesis, Photoelectric Properties and Application of a Polymer Fluorescent Probe with Quinoline and Benzene Groups. Sens. Actuators B Chem. 2017, 238, 613–618. [Google Scholar] [CrossRef]
- Yuan, C.; Chang, Y.; Mao, J.; Yu, S.; Luo, W.; Xu, Y.; Thayumanavan, S.; Dai, L. Supramolecular Assembly of Crosslinkable Monomers for Degradable and Fluorescent Polymer Nanoparticles. J. Mater. Chem. B 2015, 3, 2858–2866. [Google Scholar] [CrossRef]
- Sun, X.L.; Liu, D.M.; Lv, X.H.; Zhou, P.; Sun, M.; Wan, W.M. Thermo-Responsive Rheological Behavior of Borinic Acid Polymer in Dilute Solution. RSC Adv. 2016, 6, 83393–83398. [Google Scholar] [CrossRef]
- Wan, W.-M.; Li, S.-S.; Liu, D.-M.; Lv, X.-H.; Sun, X.-L. Synthesis of Electron-Deficient Borinic Acid Polymers with Multiresponsive Properties and Their Application in the Fluorescence Detection of Alizarin Red S and Electron-Rich 8-Hydroxyquinoline and Fluoride Ion: Substituent Effects. Macromolecules 2017, 50, 6872–6879. [Google Scholar] [CrossRef]
- Ren, Y.; Sezen, M.; Guo, F.; Jäkle, F.; Loo, Y.-L. [d]-Carbon–Carbon Double Bond Engineering in Diazaphosphepines: A Pathway to Modulate the Chemical and Electronic Structures of Heteropines. Chem. Sci. 2016, 7, 4211–4219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, Y.; Yuan, C.; Liu, C.; Mao, J.; Li, Y.; Wu, H.; Wu, Y.; Xu, Y.; Zeng, B.; Dai, L. B, N Co-Doped Carbon from Cross-Linking Induced Self-Organization of Boronate Polymer for Supercapacitor and Oxygen Reduction Reaction. J. Power Sources 2017, 365, 354–361. [Google Scholar] [CrossRef]
- Chang, Y.; Yuan, C.; Li, Y.; Liu, C.; Wu, T.; Zeng, B.; Xu, Y.; Dai, L. Controllable Fabrication of a N and B Co-Doped Carbon Shell on the Surface of TiO2 as a Support for Boosting the Electrochemical Performances. J. Mater. Chem. A 2016, 5, 1672–1678. [Google Scholar] [CrossRef]
- Ji, G.; Wang, N.; Yin, X.; Chen, P. Substituent Effect Induces Emission Modulation of Stilbene Photoswitches by Spatial Tuning of the N/B Electronic Constraints. Org. Lett. 2020, 22, 5758–5762. [Google Scholar] [CrossRef]
- Yin, X.D.; Guo, F.; Lalancette, R.A.; Jäkle, F. Luminescent Main-Chain Organoborane Polymers: Highly Robust, Electron-Deficient Poly(oligothiophene borane)s via Stille Coupling Polymerization. Macromolecules 2016, 49, 537–546. [Google Scholar] [CrossRef]
- Juárez, A.R.; Ortiz-Chi, F.; Pino-Ríos, R.; Cárdenas-Jirón, G.; Villanueva, M.S.; Anota, E.C. The Boron Nitride (B116N124) Fullerene: Stability and Electronic Properties from DFT Simulations. Chem. Phys. Lett. 2020, 741, 137097. [Google Scholar] [CrossRef]
- Sun, C.-J.; Meng, G.; Li, Y.; Wang, N.; Chen, P.; Wang, S.; Yin, X. Millisecond Time-Scale Photoluminescence of B–N-Doped Tetrathienonaphthalene with Borane/Amine Substituents. Inorg. Chem. 2020, 60, 1099–1106. [Google Scholar] [CrossRef]
- Muñoz, A.D.O.; Escobedo-Morales, A.; Skakerzadeh, E.; Anota, E.C. Effect of Homonuclear Boron Bonds in the Adsorption of DNA Nucleobases on Boron Nitride Nanosheets. J. Mol. Liq. 2020, 322, 114951. [Google Scholar] [CrossRef]
- Escobedo-Morales, A.; Tepech-Carrillo, L.; Bautista-Hernández, A.; Camacho-García, J.H.; Cortés-Arriagada, D.; Chigo-Anota, E. Effect of Chemical Order in the Structural Stability and Physicochemical Properties of B12N12 Fullerenes. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Tasseroul, J.; Lorenzo-Garcia, M.M.; Jacopo, D.; François, S.; Simone, V.; Alessandro De Vita, P.T.; Bonifazi, D. Probing Peripheral H-Bonding Functionalities in BN-Doped Polycyclic Aromatic Hydrocarbons. J. Org. Chem. 2020, 85, 3454–3464. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.; Marwitz, A.J.V.; Liu, S.-Y. Recent Advances in Azaborine Chemistry. Angew. Chem. Int. Ed. 2012, 51, 6074–6092. [Google Scholar] [CrossRef] [PubMed]
- Braunschweig, H.; Damme, A.; Jimenez-Halla, J.O.C.; Pfaffinger, B.; Radacki, K.; Wolf, J. Metal-Mediated Synthesis of 1,4-Di-tert-butyl-1,4-azaborine. Angew. Chem. Int. Ed. 2012, 51, 10034–10037. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Lalancette, R.A.; Jaekle, F. Tuning the Structure and Electronic Properties of B–N Fused Dipyridylanthracene and Implications on the Self-Sensitized Reactivity with Singlet Oxygen. J. Am. Chem. Soc. 2019, 141, 7453–7462. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Marder, T.B. B–N Versus C–C: How Similar Are They? Angew. Chem. Int. Ed. 2008, 47, 242–244. [Google Scholar] [CrossRef]
- Xu, S.M.; Zakharov, L.N.; Liu, S.Y. A 1,3-Dihydro-1,3-Azaborine Debuts. J. Am. Chem. Soc. 2011, 133, 20152–20155. [Google Scholar] [CrossRef] [Green Version]
- Wan, W.-M.; Baggett, A.W.; Cheng, F.; Lin, H.; Liu, S.-Y.; Jäkle, F. Synthesis by Free Radical Polymerization and Properties of BN-Polystyrene and BN-Poly(Vinylbiphenyl). Chem. Commun. 2016, 52, 13616–13619. [Google Scholar] [CrossRef]
- Alahmadi, A.F.; Lalancette, R.A.; Jäkle, F. Highly Luminescent Ladderized Fluorene Copolymers Based on B–N Lewis Pair Functionalization. Macromol. Rapid Commun. 2018, 39, 1800456. [Google Scholar] [CrossRef]
- Liu, K.; Lalancette, R.A.; Jäkle, F. B–N Lewis Pair Functionalization of Anthracene: Structural Dynamics, Optoelectronic Properties, and O2 Sensitization. J. Am. Chem. Soc. 2017, 139, 18170–18173. [Google Scholar] [CrossRef]
- Zhang, W.; Li, G.; Xu, L.; Zhuo, Y.; Wan, W.; Yan, N.; He, G. 9,10-Azaboraphenanthrene-Containing Small Molecules and Conjugated Polymers: Synthesis and their Application in Chemodosimeters for the Ratiometric Detection of Fluoride Ions. Chem. Sci. 2018, 9, 4444–4450. [Google Scholar] [CrossRef] [Green Version]
- Wan, W.M.; Tian, D.; Jing, Y.N.; Zhang, X.Y.; Wu, W.; Ren, H.; Bao, H.L. NBN-Doped Conjugated Polycyclic Aromatic Hydrocarbons as an AIEgen Class for Extremely Sensitive Detection of Explosives. Angew. Chem. 2018, 57, 15510–15516. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Li, T.; Sun, X.-L.; Wan, W.-M.; Bao, H.; Qian, Q.; Chen, Q. Unpredicted Concentration-Dependent Sensory Properties of Pyrene-Containing NBN-Doped Polycyclic Aromatic Hydrocarbons. Molecules 2022, 27, 327. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.-M.; Sun, X.-L.; Pan, C.-Y. Morphology Transition in RAFT Polymerization for Formation of Vesicular Morphologies in One Pot. Macromolecules 2009, 42, 4950–4952. [Google Scholar] [CrossRef]
- Cardone, G.; Carotenuto, G.; Conte, P.; Alonzo, G. Synthesis and Characterization of a Novel High Luminescent Gold-2-mercapto-1-methyl-imidazole Complex. Luminescence 2010, 26, 506–509. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Budanovic, M.; Li, T.; Liang, C.; Klein, M.; Soci, C.; Webster, R.D.; Gurzadyan, G.G.; Grimsdale, A.C. Synthesis of 5-Azatetracene and Comparison of Its Optical and Electrochemical Properties with Tetracene. Asian J. Org. Chem. 2021, 10, 2571–2579. [Google Scholar] [CrossRef]
- Li, S.-S.; Zhu, N.; Jing, Y.-N.; Li, Y.; Bao, H.; Wan, W.-M. Barbier Self-Condensing Ketyl Polymerization-Induced Emission: A Polarity Reversal Approach to Reversed Polymerizability. iScience 2020, 23, 101031. [Google Scholar] [CrossRef]
- Shi, Q.-X.; Li, Q.; Xiao, H.; Sun, X.-L.; Bao, H.; Wan, W.-M. Room-Temperature Barbier single-Atom Polymerization Induced Emission as a Versatile Approach for the Utilization of Monofunctional Carboxylic Acid Resources. Polym. Chem. 2021, 13, 592–599. [Google Scholar] [CrossRef]
- Bosdet, M.J.D.; Piers, W.E.; Sorensen, T.S.; Parvez, M. 10a-Aza-10b-borapyrenes: Heterocyclic Analogues of Pyrene with In-ternalized BN Moieties. Angew. Chem. Int. 2007, 119, 5028–5031. [Google Scholar] [CrossRef]
- Thirion, D.; Romain, M.; Berthelot, R.J.; Poriel, C. Intramolecular Excimer Emission as a Blue Light Source Influorescent Organic Light Emitting Diodes: A Promising Molecular Design. J. Mater. Chem. 2012, 22, 7149–7157. [Google Scholar] [CrossRef] [Green Version]
- Thirupathi, P.; Park, J.-Y.; Neupane, L.N.; Kishore, M.Y.L.N.; Lee, K.-H. Pyrene Excimer-Based Peptidyl Chemosensors for the Sensitive Detection of Low Levels of Heparin in 100% Aqueous Solutions and Serum Samples. ACS Appl. Mater. Interfaces 2015, 7, 14243–14253. [Google Scholar] [CrossRef]
- Arvapalli, D.M.; Sheardy, A.T.; Alapati, K.C.; Wei, J. High Quantum Yield Fluorescent Carbon Nanodots for Detection of Fe(III) Ions and Electrochemical Study of Quenching Mechanism. Talanta 2019, 209, 120538. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Sheng, Y.-J.; Sun, X.-L.; Wan, W.-M.; Liu, Y.; Qian, Q.; Chen, Q. Novel NBN-Embedded Polymers and Their Application as Fluorescent Probes in Fe3+ and Cr3+ Detection. Polymers 2022, 14, 2025. https://doi.org/10.3390/polym14102025
Li T, Sheng Y-J, Sun X-L, Wan W-M, Liu Y, Qian Q, Chen Q. Novel NBN-Embedded Polymers and Their Application as Fluorescent Probes in Fe3+ and Cr3+ Detection. Polymers. 2022; 14(10):2025. https://doi.org/10.3390/polym14102025
Chicago/Turabian StyleLi, Tao, Yu-Jing Sheng, Xiao-Li Sun, Wen-Ming Wan, Yanru Liu, Qingrong Qian, and Qinghua Chen. 2022. "Novel NBN-Embedded Polymers and Their Application as Fluorescent Probes in Fe3+ and Cr3+ Detection" Polymers 14, no. 10: 2025. https://doi.org/10.3390/polym14102025
APA StyleLi, T., Sheng, Y.-J., Sun, X.-L., Wan, W.-M., Liu, Y., Qian, Q., & Chen, Q. (2022). Novel NBN-Embedded Polymers and Their Application as Fluorescent Probes in Fe3+ and Cr3+ Detection. Polymers, 14(10), 2025. https://doi.org/10.3390/polym14102025