Novel Copper Complexes as Visible Light Photoinitiators for the Synthesis of Interpenetrating Polymer Networks (IPNs)
Abstract
:1. Introduction
2. Materials and Methods
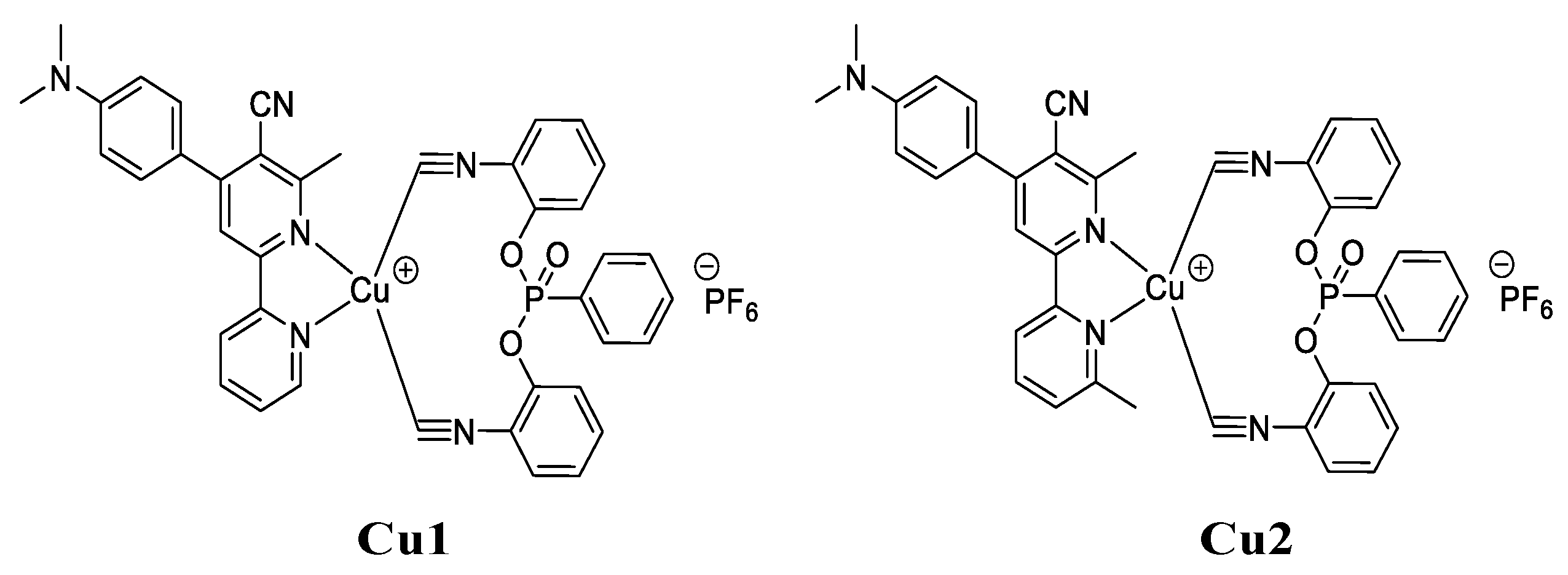
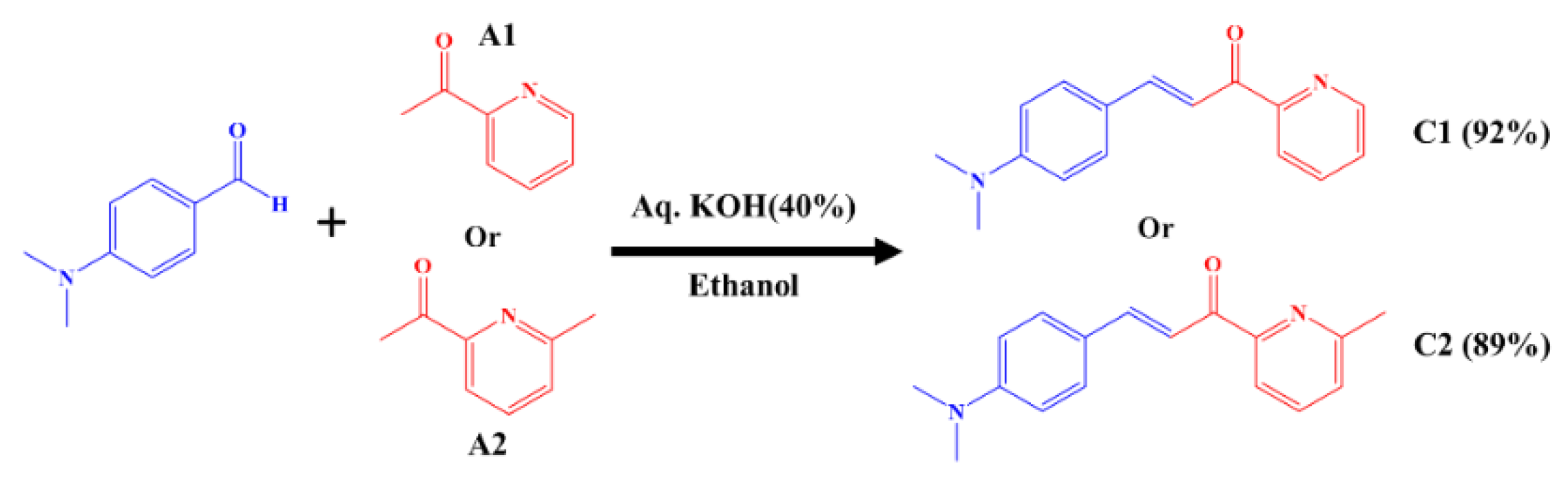
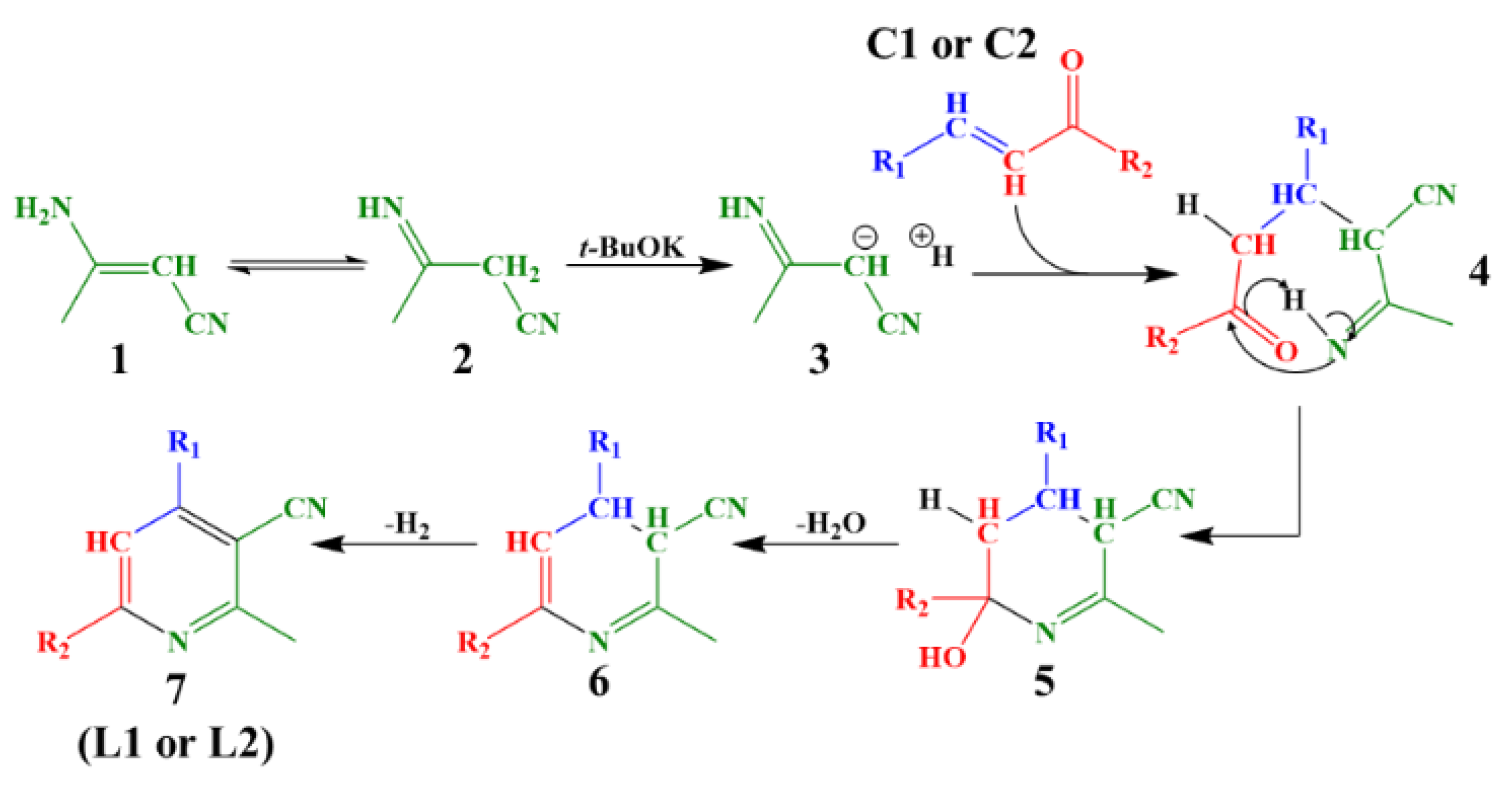
2.1. Synthesis of Chalcones, Ligands and Copper Complexes
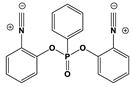
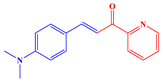
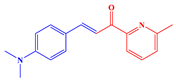
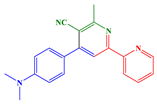
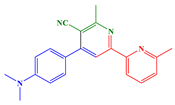
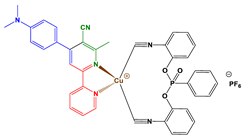
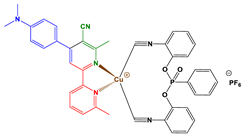
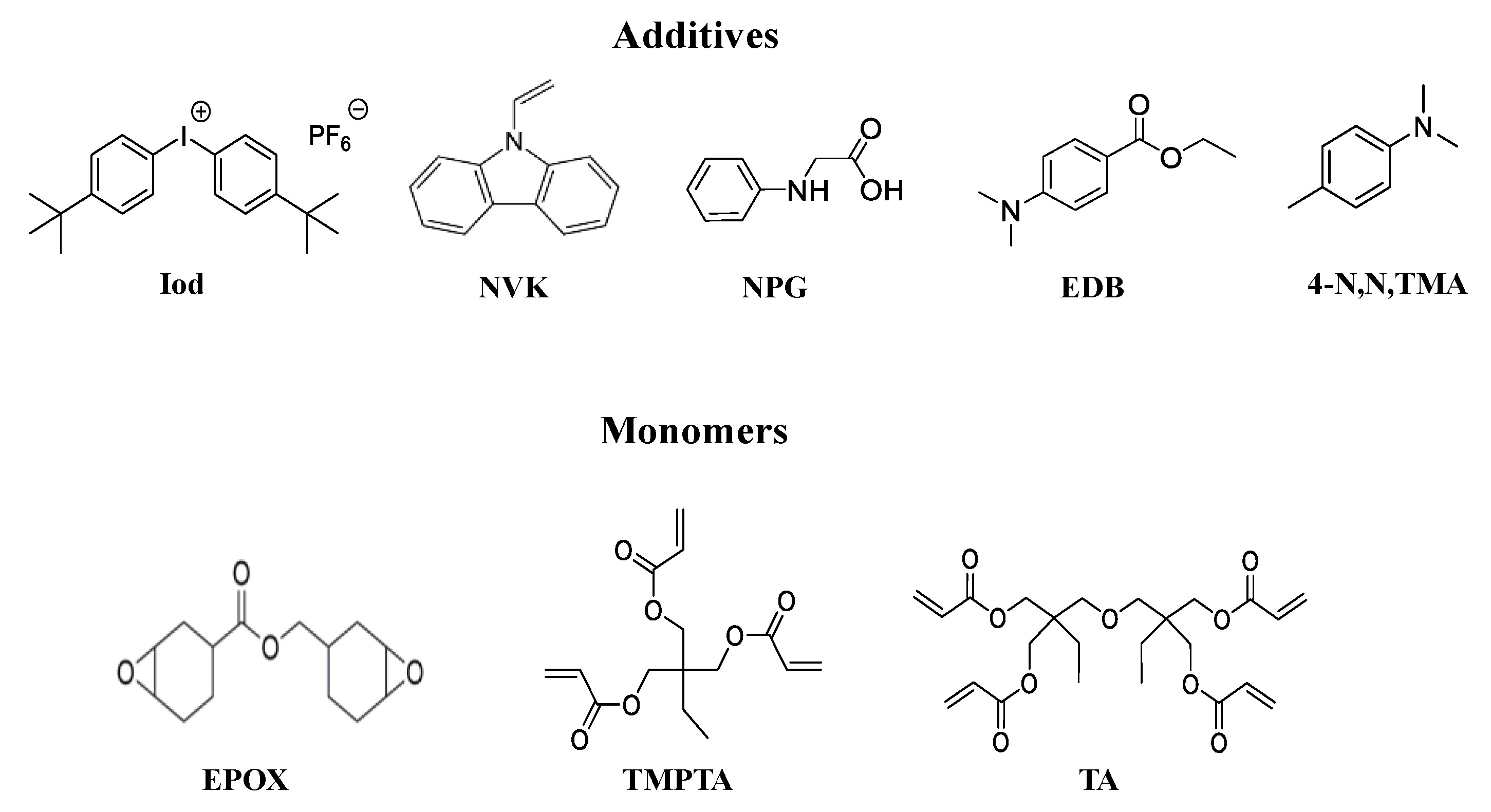
2.2. Other Chemicals
2.3. Irradiation Sources: Light-Emitting Diodes
2.4. Photopolymerization Kinetics Determination by Real-Time Fourier Transform Infrared Spectroscopy (RT-FTIR)
2.5. Redox Potentials: Electrochemical Properties
2.6. UV-Visible Absorption, Steady-State Photolysis and Luminescence Experiments
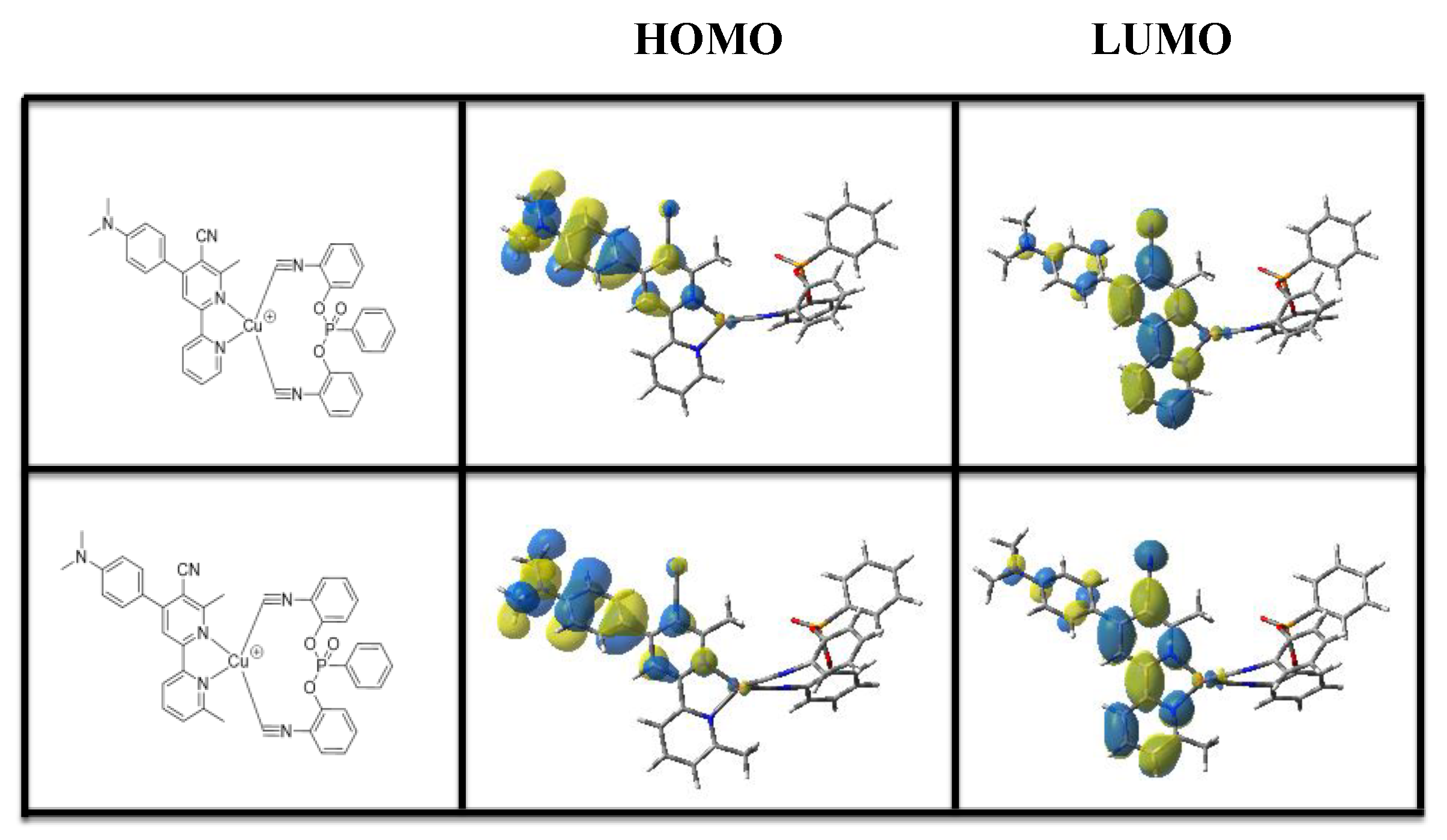
2.7. Computational Procedure
2.8. Photocomposite Access Using a Near-UV Conveyor
2.9. Direct Laser Write (DLW) Experiment
3. Results
3.1. Synthetic Routes to Copper Complexes Cu1 and Cu2
3.2. UV-Visible Absorption Spectra of Cu1 and Cu2
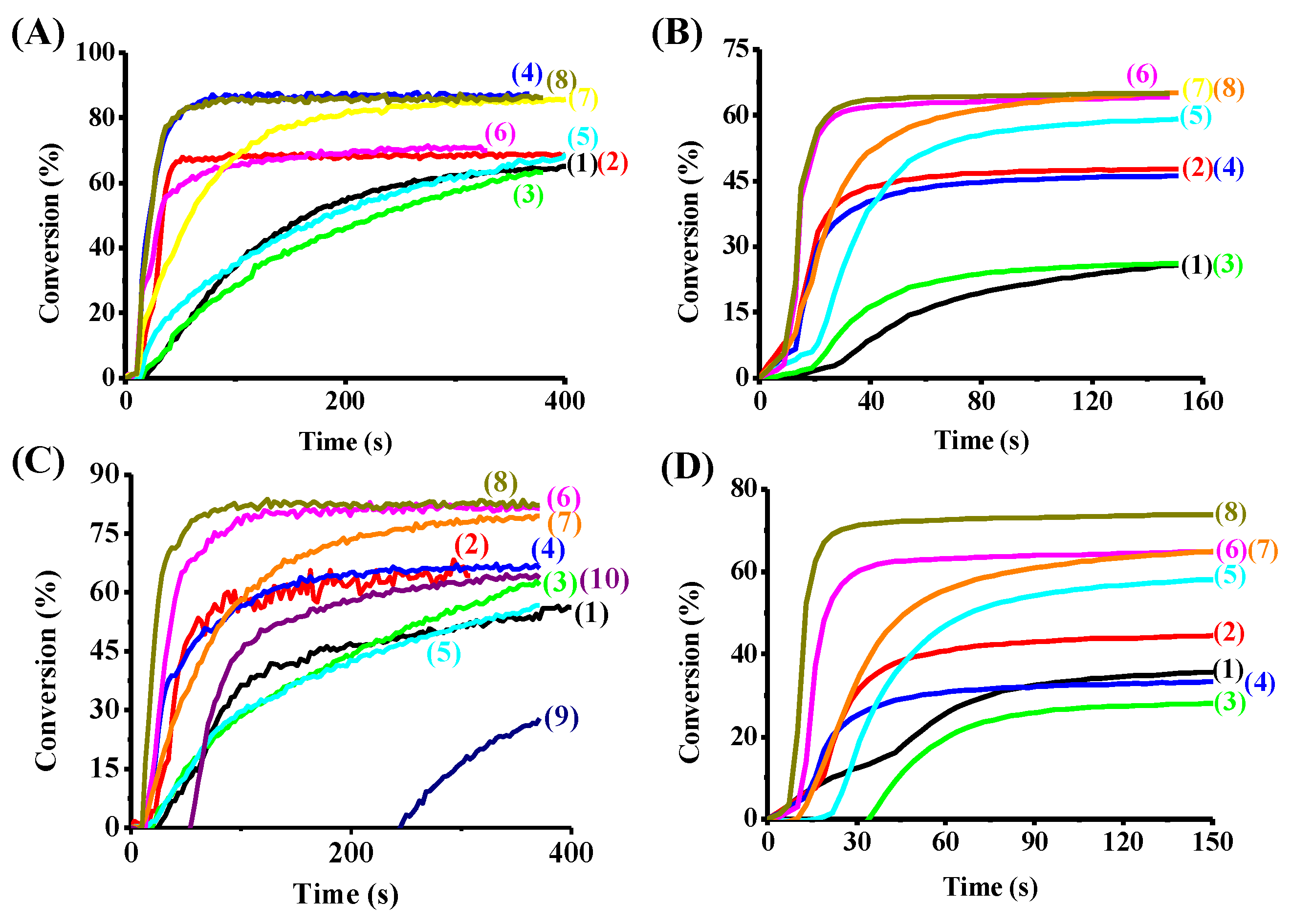
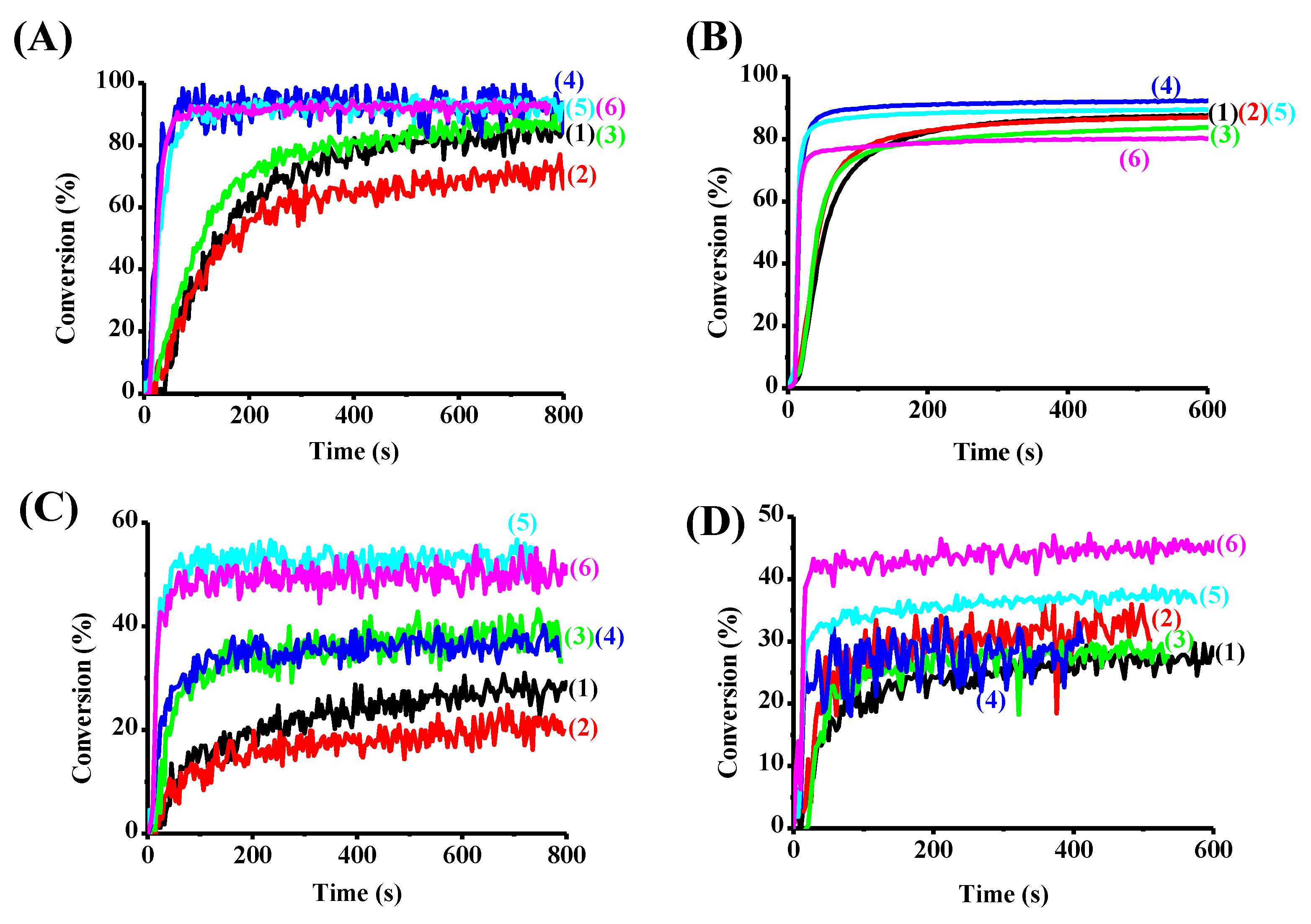
3.3. Photopolymerization Experiments
3.3.1. Free Radical Photopolymerization Using TA as a Benchmark Monomer
3.3.2. Cationic Polymerization and IPN Synthesis
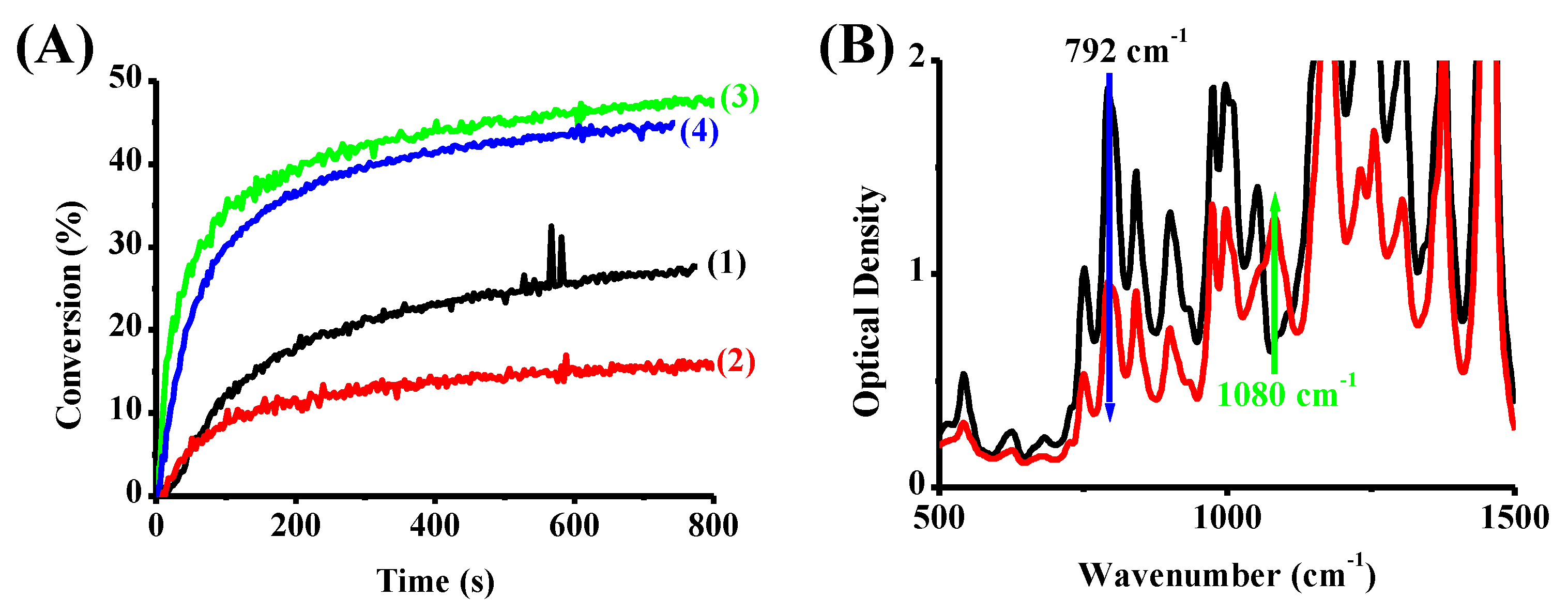
3.4. Photocomposites Synthesis
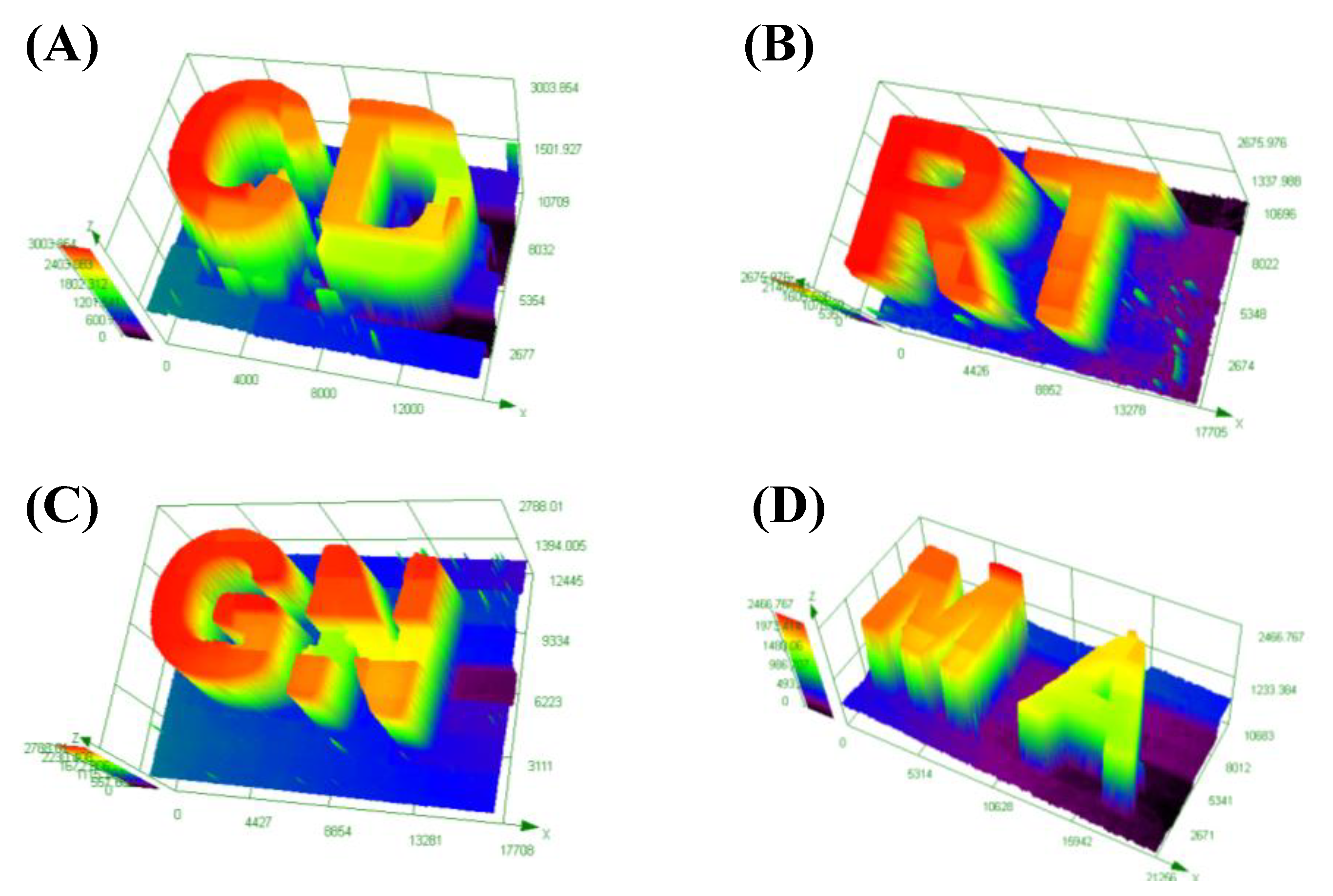
3.5. Direct Laser Write (DLW)
3.6. Mechanical Properties: Tensile Test Measurements
4. Discussion
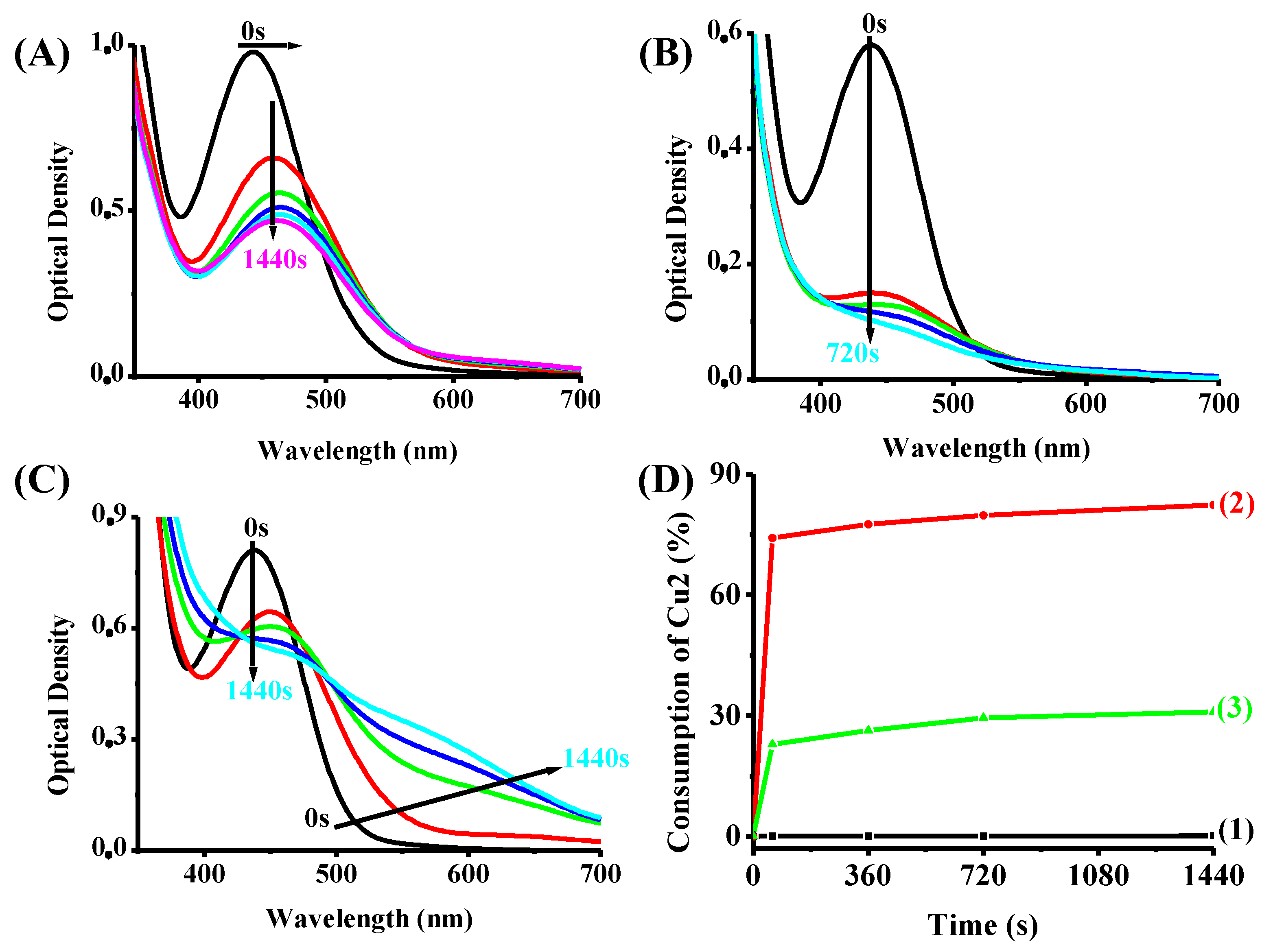
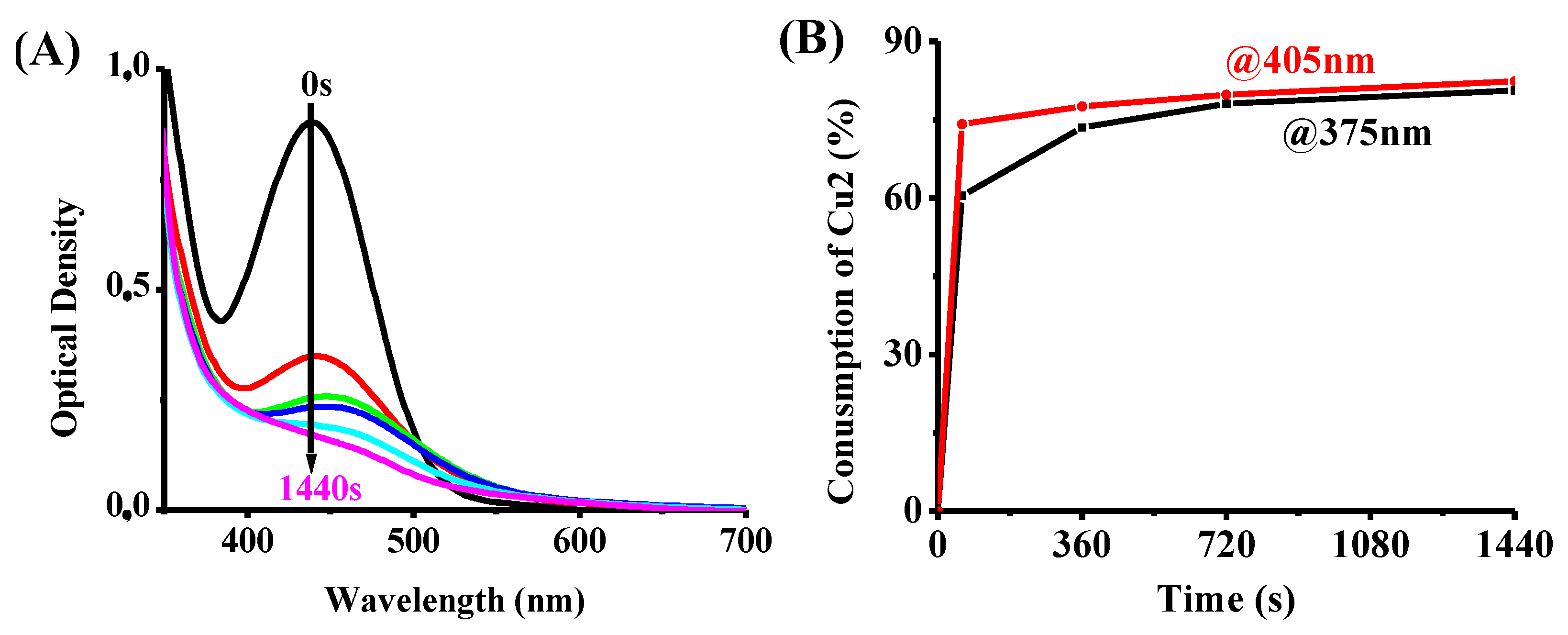
4.1. Steady-State Photolysis of the Investigated Compounds
4.2. Photoluminescence and Electrochemical Properties
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fouassier, J.P.; Lalevée, J. Photoinitiators for Polymer Synthesis, Scope, Reactivity, and Efficiency; Wiley-VCH Verlag: Weinheim, Germany, 2012. [Google Scholar]
- Fouassier, J.P. Photoinitiator, Photopolymerization and Photocuring: Fundamentals and Applications; Hanser Publishers: Munich, Germany, 1995. [Google Scholar]
- Dietliker, K.A. Compilation of Photoinitiators Commercially Available for UV Today; Sita Technology Ltd.: London, UK, 2002. [Google Scholar]
- Davidson, S. Exploring the Science, Technology and Application of UV and EB Curing; Sita Technology Ltd.: London, UK, 1999. [Google Scholar]
- Crivello, J.V.; Dietliker, K.; Bradley, G. Photoinitiators for Free Radical Cationic & Anionic Photopolymerisation; John Wiley & Sons: Chichester, UK, 1999. [Google Scholar]
- Cunningham, A.F.; Desobry, V. Radiation Curing in Polymer Science and Technology; Fouassier, J.P., Rabek, J.F., Eds.; Elsevier: Barking, UK, 1993; Volume 2, p. 323. [Google Scholar]
- Andrzejewska, E. Chapter 2—Free Radical Photopolymerization of Multifunctional Monomers. In Three-Dimensional Microfabrication Using Two-Photon Polymerization; Baldacchini, T., Ed.; Micro and Nano Technologies; William Andrew Publishing: Oxford, UK, 2016; pp. 62–81. ISBN 978-0-323-35321-2. [Google Scholar]
- Luu, T.T.H.; Jia, Z.; Kanaev, A.; Museur, L. Effect of Light Intensity on the Free-Radical Photopolymerization Kinetics of 2-Hydroxyethyl Methacrylate: Experiments and Simulations. J. Phys. Chem. B 2020, 124, 6857–6866. [Google Scholar] [CrossRef] [PubMed]
- Andrezajewska, E.; Grajek, K. Recent Advances in Photo-Induced Free-Radical Polymerization. MOJ Polym. Sci. 2017, 1, 58–60. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Zou, X.; Shi, F.; Liu, R.; Yagci, Y. Highly Efficient Dandelion-like near-Infrared Light Photoinitiator for Free Radical and Thiol-Ene Photopolymerizations. Nat. Commun. 2019, 10, 3560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sangermano, M.; Roppolo, I.; Chiappone, A. New Horizons in Cationic Photopolymerization. Polymers 2018, 10, 136. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Liao, W.; Ni, X. Cationic Photopolymerization Initiated by a Photocatalytic Complex Sensitive to Visible Light at 520 Nm. Catal. Lett. 2021, 151, 1766–1775. [Google Scholar] [CrossRef]
- Bouzrati-Zerelli, M.; Maier, M.; Dietlin, C.; Morlet-Savary, F.; Fouassier, J.P.; Klee, J.E.; Lalevée, J. A novel photoinitiating system producing germyl radicals for the polymerization of representative methacrylate resins: Camphorquinone/R3GeH/iodonium salt. Dent. Mater. 2016, 32, 1226. [Google Scholar] [CrossRef]
- Abdul-Monem, M.M. Naturally Derived Photoinitiators for Dental and Biomaterials Applications. Eur. Dent. Res. Biomater. J. 2021, 1, 72–78. [Google Scholar] [CrossRef]
- Alizadehgharib, S.; Östberg, A.-K.; Dahlstrand Rudin, A.; Dahlgren, U.; Christenson, K. The Effects of the Dental Methacrylates TEGDMA, Bis-GMA, and UDMA on Neutrophils in Vitro. Clin. Exp. Dent. Res. 2020, 6, 439–447. [Google Scholar] [CrossRef]
- Arikawa, H.; Takahashi, H.; Kanie, T.; Ban, S. Effect of Various Visible Light Photoinitiators on the Polymerization and Color of Light-Activated Resins. Dent. Mater. J. 2009, 28, 454–460. [Google Scholar] [CrossRef] [Green Version]
- Catarzi, D.; Cecchi, L.; Colotta, V.; Melani, F.; Filacchioni, G.; Martini, C.; Giusti, L.; Lucacchini, A. Structure-Activity Relationships of 1,2,4-Triazolo [1,5-a]Quinoxalines and Their 1-Deaza Analogs Imidazo [1,2-a]Quinoxalines at the Benzodiazepine Receptor. J. Med. Chem. 1994, 37, 2846–2850. [Google Scholar] [CrossRef]
- Boeira, P.O.; Meereis, C.T.W.; Suárez, C.E.C.; de Almeida, S.M.; Piva, E.; da Silveira Lima, G. Coumarin-Based Iodonium Hexafluoroantimonate as an Alternative Photoinitiator for Experimental Dental Adhesives Resin. Appl. Adhes. Sci. 2017, 5, 2. [Google Scholar] [CrossRef] [Green Version]
- Cadenaro, M.; Antoniolli, F.; Codan, B.; Agee, K.; Tay, F.R.; Dorigo, E.D.S.; Pashley, D.H.; Breschi, L. Influence of Different Initiators on the Degree of Conversion of Experimental Adhesive Blends in Relation to Their Hydrophilicity and Solvent Content. Dent. Mater. 2010, 26, 288–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dickens, S.H.; Stansbury, J.W.; Choi, K.M.; Floyd, C.J.E. Photopolymerization Kinetics of Methacrylate Dental Resins. Macromolecules 2003, 36, 6043–6053. [Google Scholar] [CrossRef]
- Ikemura, K.; Endo, T. A Review of the Development of Radical Photopolymerization Initiators Used for Designing Light-Curing Dental Adhesives and Resin Composites. Dent. Mater. J. 2010, 29, 481–501. [Google Scholar] [CrossRef] [Green Version]
- Maffezzoli, A.; Pietra, A.D.; Rengo, S.; Nicolais, L.; Valletta, G. Photopolymerization of Dental Composite Matrices. Biomaterials 1994, 15, 1221–1228. [Google Scholar] [CrossRef]
- Neumann, M.G.; Schmitt, C.C.; Ferreira, G.C.; Corrêa, I.C. The Initiating Radical Yields and the Efficiency of Polymerization for Various Dental Photoinitiators Excited by Different Light Curing Units. Dent. Mater. 2006, 22, 576–584. [Google Scholar] [CrossRef]
- Maruno, T.; Murata, N. Properties of a UV-Curable, Durable Precision Adhesive. J. Adhes. Sci. Technol. 1995, 9, 1343. [Google Scholar] [CrossRef]
- Besse, V.; Derbanne, M.A.; Pham, T.-N.; Cook, W.D.; Le Pluart, L. Photopolymerization Study and Adhesive Properties of Self-Etch Adhesives Containing Bis(Acyl)Phosphine Oxide Initiator. Dent. Mater. 2016, 32, 561–569. [Google Scholar] [CrossRef] [Green Version]
- Gziut, K.; Kowalczyk, A.; Schmidt, B.; Kowalczyk, K.; Weisbrodt, M. Epoxy-Based Structural Self-Adhesive Tapes Modified with Acrylic Syrups Prepared via a Free Radical Photopolymerization Process. Polymers 2021, 13, 189. [Google Scholar] [CrossRef]
- Zhu, M.; Cao, Z.; Zhou, H.; Xie, Y.; Li, G.; Wang, N.; Liu, Y.; He, L.; Qu, X. Preparation of Environmentally Friendly Acrylic Pressure-Sensitive Adhesives by Bulk Photopolymerization and Their Performance. RSC Adv. 2020, 10, 10277–10284. [Google Scholar] [CrossRef]
- Kilponen, L.; Uusitalo, E.; Tolvanen, M.; Varrela, J.; Vallittu, P.K. Photopolymerization of Light Curing Adhesives Used with Metal Orthodontic Brackets and Matrices. J. Biomater. Tissue Eng. 2016, 6, 659–664. [Google Scholar] [CrossRef]
- Wu, L.; Baghdachi, J. Functional Polymer Coatings: Principles, Methods, and Applications; Wiley Series on Polymer Engineering and Technology: New York, NY, USA, 2015. [Google Scholar]
- Al Rashid, A.; Ahmed, W.; Khalid, M.Y.; Koç, M. Vat Photopolymerization of Polymers and Polymer Composites: Processes and Applications. Addit. Manuf. 2021, 47, 102279. [Google Scholar] [CrossRef]
- Pagac, M.; Hajnys, J.; Ma, Q.-P.; Jancar, L.; Jansa, J.; Stefek, P.; Mesicek, J. A Review of Vat Photopolymerization Technology: Materials, Applications, Challenges, and Future Trends of 3D Printing. Polymers 2021, 13, 598. [Google Scholar] [CrossRef] [PubMed]
- Noè, C.; Hakkarainen, M.; Malburet, S.; Graillot, A.; Adekunle, K.; Skrifvars, M.; Sangermano, M. Frontal-Photopolymerization of Fully Biobased Epoxy Composites. Macromol. Mater. Eng. 2021, 2100864. [Google Scholar] [CrossRef]
- Dikova, T.; Maximov, J.; Todorov, V.; Georgiev, G.; Panov, V. Optimization of Photopolymerization Process of Dental Composites. Processes 2021, 9, 779. [Google Scholar] [CrossRef]
- Mokbel, H.; Anderson, D.; Plenderleith, R.; Dietlin, C.; Morlet-Savary, F.; Dumur, F.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Copper Photoredox Catalyst “G1”: A New High Performance Photoinitiator for near-UV and Visible LEDs. Polym. Chem. 2017, 8, 5580. [Google Scholar] [CrossRef]
- Pereira, R.F.; Bártolo, P.J. Photopolymerizable hydrogels in regenerative medicine and drug delivery. In Hot Topics in Biomaterials; Future Science Book Series Volume 6; Future Science Ltd.: London, UK, 2014. [Google Scholar]
- Xu, X.; Awad, A.; Robles-Martinez, P.; Gaisford, S.; Goyanes, A.; Basit, A.W. Vat Photopolymerization 3D Printing for Advanced Drug Delivery and Medical Device Applications. J. Control. Release 2021, 329, 743–757. [Google Scholar] [CrossRef]
- Chiulan, I.; Heggset, E.B.; Voicu, Ş.I.; Chinga-Carrasco, G. Photopolymerization of Bio-Based Polymers in a Biomedical Engineering Perspective. Biomacromolecules 2021, 22, 1795–1814. [Google Scholar] [CrossRef]
- Elisseeff, J.; Anseth, K.; Sims, D.; McIntosh, W.; Randolph, M.; Langer, R. Transdermal Photopolymerization for Minimally Invasive Implantation. Proc. Natl. Acad. Sci. USA 1999, 96, 3104–3107. [Google Scholar] [CrossRef] [Green Version]
- Bao, Y.; Paunović, N.; Leroux, J.-C. Challenges and Opportunities in 3D Printing of Biodegradable Medical Devices by Emerging Photopolymerization Techniques. Adv. Funct. Mater. 2022, 32, 2109864. [Google Scholar] [CrossRef]
- Baroli, B. Photopolymerization of Biomaterials: Issues and Potentialities in Drug Delivery, Tissue Engineering, and Cell Encapsulation Applications. J. Chem. Technol. Biotechnol. 2006, 81, 491–499. [Google Scholar] [CrossRef]
- Lee, J.Y.; An, J.; Chua, C.K. Fundamentals and Applications of 3D Printing for Novel Materials. Appl. Mater. Today. 2017, 7, 120. [Google Scholar] [CrossRef]
- Andreu, A.; Su, P.-C.; Kim, J.-H.; Ng, C.S.; Kim, S.; Kim, I.; Lee, J.; Noh, J.; Subramanian, A.S.; Yoon, Y.-J. 4D Printing Materials for Vat Photopolymerization. Addit. Manuf. 2021, 44, 102024. [Google Scholar] [CrossRef]
- Bagheri, A.; Jin, J. Photopolymerization in 3D Printing. ACS Appl. Polym. Mater. 2019, 1, 593–611. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Dai, B.; Su, B.; Shi, Y. Recent Advances of 4D Printing Technologies Toward Soft Tactile Sensors. Front. Mater. 2021, 8, 658046. [Google Scholar] [CrossRef]
- Imrie, P.; Jin, J. Polymer 4D Printing: Advanced Shape-Change and Beyond. J. Polym. Sci. 2022, 60, 149–174. [Google Scholar] [CrossRef]
- Zhang, Z.; Corrigan, N.; Bagheri, A.; Jin, J.; Boyer, C. A Versatile 3D and 4D Printing System through Photocontrolled RAFT Polymerization. Angew. Chem. 2019, 131, 18122–18131. [Google Scholar] [CrossRef]
- Schwartz, J.J.; Boydston, A.J. Multimaterial Actinic Spatial Control 3D and 4D Printing. Nat. Commun. 2019, 10, 791. [Google Scholar] [CrossRef]
- Jeong, H.Y.; Woo, B.H.; Kim, N.; Jun, Y.C. Multicolor 4D Printing of Shape-Memory Polymers for Light-Induced Selective Heating and Remote Actuation. Sci. Rep. 2020, 10, 6258. [Google Scholar] [CrossRef]
- Shan, W.; Chen, Y.; Hu, M.; Qin, S.; Liu, P. 4D Printing of Shape Memory Polymer via Liquid Crystal Display (LCD) Stereolithographic 3D Printing. Mater. Res. Express 2020, 7, 105305. [Google Scholar] [CrossRef]
- Choong, Y.Y.C.; Maleksaeedi, S.; Eng, H.; Yu, S.; Wei, J.; Su, P.-C. High Speed 4D Printing of Shape Memory Polymers with Nanosilica. Appl. Mater. Today 2020, 18, 100515. [Google Scholar] [CrossRef]
- Tedla, G.; Jarabek, A.M.; Byrley, P.; Boyes, W.; Rogers, K. Human Exposure to Metals in Consumer-Focused Fused Filament Fabrication (FFF)/ 3D Printing Processes. Sci. Total Environ. 2022, 814, 152622. [Google Scholar] [CrossRef]
- Pierau, L.; Elian, C.; Akimoto, J.; Ito, Y.; Caillol, S.; Versace, D.-L. Bio-Sourced Monomers and Cationic Photopolymerization–The Green Combination towards Eco-Friendly and Non-Toxic Materials. Prog. Polym. Sci. 2022, 127, 101517. [Google Scholar] [CrossRef]
- Leonhardt, S.; Klare, M.; Scheer, M.; Fischer, T.; Cordes, B.; Eblenkamp, M. Biocompatibility of Photopolymers for Additive Manufacturing. Curr. Dir. Biomed. Eng. 2016, 2, 113–116. [Google Scholar] [CrossRef]
- Klikovits, N.; Knaack, P.; Bomze, D.; Krossing, I.; Liska, R. Novel Photoacid Generators for Cationic Photopolymerization. Polym. Chem. 2017, 8, 4414–4421. [Google Scholar] [CrossRef] [Green Version]
- Mueller, M.; Bandl, C.; Kern, W. Surface-Immobilized Photoinitiators for Light Induced Polymerization and Coupling Reactions. Polymers 2022, 14, 608. [Google Scholar] [CrossRef]
- Zhou, J.; Allonas, X.; Ibrahim, A.; Liu, X. Progress in the Development of Polymeric and Multifunctional Photoinitiators. Prog. Polym. Sci. 2019, 99, 101165. [Google Scholar] [CrossRef]
- Tomal, W.; Ortyl, J. Water-Soluble Photoinitiators in Biomedical Applications. Polymers 2020, 12, 1073. [Google Scholar] [CrossRef]
- Photopolymerization Using Metal Oxide Semiconducting Nanoparticles for Epoxy-Based Coatings and Patterned Films. ACS Appl. Nano Mater. 2020, 3, 2875–2880. Available online: https://pubs-acs-org.lama.univ-amu.fr/doi/10.1021/acsanm.0c00147 (accessed on 24 April 2022). [CrossRef]
- Breimer, M.A.; Yevgeny, G.; Sy, S.; Sadik, O.A. Incorporation of Metal Nanoparticles in Photopolymerized Organic Conducting Polymers: A Mechanistic Insight. Nano Lett. 2001, 1, 305–308. [Google Scholar] [CrossRef]
- Guo, J.; Jian, J.; Wang, M.; Tomita, Y.; Cao, L.; Wang, D.; Zhang, X. Ag Nanoparticle-Enhanced Alkyl Radical Generation in Photopolymerization for Holographic Recording. Nanophotonics 2019, 8, 1795–1802. [Google Scholar] [CrossRef]
- He, C.; Feng, Z.; Shan, S.; Wang, M.; Chen, X.; Zou, G. Highly Enantioselective Photo-Polymerization Enhanced by Chiral Nanoparticles and in Situ Photopatterning of Chirality. Nat. Commun. 2020, 11, 1188. [Google Scholar] [CrossRef] [PubMed]
- Ibn-El-Ahrach, H.; Bachelot, R.; Lérondel, G.; Vial, A.; Grimault, A.-S.; Plain, J.; Royer, P.; Soppera, O. Controlling the Plasmon Resonance of Single Metal Nanoparticles by Near-Field Anisotropic Nanoscale Photopolymerization. J. Microsc. 2008, 229, 421–427. [Google Scholar] [CrossRef]
- Ibn El Ahrach, H.; Bachelot, R.; Vial, A.; Lérondel, G.; Plain, J.; Royer, P.; Soppera, O. Spectral Degeneracy Breaking of the Plasmon Resonance of Single Metal Nanoparticles by Nanoscale Near-Field Photopolymerization. Phys. Rev. Lett. 2007, 98, 107402. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Anyaogu, K.C.; Neckers, D.C. Photopolymerization of Gold Nanoparticles: Size-Related Charge Separation and Emission. J. Am. Chem. Soc. 2007, 129, 11324–11325. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Lu, Y.; Wang, J.; Zhao, F.; Wang, Y.; He, H.; Wu, Y. Schiff Base-Type Copper(I) Complexes Exhibiting High Molar Extinction Coefficients: Synthesis, Characterization and DFT Studies. J. Mol. Struct. 2022, 1249, 131638. [Google Scholar] [CrossRef]
- Kuang, D.; Ito, S.; Wenger, B.; Klein, C.; Moser, J.-E.; Humphry-Baker, R.; Zakeeruddin, S.M.; Grätzel, M. High Molar Extinction Coefficient Heteroleptic Ruthenium Complexes for Thin Film Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 2006, 128, 4146–4154. [Google Scholar] [CrossRef]
- Sciortino, G.; Maréchal, J.-D.; Fábián, I.; Lihi, N.; Garribba, E. Quantitative Prediction of Electronic Absorption Spectra of Copper(II)–Bioligand Systems: Validation and Applications. J. Inorg. Biochem. 2020, 204, 110953. [Google Scholar] [CrossRef]
- Faggi, E.; Gavara, R.; Bolte, M.; Fajarí, L.; Juliá, L.; Rodríguez, L.; Alfonso, I. Copper(II) Complexes of Macrocyclic and Open-Chain Pseudopeptidic Ligands: Synthesis, Characterization and Interaction with Dicarboxylates. Dalton Trans. 2015, 44, 12700–12710. [Google Scholar] [CrossRef] [Green Version]
- Iwamura, M.; Takeuchi, S.; Tahara, T. Ultrafast Excited-State Dynamics of Copper(I) Complexes. Acc. Chem. Res. 2015, 48, 782–791. [Google Scholar] [CrossRef]
- Cuttell, D.G.; Kuang, S.-M.; Fanwick, P.E.; McMillin, D.R.; Walton, R.A. Simple Cu(I) Complexes with Unprecedented Excited-State Lifetimes. J. Am. Chem. Soc. 2002, 124, 6–7. [Google Scholar] [CrossRef] [PubMed]
- Giereth, R.; Reim, I.; Frey, W.; Junge, H.; Tschierlei, S.; Karnahl, M. Remarkably Long-Lived Excited States of Copper Photosensitizers Containing an Extended π-System Based on an Anthracene Moiety. Sustain. Energy Fuels 2019, 3, 692–700. [Google Scholar] [CrossRef]
- Egly, J.; Bissessar, D.; Achard, T.; Heinrich, B.; Steffanut, P.; Mauro, M.; Bellemin-Laponnaz, S. Copper(I) Complexes with Remotely Functionalized Phosphine Ligands: Synthesis, Structural Variety, Photophysics and Effect onto the Optical Properties. Inorg. Chim. Acta 2021, 514, 119971. [Google Scholar] [CrossRef]
- Bergmann, L.; Hedley, G.J.; Baumann, T.; Bräse, S.; Samuel, I.D.W. Direct Observation of Intersystem Crossing in a Thermally Activated Delayed Fluorescence Copper Complex in the Solid State. Sci. Adv. 2016, 2, e1500889. [Google Scholar] [CrossRef] [Green Version]
- Ochiai, H.; Miura, T.; Ikoma, T.; Minoura, M.; Nakano, H.; Matano, Y. Copper(II) Complexes of 10,20-Diaryl-5,15-Diazaporphyrin: Alternative Synthesis, Excited State Dynamics, and Substituent Effect on the 1O2-Generation Efficiency. BCSJ 2022, 95, 427–432. [Google Scholar] [CrossRef]
- Fors, B.P.; Hawker, C.J. Control of a Living Radical Polymerization of Methacrylates by light. Angew. Chem. Int. Ed. 2012, 51, 8850. [Google Scholar] [CrossRef]
- Ohtsuki, A.; Goto, A.; Kaji, H. Visible-Light-Induced Reversible Complexation Mediated Living Radical Polymerization of Methacrylates with Organic Catalysts. Macromolecules 2013, 46, 96. [Google Scholar] [CrossRef]
- Cope, J.D.; Valle, H.U.; Hall, R.S.; Riley, K.M.; Goel, E.; Biswas, S.; Hendrich, M.P.; Wipf, D.O.; Stokes, S.L.; Emerson, J.P. Tuning the Copper(II)/Copper(I) Redox Potential for More Robust Copper-Catalyzed C–N Bond Forming Reactions. Eur. J. Inorg. Chem. 2020, 2020, 1278–1285. [Google Scholar] [CrossRef]
- Tano, T.; Okubo, Y.; Kunishita, A.; Kubo, M.; Sugimoto, H.; Fujieda, N.; Ogura, T.; Itoh, S. Redox Properties of a Mononuclear Copper(II)-Superoxide Complex. Inorg. Chem. 2013, 52, 10431–10437. [Google Scholar] [CrossRef]
- Asahi, M.; Yamazaki, S.; Itoh, S.; Ioroi, T. Electrochemical Reduction of Dioxygen by Copper Complexes with Pyridylalkylamine Ligands Dissolved in Aqueous Buffer Solution: The Relationship between Activity and Redox Potential. Dalton Trans. 2014, 43, 10705–10709. [Google Scholar] [CrossRef]
- Nishikawa, M.; Kakizoe, D.; Saito, Y.; Ohishi, T.; Tsubomura, T. Redox Properties of Copper(I) Complex Bearing 4,7-Diphenyl-2,9-Dimethyl-1,10-Phenanthroline and 1,4-Bis(Diphenylphosphino)Butane Ligands and Effects of Light in the Presence of Chloroform. BCSJ 2017, 90, 286–288. [Google Scholar] [CrossRef]
- Araya, L.M.; Vargas, J.A.; Costamagna, J.A. Ligand Influence on the Redox Properties of Some Copper(II) Complexes with Schiff Bases Derived from Bromosalicylaldehydes and Methyl or Chloro-Substituted Anilines. Transit. Met. Chem. 1986, 11, 312–316. [Google Scholar] [CrossRef]
- Das, A.; Ren, Y.; Hessin, C.; Murr, M.D.-E. Copper Catalysis with Redox-Active Ligands. Beilstein J. Org. Chem. 2020, 16, 858–870. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Yang, X.; Deng, Z.; Zhang, L.; An, J. Copper Piperazine Complex with a High Diffusion Coefficient for Dye-Sensitized Solar Cells. ACS Appl. Energy Mater. 2021, 4, 14004–14013. [Google Scholar] [CrossRef]
- Dragonetti, C.; Magni, M.; Colombo, A.; Fagnani, F.; Roberto, D.; Melchiorre, F.; Biagini, P.; Fantacci, S. Towards Efficient Sustainable Full-Copper Dye-Sensitized Solar Cells. Dalton Trans. 2019, 48, 9703–9711. [Google Scholar] [CrossRef]
- Giordano, M.; Volpi, G.; Bonomo, M.; Mariani, P.; Garino, C.; Viscardi, G. Methoxy-Substituted Copper Complexes as Possible Redox Mediators in Dye-Sensitized Solar Cells. New J. Chem. 2021, 45, 15303–15311. [Google Scholar] [CrossRef]
- Conradie, J. Polypyridyl Copper Complexes as Dye Sensitizer and Redox Mediator for Dye-Sensitized Solar Cells. Electrochem. Commun. 2022, 134, 107182. [Google Scholar] [CrossRef]
- Magni, M.; Biagini, P.; Colombo, A.; Dragonetti, C.; Roberto, D.; Valore, A. Versatile Copper Complexes as a Convenient Springboard for Both Dyes and Redox Mediators in Dye Sensitized Solar Cells. Coord. Chem. Rev. 2016, 322, 69–93. [Google Scholar] [CrossRef]
- Michaels, H.; Benesperi, I.; Edvinsson, T.; Muñoz-Garcia, A.B.; Pavone, M.; Boschloo, G.; Freitag, M. Copper Complexes with Tetradentate Ligands for Enhanced Charge Transport in Dye-Sensitized Solar Cells. Inorganics 2018, 6, 53. [Google Scholar] [CrossRef] [Green Version]
- Rui, H.; Shen, J.; Yu, Z.; Li, L.; Han, H.; Sun, L. Stable Dye-Sensitized Solar Cells Based on Copper(II/I) Redox Mediators Bearing a Pentadentate Ligand. Angew. Chem. Int. Ed. 2021, 60, 16156–16163. [Google Scholar] [CrossRef]
- Dumur, F. Recent advances on visible light metal-based photocatalysts for polymerization under low light intensity. Catalysts 2019, 9, 736. [Google Scholar] [CrossRef] [Green Version]
- Noirbent, G.; Dumur, F. Recent advances on copper complexes as visible light photoinitiators and (photo)redox initiators of polymerization. Catalysts 2020, 10, 953. [Google Scholar] [CrossRef]
- Zivic, N.; Kuroishi, P.K.; Dumur, F.; Gigmes, D.; Dove, A.P.; Sardon, H. Recent advances and challenges in the design of organic photoacid and photobase generators for polymerizations. Angew. Chem. Int. Ed. 2019, 58, 10410–10422. [Google Scholar] [CrossRef] [PubMed]
- Tehfe, M.-A.; Lalevée, J.; Dumur, F.; Telitel, S.; Gigmes, D.; Contal, E.; Bertin, D.; Fouassier, J.-P. Zinc-based metal complexes as new photocatalysts in polymerization initiating systems. Eur. Polym. J. 2013, 49, 1040–1049. [Google Scholar] [CrossRef]
- Lalevée, J.; Telitel, S.; Xiao, P.; Lepeltier, M.; Dumur, F.; Morlet-Savary, F.; Gigmes, D.; Fouassier, J.-P. Metal and metal free photocatalysts: Mechanistic approach and application as photoinitiators of photopolymerization. Beilstein J. Org. Chem. 2014, 10, 863–876. [Google Scholar] [CrossRef] [Green Version]
- Baralle, A.; Fensterbank, L.; Goddard, J.P.; Olivier, C. Aryl Radical Formation by Copper (I) Photocatalyzed Reduction of Diaryliodonium Salts. NMR Evidences for a Cu (II)/Cu (I) Mechanism. Chem. Eur. J. 2013, 19, 10809. [Google Scholar] [CrossRef]
- Paria, S.; Reiser, O. Copper in Photocatalysis. ChemCatChem 2014, 6, 2477. [Google Scholar] [CrossRef]
- Reiser, O. Shining Light on Copper: Unique Opportunities for Visible-Light-Catalyzed Atom Transfer Radical Addition Reactions and Related Processes. Acc. Chem. Res. 2016, 49, 1990. [Google Scholar] [CrossRef]
- Hernandez-Perez, A.C.; Collins, S.K. Heteroleptic Cu-Based Sensitizers in Photoredox Catalysis. Acc. Chem. Res. 2016, 49, 1557. [Google Scholar] [CrossRef]
- Hernandez-Perez, A.C.; Collins, S.K. A visible-light-mediated synthesis of carbazoles. Angew. Chem. Int. Ed. 2013, 52, 12696. [Google Scholar] [CrossRef]
- Mau, A.; Dietlin, C.; Dumur, F.; Lalevée, J. Concomitant initiation of radical and cationic polymerisations using new copper complexes as photoinitiators: Synthesis and characterisation of acrylate/epoxy interpenetrated polymer networks. Eur. Polym. J. 2021, 152, 110457. [Google Scholar] [CrossRef]
- Mau, A.; Noirbent, G.; Dietlin, C.; Graff, B.; Gigmes, D.; Dumur, F.; Lalevée, J. Panchromatic Copper Complexes for Visible Light Photopolymerization. Photochem 2021, 1, 167–189. [Google Scholar] [CrossRef]
- Mokbel, H.; Anderson, D.; Plenderleith, R.; Dietlin, C.; Morlet-Savary, F.; Dumur, F.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Simultaneous initiation of radical and cationic polymerization reactions using the “G1” copper complex as photoredox catalyst: Applications of free radical/cationic hybrid photopolymerization in the composites and 3D printing fields. Prog. Org. Coat. 2019, 132, 50. [Google Scholar] [CrossRef]
- AL Mousawi, A.; Kermagoret, A.; Versace, D.L.; Toufaily, J.; Hamieh, T.; Graff, B.; Dumur, F.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Copper photoredox catalysts for polymerization upon near UV or visible light: Structure/reactivity/efficiency relationships and use in LED projector 3D printing resins. Polym. Chem. 2017, 8, 568. [Google Scholar] [CrossRef]
- Xiao, P.; Dumur, F.; Zhang, J.; Fouassier, J.-P.; Gigmes, D.; Lalevée, J. Copper complexes in radical photoinitiating systems: Applications to free radical and cationic polymerization under visible lights. Macromolecules 2014, 47, 3837–3844. [Google Scholar] [CrossRef]
- Matsui, M.; Matsumoto, K.; Shibata, K.; Muramatsu, H. Synthesis and Characterization of 5-Cyano-6-Methyl-2,2′-Bipyridine Metal-Complex Dyes. Dyes Pigments 1992, 18, 47–55. [Google Scholar] [CrossRef]
- Matsui, M.; Oji, A.; Hiramatsu, K.; Shibata, K.; Muramatsu, H. Synthesis and Characterization of Fluorescent 4,6-Disubstituted-3-Cyano-2-Methylpyridines. J. Chem. Soc. Perkin Trans. 2 1992, 2, 201–206. [Google Scholar] [CrossRef]
- Knorn, M.; Rawner, T.; Czerwieniec, R.; Reiser, O. [Copper(phenanthroline)(bisisonitrile)]+-Complexes for the Visible-Light-Mediated Atom Transfer Radical Addition and Allylation Reactions. ACS Catal. 2015, 5, 5186–5193. [Google Scholar] [CrossRef]
- Kaeser, A.; Mohankumar, M.; Mohanraj, J.; Monti, F.; Holler, M.; Cid, J.J.; Moudam, O.; Nierengarten, I.; Karmazin-Brelot, L.; Duhayon, C.; et al. Heteroleptic Copper(I) Complexes Prepared from Phenanthroline and Bis-Phosphine Ligands. Inorg. Chem. 2013, 52, 12140–12151. [Google Scholar] [CrossRef]
- Wang, X.; Bai, X.; Su, D.; Zhang, Y.; Li, P.; Lu, S.; Gong, Y.; Zhang, W.; Tang, B. Simultaneous Fluorescence Imaging Reveals N-Methyl-d-aspartic Acid Receptor Dependent Zn2+/H+ Flux in the Brains of Mice with Depression. Anal. Chem. 2020, 92, 4101. [Google Scholar] [CrossRef]
- Foresman, J.B.; Frisch, A. Exploring Chemistry with Electronic Structure Methods, 2nd ed.; Gaussian Inc.: Pittsburgh, PA, USA, 1996. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Zakrzewski, V.G.; Montgomery, J.A.; Stratmann, J.R.E.; Burant, J.C.; et al. Gaussian 03, Revision B-2; Gaussian Inc.: Pittsburgh, PA, USA, 2003. [Google Scholar]
- Al Mousawi, A.; Dumur, F.; Garra, P.; Toufaily, J.; Hamieh, T.; Goubard, F.; Bui, T.T.; Graff, B.; Gigmes, D.; Fouassier, J.P.; et al. Azahelicenes as visible light photoinitiators for cationic and radical polymerization: Preparation of photoluminescent polymers and use in high performance LED projector 3D printing resins. J. Polym. Sci. A Polym. Chem. 2017, 55, 1189. [Google Scholar] [CrossRef]
- Dong, F.; Jian, C.; Zhenghao, F.; Kai, G.; Zuliang, L. Synthesis of Chalcones via Claisen–Schmidt Condensation Reaction Catalyzed by Acyclic Acidic Ionic Liquids. Catal. Commun. 2008, 9, 1924–1927. [Google Scholar] [CrossRef]
- Rafiee, E.; Rahimi, F. A Green Approach to the Synthesis of Chalcones via Claisen-Schmidt Condensation Reaction Using Cesium Salts of 12-Tungstophosphoric Acid as a Reusable Nanocatalyst. Monatsh. Chem. 2013, 144, 361–367. [Google Scholar] [CrossRef]
- Mousavi, S.R. Claisen–Schmidt Condensation: Synthesis of (1S,6R)/(1R,6S)-2-Oxo-N,4,6-Triarylcyclohex-3-Enecarboxamide Derivatives with Different Substituents in H2O/EtOH. Chirality 2016, 28, 728–736. [Google Scholar] [CrossRef]
- Kumar, D.; Suresh; Sandhu, J.S. An Efficient Green Protocol for the Synthesis of Chalcones by a Claisen–Schmidt Reaction Using Bismuth(III)Chloride as a Catalyst under Solvent-Free Condition. Green Chem. Lett. Rev. 2010, 3, 283–286. [Google Scholar] [CrossRef]
- Ekanayake, U.G.M.; Weerathunga, H.; Weerasinghe, J.; Waclawik, E.R.; Sun, Z.; MacLeod, J.M.; O’Mullane, A.P.; Ostrikov, K. (Ken) Sustainable Claisen-Schmidt Chalcone Synthesis Catalysed by Plasma-Recovered MgO Nanosheets from Seawater. Sustain. Mater. Technol. 2022, 32, e00394. [Google Scholar] [CrossRef]
- Parekh, A.K.; Desai, K.K. Synthesis and Antibacterial Activity of Chalcones and Pyrimidine-2-Ones. E-J. Chem. 1900, 2, 134830. [Google Scholar] [CrossRef] [Green Version]
- Jesus, A.R.; Marques, A.P.; Rauter, A.P. An Easy Approach to Dihydrochalcones via Chalcone in Situ Hydrogenation. Pure Appl. Chem. 2016, 88, 349–361. [Google Scholar] [CrossRef]
- Julakanti, S.R.; Patel, M.; Ponneri, V. Highly Efficient Synthesis of Chalcones from Poly Carbonyl Aromatic Compounds Using BF3–Et2O via a Regioselective Condensation Reaction. Chem. Pharm. Bull. 2016, 64, 570–576. [Google Scholar] [CrossRef] [Green Version]
- Winter, C.; Caetano, J.N.; Araújo, A.B.C.; Chaves, A.R.; Ostroski, I.C.; Vaz, B.G.; Pérez, C.N.; Alonso, C.G. Activated Carbons for Chalcone Production: Claisen-Schmidt Condensation Reaction. Chem. Eng. J. 2016, 303, 604–610. [Google Scholar] [CrossRef]
- Qian, H.; Liu, D.; Lv, C. Synthesis of Chalcones via Claisen-Schmidt Reaction Catalyzed by Sulfonic Acid-Functional Ionic Liquids. Ind. Eng. Chem. Res. 2011, 50, 1146–1149. [Google Scholar] [CrossRef]
- Calvino, V.; Picallo, M.; López-Peinado, A.J.; Martín-Aranda, R.M.; Durán-Valle, C.J. Ultrasound Accelerated Claisen–Schmidt Condensation: A Green Route to Chalcones. Appl. Surf. Sci. 2006, 252, 6071–6074. [Google Scholar] [CrossRef]
- Li, J.-T.; Yang, W.-Z.; Wang, S.-X.; Li, S.-H.; Li, T.-S. Improved Synthesis of Chalcones under Ultrasound Irradiation. Ultrason. Sonochem. 2002, 9, 237–239. [Google Scholar] [CrossRef]
- Bui, T.H.; Nguyen, N.T.; Dang, P.H.; Nguyen, H.X.; Nguyen, M.T.T. Design and Synthesis of Chalcone Derivatives as Potential Non-Purine Xanthine Oxidase Inhibitors. SpringerPlus 2016, 5, 1789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
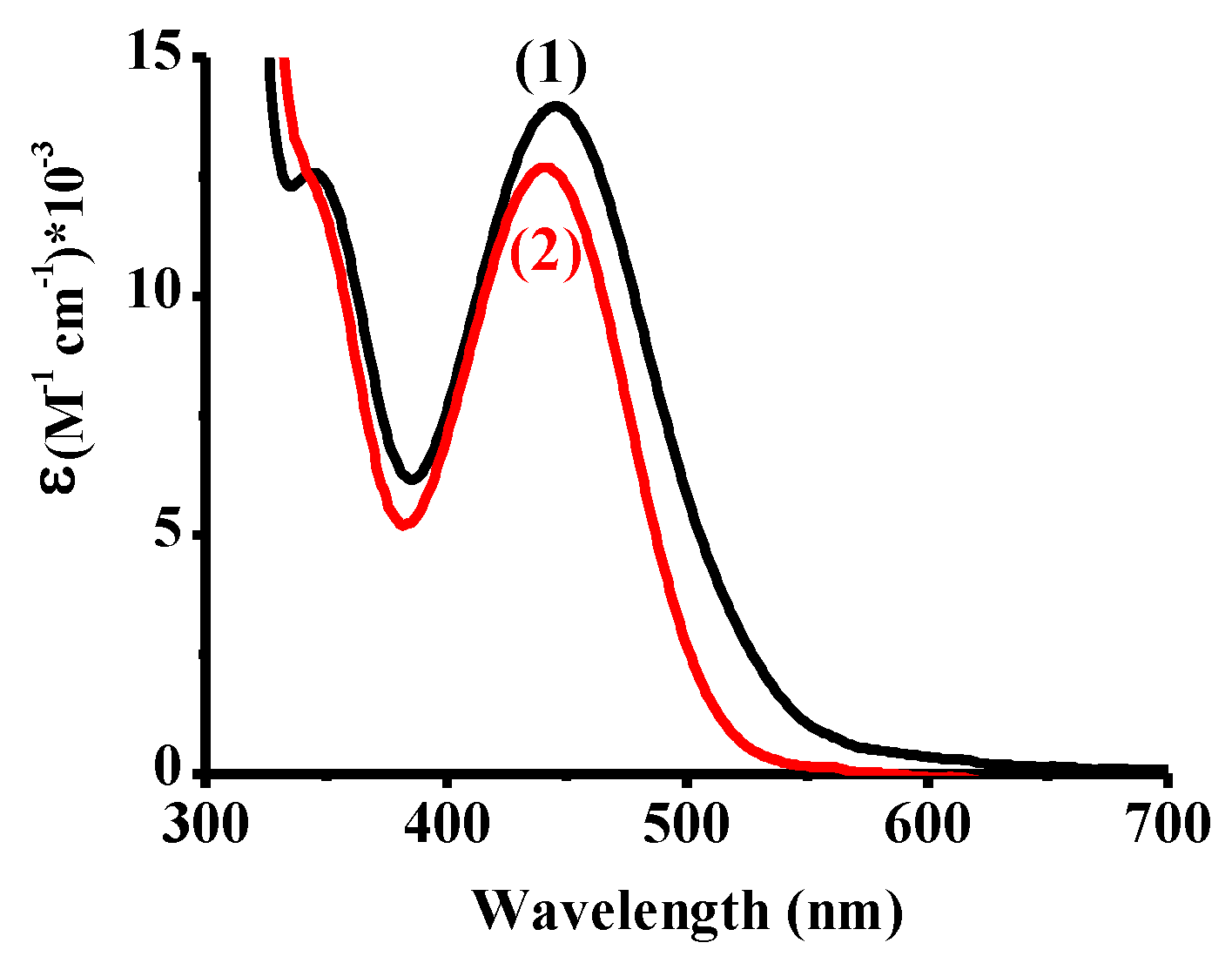
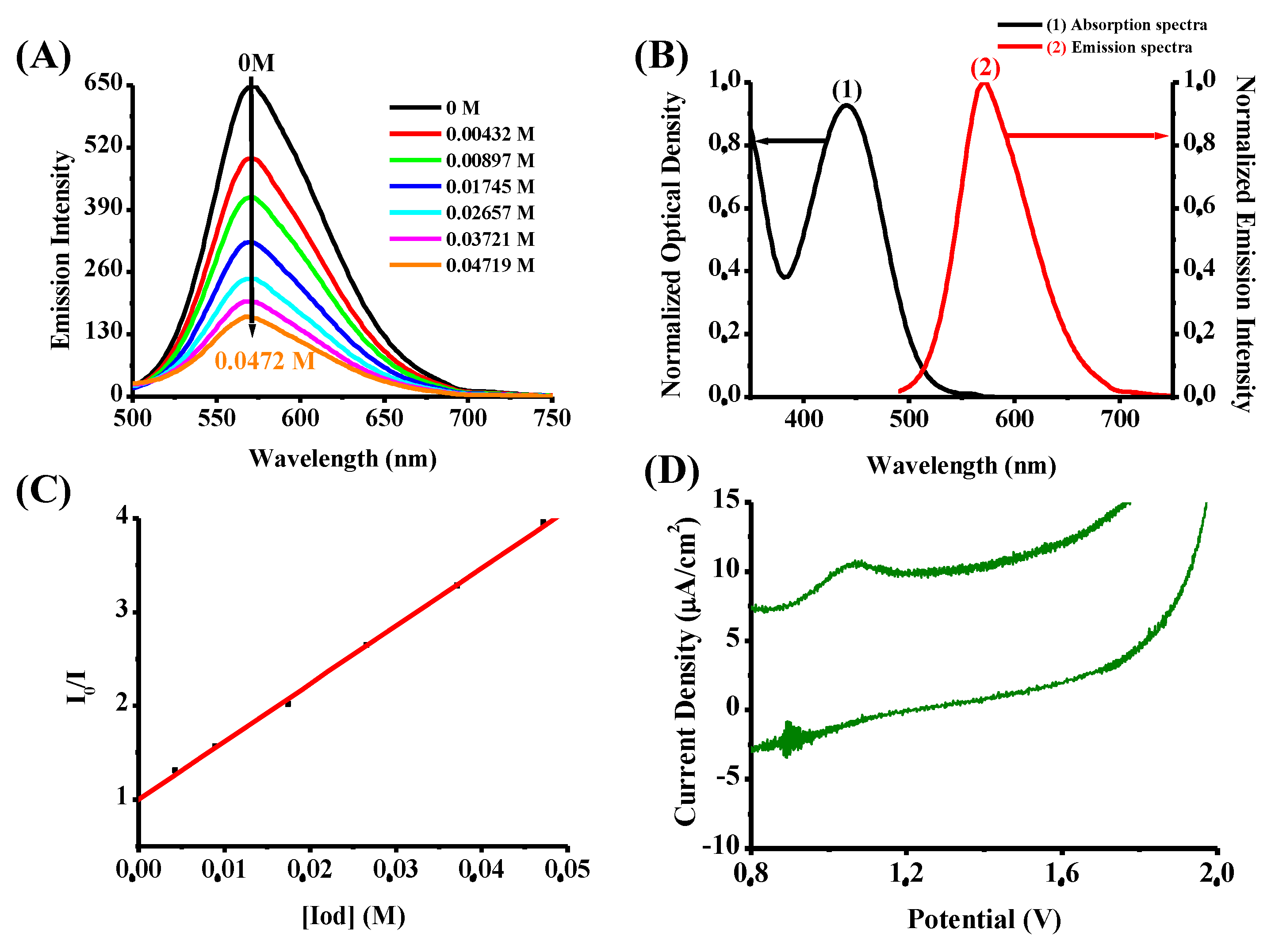
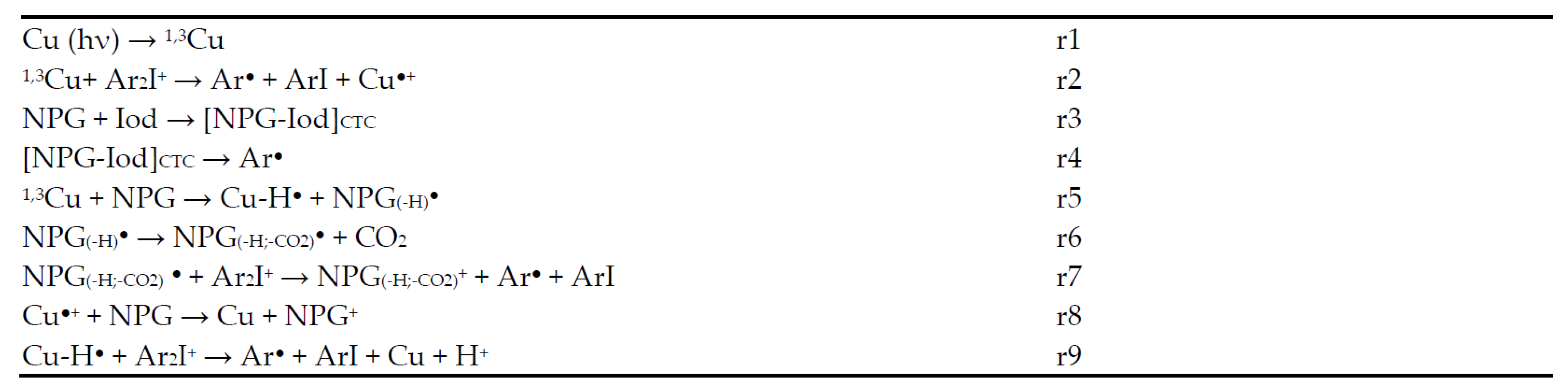
| λmax (nm) | εmax (M−1∙cm−1) | ε375nm (M−1∙cm−1) | ε405nm (M−1∙cm−1) | ε455nm (M−1∙cm−1) | |
|---|---|---|---|---|---|
| Cu1 | 445 | 14,000 | 7150 | 8460 | 13,600 |
| Cu2 | 441 | 12,700 | 5760 | 7950 | 11,740 |
| At 405 nm | At 455 nm | |||||||
|---|---|---|---|---|---|---|---|---|
| Thick Sample | Thin Sample | Thick Sample | Thin Sample | |||||
| Cu1/Iod | 56% a | 62% b | 36% a | 28% b | 64% a | 64% b | 26% a | 26% b |
| Cu2/Iod | 64% a | 67% b | 45% a | 33% b | 70% a | 87% b | 48% a | 46% b |
| Cu1/Iod/amine | 57% c | 80% d | 58% c | 65% d | 69% c | 85% d | 59% c | 65% d |
| Cu2/Iod/amine | 82% c | 83% d | 65% c | 74% d | 71% c | 87% d | 64% c | 65% d |
| Cu/Iod (0.1%/1% w/w) | Cu/Iod/NVK (0.1%/2%/3% w/w/w) | |
|---|---|---|
| Cu1 | 27% | 49% |
| Cu2 | 16% | 45% |
| IPN Synthesis of TA/EPOX Blend Performed in Thick Sample at 405 nm | IPN Synthesis of TA/EPOX Blend Performed in Thin Sample at 405 nm | |||||
|---|---|---|---|---|---|---|
| 30%/70% | 50%/50% | 30%/70% | 30%/70% | 50%/50% | 70%/30% | |
| Cu1 | 90%/25% | 90%/15% | 93%/27% | 88%/25% | 87%/31% | 84%/47% |
| Cu2 | 99%/30% | 98%/20% | 96%/38% | 92%/22% | 90%/35% | 80%/32% |
| IPN Synthesis of TA/EPOX Blend Performed in Thick Sample @455 nm | IPN Synthesis of TA/EPOX Blend Performed in Thin Sample @455 nm | |||||
|---|---|---|---|---|---|---|
| 30%/70% | 50%/50% | 30%/70% | 30%/70% | 50%/50% | 70%/30% | |
| Cu1 | 90%/30% | 90%/23% | 90%/41% | 90%/15% | 90%/25% | 90%/15% |
| Cu2 | 100%/38% | 98%/55% | 99%/51% | 98%/22% | 99%/30% | 98%/22% |
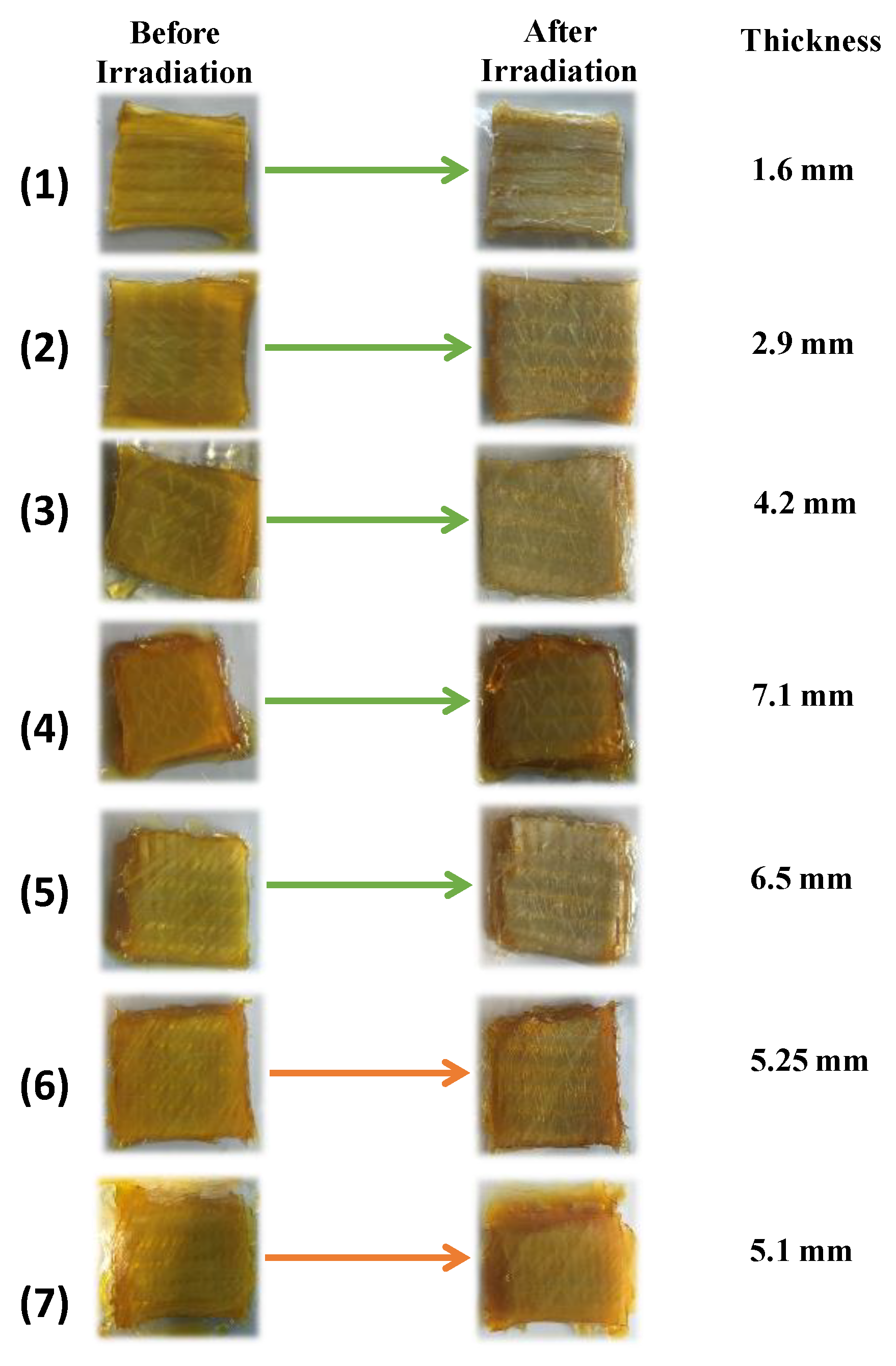
| Thickness | Number of Passes to Reach Tack-Free Character on the Surface | Number of Passes to Reach Tack-Free Character on the Bottom | |
|---|---|---|---|
| Cu2/Iod/NPG | 1.6 mm | 1 | 1 |
| Cu2/Iod/NPG | 2.9 mm | 1 | 2 |
| Cu2/Iod/NPG | 4.2 mm | 1 | 6 |
| Cu2/Iod/NPG (TMPTA) | 7.1 mm | 1 | 25 |
| Cu2/Iod/NPG | 6.5 mm | 1 | 30 |
| Cu1/Iod/NPG | 5.25 mm | 1 | 40 |
| Cu1/Iod/NPG (TMPTA) | 5.1 mm | 1 | 45 |
| Tensile Strength [MPa] | 0.1%PA/Iod/NPG TA/EPOX (30% 70%) @ 395 nm | 0.1% PA/Iod/NPG TA/EPOX (50% 50%) @ 395 nm | 0.1% PA/Iod/NPG TA/EPOX (70% 30%) @ 395 nm |
|---|---|---|---|
| Cu1 | 6.5 | 7.3 | 34.3 |
| Cu 2 | 7.2 | 26.3 | 37.2 |
| Eox (V) | ES1 (eV) | ΔG(Cu/Iod) (eV) | ΚSV(Cu/Iod) | Φ(Cu/Iod) | Eox (V) | |
|---|---|---|---|---|---|---|
| Cu1 | 1.07 | 2.34 | −0.62 | 13.58 | 0.55 | Cu1 |
| Cu2 | 0.97 | 2.41 | −0.74 | 61.83 | 0.74 | Cu2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahal, M.; Noirbent, G.; Graff, B.; Toufaily, J.; Hamieh, T.; Gigmes, D.; Dumur, F.; Lalevée, J. Novel Copper Complexes as Visible Light Photoinitiators for the Synthesis of Interpenetrating Polymer Networks (IPNs). Polymers 2022, 14, 1998. https://doi.org/10.3390/polym14101998
Rahal M, Noirbent G, Graff B, Toufaily J, Hamieh T, Gigmes D, Dumur F, Lalevée J. Novel Copper Complexes as Visible Light Photoinitiators for the Synthesis of Interpenetrating Polymer Networks (IPNs). Polymers. 2022; 14(10):1998. https://doi.org/10.3390/polym14101998
Chicago/Turabian StyleRahal, Mahmoud, Guillaume Noirbent, Bernadette Graff, Joumana Toufaily, Tayssir Hamieh, Didier Gigmes, Frédéric Dumur, and Jacques Lalevée. 2022. "Novel Copper Complexes as Visible Light Photoinitiators for the Synthesis of Interpenetrating Polymer Networks (IPNs)" Polymers 14, no. 10: 1998. https://doi.org/10.3390/polym14101998
APA StyleRahal, M., Noirbent, G., Graff, B., Toufaily, J., Hamieh, T., Gigmes, D., Dumur, F., & Lalevée, J. (2022). Novel Copper Complexes as Visible Light Photoinitiators for the Synthesis of Interpenetrating Polymer Networks (IPNs). Polymers, 14(10), 1998. https://doi.org/10.3390/polym14101998











