Polymers in High-Efficiency Solar Cells: The Latest Reports
Abstract
:1. Introduction
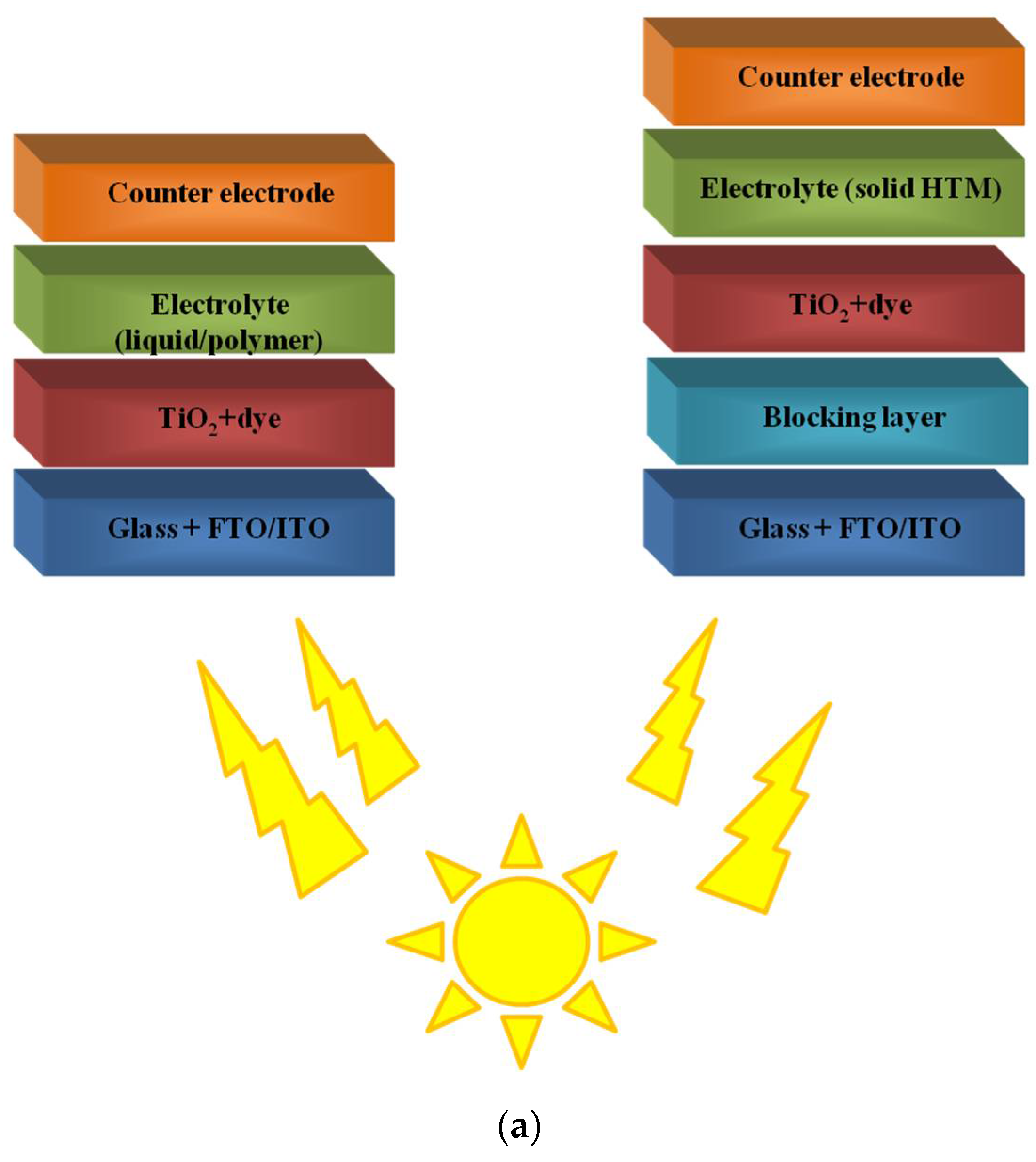
2. Polymers in Dye-Sensitized Solar Cells (DSSCs)
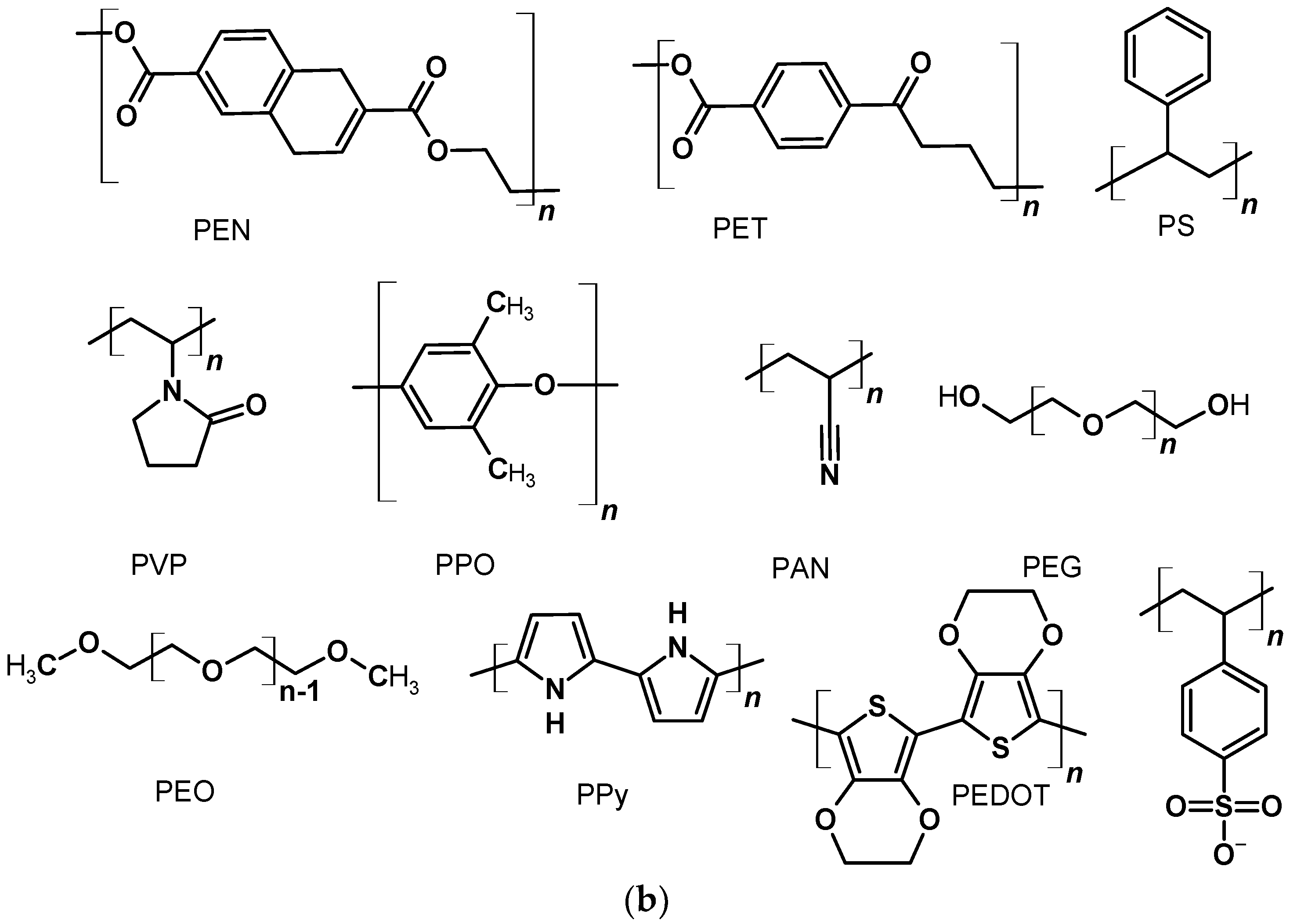
2.1. Polymers as a Flexible Substrates
2.2. Polymers in Mesoporous Layer of Photoanode
2.3. Polymers as Dyes in DSSCs
2.4. Polymers in Gel Electrolyte to Quasi-Solid State DSSCs
2.5. Conductive Polymers as Counter-Electrodes
2.5.1. Polypyrrole as Counter-Electrode
2.5.2. Counter-Electrodes Based on Polypyrrole Nanocomposites
2.5.3. Counter-Electrodes Based on Polyaniline
2.5.4. Counter-Electrodes Based on PANI-Nanocomposites
2.5.5. Counter-Electrodes Based on Poly(3,4-Ethylenedioxythiophene)
2.5.6. Counter-Electrode Based on PEDOT-Nanocomposites
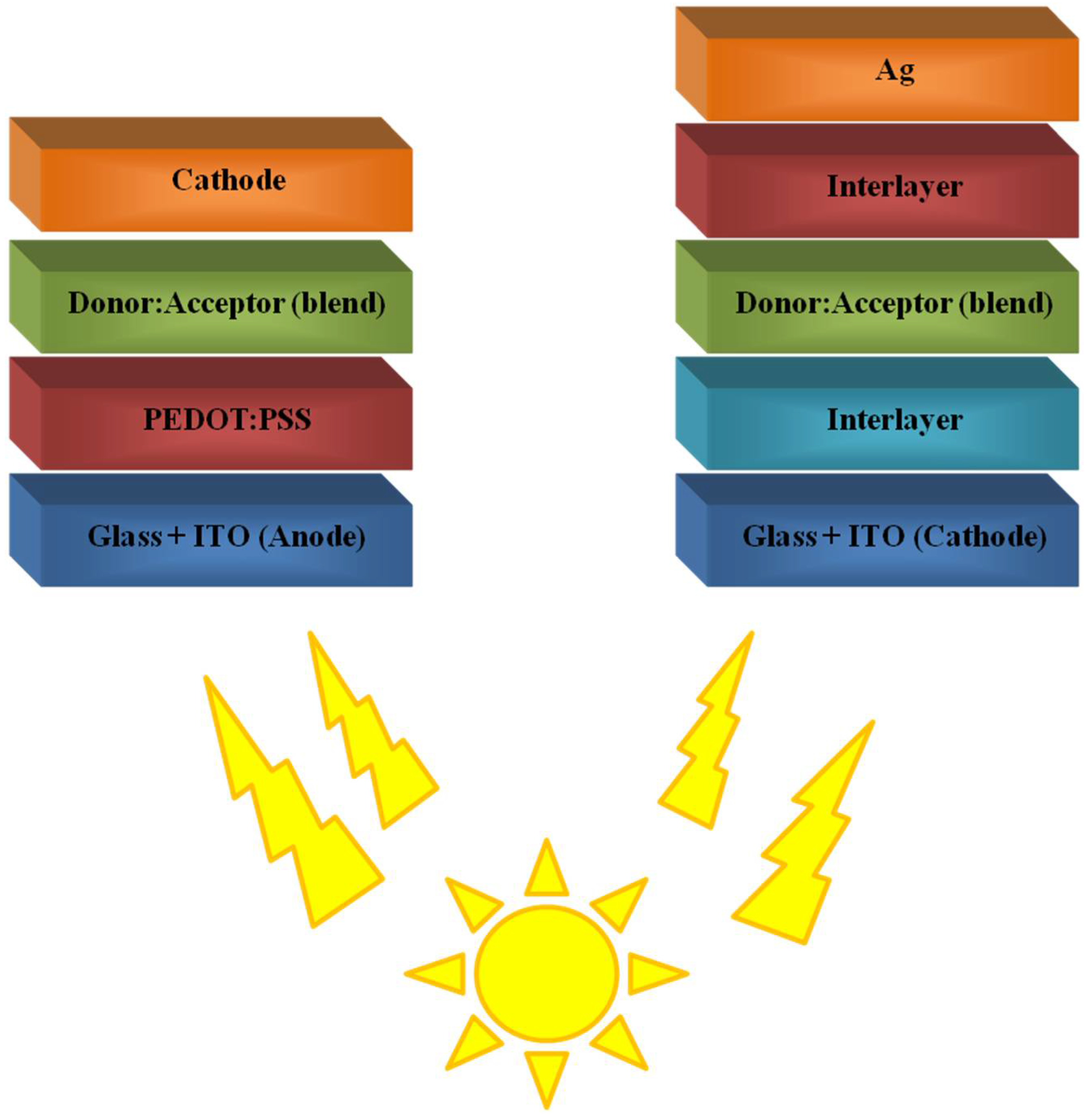
3. Polymers in Bulk-Heterojunction Solar Cells (BHJ)
3.1. Polymers as Donors Materials
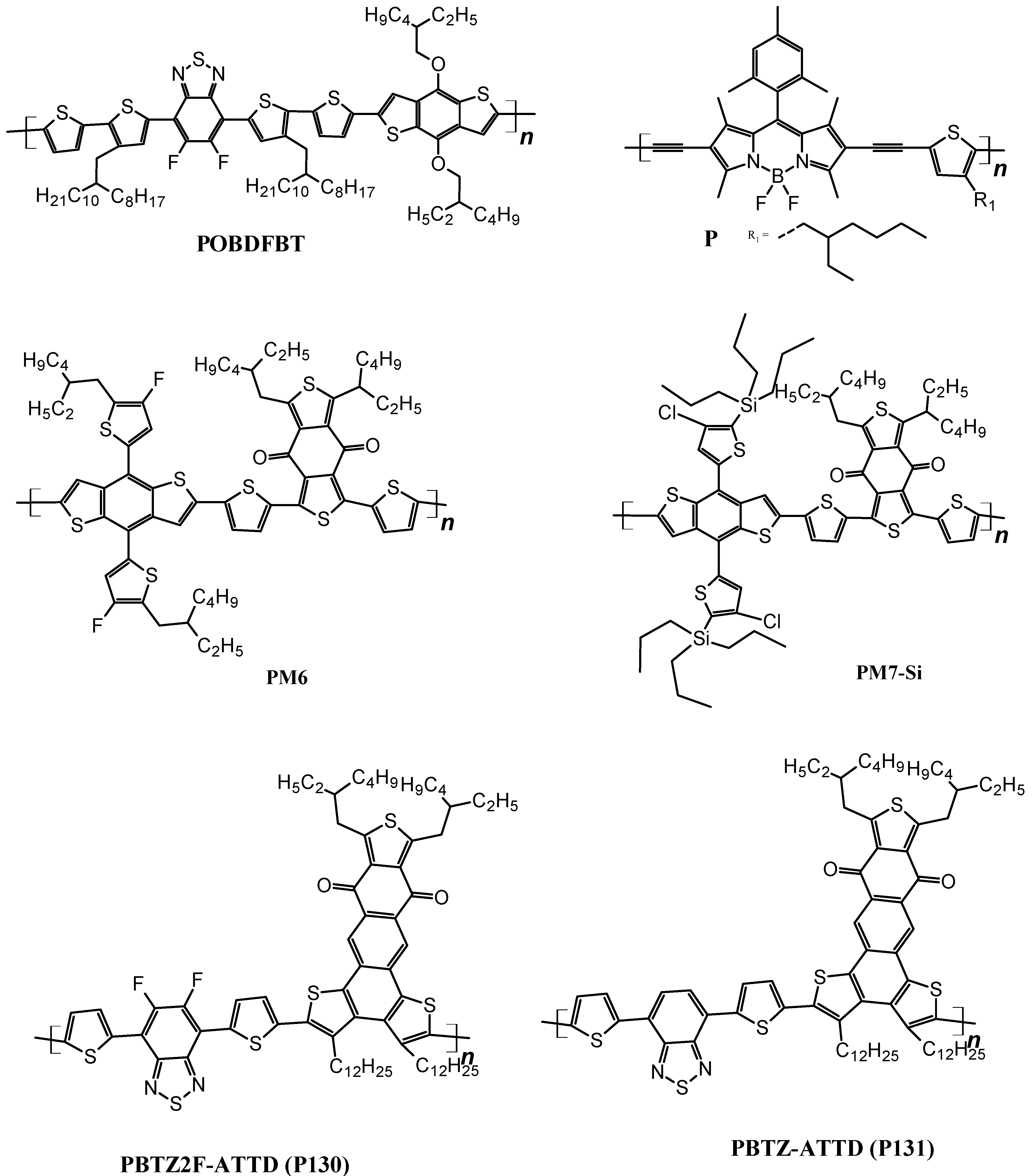
3.1.1. Wide-Bandgap Polymer Donors
3.1.2. Medium-Bandgap Polymer Donors
3.1.3. Narrow Bandgap Polymers
3.2. Polymers as Acceptor Materials
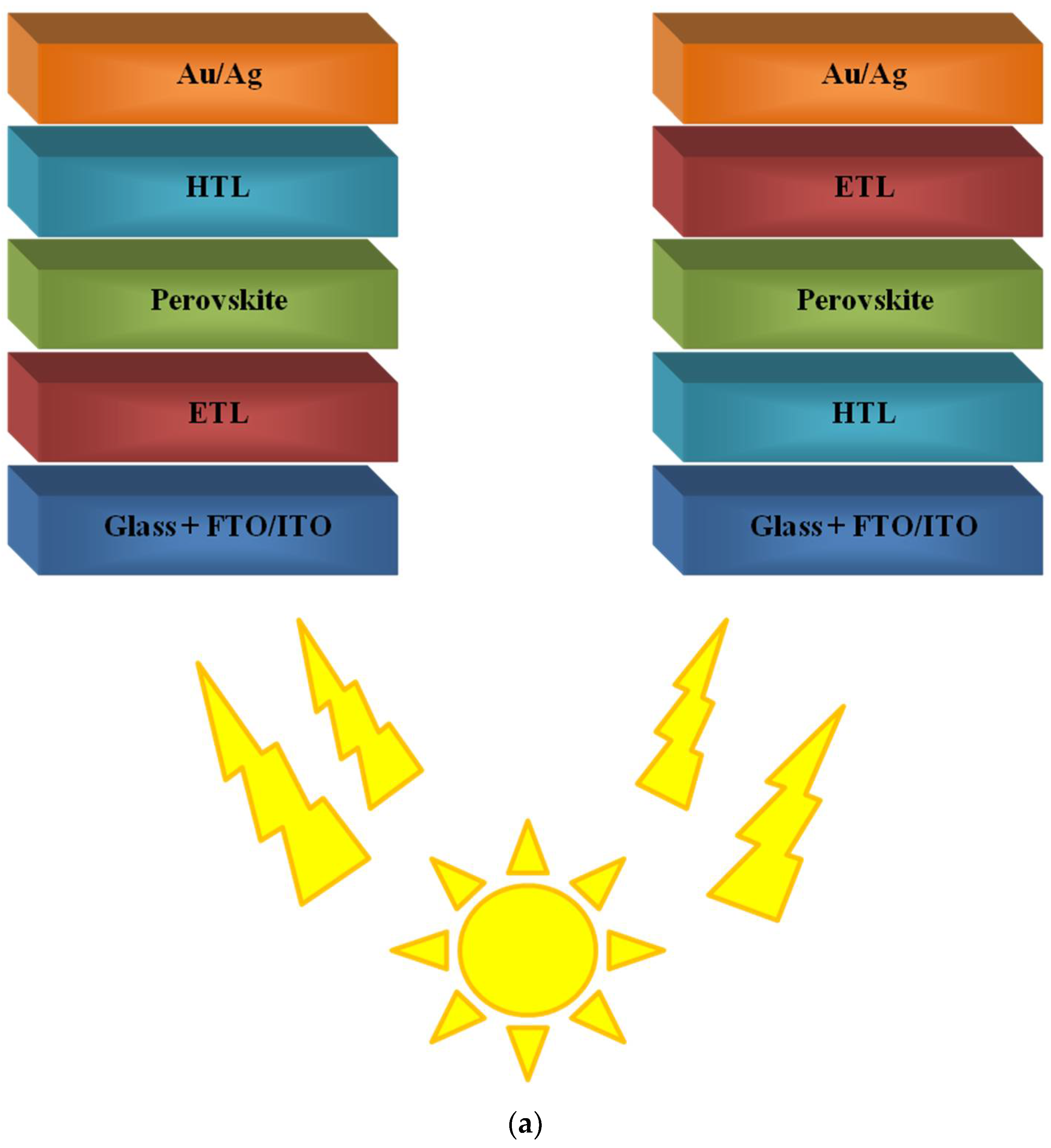
4. Polymers in Perovskite Solar Cells (PSCs)
4.1. Polymers in Improving Perovskite Morphology
4.2. Polymers as Hole-Transporting Materials (HTM)
4.3. Polymers as Additives of Electron Transport Layers (ETL) and Electron-Transporting Materials (ETM)
4.4. Polymeric Interlayer/s
5. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grätzel, M. Recent advances in sensitized mesoscopic solar cells. Acc. Chem. Res. 2009, 42, 1788–1798. [Google Scholar] [CrossRef] [PubMed]
- Khatibi, A.; Razi Astaraei, F.; Ahmadi, M.H. Generation and combination of the solar cells: A current model review. Energy Sci. Eng. 2019, 7, 305–322. [Google Scholar] [CrossRef] [Green Version]
- Palm, J.; Probst, V.; Karg, F.H. Second generation CIS solar modules. Sol. Energy 2004, 77, 757–765. [Google Scholar] [CrossRef]
- El Chaar, L.; Lamont, L.A.; El Zein, N. Review of photovoltaic technologies. Renew. Sustain. Energy Rev. 2011, 15, 2165–2175. [Google Scholar] [CrossRef]
- Parida, B.; Iniyan, S.; Goic, R. A review of solar photovoltaic technologies. Renew. Sustain. Energy Rev. 2011, 15, 1625–1636. [Google Scholar] [CrossRef]
- Polman, A.; Knight, M.; Garnett, E.C.; Ehrler, B.; Sinke, W.C. Photovoltaic materials: Present efficiencies and future challenges. Science 2016, 352, aad4424. [Google Scholar] [CrossRef] [Green Version]
- NREL. Best Research-Cell Efficiencies: Rev. 04-06-2020. Best Res. Effic. Chart|Photovolt. Res.|NREL. 2020. Available online: https://www.nrel.gov/pv/cell-efficiency.html (accessed on 15 February 2022).
- Roncali, J.; Leriche, P.; Blanchard, P. Molecular materials for organic photovoltaics: Small is beautiful. Adv. Mater. 2014, 26, 3821–3838. [Google Scholar] [CrossRef] [Green Version]
- Ye, M.; Wen, X.; Wang, M.; Iocozzia, J.; Zhang, N.; Lin, C.; Lin, Z. Recent advances in dye-sensitized solar cells: From photoanodes, sensitizers and electrolytes to counter electrodes. Mater. Today 2015, 18, 155–162. [Google Scholar] [CrossRef]
- Gnida, P.; Libera, M.; Pająk, A.; Schab-Balcerzak, E. Examination of the Effect of Selected Factors on the Photovoltaic Response of Dye-Sensitized Solar Cells. Energy Fuels 2020, 34, 14344–14355. [Google Scholar] [CrossRef]
- Song, L.; Du, P.; Xiong, J.; Ko, F.; Cui, C. Efficiency enhancement of dye-sensitized solar cells by optimization of electrospun ZnO nanowire/nanoparticle hybrid photoanode and combined modification. Electrochim. Acta 2015, 163, 330–337. [Google Scholar] [CrossRef]
- Nath, B.; Pradhan, B.; Panda, S.K. Optical tunability of lead free double perovskite Cs2AgInCl6: Via composition variation. New J. Chem. 2020, 44, 18656–18661. [Google Scholar] [CrossRef]
- Ji, J.M.; Zhou, H.; Eom, Y.K.; Kim, C.H.; Kim, H.K. 14.2% Efficiency Dye-Sensitized Solar Cells by Co-sensitizing Novel Thieno[3,2-b]indole-Based Organic Dyes with a Promising Porphyrin Sensitizer. Adv. Energy Mater. 2020, 10, 2000124. [Google Scholar] [CrossRef]
- Kakiage, K.; Aoyama, Y.; Yano, T.; Oya, K.; Fujisawa, J.I.; Hanaya, M. Highly-efficient dye-sensitized solar cells with collaborative sensitization by silyl-anchor and carboxy-anchor dyes. Chem. Commun. 2015, 51, 15894–15897. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Jiang, Y.; Jin, K.; Qin, J.; Xu, J.; Li, W.; Xiong, J.; Liu, J.; Xiao, Z.; Sun, K.; et al. 18% Efficiency organic solar cells. Sci. Bull. 2020, 65, 272–275. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.W.; Jeon, S.J.; Lee, H.S.; Park, H.; Kim, K.S.; Lee, H.W.; Moon, D.K. Evaporation-Free Nonfullerene Flexible Organic Solar Cell Modules Manufactured by An All-Solution Process. Adv. Energy Mater. 2019, 9, 1902065. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, S.; Lee, S.; Ahn, H.; Joe, S.Y.; Kim, B.J.; Son, H.J. Low-Temperature Processable High-Performance D–A-Type Random Copolymers for Nonfullerene Polymer Solar Cells and Application to Flexible Devices. Adv. Energy Mater. 2018, 8, 1801601. [Google Scholar] [CrossRef]
- Gong, S.C.; Jang, S.K.; Ryu, S.O.; Jeon, H.; Park, H.H.; Chang, H.J. Post annealing effect of flexible polymer solar cells to improve their electrical properties. Curr. Appl. Phys. 2010, 10, e192–e196. [Google Scholar] [CrossRef]
- Jung, H.S.; Han, G.S.; Park, N.G.; Ko, M.J. Flexible Perovskite Solar Cells. Joule 2019, 3, 1850–1880. [Google Scholar] [CrossRef]
- Feng, J.; Zhu, X.; Yang, Z.; Zhang, X.; Niu, J.; Wang, Z.; Zuo, S.; Priya, S.; Liu, S.F.; Yang, D. Record Efficiency Stable Flexible Perovskite Solar Cell Using Effective Additive Assistant Strategy. Adv. Mater. 2018, 30, e1801418. [Google Scholar] [CrossRef]
- Yang, D.; Yang, R.; Priya, S.; Liu, S.F. Recent Advances in Flexible Perovskite Solar Cells: Fabrication and Applications. Angew. Chemie—Int. Ed. 2019, 58, 4466–4483. [Google Scholar] [CrossRef]
- Noorasid, N.S.; Arith, F.; Mustafa, A.N.; Azam, M.A.; Mahalingam, S.; Chelvanathan, P.; Amin, N. Current advancement of flexible dye sensitized solar cell: A review. Optik 2022, 254, 168089. [Google Scholar] [CrossRef]
- Devadiga, D.; Selvakumar, M.; Shetty, P.; Santosh, M.S. The integration of flexible dye-sensitized solar cells and storage devices towards wearable self-charging power systems: A review. Renew. Sustain. Energy Rev. 2022, 159, 112252. [Google Scholar] [CrossRef]
- Fan, X. Flexible dye-sensitized solar cells assisted with lead-free perovskite halide. J. Mater. Res. 2022, 37, 866–875. [Google Scholar] [CrossRef]
- Khan, A.; Liang, C.; Huang, Y.T.; Zhang, C.; Cai, J.; Feng, S.P.; Li, W. Di Template-Electrodeposited and Imprint-Transferred Microscale Metal-Mesh Transparent Electrodes for Flexible and Stretchable Electronics. Adv. Eng. Mater. 2019, 21, 1801363. [Google Scholar] [CrossRef]
- Mustafa, M.N.; Sulaiman, Y. Fully flexible dye-sensitized solar cells photoanode modified with titanium dioxide-graphene quantum dot light scattering layer. Sol. Energy 2020, 212, 332–338. [Google Scholar] [CrossRef]
- Wante, H.P.; Yap, S.L.; Aidan, J.; Saikia, P. Efficiency Enhancement of Dye Sensitized Solar cells (DSSCs) by Atmospheric DBD Plasma Modification of Polyetherimide (PEI) Polymer Substrate. J. Mater. Environ. Sci. 2020, 2020, 713–722. [Google Scholar]
- Hellert, C.; Wortmann, M.; Frese, N.; Grötsch, G.; Cornelißen, C.; Ehrmann, A. Adhesion of electrospun poly(Acrylonitrile) nanofibers on conductive and isolating foil substrates. Coatings 2021, 11, 249. [Google Scholar] [CrossRef]
- Baiju, K.G.; Murali, B.; Subba Rao, R.; Jayanarayanan, K.; Kumaresan, D. Heat sink assisted elevated temperature sintering process of TiO2 on polymer substrates for producing high performance flexible dye-sensitized solar cells. Chem. Eng. Process. 2020, 149, 107817. [Google Scholar] [CrossRef]
- Hou, W.; Xiao, Y.; Han, G.; Zhou, H.; Chang, Y.; Zhang, Y. Preparation of mesoporous titanium dioxide anode by a film- and pore-forming agent for the dye-sensitized solar cell. Mater. Res. Bull. 2016, 76, 140–146. [Google Scholar] [CrossRef]
- Bharwal, A.K.; Manceriu, L.; Alloin, F.; Iojoiu, C.; Dewalque, J.; Toupance, T.; Henrist, C. Tuning bimodal porosity in TiO2 photoanodes towards efficient solid-state dye-sensitized solar cells comprising polysiloxane-based polymer electrolyte. Microporous Mesoporous Mater. 2019, 273, 226–234. [Google Scholar] [CrossRef]
- Zukalovà, M.; Zukal, A.; Kavan, L.; Nazeeruddin, M.K.; Liska, P.; Grätzel, M. Organized mesoporous TiO2 films exhibiting greatly enhanced performance in dye-sensitized solar cells. Nano Lett. 2005, 5, 1789–1792. [Google Scholar] [CrossRef]
- Agarwala, S.; Kevin, M.; Wong, A.S.W.; Peh, C.K.N.; Thavasi, V.; Ho, G.W. Mesophase ordering of TiO2 film with high surface area and strong light harvesting for dye-sensitized solar cell. ACS Appl. Mater. Interfaces 2010, 2, 1844–1850. [Google Scholar] [CrossRef]
- Li, C.; Xin, C.; Xu, L.; Zhong, Y.; Wu, W. Components control for high-voltage quasi-solid state dye-sensitized solar cells based on two-phase polymer gel electrolyte. Sol. Energy 2019, 181, 130–136. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, F.; Tang, W.; Deng, Z.; Zhang, M.; Ruan, W. Azobenzene-dyed, nanofibrous microstructure for improving photothermal effect of polymer gel electrolyte. Sol. Energy 2021, 230, 1–9. [Google Scholar] [CrossRef]
- Kumar Shah, D.; Son, Y.H.; Lee, H.R.; Shaheer Akhtar, M.; Kim, C.Y.; Yang, O.B. A stable gel electrolyte based on poly butyl acrylate (PBA)-co-poly acrylonitrile (PAN) for solid-state dye-sensitized solar cells. Chem. Phys. Lett. 2020, 754, 137756. [Google Scholar] [CrossRef]
- Khan, S.A.; Li, L.; Zhao, D.; Chen, S. Increased power conversion efficiency of dye-sensitized solar cells with counter electrodes based on porous polypyrrole. React. Funct. Polym. 2020, 148, 104483. [Google Scholar] [CrossRef]
- Wu, J.; Wu, S.; Sun, W. Electropolymerization and application of polyoxometalate-doped polypyrrole film electrodes in dye-sensitized solar cells. Electrochem. Commun. 2021, 122, 106879. [Google Scholar] [CrossRef]
- Karakuş, M.Ö.; Yakışıklıer, M.E.; Delibaş, A.; Ayyıldız, E.; Çetin, H. Anionic and cationic polymer-based quasi-solid-state dye-sensitized solar cell with poly(aniline) counter electrode. Sol. Energy 2020, 195, 565–572. [Google Scholar] [CrossRef]
- Farooq, S.; Tahir, A.A.; Krewer, U.; Shah, A.U.H.A.; Bilal, S. Efficient photocatalysis through conductive polymer coated FTO counter electrode in platinum free dye sensitized solar cells. Electrochim. Acta 2019, 320, 134544. [Google Scholar] [CrossRef]
- Mazloum-Ardakani, M.; Arazi, R. Enhancement ofphotovoltaic performance using a novel photocathode based on poly(3,4-ethylenedioxythiophene)/Ag–CuO nanocomposite in dye-sensitized solar cells. Comptes Rendus 2020, 23, 105–115. [Google Scholar]
- Sathiyan, G.; Sivakumar, E.K.T.; Ganesamoorthy, R.; Thangamuthu, R.; Sakthivel, P. Review of carbazole based conjugated molecules for highly efficient organic solar cell application. Tetrahedron Lett. 2016, 57, 243–252. [Google Scholar] [CrossRef]
- Sakthivel, P.; Ban, T.W.; Kim, S.; Kim, S.; Gal, Y.S.; Chae, E.A.; Shin, W.S.; Moon, S.J.; Lee, J.C.; Jin, S.H. Synthesis and studies of methyl ester substituted thieno-o-quinodimethane fullerene multiadducts for polymer solar cells. Sol. Energy Mater. Sol. Cells 2013, 113, 13–19. [Google Scholar] [CrossRef]
- Brabec, C.J.; Winder, C.; Sariciftci, N.S.; Hummelen, J.C.; Dhanabalan, A.; Van Hal, P.A.; Janssen, R.A.J. A low-bandgap semiconducting polymer for photovoltaic devices and infrared emitting diodes. Adv. Funct. Mater. 2002, 12, 709–712. [Google Scholar] [CrossRef] [Green Version]
- Watson, B.W.; Meng, L.; Fetrow, C.; Qin, Y. Core/shell conjugated polymer/quantum dot composite nanofibers through orthogonal non-covalent interactions. Polymers 2016, 8, 408. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Qin, Y. Structurally Diverse Poly(thienylene vinylene)s (PTVs) with Systematically Tunable Properties through Acyclic Diene Metathesis (ADMET) and Postpolymerization Modification. Macromolecules 2016, 49, 3318–3327. [Google Scholar] [CrossRef]
- Zhu, L.; Zhong, W.; Qiu, C.; Lyu, B.; Zhou, Z.; Zhang, M.; Song, J.; Xu, J.; Wang, J.; Ali, J.; et al. Aggregation-Induced Multilength Scaled Morphology Enabling 11.76% Efficiency in All-Polymer Solar Cells Using Printing Fabrication. Adv. Mater. 2019, 31, e1902899. [Google Scholar] [CrossRef]
- Guo, Y.; Li, Y.; Awartani, O.; Han, H.; Zhao, J.; Ade, H.; Yan, H.; Zhao, D. Improved Performance of All-Polymer Solar Cells Enabled by Naphthodiperylenetetraimide-Based Polymer Acceptor. Adv. Mater. 2017, 29, 1700309. [Google Scholar] [CrossRef]
- Kolhe, N.B.; Tran, D.K.; Lee, H.; Kuzuhara, D.; Yoshimoto, N.; Koganezawa, T.; Jenekhe, S.A. New Random Copolymer Acceptors Enable Additive-Free Processing of 10.1% Efficient All-Polymer Solar Cells with Near-Unity Internal Quantum Efficiency. ACS Energy Lett. 2019, 4, 1162–1170. [Google Scholar] [CrossRef]
- Liao, J.F.; Wu, W.Q.; Zhong, J.X.; Jiang, Y.; Wang, L.; Kuang, D. Bin Enhanced efficacy of defect passivation and charge extraction for efficient perovskite photovoltaics with a small open circuit voltage loss. J. Mater. Chem. A 2019, 7, 9025–9033. [Google Scholar] [CrossRef]
- Liu, G.; Liu, C.; Lin, Z.; Yang, J.; Huang, Z.; Tan, L.; Chen, Y. Regulated Crystallization of Efficient and Stable Tin-Based Perovskite Solar Cells via a Self-Sealing Polymer. ACS Appl. Mater. Interfaces 2020, 12, 14049–14056. [Google Scholar] [CrossRef]
- Han, T.H.; Lee, J.W.; Choi, C.; Tan, S.; Lee, C.; Zhao, Y.; Dai, Z.; De Marco, N.; Lee, S.J.; Bae, S.H.; et al. Perovskite-polymer composite cross-linker approach for highly-stable and efficient perovskite solar cells. Nat. Commun. 2019, 10, 520. [Google Scholar] [CrossRef] [Green Version]
- Fairfield, D.J.; Sai, H.; Narayanan, A.; Passarelli, J.V.; Chen, M.; Palasz, J.; Palmer, L.C.; Wasielewski, M.R.; Stupp, S.I. Structure and chemical stability in perovskite-polymer hybrid photovoltaic materials. J. Mater. Chem. A 2019, 7, 1687–1699. [Google Scholar] [CrossRef]
- Chen, W.; Shi, Y.; Wang, Y.; Feng, X.; Djurišić, A.B.; Woo, H.Y.; Guo, X.; He, Z. N-type conjugated polymer as efficient electron transport layer for planar inverted perovskite solar cells with power conversion efficiency of 20.86%. Nano Energy 2020, 68, 104363. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, X.; Wang, K.; Wu, C.; Yang, R.; Hou, Y.; Jiang, Y.; Liu, S.; Priya, S. Stable Efficiency Exceeding 20.6% for Inverted Perovskite Solar Cells through Polymer-Optimized PCBM Electron-Transport Layers. Nano Lett. 2019, 19, 3313–3320. [Google Scholar] [CrossRef]
- Syed, A.A.; Poon, C.Y.; Li, H.W.; Zhu, F. A sodium citrate-modified-PEDOT:PSS hole transporting layer for performance enhancement in inverted planar perovskite solar cells. J. Mater. Chem. C 2019, 7, 5260–5266. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Zhou, X.; Zhong, X.; Cheng, C.; Tian, Y.; Xu, B. Hole-transporting layer based on a conjugated polyelectrolyte with organic cations enables efficient inverted perovskite solar cells. Nano Energy 2019, 57, 248–255. [Google Scholar] [CrossRef]
- Zhang, F.; Yao, Z.; Guo, Y.; Li, Y.; Bergstrand, J.; Brett, C.J.; Cai, B.; Hajian, A.; Guo, Y.; Yang, X.; et al. Polymeric, cost-effective, dopant-free hole transport materials for efficient and stable perovskite solar cells. J. Am. Chem. Soc. 2019, 141, 19700–19707. [Google Scholar] [CrossRef]
- Lee, J.; Kim, G.W.; Kim, M.; Park, S.A.; Park, T. Nonaromatic Green-Solvent-Processable, Dopant-Free, and Lead-Capturable Hole Transport Polymers in Perovskite Solar Cells with High Efficiency. Adv. Energy Mater. 2020, 10, 290. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, C.; Wang, X.; Tian, Y.; Jen, A.K.Y.; Xu, B. Side-Chain Engineering on Dopant-Free Hole-Transporting Polymers toward Highly Efficient Perovskite Solar Cells (20.19%). Adv. Funct. Mater. 2019, 29, 1904856. [Google Scholar] [CrossRef]
- Wang, M.; Wang, H.; Li, W.; Hu, X.; Sun, K.; Zang, Z. Defect passivation using ultrathin PTAA layers for efficient and stable perovskite solar cells with a high fill factor and eliminated hysteresis. J. Mater. Chem. A 2019, 7, 26421–26428. [Google Scholar] [CrossRef]
- Tian, J.; Xue, Q.; Tang, X.; Chen, Y.; Li, N.; Hu, Z.; Shi, T.; Wang, X.; Huang, F.; Brabec, C.J.; et al. Dual Interfacial Design for Efficient CsPbI2Br Perovskite Solar Cells with Improved Photostability. Adv. Mater. 2019, 31, 1901152. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, L.; Li, Y.; Zhou, X.; She, S.; Wang, X.; Tian, Y.; Jen, A.K.Y.; Xu, B. Highly Stable and Efficient Perovskite Solar Cells with 22.0% Efficiency Based on Inorganic–Organic Dopant-Free Double Hole Transporting Layers. Adv. Funct. Mater. 2020, 30, 1908462. [Google Scholar] [CrossRef]
- Tan, F.; Tan, H.; Saidaminov, M.I.; Wei, M.; Liu, M.; Mei, A.; Li, P.; Zhang, B.; Tan, C.S.; Gong, X.; et al. In Situ Back-Contact Passivation Improves Photovoltage and Fill Factor in Perovskite Solar Cells. Adv. Mater. 2019, 31, e1807435. [Google Scholar] [CrossRef]
- Weerasinghe, H.C.; Huang, F.; Cheng, Y.B. Fabrication of flexible dye sensitized solar cells on plastic substrates. Nano Energy 2013, 2, 174–189. [Google Scholar] [CrossRef]
- Kang, M.G.; Park, N.G.; Ryu, K.S.; Chang, S.H.; Kim, K.J. A 4.2% efficient flexible dye-sensitized TiO2 solar cells using stainless steel substrate. Sol. Energy Mater. Sol. Cells 2006, 90, 574–581. [Google Scholar] [CrossRef]
- Hou, W.; Xiao, Y.; Han, G.; Lin, J.Y. The applications of polymers in solar cells: A review. Polymers 2019, 11, 143. [Google Scholar] [CrossRef] [Green Version]
- Zardetto, V.; Di Giacomo, F.; Garcia-Alonso, D.; Keuning, W.; Creatore, M.; Mazzuca, C.; Reale, A.; Di Carlo, A.; Brown, T.M. Fully plastic dye solar cell devices by low-temperature UV-irradiation of both the mesoporous TiO2 photo- and platinized counter-electrodes. Adv. Energy Mater. 2013, 3, 1292–1298. [Google Scholar] [CrossRef]
- Fu, Y.; Lv, Z.; Hou, S.; Wu, H.; Wang, D.; Zhang, C.; Zou, D. TCO-free, flexible, and bifacial dye-sensitized solar cell based on low-cost metal wires. Adv. Energy Mater. 2012, 2, 37–41. [Google Scholar] [CrossRef]
- Barbé, C.J.; Arendse, F.; Comte, P.; Jirousek, M.; Lenzmann, F.; Shklover, V.; Grätzel, M. Nanocrystalline titanium oxide electrodes for photovoltaic applications. J. Am. Ceram. Soc. 1997, 80, 3157–3171. [Google Scholar] [CrossRef]
- Park, J.T.; Moon, J.; Choi, G.H.; Lim, S.M.; Kim, J.H. Facile graft copolymer template synthesis of mesoporous polymeric metal-organic frameworks to produce mesoporous TiO2: Promising platforms for photovoltaic and photocatalytic applications. J. Ind. Eng. Chem. 2020, 84, 384–392. [Google Scholar] [CrossRef]
- Prakash, G.; Subramanian, K. Interaction of pyridine π-bridge-based poly(methacrylate) dyes for the fabrication of dye-sensitized solar cells with the influence of different strength phenothiazine, fluorene and anthracene sensitizers as donor units with new anchoring mode. New J. Chem. 2018, 42, 17939–17949. [Google Scholar] [CrossRef]
- Ramasamy, S.; Boopathy, M.; Johnsanthoshkumar, S.; Subramanian, K. Structural engineering of poly-(methacrylate) bearing push-pull type pendants oxindole-phenothiazine with tetrazole anchoring acceptor for efficient organic photovoltaic cells. Polymer 2017, 115, 128–136. [Google Scholar] [CrossRef]
- Wang, G.; Wu, Y.; Ding, W.; Yu, G.; Hu, Z.; Wang, H.; Liu, S.; Zou, Y.; Pan, C. Photovoltaic performance of long-chain poly(triphenylamine-phenothiazine) dyes with a tunable π-bridge for dye-sensitized solar cells. J. Mater. Chem. A 2015, 3, 14217–14227. [Google Scholar] [CrossRef]
- Nusbaumer, H.; Zakeeruddin, S.M.; Moser, J.E.; Grätzel, M. An alternative efficient redox couple for the dye-sensitized solar cell system. Chemistry 2003, 9, 3756–3763. [Google Scholar] [CrossRef]
- Shakeel Ahmad, M.; Pandey, A.K.; Abd Rahim, N. Advancements in the development of TiO2 photoanodes and its fabrication methods for dye sensitized solar cell (DSSC) applications. A review. Renew. Sustain. Energy Rev. 2017, 77, 89–108. [Google Scholar] [CrossRef]
- Xiao, B.C.; Lin, L.Y. Tuning electrolyte configuration and composition for fiber-shaped dye-sensitized solar cell with poly(vinylidene fluoride-co-hexafluoropropylene) gel electrolyte. J. Colloid Interface Sci. 2020, 571, 126–133. [Google Scholar] [CrossRef]
- Abdul Azeez, U.H.; Gunasekaran, A.; Sorrentino, A.; Syed, A.; Marraiki, N.; Anandan, S. Synthesis and characterization of poly-3-(9H-carbazol-9-yl)propylmethacrylate as a gel electrolyte for dye-sensitized solar cell applications. Polym. Bull. 2022, 79, 921–934. [Google Scholar] [CrossRef]
- Cheng, F.; Wu, C.; Wang, S.; Wen, S. Polydopamine-Modified Electrospun Polyvinylidene Fluoride Nanofiber Based Flexible Polymer Gel Electrolyte for Highly Stable Dye-Sensitized Solar Cells. ACS Omega 2021, 6, 28663–28670. [Google Scholar] [CrossRef] [PubMed]
- Elayappan, V.; Murugadoss, V.; Fei, Z.; Dyson, P.J.; Angaiah, S. Influence of polypyrrole incorporated electrospun poly (vinylidene fluoride-co-hexafluoropropylene) nanofibrous composite membrane electrolyte on the photovoltaic performance of dye sensitized solar cell. Eng. Sci. 2020, 10, 78–84. [Google Scholar] [CrossRef]
- Gunasekaran, A.; Chen, H.Y.; Ponnusamy, V.K.; Sorrentino, A.; Anandan, S. Synthesis of high polydispersity index polylactic acid and its application as gel electrolyte towards fabrication of dye-sensitized solar cells. J. Polym. Res. 2021, 28, 252. [Google Scholar] [CrossRef]
- Park, J.W.; Jang, J. Fabrication of graphene/free-standing nanofibrillar PEDOT/P(VDF-HFP) hybrid device for wearable and sensitive electronic skin application. Carbon N. Y. 2016, 87, 275–281. [Google Scholar] [CrossRef]
- Zhou, L.; Yu, M.; Chen, X.; Nie, S.; Lai, W.Y.; Su, W.; Cui, Z.; Huang, W. Screen-Printed Poly(3,4-Ethylenedioxythiophene):Poly(Styrenesulfonate) Grids as ITO-Free Anodes for Flexible Organic Light-Emitting Diodes. Adv. Funct. Mater. 2018, 28, 1705955. [Google Scholar] [CrossRef]
- Tang, Q.; Cai, H.; Yuan, S.; Wang, X. Counter electrodes from double-layered polyaniline nanostructures for dye-sensitized solar cell applications. J. Mater. Chem. A 2013, 1, 317–323. [Google Scholar] [CrossRef]
- Calogero, G.; Calandra, P.; Irrera, A.; Sinopoli, A.; Citro, I.; Di Marco, G. A new type of transparent and low cost counter-electrode based on platinum nanoparticles for dye-sensitized solar cells. Energy Environ. Sci. 2011, 4, 1838–1844. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Senthil, R.A.; Buraidah, M.H.; Raghavender, M.; Madhavan, J.; Arof, A.K. Synthesis and characterization of (Ni1-xCox)Se2 based ternary selenides as electrocatalyst for triiodide reduction in dye-sensitized solar cells. J. Solid State Chem. 2016, 238, 113–120. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, X.; Zhang, K.; Pang, S.; Zhou, X.; Xu, H.; Dong, S.; Han, P.; Zhang, Z.; Zhang, C.; et al. Transition-metal nitride nanoparticles embedded in N-doped reduced graphene oxide: Superior synergistic electrocatalytic materials for the counter electrodes of dye-sensitized solar cells. J. Mater. Chem. A 2013, 1, 3340–3346. [Google Scholar] [CrossRef]
- Jing, H.; Shi, Y.; Wu, D.; Liang, S.; Song, X.; An, Y.; Hao, C. Well-defined heteroatom-rich porous carbon electrocatalyst derived from biowaste for high-performance counter electrode in dye-sensitized solar cells. Electrochim. Acta 2018, 281, 646–653. [Google Scholar] [CrossRef]
- Zheng, H.; Neo, C.Y.; Mei, X.; Qiu, J.; Ouyang, J. Reduced graphene oxide films fabricated by gel coating and their application as platinum-free counter electrodes of highly efficient iodide/triiodide dye-sensitized solar cells. J. Mater. Chem. 2012, 22, 14465–14474. [Google Scholar] [CrossRef]
- Hou, W.; Xiao, Y.; Han, G. The dye-sensitized solar cells based on the interconnected ternary cobalt diindium sulfide nanosheet array counter electrode. Mater. Res. Bull. 2018, 107, 204–212. [Google Scholar] [CrossRef]
- Yang, W.; Li, Z.; Xu, X.; Hou, L.; Tang, Y.; Deng, B.; Yang, F.; Wang, Y.; Li, Y. Atomic N-coordinated cobalt sites within nanomesh graphene as highly efficient electrocatalysts for triiodide reduction in dye-sensitized solar cells. Chem. Eng. J. 2018, 349, 782–790. [Google Scholar] [CrossRef]
- Tai, Q.; Chen, B.; Guo, F.; Xu, S.; Hu, H.; Sebo, B.; Zhao, X.Z. In situ prepared transparent polyaniline electrode and its application in bifacial dye-sensitized solar cells. ACS Nano 2011, 5, 3795–3799. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.S.; Kim, C.; Ko, J.; Im, S.S. Spherical polypyrrole nanoparticles as a highly efficient counter electrode for dye-sensitized solar cells. J. Mater. Chem. 2011, 21, 8146–8151. [Google Scholar] [CrossRef]
- Tang, Z.; Wu, J.; Zheng, M.; Tang, Q.; Liu, Q.; Lin, J.; Wang, J. High efficient PANI/Pt nanofiber counter electrode used in dye-sensitized solar cell. RSC Adv. 2012, 2, 4062–4064. [Google Scholar] [CrossRef]
- Li, G.R.; Song, J.; Pan, G.L.; Gao, X.P. Highly Pt-like electrocatalytic activity of transition metal nitrides for dye-sensitized solar cells. Energy Environ. Sci. 2011, 4, 1680–1683. [Google Scholar] [CrossRef]
- Xiao, Y.; Han, G.; Li, Y.; Li, M.; Lin, J.Y. Three-dimensional hollow platinum-nickel bimetallic nanoframes for use in dye-sensitized solar cells. J. Power Sources 2015, 278, 149–155. [Google Scholar] [CrossRef]
- Tang, Q.; Zhang, H.; Meng, Y.; He, B.; Yu, L. Dissolution Engineering of Platinum Alloy Counter Electrodes in Dye-Sensitized Solar Cells. Angew. Chemie—Int. Ed. 2015, 54, 11448–11452. [Google Scholar] [CrossRef]
- Kim, S.S.; Nah, Y.C.; Noh, Y.Y.; Jo, J.; Kim, D.Y. Electrodeposited Pt for cost-efficient and flexible dye-sensitized solar cells. Electrochim. Acta 2006, 51, 3814–3819. [Google Scholar] [CrossRef]
- Xia, J.; Chen, L.; Yanagida, S. Application of polypyrrole as a counter electrode for a dye-sensitized solar cell. J. Mater. Chem. 2011, 21, 4644–4649. [Google Scholar] [CrossRef]
- Sangiorgi, N.; Sangiorgi, A.; Tarterini, F.; Sanson, A. Molecularly imprinted polypyrrole counter electrode for gel-state dye-sensitized solar cells. Electrochim. Acta 2019, 305, 322–328. [Google Scholar] [CrossRef]
- Saberi Motlagh, M.; Mottaghitalab, V.; Rismanchi, A.; Rafieepoor Chirani, M.; Hasanzadeh, M. Performance modelling of textile solar cell developed by carbon fabric/polypyrrole flexible counter electrode. Int. J. Sustain. Energy 2022, 1–21. [Google Scholar] [CrossRef]
- Ohtani, Y.; Kumano, K.; Saneshige, M.; Takami, K.; Hoshi, H. Effect of electropolymerization duration on the structure and performance of polypyrrole/graphene nanoplatelet counter electrode for dye-sensitized solar cells. J. Solid State Electrochem. 2021, 25, 2107–2113. [Google Scholar] [CrossRef]
- Ahmed, U.; Shahid, M.M.; Shahabuddin, S.; Rahim, N.A.; Alizadeh, M.; Pandey, A.K.; Sagadevan, S. An efficient platform based on strontium titanate nanocubes interleaved polypyrrole nanohybrid as counter electrode for dye-sensitized solar cell. J. Alloys Compd. 2021, 860, 158228. [Google Scholar] [CrossRef]
- Rafique, S.; Rashid, I.; Sharif, R. Cost effective dye sensitized solar cell based on novel Cu polypyrrole multiwall carbon nanotubes nanocomposites counter electrode. Sci. Rep. 2021, 11, 14830. [Google Scholar] [CrossRef]
- Yao, X.; He, B.; Cui, L.; Ti, J.; Chen, H.; Duan, Y.; Tang, Q. Polypyrrole-molybdenum sulfide complex as an efficient and transparent catalytic electrode for bifacial dye-sensitized solar cells. Catal. Commun. 2022, 163, 106403. [Google Scholar] [CrossRef]
- Utami, A.N.; Reza, M.; Benu, D.P.; Fatya, A.I.; Yuliarto, B.; Suendo, V. Reverse micelle facilitated synthesis of nanostructured polyaniline as the counter electrode materials in dye-sensitized solar cells. Polym. Technol. Mater. 2020, 59, 1350–1358. [Google Scholar] [CrossRef]
- Jiao, S.; Wen, J.; Zhou, Y.; Sun, Z.; Liu, Y.; Liu, R. Preparation and Property Studies of Polyaniline Film for Flexible Counter Electrode of Dye-Sensitized Solar Cells by Cyclic Voltammetry. ChemistrySelect 2021, 6, 230–233. [Google Scholar] [CrossRef]
- Zatirostami, A. A new electrochemically prepared composite counter electrode for dye-sensitized solar cells. Thin Solid Films 2020, 701, 137926. [Google Scholar] [CrossRef]
- Ravichandran, S.; Varthamanan, Y.; Akilandeswari; Elangoven, T.; Ragupathi, C.; Murugesan, S. Effect of polyaniline/FeS2 composite and usages of alternates counter electrode for dye-sensitized solar cells. Mater. Today Proc. 2021, 49, 2615–2619. [Google Scholar] [CrossRef]
- Bella, F.; Porcarelli, L.; Mantione, D.; Gerbaldi, C.; Barolo, C.; Grätzel, M.; Mecerreyes, D. A water-based and metal-free dye solar cell exceeding 7% efficiency using a cationic poly(3,4-ethylenedioxythiophene) derivative. Chem. Sci. 2020, 11, 1485–1493. [Google Scholar] [CrossRef] [Green Version]
- Pradhan, S.C.; Soman, S. Effect of thickness on charge transfer properties of conductive polymer based PEDOT counter electrodes in DSSC. Results Surf. Interfaces 2021, 5, 100030. [Google Scholar] [CrossRef]
- Venkatesan, S.; Lin, W.-H.; Hsu, T.-H.; Teng, H.; Lee, Y.-L. Indoor Dye-Sensitized Solar Cells with Efficiencies Surpassing 26% Using Polymeric Counter Electrodes. ACS Sustain. Chem. Eng. 2022, 10, 2473–2483. [Google Scholar] [CrossRef]
- Xu, T.; Cao, W.; Kong, D.; Qin, X.; Song, J.; Kou, K.; Chen, L.; Qiao, Q.; Huang, W. Enhanced catalytic property of transparent PEDOT counter electrodes for bifacial dye sensitized solar cells. Mater. Today Commun. 2020, 25, 101313. [Google Scholar] [CrossRef]
- Gemeiner, P.; Pavličková, M.; Hatala, M.; Hvojnik, M.; Homola, T.; Mikula, M. The effect of secondary dopants on screen-printed PEDOT:PSS counter-electrodes for dye-sensitized solar cells. J. Appl. Polym. Sci. 2022, 139, 51929. [Google Scholar] [CrossRef]
- Yeh, N.; Yeh, P. Organic solar cells: Their developments and potentials. Renew. Sustain. Energy Rev. 2013, 21, 421–431. [Google Scholar] [CrossRef]
- Blom, P.W.M.; Mihailetchi, V.D.; Koster, L.J.A.; Markov, D.E. Device physics of polymer:Fullerene bulk heterojunction solar cells. Adv. Mater. 2007, 19, 1551–1566. [Google Scholar] [CrossRef] [Green Version]
- Gao, F.; Inganäs, O. Charge generation in polymer-fullerene bulk-heterojunction solar cells. Phys. Chem. Chem. Phys. 2014, 16, 20291–20304. [Google Scholar] [CrossRef] [Green Version]
- Dimitrov, S.D.; Durrant, J.R. Materials design considerations for charge generation in organic solar cells. Chem. Mater. 2014, 26, 616–630. [Google Scholar] [CrossRef]
- Mayer, A.C.; Scully, S.R.; Hardin, B.E.; Rowell, M.W.; McGehee, M.D. Polymer-based solar cells. Mater. Today 2007, 10, 28–33. [Google Scholar] [CrossRef]
- Yu, G.; Gao, J.; Hummelen, J.C.; Wudl, F.; Heeger, A.J. Internal Donor-Acceptor. Science 1995, 270, 1789–1791. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Qin, J.; Xiao, Z.; Meng, X.; Zuo, C.; Yang, B.; Tan, H.; Yang, J.; Yang, S.; Sun, K.; et al. A 2.16 eV bandgap polymer donor gives 16% power conversion efficiency. Sci. Bull. 2020, 65, 179–181. [Google Scholar] [CrossRef] [Green Version]
- Cao, Z.; Chen, J.; Liu, S.; Qin, M.; Jia, T.; Zhao, J.; Li, Q.; Ying, L.; Cai, Y.P.; Lu, X.; et al. Understanding of Imine Substitution in Wide-Bandgap Polymer Donor-Induced Efficiency Enhancement in All-Polymer Solar Cells. Chem. Mater. 2019, 31, 8533–8542. [Google Scholar] [CrossRef]
- Xue, C.; Tang, Y.; Liu, S.; Feng, H.; Li, S.; Xia, D. Achieving efficient polymer solar cells based on benzodithiophene-thiazole-containing wide band gap polymer donors by changing the linkage patterns of two thiazoles. New J. Chem. 2020, 44, 13100–13107. [Google Scholar] [CrossRef]
- Qin, J.; Zhang, L.; Xiao, Z.; Chen, S.; Sun, K.; Zang, Z.; Yi, C.; Yuan, Y.; Jin, Z.; Hao, F.; et al. Over 16% efficiency from thick-film organic solar cells. Sci. Bull. 2020, 65, 1979–1982. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Q.; Liu, S.; Cao, Z.; Jiao, X.; Cai, Y.P.; Huang, F. Bithieno[3,4-c]pyrrole-4,6-dione-Mediated Crystallinity in Large-Bandgap Polymer Donors Directs Charge Transportation and Recombination in Efficient Nonfullerene Polymer Solar Cells. ACS Energy Lett. 2020, 5, 367–375. [Google Scholar] [CrossRef]
- Keshtov, M.L.; Konstantinov, I.O.; Kuklin, S.A.; Zou, Y.; Agrawal, A.; Chen, F.C.; Sharma, G.D. Binary and Ternary Polymer Solar Cells Based on a Wide Bandgap D-A Copolymer Donor and Two Nonfullerene Acceptors with Complementary Absorption Spectral. ChemSusChem 2021, 14, 4731–4740. [Google Scholar] [CrossRef]
- Gokulnath, T.; Feng, K.; Park, H.-Y.; Do, Y.; Park, H.; Gayathri, R.D.; Reddy, S.S.; Kim, J.; Guo, X.; Yoon, J.; et al. Facile Strategy for Third Component Optimization in Wide-Band-Gap π-Conjugated Polymer Donor-Based Efficient Ternary All-Polymer Solar Cells. ACS Appl. Mater. Interfaces 2022, 14, 11211–11221. [Google Scholar] [CrossRef]
- Liang, Y.; Wu, Y.; Feng, D.; Tsai, S.T.; Son, H.J.; Li, G.; Yu, L. Development of new semiconducting polymers for high performance solar cells. J. Am. Chem. Soc. 2009, 131, 56–57. [Google Scholar] [CrossRef]
- Liang, Y.; Xu, Z.; Xia, J.; Tsai, S.T.; Wu, Y.; Li, G.; Ray, C.; Yu, L. For the bright future-bulk heterojunction polymer solar cells with power conversion efficiency of 7.4%. Adv. Mater. 2010, 22, 135–138. [Google Scholar] [CrossRef]
- Chen, Y.; You, G.; Zou, D.; Zhuang, Q.; Zhen, H.; Ling, Q. Enhanced photovoltaic performances via ternary blend strategy employing a medium-bandgap D-A type alternating copolymer as the single donor. Sol. Energy 2019, 183, 350–355. [Google Scholar] [CrossRef]
- Sharma, G.D.; Bucher, L.; Desbois, N.; Gros, C.P.; Gupta, G.; Malhotra, P. Polymer solar cell based on ternary active layer consists of medium bandgap polymer and two non-fullerene acceptors. Sol. Energy 2020, 207, 1427–1433. [Google Scholar] [CrossRef]
- An, Q.; Wang, J.; Gao, W.; Ma, X.; Hu, Z.; Gao, J.; Xu, C.; Hao, M.; Zhang, X.; Yang, C.; et al. Alloy-like ternary polymer solar cells with over 17.2% efficiency. Sci. Bull. 2020, 65, 538–545. [Google Scholar] [CrossRef] [Green Version]
- Yuan, J.; Zhang, Y.; Zhou, L.; Zhang, G.; Yip, H.L.; Lau, T.K.; Lu, X.; Zhu, C.; Peng, H.; Johnson, P.A.; et al. Single-Junction Organic Solar Cell with over 15% Efficiency Using Fused-Ring Acceptor with Electron-Deficient Core. Joule 2019, 3, 1140–1151. [Google Scholar] [CrossRef]
- Yan, T.; Song, W.; Huang, J.; Peng, R.; Huang, L.; Ge, Z. 16.67% Rigid and 14.06% Flexible Organic Solar Cells Enabled by Ternary Heterojunction Strategy. Adv. Mater. 2019, 31, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Lin, Y.; Jeong, S.Y.; Firdaus, Y.; Genene, Z.; Nikitaras, A.; Tsetseris, L.; Woo, H.Y.; Zhu, W.; Anthopoulos, T.D.; et al. Using Two Compatible Donor Polymers Boosts the Efficiency of Ternary Organic Solar Cells to 17.7%. Chem. Mater. 2021, 33, 7254–7262. [Google Scholar] [CrossRef]
- Keshtov, M.L.; Kuklin, S.A.; Khokhlov, A.R.; Xie, Z.; Alekseev, V.G.; Dahiya, H.; Singhal, R.; Sharma, G.D. New Medium Bandgap Donor D-A1-D-A2 Type Copolymers Based on Anthra[1,2-b: 4,3-b“:6,7-c”’] Trithiophene-8,12-dione Groups for High-Efficient Non-Fullerene Polymer Solar Cells. Macromol. Rapid Commun. 2022, 43, 2100839. [Google Scholar] [CrossRef]
- Pan, L.; Liu, T.; Wang, J.; Ye, L.; Luo, Z.; Ma, R.; Pang, S.; Chen, Y.; Ade, H.; Yan, H.; et al. Efficient Organic Ternary Solar Cells Employing Narrow Band Gap Diketopyrrolopyrrole Polymers and Nonfullerene Acceptors. Chem. Mater. 2020, 32, 7309–7317. [Google Scholar] [CrossRef]
- Rech, J.J.; Neu, J.; Qin, Y.; Samson, S.; Shanahan, J.; Josey, R.F.; Ade, H.; You, W. Designing Simple Conjugated Polymers for Scalable and Efficient Organic Solar Cells. ChemSusChem 2021, 14, 3561–3568. [Google Scholar] [CrossRef]
- Caliskan, M.; Erer, M.C.; Aslan, S.T.; Udum, Y.A.; Toppare, L.; Cirpan, A. Narrow band gap benzodithiophene and quinoxaline bearing conjugated polymers for organic photovoltaic applications. Dye. Pigment. 2020, 180, 108479. [Google Scholar] [CrossRef]
- Guo, L.; Huang, X.; Luo, Y.; Liu, S.; Cao, Z.; Chen, J.; Luo, Y.; Li, Q.; Zhao, J.; Cai, Y.P. Novel narrow bandgap polymer donors based on ester-substituted quinoxaline unit for organic photovoltaic application. Sol. Energy 2021, 220, 425–431. [Google Scholar] [CrossRef]
- Can, A.; Choi, G.-S.; Ozdemir, R.; Park, S.; Park, J.S.; Lee, Y.; Deneme, İ.; Mutlugun, E.; Kim, C.; Kim, B.J.; et al. Meso-π-Extended/Deficient BODIPYs and Low-Band-Gap Donor–Acceptor Copolymers for Organic Optoelectronics. ACS Appl. Polym. Mater. 2022, 4, 1991–2005. [Google Scholar] [CrossRef]
- Cruciani, F.; Babics, M.; Liu, S.; Carja, D.; Mantione, D.; Beaujuge, P.M. N-Acylisoindigo Derivatives as Polymer Acceptors for “All-Polymer” Bulk-Heterojunction Solar Cells. Macromol. Chem. Phys. 2019, 220, 1900029. [Google Scholar] [CrossRef]
- Wu, Y.; Guo, J.; Wang, W.; Chen, Z.; Chen, Z.; Sun, R.; Wu, Q.; Wang, T.; Hao, X.; Zhu, H.; et al. A conjugated donor-acceptor block copolymer enables over 11% efficiency for single-component polymer solar cells. Joule 2021, 5, 1800–1815. [Google Scholar] [CrossRef]
- Aydin, E.; De Bastiani, M.; De Wolf, S. Defect and Contact Passivation for Perovskite Solar Cells. Adv. Mater. 2019, 31, e1900428. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, M. Organic hole-transporting materials for efficient perovskite solar cells. Mater. Today Energy 2018, 7, 208–220. [Google Scholar] [CrossRef]
- Bakr, Z.H.; Wali, Q.; Fakharuddin, A.; Schmidt-Mende, L.; Brown, T.M.; Jose, R. Advances in hole transport materials engineering for stable and efficient perovskite solar cells. Nano Energy 2017, 34, 271–305. [Google Scholar] [CrossRef] [Green Version]
- Yin, X.; Song, Z.; Li, Z.; Tang, W. Toward ideal hole transport materials: A review on recent progress in dopant-free hole transport materials for fabricating efficient and stable perovskite solar cells. Energy Environ. Sci. 2020, 13, 4057–4086. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, P.; Wang, M.; Huang, S.; Zhao, Z.; Tan, S.; Han, T.H.; Lee, J.W.; Huang, T.; Wang, R.; et al. A Polymerization-Assisted Grain Growth Strategy for Efficient and Stable Perovskite Solar Cells. Adv. Mater. 2020, 32, e1907769. [Google Scholar] [CrossRef]
- Yousif, Q.A.; Agbolaghi, S. A Comparison Between Functions of Carbon Nanotube and Reduced Graphene Oxide and Respective Ameliorated Derivatives in Perovskite Solar Cells. Macromol. Res. 2020, 28, 425–432. [Google Scholar] [CrossRef]
- Yu, B.; Zhang, L.; Wu, J.; Liu, K.; Wu, H.; Shi, J.; Luo, Y.; Li, D.; Bo, Z.; Meng, Q. Application of a new π-conjugated ladder-like polymer in enhancing the stability and efficiency of perovskite solar cells. J. Mater. Chem. A 2020, 8, 1417–1424. [Google Scholar] [CrossRef]
- Fu, Q.; Xiao, S.; Tang, X.; Chen, Y.; Hu, T. Amphiphilic Fullerenes Employed to Improve the Quality of Perovskite Films and the Stability of Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2019, 11, 24782–24788. [Google Scholar] [CrossRef]
- Chen, W.; Wang, Y.; Pang, G.; Koh, C.W.; Djurišić, A.B.; Wu, Y.; Tu, B.; Liu, F.Z.; Chen, R.; Woo, H.Y.; et al. Conjugated Polymer–Assisted Grain Boundary Passivation for Efficient Inverted Planar Perovskite Solar Cells. Adv. Funct. Mater. 2019, 29, 1808855. [Google Scholar] [CrossRef]
- Yao, Z.; Qu, D.; Guo, Y.; Huang, H. Grain boundary regulation of flexible perovskite solar cells via a polymer alloy additive. Org. Electron. 2019, 70, 205–210. [Google Scholar] [CrossRef]
- Suwa, K.; Oyaizu, K.; Segawa, H.; Nishide, H. Anti-Oxidizing Radical Polymer-Incorporated Perovskite Layers and their Photovoltaic Characteristics in Solar Cells. ChemSusChem 2019, 12, 5207–5212. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Yi, X.; Zhuang, J.; Wei, Y.; Zhang, Y.; Wang, F.; Cao, S.; Li, C.; Wang, J. An Efficient Trap Passivator for Perovskite Solar Cells: Poly(propylene glycol) bis(2-aminopropyl ether). Nano-Micro Lett. 2020, 12, 177. [Google Scholar] [CrossRef]
- Garai, R.; Gupta, R.K.; Tanwar, A.S.; Hossain, M.; Iyer, P.K. Conjugated Polyelectrolyte-Passivated Stable Perovskite Solar Cells for Efficiency beyond 20%. Chem. Mater. 2021, 33, 5709–5717. [Google Scholar] [CrossRef]
- Zarenezhad, H.; Balkan, T.; Solati, N.; Halali, M.; Askari, M.; Kaya, S. Efficient carrier utilization induced by conductive polypyrrole additives in organic-inorganic halide perovskite solar cells. Sol. Energy 2020, 207, 1300–1307. [Google Scholar] [CrossRef]
- Zhong, M.; Chai, L.; Wang, Y.; Di, J. Enhanced efficiency and stability of perovskite solar cell by adding polymer mixture in perovskite photoactive layer. J. Alloys Compd. 2021, 864, 158793. [Google Scholar] [CrossRef]
- Wang, F.; Cao, Y.; Chen, C.; Chen, Q.; Wu, X.; Li, X.; Qin, T.; Huang, W. Materials toward the Upscaling of Perovskite Solar Cells: Progress, Challenges, and Strategies. Adv. Funct. Mater. 2018, 28, 1803753. [Google Scholar] [CrossRef]
- Chawanpunyawat, T.; Funchien, P.; Wongkaew, P.; Henjongchom, N.; Ariyarit, A.; Ittisanronnachai, S.; Namuangruk, S.; Cheacharoen, R.; Sudyoadsuk, T.; Goubard, F.; et al. A Ladder-like Dopant-free Hole-Transporting Polymer for Hysteresis-less High-Efficiency Perovskite Solar Cells with High Ambient Stability. ChemSusChem 2020, 13, 5058–5066. [Google Scholar] [CrossRef]
- Liao, Q.; Wang, Y.; Yao, X.; Su, M.; Li, B.; Sun, H.; Huang, J.; Guo, X. A Dual-Functional Conjugated Polymer as an Efficient Hole-Transporting Layer for High-Performance Inverted Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2021, 13, 16744–16753. [Google Scholar] [CrossRef]
- Qi, F.; Deng, X.; Wu, X.; Huo, L.; Xiao, Y.; Lu, X.; Zhu, Z.; Jen, A.K.Y. A Dopant-Free Polymeric Hole-Transporting Material Enabled High Fill Factor Over 81% for Highly Efficient Perovskite Solar Cells. Adv. Energy Mater. 2019, 9, 1902600. [Google Scholar] [CrossRef]
- Jeong, S.; Lee, I.; Kim, T.S.; Lee, J.Y. An Interlocking Fibrillar Polymer Layer for Mechanical Stability of Perovskite Solar Cells. Adv. Mater. Interfaces 2020, 7, 2001425. [Google Scholar] [CrossRef]
- Yao, Z.; Zhang, F.; Guo, Y.; Wu, H.; He, L.; Liu, Z.; Cai, B.; Guo, Y.; Brett, C.J.; Li, Y.; et al. Conformational and Compositional Tuning of Phenanthrocarbazole-Based Dopant-Free Hole-Transport Polymers Boosting the Performance of Perovskite Solar Cells. J. Am. Chem. Soc. 2020, 142, 17681–17692. [Google Scholar] [CrossRef] [PubMed]
- You, G.; Zhuang, Q.; Wang, L.; Lin, X.; Zou, D.; Lin, Z.; Zhen, H.; Zhuang, W.; Ling, Q. Dopant-Free, Donor–Acceptor-Type Polymeric Hole-Transporting Materials for the Perovskite Solar Cells with Power Conversion Efficiencies over 20%. Adv. Energy Mater. 2020, 10, 1903146. [Google Scholar] [CrossRef]
- Shalan, A.E.; Sharmoukh, W.; Elshazly, A.N.; Elnagar, M.M.; Al Kiey, S.A.; Rashad, M.M.; Allam, N.K. Dopant-free hole-transporting polymers for efficient, stable, and hysteresis-less perovskite solar cells. Sustain. Mater. Technol. 2020, 26, e00226. [Google Scholar] [CrossRef]
- Kranthiraja, K.; Arivunithi, V.M.; Aryal, U.K.; Park, H.Y.; Cho, W.; Kim, J.; Reddy, S.S.; Kim, H.K.; Kang, I.N.; Song, M.; et al. Efficient and hysteresis-less perovskite and organic solar cells by employing donor-acceptor type π-conjugated polymer. Org. Electron. 2019, 72, 18–24. [Google Scholar] [CrossRef]
- Kong, X.; Jiang, Y.; Wu, X.; Chen, C.; Guo, J.; Liu, S.; Gao, X.; Zhou, G.; Liu, J.M.; Kempa, K.; et al. Dopant-free F-substituted benzodithiophene copolymer hole-Transporting materials for efficient and stable perovskite solar cells. J. Mater. Chem. A 2020, 8, 1858–1864. [Google Scholar] [CrossRef]
- You, G.; Li, L.; Wang, S.; Cao, J.; Yao, L.; Cai, W.; Zhou, Z.; Li, K.; Lin, Z.; Zhen, H.; et al. Donor–Acceptor Type Polymer Bearing Carbazole Side Chain for Efficient Dopant-Free Perovskite Solar Cells. Adv. Energy Mater. 2022, 12, 2102697. [Google Scholar] [CrossRef]
- Liu, W.; Ma, Y.; Wang, Z.; Zhu, M.; Wang, J.; Khalil, M.; Wang, H.; Gao, W.; Fan, W.J.; Li, W.S.; et al. Improving the fill factor of perovskite solar cells by employing an amine-tethered diketopyrrolopyrrole-based polymer as the dopant-free hole transport layer. ACS Appl. Energy Mater. 2020, 3, 9600–9609. [Google Scholar] [CrossRef]
- Liu, W.; Ma, Y.; Wang, Z.; Mu, Z.; Gao, W.; Fan, W.; Li, W.S.; Zhang, Q. Improving the hole transport performance of perovskite solar cells through adjusting the mobility of the as-synthesized conjugated polymer. J. Mater. Chem. C 2021, 9, 3421–3428. [Google Scholar] [CrossRef]
- Ma, J.; Li, Y.; Li, J.; Qin, M.; Wu, X.; Lv, Z.; Hsu, Y.J.; Lu, X.; Wu, Y.; Fang, G. Constructing highly efficient all-inorganic perovskite solar cells with efficiency exceeding 17% by using dopant-free polymeric electron-donor materials. Nano Energy 2020, 75, 104933. [Google Scholar] [CrossRef]
- Xiong, J.; Qi, Y.; Zhang, Q.; Box, D.; Williams, K.; Tatum, J.; Das, P.; Pradhan, N.R.; Dai, Q. Enhanced Moisture and Water Resistance in Inverted Perovskite Solar Cells by Poly(3-hexylthiophene). ACS Appl. Energy Mater. 2021, 4, 1815–1823. [Google Scholar] [CrossRef]
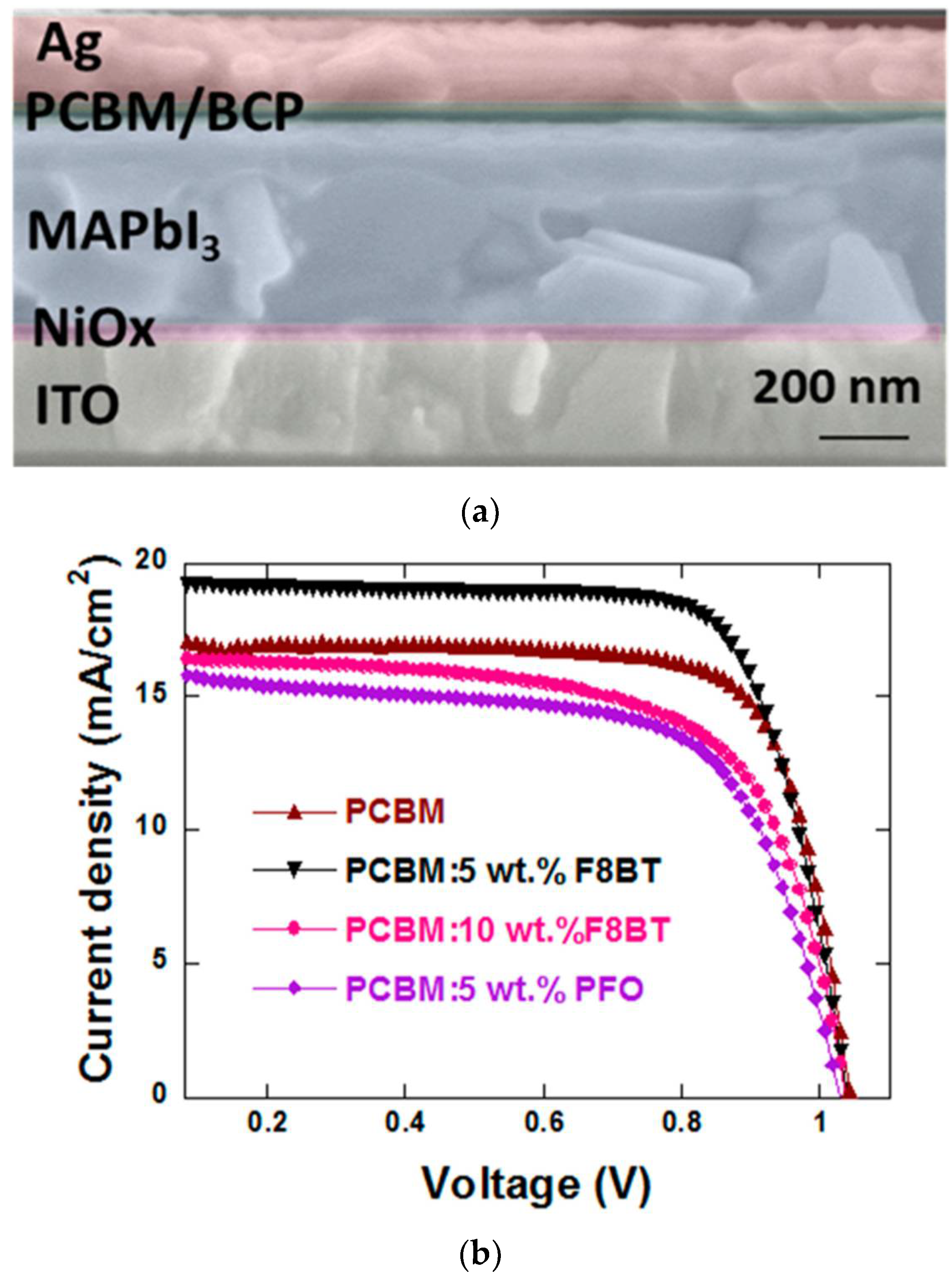
- Jiang, M.; Niu, Q.; Tang, X.; Zhang, H.; Xu, H.; Huang, W.; Yao, J.; Yan, B.; Xia, R. Improving the performances of perovskite solar cells via modification of electron transport layer. Polymers 2019, 11, 147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- You, S.; Zeng, H.; Ku, Z.; Wang, X.; Wang, Z.; Rong, Y.; Zhao, Y.; Zheng, X.; Luo, L.; Li, L.; et al. Multifunctional Polymer-Regulated SnO2 Nanocrystals Enhance Interface Contact for Efficient and Stable Planar Perovskite Solar Cells. Adv. Mater. 2020, 32, 2003990. [Google Scholar] [CrossRef]
- Liu, G.Z.; Du, C.S.; Wu, J.Y.; Liu, B.T.; Wu, T.M.; Huang, C.F.; Lee, R.H. Enhanced photovoltaic properties of perovskite solar cells by employing bathocuproine/hydrophobic polymer films as hole-blocking/electron-transporting interfacial layers. Polymers 2021, 13, 42. [Google Scholar] [CrossRef]
- Said, A.A.; Xie, J.; Wang, Y.; Wang, Z.; Zhou, Y.; Zhao, K.; Gao, W.B.; Michinobu, T.; Zhang, Q. Efficient Inverted Perovskite Solar Cells by Employing N-Type (D–A1–D–A2) Polymers as Electron Transporting Layer. Small 2019, 15, e1803339. [Google Scholar] [CrossRef]
- Tian, L.; Hu, Z.; Liu, X.; Liu, Z.; Guo, P.; Xu, B.; Xue, Q.; Yip, H.L.; Huang, F.; Cao, Y. Fluoro- and Amino-Functionalized Conjugated Polymers as Electron Transport Materials for Perovskite Solar Cells with Improved Efficiency and Stability. ACS Appl. Mater. Interfaces 2019, 11, 5289–5297. [Google Scholar] [CrossRef]
- Elnaggar, M.M.; Frolova, L.A.; Gordeeva, A.M.; Ustinova, M.I.; Laurenzen, H.; Akkuratov, A.V.; Nikitenko, S.L.; Solov’eva, E.A.; Luchkin, S.Y.; Fedotov, Y.S.; et al. Improving stability of perovskite solar cells using fullerene-polymer composite electron transport layer. Synth. Met. 2022, 286, 117028. [Google Scholar] [CrossRef]
- Yan, W.; Wang, Z.; Gong, Y.; Guo, S.; Jiang, J.; Chen, J.; Tang, C.; Xia, R.; Huang, W.; Xin, H. Naphthalene-diimide selenophene copolymers as efficient solution-processable electron-transporting material for perovskite solar cells. Org. Electron. 2019, 67, 208–214. [Google Scholar] [CrossRef]
- Ding, Y.; He, B.; Zhu, J.; Zhang, W.; Su, G.; Duan, J.; Zhao, Y.; Chen, H.; Tang, Q. Advanced Modification of Perovskite Surfaces for Defect Passivation and Efficient Charge Extraction in Air-Stable CsPbBr3 Perovskite Solar Cells. ACS Sustain. Chem. Eng. 2019, 7, 19286–19294. [Google Scholar] [CrossRef]
- Zhao, H.; Yang, S.; Han, Y.; Yuan, S.; Jiang, H.; Duan, C.; Liu, Z.; Liu, S. A High Mobility Conjugated Polymer Enables Air and Thermally Stable CsPbI2Br Perovskite Solar Cells with an Efficiency Exceeding 15%. Adv. Mater. Technol. 2019, 4, 1900311. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, S.; Song, X.; Rosas Villalva, D.; Troughton, J.; Corzo, D.; Toppare, L.; Gunbas, G.; Schroeder, B.C.; Baran, D. A Nonionic Alcohol Soluble Polymer Cathode Interlayer Enables Efficient Organic and Perovskite Solar Cells. Chem. Mater. 2021, 33, 8602–8611. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Zhu, T.; Zheng, L.; Zhang, D.; Xu, W.; Liu, L.; Cheng, G.; Zheng, J.; Gong, X. A zwitterionic polymer as an interfacial layer for efficient and stable perovskite solar cells. RSC Adv. 2019, 9, 30317–30324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, D.; Dai, S.; Li, M.; Wu, Y.; Zheng, L.; Wang, Y.; Yun, D.Q. Improved Efficiency and Stability of Perovskite Solar Cells Using a Difluorobenzothiadiazole-Based Interfacial Material. ACS Appl. Energy Mater. 2021, 4, 10646–10655. [Google Scholar] [CrossRef]
- Liu, Z.; Li, S.; Wang, X.; Cui, Y.; Qin, Y.; Leng, S.; Xu, Y.X.; Yao, K.; Huang, H. Interfacial engineering of front-contact with finely tuned polymer interlayers for high-performance large-area flexible perovskite solar cells. Nano Energy 2019, 62, 734–744. [Google Scholar] [CrossRef]
- Kim, J.; Lee, Y.; Yun, A.J.; Gil, B.; Park, B. Interfacial Modification and Defect Passivation by the Cross-Linking Interlayer for Efficient and Stable CuSCN-Based Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2019, 11, 46818–46824. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, F.; Li, Z.; Zhang, J.; Lian, J.; Song, J.; Qu, J.; Wong, W.Y. Naphthalene imide dimer as interface engineering material: An efficient strategy for achieving high-performance perovskite solar cells. Chem. Eng. J. 2020, 395, 125062. [Google Scholar] [CrossRef]

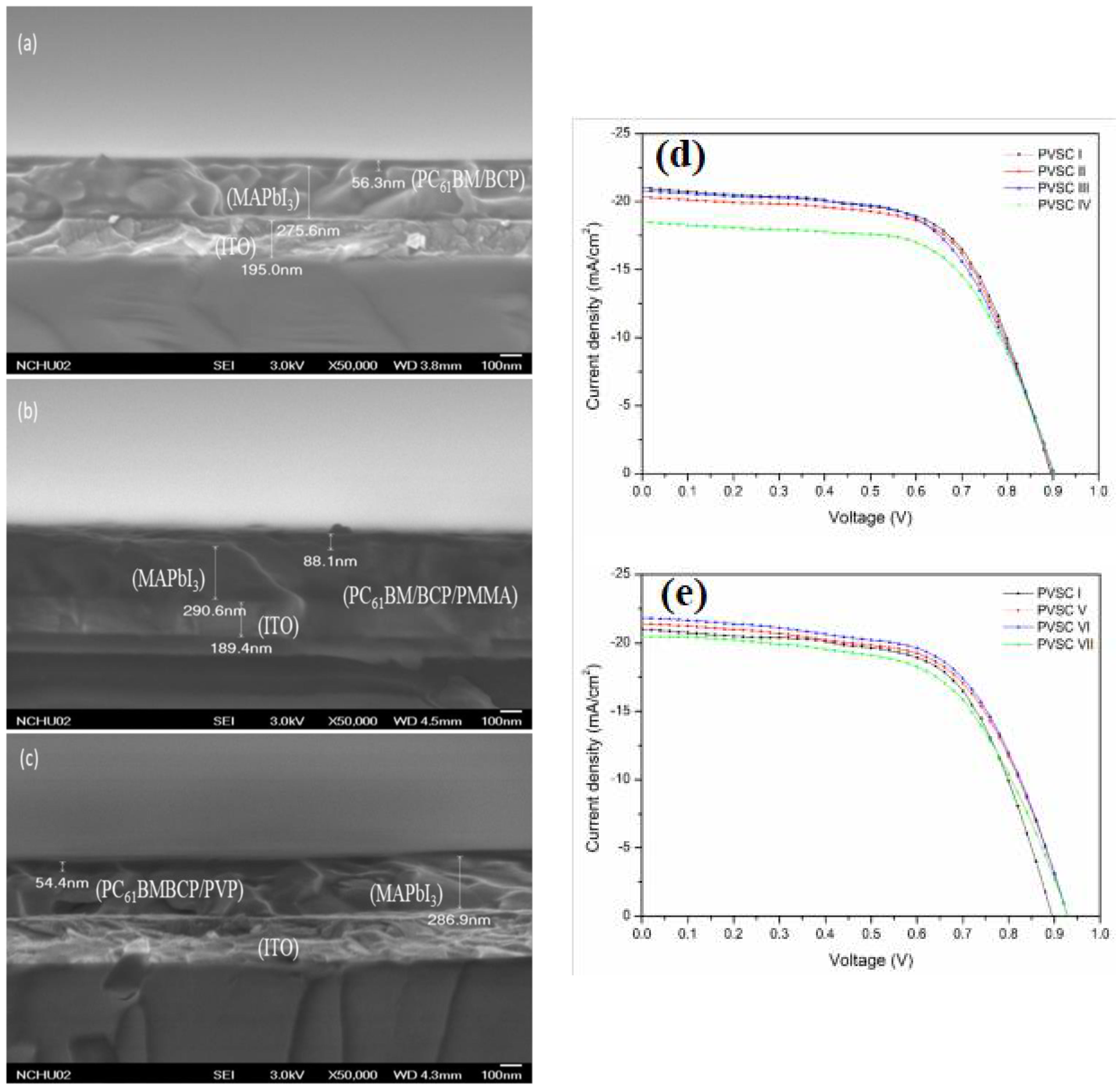
| Substrate | Voc (mV) | Jsc (mA/cm2) | FF (-) | PCE (%) | Ref. |
|---|---|---|---|---|---|
| ITO/PEN | 660 | 8.97 | 0.45 | 2.65 | [26] |
| ITO/PEN + G LSL | 680 | 10.62 | 0.48 | 3.47 | |
| ITO/PEN + T LSL | 690 | 14.65 | 0.43 | 4.33 | |
| ITO/PEN + TG LSL | 680 | 14.32 | 0.53 | 5.18 | |
| ITO/PEN (100 mW/cm2) | 400 | 8.70 | 0.46 | 2.60 | [68] |
| ITO/PEN (18 mW/cm2) | 380 | 1.8 | 0.53 | 3.30 | |
| ITO/PET (Pt CE) | 685 | 5.53 | 0.78 | 2.95 | [69] |
| ITO/PET (PEDOT:PSS CE) | 695 | 6.09 | 0.52 | 2.18 |
| Gel Electrolyte Structure | Solvent | Voc (mV) | Jsc (mA/cm2) | FF (-) | PCE (%) | Ref. |
|---|---|---|---|---|---|---|
| PEO + PGEDME/I−/I3− (0.1 M) | EtOH | 730 | 12.91 | 0.66 | 6.34 | [34] |
| ACN | 752 | 13.45 | 0.67 | 6.87 | ||
| ACN/VN | 785 | 11.56 | 0.76 | 6.88 | ||
| ACN/3-MPN | 785 | 12.05 | 0.76 | 7.16 | ||
| PEO + PGEDME/I−/I3− (0.2 M) | ACN/VN | 785 | 12.49 | 0.75 | 7.39 | |
| PEO + PGEDME/I−/I3− (0.4 M) | 784 | 11.94 | 0.75 | 7.03 | ||
| PEO + PGEDME/I−/I3− (0.2 M) + GuSCN (0 M) | 778 | 11.94 | 0.75 | 7.00 | ||
| PEO + PGEDME/I−/I3− (0.2 M) + GuSCN (0.05 M) | 787 | 12.48 | 0.75 | 7.35 | ||
| PEO + PGEDME/I−/I3− (0.2 M) + GuSCN (0.1 M) | 793 | 12.56 | 0.77 | 7.66 | ||
| PEO + PGEDME/I−/I3− (0.2 M) + GuSCN (0.2 M) | 758 | 12.85 | 0.73 | 7.07 | ||
| PAN (I−/I3−) | DMF | 790 | 6.85 | 0.67 | 4.19 | [35] |
| C11-AZO-C11/PAN (I−/I3−) | 780 | 11.96 | 0.75 | 6.28 | ||
| 3 wt.% PAN-co-PBA (I−/I3−) | ACN | 593 | 9.86 | 0.64 | 3.77 | [36] |
| 5 wt.% PAN-co-PBA (I−/I3−) | 587 | 11.60 | 0.61 | 4.13 | ||
| 7 wt.% PAN-co-PBA (I−/I3−) | 646 | 13.16 | 0.62 | 5.23 | ||
| 9 wt.% PAN-co-PBA (I−/I3−) | 618 | 10.41 | 0.65 | 4.35 | ||
| 10 wt.% PVdF-HFP (I−/I3−) | ACN | 6920 | 10.34 | 0.66 | 4.74 | [77] |
| 9 wt.% PVdF-HFP (I−/I3−) | 690 | 13.75 | 0.63 | 6.02 | ||
| 8 wt.% PVdF-HFP (I−/I3−) | 670 | 12.04 | 0.62 | 5.03 | ||
| 7 wt.% PVdF-HFP (I−/I3−) | 660 | 10.04 | 0.58 | 3.97 | ||
| pCMA-PGE (I−/I3−) | PC:ACN | 545 | 10.30 | 0.34 | 2.20 | [78] |
| PVP-PGE (I−/I3−) | 640 | 6.67 | 0.60 | 3.00 | ||
| PVDF (I−/I3−) | ACN | 730 | 17.79 | 0.64 | 8.36 | [79] |
| PDA@PVDF (I−/I3−) | 720 | 17.95 | 0.64 | 8.26 | ||
| esPME (I−/I3−) | ACN | 710 | 13.10 | 0.69 | 6.42 | [80] |
| esCPME (2 wt.% PPy) (I−/I3−) | 720 | 13.90 | 0.70 | 7.02 |
| Counter-Electrodes | Fabrication Methods of PPy-Based Counter-Electrodes | Voc (mV) | Jsc (mA cm−2) | FF (-) | PCE (%) | Ref. |
|---|---|---|---|---|---|---|
| Polypyrrole | Doctor Blade Technique | 749 | 15.75 | 0.69 | 8.13 | [37] |
| Poylpyrrole | Electropolymerization | 727 | 10.20 | 0.42 | 3.12 | [100] |
| Polypyrrole | Electropolymerization | 630 | 12.00 | 0.51 | 3.86 | [101] |
| PPy-POM | Electropolymerization | 765 | 11.68 | 0.56 | 5.04 | [38] |
| PPy-SrTiO3 | Doctor Blade Technique | 671 | 10.45 | 0.36 | 2.52 | [103] |
| PPy-MoS | In-situ Polymerization | 708 | 18.90 | 0.62 | 8.28 | [105] |
| Counter-Electrode | Fabrication Methods of PANI-Based Electrodes | Voc (mV) | Jsc (mA cm−2) | FF (-) | PCE (%) | Ref. |
|---|---|---|---|---|---|---|
| PANI | Doctor Blade Technique | 645 | 20.8 | 0.41 | 4.20 | [39] |
| PANI | Screen Printing Technique | 630 | 5.10 | 0.48 | 1.71 | [106] |
| PANI | Cyclic Voltametric-Electrochemical Method | 740 | 15.34 | 0.64 | 7.27 | [107] |
| Pristine PANI | Doctor Blade Technique | 480 | 4.71 | 0.45 | 1.14 | [40] |
| H2SO4-doped PANI | Doctor Blade Technique | 530 | 7.86 | 0.43 | 1.78 | |
| ALS-doped PANI | Doctor Blade Technique | 603 | 10.84 | 0.43 | 2.79 | |
| ALS-H2SO4-doped PANI | Doctor Blade Technique | 603 | 15.13 | 0.53 | 4.54 | |
| WO3-PANI | Cyclic voltammetry Technique | 685 | 18.00 | 0.55 | 6.78 | [108] |
| Structure of Solar Cell | Voc (mV) | Jsc (mA/cm2) | FF (-) | PCE (%) | Ref. |
|---|---|---|---|---|---|
| ITO/PEDOT:PSS/W1:Y6 (1:1)/PDIN/Ag | 890 | 25.36 | 0.68 | 15.39 (14.95) a | [121] |
| ITO/PEDOT:PSS/W1:Y6 (1:1.2)/PDIN/Ag | 890 | 25.92 | 0.69 | 15.95 (15.69) | |
| ITO/PEDOT:PSS/W1:Y6 (1:1.4)/PDIN/Ag | 890 | 25.06 | 0.71 | 15.87 (15.64) | |
| ITO/PEDOT:PSS/W1:Y6 (1:1.6)/PDIN/Ag | 880 | 24.59 | 0.71 | 15.65 (15.35) | |
| ITO/PEDOT:PSS/PBDT- TTZ:N2200/PFN-Br/Ag | 870 | 14.4 | 0.67 | 8.40 | [122] |
| ITO/PEDOT:PSS/PBDT-TT:N2200/PFN-Br/Ag | 750 | 2.0 | 0.46 | 0.70 | |
| ITO/PEDOT:PSS/PBDT-TTz:PC61BM/PFN-Br/Ag | 890 | 10.3 | 0.73 | 6.70 | |
| ITO.PEDOT:PSS/PBTz:IT-4F/PFN-Br/Al | 840 | 17.68 | 0.59 | 8.76 | [123] |
| ITO/PEDOT:PSS/PTzTz:IT-4F/PFN-Br/Al | 820 | 18.81 | 0.69 | 10.63 | |
| ITO/PEDOT:PSS/D18:Y6 (1:0.8)/PDIN/Ag | 861 | 27.16 | 0.72 | 16.98 (16.76) a | [15] |
| ITO/PEDOT:PSS/D18:Y6 (1:1.2)/PDIN/Ag | 863 | 27.05 | 0.75 | 17.51 (17.34) | |
| ITO/PEDOT:PSS/D18:Y6 (1:1.6)/PDIN/Ag | 865 | 27.31 | 0.75 | 17.84 (17.67) | |
| ITO/PEDOT:PSS/D18:Y6 (1:2)/PDIN/Ag | 870 | 26.20 | 0.75 | 17.16 (16.87) | |
| ITO/PEDOT:PSS/D18:Y6/PDIN/Ag (170 nm) b | 864 | 25.89 | 0.73 | 16.38 (16.34) a | |
| ITO/PEDOT:PSS/D18:Y6/PDIN/Ag (130 nm) | 864 | 26.39 | 0.74 | 16.92 (16.77) | |
| ITO/PEDOT:PSS/D18:Y6/PDIN/Ag (112 nm) | 866 | 27.16 | 0.75 | 17.65 (17.38) | |
| ITO/PEDOT:PSS/D18:Y6/PDIN/Ag (103 nm) | 865 | 27.31 | 0.75 | 17.84 (17.67) | |
| ITO/PEDOT:PSS/D18:Y6/PDIN/Ag (91 nm) | 869 | 26.75 | 0.76 | 17.73 (17.64) | |
| ITO/PEDOT:PSS/D18:Y6:PC61BM (1:1.6:0)/PDIN/Ag | 862 | 26.09 | 0.76 | 17.23 (17.01) b | [124] |
| ITO/PEDOT:PSS/D18:Y6:PC61BM (1:1.6:0.1)/PDIN/Ag | 865 | 26.33 | 0.76 | 17.42 (17.16) | |
| ITO/PEDOT:PSS/D18:Y6:PC61BM (1:1.6:0.2)/PDIN/Ag | 870 | 26.48 | 0.77 | 17.89 (17.57) | |
| ITO/PEDOT:PSS/D18:Y6:PC61BM (1:1.6:0.4)/PDIN/Ag | 874 | 25.70 | 0.75 | 16.94 (16.78) | |
| ITO/PEDOT:PSS/D18:Y6:PC61BM (1:1.6:0.6)/PDIN/Ag | 882 | 25.64 | 0.71 | 16.05 (15.82) | |
| ITO/PEDOT:PSS/D18:Y6:PC61BM (1:1.6:0.2 c)/PDIN/Ag | 865 | 25.90 | 0.76 | 17.09 (16.92) | |
| ITO/PEDOT:PSS/D18:Y6:PC61BM (1:1.6:0 d)/PDIN/Ag | 865 | 27.31 | 0.75 | 17.84 (17.67) | |
| ITO/PEDOT:PSS/D18:Y6:PC61BM (1:1.6:0 e)/PDIN/Ag | 859 | 27.70 | 0.76 | 18.22 (18.01) | |
| ITO/PEDOT:PSS/D18:Y6:PC61BM/PDIN/Ag (90 nm) b | 870 | 25.94 | 0.75 | 17.10 (16.97) b | |
| ITO/PEDOT:PSS/D18:Y6:PC61BM/PDIN/Ag (110 nm) | 870 | 26.48 | 0.77 | 17.89 (17.57) | |
| ITO/PEDOT:PSS/D18:Y6:PC61BM/PDIN/Ag (130 nm) | 864 | 26.44 | 0.75 | 17.12 (16.72) | |
| ITO/PEDOT:PSS/PTPD:Y6/PFN-Br/Ag | 660 | 19.5 | 0.46 | 5.90 | [125] |
| ITO/PEDOT:PSS/PBiTPD:Y6/PFN-Br/Ag | 830 | 25.6 | 0.66 | 14.20 | |
| ITO/PEDOT:PSS/P106:Y18-DMO/PFN/Al | 870 | 22.78 (22.62) f | 0.71 | 14.07 (13.91) b | [126] |
| ITO/PEDOT:PSS/P106:DBTBT-IC/PFN/Al | 960 | 18.56 (18.41) | 0.66 | 11.76 (11.59) | |
| ITO/PEDOT:PSS/P106:DBTBT-IC:Y18-DMO/PFN/Al | 910 | 24.82 (24.66) | 0.73 | 16.49 (16.32) | |
| ITO/ZnO/[PTB7-Th(1):Si-BDT(0):DCNBT-TPIC(0.6)/MoO3/Ag | 850 | 18.00 (18.07) f | 0.64 | 10.11 | [127] |
| ITO/ZnO/[PTB7-Th(0.8):Si-BDT(0.2):DCNBT-TPIC(0.6)/MoO3/Ag | 850 | 19.32 (19.74) f | 0.65 | 11.20 | |
| ITO/ZnO/[PTB7-Th(0.6):Si-BDT(0.4):DCNBT-TPIC(0.6)/MoO3/Ag | 860 | 22.32 (22.06) f | 0.68 | 13.45 | |
| ITO/ZnO/[PTB7-Th(0.4):Si-BDT(0.6):DCNBT-TPIC(0.6)/MoO3/Ag | 820 | 19.21 (19.23) f | 0.66 | 10.88 | |
| ITO/ZnO/[PTB7-Th(0.2):Si-BDT(0.8):DCNBT-TPIC(0.6)/MoO3/Ag | 820 | 16.00 (16.01) f | 0.54 | 7.53 | |
| ITO/ZnO/[PTB7-Th(0):Si-BDT(1):DCNBT-TPIC(0.6)/MoO3/Ag | 820 | 17.58 (17.59) f | 0.65 | 9.92 |
| Structure of Solar Cell | Voc (mV) | Jsc (mA/cm2) | FF (-) | PCE (%) | Ref. |
|---|---|---|---|---|---|
| ITO/PEDOT:PSS/POBDFBT(1):ITIC(1): PCBM(0)/PFN/Al | 820 | 16.59 | 0.46 | 6.16 | [130] |
| ITO/PEDOT:PSS/POBDFBT(1):ITIC(1): PCBM(0.5)/PFN/Al | 780 | 12.7 | 0.64 | 6.26 | |
| ITO/PEDOT:PSS/POBDFBT(1):ITIC(0.75): PCBM(0.75)/PFN/Al | 760 | 13.8 | 0.61 | 6.39 | |
| ITO/PEDOT:PSS/POBDFB(1):ITIC(0.5): PCBM(1)/PFN/Al | 720 | 17.65 | 0.62 | 7.91 | |
| ITO/PEDOT:PSS/POBDFB(1):ITIC(0.25): PCBM(1.25)/PFN/Al | 790 | 13.78 | 0.61 | 6.66 | |
| ITO/PEDOT:PSS/POBDFB(1):ITIC(0): PCBM(1.5)/PFN/Al | 710 | 13.67 | 0.64 | 6.23 | |
| ITO/PEDOT:PSS/P:ITIC-m/PFN/Al | 1040 | 16.86 | 0.69 | 12.10 | [131] |
| ITO/PEDOT:PSS/P:Y6/PFN/Al | 940 | 19.72 | 0.71 | 13.16 | |
| ITO/PEDOT:PSS/P:ITIC-m:Y6/PFN/Al | 990 | 20.65 | 0.74 | 15.13 | |
| ITO/PEDOT:PSS/PM6:MF1(0):Y6/PDIN/Al | 843 | 25.11 | 0.75 | 15.93 | [132] |
| ITO/PEDOT:PSS/PM6:MF1(10):Y6/PDIN/Al | 853 | 25.68 | 0.77 | 17.22 | |
| ITO/PEDOT:PSS/PM6:MF1(50):Y6/PDIN/Al | 867 | 23.53 | 0.71 | 14.40 | |
| ITO/PEDOT:PSS/PM6:MF1(100):Y6/PDIN/Al | 914 | 16.67 | 0.79 | 12.09 | |
| ITO/PEDOT:PSS/PM6:Y6/PDINO/Al (150) | 860 | 24.3 | 0.73 | 15.3(15.2 ± 0.1) | [133] |
| ITO/PEDOT:PSS/PM6:Y6/PDINO/Al (150) | 830 | 25.3 | 0.75 | 15.7(15.6 ± 0.1) | |
| ITO/PEDOT:PSS/PM6:Y6/PDINO/Al (200) | 830 | 25.8 | 0.67 | 14.3(14.2 ± 0.1) | |
| ITO/PEDOT:PSS/PM6:Y6/PDINO/Al (250) | 820 | 27.1 | 0.63 | 14.1(13.9 ± 0.2) | |
| ITO/PEDOT:PSS/PM6:Y6/PDINO/Al (300) | 820 | 26.5 | 0.62 | 13.6(13.3 ± 0.3) | |
| ITO/ZnO/PM6:Y6/MoO3/Ag (100) | 820 | 25.2 | 0.76 | 15.7(15.5 ± 0.2) | |
| ITO/PEDOT:PSS/PM6:Y6/PDINO/Al | 830 | 23.2 | 0.77 | 14.90 | |
| ITO/PEDOT:PSS/PM6(1):Y6 (1.2): PC71BM(0)/PDINO/Al | 8450 | 24.89 | 0.74 | 15.75 (15.70) | [134] |
| ITO/PEDOT:PSS/PM6(1):Y6 (1.1): PC71BM(0.1)/PDINO/Al | 850 | 25.36 | 0.76 | 16.30 (16.26) | |
| ITO/PEDOT:PSS/PM6(1):Y6 (1.05): PC71BM(0.15)/PDINO/Al | 850 | 25.8 | 0.75 | 16.38 (16.32) | |
| ITO/PEDOT:PSS/PM6(1):Y6 (1.0): PC71BM(0.2)/PDINO/Al | 850 | 25.7 | 0.76 | 16.67 (16.61) | |
| ITO/PEDOT:PSS/PM6(1):Y6 (0.9): PC71BM(0.3)/PDINO/Al | 853 | 25.05 | 0.75 | 16.05 (16.0) | |
| ITO/PEDOT:PSS/PM6(1):Y6 (0.7): PC71BM(0.5)/PDINO/Al | 865 | 23.94 | 0.74 | 15.30 (15.23) | |
| ITO/PEDOT:PSS/PM6(1):Y6 (0.4): PC71BM(0.8)/PDINO/Al | 876 | 19.24 | 0.49 | 8.39 (8.27) | |
| ITO/PEDOT:PSS/PM6(1):Y6 (0.1): PC71BM(1.2)/PDINO/Al | 965 | 11.56 | 0.53 | 6.01 (5.94) | |
| ITO/PEDOT:PSS/PM6(1):PM7-Si(0):C9(1.2)/PFN-Br/Ag | 841 | 26.36 | 0.76 | 17.0 | [135] |
| ITO/PEDOT:PSS/PM6(0.9):PM7-Si(0.1):C9(1.2)/PFN-Br/Ag | 864 | 26.35 | 0.77 | 17.7 | |
| ITO/PEDOT:PSS/PM6(0):PM7-Si(1):C9(1.2)/PFN-Br/Ag | 895 | 14.43 | 0.41 | 5.4 | |
| ITO/PEDOT:PSS/P130:Y6/PFN/Al | 890 (±5) | 23.84 (±0.32) | 0.72 (±0.05) | 15.28 (±0.21) | [136] |
| ITO/PEDOT:PSS/P131:Y6/PFN/Al | 780 (±3) | 21.96 (0.22) | 0.65 (±0.03) | 11.13 (±0.18) |
| Device Structure | Voc (mV) | Jsc (mA cm−2) | FF (-) | PCE (%) | Ref. |
|---|---|---|---|---|---|
| ITO/PEDOT:PSS/PffBT-DPP(1)/[70] PCBM(3)/MeIC(1)/ZrAcAc/Al | 740 | 12.5 | 0.74 | 6.8 | [137] |
| ITO/PEDOT:PSS/PffBT-DPP(1)/[70] PCBM(0)/MeIC(1)/ZrAcAc/Al | 780 | 4.5 | 0.58 | 2.0 | |
| ITO/PEDOT:PSS/PffBT-DPP(1)/[70] PCBM(2)/MeIC(1)/ZrAcAc/Al | 760 | 16.1 | 0.73 | 9.0 | |
| (ITO)/PEDOT:PSS/PTQ10:Y6/PFN-Br/Al | 820 ± 1 | 23.9 ± 0.1 | 0.73 | 14.5 ± 0.1 | [138] |
| ITO/PEDOT:PSS/P1(1):PC71BM(2)/LiF/Al (500 rpm) i | 770 | 5.76 | 0.43 | 1.92 | [139] |
| ITO/PEDOT:PSS/P1(1):PC71BM(3)/LiF/Al (500 rpm) i | 770 | 7.32 | 0.39 | 2.21 | |
| ITO/PEDOT:PSS/P1(1):PC71BM(4)/LiF/Al (500 rpm) i | 770 | 7.10 | 0.39 | 1.97 | |
| ITO/PEDOT:PSS/P1(1):PC71BM(3)/LiF/Al (500 rpm) i | 580 | 3.07 | 0.30 | 0.55 | |
| ITO/PEDOT:PSS/P1(1):PC71BM(3)/LiF/Al (500 rpm) i | 770 | 8.19 | 0.35 | 2.21 | |
| ITO/PEDOT:PSS/P1(1):PC71BM(3)/LiF/Al (350 rpm)i | 790 | 7.29 | 0.41 | 2.36 | |
| ITO/PEDOT:PSS/P1(1):PC71BM(3)/LiF/Al (750 rpm) i | 790 | 6.94 | 0.35 | 1.92 | |
| ITO/PEDOT:PSS/P2(1):PC71BM(2)/LiF/Al (500 rpm) i | 710 | 5.27 | 0.55 | 2.07 | |
| ITO/PEDOT:PSS/P2(1):PC71BM(3)/LiF/Al (500 rpm) i | 700 | 5.30 | 0.37 | 1.38 | |
| ITO/PEDOT:PSS/P3(1):PC71BM(1)/LiF/Al (500 rpm) i | 750 | 2.50 | 0.49 | 0.92 | |
| ITO/PEDOT:PSS/P3(1):PC71BM(2)/LiF/Al (500 rpm) i | 750 | 3.95 | 0.46 | 1.38 | |
| ITO/PEDOT:PSS/P3(1):PC71BM(3)/LiF/Al (500 rpm) i | 750 | 3.85 | 0.49 | 1.43 | |
| ITO/PEDOT:PSS/P3(1):PC71BM(4)/LiF/Al (500 rpm) i | 760 | 5.14 | 0.42 | 1.65 | |
| ITO/PEDOT:PSS/P3(1):PC71BM(4)/LiF/Al (500 rpm) i | 740 | 7.13 | 0.34 | 1.83 | |
| ITO/PEDOT:PSS/P3(1):PC71BM(4)/LiF/Al (500 rpm)i | 750 | 7.63 | 0.35 | 2.02 | |
| ITO/PEDOT:PSS/P3(1):PC71BM(4)/LiF/Al (350 rpm) i | 770 | 7.59 | 0.41 | 2.45 | |
| ITO/PEDOT:PSS/P3(1):PC71BM(4)/LiF/Al (750 rpm) i | 740 | 5.9 | 0.33 | 1.48 | |
| ITO/PEDOT:PSS/PTT-EFQX:PCBM/PFN-Br/Ag | 690 | 11.19 | 0.68 | 5.37 | [140] |
| ITO/PEDOT:PSS/PT-DFBT-T-EFQX:PCBM/PFN-Br/Ag | 870 | 5.62 | 0.54 | 2.69 | |
| ITO/PEDOT:PSS/P(T2BDY−TBDT)/PNDIT-F3N−Br/Ag | 780 | 12.07 | 0.47 | 4.40 | [141] |
| ITO/PEDOT:PSS/P(TTzBDY−TBDT)/PNDIT-F3N−Br/Ag | 800 | 7.71 | 0.40 | 2.49 | |
| ITO/PEDOT:PSS/P(T2BDY−TBDT0.7−OBDT0.3)/PNDIT-F3N−Br/Ag | 750 | 3.80 | 0.37 | 1.06 | |
| ITO/PEDOT:PSS/P(TTzBDY−TBDT0.7−OBDT0.3)/PNDIT-F3N−Br/Ag | 770 | 5.23 | 0.39 | 1.58 |
| Structure of Solar Cell | Voc (mV) | Jsc (mA/cm2) | FF (-) | PCE (%) | Ref. |
|---|---|---|---|---|---|
| ITO/PEDOT:PSS/PTzBISi:N2200/C60N/Ag CB- as print | 930 | 2.76 | 0.43 | 1.01 | [47] |
| ITO/PEDOT:PSS/PTzBISi:N2200/C60N/Ag CB-TA | 890 | 3.98 | 0.48 | 1.57 | |
| ITO/PEDOT:PSS/PTzBISi:N2200/C60N/Ag CB-TA+SVA | 870 | 4.58 | 0.51 | 1.83 | |
| ITO/PEDOT:PSS/PTzBISi:N2200/C60N/Ag MTHF-as print | 890 | 15.41 | 0.70 | 9.01 | |
| ITO/PEDOT:PSS/PTzBISi:N2200/C60N/Ag MTHF-TA | 880 | 16.19 | 0.73 | 9.96 | |
| ITO/PEDOT:PSS/PTzBISi:N2200/C60N/Ag MTHF-TA+SVA | 880 | 17.62 | 0.76 | 11.25 | |
| ITO/ZnO/PTB7-Th:NDP-V/V2O5/Al | 740 | 17.07 | 0.67 | 8.59 | [48] |
| ITO/ZnO/PTB7-Th:PDI-V/V2O5/Al | 740 | 15.39 | 0.64 | 7.38 | |
| ITO/ZnO/PEI/BSS0:PBDB-T/MoO3/Ag | 820 | 15.74 | 0.57 | 7.38 | [49] |
| ITO/ZnO/PEI/BSS10:PBDB-T/MoO3/Ag | 860 | 18.55 | 0.64 | 10.10 | |
| ITO/ZnO/PEI/BSS20:PBDB-T/MoO3/Ag | 860 | 17.07 | 0.65 | 9.58 | |
| ITO/ZnO/PEI/BSS50:PBDB-T/MoO3/Ag | 850 | 17.50 | 0.65 | 9.69 | |
| ITO/ZnO/PBDBT:PIID(CO) 2FT/MoO3/Ag | 640 | 8.30 | 0.50 | 2.65 | [142] |
| ITO/ZnO/PBDBT:PIID(CO) BTIA/MoO3/Ag | 630 | 1.80 | 0.50 | 0.37 | |
| ITO/PEDOT:PSS/PBDB-Tb-PYT/PDINN50/Ag (CF; area 5 mm2) | 919 | 16.90 | 0.46 | 7.18 | [143] |
| ITO/PEDOT:PSS/PBDB-Tb-PYT/PDINN50/Ag (CF, 4% CN; area 5 mm2) | 916 | 19.60 | 0.63 | 11.32 | |
| ITO/PEDOT:PSS/PBDB-Tb-PYT/PDINN50/Ag (CB, 4% CN; area 5 mm2) | 908 | 19.31 | 0.60 | 10.53 | |
| ITO/PEDOT:PSS/PBDB-Tb-PYT/PDINN50/Ag (ODCB, 4% CN; area 5 mm2) | 917 | 18.67 | 0.59 | 10.08 | |
| ITO/PEDOT:PSS/PBDB-Tb-PYT/PDINN50/Ag (THF, 4% CN; area 5 mm2) | 914 | 19.25 | 0.63 | 11.13 | |
| ITO/PEDOT:PSS/PBDB-Tb-PYT/PDINN50/Ag (Toluene, 4% CN; area 5 mm2) | 912 | 19.38 | 0.62 | 11.07 | |
| ITO/PEDOT:PSS/PBDB-Tb-PYT/PDINN50/Ag (CF, 4% CN; area 2.2 mm2) | 867 | 19.71 | 0.63 | 10.80 | |
| ITO/PEDOT:PSS/PBDB-T:PYT/PDINN50/Ag (CF; area 5 mm2) | 883 | 22.70 | 0.72 | 14.57 |
| Device Structure | Voc (mV) | Jsc (mA cm−2) | FF (-) | PCE (%) | Ref. |
|---|---|---|---|---|---|
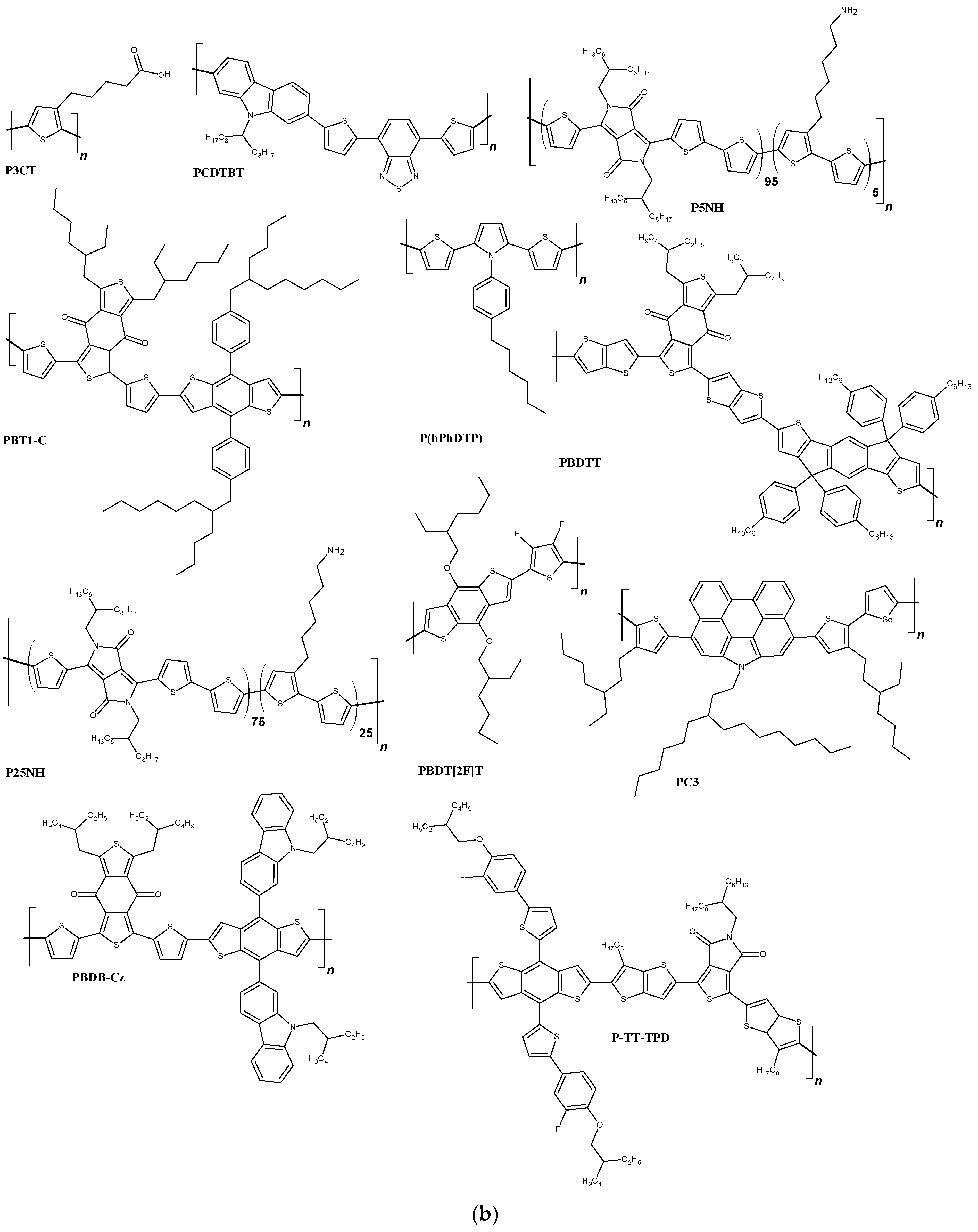
| FTO/TiO2/SnO2/[Cs0.05FA0.8MA0.15PbI2.55Br0.45]/IDTB/Au | 1107 | 23.06 | 0.76 | 19.38 | [160] |
| ITO/P3CT/[(FA0.17MA0.94PbI3.11)0.95(PbCl2)0.05]/C60/ZrAcac/Ag | 1120 | 22.88 | 0.82 | 21.09 | [161] |
| FTO/TiO2/[0.001 M FAI, 0.001 M PbI2, 0.0002 M MABr, 0.0002 M PbBr2 + CsI solution (1.5 M in DMSO)]/PBT1-C/-C/MoO3/Ag | 1030 | 22.10 | 0.79 | 19.06 | [162] |
| ITO/SnO2/[CH3NH3PbI3]/PCDTBT/Ag | 970 | 19.90 | 0.73 | 14.08 | [163] |
| FTO/SnO2/[0.001 M MAI,0.001 M PbI2 + EACl (0.0002 M (15% molar ratio))]/PC3/Au | 1110 | 23.50 | 0.80 | 20.80 | [164] |
| ITO/SnO2/[1.1 M PbI2, 1.0 M FAI, 0.22 M PbBr2, 0.2 M MABr + 1.5 M CsI]/PBDTT/MoO3/Ag | 1120 | 23.64 | 0.77 | 20.28 | [165] |
| FTO/b-TiO2/m-TiO2/[CH3NH3PbI3]/P(hPhDTP)/Ag | 960 | 20.82 | 0.79 | 15.71 | [166] |
| FTO/TiO2/[0.0006 M PbI2, 0.0001 M PbBr2, 0.0001 M MABr, 0.0005 M FAI]/P-TT-TPD/Au | 1040 | 21.68 | 0.73 | 16.82 | [167] |
| FTO/SnO2/[CH3NH3PbI3]/PBDT[2F]T/Ag | 1060 | 22.64 | 0.73 | 17.52 | [168] |
| ITO/SnO2/[1.1 M PbI2, 1.0 M FAI, 0.22 M PbBr2, 0.2 M MABr]/PBDB-Cz/MoO3/Ag | 1135 | 24.34 | 0.76 | 21.11 | [169] |
| ITO/SnO2/[0.26 M FAI,1.26 M PbI2, 1.08 M MAI, 0.14 M PbCl2]/P25NH/Ag | 1049 | 19.81 | 0.83 | 17.30 | [170] |
| ITO/SnO2/[(MA0.8FA0.2)Pb(I0.93Cl0.07)3]/P5NH/Ag | 1041 | 20.95 | 0.83 | 18.10 | [171] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gnida, P.; Amin, M.F.; Pająk, A.K.; Jarząbek, B. Polymers in High-Efficiency Solar Cells: The Latest Reports. Polymers 2022, 14, 1946. https://doi.org/10.3390/polym14101946
Gnida P, Amin MF, Pająk AK, Jarząbek B. Polymers in High-Efficiency Solar Cells: The Latest Reports. Polymers. 2022; 14(10):1946. https://doi.org/10.3390/polym14101946
Chicago/Turabian StyleGnida, Paweł, Muhammad Faisal Amin, Agnieszka Katarzyna Pająk, and Bożena Jarząbek. 2022. "Polymers in High-Efficiency Solar Cells: The Latest Reports" Polymers 14, no. 10: 1946. https://doi.org/10.3390/polym14101946
APA StyleGnida, P., Amin, M. F., Pająk, A. K., & Jarząbek, B. (2022). Polymers in High-Efficiency Solar Cells: The Latest Reports. Polymers, 14(10), 1946. https://doi.org/10.3390/polym14101946







