Rheometer Evidences for the Co-Curing Effect of a Bismaleimide in Conjunction with the Accelerated Sulfur on Natural Rubber/Chloroprene Rubber Blends
Abstract
1. Introduction
2. Materials
2.1. Preparation of Rubber Compounds
2.2. Characterization
2.2.1. Cure Characteristics
2.2.2. Swelling Behavior
2.2.3. Mechanical (Tensile) Properties
2.2.4. Hardness Testing
3. Results and Discussion
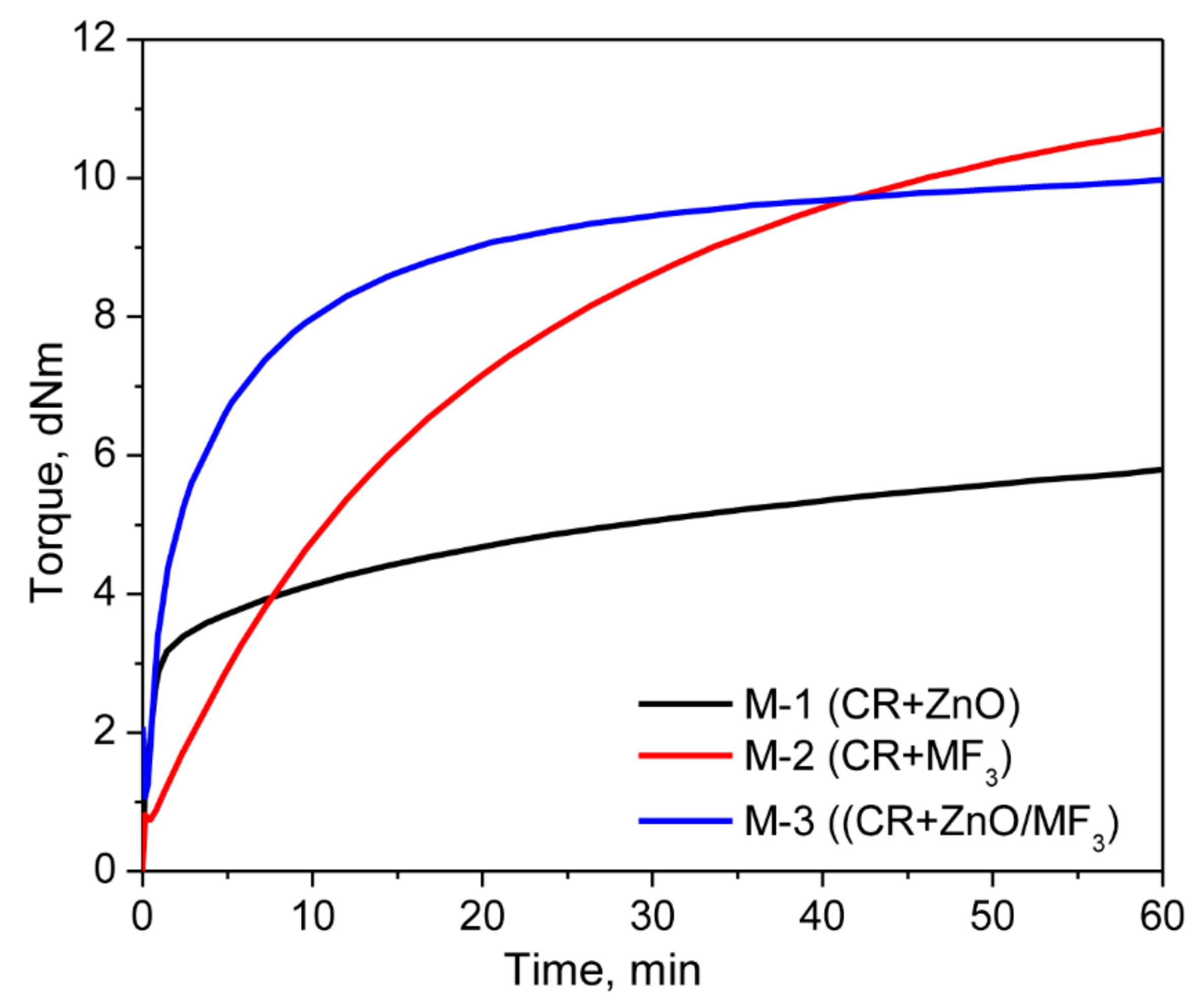
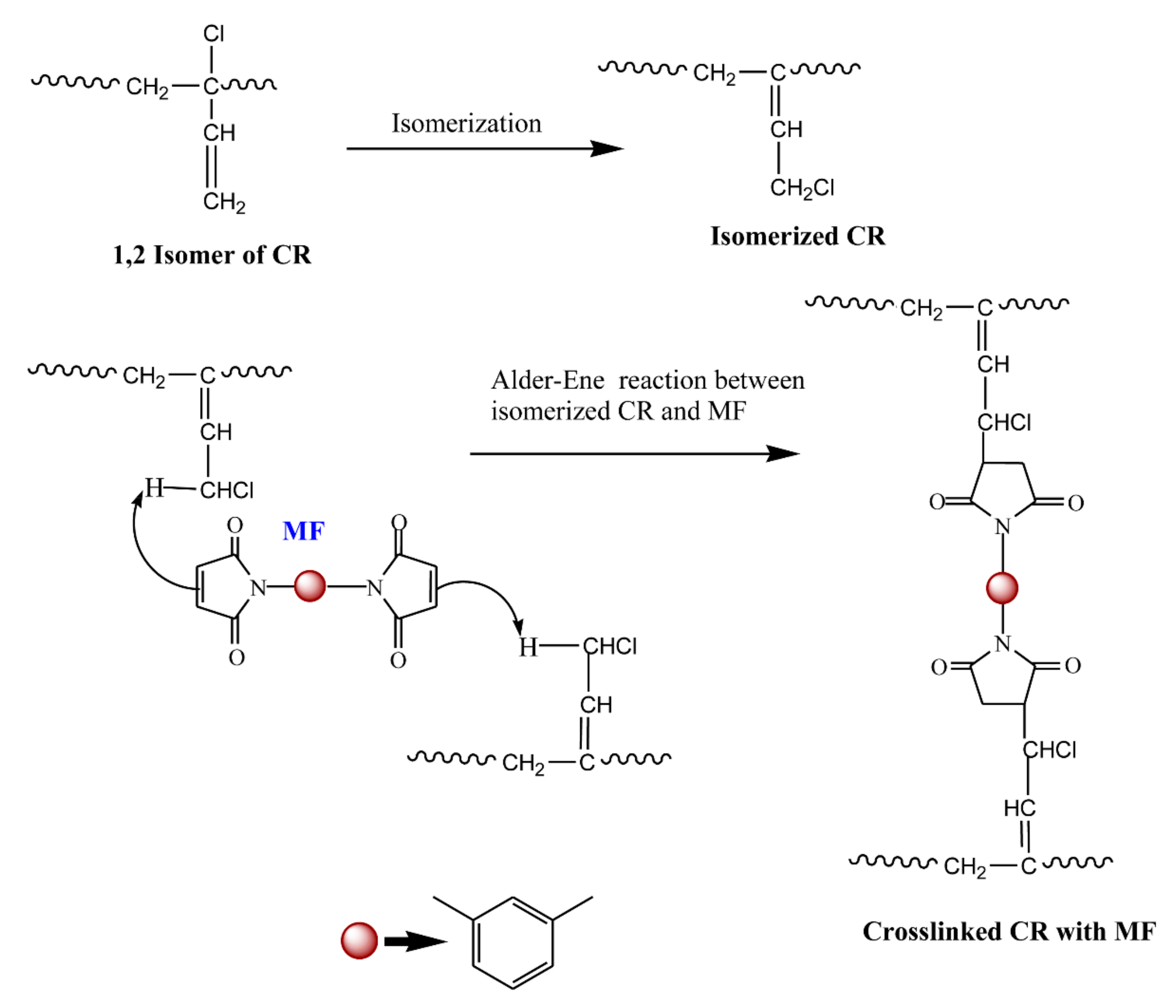
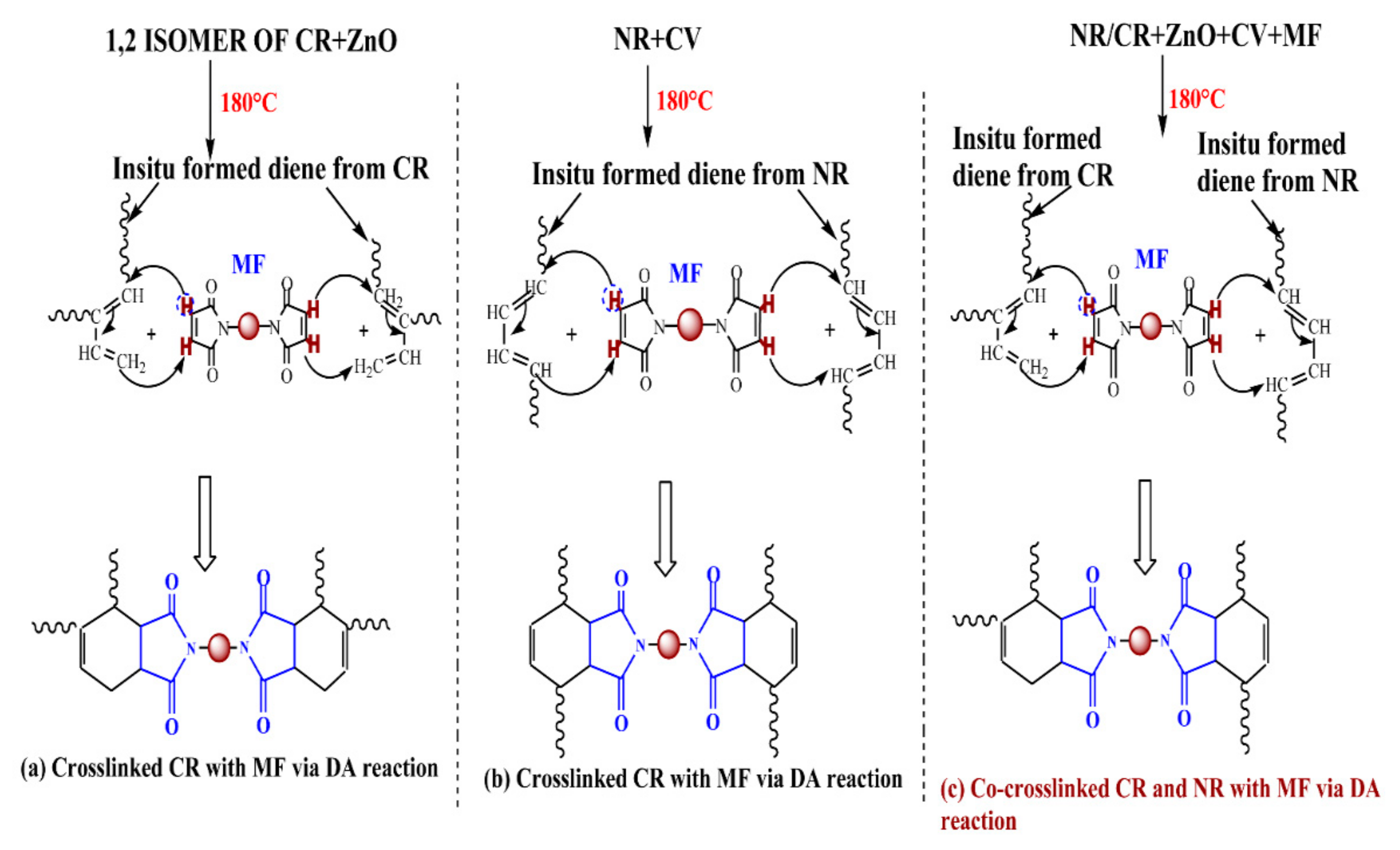
3.1. Curing Behavior of Neat CR with ZnO and MF
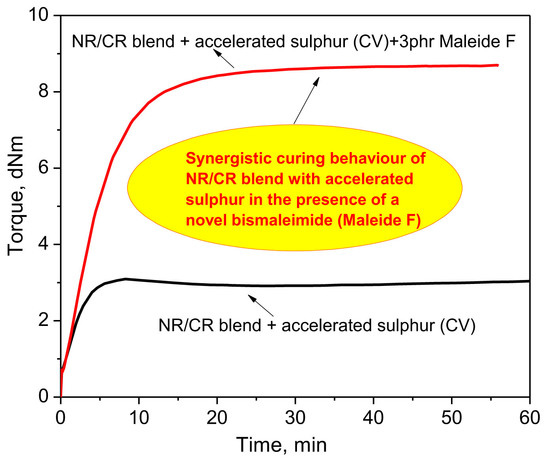
3.2. Curing Behavior of NR/CR Blend in the Presence of MF in Conjunction with Accelerated Sulfur
3.3. Swelling Behavior
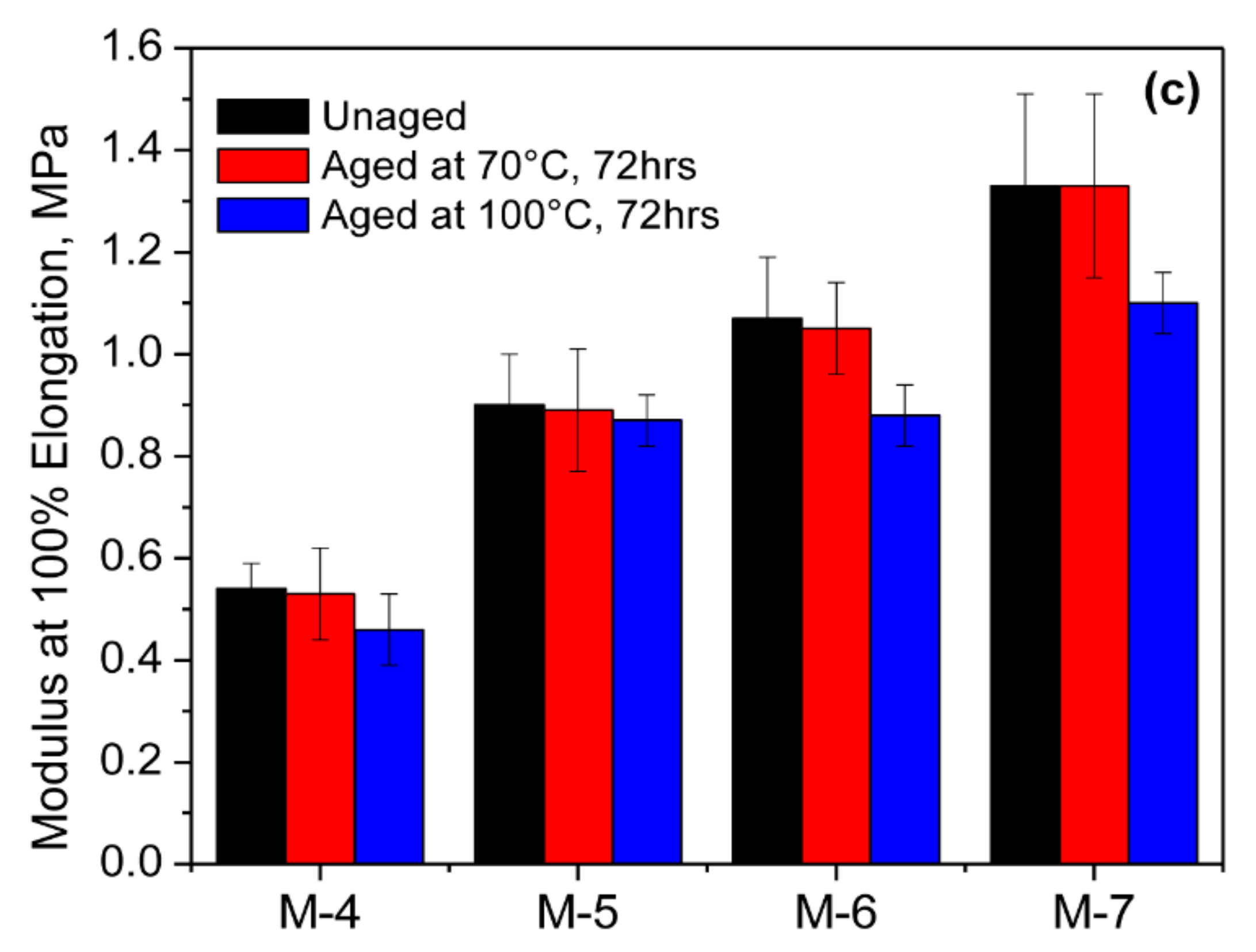
3.4. Mechanical Properties of the Blends
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morton, M. Rubber Technology; Van Nostrand Reinhold Company: New York, NY, USA, 1987; pp. 1–20. [Google Scholar]
- Hofmann, W. Rubber Technology Hand Book; Hanser Publishers: New York, NY, USA, 1989. [Google Scholar]
- Ferguson, R.C. Infrared and nuclear magnetic resonance studies of the microstructures of polychlo-roprenes. J. Polym. Sci. Part A Gen. Pap. 1964, 2, 4735–4741. [Google Scholar] [CrossRef]
- Tabb, D.L.; Koenig, J.L.; Coleman, M.M. Infrared spectroscopic evidence of structural defects in the crystalline regions of trans-1,4-polychloroprene. J. Polym. Sci. Polym. Phys. Ed. 1975, 13, 1145–1158. [Google Scholar] [CrossRef]
- Aufdermarsh, C.A.; Pariser, R. Cis-polychloroprene. J. Polym. Sci. Part A Gen. Pap. 1964, 2, 4727–4733. [Google Scholar] [CrossRef]
- Sathasivam, K.; Haris, M.R.H.M.; Mohan, S. Vibrational spectroscopic studies on cis-1, 4-polychloroprene. Int. J. Chemtech Res. 2010, 2, 1780–1785. [Google Scholar]
- Ferguson, R.C. Determination of Polychloroprene Isomers by High Resolution Infrared Spectrometry. Anal. Chem. 1964, 36, 2204–2205. [Google Scholar] [CrossRef]
- Petcavich, R.J.; Painter, P.C.; Coleman, M.M. Application of infra-red digital subtraction techniques to the microstructure of polychloroprenes: 2. Mechanism of oxidative degradation at 60 °C. Polymer 1978, 19, 1249–1252. [Google Scholar] [CrossRef]
- Alliger, G.; Sjothun, I.J. Vulcanization of Elastomers; Robert E. Krieger Publishers: New York, NY, USA, 1978. [Google Scholar]
- Hofmann, W. Vulcanization and Vulcanizing Agents; Maclaren and Sons: London, UK, 1967. [Google Scholar]
- Desai, H.; Hendrikse, K.G.; Woolard, C.D. Vulcanization of polychloroprene rubber. I. A revised cationic mechanism for ZnO crosslinking. J. Appl. Polym. Sci. 2007, 105, 865–876. [Google Scholar] [CrossRef]
- Berry, K.; Liu, M.; Chakraborty, K.; Pullan, N.; West, A.; Sammon, C.; Topham, P.D. Mechanism for Cross-Linking Polychloroprene with Ethylene Thiourea and Zinc Oxide. Rubber Chem. Technol. 2015, 88, 80–97. [Google Scholar] [CrossRef]
- Miyata, Y.; Atsumi, M. Zinc Oxide Crosslinking Reaction of Polychloroprene Rubber. Rubber Chem. Technol. 1989, 62, 1–12. [Google Scholar] [CrossRef]
- Kovacic, P. Bisalkylation Theory of Neoprene Vulcanization. Ind. Eng. Chem. 1955, 47, 1090–1094. [Google Scholar] [CrossRef]
- Mallon, P.E.; McGill, W.J.; Shillington, D.P. A DSC study of the crosslinking of polychloroprene with ZnO and MgO. J. Appl. Polym. Sci. 1995, 55, 705–721. [Google Scholar] [CrossRef]
- Vukov, R. Zinc oxide cross-linking chemistry of halobutyl elastomers—A model compound approach. Rubber Chem. Technol. 1984, 57, 284–290. [Google Scholar] [CrossRef]
- Kuntz, I.; Zapp, R.L.; Pancirov, R.J. The Chemistry of the Zinc Oxide Cure of Halobutyl. Rubber Chem. Technol. 1984, 57, 813–825. [Google Scholar] [CrossRef]
- Joseph, R.; George, K.E.; Francis, D.J. Tribasic lead sulphate as efficient curing agent for Polychlo-roprene. Angew. Makromol. Chem. 1987, 148, 19–26. [Google Scholar] [CrossRef]
- Das, A.; Naskar, N.; Datta, R.N.; Bose, P.P.; Debnath, S.C. Naturally occurring amino acid: Novel curatives for chloroprene rubber. J. Appl. Polym. Sci. 2006, 100, 3981–3986. [Google Scholar] [CrossRef]
- Das, A.; Naskar, N.; Basu, D.K. Thiophosphoryl disulfides as crosslinking agents for chloroprene rubber. J. Appl. Polym. Sci. 2003, 91, 1913–1919. [Google Scholar] [CrossRef]
- Ismail, H.; Ahmad, Z.; Ishak, Z.M. Effects of cetyltrimethylammonium maleate on curing characteristics and mechanical properties of polychloroprene rubber. Polym. Test. 2003, 22, 179–183. [Google Scholar] [CrossRef]
- Dziemidkiewicz, A.; Pingot, M.; Maciejewska, M. Metal complexes as a new pro-ecological cross-linking agents for chloroprene rubber based on Heck coupling reaction. Rubber Chem. Technol. 2019, 92, 589–597. [Google Scholar] [CrossRef]
- Gros, A.; Tosaka, M.; Huneau, B.; Verron, E.; Poompradub, S.; Senoo, K. Dominating factor of strain-induced crystallization in natural rubber. Polymer 2015, 76, 230–236. [Google Scholar] [CrossRef][Green Version]
- Sotta, P.; Albouy, P.-A. Strain-Induced Crystallization in Natural Rubber: Flory’s Theory Revisited. Macromolecules 2020, 53, 3097–3109. [Google Scholar] [CrossRef]
- Albouy, P.A.; Sotta, P. Draw ratio at the onset of strain-induced crystallization in cross-linked natural rubber. Macromolecules 2020, 53, 992–1000. [Google Scholar] [CrossRef]
- Sathi, S.G.; Jang, J.Y.; Jeong, K.U.; Nah, C. Thermally stable bromobutyl rubber with a high cross-linking density based on a 4,4′ bismaleimidodiphenylmethane curing agent. J. Appl. Polym. Sci. 2016, 133, 44092. [Google Scholar] [CrossRef]
- Sathi, S.G.; Jeon, J.; Won, J.; Nah, C. Enhancing the efficiency of zinc oxide vulcanization in brominated poly (isobutylene-co-isoprene) rubber using structurally different bismaleimides. J. Polym. Res. 2018, 25, 108–121. [Google Scholar] [CrossRef]
- Sathi, S.G.; Jang, J.Y.; Jeong, K.U.; Nah, C. Synergistic effect of 4,4′-bis(maleimido) diphenylme-thane and zinc oxide on the vulcanization behavior and thermo-mechanical properties of chlorinated isobutylene–isoprene rubber. Polym. Adv. Technol. 2017, 28, 742–753. [Google Scholar]
- Sathi, S.G.; Park, C.; Huh, Y.I.; Jeon, J.; Yun, C.H.; Won, J.; Jeong, K.U.; Nah, C. Enhancing the reversion resistance, crosslinking density and thermo-mechanical properties of accelerated sulfur cured chlorobutyl rubber using 4,4′-bis (maleimido) diphenyl methane. Rubber Chem. Technol. 2019, 92, 110–128. [Google Scholar]
- Shibulal, G.S.; Jang, J.; Yu, H.C.; Huh, Y.I.; Nah, C. Cure characteristics and physico-mechanical properties of a conventional sulphur-cured natural rubber with a novel anti-reversion agent. J. Polym. Res. 2016, 23, 1–12. [Google Scholar] [CrossRef]
- Sathi, S.G.; Harea, E.; Machů, A.; Stoček, R. Facilitating high-temperature curing of natural rubber with a conventional accelerated-sulfur system using a synergistic combination of bismaleimides. EXPRESS Polym. Lett. 2021, 15, 16–27. [Google Scholar] [CrossRef]
- Sathi, S.G.; Stocek, R.; Kratina, O. Reversion free high-temperature vulcanization of cis-polybutadiene rubber with the accelerated-sulfur system. Express Polym. Lett. 2020, 14, 823–837. [Google Scholar] [CrossRef]
- Inoue, S. Chloroprene Rubber Composition. JPS59109541A, 1984. [Google Scholar]
- Jain, S.R.; Sekkar, V.; Krishnamurthy, V.N. Mechanical and swelling properties of HTPB-based copolyurethane networks. J. Appl. Polym. Sci. 1993, 48, 1515–1523. [Google Scholar] [CrossRef]
- Hayeemasae, N.; Salleh, S.Z.; Ismail, H. Utilization of chloroprene rubber waste as blending compo-nents with natural rubber: Aspect on metal oxide contents. J. Mater. Cycles Waste Manag. 2019, 21, 1095–1105. [Google Scholar] [CrossRef]
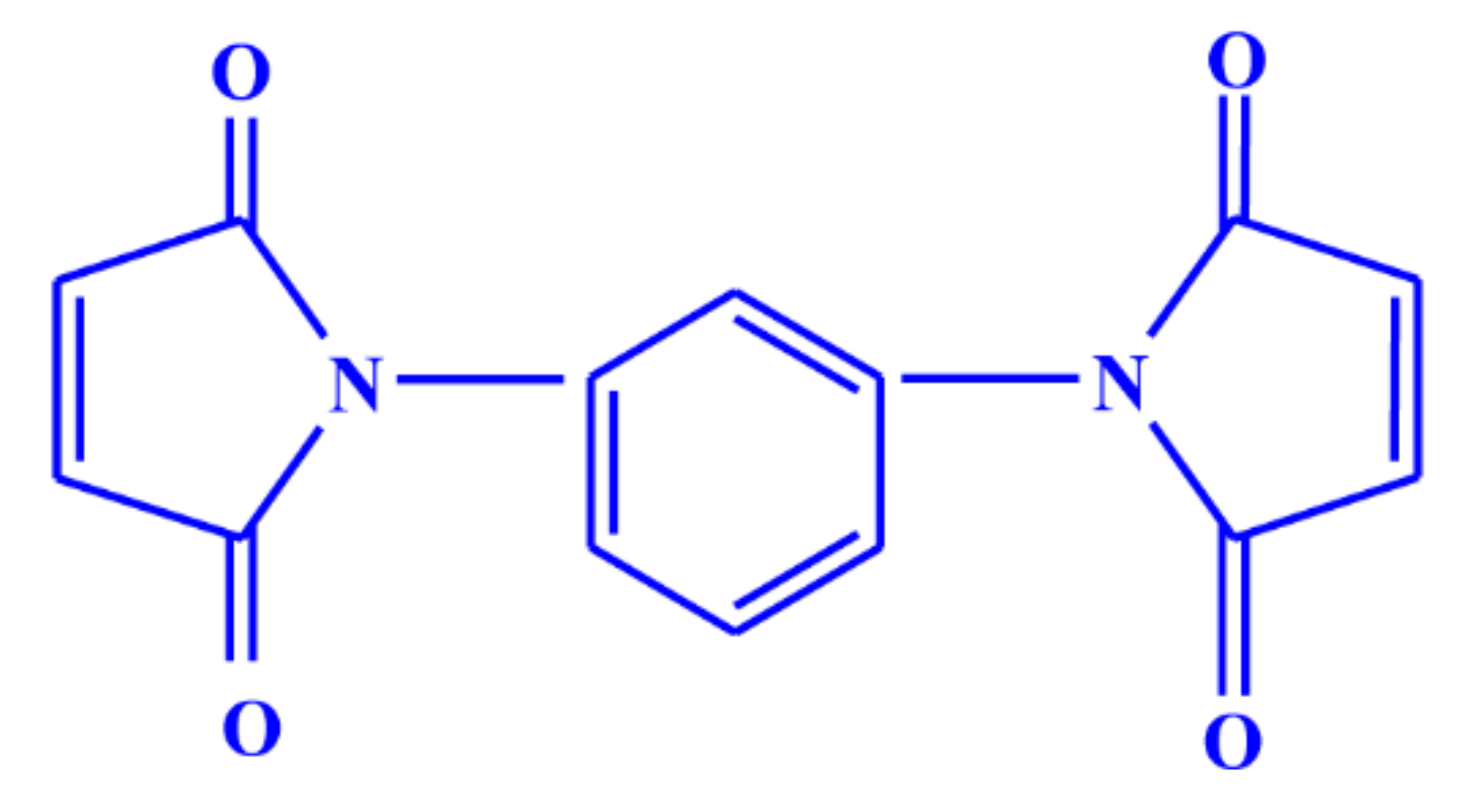


| Ingredients |  | NR | CR | ZnO | MgO | Stearic Acid | Sulfur | CBS | Maleide F |
|---|---|---|---|---|---|---|---|---|---|
| Mix Code |  | ||||||||
| M-1 | - | 100 | 5 | 4 | 0.5 | - | - | - | |
| M-2 | - | 100 | - | - | - | - | - | 3 | |
| M-3 | - | 100 | 5 | 4 | 0.5 | - | - | 3 | |
| M-4 | 50 | 50 | 5 | 2 | 1.25 | 1.25 | 0.25 | - | |
| M-5 | 50 | 50 | 5 | 2 | 1.25 | 1.25 | 0.25 | 1 | |
| M-6 | 50 | 50 | 5 | 2 | 1.25 | 1.25 | 0.25 | 3 | |
| M-7 | 50 | 50 | 5 | 2 | 1.25 | 1.25 | 0.25 | 5 |
| Mix Code | ML(dNm) | MH (dNm) | ΔM (dNm) | TS1 (min) | TS2 (min) | T90 (min) | Cure Rate Index (min−1) |
|---|---|---|---|---|---|---|---|
| M-1 | 1.38 | 5.80 | 4.42 | 0.65 | 0.65 | 40.23 | 2.52 |
| M-2 | 0.74 | 10.69 | 9.95 | 2.43 | 4.52 | 41.58 | 2.69 |
| M-3 | 1.07 | 9.97 | 8.90 | 0.53 | 0.70 | 20.63 | 4.99 |
| M-4 | 0.70 | 3.09 | 2.39 | 1.65 | 3.86 | 4.62 | 131.57 |
| M-5 | 0.55 | 4.43 | 3.88 | 1.55 | 2.54 | 5.55 | 33.22 |
| M-6 | 0.66 | 8.70 | 8.04 | 1.34 | 2.30 | 12.44 | 9.86 |
| M-7 | 0.49 | 9.80 | 9.31 | 1.51 | 2.38 | 18.36 | 6.25 |
| Mix Code | Swelling (%) | Swell Ratio (Q) |
|---|---|---|
| M-1 | 281.7 ± 0.1 | 2.94 ± 0.1 |
| M-2 | 237.2 ± 0.02 | 2.35 ± 0.003 |
| M-3 | 177.0 ± 0.07 | 1.78 ± 0.08 |
| M-4 | 562.9 ± 0.08 | 6.55 ± 0.13 |
| M-5 | 428.2 ± 0.03 | 4.57 ± 0.12 |
| M-6 | 314.9 ± 0.13 | 3.29 ± 0.1 |
| M-7 | 267.9 ± 0.06 | 2.79 ± 0.06 |
| Mie | Tensile Strength (MPa) | Elongation at Break (%) | Modulus at 50% (MPa) | Modulus at 100% (MPa) | Modulus at 300% (MPa) | Hardness (Shore A) |
|---|---|---|---|---|---|---|
| M-1 | 5.32 ± 0.98 | 292 ± 63 | 0.91 ± 0.13 | 1.56 ± 0.26 | 4.03 ± 1.87 | 39 |
| M-3 | 2.84 ± 0.20 | 155 ± 9.0 | 1.08 ± 0.08 | 1.83 ± 0.19 | - | 52 |
| M-4 | 3.07 ± 0.74 | 490 ± 98 | 0.37 ± 0.03 | 0.54 ± 0.05 | 1.38 ± 0.26 | 24 |
| M-5 | 9.25 ± 1.40 | 566 ± 89 | 0.62 ± 0.13 | 0.90 ± 0.10 | 2.55 ± 0.67 | 34 |
| M-6 | 9.34 ± 2.93 | 482 ± 77 | 0.70 ± 0.11 | 1.07 ± 0.12 | 3.08 ± 0.48 | 42 |
| M-7 | 9.89 ± 2.81 | 425 ± 45 | 0.85 ± 0.11 | 1.33 ± 0.18 | 4.32 ± 1.34 | 47 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pöschl, M.; Gopi Sathi, S.; Stoček, R.; Kratina, O. Rheometer Evidences for the Co-Curing Effect of a Bismaleimide in Conjunction with the Accelerated Sulfur on Natural Rubber/Chloroprene Rubber Blends. Polymers 2021, 13, 1510. https://doi.org/10.3390/polym13091510
Pöschl M, Gopi Sathi S, Stoček R, Kratina O. Rheometer Evidences for the Co-Curing Effect of a Bismaleimide in Conjunction with the Accelerated Sulfur on Natural Rubber/Chloroprene Rubber Blends. Polymers. 2021; 13(9):1510. https://doi.org/10.3390/polym13091510
Chicago/Turabian StylePöschl, Marek, Shibulal Gopi Sathi, Radek Stoček, and Ondřej Kratina. 2021. "Rheometer Evidences for the Co-Curing Effect of a Bismaleimide in Conjunction with the Accelerated Sulfur on Natural Rubber/Chloroprene Rubber Blends" Polymers 13, no. 9: 1510. https://doi.org/10.3390/polym13091510
APA StylePöschl, M., Gopi Sathi, S., Stoček, R., & Kratina, O. (2021). Rheometer Evidences for the Co-Curing Effect of a Bismaleimide in Conjunction with the Accelerated Sulfur on Natural Rubber/Chloroprene Rubber Blends. Polymers, 13(9), 1510. https://doi.org/10.3390/polym13091510