Highly Functionalized Modified Metal Oxides Polymeric Sensors for Potentiometric Determination of Letrozole in Commercial Oral Tablets and Biosamples
Abstract
1. Introduction
2. Experimental
2.1. Chemicals
2.2. Instruments
2.3. Synthesis of Magnesium Oxide and Copper Oxide Nanoparticles
2.4. Characterization of Nanoparticles
2.5. Preparation of Standard Drug Solution
2.6. Preparation of Electroactive Complex
2.7. Membrane Composition and Sensor Fabrication
2.8. Calibration Graphs
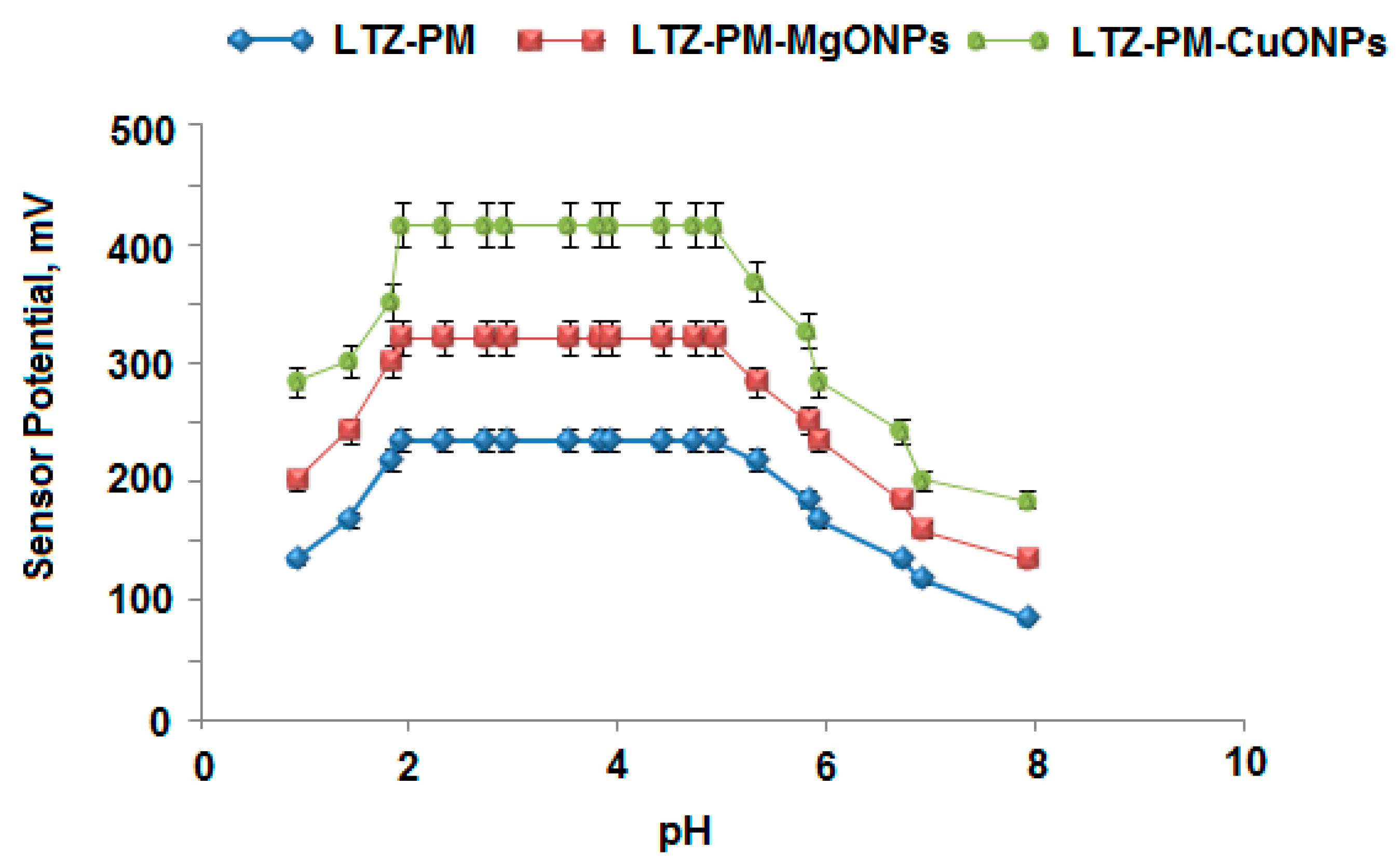
2.9. Optimization of Potential Readings Condition
2.10. Analysis of LTZ in Femara® Tablets
2.11. Analysis of LTZ in Biosamples
2.12. Statistical Analysis
3. Results and Discussion
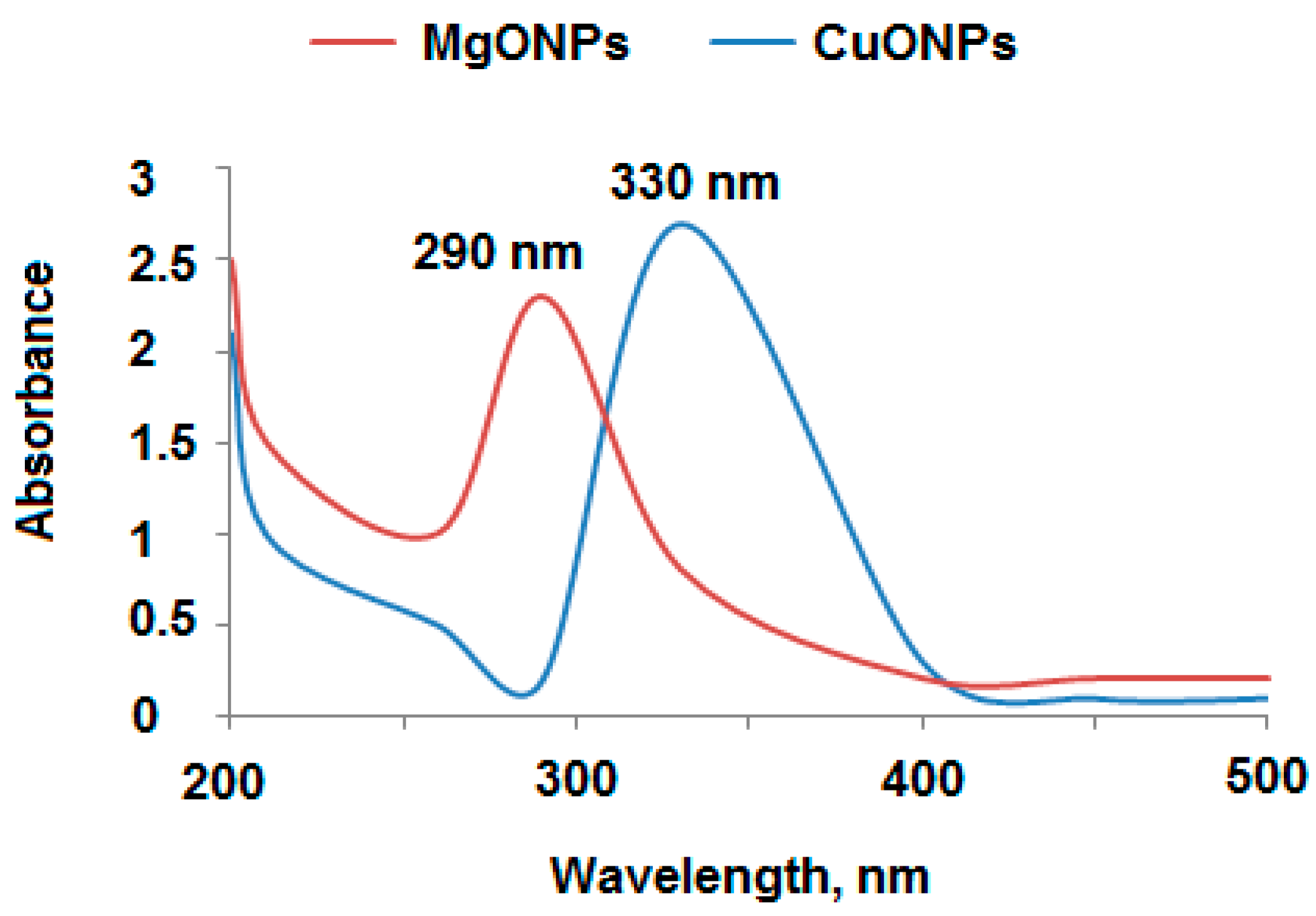
3.1. Characterization of MgO and CuO Nanoparticles
3.2. The Nature of the Fabricated Sensors
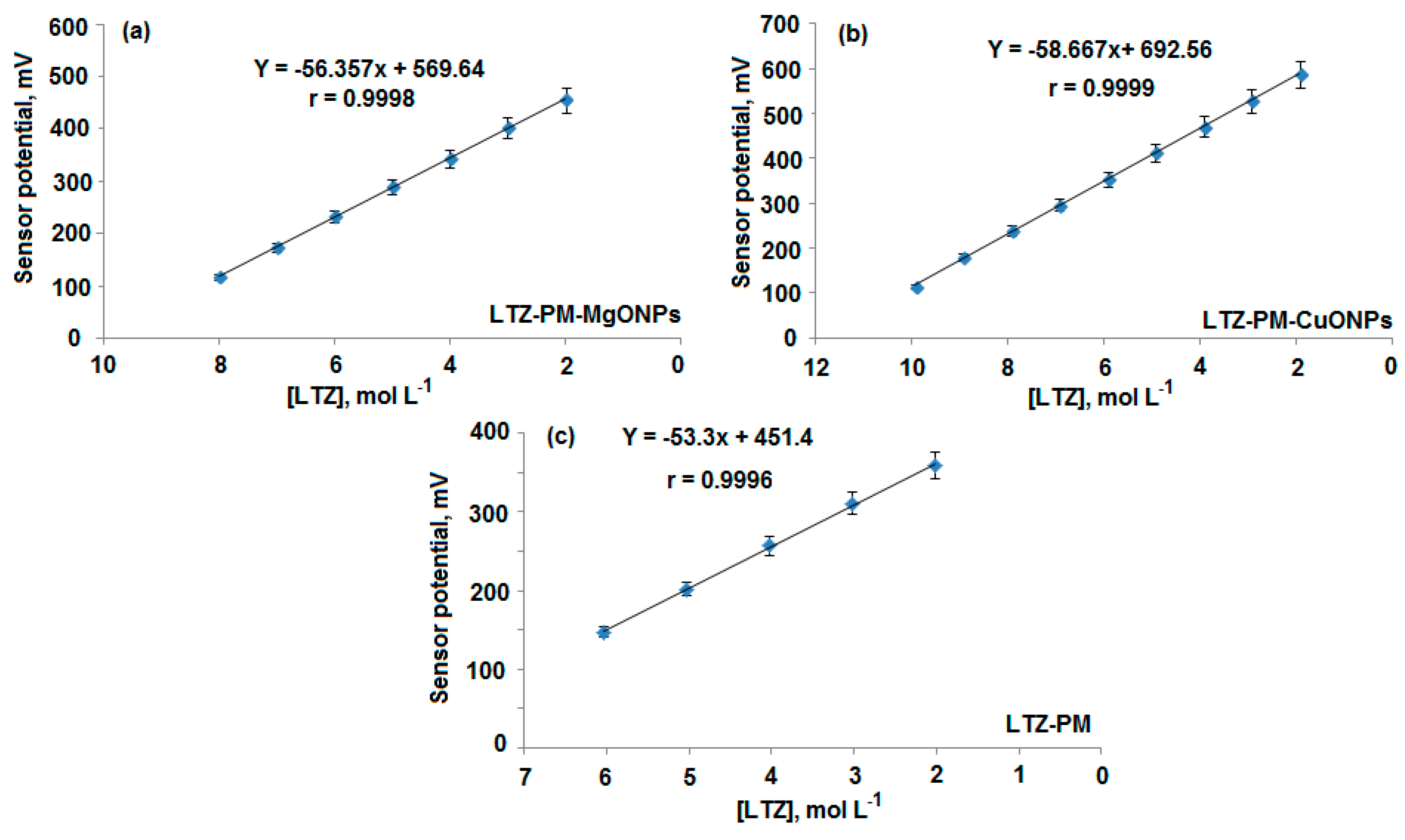
3.3. Quantification of Letrozole
3.4. Method Validation
3.5. Determination of LTZ in Tablets
3.6. Quantification of LTZ in Biosamples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mousavi, S.M.; Hashemi, S.A.; Zarei, M.; Amani, A.M.; Babapoor, A. Nanosensors for chemical and biological and medical applications. Med. Chem. Los Angeles 2018, 8, 205–217. [Google Scholar] [CrossRef]
- Evanoff, D.D., Jr.; Chumanov, G. Synthesis and optical properties of silver nanoparticles and arrays. ChemPhys. Chem. 2005, 6, 1221–1231. [Google Scholar] [CrossRef]
- Whitesides, G.M. Nanoscience, nanotechnology, and chemistry. Small 2005, 1, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Guo, D.; Liu, Y.; Wang, H.; Wang, L. Recent advances and challenges in biomass-derived porous carbon nanomaterials for supercapacitors. Chem. Eng. J. 2020, 397, 125418. [Google Scholar] [CrossRef]
- Oprea, M.; Panaitescu, D.M. Nanocellulose hybrids with metal oxides nanoparticles for biomedical applications. Molecules 2020, 25, 4045. [Google Scholar] [CrossRef] [PubMed]
- Abinaya, S.; Kavitha, H.P.; Prakash, M.; Muthukrishnaraj, A. Green synthesis of magnesium oxide nanoparticles and its applications: A review. Sustain. Chem. Pharm. 2021, 19, 100368. [Google Scholar] [CrossRef]
- Fouda, A.; Hassan, S.E.D.; Abdel-Rahman, M.A.; Farag, M.M.; Shehal-deen, A.; Mohamed, A.A.; Alsharif, S.M.; Saied, E.; Moghanim, S.A.; Azab, M.S. Catalytic degradation of wastewater from the textile and tannery industries by green synthesized hematite (α-Fe2O3) and magnesium oxide (MgO) nanoparticles. Curr. Res. Biotechnol. 2021, 3, 29–41. [Google Scholar] [CrossRef]
- Yan, J.C.; Tianyi, W.; Liu, M.Y.; Yuan, Z. Synthesis of porous hematite nanorods loaded with Cuo nanocrystals as catalysts for CO oxidation. J. Nat. Gas Chem. 2011, 20, 669–676. [Google Scholar] [CrossRef]
- Jess, K.; Nicolas, G.; Richard, R.; Miller, E. Advances in copper-chalcopyrite thin films for solar energy conversion. Sol. Energ. Mat. Sol. C 2009, 94, 12–16. [Google Scholar] [CrossRef]
- Yang, Z.; He, X.; Jianping, L.; Huigang, Z.; Xiaoguang, G. Gas-sensing properties of hollow and hierarchical copper oxide microspheres. Sensors 2007, 128, 293–298. [Google Scholar] [CrossRef]
- Bohr, R.H.; Chun, S.Y.; Dau, C.W.; Tan, J.T.; Sung, J. Field emission studies of amorphous carbon deposited on copper nanowires grown by cathodic arc plasma deposition. New Carbon Mater. 2009, 24, 97–101. [Google Scholar] [CrossRef]
- Manmeet, K.; Muthea, K.P.; Despandeb, S.K.; Shipra, C.H.; Singhd, J.B.; Neetika, V.; Gupta, S.K.; Yakhmi, J.V. Growth and branching of CuO nanowires by thermal oxidation of copper. J. Cryst. Growth 2011, 289, 670–675. [Google Scholar] [CrossRef]
- Alothman, A.A.; Albaqami, M.D. Nano sized Cu (II) and Zn (II) complexes and their use as a precursor for synthesis of CuO and ZnO nanoparticles: A study on their sonochemical synthesis, characterization, and DNA/binding/cleavage, anticancer, and antimicrobial activities. Appl. Organometal. Chem. 2020, 34, e5827. [Google Scholar] [CrossRef]
- Yamukyan, M.H.; Manukyan, K.V.; Kharatyan, S.L. Copper oxide reduction by combined reducers under the combustion mode. Chem. Eng. J. 2008, 137, 636–642. [Google Scholar] [CrossRef]
- Wu, R.; Ma, Z.; Gu, Z.; Yang, Y. Preparation and characterization of CuO nanoparticles with different morphology through a simple quick-precipitation method in DMAC–water mixed solvent. J. Alloys Compd. 2010, 504, 45–49. [Google Scholar] [CrossRef]
- Shuai, H.-L.; Huang, K.-J.; Zhang, W.-J.; Cao, X.; Jia, M.-P. Sanwich-type microRNA biosensor based on magnesium oxide nanoflower and graphene oxide-gold nanoparticles hybrids coupling with enzyme signal amplification. Sens. Actuators B 2017, 243, 403–411. [Google Scholar] [CrossRef]
- Al-Mohaimeed, A.M.; Alarfaj, N.A.; El-Tohamy, M.F.; Al-Harbi, H. Prospective of ultrasenstive nanometal oxides electrochemical sensors for pharmaceutical analysis of antihistamine drug fexofenadine hydrochloride. Int. J. Electrochem. Sci. 2020, 15, 4774–4788. [Google Scholar] [CrossRef]
- Alarfaj, N.A.; Amina, M.; Al Musayeib, N.M.; El‑Tohamy, M.F.; Oraby, H.F.; Bukhari, S.I.; Moubayed, N.M.S. Prospective of green synthesized Oleum cumini Oil/PVP/MgO bionanocomposite film for its antimicrobial, antioxidant and anticancer Applications. J. Polymer. Environ. 2020, 28, 2108–2124. [Google Scholar] [CrossRef]
- Rabiee, N.; Bagherzadeh, M.; Kiani, M.; Ghadiri, A.M.; Etessamifar, F.; Jaberizadeh, A.H.; Shakeri, A. Biosynthesis of copper oxide nanoparticles with potential biomedical applications. Int. J. Nanomed. 2020, 15, 3983. [Google Scholar] [CrossRef] [PubMed]
- Alfaro, A.; Leon, A.; Guajardo-Correa, E.; Reuquen, P.; Torres, F.; Mery, M.; Segura, R.; Zapata, P.A.; Orihuela, P.A. MgO nanoparticles coated with polyethylene glycol as carrier for 2-Methoxyestradiol anticancer drug. PLoS ONE 2019, 14, e0214900. [Google Scholar] [CrossRef]
- Javed, R.; Rais, F.; Kaleem, M.; Jamil, B.; Ahmad, M.A.; Yu, T.; Qureshi, S.W.; Ao, Q. Chitosan capping of CuO nanoparticles: Facile chemical preparation, biological analysis, and applications in dentistry. Int. J. Biol. Macromol. 2021, 167, 1452–1467. [Google Scholar] [CrossRef]
- Israr-Qadir, M.; Jamil-Rana, S.; Nur, O.; Willander, M. Zinc oxide-based self-powered potentiometric chemical sensors for biomolecules and metal ions. Sensors 2017, 17, 1645. [Google Scholar] [CrossRef]
- Alarfaj, N.A.; El-Tohamy, M.F. New functionalized polymeric sensor based NiO/MgO nanocomposite for potentiometric determination of doxorubicin hydrochloride in commercial injections and human plasma. Polymers 2020, 12, 3066. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Sheikhshoaie, M.; Sheikhshoaie, I.; Ranjbar, M.; Alizadeh, J.; Maxakato, N.W.; Abbaspourrad, A. A novel electrochemical epinine sensor using amplified CuO nanoparticles and an-hexyl-3-methylimidazolium hexafluorophosphate electrode. New J. Chem. 2019, 43, 2362–2367. [Google Scholar] [CrossRef]
- Lamb, H.M.; Adkins, J.C. Letrozole. Drugs 1998, 56, 1125–1140. [Google Scholar] [CrossRef]
- Tamilselvi, N.; Arivukkarasu, R.; Antony, A.; Anitha, T.J.; Boominathan, A.; Chandrasekar, R.; Visakh, D. Development and validation of HPTLC method for the determination of Letrozole in bulk and tablet dosage form. World J. Pharm. Res. 2015, 5, 884–893. [Google Scholar]
- Dhakne, P.; Sahu, A.K.; Sharma, M.K.; Sengupta, P. Simultaneous quantification of abemaciclib and letrozole in rat plasma: Method development, validation and pharmacokinetic application. Biomed. Chromatogr. 2020, 34, e4825. [Google Scholar] [CrossRef]
- Mondal, N.; Pal, T.K.; Ghosal, S.K. Development and validation of RP-HPLC method to determine letrozole in different pharmaceutical formulations and its application to studies of drug release from nanoparticles. Acta Pol. Pharm. 2009, 66, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Ameline, A.; Gheddar, L.; Raul, J.S.; Kintz, P. Characterization of letrozole in human hair using LC-MS/MS and confirmation by LC-HRMS: Application to a doping case. J. Chromatogr. B 2021, 1162, 122495. [Google Scholar] [CrossRef] [PubMed]
- Joshi, C.; Vishnubhatla, S.; Chakkirala, S.; Mannam, S. Validation and application of a high-performance liquid chromatography- tandem mass spectrometry assay for Letrozole in human plasma. Asian J. Pharm. Clin. Res. 2011, 4, 107–112. [Google Scholar]
- Rusu, A.; Sbanca, M.A.; Todoran, N.; Vari, C.E. Letrozole determination by capillary zone electrophoresis and UV spectrophotometry methods. Acta Med. Marisiensis 2017, 63, 80–86. [Google Scholar] [CrossRef]
- Patil, S.M.; Galatage, S.T.; Choudhary, A.U. Development of UV spectrophotometric method for estimation of letrozole in pure and pharmaceutical dosage form. Indo Am. J. Pharm. Res. 2013, 3, 5541–5548. [Google Scholar] [CrossRef]
- Mondal, N.; Pal, T.K.; Ghosal, S.K. Development and validation of a spectrophotometric method for estimation of letrozole in bulk and pharmaceutical formulation. Die Pharmazie-An Int. J. Pharm. Sci. 2007, 62, 597–598. [Google Scholar] [CrossRef]
- Swain, S.; Jena, B.R.; Sitty, M.B.; Pradhan, D.P. Validated UV spectrophotometric methods for the estimation of Letrozole in solid dosage forms. Int. J. Pharm. Sci. Nanotechnol. 2018, 11, 4129–4135. [Google Scholar] [CrossRef]
- Dange, Y.D.; Salunkhe, V.R.; Bhinge, S.D.; Bhutkar, B.R.; Momin, Y.H. Simultaneous equation method for the estimation of Palbociclib and Letrozole by UV-visible spectrophotometry. Ind. Drugs 2017, 54, 61–66. [Google Scholar]
- Acharjya, S.K.; Mallick, P.; Panda, P.; Kumar, K.R.; Annapurna, M.M. Spectrophotometric methods for the determination of Letrozole in bulk and pharmaceutical dosage forms. J. Adv. Pharm. Technol. Res. 2010, 1, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Ganjali, M.R.; Karimi, A.; Norouzi, P. Letrozole potentiometric PVC membrane and nano-composite carbon paste electrodes. Int. J. Electrochem. Sci. 2012, 7, 3681–3692. [Google Scholar]
- Dais, S.L.; Mary, A.C.C.; Devi, K.; Prabha, S.S.; Rajendran, S.; Zahirullah, S.S. Magnesium oxide nanoparticles-synthesis and characterization. Int. J. Nano Corr. Sci. Eng. 2015, 2, 64–69. [Google Scholar]
- Phiwdang, K.; Suphankij, S.; Mekprasart, W.; Pecharapa, W. Synthesis of CuO nanoparticles by precipitation method using different precursors. Energ. Procedia 2013, 34, 740–745. [Google Scholar] [CrossRef]
- Alarfaj, N.A.; El-Tohamy, M.F. Ultrasensitve modified carbon paste inclusion β-cyclodextrin and carbon nanotubes sensors for electrochemical detection of anticancer nimustine hydrochloride. Int. J. Electrochem. Sci. 2016, 11, 1184–1198. [Google Scholar]
- Mishra, P.; Singh, U.; Pandey, C.M.; Mishra, P.; Pandey, G. Application of student’s t-test, analysis of variance, and covariance. Ann. Card. Anaesth. 2019, 22, 407. [Google Scholar] [CrossRef] [PubMed]
- UV/VIS/IR Spectroscopy Analysis of Nanoparticles. 2012. Available online: http://50.87.149.212/sites/default/files/nanoComposix%20Guidelines%20for%20UV-vis%20Analysis (accessed on 10 May 2015).
- Moorthy, S.K.; Ashok, C.H.; Rao, K.V.; Viswanathan, C. Synthesis and characterization of MgO nanoparticles by Neem leaves through Green method. Mater. Today Proc. 2015, 2, 4360–4368. [Google Scholar] [CrossRef]
- Varughese, G.; Rini, V.; Suraj, S.P.; Usha, K.T. Characterization and optical studies of copper oxide nanostructures doped with lanthanum ions. Adv. Mater. Sci. 2014, 14, 49–60. [Google Scholar] [CrossRef]
- Mageshwari, K.; Mali, S.S.; Sathyamoorthy, R.; Patil, P.S. Template-free synthesis of MgO nanoparticles for effective photocatalytic applications. Powder Technol. 2013, 249, 456–462. [Google Scholar] [CrossRef]
- Ogunyemia, S.O.; Zhanga, F.; Abdallaha, Y.; Zhanga, M.; Wangc, Y.; Sunc, G.; Qiua, W.; Lia, B. Biosynthesis and characterization of magnesium oxide and manganese dioxide nanoparticles using Matricaria chamomilla L. extract and its inhibitory effect on Acidovorax oryzae strain RS-2. Artificial Cells. Nanomed. Biotechnol. 2019, 47, 2230–2239. [Google Scholar] [CrossRef]
- Manyasree, D.; Peddi, K.M.; Ravikumar, R. CuO nanoparticles: Synthesis, characterization and their bactericidal efficacy. Int. J. App. Pharm. 2017, 9, 71–74. [Google Scholar] [CrossRef]
- Al-Tamimi, S.A. Biogenic green synthesis of metal oxide nanoparticles using oat biomass for ultrasensitive modified polymeric sensors. Green Chem. Lett. Rev. 2021, 14, 165–178. [Google Scholar] [CrossRef]
- Hornak, J.; Trnka, P.; Kadlec, P.; Michal, O.; Mentlík, V.; Sutta, P.; Csanyi, G.M.; Tamus, Z.A. Magnesium oxide nanoparticles: Dielectric properties, surface functionalization and improvement of epoxy-based composites insulating properties. Nanomaterials 2018, 8, 381. [Google Scholar] [CrossRef]
- Sarkar, S.; Jana, P.K.; Chaudhuri, B.K.; Sakata, H. Copper (II) oxide as a giant dielectric material. Appl. Phys. Lett. 2006, 89, 212905. [Google Scholar] [CrossRef]
- Pingarron, J.M.; Labuda, J.; Barek, J.; Brett, C.M.; Camões, M.F.; Fojta, M.; Hibbert, D.B. Terminology of electrochemical methods of analysis (IUPAC Recommendations 2019). Pure Appl. Chem. 2020, 92, 641–694. [Google Scholar] [CrossRef]
- Kakhkiz, R.M. Application of nanoparticles in the potentiometric ion selective electrodes. Russ. J. Electrochem. 2013, 49, 458–465. [Google Scholar] [CrossRef]
- Isa, I.M.; Sohaimi, N.M.; Hashim, N.; Kamari, A.; Mohamed, A.; Ahmad, M.; Ghani, S.A. Determination of salicylate ion by potentiometric membrane electrode based on zinc aluminium layered double hydroxides-4 (2, 4-dichlorophenoxy) butyrate nanocomposites. Int. J. Electrochem. Sci. 2013, 8, 2112–2121. [Google Scholar]
- ICH-Q2 (R1) Validation and Analytical Procedures: Text and Methodology. In Proceedings of the International Conference on Harmonization Guidelines, Geneva, Switzerland, 4 November 2005.
- Liu, Y.; Zhang, Y.; Wang, Q.; Yang, W.; Chen, X.; Jing, S.; Zhao, L.; Zhang, C.; Wei, L.; Li, X.; et al. Bioequivalence and safety study of letrozole tablet in healthy Chinese postmenopausal women volunteers. J. Chin. Pharm. Sci. 2013, 22, 190–196. [Google Scholar] [CrossRef]





| Parameter | Conventional Coated Wire LTZ-PM Sensor | Modified LTZ-PM-MgONPs Sensor | Modified LTZ-PM-CuONPs Sensor |
|---|---|---|---|
| Slope (mV. Decade−1) | 53.3 ± 0.5 | 56.4 ± 0.7 | 58.7 ± 0.5 |
| Intercept | 451.4 | 569.6 | 692.6 |
| Regression equation | EmV = (53.3 ± 0.5) log [LTZ] + 451.4 | EmV = (56.4 ± 0.7) log [LTZ] + 569.6 | EmV = (58.7 ± 0.5) log [LTZ] + 692.6 |
| Correlation coefficient, r | 0.9996 | 0.9998 | 0.9999 |
| Linear range (mol L−1) | 10 × 10−6–1.0 × 10−2 | 1.0 × 10−8–1.0 × 10−2 | 1.0 × 10−10–1.0 × 10−2 |
| LOD | 5.0 × 10−7 | 5.9 × 10−9 | 5.6 × 10−11 |
| Response time/s | 75 | 45 | 30 |
| Working pH range | 2–5 | 2–5 | 2–5 |
| Lifetime/day | 20 | 50 | 65 |
| Temperature, °C | 25 | 25 | 25 |
| Accuracy (%) | 99.3 ± 0.4 | 99.6 ± 0.3 | 99.8 ± 0.3 |
| Interferences | Conventional Coated Wire LTZ-PM Sensor (KpotLTZ+) | Modified LTZ-PM-MgONPs Sensor (KpotLTZ+) | Modified LTZ-PM-CuONPs Sensor (KpotLTZ+) |
|---|---|---|---|
| Na+ | 5.4 × 10−3 | 4.8 × 10−4 | 9.2 × 10−5 |
| K+ | 1.9 × 10−3 | 3.3 × 10−4 | 4.8 × 10−4 |
| Ag+ | 3.1 × 10−3 | 1.5 × 10−3 | 8.4 × 10−4 |
| Ni2+ | 5.6 × 10−3 | 4.2 × 10−3 | 2.2 × 10−3 |
| Mg2+ | 6.8 × 10−3 | 7.9 × 10−4 | 8.7 × 10−4 |
| Cu2+ | 6.6 × 10−3 | 1.4 × 10−4 | 2.3 × 10−5 |
| Zn2+ | 4.9 × 10−3 | 4.9 × 10−4 | 6.3 × 10−4 |
| Glucose | 3.6 × 10−3 | 9.9 × 10−4 | 4.2 × 10−4 |
| Lactose | 3.9 × 10−3 | 6.7 × 10−4 | 5.6 × 10−4 |
| Starch | 4.8 × 10−3 | 2.3 × 10−4 | 2.1 × 10−3 |
| Lysine | 1.4 × 10−3 | 8.9 × 10−3 | 9.5 × 10−4 |
| L-histidine | 2.6 × 10−3 | 2.7 × 10−4 | 3.2 × 10−5 |
| Tryptophan | 5.5 × 10−3 | 5.8 × 10−4 | 5.4 × 10−5 |
| Glycine | 8.4 × 10−3 | 3.6 × 10−3 | 6.6 × 10−4 |
| Valine | 2.6 × 10−3 | 8.4 × 10−3 | 2.5 × 10−4 |
| Leucine | 2.5 × 10−3 | 3.5 × 10−3 | 2.9 × 10−4 |
| Talc | 5.2 × 10−3 | 2.2 × 10−4 | 9.1 × 10−5 |
| SiO2 | 3.7 × 10−3 | 7.7 × 10−3 | 8.2 × 10−4 |
| TiO2 | 7.8 × 10−3 | 7.8 × 10−4 | 7.8 × 10−4 |
| Magnesium stearate | 3.9 × 10−3 | 4.5 × 10−3 | 7.3 × 10−5 |
| Microcrystalline cellulose | 4.5 × 10−3 | 1.8 × 10−4 | 3.6 × 10−4 |
| Statistical analysis | Conventional LTZ-PM Coated Wire Sensor | Modified LTZ-PM MgONPs Sensor | Modified LTZ-PM CuONPs Sensor | |||
| Test Solution | % Recovery | Test Solution * | % Recovery | Test Solution * | % Recovery | |
| 6 | 98.5 | 8 | 99.8 | 10 | 100.0 | |
| 5.3 | 99.2 | 7 | 99.7 | 9 | 99.7 | |
| 5 | 99.8 | 6 | 100.0 | 8 | 99.9 | |
| 4 | 99.5 | 5 | 100.2 | 7 | 100.0 | |
| 3 | 98.0 | 4 | 99.8 | 6 | 99.7 | |
| 2 | 98.7 | 3 | 98.7 | 5 | 99.2 | |
| 2 | 99.0 | 4 | 99.8 | |||
| 3 | 99.3 | |||||
| 2 | 99.5 | |||||
| Mean ± SD | 98.9 ± 0.7 | 99.6 ± 0.5 | 99.7 ± 0.3 | |||
| n | 6 | 7 | 9 | |||
| Variance | 0.49 | 0.25 | 0.09 | |||
| RSD % | 0.71 | 0.50 | 0.30 | |||
| SE ** | 0.29 | 0.18 | 0.12 | |||
| Statistical Analysis | Modified LTZ-PM-MgONPs Coated Wire Sensor | |||||
| Intra-Day Assay | Inter-Day Assay | |||||
| Test Solution * | Found * | % Recovery | Test Solution * | Found * | % Recovery | |
| 8.0 | 7.98 | 99.50 | 8.0 | 7.97 | 99.60 | |
| 6.0 | 6.00 | 100.00 | 6.0 | 5.99 | 99.80 | |
| 4.0 | 3.98 | 99.50 | 4.0 | 3.96 | 99.00 | |
| Mean ± SD | 99.8 ± 0.3 | 99.5 ± 0.4 | ||||
| n | 3 | 3 | ||||
| Variance | 0.09 | 0.16 | ||||
| RSD % | 0.30 | 0.40 | ||||
| SE ** | 0.17 | 0.23 | ||||
| Statistical Analysis | Modified LTZ-PM-CuONPs Sensor | |||||
| Intra-day assay | Inter-day assay | |||||
| Test Solution * | Found * | % Recovery | Test solution * | Found * | % Recovery | |
| 10 | 10.00 | 100.00 | 10 | 9.99 | 99.90 | |
| 8 | 7.99 | 99.90 | 8 | 8.00 | 100.00 | |
| 6 | 5.98 | 99.70 | 6 | 5.97 | 99.50 | |
| Mean ± SD | 99.9 ± 0.1 | 99.8 ± 0.2 | ||||
| n | 3 | 3 | ||||
| Variance | 0.01 | 0.04 | ||||
| RSD % | 0.10 | 0.20 | ||||
| SE ** | 0.06 | 0.12 | ||||
| Statistical Analysis | Conventional LTZ-PM Coated Wire Sensor | Modified LTZ-PM MgONPs Sensor | Modified LTZ-PM CuONPs Sensor | Reported Method [37] | |||
| Test Solution * | % Recovery | Test Solution * | % Recovery | Test Solution * | % Recovery | ||
| 6 | 99.3 | 8 | 99.9 | 10 | 100.0 | 99.5 ± 0.4 | |
| 5.3 | 99.4 | 7 | 100.01 | 8 | 99.9 | ||
| 5 | 99.4 | 6 | 99.7 | 6 | 99.7 | ||
| 4 | 99.8 | 4 | 99.8 | 4 | 99.8 | ||
| 3 | 99.3 | 3 | 99.3 | 3 | 100.3 | ||
| 2 | 98.5 | 2 | 99.0 | 2 | 100.0 | ||
| Mean ± SD | 99.3 ± 0.4 | 99.6 ± 0.3 | 99.9 ± 0.2 | ||||
| n | 6 | 6 | 6 | ||||
| Variance | 0.16 | 0.09 | 0.04 | ||||
| RSD % | 0.40 | 0.30 | 0.20 | ||||
| SE ** | 0.16 | 0.12 | 0.08 | ||||
| t-test | 0.884 (2.228) *** | 0.500 (2.228) *** | 2.236 (2.228) *** | ||||
| F-test | 1.00 (5.05) *** | 1.78 (5.05) *** | 4.00 (5.05) *** | ||||
| Initial [LTZ], mol L−1 | Added [LTZ] mol L−1 | LTZ-PM-MgONPs | LTZ-PM-CuONPs | Reported Method [55] |
|---|---|---|---|---|
| % Recovery ± %RSD | % Recovery ± %RSD | % Recovery ± %RSD | ||
| 8.9 | 0.5 | 98.2 ± 0.8 | 99.3 ± 0.7 | 97.3 ± 0.6 |
| 6.8 | 0.5 | 98.4 ± 0.6 | 99.2 ± 0.1 | 96.8 ± 0.9 |
| 8.5 | 0.5 | 98.8 ± 0.5 | 99.5 ± 0.2 | 97.2 ± 1.2 |
| 6.6 | 0.5 | 98.2 ± 0.4 | 98.9 ± 0.6 | 96.8 ± 0.9 |
| 8.4 | 0.5 | 98.3 ± 0.5 | 99.4 ± 0.3 | 97.3 ± 0.7 |
| 6.3 | 0.5 | 98.7 ± 0.9 | 99.8 ± 0.2 | 98.2 ± 1.2 |
| 6.7 | 0.5 | 99.3 ± 0.7 | 99.5 ± 0.3 | 96.9 ± 1.4 |
| 8.7 | 0.5 | 99.2 ± 0.4 | 99.7 ± 0.9 | 97.6 ± 0.9 |
| 7.2 | 0.5 | 98.6 ± 1.2 | 99.9 ± 0.1 | 98.1 ± 0.4 |
| 8.3 | 0.5 | 98.6 ± 0.8 | 99.7 ± 0.3 | 96.7 ± 1.1 |
| 7.4 | 0.5 | 98.9 ± 1.2 | 99.2 ± 0.6 | 97.5 ± 0.6 |
| 8.1 | 0.5 | 98.2 ± 0.7 | 98.9 ± 0.7 | 98.1 ± 1.2 |
| 7.9 | 0.5 | 98.6 ± 0.4 | 99.4 ± 0.2 | 96.8 ± 0.9 |
| 8.8 | 0.5 | 99.3 ± 0.8 | 99.8 ± 0.4 | 97.8 ± 0.6 |
| 7.5 | 0.5 | 98.4 ± 1.4 | 99.6 ± 0.1 | 98.3 ± 0.4 |
| 8.4 | 0.5 | 99.3 ± 0.7 | 99.9 ± 0.3 | 96.9 ± 1.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shawky, A.M.; El-Tohamy, M.F. Highly Functionalized Modified Metal Oxides Polymeric Sensors for Potentiometric Determination of Letrozole in Commercial Oral Tablets and Biosamples. Polymers 2021, 13, 1384. https://doi.org/10.3390/polym13091384
Shawky AM, El-Tohamy MF. Highly Functionalized Modified Metal Oxides Polymeric Sensors for Potentiometric Determination of Letrozole in Commercial Oral Tablets and Biosamples. Polymers. 2021; 13(9):1384. https://doi.org/10.3390/polym13091384
Chicago/Turabian StyleShawky, Ahmed Mahmoud, and Maha Farouk El-Tohamy. 2021. "Highly Functionalized Modified Metal Oxides Polymeric Sensors for Potentiometric Determination of Letrozole in Commercial Oral Tablets and Biosamples" Polymers 13, no. 9: 1384. https://doi.org/10.3390/polym13091384
APA StyleShawky, A. M., & El-Tohamy, M. F. (2021). Highly Functionalized Modified Metal Oxides Polymeric Sensors for Potentiometric Determination of Letrozole in Commercial Oral Tablets and Biosamples. Polymers, 13(9), 1384. https://doi.org/10.3390/polym13091384







