Synergistic Effects of Ladder and Cage Structured Phosphorus-Containing POSS with Tetrabutyl Titanate on Flame Retardancy of Vinyl Epoxy Resins
Abstract
1. Introduction
2. Experimental
2.1. Materials
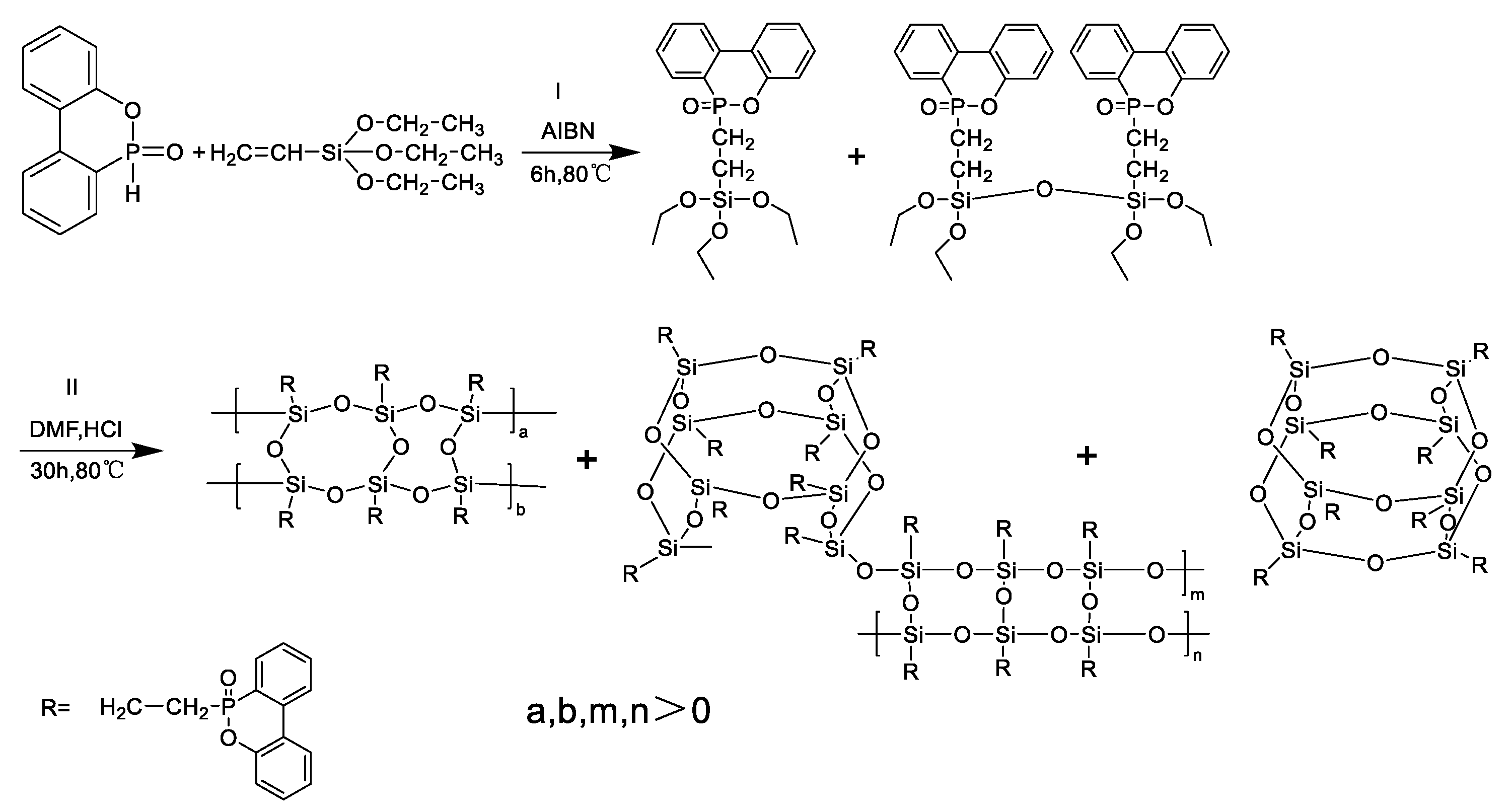
2.2. Synthesis of DOPO-VTES and DOPO-POSS
2.2.1. Synthesis of DOPO-VTES
2.2.2. Synthesis of DOPO-POSS
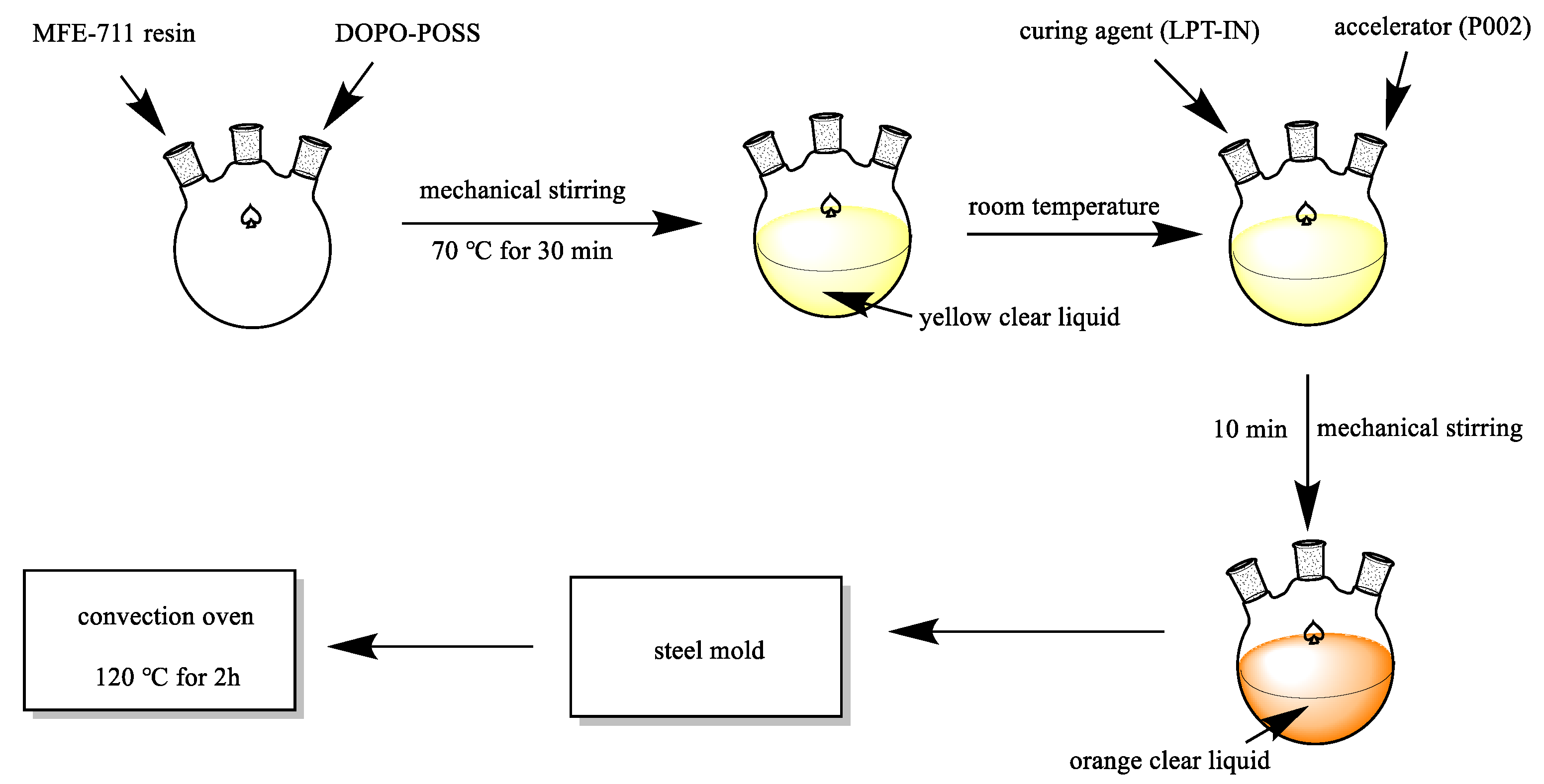
2.3. Preparation of the VE Composites
2.4. Characterization
3. Results and Discussion
3.1. Characterization of DOPO-VTES and DOPO-POSS
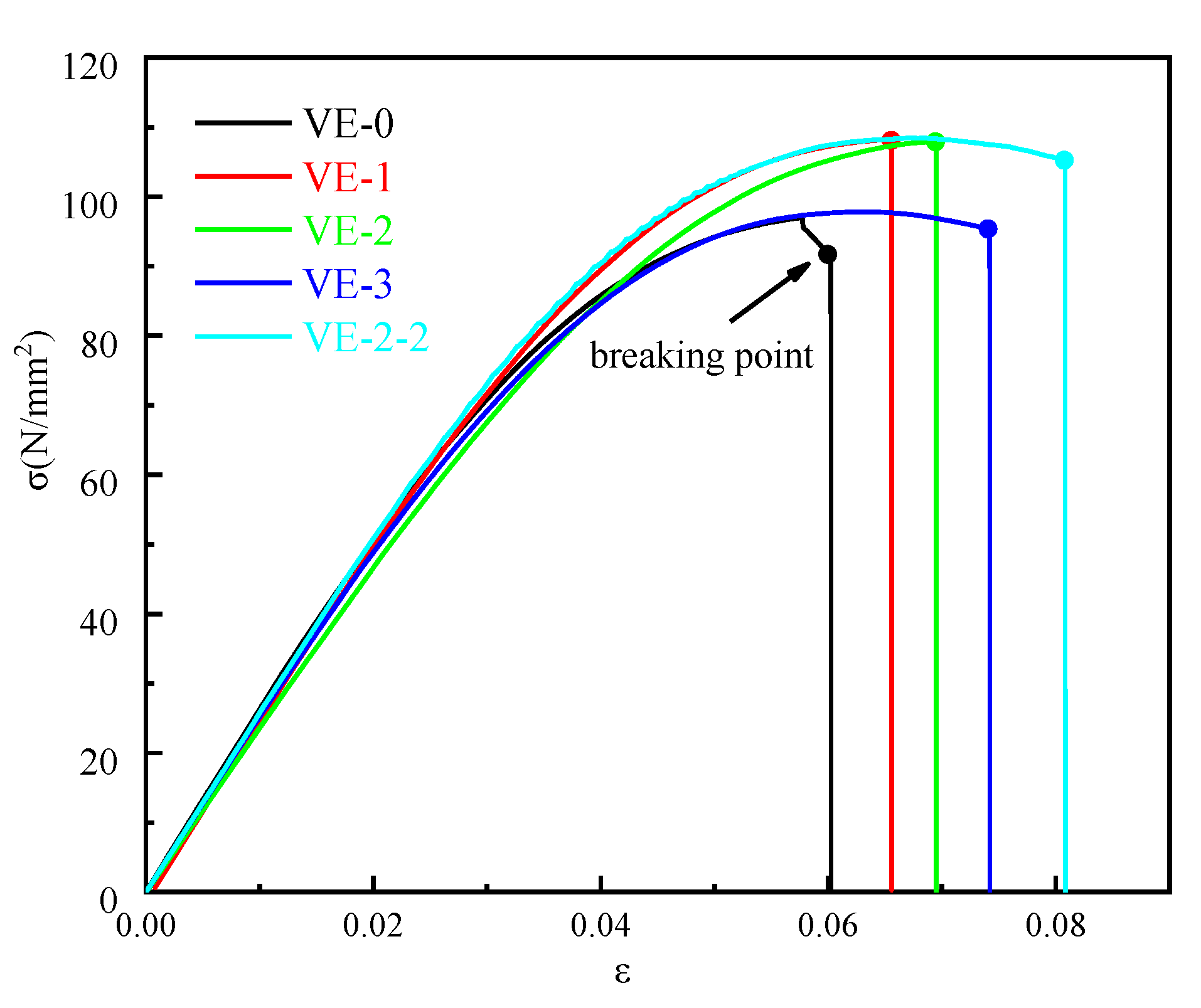
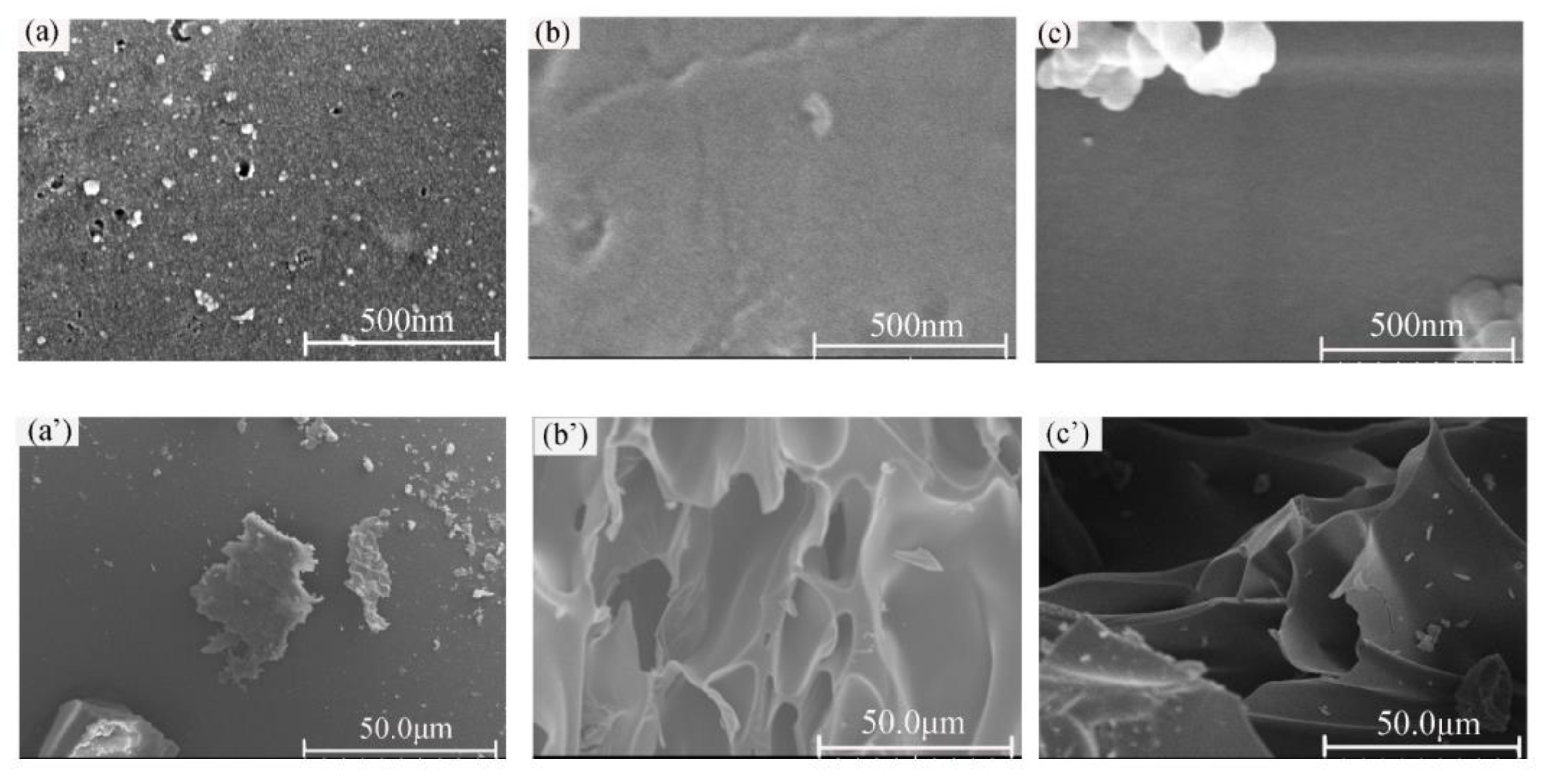
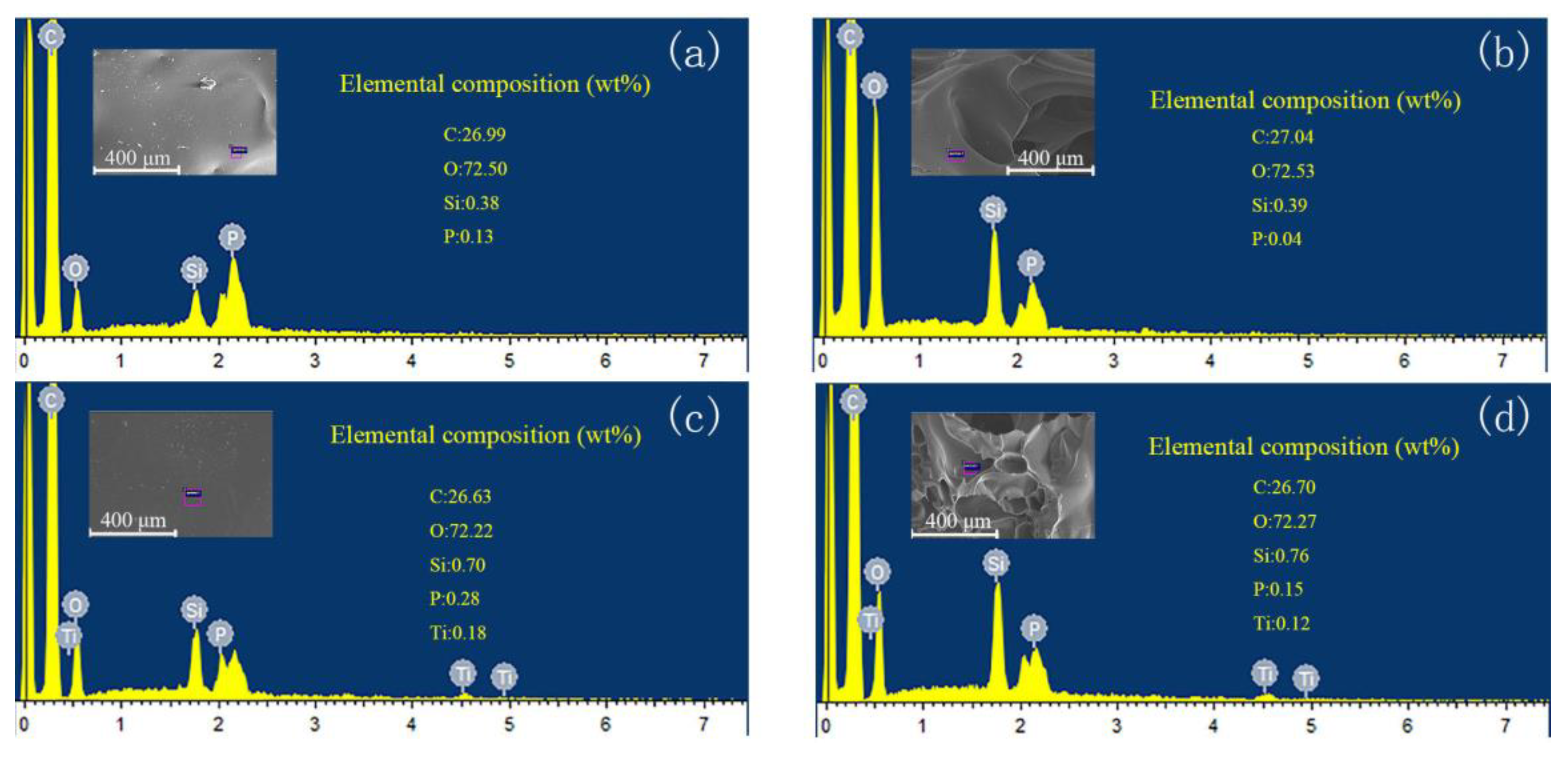
3.2. Morphologies and Mechanical Properties of the VE Composites
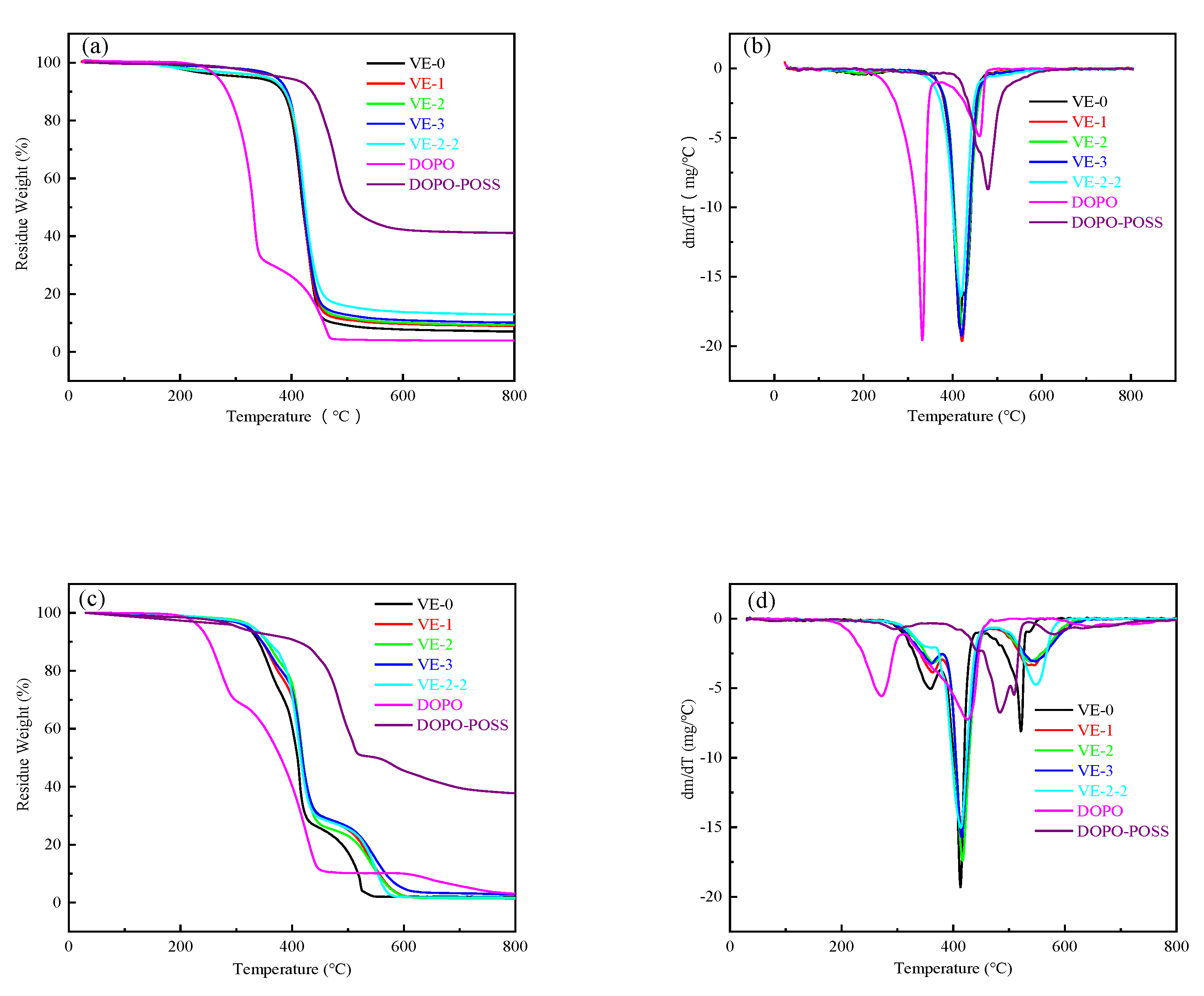
3.3. Thermal Stability
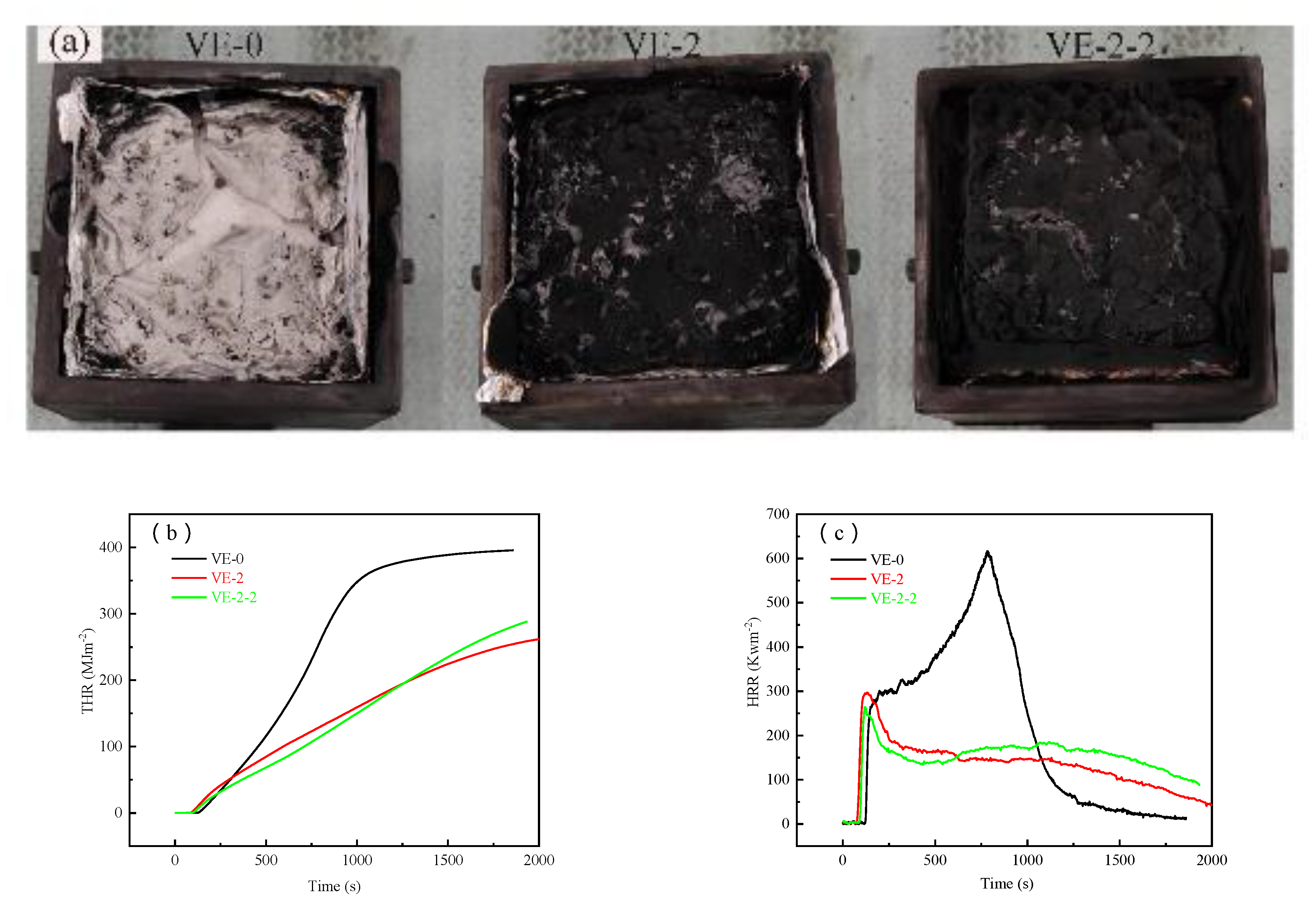
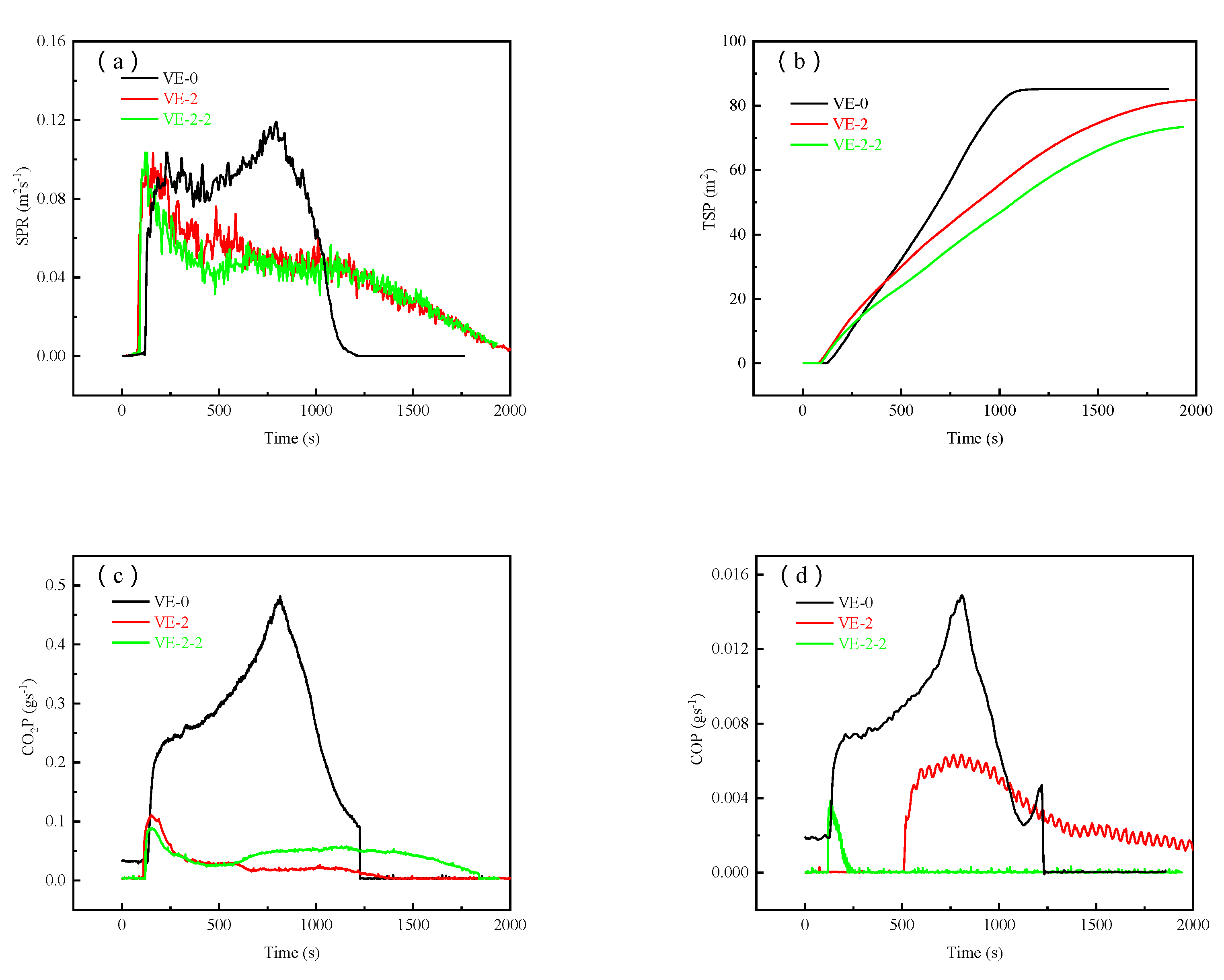
3.4. Flame Retardant Properties of the VE Composites
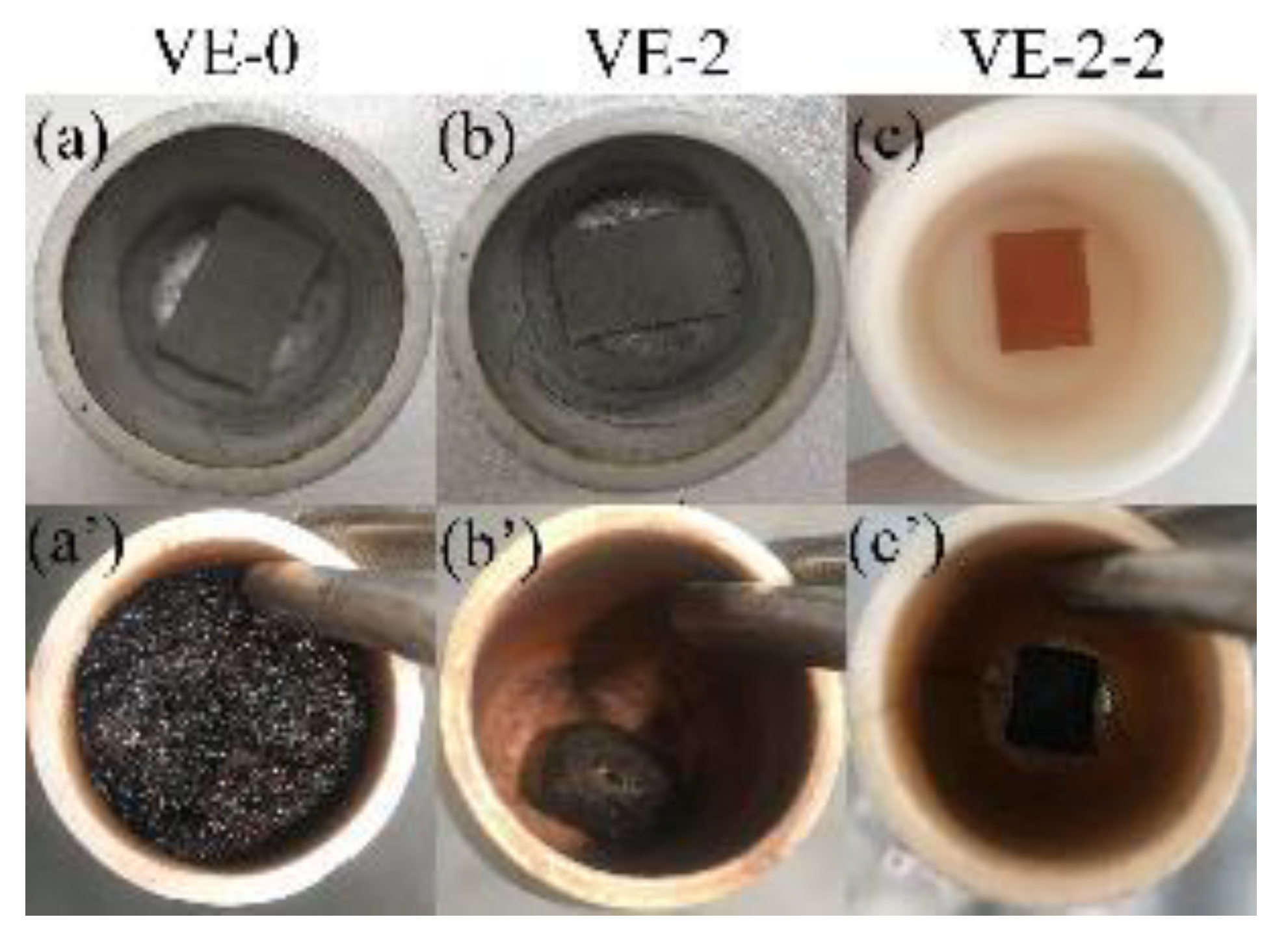
3.5. Flame-Retardant Mechanism
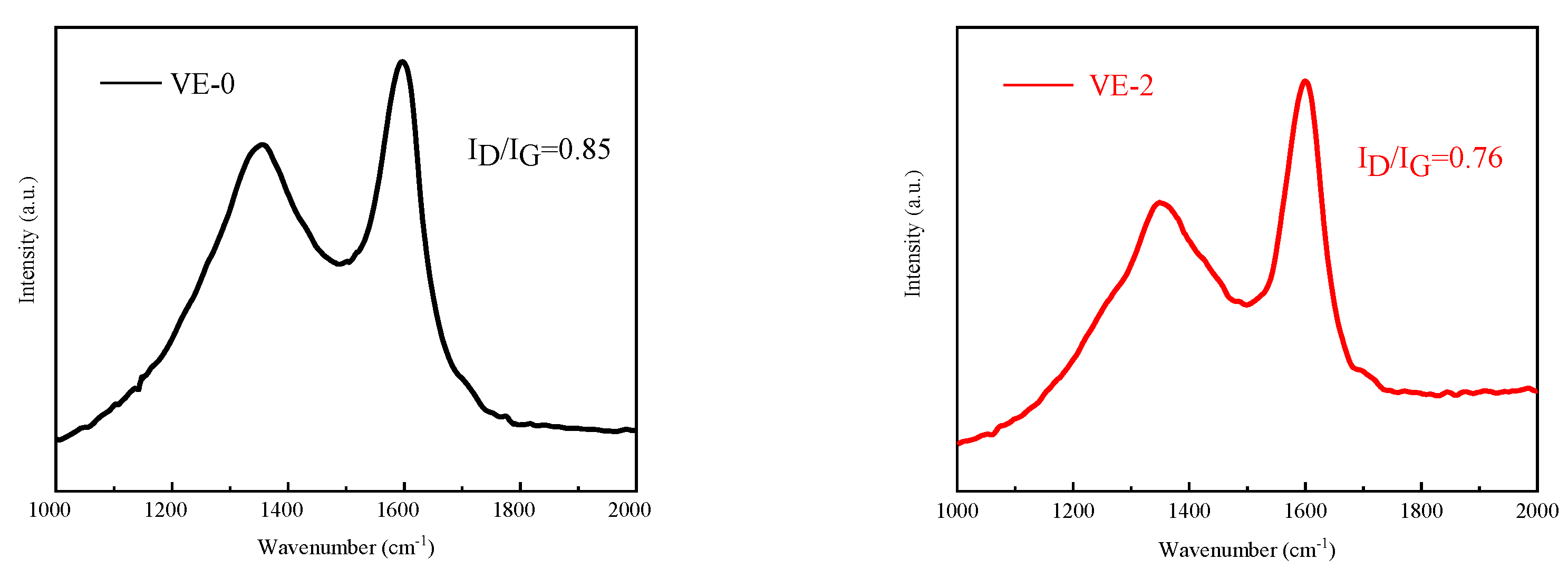
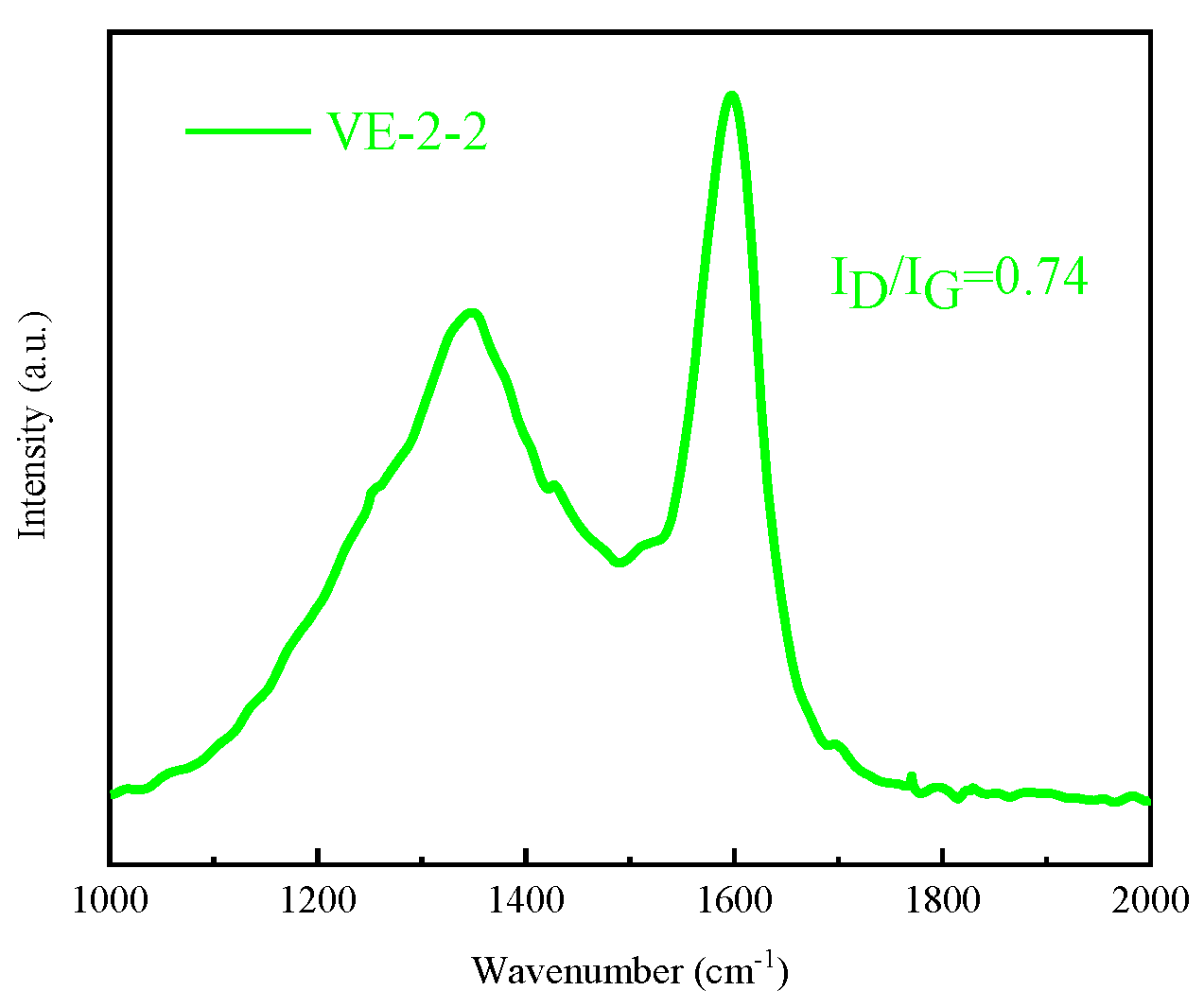
3.5.1. Condensed Phase Analysis
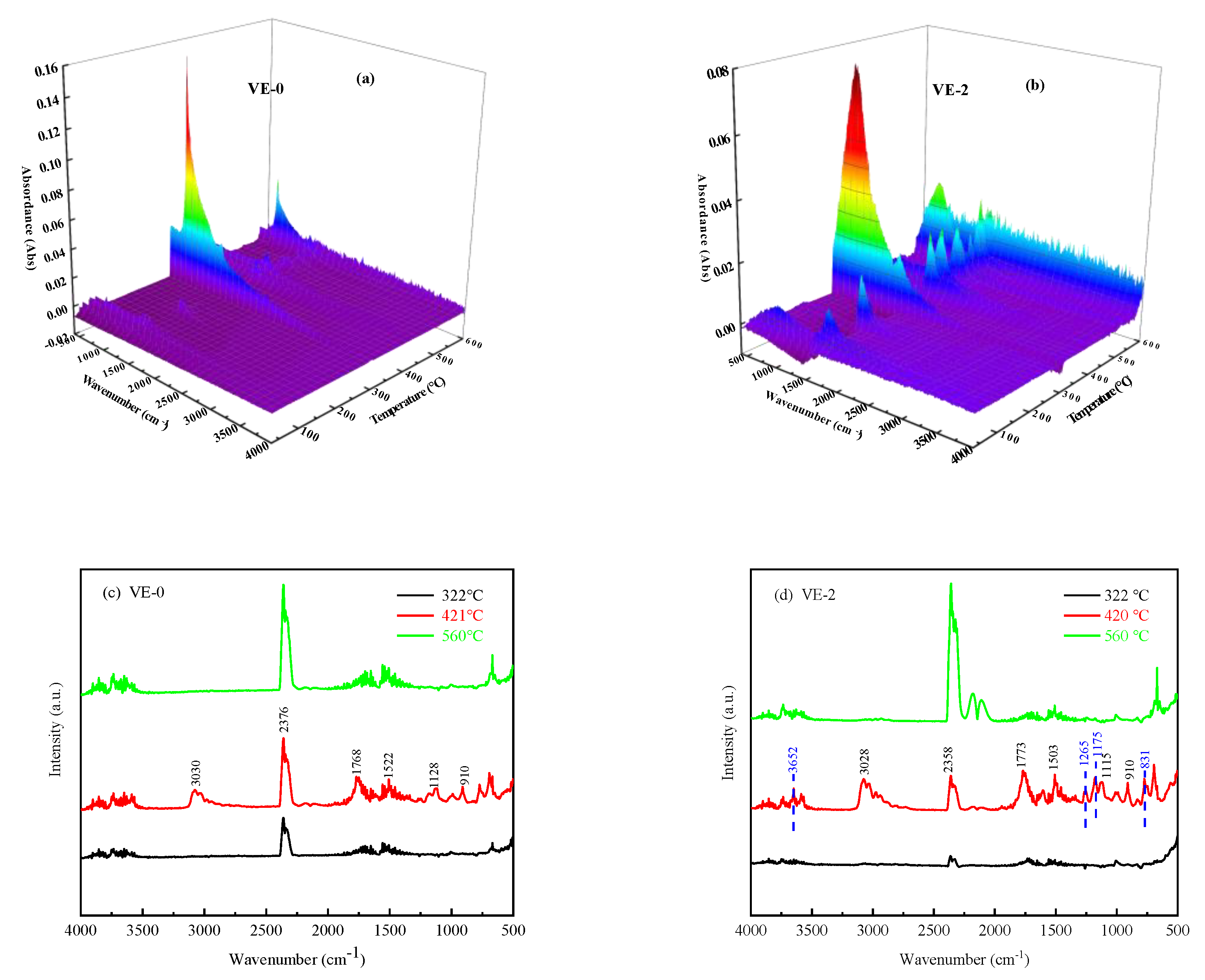
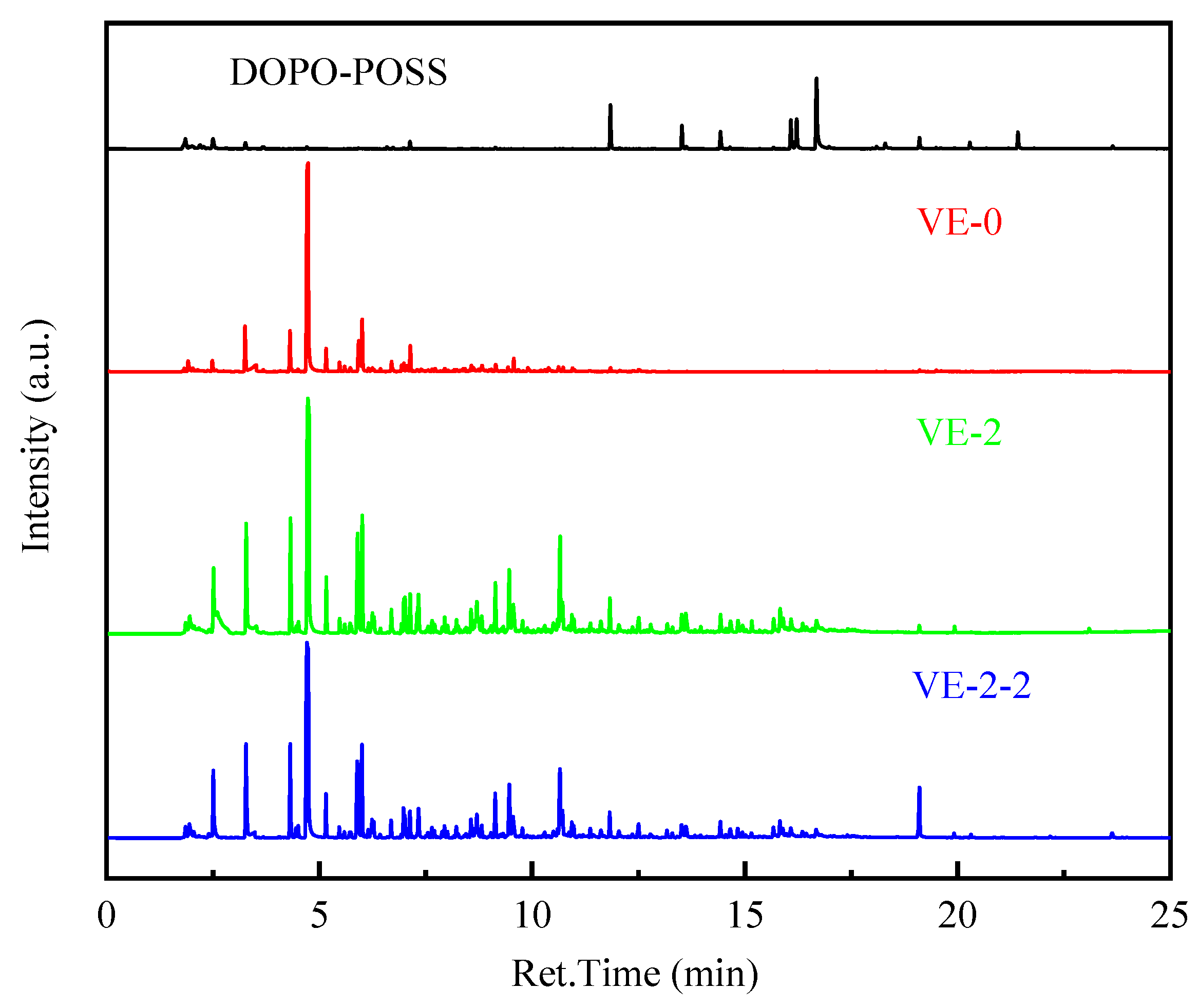
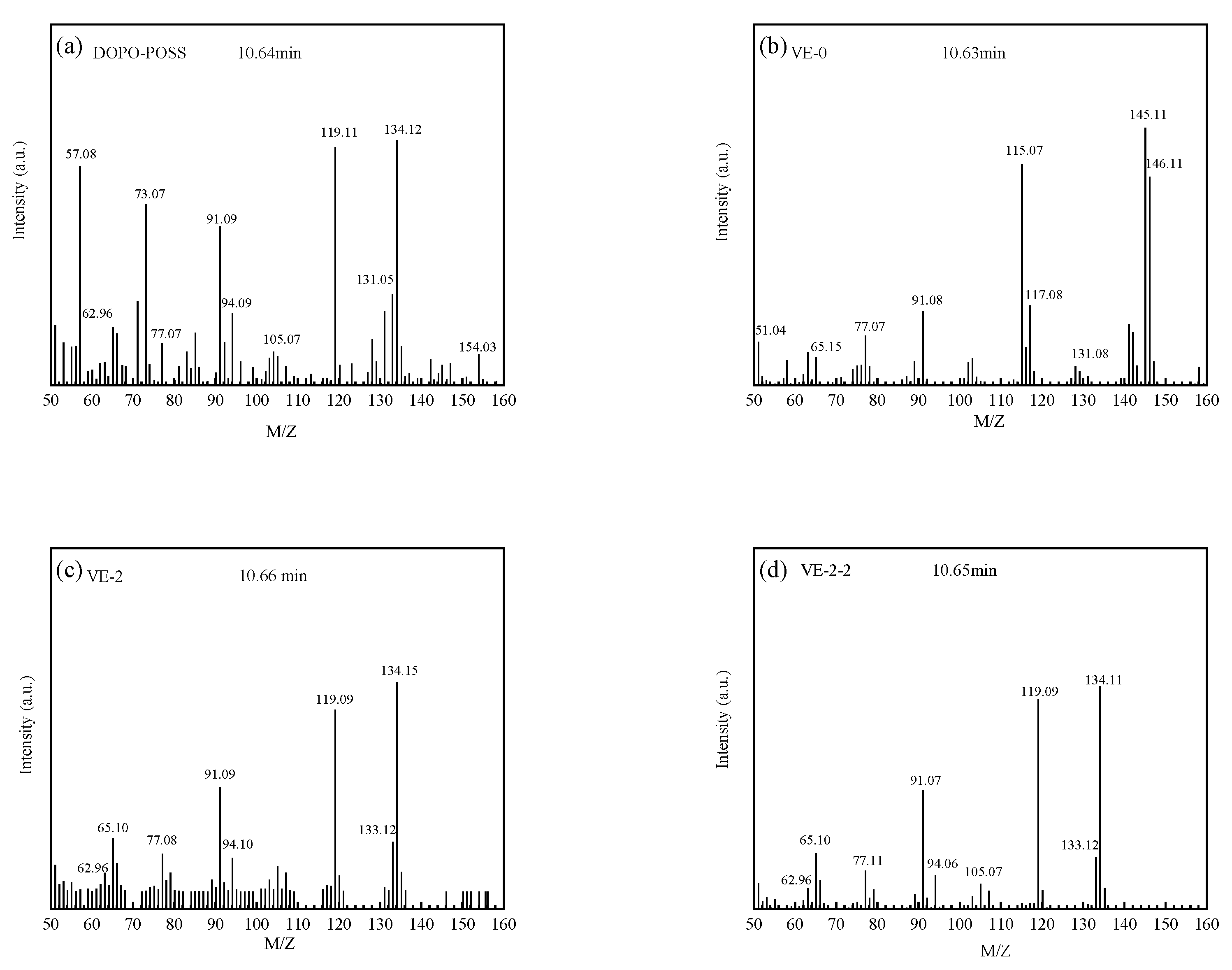
3.5.2. Pyrolysis Behaviors of the VE Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pham, S.; Burchill, P.J. Toughening of vinyl esters resins with modified polybutadienes. Polymer 1995, 36, 3279–3285. [Google Scholar] [CrossRef]
- Siva, P.; Varma, I.K.; Patel, D.M.; Sinha, T.J.M. Effect of structure on properties of vinyl ester resins. Bull. Mater. Sci. 1994, 17, 1095–1101. [Google Scholar] [CrossRef]
- da Silva, A.L.N.; Teixeira, S.C.S.; Widal, A.C.C.; Coutinho, F.M.B. Mechanical properties of polymer composites based on commercial epoxy vinyl ester resin and glass fiber. Polym. Test. 2001, 20, 895–899. [Google Scholar] [CrossRef]
- Tao, X.; Duan, H.; Dong, W.; Wang, X.; Yang, S. Synthesis of an acrylate constructed by phosphaphenanthrene and triazine-trione and its application in intrinsic flame retardant vinyl ester resin. Polym. Degrad. Stab. 2018, 154, 285–294. [Google Scholar] [CrossRef]
- Wu, C.S.; Liu, Y.L.; Chiu, Y.C.; Chiu, Y.S. Thermal stability of epoxy resins containing flame retardant components: An evaluation with thermogravimetric analysis. Polym. Degrad. Stab. 2002, 78, 41–48. [Google Scholar] [CrossRef]
- Lu, S.Y.; Hamerton, I. Recent developments in the chemistry of halogen-free flame retardant polymers. Prog. Polym. Sci. 2002, 27, 1661–1712. [Google Scholar] [CrossRef]
- Cong, P.L.; Chen, S.F.; Yu, J.Y. Investigation of the Properties of Epoxy Resin-Modified Asphalt Mixtures for Application to Orthotropic Bridge Decks. J. Appl. Polym. Sci. 2011, 121, 2310–2316. [Google Scholar] [CrossRef]
- Huang, W.J.; He, W.T.; Long, L.J.; Yan, W.; He, M.; Qin, S.H.; Yu, J. Thermal degradation kinetics of flame-retardant glass-fiber-reinforced polyamide 6T composites based on bridged DOPO derivatives. Polym. Bull. 2018, 76, 2061–2080. [Google Scholar] [CrossRef]
- Yang, S.; Hu, Y.F.; Zhang, Q.X. Synthesis of a phosphorus–nitrogen-containing flame retardant and its application in epoxy resin. High. Perform. Polym. 2018, 31, 186–196. [Google Scholar] [CrossRef]
- Tang, B.; Hu, G.X.; Gao, H.Y.; Hai, L.Y. Application of graphene as filler to improve thermal transport property of epoxy resin for thermal interface materials. Int. J. Heat Mass Transfer. 2015, 85, 420–429. [Google Scholar] [CrossRef]
- Velencoso, M.M.; Battig, A.; Markwart, J.C.; Schartel, B.; Wurm, F.R. Molecular Firefighting-How Modern Phosphorus Chemistry Can Help Solve the Challenge of Flame Retardancy. Angew. Chem. Int. Ed. 2018, 57, 10450–10467. [Google Scholar] [CrossRef] [PubMed]
- Ciesielski, M.; Schafer, A.; Doring, M. Novel efficient DOPO-based flame-retardants for PWB relevant epoxy resins with high glass transition temperatures. Polym. Adv. Technol. 2008, 19, 507–515. [Google Scholar] [CrossRef]
- Jain, P.; Choudhary, V.; Varma, I.K. Flame retarding epoxies with phosphorus. J. Macromol. Sci. Polym. Rev. 2002, 42, 139–183. [Google Scholar] [CrossRef]
- Huang, W.J.; He, W.T.; Long, L.J.; Yan, W.; He, M.; Qin, S.H.; Yu, J. Highly efficient flame-retardant glass-fiber-reinforced polyamide 6T system based on a novel DOPO-based derivative: Flame retardancy, thermal decomposition, and pyrolysis behavior. Polym. Degrad. Stab. 2018, 148, 26–41. [Google Scholar] [CrossRef]
- Hu, Z.; Chen, L.; Zhao, B.; Luo, Y.; Wang, D.Y.; Wang, Y.Z. A novel efficient halogen-free flame retardant system for polycarbonate. Polym. Degrad. Stab. 2011, 96, 320–327. [Google Scholar] [CrossRef]
- Hamciuc, C.; Serbezeanu, D.; Carja, I.D.; Vlad-Bubulac, T.; Musteata, V.E.; Perez, V.F.; Lopez, C.G.; Buendia, A.M.L. Effect of DOPO units and of polydimethylsiloxane segments on the properties of epoxy resins. J. Mater. Sci. 2013, 48, 8520–8529. [Google Scholar] [CrossRef]
- Artner, J.; Ciesielski, M.; Walter, O.; Doring, M.; Perez, R.M.; Sandler, J.K.W.; Altstadt, V.; Schartel, B. A novel DOPO-based diamine as hardener and flame retardant for epoxy resin systems. Macromol. Mater. Eng. 2008, 293, 503–514. [Google Scholar] [CrossRef]
- Antonov, A.; Yablokova, M.; Costa, L.; Balabanovich, A.; Levchik, G.; Levchik, S. The Effect of Nanometals on the Flammability and Thermooxidative Degradation of Polymer Materials. Mol. Cryst. Liq. Cryst. Sci. Technol. Sect. A 2006, 353, 203–210. [Google Scholar] [CrossRef]
- Qian, X.D.; Song, L.; Yuan, B.H.; Yu, B.; Shi, Y.Q.; Hu, Y.; Yuen, R.K.K. Organic/inorganic flame retardants containing phosphorus, nitrogen and silicon: Preparation and their performance on the flame retardancy of epoxy resins as a novel intumescent flame retardant system. Mater. Chem. Phys. 2014, 143, 1243–1252. [Google Scholar] [CrossRef]
- Svetlichnyi, V.M.; Romashkova, K.A.; Subbotina, L.I.; Yudin, V.E.; Popova, E.V.; Gofman, I.V.; Sukhanova, T.E.; Vlasova, E.N.; Afanas‘eva, N.V. Nanocomposites Based on Polyamidoimide and Octahedral Silsesquioxanes. Russ. J. Appl. Chem. 2013, 86, 415–422. [Google Scholar] [CrossRef]
- Pielichowski, K.; Njuguna, J.; Janowski, B.; Pielichowski, J. Polyhedral oligomeric silsesquioxanes (POSS)-containing nanohybrid polymers. Adv. Polym. Sci. 2006, 201, 225–296. [Google Scholar]
- Gushikem, Y.; Benvenutti, E.V.; Kholin, Y.V. Synthesis and applications of functionalized silsesquioxane polymers attached to organic and inorganic matrices. Pure Appl. Chem. 2008, 80, 1593–1611. [Google Scholar] [CrossRef]
- Zhao, B.J.; Mei, H.G.; Liu, N.; Zheng, S.X. Organic-Inorganic Polycyclooctadienes with Double-Decker Silsesquioxanes in the Main Chains: Synthesis, Self-Healing, and Shape Memory Properties Regulated with Quadruple Hydrogen Bonds. Macromolecules 2020, 53, 7119–7131. [Google Scholar] [CrossRef]
- Song, G.H.; Li, X.S.; Jiang, Q.Y.; Mu, J.X.; Jiang, Z.H. A novel structural polyimide material with synergistic phosphorus and POSS for atomic oxygen resistance. RSC Adv. 2015, 5, 11980–11988. [Google Scholar] [CrossRef]
- Chao, P.J.; Li, Y.J.; Gu, X.Y.; Han, D.D.; Jia, X.Q.; Wang, M.Q.; Zhou, T.F.; Wang, T. Novel phosphorus-nitrogen-silicon flame retardants and their application in cycloaliphatic epoxy systems. Polym. Chem. 2015, 6, 2977–2985. [Google Scholar] [CrossRef]
- Liu, C.; Chen, T.; Yuan, C.H.; Song, C.F.; Chang, Y.; Chen, G.R.; Xu, Y.T.; Dai, L.Z. Modification of epoxy resin through the self-assembly of a surfactant like multi-element flame retardant. J. Mater. Chem. A 2016, 4, 3462–3470. [Google Scholar] [CrossRef]
- Szolyga, M.; Dutkiewicz, M.; Nowicki, M.; Salasinska, K.; Celinski, M.; Marciniec, B. Phosphorus-Containing Silsesquioxane Derivatives as Additive or Reactive Components of Epoxy Resins. Materials 2020, 13, 5373. [Google Scholar] [CrossRef]
- Zhang, W.C.; Li, X.M.; Yang, R.J. Flame retardant mechanisms of phosphorus-containing polyhedral oligomeric silsesquioxane (DOPO-POSS) in polycarbonate composites. J. Appl. Polym. Sci. 2012, 124, 1848–1857. [Google Scholar] [CrossRef]
- Qi, Z.; Zhang, W.C.; He, X.D.; Yang, R.J. High-efficiency flame retardency of epoxy resin composites with perfect T-8 caged phosphorus containing polyhedral oligomeric silsesquioxanes (P-POSSs). Compos. Sci. Technol. 2016, 127, 8–19. [Google Scholar] [CrossRef]
- Zhang, W.C.; Li, X.M.; Yang, R.J. Novel flame retardancy effects of DOPO-POSS on epoxy resins. Polym. Degrad. Stab. 2011, 96, 2167–2173. [Google Scholar] [CrossRef]
- Zhang, W.C.; Li, X.M.; Yang, R.J. Pyrolysis and fire behaviour of epoxy resin composites based on a phosphorus-containing polyhedral oligomeric silsesquioxane (DOPO-POSS). Polym. Degrad. Stab. 2011, 96, 1821–1832. [Google Scholar] [CrossRef]
- Zhang, W.C.; Li, X.M.; Yang, R.J. Blowing-out effect in epoxy composites flame retarded by DOPO-POSS and its correlation with amide curing agents. Polym. Degrad. Stab. 2012, 97, 1314–1324. [Google Scholar] [CrossRef]
- Zhang, W.C.; Li, X.M.; Yang, R.J. Study on flame retardancy of TGDDM epoxy resins loaded with DOPO-POSS compound and OPS/DOPO mixture. Polym. Degrad. Stab. 2014, 99, 118–126. [Google Scholar] [CrossRef]
- Zhang, W.C.; Li, X.M.; Yang, R.J. Flame retardancy mechanisms of phosphorus-containing polyhedral oligomeric silsesquioxane (DOPO-POSS) in polycarbonate/acrylonitrile-butadiene-styrene blends. Polym. Adv. Technol. 2012, 23, 588–595. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, W.W.; Zhang, W.C.; Yang, R.J. Enhanced mechanical and flame retardancy properties of vinyl ester resin systems with the synthesis of two flame retardants with vinyl group. Polym. Int. 2020, 69, 1196–1206. [Google Scholar] [CrossRef]
- Fina, A.; Tabuani, D.; Camino, G. Polypropylene-polysilsesquioxane blends. Eur. Polym. J. 2010, 46, 14–23. [Google Scholar] [CrossRef]
- Fox, D.M.; Lee, J.; Zammarano, M.; Katsoulis, D.; Eldred, D.V.; Haverhals, L.M.; Trulove, P.C.; De Long, H.C.; Gilman, J.W. Char-forming behavior of nanofibrillated cellulose treated with glycidyl phenyl POSS. Carbohydr. Polym. 2012, 88, 847–858. [Google Scholar] [CrossRef]
- Zhang, P.; Hu, Y.; Song, L.; Lu, H.D.; Wang, J.; Liu, Q.Q. Synergistic effect of iron and intumescent flame retardant on shape-stabilized phase change material. Thermochim. Acta 2009, 487, 74–79. [Google Scholar] [CrossRef]
- Chen, X.L.; Li, M.; Zhuo, J.L.; Ma, C.Y.; Jiao, C.M. Influence of Fe2O3 on smoke suppression and thermal degradation properties in intumescent flame-retardant silicone rubber. J. Therm. Anal. Calorim. 2015, 123, 439–448. [Google Scholar] [CrossRef]
- Zeng, B.R.; Liu, Y.Z.; Yang, L.; Zheng, W.; Chen, T.; Chen, G.R.; Xu, Y.T.; Yuan, C.H.; Dai, L.Z. Flame retardant epoxy resin based on organic titanate and polyhedral oligomeric silsesquioxane-containing additives with synergistic effects. RSC Adv. 2017, 7, 26082–26088. [Google Scholar] [CrossRef]
- Zeng, G.F.; Zhang, W.W.; Zhang, X.; Zhang, W.C.; Du, J.X.; He, J.Y.; Yang, R.J. Study on flame retardancy of APP/PEPA/MoO3 synergism in vinyl ester resins. J. Appl. Polym. Sci. 2020, 137, 49026. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, W.W.; Zeng, G.F.; Du, J.X.; Zhang, W.C.; Yang, R.J. The Effect of Different Smoke Suppressants with APP for Enhancing the Flame Retardancy and Smoke Suppression on Vinyl Ester Resin. Polym. Eng. Sci. 2020, 60, 314–322. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, X.; He, J.; Yu, L.; Yang, R. Effect of polyphenylsilsesquioxane on the ablative and flame-retardation properties of ethylene propylene diene monomer (EPDM) composite. Polym. Degrad. Stab. 2011, 96, 949–954. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, X.; Yang, R. Polycarbonate composites flame-retarded by polyphenylsilsesquioxane of ladder structure. J. Appl. Polym. Sci. 2012, 124, 4381–4388. [Google Scholar] [CrossRef]
- Zhang, W.C.; Li, X.M.; Jiang, Y.Y.; Yang, R.J. Investigations of epoxy resins flame-retarded by phenyl silsesquioxanes of cage and ladder structures. Polym. Degrad. Stab. 2013, 98, 246–254. [Google Scholar] [CrossRef]
- Zhang, Z.; Liang, G.; Lu, T. Synthesis and characterization of cage octa(aminopropylsilsesquioxane). J. Appl. Polym. Sci. 2007, 103, 2608–2614. [Google Scholar] [CrossRef]
- Li, S.N.; Zhao, X.J.; Zhang, Y.; Yang, X.; Yu, R.; Zhang, Y.; Deng, K.L.; Huang, W. A facile approach to prepare cage-ladder-structure phosphorus-containing amino-functionalized POSS for enhancing flame retardancy of epoxy resins. J. Appl. Polym. Sci. 2020, 138, 49870. [Google Scholar] [CrossRef]
- Li, S.N.; Zhao, X.J.; Liu, X.H.; Yang, X.; Yu, R.; Zhang, Y.; Huang, W.; Deng, K.L. Cage-ladder-structure, phosphorus-containing polyhedral oligomeric silsesquinoxanes as promising reactive-type flame retardants for epoxy resin. J. Appl. Polym. Sci. 2019, 136, 47607. [Google Scholar] [CrossRef]
- Nowacka, M.; Kowalewska, A.; Makowski, T. Structural studies on ladder phenylsilsesquioxane oligomers formed by polycondensation of cyclotetrasiloxanetetraols. Polymer 2016, 87, 81–89. [Google Scholar] [CrossRef]
- Zhang, W.C.; Yang, R.J. Synthesis of Phosphorus-Containing Polyhedral Oligomeric Silsesquioxanes via Hydrolytic Condensation of a Modified Silane. J. Appl. Polym. Sci. 2011, 122, 3383–3389. [Google Scholar] [CrossRef]
- Liu, C.; Chen, T.; Yuan, C.H.; Chang, Y.; Chen, G.R.; Zeng, B.R.; Xu, Y.T.; Luo, W.A.; Dai, L.Z. Highly transparent and flame-retardant epoxy composites based on a hybrid multi-element containing POSS derivative. RSC Adv. 2017, 7, 46139–46147. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, Y.; Zhao, Y.; Liu, S.; Zhao, J. Flame–retarded epoxy resin with high glass transition temperature cured by DOPO-containing H-benzimidazole. High. Perform. Polym. 2016, 29, 94–103. [Google Scholar] [CrossRef]
- Wu, H.Y.; Zeng, B.R.; Chen, J.M.; Wu, T.; Li, Y.T.; Liu, Y.Z.; Dai, L.Z. An intramolecular hybrid of metal polyhedral oligomeric silsesquioxanes with special titanium-embedded cage structure and flame retardant functionality. Chem. Eng. J. 2019, 374, 1304–1316. [Google Scholar] [CrossRef]
- Huo, S.; Yang, S.; Wang, J.; Cheng, J.; Zhang, Q.; Hu, Y.; Ding, G.; Zhang, Q.; Song, P. A liquid phosphorus-containing imidazole derivative as flame-retardant curing agent for epoxy resin with enhanced thermal latency, mechanical, and flame-retardant performances. J. Hazard. Mater. 2020, 386, 121984. [Google Scholar] [CrossRef]
- Sun, Z.; Hou, Y.; Hu, Y.; Hu, W. Effect of additive phosphorus-nitrogen containing flame retardant on char formation and flame retardancy of epoxy resin. Mater. Chem. Phys. 2018, 214, 154–164. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Y.; Song, L.; Xing, W.Y.; Lu, H.D. Thermal Degradation Behaviors of Epoxy Resin/POSS Hybrids and Phosphorus-Silicon Synergism of Flame Retardancy. J. Polym. Sci. Part B Polym. Phys. 2010, 48, 693–705. [Google Scholar] [CrossRef]
- Mercado, L.A.; Reina, J.A.; Galia, M. Flame retardant epoxy resins based on diglycidyloxymethylphenylsilane. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 5580–5587. [Google Scholar] [CrossRef]
- Zhang, W.C.; Camino, G.; Yang, R.J. Polymer/polyhedral oligomeric silsesquioxane (POSS) nanocomposites: An overview of fire retardance. Prog. Polym. Sci. 2017, 67, 77–125. [Google Scholar] [CrossRef]
- Wang, P.; Yang, F.S.; Li, L.; Cai, Z.S. Flame retardancy and mechanical properties of epoxy thermosets modified with a novel DOPO-based oligomer. Polym. Degrad. Stab. 2016, 129, 156–167. [Google Scholar] [CrossRef]
- Jiang, S.D.; Bai, Z.M.; Tang, G.; Song, L.; Stec, A.A.; Hull, T.R.; Hu, Y.; Hu, W.Z. Synthesis of mesoporous silica@Co-Al layered double hydroxide spheres: Layer-by-layer method and their effects on the flame retardancy of epoxy resins. ACS Appl. Mater. Interfaces 2014, 6, 14076–14086. [Google Scholar] [CrossRef]
- Fei, P.; Chen, X.; Xiong, H.G.; Ud Din, Z.; Chen, L.; Cai, J. Synthesis of H2Ti2O3 center dot H2O nanotubes and their effects on the flame retardancy of bamboo fiber/high-density polyethylene composites. Compos. Part A Appl. Sci. Manuf. 2016, 90, 225–233. [Google Scholar] [CrossRef]
- Hashemikia, S.; Montazer, M. Sodium hypophosphite and nano TiO2 inorganic catalysts along with citric acid on textile producing multi-functional properties. Appl. Catal. A Gen. 2012, 417, 200–208. [Google Scholar] [CrossRef]
- Zhang, W.C.; Li, X.M.; Fan, H.B.; Yang, R.J. Study on mechanism of phosphorus-silicon synergistic flame retardancy on epoxy resins. Polym. Degrad. Stab. 2012, 97, 2241–2248. [Google Scholar] [CrossRef]
- Su, C.H.; Chiu, Y.P.; Teng, C.C.; Chiang, C.L. Preparation, characterization and thermal properties of organic-inorganic composites involving epoxy and polyhedral oligomeric silsesquioxane (POSS). J. Polym. Res. 2010, 17, 673–681. [Google Scholar] [CrossRef]
- Tan, Y.; Shao, Z.-B.; Chen, X.-F.; Long, J.-W.; Chen, L.; Wang, Y.-Z. Novel Multifunctional Organic Inorganic Hybrid Curing Agent with High Flame-Retardant Efficiency for Epoxy Resin. ACS Appl. Mater. Interfaces 2015, 7, 17919–17928. [Google Scholar] [CrossRef]
- Shi, Y.Q.; Yu, B.; Zheng, Y.Y.; Guo, J.; Chen, B.H.; Pan, Z.M.; Hu, Y. A combination of POSS and polyphosphazene for reducing fire hazards of epoxy resin. Polym. Adv. Technol. 2018, 29, 1242–1254. [Google Scholar] [CrossRef]
- Liu, J.; Yu, Q.; Yu, M.; Li, S.; Zhao, K.; Xue, B.; Zu, H. Silane modification of titanium dioxide-decorated graphene oxide nanocomposite for enhancing anticorrosion performance of epoxy coatings on AA-2024. J. Alloys Compd. 2018, 744, 728–739. [Google Scholar] [CrossRef]
- Babelon, P.; Dequiedt, A.S.; Mostefa-Sba, H.; Bourgeois, S.; Sibillot, P.; Sacilotti, M. SEM and XPS studies of titanium dioxide thin films grown by MOCVD. Thin Solid Film. 1998, 322, 63–67. [Google Scholar] [CrossRef]
- Zheng, R.Y.; Lin, L.; Xie, J.L.; Zhu, Y.X.; Me, Y.C. State of doped phosphorus and its influence on the physicochemical and photocatalytic properties of P-doped titania. J. Phys. Chem. C 2008, 112, 15502–15509. [Google Scholar] [CrossRef]
- Hong, N.; Pan, Y.; Zhan, J.; Wang, B.; Zhou, K.; Song, L.; Hu, Y. Fabrication of graphene/Ni-Ce mixed oxide with excellent performance for reducing fire hazard of polypropylene. RSC Adv. 2013, 3, 16440–16448. [Google Scholar] [CrossRef]
- Wang, B.; Xu, Y.J.; Li, P.; Zhang, F.Q.; Liu, Y.; Zhu, P. Flame-retardant polyester/cotton blend with phosphorus/nitrogen/silicon-containing nano-coating by layer-by-layer assembly. Appl. Surf. Sci. 2020, 509, 145323. [Google Scholar] [CrossRef]
- Wang, S.; Du, X.S.; Jiang, Y.X.; Xu, J.H.; Zhou, M.; Wang, H.B.; Cheng, X.; Du, Z.L. Synergetic enhancement of mechanical and fire-resistance performance of waterborne polyurethane by introducing two kinds of phosphorus-nitrogen flame retardant. J. Colloid Interface Sci. 2019, 537, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Li, D.S.; Xu, M.J.; Li, B. Synthesis of a novel phosphorus and nitrogen-containing flame retardant and its application in rigid polyurethane foam with expandable graphite. Polym. Degrad. Stab. 2020, 173, 109077. [Google Scholar] [CrossRef]
- Wu, H.Y.; Li, Y.T.; Zeng, B.R.; Chen, G.R.; Wu, Y.Z.; Chen, T.; Dai, L.Z. A high synergistic P/N/Si-containing additive with dandelion-shaped structure deriving from self-assembly for enhancing thermal and flame retardant property of epoxy resins. React. Funct. Polym. 2018, 131, 89–99. [Google Scholar] [CrossRef]
- Jin, S.L.; Qian, L.J.; Qiu, Y.; Chen, Y.J.; Xin, F. High-efficiency flame retardant behavior of bi-DOPO compound with hydroxyl group on epoxy resin. Polym. Degrad. Stab. 2019, 166, 344–352. [Google Scholar] [CrossRef]
- Huo, S.Q.; Wang, J.; Yang, S.; Wang, J.P.; Zhang, B.; Zhang, B.; Chen, X.; Tang, Y.S. Synthesis of a novel phosphorus-nitrogen type flame retardant composed of maleimide, triazine-trione, and phosphaphenanthrene and its flame retardant effect on epoxy resin. Polym. Degrad. Stab. 2016, 131, 106–113. [Google Scholar] [CrossRef]



| Samples | MFE-711 (g) | LPT-IN (g) | P002(g) | DOPO-POSS(g) | TBT(g) |
|---|---|---|---|---|---|
| VE-0 | 100 | 1 | 0.08 | - | - |
| VE-1 | 100 | 1 | 0.08 | 2 | - |
| VE-2 | 100 | 1 | 0.08 | 4 | - |
| VE-3 | 100 | 1 | 0.08 | 5 | - |
| VE-2-1 | 100 | 1 | 0.08 | 4 | 0.25 |
| VE-2-2 | 100 | 1 | 0.08 | 4 | 0.50 |
| VE-2-3 | 100 | 1 | 0.08 | 4 | 0.75 |
| m/z |  | Number of Si (28) | Number of -OH (17) | Note |
|---|---|---|---|---|
| 1845(K+) | 6 | 6 | 4 | ladder |
| 2057 | 7 | 7 | 1 | cage |
| 2149(K+) | 7 | 7 | 5 | ladder |
| 2361(H+) | 8 | 8 | 0 | cage |
| 2435(K+) | 8 | 8 | 4 | ladder |
| 2648(H+) | 9 | 9 | 1 | cage-ladder |
| 2739(K+) | 9 | 9 | 5 | ladder |
| 2951 | 10 | 10 | 2 | cage-ladder |
| 3025(K+) | 10 | 10 | 4 | ladder |
| Samples | Flexural Modulus (GPa) | Flexural Strength (MPa) | Fracture Strain (%) |
|---|---|---|---|
| VE-0 | 2.39 | 76.54 | 6.02 |
| VE-1 | 2.46 | 81.56 | 6.55 |
| VE-2 | 2.32 | 77.46 | 6.94 |
| VE-3 | 2.21 | 71.77 | 7.42 |
| VE-2-2 | 2.52 | 79.17 | 8.08 |
| Samples | N2 | Air | |||||
|---|---|---|---|---|---|---|---|
| T5% a (°C) | Tmax1 b (°C) | Residue (800 °C) | T5% a (°C) | Tmax1 b (°C) | Tmax2 b (°C) | Residue (800 °C) | |
| VE-0 | 321.2 | 416.1 | 7.07% | 320.6 | 413.1 | 521.4 | 1.96% |
| VE-1 | 352.7 | 421.2 | 9.04% | 322.6 | 414.1 | 545.2 | 1.35% |
| VE-2 | 352.9 | 422.7 | 9.35% | 330.3 | 416.7 | 538.3 | 1.34% |
| VE-3 | 371.9 | 420.0 | 10.15% | 320.6 | 414.7 | 547.1 | 2.84% |
| VE-2-2 | 353.3 | 418.6 | 14.38% | 330.1 | 413.6 | 548.1 | 1.69% |
| DOPO | 267.0 | 332.3 | 3.92% | 234.7 | 271.7 | 425.4 | 3.07% |
| DOPO-POSS | 384.0 | 479.4 | 41.1% | 304.3 | 483.7 | 509.0 | 37.71% |
| Samples | MFE-711 | DOPO-POSS (wt%) | TBT (wt%) | LOI |
|---|---|---|---|---|
| VE-0 | 100 | - | - | 19.5 |
| VE-1 | 100 | 2 | - | 21.1 |
| VE-2 | 100 | 4 | - | 22.1 |
| VE-3 | 100 | 5 | - | 21.3 |
| VE-2-1 | 100 | 4 | 0.25 | 22.7 |
| VE-2-2 | 100 | 4 | 0.50 | 24.2 |
| VE-2-3 | 100 | 4 | 0.75 | 22.9 |
| Samples | PHRR (Kwm−2) | THR (MJm−2) | SPR (m2s−1) | TSP (m2) | av-CO2Y (kgkg−1) | av-COY (kgkg−1) |
|---|---|---|---|---|---|---|
| VE-0 | 616.7 | 395.8 | 0.127 | 85.15 | 2.15 | 0.06 |
| VE-2 | 299.9 | 267.1 | 0.103 | 82.05 | 0.28 | 0.01 |
| VE-2-2 | 264.5 | 289.2 | 0.104 | 73.45 | 0.69 | 0.002 |
| M/Z | Structures | M/Z | Structures |
|---|---|---|---|
| 146 | 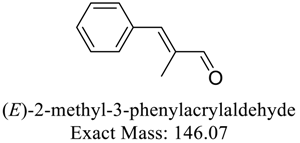 | 132 |  |
| 118 | 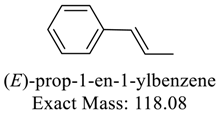 | 104 | 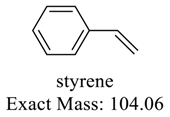 |
| 92 | 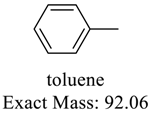 | 78 | 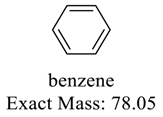 |
| 66 |  | 52 |  |
| 134 | 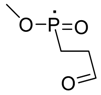 | 119 | 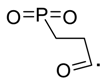 |
| 91 | 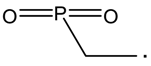 | 77 | 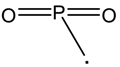 |
| 63 | 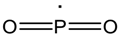 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, X.; Zhang, X.; Guo, Y.; Liu, X.; Zhao, X.; Zhou, H.; Zhang, S.; Zhao, T. Synergistic Effects of Ladder and Cage Structured Phosphorus-Containing POSS with Tetrabutyl Titanate on Flame Retardancy of Vinyl Epoxy Resins. Polymers 2021, 13, 1363. https://doi.org/10.3390/polym13091363
Han X, Zhang X, Guo Y, Liu X, Zhao X, Zhou H, Zhang S, Zhao T. Synergistic Effects of Ladder and Cage Structured Phosphorus-Containing POSS with Tetrabutyl Titanate on Flame Retardancy of Vinyl Epoxy Resins. Polymers. 2021; 13(9):1363. https://doi.org/10.3390/polym13091363
Chicago/Turabian StyleHan, Xu, Xiaohua Zhang, Ying Guo, Xianyuan Liu, Xiaojuan Zhao, Heng Zhou, Songli Zhang, and Tong Zhao. 2021. "Synergistic Effects of Ladder and Cage Structured Phosphorus-Containing POSS with Tetrabutyl Titanate on Flame Retardancy of Vinyl Epoxy Resins" Polymers 13, no. 9: 1363. https://doi.org/10.3390/polym13091363
APA StyleHan, X., Zhang, X., Guo, Y., Liu, X., Zhao, X., Zhou, H., Zhang, S., & Zhao, T. (2021). Synergistic Effects of Ladder and Cage Structured Phosphorus-Containing POSS with Tetrabutyl Titanate on Flame Retardancy of Vinyl Epoxy Resins. Polymers, 13(9), 1363. https://doi.org/10.3390/polym13091363






