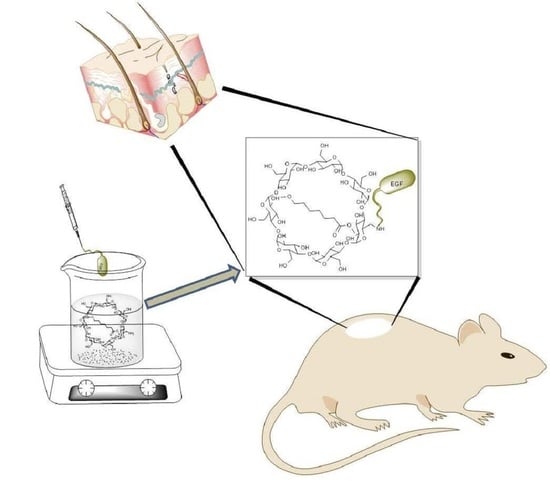
Epithelial Growth Factor-Anchored on Polycaprolactone/6-deoxy-6-amino-β-cyclodextrin Nanofibers: In Vitro and In Vivo Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. EGF Immobilization on Surface of Aminated Nanofiber
2.2. Aminated Nanofibers with/without EGF Characterization
2.3. In Vitro Biocompatibility Study (3T3 Cell Culture)
2.4. T3 Cell Proliferation in Contact with the Scaffold
2.5. In Vivo Study: Wound Healing Test
3. Results and Discussion
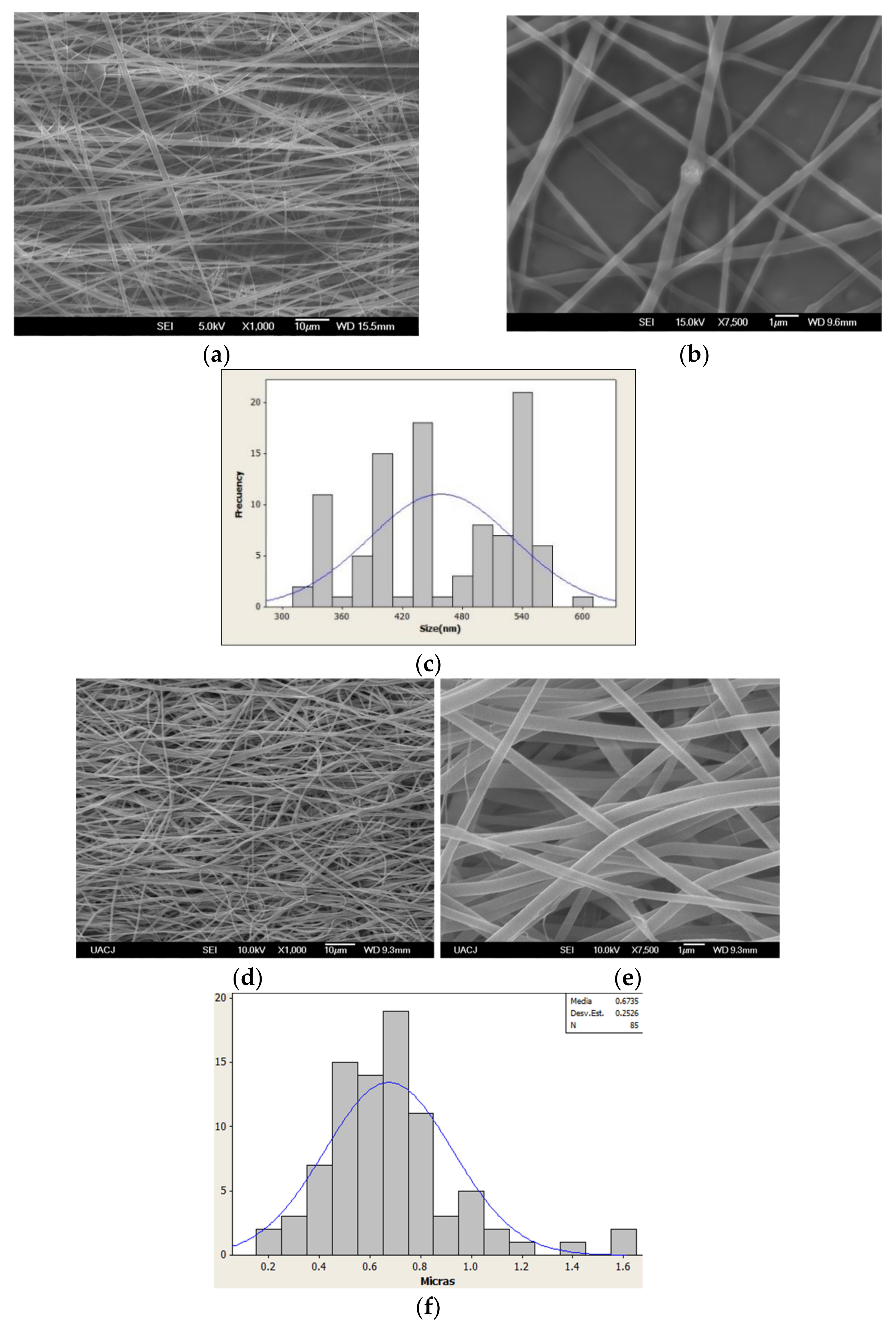
3.1. EGF Immobilization on Surface of Aminated Nanofiber Scaffolds and Characterization
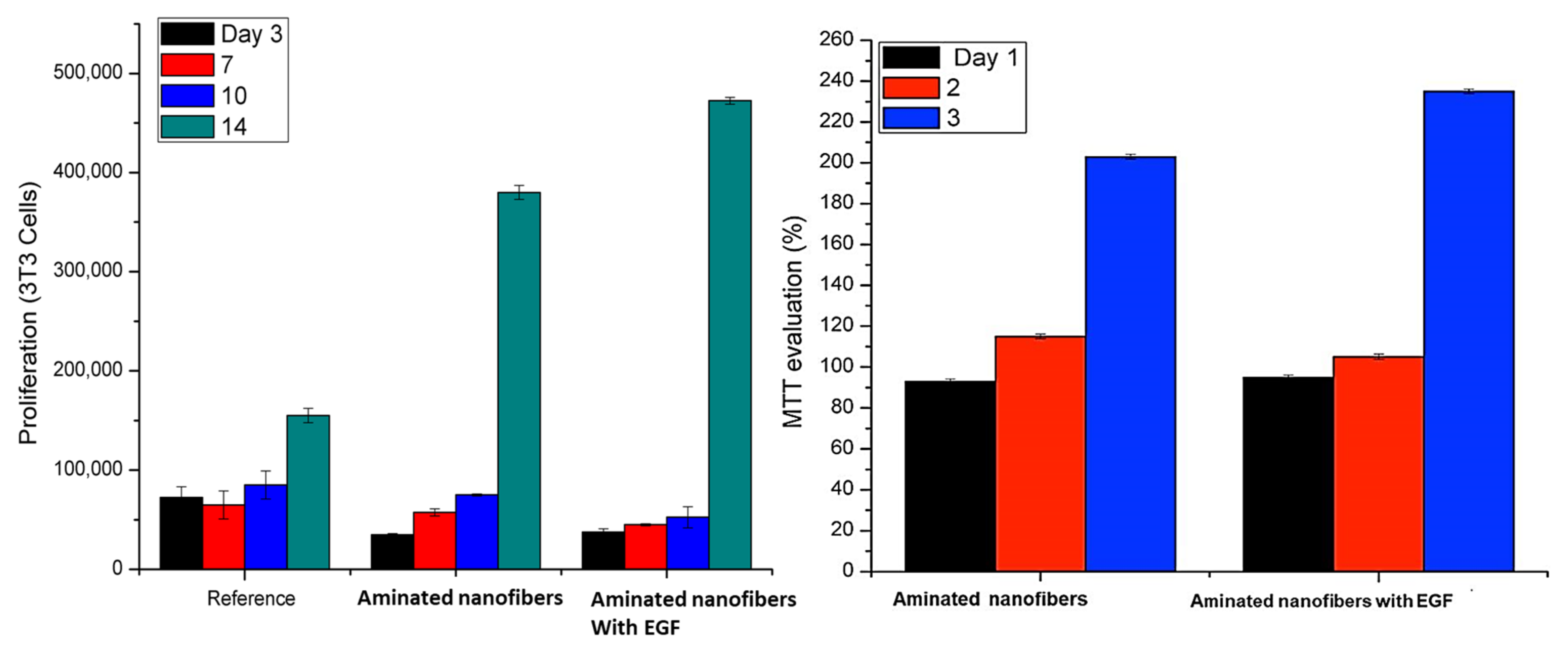
3.2. In Vitro Biocompatibility Study (3T3 Cell Culture)
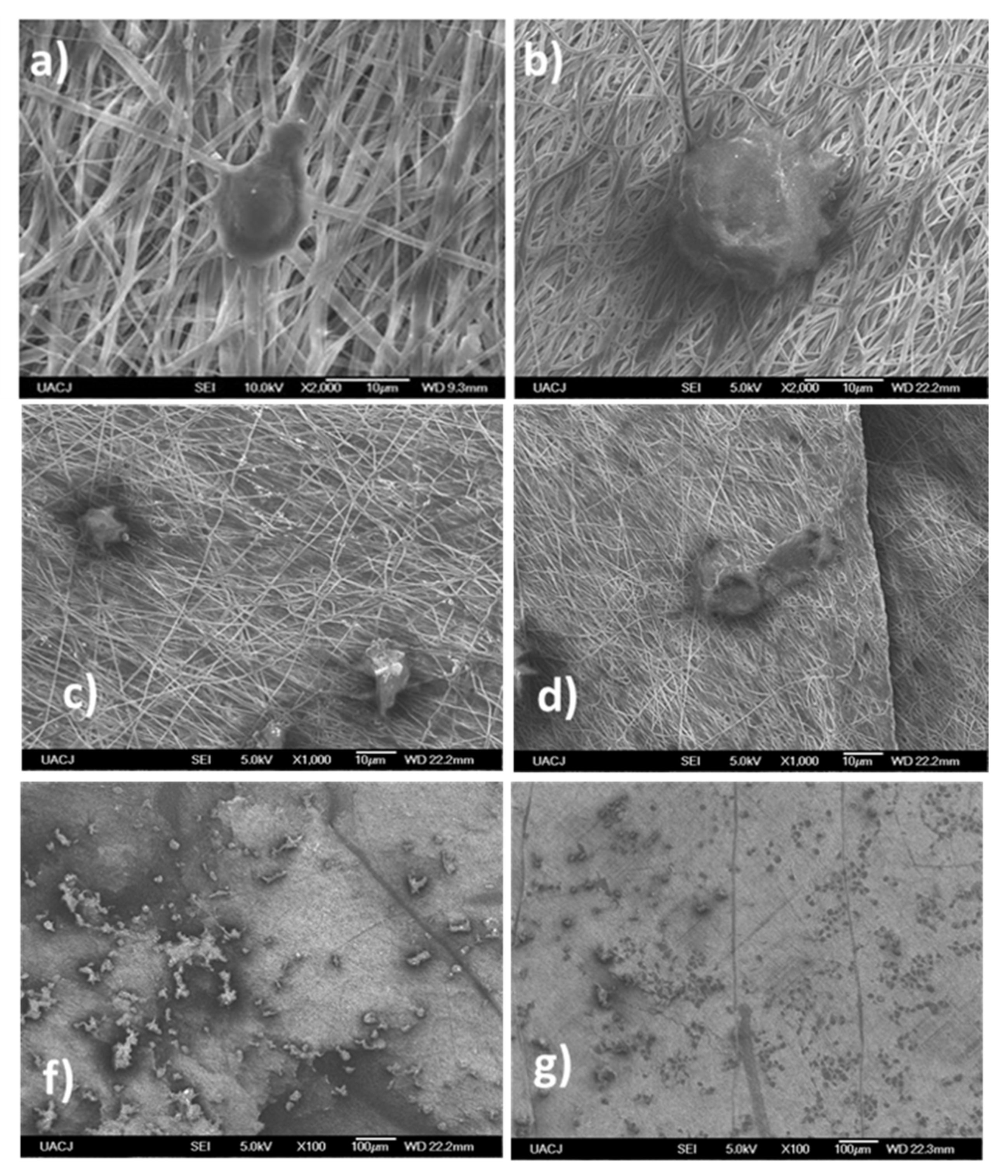
3.3. In Vivo Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jeckson, T.A.; Neo, Y.P.; Sisinthy, S.P.; Gorain, B. Delivery of therapeutics from layer-by-layer electrospun nanofiber matrix for wound healing: An update. J. Pharm. Sci. 2021, 110, 635–653. [Google Scholar] [CrossRef]
- Johnson, N.R.; Wang, Y. Controlled delivery of heparin-binding EGF-like growth factor yields fast and comprehensive wound healing. J. Control. Release 2013, 166, 124–129. [Google Scholar] [CrossRef]
- Martínez-Pérez, C.A. Electrospinning: A promising technique for drug delivery systemsm. Rev. Adv. Mater. Sci. 2020, 59, 441–454. [Google Scholar] [CrossRef]
- Hasan, A.; Soliman, S.; El Hajj, F.; Tseng, Y.T.; Yalcin, H.C.; Marei, H.E. Fabrication and in Vitro Characterization of a Tissue Engineered PCL-PLLA Heart Valve. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]
- Malikmammadov, E.; Tanir, T.E.; Kiziltay, A.; Hasirci, V.; Hasirci, N. PCL and PCL-based materials in biomedical applications. J. Biomater. Sci. Polym. Ed. 2018, 29, 863–893. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Blum, K.; Musgrave, A.; Onwuka, K.; Yi, T.; Reinhardt, J.; Best, C.; Breuer, C. Degradation and in vivo evaluation of polycaprolactone, poly(ε-caprolactone-co-L-lactide), and poly-L-lactic acid as scaffold sealant polymers for murine tissue-engineered vascular grafts. Regen Med. 2019, 14, 627–637. [Google Scholar] [CrossRef]
- Shuai, C.; Yang, W.; Feng, P.; Peng, S.; Pan, H. Accelerated degradation of HAP/PLLA bone scaffold by PGA blending facilitates bioactivity and osteoconductivity. Bioact. Mater. 2021, 6, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.T.; Gonçalves, A.I.; Babo, P.S.; Gomez-Florit, M.; Reis, R.L.; Gomes, M.E. Bioinspired materials and tissue engineering approaches applied to the regeneration of musculoskeletal tissues. In Engineering Strategies for Regenerative Medicine; Elsevier: London, UK, 2020; pp. 73–105. [Google Scholar]
- Hall, H. Modified Fibrin Hydrogel Matrices: Both, 3D-Scaffolds and Local and Controlled Release Systems to Stimulate Angiogenesis. Curr. Pharm. Des. 2007, 13, 3597–3607. [Google Scholar] [CrossRef]
- Rice, J.J.; Martino, M.M.; De Laporte, L.; Tortelli, F.; Briquez, P.S.; Hubbell, J.A. Engineering the Regenerative Microenvironment with Biomaterials. Adv. Healthc. Mater. 2013, 2, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Kai, D.; Liu, S.; Huang, X.; Heng, S.; Zhao, J.; Chan, B.Q.Y.; Loh, X.J.; Zhu, Y.; Mao, C.; et al. Mechanically cartilage-mimicking poly(PCL-PTHF urethane)/collagen nanofibers induce chondrogenesis by blocking NF-kappa B signaling pathway. Biomaterials 2018, 178, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Wang, P.; Zhang, X.; Xiang, C.; Li, L. Preparation of hierarchically structured PCL superhydrophobic membrane via alternate electrospinning/electrospraying techniques. J. Polym. Sci. Part. B Polym. Phys. 2019, 57, 421–430. [Google Scholar] [CrossRef]
- Moyers-Montoya, E.; García-Casillas, P.; Vargas-Requena, C.; Escobedo-González, R.; Martel-Estrada, S.-A.; Martínez-Pérez, C.A. Polycaprolactone/amino-β-cyclodextrin inclusion complex prepared by an electrospinning technique. Polymers 2016, 8, 395. [Google Scholar] [CrossRef]
- Suk, J.; Kam, C.; Leong, W.; Sang Yooa, H. In vivo wound healing of diabetic ulcers using electrospun nanofibers immobilized with human epidermal growth factor (EGF). Biomaterials 2008, 29, 587–596. [Google Scholar]
- Kawaguchi, Y.; Nishiyama, T.; Okada, M.; Kamachi, M.; Harada, A. Complex Formation of Poly (e-caprolactone) with Cyclodextrins. Macromolecules 2000, 33, 4472–4477. [Google Scholar] [CrossRef]
- Zhan, J.; Singh, A.; Zhang, Z.; Huang, L.; Elisseeff, J.H. Multifunctional aliphatic polyester nanofibers for tissue engineering. Biomatter 2012, 2, 202–212. [Google Scholar] [CrossRef]
- Poli, E.; Chaleix, V.; Damia, C.; Hjezi, Z.; Champion, E.; Sol, V. Efficient quantification of primary amine functions grafted onto apatite ceramics by using two UV-Vis spectrophotometric methods. Anal. Methods 2014, 6, 9622–9627. [Google Scholar] [CrossRef]
- Zhu, Y.; Gao, C.; Liu, X.; Shen, J. Surface Modification of Polycaprolactone Membrane via Aminolysis and Biomacromolecule Immobilization for Promoting Cytocompatibility of Human Endothelial Cells. Biomacromolecules 2002, 3, 1312–1319. [Google Scholar] [CrossRef]
- Singh, R.S.; Chauhan, K.; Kennedy, J.F. Immobilization of fungal inulinase on hetero-functionalized carbon nanofibers for the production of fructose from inulin. LWT 2019, 116, 108569. [Google Scholar] [CrossRef]
- Gomes, S.R.; Rodrigues, G.; Martins, G.G.; Roberto, M.A.; Mafra, M.; Henriques, C.M.R.; Silva, J.C. In vitro and in vivo evaluation of electrospun nanofibers of PCL, chitosan and gelatin: A comparative study. Mater. Sci. Eng. C 2015, 46, 348–358. [Google Scholar] [CrossRef]
- Wakabayashi, R.; Yahiro, K.; Hayashi, K.; Goto, M.; Kamiya, N. Protein-Grafted Polymers Prepared Through a Site-Specific Conjugation by Microbial Transglutaminase for an Immunosorbent Assay. Biomacromolecules 2017, 18, 422–430. [Google Scholar] [CrossRef]
- Tiǧli, R.S.; Gümüşderelioǧlu, M. Evaluation of RGD- or EGF-immobilized chitosan scaffolds for chondrogenic activity. Int. J. Biol. Macromol. 2008, 43, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Tiǧli, R.S.; Kazaroǧlu, N.M.; Maviş, B.; Gumusderelioglu, M. Cellular behavior on epidermal growth factor (EGF)-immobilized PCL/gelatin nanofibrous scaffolds. J. Biomater. Sci. Polym. Ed. 2011, 22, 207–223. [Google Scholar] [CrossRef] [PubMed]
- Ogino, S.; Morimoto, N.; Sakamoto, M.; Jinno, C.; Taira, T.; Suzuki, S. Efficacy of gelatin gel sheets sustaining epidermal growth factor for murine skin defects. J. Surg. Res. 2016, 201, 446–454. [Google Scholar] [CrossRef]
- Anklesaria, P.; Teixidó, J.; Laiho, M.; Pierce, J.H.; Greenberger, J.S.; Massagué, J. Cell-cell adhesion mediated by binding of membrane-anchored transforming growth factor alpha to epidermal growth factor receptors promotes cell proliferation. Proc. Natl. Acad. Sci. USA 1990, 87, 3289–3293. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.; Fredericks, P. Surface-enhanced Raman spectroscopy of amino acids adsorbed on an electrochemically prepared silver surface. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 1999, 55, 1641–1660. [Google Scholar] [CrossRef]
- Rygula, A.; Majzner, K.; Marzec, K.M.; Kaczor, A.; Pilarczyk, M.; Baranska, M. Raman spectroscopy of proteins: A review. J. Raman Spectrosc. 2013, 44, 1061–1076. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Escobedo-González, R.G.; Pérez Martínez, H.; Nicolás-Vázquez, M.I.; Martínez, J.; Gómez, G.; Serrano, J.N.; Carranza Téllez, V.; Vargas-Requena, C.L.; Miranda Ruvalcaba, R. Green production of indolylquinones, derivatives of perezone, and related molecules, promising antineoplastic compounds. J. Chem. 2016, 2016, 1–10. [Google Scholar] [CrossRef]
- Keay, S.; Zhang, C.O.; Shoenfelt, J.L.; Chai, T.C. Decreased in vitro proliferation of bladder epithelial cells from patients with interstitial cystitis. Urology 2003, 61, 1278–1284. [Google Scholar] [CrossRef]
- Narayanan, R.; Kenney, M.C.; Kamjoo, S.; Trinh, T.H.T.; Seigel, G.M.; Resende, G.P.; Kuppermann, B.D. Trypan blue: Effect on retinal pigment epithelial and neurosensory retinal cells. Investig. Ophthalmol. Vis. Sci. 2005, 46, 304–309. [Google Scholar] [CrossRef]
- Gelain, F.; Bottai, D.; Vescovi, A.; Zhang, S. Designer Self-Assembling Peptide Nanofiber Scaffolds for Adult Mouse Neural Stem Cell 3-Dimensional Cultures. PLoS ONE 2006, 1, e119. [Google Scholar] [CrossRef]
- Löbler, M.; Saß, M.; Kunze, C.; Schmitz, K.P.; Hopt, U.T. Biomaterial patches sutured onto the rat stomach induce a set of genes encoding pancreatic enzymes. Biomaterials 2002, 23, 577–583. [Google Scholar] [CrossRef]
- Kang, X.; Xie, Y.; Powell, H.M.; James Lee, L.; Belury, M.A.; Lannutti, J.J.; Kniss, D.A. Adipogenesis of murine embryonic stem cells in a three-dimensional culture system using electrospun polymer scaffolds. Biomaterials 2007, 28, 450–458. [Google Scholar] [CrossRef]
- Yang, Y.; Xia, T.; Zhi, W.; Wei, L.; Weng, J.; Zhang, C.; Li, X. Promotion of skin regeneration in diabetic rats by electrospun core-sheath fibers loaded with basic fibroblast growth factor. Biomaterials 2011, 32, 4243–4254. [Google Scholar] [CrossRef] [PubMed]
- Klinger, T. Image Processing with LabVIEW and IMAQ Vision; Prentice Hall Professional: Upper Saddle River, NJ, USA, 2003. [Google Scholar]
- Vazquez, N.; Kodosky, J.; Kudukoli, R.; Schultz, K.L.; Nair, D.; Caltagirone, C. System and Method for Automatically Generating a Graphical Program to Perform an Image Processing Algorithm. U.S. Patent US20010034881A1, 13 June 2006. [Google Scholar]
- Pandit, R.B.; Tang, J.; Liu, F.; Pitts, M. Development of a novel approach to determine heating pattern using computer vision and chemical marker (M-2) yield. J. Food Eng. 2007, 78, 522–528. [Google Scholar] [CrossRef]
- Suk Choi, J.; Sang Yoo, H. Electrospun Nanofibers Surface-modified with Fluorescent Proteins. J. Bioact. Compat. Polym. 2007, 22, 508–524. [Google Scholar] [CrossRef]
- Tobin, M.J.; Chesters, M.A.; Chalmers, J.M.; Rutten, F.J.M.; Fisher, S.E.; Symonds, I.M.; Hitchcock, A.; Allibone, R.; Dias-Gunasekara, S. Infrared microscopy of epithelial cancer cells in whole tissues and in tissue culture, using synchrotron radiation. Faraday Discuss. 2004, 126, 27. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Duan, Y.; Yu, J.; Lu, J. Preparation and immobilization of soluble eggshell membrane protein on the electrospun nanofibers to enhance cell adhesion and growth. J. Biomed. Mater. Res. Part A 2008, 86A, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Venyaminov, S.Y.; Kalnin, N. Quantitative IR spectrophotometry of peptide compounds in water (H2O) solutions. I. Spectral parameters of amino acid residue absorption bands. Biopolym. Orig. Res. Biomol. 1990, 30, 1243–1257. [Google Scholar] [CrossRef]
- Elumalai, S.; Managó, S.; De Luca, A.C. Raman Microscopy: Progress in Research on Cancer Cell Sensing. Sensors 2020, 20, 5525. [Google Scholar] [CrossRef]
- Razmshoar, P.; Bahrami, S.H.; Akbari, S. Functional hydrophilic highly biodegradable PCL nanofibers through direct aminolysis of PAMAM dendrimer. Int. J. Polym. Mater. Polym. Biomater. 2019, 69, 1069–1080. [Google Scholar] [CrossRef]
- Lee, K.-W.; Paek, S.-H.; Lien, A.; Durning, C.; Fukuro, H. Microscopic Molecular Reorientation of Alignment Layer Polymer Surfaces Induced by Rubbing and Its Effects on LC Pretilt Angles. Macromolecules 1996, 29, 8894–8899. [Google Scholar] [CrossRef]
- Rajesh, S.; Crandall, C.; Schneiderman, S.; Menkhaus, T.J. Cellulose-graft-polyethyleneamidoamine Anion-Exchange Nanofiber Membranes for Simultaneous Protein Adsorption and Virus Filtration. ACS Appl. Nano Mater. 2018, 1, 3321–3330. [Google Scholar] [CrossRef]
- Ma, Z.; Kotaki, M.; Yong, T.; He, W.; Ramakrishna, S. Surface engineering of electrospun polyethylene terephthalate (PET) nanofibers towards development of a new material for blood vessel engineering. Biomaterials 2005, 26, 2527–2536. [Google Scholar] [CrossRef]
- Yan, E.; Fan, Y.; Sun, Z.; Gao, J.; Hao, X.; Pei, S.; Wang, C.; Sun, L.; Zhang, D. Biocompatible core-shell electrospun nanofibers as potential application for chemotherapy against ovary cancer. Mater. Sci. Eng. C 2014, 41, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Freier, T.; Koh, H.S.; Kazazian, K.; Shoichet, M.S. Controlling cell adhesion and degradation of chitosan films by N-acetylation. Biomaterials 2005, 26, 5872–5878. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.G.; Kim, Y.J.; Park, W.H.; Cho, D.; Chung, H.Y.; Kwon, O.H. Surface modification of PHBV nanofiber mats for rapid cell cultivation and harvesting. J. Biomater. Sci. Polym. Ed. 2018, 29, 1026–1041. [Google Scholar] [CrossRef] [PubMed]
- Daamen, W.F.; Van Moerkerk, H.T.B.; Hafmans, T.; Buttafoco, L.; Poot, A.A.; Veerkamp, J.H.; Van Kuppevelt, T.H. Preparation and evaluation of molecularly-defined collagen-elastin-glycosaminoglycan scaffolds for tissue engineering. Biomaterials 2003, 24, 4001–4009. [Google Scholar] [CrossRef]
- Karakeçili, A.G.; Satriano, C.; Gümüşderelioǧlu, M.; Marletta, G. Enhancement of fibroblastic proliferation on chitosan surfaces by immobilized epidermal growth factor. Acta Biomater. 2008, 4, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Sarhan, W.A.; Azzazy, H.M.E. High concentration honey chitosan electrospun nanofibers: Biocompatibility and antibacterial effects. Carbohydr. Polym. 2015, 122, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.C.; Yeh, I.T.; Hsiao, H.Y. Development and Evaluation of Multistructured and Hierarchical Epidermal Growth Factor-Poly (epsilon-Caprolactone) Scaffolds. IEEE Trans. Nanobiosci. 2018, 18, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Xiuchun, L.I. Human lung epithelial cells A549 epithelial-mesenchymal transition induced by PVA/Collagen nanofiber. Colloids Surf. B Biointerfaces 2018, 162, 390–397. [Google Scholar]
- Arima, Y.; Iwata, H. Effect of wettability and surface functional groups on protein adsorption and cell adhesion using well-defined mixed self-assembled monolayers. Biomaterials 2007, 28, 3074–3082. [Google Scholar] [CrossRef] [PubMed]
- Min, B.M.; Lee, G.; Kim, S.H.; Nam, Y.S.; Lee, T.S.; Park, W.H. Electrospinning of silk fibroin nanofibers and its effect on the adhesion and spreading of normal human keratinocytes and fibroblasts in vitro. Biomaterials 2004, 25, 1289–1297. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moyers-Montoya, E.D.; Escobedo-González, R.G.; Vargas-Requena, C.L.; Garcia-Casillas, P.E.; Martínez-Pérez, C.A. Epithelial Growth Factor-Anchored on Polycaprolactone/6-deoxy-6-amino-β-cyclodextrin Nanofibers: In Vitro and In Vivo Evaluation. Polymers 2021, 13, 1303. https://doi.org/10.3390/polym13081303
Moyers-Montoya ED, Escobedo-González RG, Vargas-Requena CL, Garcia-Casillas PE, Martínez-Pérez CA. Epithelial Growth Factor-Anchored on Polycaprolactone/6-deoxy-6-amino-β-cyclodextrin Nanofibers: In Vitro and In Vivo Evaluation. Polymers. 2021; 13(8):1303. https://doi.org/10.3390/polym13081303
Chicago/Turabian StyleMoyers-Montoya, Edgar D., René Gerardo Escobedo-González, Claudia L. Vargas-Requena, Perla Elvia Garcia-Casillas, and Carlos A. Martínez-Pérez. 2021. "Epithelial Growth Factor-Anchored on Polycaprolactone/6-deoxy-6-amino-β-cyclodextrin Nanofibers: In Vitro and In Vivo Evaluation" Polymers 13, no. 8: 1303. https://doi.org/10.3390/polym13081303
APA StyleMoyers-Montoya, E. D., Escobedo-González, R. G., Vargas-Requena, C. L., Garcia-Casillas, P. E., & Martínez-Pérez, C. A. (2021). Epithelial Growth Factor-Anchored on Polycaprolactone/6-deoxy-6-amino-β-cyclodextrin Nanofibers: In Vitro and In Vivo Evaluation. Polymers, 13(8), 1303. https://doi.org/10.3390/polym13081303