Demineralized Dentin Matrix Particle-Based Bio-Ink for Patient-Specific Shaped 3D Dental Tissue Regeneration
Abstract
1. Introduction
2. Materials and Methods
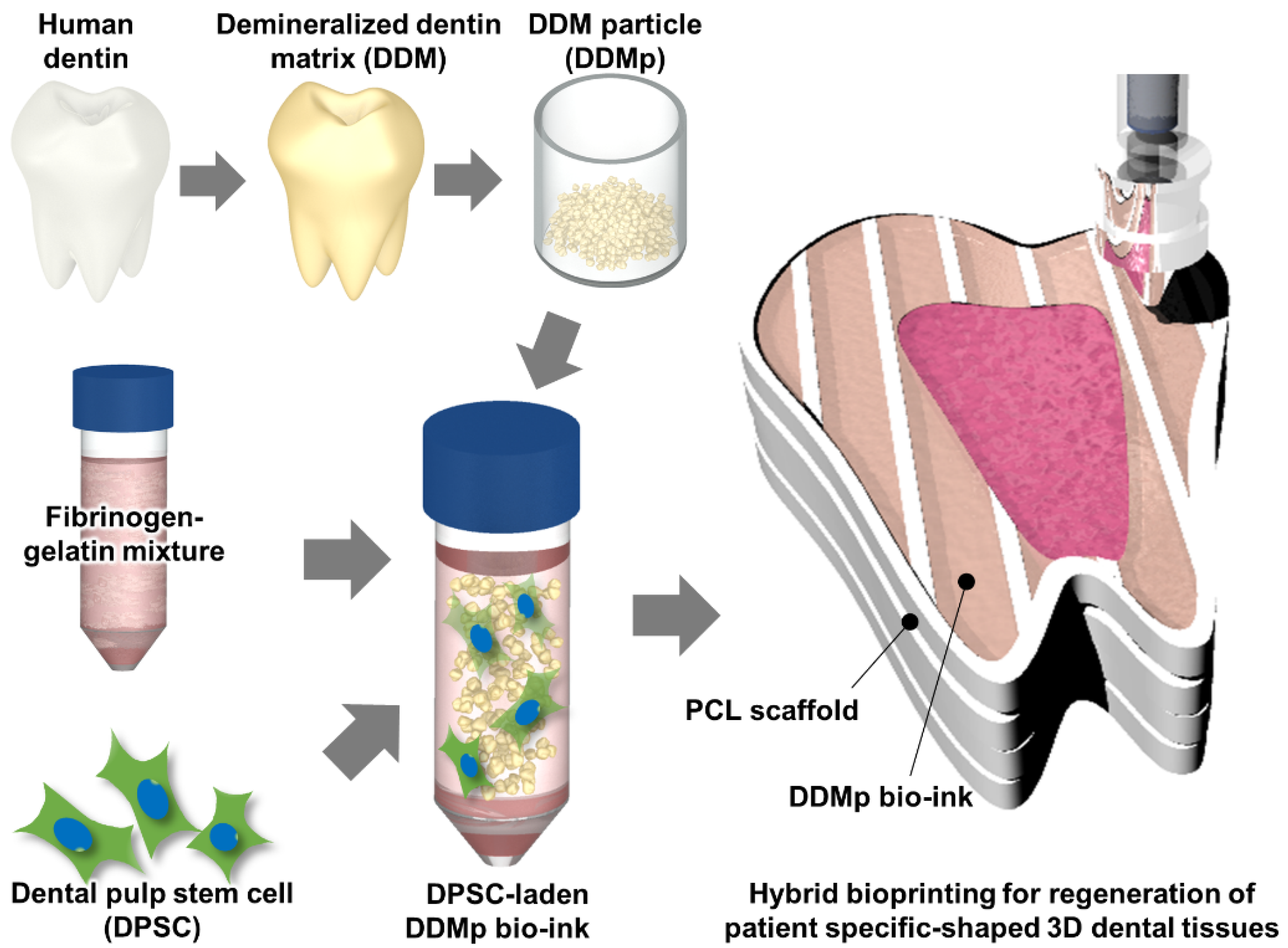
2.1. Preparation of DDMp Bio-Ink
2.2. Cell Culture and Cell-Laden Bio-Ink Preparation
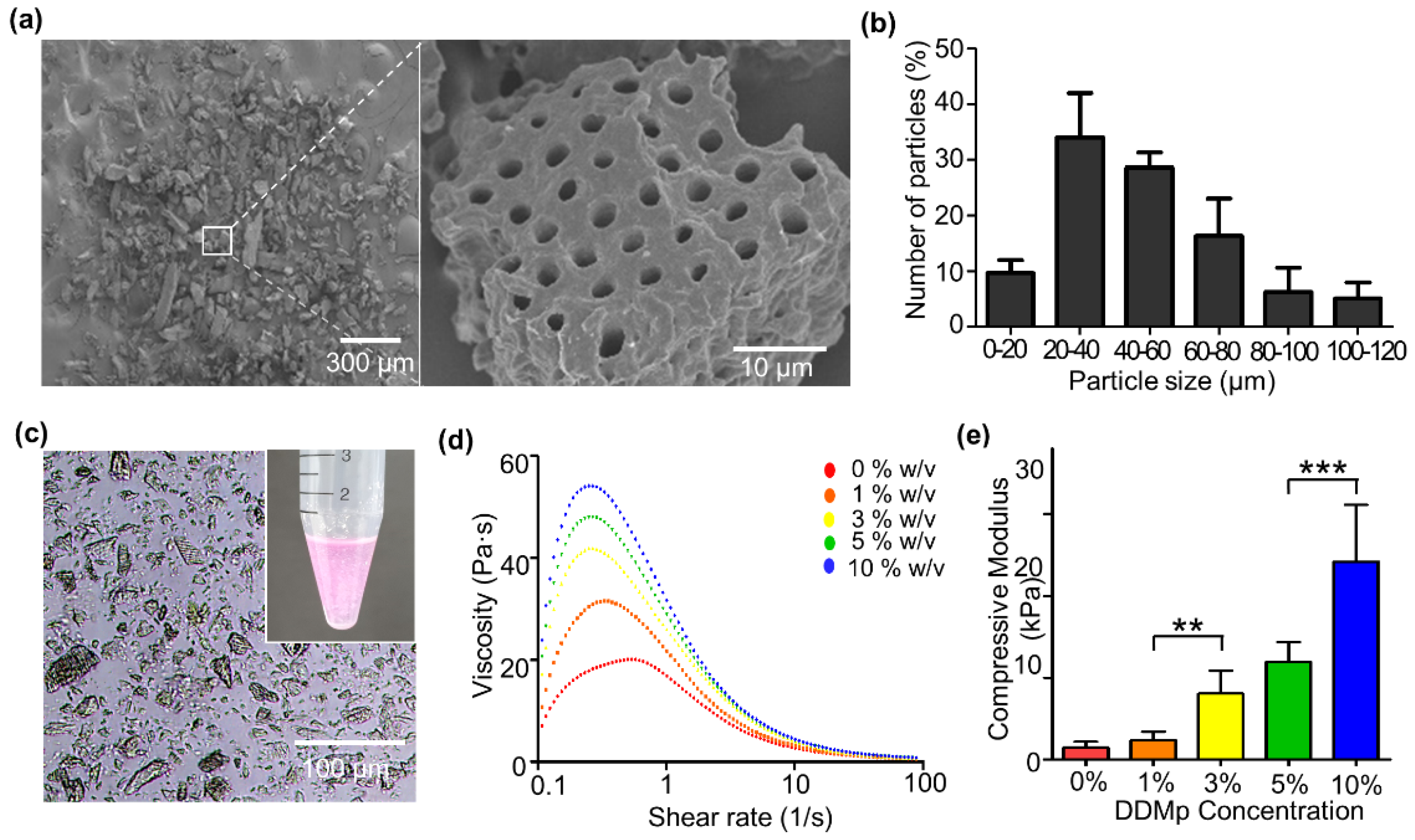
2.3. Compressive Modulus and Rheological Property Measurement
2.4. Scanning Electron Microscopy (SEM) Imaging
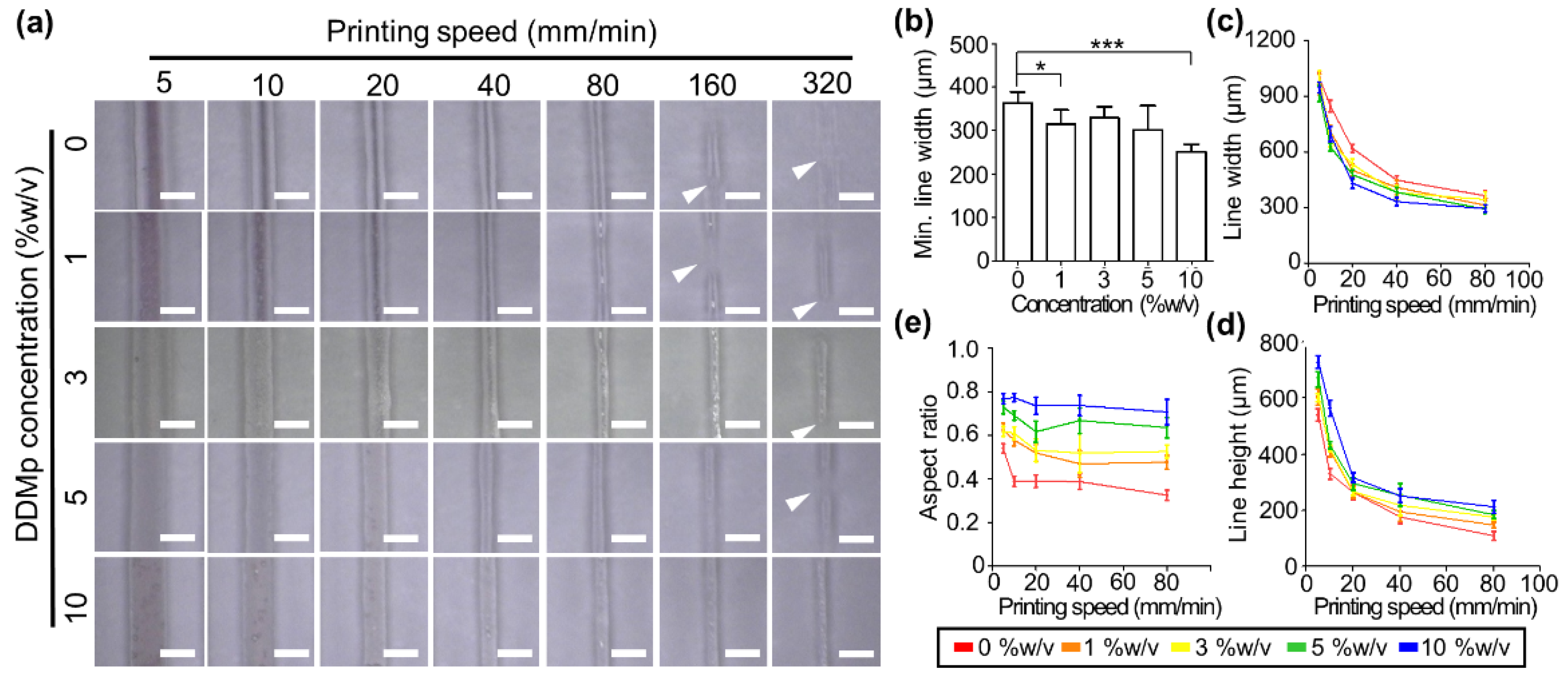
2.5. Bioprinting System and Printability Test
2.6. Cytocompatibility
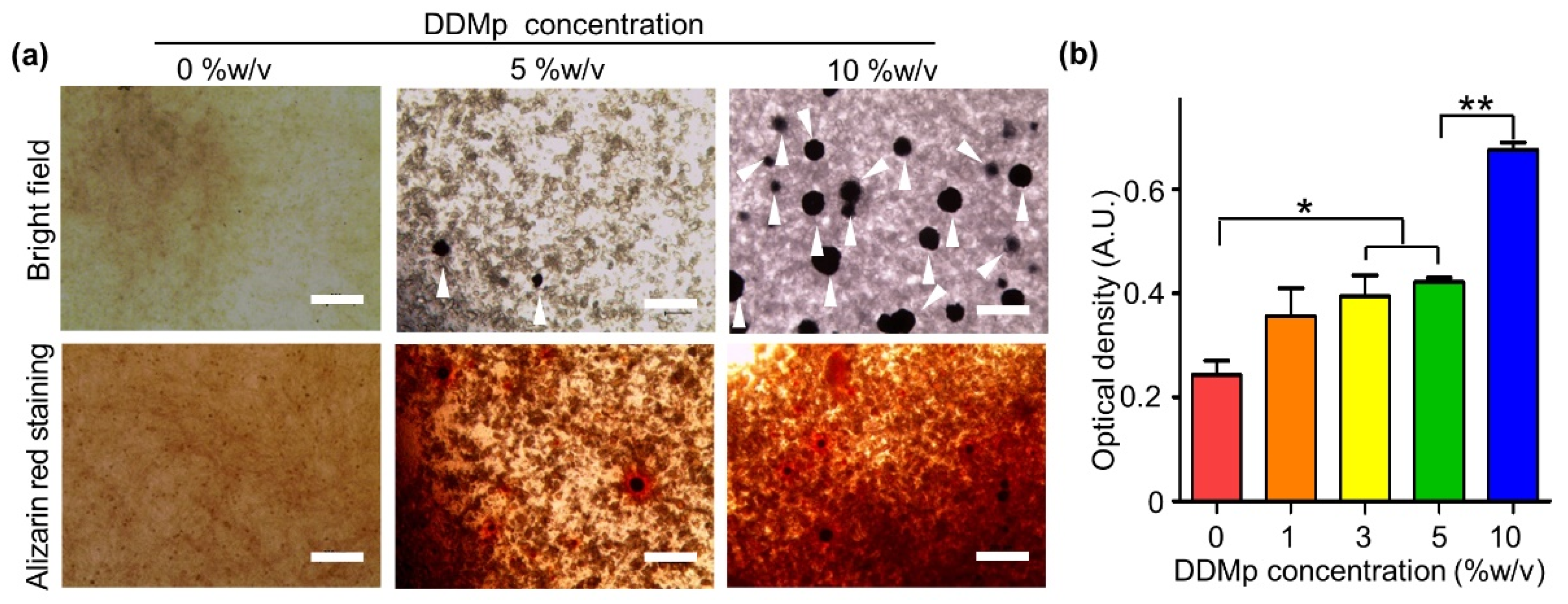
2.7. Alizarin Red and Alkaline Phosphate (AP) Staining
2.8. Immunofluorescence Staining
2.9. RT-qPCR
2.10. Bioprinting of 3D Tooth-Shaped Cellular Construct with DDMp Bio-Ink
2.11. Statistical Analysis
3. Results
3.1. Preparation and Characterization of DDMp Bio-Ink
3.2. Cytocompatibility of DDMp Bio-Ink
3.3. Odontogenic Differentiation of DPSCs in DDMp Bio-Ink
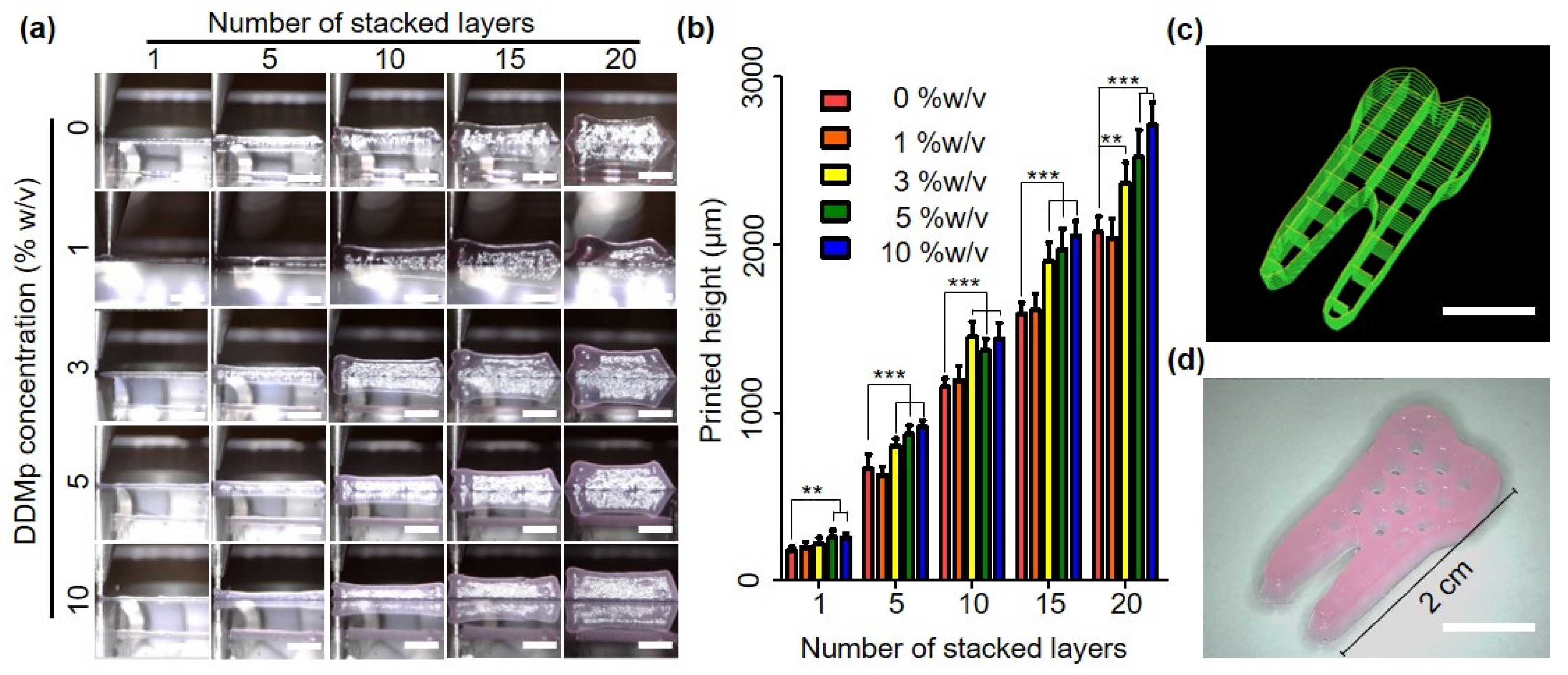
3.4. Printability of DDMp Bio-Ink
3.5. Bioprinting of Human Tooth-Shaped 3D Construct with DPSCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jung, R.E.; Pjetursson, B.E.; Glauser, R.; Zembic, A.; Zwahlen, M.; Lang, N.P. A systematic review of the 5-year survival and complication rates of implant-supported single crowns. Clin. Oral Implant. Res. 2008, 19, 119–130. [Google Scholar] [CrossRef]
- Greenstein, G.; Cavallaro, J.; Romanos, G.; Tarnow, D. Clinical recommendations for avoiding and managing surgical complications associated with implant dentistry: A review. J. Periodontol. 2008, 79, 1317–1329. [Google Scholar] [CrossRef]
- Cassetta, M.; Pranno, N.; Calasso, S.; Di Mambro, A.; Giansanti, M. Early peri-implant bone loss: A prospective cohort study. Int. J. Oral Maxillofac. Surg. 2015, 44, 1138–1145. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Jin, X.; Ma, H.; Hu, J.; Ni, L.; Ma, P.X. The odontogenic differentiation of human dental pulp stem cells on nanofibrous poly(L-lactic acid) scaffolds in vitro and in vivo. Acta Biomater. 2010, 6, 3856–3863. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.W.; Zhang, Y.F.; Sun, Z.Y.; Song, G.T.; Chen, Z. Dental pulp tissue engineering with bFGF-incorporated silk fibroin scaffolds. J. Biomater. Appl. 2015, 30, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Galler, K.M.; D’Souza, R.N.; Hartgerink, J.D.; Schmalz, G. Scaffolds for dental pulp tissue engineering. Adv. Dent. Res. 2011, 23, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Young, C.S.; Terada, S.; Vacanti, J.P.; Honda, M.; Bartlett, J.D.; Yelick, P.C. Tissue engineering of complex tooth structures on biodegradable polymer scaffolds. J. Dent. Res. 2002, 81, 695–700. [Google Scholar] [CrossRef]
- Yamamoto, H.; Kim, E.J.; Cho, S.W.; Jung, H.S. Analysis of tooth formation by reaggregated dental mesenchyme from mouse embryo. J. Electron. Microsc. 2003, 52, 559–566. [Google Scholar] [CrossRef]
- Duailibi, M.T.; Duailibi, S.E.; Young, C.S.; Bartlett, J.D.; Vacanti, J.P.; Yelick, P.C. Bioengineered teeth from cultured rat tooth bud cells. J. Dent. Res. 2004, 83, 523–528. [Google Scholar] [CrossRef]
- Hu, B.; Nadiri, A.; Kuchler-Bopp, S.; Perrin-Schmitt, F.; Peters, H.; Lesot, H. Tissue engineering of tooth crown, root, and periodontium. Tissue Eng. 2006, 12, 2069–2075. [Google Scholar] [CrossRef]
- Yelick, P.C.; Vacanti, J.P. Bioengineered teeth from tooth bud cells. Dent. Clin. N. Am. 2006, 50, 191–203. [Google Scholar] [CrossRef]
- Honda, M.J.; Tsuchiya, S.; Sumita, Y.; Sagara, H.; Ueda, M. The sequential seeding of epithelial and mesenchymal cells for tissue-engineered tooth regeneration. Biomaterials 2007, 28, 680–689. [Google Scholar] [CrossRef]
- Ono, M.; Oshima, M.; Ogawa, M.; Sonoyama, W.; Hara, E.S.; Oida, Y.; Shinkawa, S.; Nakajima, R.; Mine, A.; Hayano, S.; et al. Practical whole-tooth restoration utilizing autologous bioengineered tooth germ transplantation in a postnatal canine model. Sci. Rep. 2017, 7, 44522. [Google Scholar] [CrossRef]
- Schmidt-Schultz, T.H.; Schultz, M. Intact growth factors are conserved in the extracellular matrix of ancient human bone and teeth: A storehouse for the study of human evolution in health and disease. Biol. Chem. 2005, 386, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Um, I.W.; Kim, Y.K.; Mitsugi, M. Demineralized dentin matrix scaffolds for alveolar bone engineering. J. Indian Prosthodont. Soc. 2017, 17, 120–127. [Google Scholar] [CrossRef]
- Chen, J.; Cui, C.; Qiao, X.; Yang, B.; Yu, M.; Guo, W.; Tian, W. Treated dentin matrix paste as a novel pulp capping agent for dentin regeneration. J. Tissue Eng. Regen. Med. 2017, 11, 3428–3436. [Google Scholar] [CrossRef]
- Gao, X.L.; Qin, W.; Wang, P.; Wang, L.; Weir, M.D.; Reynolds, M.A.; Zhao, L.; Lin, Z.M.; Xu, H.H.K. Nano-structured demineralized human dentin matrix to enhance bone and dental repair and regeneration. Appl. Sci. 2019, 9, 1013. [Google Scholar] [CrossRef]
- Avery, S.J.; Sadaghiani, L.; Sloan, A.J.; Waddington, R.J. Analysing the bioactive makeup of demineralised dentine matrix on bone marrow mesenchymal stem cells for enhanced bone repair. Eur. Cell Mater. 2017, 34, 1–14. [Google Scholar] [CrossRef]
- Li, R.; Guo, W.; Yang, B.; Guo, L.; Sheng, L.; Chen, G.; Li, Y.; Zou, Q.; Xie, D.; An, X.; et al. Human treated dentin matrix as a natural scaffold for complete human dentin tissue regeneration. Biomaterials 2011, 32, 4525–4538. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Chen, J.; Yang, B.; Li, L.; Luo, X.; Zhang, X.; Feng, L.; Jiang, Z.; Yu, M.; Guo, W.; et al. Combination of aligned PLGA/Gelatin electrospun sheets, native dental pulp extracellular matrix and treated dentin matrix as substrates for tooth root regeneration. Biomaterials 2015, 52, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Athirasala, A.; Tahayeri, A.; Thrivikraman, G.; Franca, C.M.; Monteiro, N.; Tran, V.; Ferracane, J.; Bertassoni, L.E. A dentin-derived hydrogel bioink for 3D bioprinting of cell laden scaffolds for regenerative dentistry. Biofabrication 2018, 10, 024101. [Google Scholar] [CrossRef]
- Chun, S.Y.; Lee, H.J.; Choi, Y.A.; Kim, K.M.; Baek, S.H.; Park, H.S.; Kim, J.Y.; Ahn, J.M.; Cho, J.Y.; Cho, D.W.; et al. Analysis of the soluble human tooth proteome and its ability to induce dentin/tooth regeneration. Tissue Eng. Part A 2011, 17, 181–191. [Google Scholar] [CrossRef]
- Chun, S.Y.; Acharya, B.; Lee, H.J.; Kwon, T.G.; Oh, S.H.; Lee, J.H.; Shin, H.I.; Park, E.K. Composite Scaffold with Demineralized Dentin Particle and Poly (Lactic Co-Glycolic Acid) for Cranial Bone Regeneration. Tissue Eng. Regen. Med. 2011, 8, 306–313. [Google Scholar]
- Kang, H.W.; Lee, S.J.; Ko, I.K.; Kengla, C.; Yoo, J.J.; Atala, A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 2016, 34, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Barone, S.; Neri, P.; Paoli, A.; Razionale, A.V. Design and manufacturing of patient-specific orthodontic appliances by computer-aided engineering techniques. Proc. Inst. Mech. Eng. H 2018, 232, 54–66. [Google Scholar] [CrossRef]
- Thilander, B.; Pena, L.; Infante, C.; Parada, S.S.; de Mayorga, C. Prevalence of malocclusion and orthodontic treatment need in children and adolescents in Bogota, Colombia. An epidemiological study related to different stages of dental development. Eur. J. Orthod. 2001, 23, 153–167. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Sueishi, K. Malocclusion associated with abnormal posture. Bull. Tokyo Dent. Coll. 2003, 44, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Honigmann, P.; Sharma, N.; Okolo, B.; Popp, U.; Msallem, B.; Thieringer, F.M. Patient-specific surgical implants made of 3D printed PEEK: Material, technology, and scope of surgical application. Biomed. Res. Int. 2018, 2018. [Google Scholar] [CrossRef]
- Visscher, D.O.; Farre-Guasch, E.; Helder, M.N.; Gibbs, S.; Forouzanfar, T.; van Zuijlen, P.P.; Wolff, J. Advances in bioprinting technologies for craniofacial reconstruction. Trends Biotechnol. 2016, 34, 700–710. [Google Scholar] [CrossRef]
- Liu, G.; Xu, G.; Gao, Z.; Liu, Z.; Xu, J.; Wang, J.; Zhang, C.; Wang, S. Demineralized dentin matrix induces odontoblastic differentiation of dental pulp stem cells. Cells Tissues Organs 2016, 201, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Bakopoulou, A.; Leyhausen, G.; Yolk, J.; Tsiftsoglou, A.; Garefis, P.; Koidis, P.; Geurtsen, W. Comparative analysis of in vitro osteo/odontogenic differentiation potential of human dental pulp stem cells (DPSCs) and stem cells from the apical papilla (SCAP). Arch. Oral Biol. 2011, 56, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Ching, H.S.; Luddin, N.; Ab Rahman, I.; Ponnuraj, K.T. Expression of odontogenic and osteogenic markers in DPSCs and SHED: A review. Curr. Stem Cell Res. Ther. 2017, 12, 71–79. [Google Scholar] [CrossRef]
- Eslaminejad, M.B.; Bordbar, S.; Nazarian, H. Odontogenic differentiation of dental pulp-derived stem cells on tricalcium phosphate scaffolds. J. Dent. Sci. 2013, 8, 306–313. [Google Scholar] [CrossRef]
- Gibson, M.P.; Zhu, Q.L.; Wang, S.Z.; Liu, Q.L.; Liu, Y.; Wang, X.F.; Yuan, B.Z.; Ruest, L.B.; Feng, J.Q.; D’Souza, R.N.; et al. The rescue of dentin matrix protein 1 (DMP1)-deficient tooth defects by the transgenic expression of dentin sialophosphoprotein (DSPP) indicates that DSPP is a downstream effector molecule of DMP1 in dentinogenesis. J. Biol. Chem. 2013, 288, 7204–7214. [Google Scholar] [CrossRef]
- Sabbagh, J.; Ghassibe-Sabbagh, M.; Fayyad-Kazan, M.; Al-Nemer, F.; Fahed, J.C.; Berberi, A.; Badran, B. Differences in osteogenic and odontogenic differentiation potential of DPSCs and SHED. J. Dent. 2020, 101. [Google Scholar] [CrossRef]
- Zhu, Q.L.; Gibson, M.P.; Liu, Q.L.; Liu, Y.; Lu, Y.B.; Wang, X.F.; Feng, J.Q.; Qin, C.L. Proteolytic processing of dentin sialophosphoprotein (DSPP) is essential to dentinogenesis. J. Biol. Chem. 2012, 287, 30426–30435. [Google Scholar] [CrossRef]
- Al-Sharabi, N.; Xue, Y.; Fujio, M.; Ueda, M.; Gjerde, C.; Mustafa, K.; Fristad, I. Bone marrow stromal cell paracrine factors direct osteo/odontogenic differentiation of dental pulp cells. Tissue Eng. Part A 2014, 20, 3063–3072. [Google Scholar] [CrossRef]
- Galler, K.M.; Widbiller, M.; Buchalla, W.; Eidt, A.; Hiller, K.A.; Hoffer, P.C.; Schmalz, G. EDTA conditioning of dentine promotes adhesion, migration and differentiation of dental pulp stem cells. Int. Endod. J. 2016, 49, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Raveendran, N.T.; Vaquette, C.; Meinert, C.; Ipe, D.S.; Ivanovski, S. Optimization of 3D bioprinting of periodontal ligament cells. Dent. Mater. 2019, 35, 1683–1694. [Google Scholar] [CrossRef] [PubMed]
- Miri, A.K.; Mirzaee, I.; Hassan, S.; Oskui, S.M.; Nieto, D.; Khademhosseini, A.; Zhang, Y.S. Effective bioprinting resolution in tissue model fabrication. Lab. Chip. 2019, 19, 2019–2037. [Google Scholar] [CrossRef]
- Morgan, F.L.C.; Moroni, L.; Baker, M.B. Dynamic bioinks to advance bioprinting. Adv. Healthc. Mater. 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Shelton, R.M.; Perrie, Y.; Harris, J.J. An initial evaluation of gellan gum as a material for tissue engineering applications. J. Biomater. Appl. 2007, 22, 241–254. [Google Scholar] [CrossRef]
- Akkineni, A.R.; Ahlfeld, T.; Funk, A.; Waske, A.; Lode, A.; Gelinsky, M. Highly concentrated alginate-gellan gum composites for 3D plotting of complex tissue engineering scaffolds. Polymers 2016, 8, 170. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Kim, D.; Jang, I.; Kim, H.R.; Kang, H.W. Bioprinting of three-dimensional dentin-pulp complex with local differentiation of human dental pulp stem cells. J. Tissue Eng. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Noh, S.; Kim, K.; Kim, J.I.; Shin, J.H.; Kang, H.W. Direct-write printing for producing biomimetic patterns with self-aligned neurites. Addit. Manuf. 2020, 32. [Google Scholar] [CrossRef]
- Jeon, S.; Heo, J.H.; Kim, M.K.; Jeong, W.; Kang, H.W. High-precision 3D bio-dot printing to improve paracrine interaction between multiple types of cell spheroids. Adv. Funct. Mater. 2020, 30. [Google Scholar] [CrossRef]
- Craig, R.G.; Peyton, F.A. Elastic and mechanical properties of human dentin. J. Dent. Res. 1958, 37, 710–718. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, J.; Jeong, W.; Kim, M.-K.; Nam, S.-H.; Park, E.-K.; Kang, H.-W. Demineralized Dentin Matrix Particle-Based Bio-Ink for Patient-Specific Shaped 3D Dental Tissue Regeneration. Polymers 2021, 13, 1294. https://doi.org/10.3390/polym13081294
Han J, Jeong W, Kim M-K, Nam S-H, Park E-K, Kang H-W. Demineralized Dentin Matrix Particle-Based Bio-Ink for Patient-Specific Shaped 3D Dental Tissue Regeneration. Polymers. 2021; 13(8):1294. https://doi.org/10.3390/polym13081294
Chicago/Turabian StyleHan, Jonghyeuk, Wonwoo Jeong, Min-Kyeong Kim, Sang-Hyeon Nam, Eui-Kyun Park, and Hyun-Wook Kang. 2021. "Demineralized Dentin Matrix Particle-Based Bio-Ink for Patient-Specific Shaped 3D Dental Tissue Regeneration" Polymers 13, no. 8: 1294. https://doi.org/10.3390/polym13081294
APA StyleHan, J., Jeong, W., Kim, M.-K., Nam, S.-H., Park, E.-K., & Kang, H.-W. (2021). Demineralized Dentin Matrix Particle-Based Bio-Ink for Patient-Specific Shaped 3D Dental Tissue Regeneration. Polymers, 13(8), 1294. https://doi.org/10.3390/polym13081294






