Exploring the Potential Application of Matrimid® and ZIFs-Based Membranes for Hydrogen Recovery: A Review
Abstract
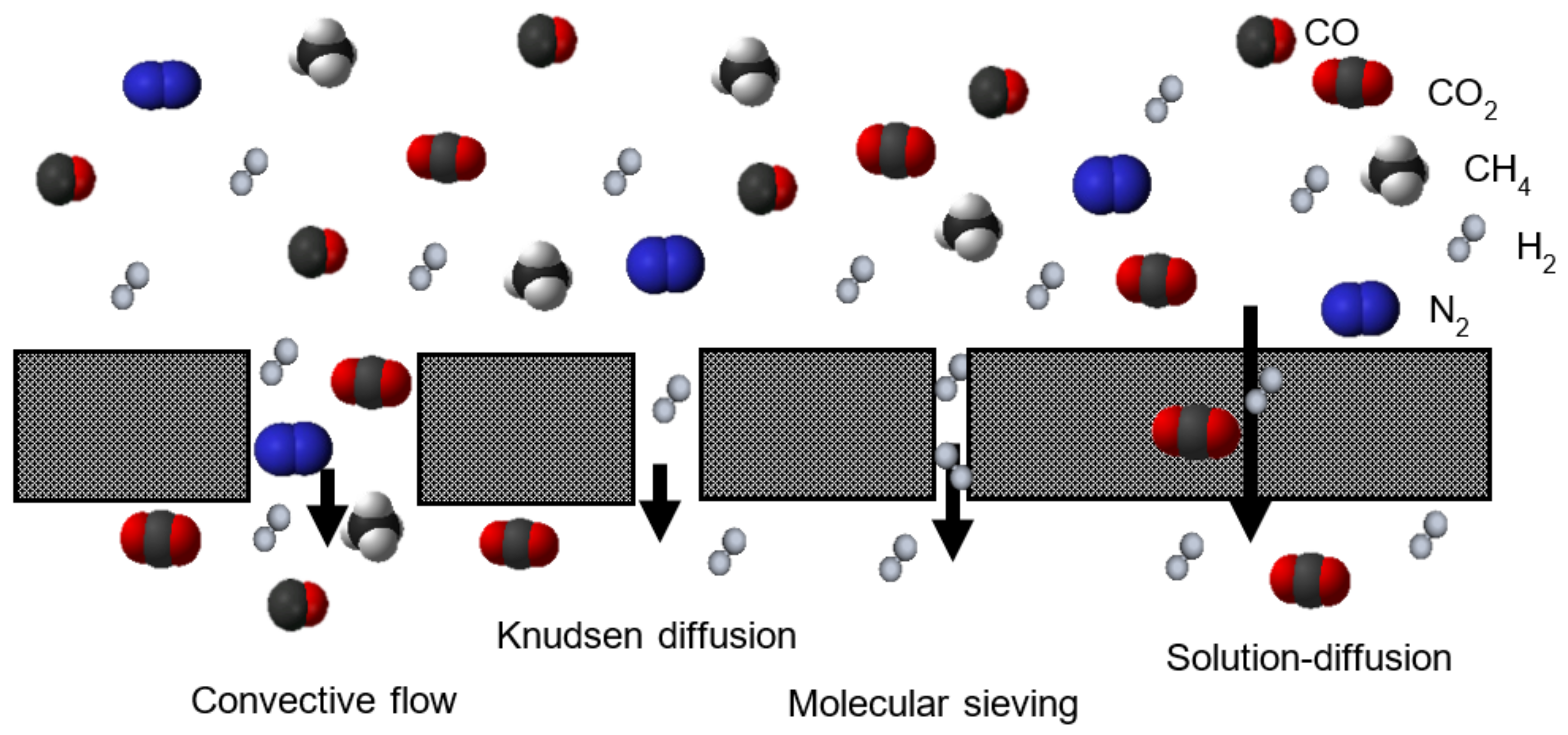
1. Introduction
2. Pristine and Mixed Matrix Membranes Based on Matrimid®/ZIF
2.1. Membrane Preparation Techniques
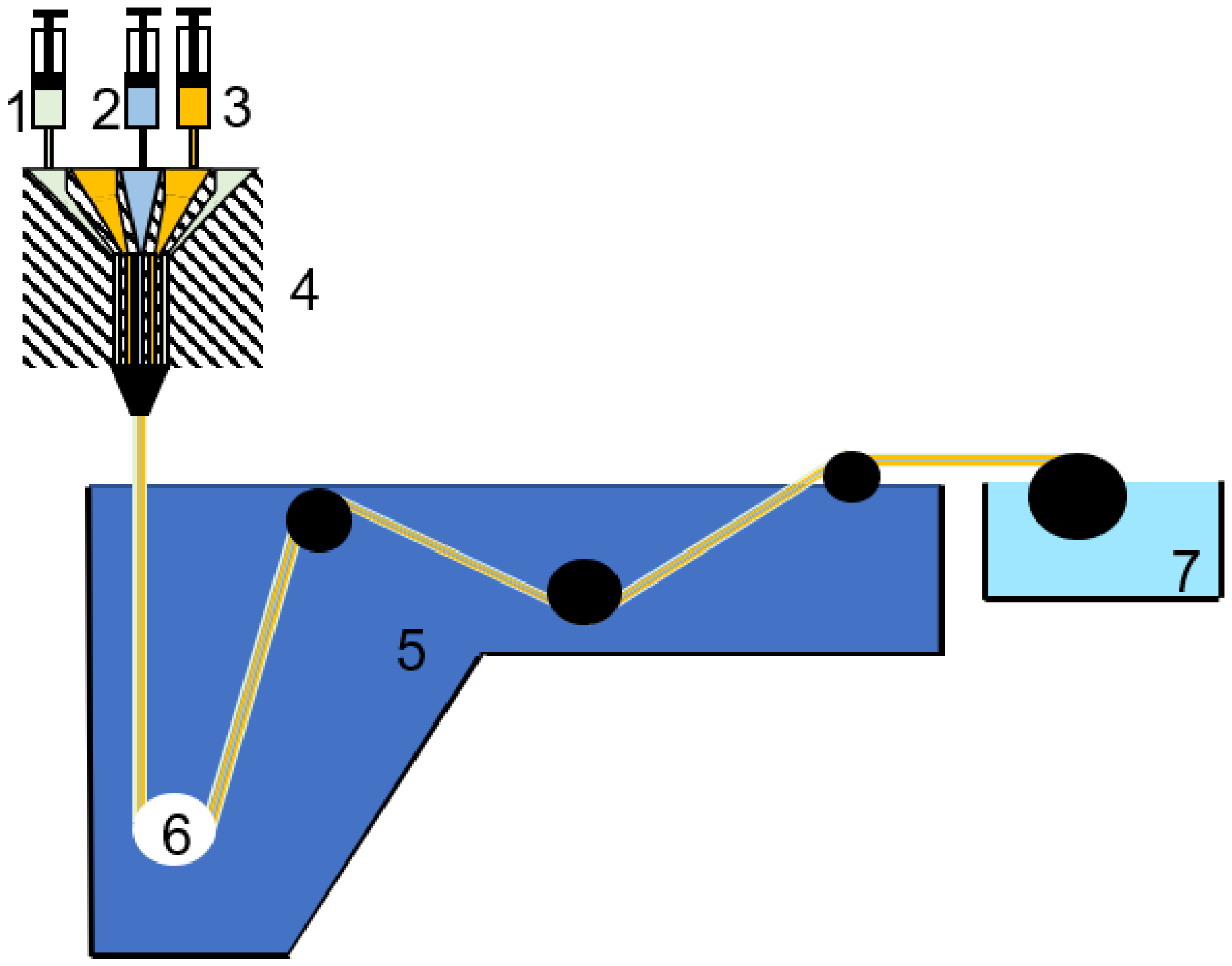
2.1.1. Flat Sheet Membranes
2.1.2. Hollow Fiber Membranes
2.2. Material and Thermo-Mechanical Characterization
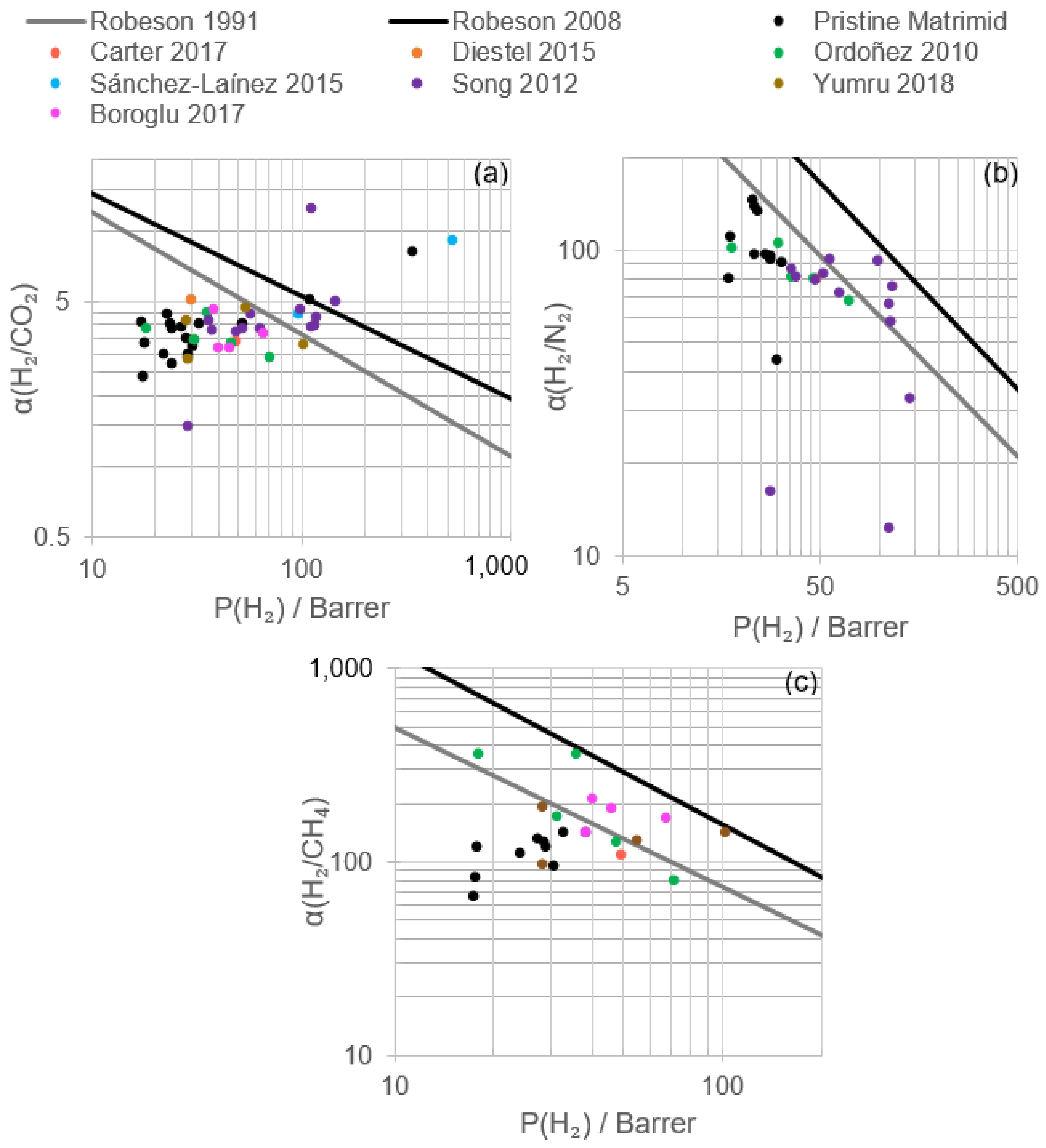
2.3. Properties and Permeation Results of Pristine Matrimid® Membrane
2.3.1. Flat Sheet Membranes
| Ref. | T (°C) | ΔP (Bar) | PH2(Barrer) | PN2(Barrer) | PCO2(Barrer) | PCH4(Barrer) | αH2/N2 | αH2/CO2 | αH2/CH4 | Solvent Used for Casting/Permeation Method |
|---|---|---|---|---|---|---|---|---|---|---|
| Carter (2017) [48] | 35 | 3.0 | 30.3 | 0.70 | 9.5 | 0.32 | 43.2 | 3.2 | 94.6 | DMF/time-lag |
| David (2011) [31,47] | 30 | 2.0 | 24.1 | 0.18 | 6.4 | 133.9 | 3.8 | DCM/continuous permeation with sweep gas | ||
| 30 | 4.0 | 23.7 | 0.17 | 5.7 | 138.8 | 4.0 | ||||
| 30 | 6.0 | 23.1 | 0.16 | 5.2 | 144.4 | 4.4 | ||||
| Diestel (2015) [46] | 25 | 0.2 | 28.0 | 8.0 | 3.5 | DCM/continuous permeation with sweep gas | ||||
| Esposito (2019) [63] | 25 | 1.0 | 21.9 | 0.19 | 8.6 | 115.3 | 2.5 | 0.2 | 128.8 | DCM/time-lag |
| Hosseini (2008) [45] | 35 | H2: 3.5 | 27.2 | 0.28 | 7.0 | 0.21 | 97.0 | 3.9 | 129.3 | NMP/time-lag |
| Other gases: 10.0 | ||||||||||
| Mirzaei (2020) [44] | 25 | 4.0 | 28.7 | 0.31 | 9.8 | 0.23 | 92.4 | 2.9 | 124.8 | NMP/time-lag |
| Ordoñez (2010) [43] | 35 | 1.7 | 28.9 | 0.31 | 9.5 | 0.24 | 95.1 | 3.0 | 120.4 | chloroform/time-lag |
| Sánchez-Laínez (2015) [58] | 35 | 2.0 | 22.0 | 7.3 | 3.0 | chloroform/continuous permeation with sweep gas | ||||
| 100 | 53.0 | 13.3 | 4.0 | |||||||
| 150 | 110.0 | 22.0 | 5.0 | |||||||
| 200 | 340.0 | 42.5 | 8.0 | |||||||
| Shishatskiy (2006) [50] | 20–80 | 0.3 | 24.0 | 0.25 | 9.8 | 0.22 | 96.0 | 2.7 | 109.1 | THF, NMP, G-BL, i-propanol, n-butanol, acetic acid and toluene/time-lag |
| Song (2012) [42] | 22 | 4.0 | 32.7 | 0.36 | 8.1 | 0.23 | 90.9 | 4.1 | 142.1 | chloroform/time-lag. Annealed at 230 °C |
| Weigelt 2018 [64] | 30 | 1.0 | 31.6 | 0.3 | 12.3 | 105.3 | 2.6 | 0.3 | 92.9 | chloroform/time-lag |
| Yumru (2018) [62] | 35 | 4.0 | 17.3 | 4.2 | 0.30 | 4.1 | 66.5 | NMP/time-lag | ||
| Zhang (2008) [49] | 25 | 2.0 | 17.5 | 0.22 | 7.3 | 0.21 | 79.6 | 2.4 | 83.3 | TCE/time-lag |
| Zhao (2008) [65] | 35 | 2.0 | 17.8 | 0.16 | 8.9 | 0.15 | 110.9 | 3.3 | 118.7 | THF/time-lag |
2.3.2. Hollow Fiber Membranes
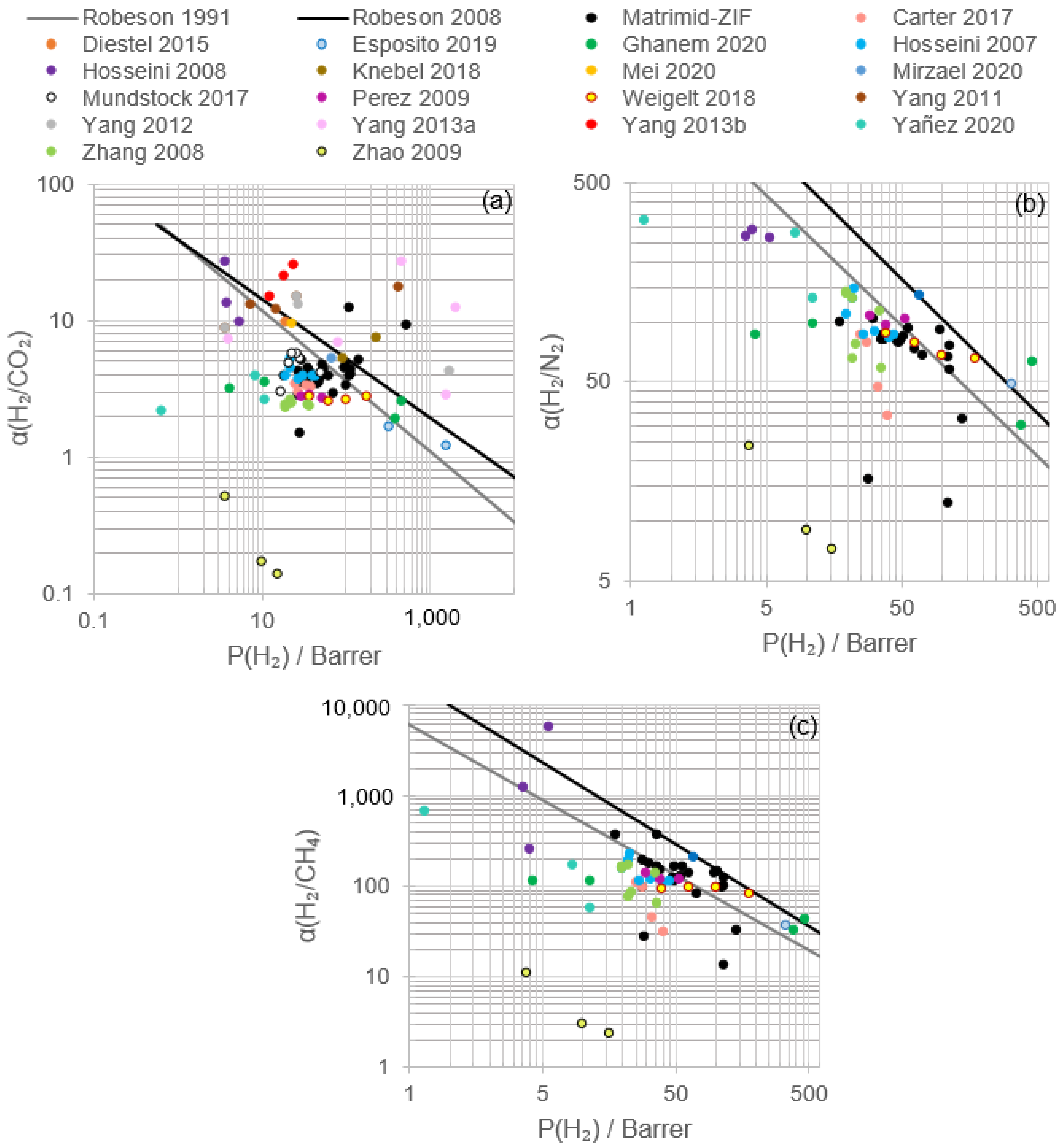
2.4. Effect of ZIF Addition on Matrimid® MMM’s Performance
2.4.1. Flat Sheet Membranes
| Ref. | T (°C) | ΔP (Bar) | PH2 (Barrer) | PN2 (Barrer) | PCO2 (Barrer) | PCH4 (Barrer) | αH2/N2 | αH2/CO2 | αH2/CH4 | ZIF Load (wt.%/v.%) | ZIF | Comments |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Boroglu (2017) [77] | 35 | 4.0 | 38.3 | 8.3 | 0.27 | 4.6 | 141.9 | 10 | ZIF-12 | |||
| 67.2 | 18.6 | 0.40 | 3.6 | 168.0 | 20 | |||||||
| 46.2 | 14.6 | 0.25 | 3.2 | 184.8 | 30 | |||||||
| 40.2 | 12.7 | 0.19 | 3.2 | 211.6 | 40 | |||||||
| Carter (2017) [48] | 35 | 3.0 | 48.7 | 0.61 | 14.3 | 0.45 | 79.8 | 3.4 | 108.2 | 10 | ZIF-8 | |
| Diestel (2015) [46] | 25 | 0.2 | 31.0 | 9.0 | 3.4 | 25 | ZIF-8 | |||||
| 30.0 | 6.0 | 5.0 | 25 | ZIF-90 | ||||||||
| Ordoñez (2010) [43] | 35 | 1.7 | 31.2 | 0.30 | 9.0 | 0.18 | 104.0 | 3.5 | 173.3 | 20 | ZIF-8 | |
| 47.2 | 0.59 | 14.2 | 0.38 | 80.0 | 3.3 | 124.2 | 30 | |||||
| 71.2 | 1.05 | 24.6 | 0.89 | 67.8 | 2.9 | 80.0 | 40 | |||||
| 18.1 | 0.18 | 4.7 | 0.05 | 100.6 | 3.8 | 362.0 | 50 | |||||
| 35.8 | 0.44 | 8.1 | 0.10 | 81.4 | 4.4 | 358.0 | 60 | |||||
| Sánchez-Laínez (2015) [58] | 35 | 2.0 | 95.9 | 21.8 | 4.4 | 25 | ZIF-11 | |||||
| 200 | 535.0 | 58.8 | 9.1 | 15 | ||||||||
| Song (2012) [42] | 22 | 4.0 | 38.1 | 0.47 | 10.1 | 0.26 | 81.0 | 3.8 | 146.3 | 5 | ZIF-8 | Annealing 230 °C |
| 52.6 | 0.63 | 13.7 | 0.45 | 83.4 | 3.8 | 116.8 | 10 | |||||
| 63.5 | 0.88 | 16.6 | 0.46 | 72.2 | 3.8 | 138.1 | 20 | |||||
| 112.1 | 1.68 | 28.7 | 1.16 | 66.7 | 3.9 | 96.6 | 30 | |||||
| 28.9 | 1.77 | 19.8 | 1.06 | 16.3 | 1.5 | 27.3 | 20 | Annealing 60 °C | ||||
| 36.4 | 0.42 | 8.8 | 0.23 | 86.6 | 4.1 | 158.2 | 20 | Annealing 150 °C | ||||
| 48.2 | 0.61 | 13.0 | 0.31 | 79.1 | 3.7 | 155.6 | 20 | Annealing 180 °C | ||||
| 56.5 | 0.61 | 12.9 | 0.36 | 92.7 | 4.4 | 157.0 | 20 | Annealing 200 °C | ||||
| 113.3 | 9.21 | 9.1 | 8.70 | 12.3 | 12.5 | 13.0 | 30 | Annealing 150 °C | ||||
| 115.8 | 2.00 | 29.2 | 1.17 | 57.9 | 4.0 | 99.0 | 30 | Annealing 180 °C | ||||
| 117.3 | 1.54 | 27.5 | 0.97 | 76.2 | 4.3 | 121.0 | 30 | Annealing 200 °C | ||||
| 98.9 | 1.08 | 21.4 | 0.73 | 91.6 | 4.6 | 135.5 | 30 | Annealing 260 °C | ||||
| 144.5 | 4.43 | 29.2 | 4.60 | 32.6 | 5.0 | 31.4 | 30 | Annealing 300 °C | ||||
| Yumru (2018) [62] | 35 | 4 | 28.1 | 6.8 | 0.29 | 4.2 | 96.9 | 10 | ZIF-11 | |||
| 54.9 | 11.8 | 0.43 | 4.7 | 127.8 | 20 | |||||||
| 102.8 | 31.4 | 0.73 | 3.3 | 140.8 | 30 | |||||||
| 28.4 | 10.0 | 0.15 | 2.8 | 189.2 | 40 |
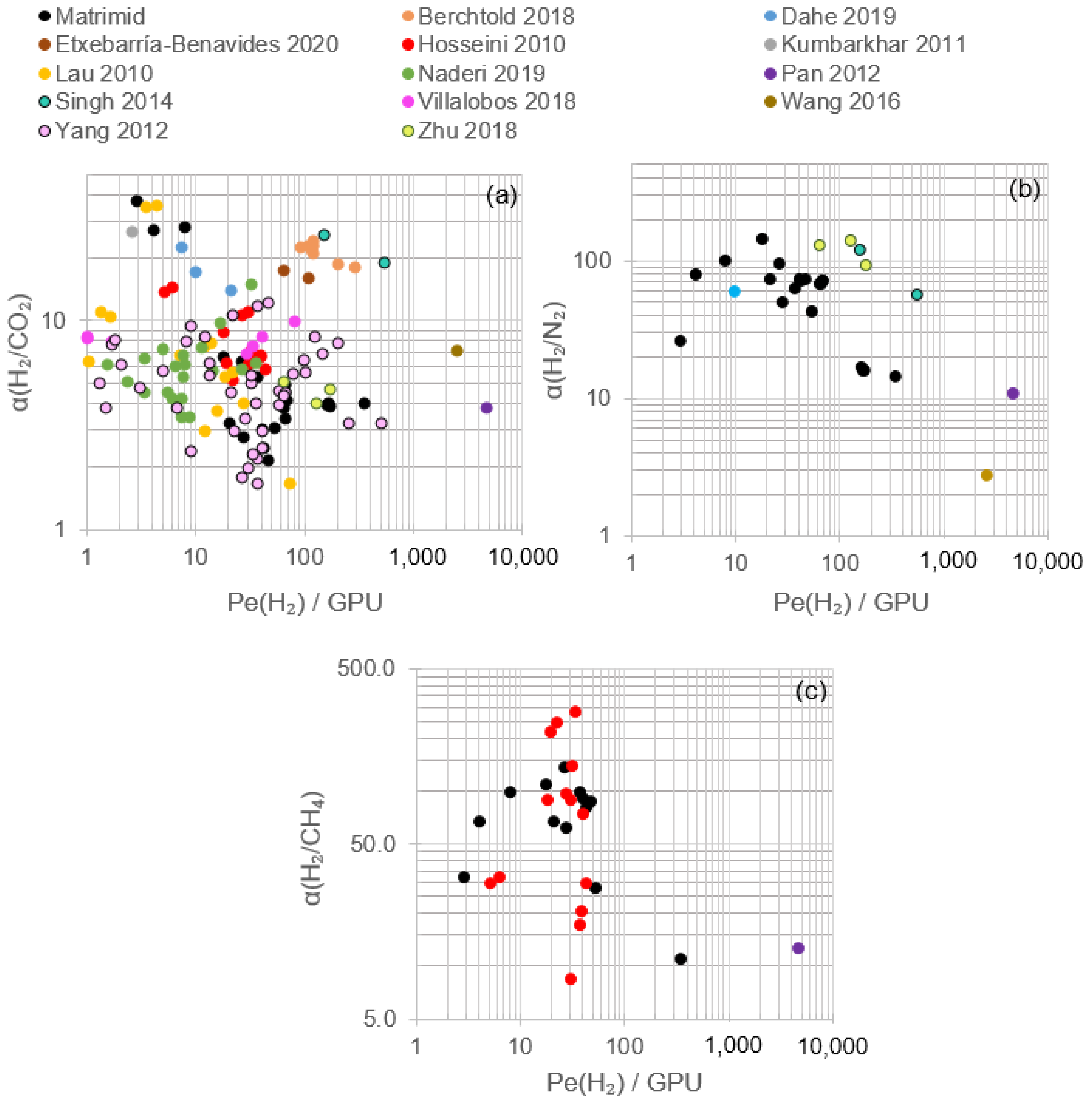
2.4.2. Mixed Matrix Hollow Fiber Membranes (MMHFMs)
2.5. Towards the Improvement of Matrimid®/ZIF Hydrogen Recovery Performance by Polymeric Substitution, Polymeric Blending, Chemical Modification and Filler Substitution or Functionalization
2.5.1. Flat Sheet Membranes
| Ref. | T (°C) | ΔP (Bar) | PH2 (Barrer) | PN2 (Barrer) | PCO2 (Barrer) | PCH4 (Barrer) | αH2/N2 | αH2/CO2 | αH2/CH4 | Polymer | Modification |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Carter (2017) [48] | 35 | 3.0 | 30.3 | 0.70 | 9.5 | 0.32 | 43.3 | 3.2 | 94.7 | Matrimid® | pristine Matrimid |
| 34.0 | 0.73 | 10.5 | 0.79 | 46.6 | 3.2 | 43.0 | Silicalite calcined (10 wt.%) | ||||
| 28.3 | 0.36 | 9.5 | 0.30 | 78.6 | 3.0 | 94.3 | Silicalite uncalcined (10 wt.%) | ||||
| 40.2 | 1.19 | 12.5 | 1.34 | 33.8 | 3.2 | 30.0 | SAPO-34 calcined (10 wt.%) | ||||
| 25.2 | 0.29 | 7.6 | 0.24 | 86.9 | 3.3 | 105.0 | SAPO-34 uncalcined (10 wt.%) | ||||
| Diestel (2015) [46] | 25 | 0.2 | 19.0 | 2.0 | 9.5 | Matrimid® | ZIF-90 + ethylendiamine | ||||
| Esposito (2019) [63] | 25 | 328 | 6.83 | 198 | 9.14 | 48.0 | 1.7 | 35.9 | Matrimid®/PIM | ||
| 1630 | 62.8 | 1380 | 77.6 | 26.0 | 1.2 | 21.0 | PIM | ||||
| Ghanem (2020) [84] | 35 | 2.0 | 4.3 | 0.05 | 1.4 | 0.04 | 87.1 | 3.1 | 108.9 | Commercial polyimide from Alfa Aesar | d-PI |
| 11.2 | 0.12 | 3.2 | 0.10 | 96.9 | 3.5 | 110.2 | 5 wt.% ZIF-302 d-PI | ||||
| 386.1 | 12.9 | 207.3 | 12.3 | 29.9 | 1.9 | 31.4 | s-PI | ||||
| 469.2 | 7.5 | 186.0 | 11.1 | 62.6 | 2.5 | 42.3 | 5 wt.% ZIF-302 s-PI | ||||
| Hosseini (2007) [56] | 35 | 32.2 | 0.36 | 8.3 | 0.28 | 89.4 | 3.9 | 115.8 | Matrimid® | 20 wt.% MgO untreated | |
| 25.3 | 0.32 | 7.4 | 0.25 | 79.3 | 3.4 | 103.3 | 20 wt.% MgO, 240 °C (12 h) | ||||
| 37.6 | 0.50 | 10.8 | 0.39 | 74.8 | 3.5 | 96.7 | 20 wt.% MgO, 350 °C (1 h) | ||||
| 41.1 | 0.52 | 11.6 | 0.21 | 79.0 | 3.5 | 199.5 | 20 wt.% MgO, 350 °C (0.5 h) | ||||
| 19.8 | 0.18 | 5.1 | 0.13 | 108.2 | 3.9 | 152.3 | 20 wt.% MgO, silver treatment 2 days | ||||
| 22.5 | 0.17 | 5.1 | 0.12 | 130.1 | 4.5 | 186.0 | 20 wt.% MgO, silver treatment 5 days | ||||
| 22.7 | 0.16 | 4.3 | 0.10 | 146.5 | 5.3 | 222.5 | 20 wt.% MgO, silver treatment 10 days | ||||
| Hosseini (2018) [45] | 35 | H2: 3.5 Other gases: 10.0 | 5.5 | 0.021 | 0.6 | 0.001 | 260.5 | 9.4 | 5500.0 | Matrimid®/PBI (25/75 wt.%) | |
| 4.0 | 0.014 | 0.3 | 0.016 | 288.6 | 13.1 | 253.2 | p-xylene dichloride | ||||
| 3.6 | 0.013 | 0.1 | 0.003 | 271.2 | 26.1 | 1200.0 | p-xylene diamine | ||||
| Knebel (2018) [83] | 25 | 0.5 | 8.9 | 6.5 | 5.5 | Ceramic support of α-Al2O3 | ZIF-67 | ||||
| 10.4 | 12.9 | 11.4 | ZIF-67 on ZIF-8 | ||||||||
| 9.3 | 13.2 | 11.1 | ZIF-8 on ZIF-67 | ||||||||
| 94.0 | 17.7 | 5.3 | Matrimid® | ZIF-8 and ZIF-67 | |||||||
| 150 | 237.0 | 32.5 | 7.3 | ||||||||
| Mei (2020) [85] | 30 | 4.0 | 23.3 | 2.5 | 9.3 | Polysulfone | 10 wt.% ZIF-8 with PDA coating | ||||
| Mirzaei (2020) [44] | 25 | 5.0 | 68.9 | 0.51 | 13.6 | 0.34 | 135.9 | 5.1 | 201.1 | Matrimid® | 20 wt.% Pd@ZIF-8 |
| Mundstock (2017) [86] | 20 | 17.0 | 5.7 | 3.0 | Matrimid® supported over Al2O3 | ||||||
| 50.8 | 12.9 | 4.0 | NaX | ||||||||
| 1.0 | 21.2 | 4.5 | 4.8 | PbX | |||||||
| 29.3 | 5.7 | 5.2 | CuX | ||||||||
| 26.0 | 4.8 | 5.6 | NiX | ||||||||
| 23.0 | 4.2 | 5.6 | Cox | ||||||||
| Perez (2009) [87] | 35 | 2.0 | 29.9 | 0.28 | 11.1 | 0.22 | 106.8 | 2.7 | 135.9 | Matrimid® | 10 wt.% MOF-5 |
| 38.3 | 0.40 | 13.8 | 0.34 | 95.8 | 2.8 | 112.6 | 20 wt.% MOF-5 | ||||
| 53.8 | 0.52 | 20.2 | 0.45 | 103.5 | 2.7 | 119.6 | 30 wt.% MOF-5 | ||||
| Sánchez-Laínez (2018) [88] | 35 | 2.0 | 3.8 | Polyamide on P84® support | ZIF-8 (0%w/v) | ||||||
| 180 | 2.0 | 7.9 | |||||||||
| 250 | 2.0 | 8.4 | |||||||||
| 35 | 2.0 | 4.4 | ZIF-8 (0.2%w/v) | ||||||||
| 180 | 2.0 | 9.2 | |||||||||
| 250 | 2.0 | 11.5 | |||||||||
| 35 | 2.0 | 9.0 | ZIF-8 (0.4%w/v) | ||||||||
| 180 | 2.0 | 14.6 | |||||||||
| 250 | 2.0 | 13.4 | |||||||||
| 180 | 2.0 | 7.2 | ZIF-8 (0.8%w/v) | ||||||||
| Weigelt (2018) [64] | 30 | 1 | 39.0 | 0.44 | 14.5 | 0.43 | 88.6 | 2.7 | 90.7 | Matrimid® | 8% Activated Carbon |
| 63.8 | 0.81 | 25.6 | 0.67 | 78.8 | 2.5 | 95.2 | 31% AC | ||||
| 101 | 1.5 | 39.5 | 1.06 | 67.3 | 2.6 | 95.3 | 44% AC | ||||
| 180 | 2.8 | 66.7 | 2.25 | 64.3 | 2.7 | 80.0 | 50% AC | ||||
| Yang (2011) [89] | 35 | 7.1 | 3.7 | 0.4 | 8.7 | PBI | pristine PBI | ||||
| 7.7 | 0.6 | 12.9 | 10 wt.% ZIF-7 | ||||||||
| 15.4 | 1.3 | 11.9 | 25 wt.% ZIF-7 | ||||||||
| 26.2 | 1.8 | 14.9 | 50 wt.% ZIF-7 50 wt.% ZIF-7 | ||||||||
| 180 | 440.0 | 25.4 | 14.6 | ||||||||
| Yang (2012) [90] | 35 | 3.5 | 3.7 | 0.4 | 8.6 | PBI | pristine PBI | ||||
| 28.5 | 2.2 | 13.0 | 15 wt.% ZIF-8 | ||||||||
| 1750 | 426.6 | 4.1 | 60 wt.% ZIF-8 | ||||||||
| 26.2 | 1.8 | 14.6 | ZIF-7 | ||||||||
| Yang (2013) [91] | 35 | 3.5 | 4.1 | 0.5 | 7.1 | PBI | pristine PBI | ||||
| 82.5 | 6.9 | 6.8 | 30 wt.% ZIF-8 | ||||||||
| 1612.8 | 397.6 | 2.8 | 60 wt.% ZIF-8 | ||||||||
| 230 | 470.0 | 17.9 | 26.3 | 30 wt.% ZIF-8 | |||||||
| 2015.0 | 163.8 | 12.3 | 60 wt.% ZIF-8 | ||||||||
| Yang (2013) [92] | 35 | 3.5 | 12.7 | 0.9 | 14.6 | PBI | 10 wt.% ZIF-90 | ||||
| 18.3 | 0.9 | 20.6 | 25 wt.% ZIF-90 | ||||||||
| 24.5 | 1.0 | 25.0 | 45 wt.% ZIF-90 | ||||||||
| Yáñez (2020) [69] | 35 | 5.5 | 8.4 | 0.03 | 2.2 | 0.05 | 280.0 | 3.8 | 168.0 | PEI ULTEM® 1000B | |
| 11.3 | 0.09 | 4.4 | 0.20 | 132.4 | 2.6 | 56.3 | PES ULTRASON® E | ||||
| 0.6 | 0.002 | 0.3 | 0.001 | 322.5 | 2.2 | 645.0 | PBI Celazole® | ||||
| Zhang (2008) [49] | 25 | 2.0 | 17.5 | 0.22 | 7.3 | 0.21 | 79.6 | 2.4 | 83.3 | Matrimid® | pristine Matrimid |
| 2.0 | 19.8 | 0.14 | 8.3 | 0.12 | 141.3 | 2.4 | 164.8 | 10 wt.% Meso-ZSM-5 | |||
| 1.5 | 19.6 | 0.14 | 8.5 | 0.13 | 139.7 | 2.3 | 150.5 | 10 wt.% Meso-ZSM-5 | |||
| 2.0 | 22.2 | 0.170 | 8.7 | 0.130 | 130.8 | 2.6 | 171.0 | 20 wt.% Meso-ZSM-5 | |||
| 35.4 | 0.31 | 14.6 | 0.26 | 114.1 | 2.4 | 136.0 | 30 wt.% Meso-ZSM-5 | ||||
| 36.3 | 0.62 | 15.4 | 0.56 | 58.6 | 2.4 | 64.8 | 10 wt.% Meso-ZSM-5 (uncalcined) | ||||
| 22.0 | 0.34 | 9.0 | 0.30 | 64.8 | 2.4 | 73.5 | 10 wt.% ZSM-5 | ||||
| 23.1 | 0.30 | 9.4 | 0.28 | 77.1 | 2.5 | 82.6 | 10 wt.% MCM-48 | ||||
| Zhao (2008) [65,93] | 35 | 1.0 | 3.8 | 0.16 | 7.5 | 0.35 | 23.7 | 0.5 | 10.8 | Matrimid® | 1:0.2 PPG/PEG/PPGDA |
| 10.0 | 1.13 | 59.2 | 3.36 | 8.9 | 0.2 | 3.0 | 1:0.5 PPG/PEG/PPGDA | ||||
| 15.8 | 2.19 | 115.8 | 6.80 | 7.2 | 0.1 | 2.3 | 1:1 PPG/PEG/PPGDA |
2.5.2. Hollow Fiber Membranes
| Ref. | T (°C) | ΔP (Bar) | PeH2 (GPU) | PeN2 (GPU) | PeCO2 (GPU) | PeCH4 (GPU) | αH2/N2 | αH2/CO2 | αH2/CH4 | Polymer or Ceramic Material | Comments |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Berchtold (2016) [104] | 250 | 1.4 | 118 | 4.9 | 24.0 | PBI/polysulfone | Feed pressure influence | ||||
| 6.9 | 110 | 4.8 | 23.0 | ||||||||
| 10.3 | 120 | 5.2 | 23.0 | ||||||||
| 13.7 | 120 | 5.7 | 21.0 | ||||||||
| 225 | 92 | 4.1 | 22.4 | Temperature influence | |||||||
| 250 | 116 | 5.3 | 22.0 | ||||||||
| 300 | 198 | 10.5 | 18.8 | ||||||||
| 350 | 285 | 15.9 | 17.9 | ||||||||
| Dahe (2019) [105] | 250 | 1.4 | 9.7 | 0.4 | 0.6 | 24.1 | 17.1 | PBI | HFM-1 21.3% PBI (acetone); % outer coagulant 0.5 v.% water (acetone) | ||
| 21.0 | 1.1 | 1.5 | 18.4 | 14.0 | HFM-1 20.0% PBI (acetone); % outer coagulant 2.0 v.% water (acetone) | ||||||
| 7.6 | 0.1 | 0.3 | 62.0 | 22.4 | HFM-1 21.5% PBI (acetone/ethanol 15/85); % outer coagulant 2.0 v.% water (acetone) | ||||||
| Etxebarría (2020) [106] | 150 | 7.0 | 65 | 3.7 | 17.6 | PBI | no fillers | ||||
| 107 | 6.6 | 16.1 | 10 wt.% ZIF-8 | ||||||||
| Hosseini (2010) [99] | 35 | H2: 3.5 other gases: 10 | 43.2 | 7.3 | 1.46 | 5.9 | 29.6 | Matrimid®/PBI | A before silicone rubber coating | ||
| 30.3 | 4.9 | 3.54 | 6.2 | 8.6 | C before silicone rubber coating | ||||||
| 36.5 | 5.5 | 2.13 | 6.7 | 17.2 | X before silicone rubber coating | ||||||
| 38.7 | 5.7 | 1.85 | 6.8 | 20.9 | Y before silicone rubber coating | ||||||
| 31.6 | 4.4 | 0.22 | 7.2 | 141.5 | A after silicone rubber coating | ||||||
| 17.8 | 2.0 | 0.20 | 9.0 | 89.6 | C After silicone rubber coating | ||||||
| 26.5 | 2.5 | 0.27 | 10.6 | 96.9 | X After silicone rubber coating | ||||||
| 29.3 | 2.6 | 0.33 | 11.1 | 89.2 | Y After silicone rubber coating | ||||||
| 39.0 | 5.8 | 0.53 | 6.8 | 74.0 | D before silicone rubber coating | ||||||
| 32.7 | 4.8 | 0.12 | 6.8 | 284.0 | D after silicone rubber coating | ||||||
| 22.1 | 4.2 | 0.09 | 5.2 | 245.2 | B before silicone rubber coating | ||||||
| 18.9 | 3.0 | 0.09 | 6.4 | 222.2 | B after silicone rubber coating | ||||||
| 6.1 | 0.42 | 0.19 | 14.5 | 32.6 | Y crosslinking 0.5 s | ||||||
| 5.1 | 0.37 | 0.17 | 13.9 | 29.7 | Y crosslinking 1.0 min | ||||||
| 0.6 | 0.06 | 0.04 | 9.2 | 16.1 | Y crosslinking 5.0 min | ||||||
| Kumbharkar (2011) [107] | 100 | 5–8 | 0.3 | 0.046 | 7.2 | PBI | |||||
| 200 | 0.6 | 0.048 | 12.9 | ||||||||
| 300 | 1.0 | 0.046 | 21.5 | ||||||||
| 400 | 2.6 | 0.096 | 27.1 | ||||||||
| Lau (2010) [101] | 35 | 1.4 | 72.6 | 42.97 | 1.7 | 6FDA-NDA/PES dual layer | Original | ||||
| 12.1 | 4.05 | 3.0 | Vapor phase modification (VPM) Method A 2 min | ||||||||
| 3.4 | 0.10 | 34.8 | VPM Method A 5 min | ||||||||
| 27.7 | 6.88 | 4.0 | Matrimid®/PBI | Original | |||||||
| 18.6 | 3.42 | 5.4 | VPM Method A 2 min | ||||||||
| 11.9 | 1.56 | 7.6 | VPM Method A 5 min | ||||||||
| 7.1 | 1.03 | 6.9 | Torlon® | Original | |||||||
| 1.6 | 0.16 | 10.4 | VPM Method A 2 min | ||||||||
| 0.1 | 0.03 | 4.8 | VPM Method A 5 min | ||||||||
| 15.4 | 4.13 | 3.7 | 6FDA-NDA/PES dual layer | VPM Method B 2 min | |||||||
| 4.4 | 0.13 | 35.5 | VPM Method B 5 min | ||||||||
| 21.7 | 3.77 | 5.8 | Matrimid®/PBI | VPM Method B 2 min | |||||||
| 13.8 | 1.77 | 7.8 | VPM Method B 5 min | ||||||||
| 1.3 | 0.12 | 11.0 | Torlon® | VPM Method B 2 min | |||||||
| 1.0 | 0.16 | 6.4 | VPM Method B 5 min | ||||||||
| Naderi (2019) [108] | 25 | 7.0 | 2.36 | 0.46 | 5.1 | Dual layer Inner layer: polysulfone Outer layer: Polyphenylsulfone/PBI | HSP-0: PBI/DMAc/LiCl 22/79.8/1.2 (wt.%). Before silicon rubber coating | ||||
| 5.50 | 1.22 | 4.5 | HSP-5: (PBI/sPPSU 95:5)/DMAc/LiCl 22/79.8/1.2 (wt.%). Before silicon rubber coating | ||||||||
| 7.52 | 1.75 | 4.3 | HSP-10: (PBI/sPPSU 90:10)/DMAc/LiCl 22/79.8/1.2 (wt.%). Before silicon rubber coating | ||||||||
| 8.78 | 2.53 | 3.5 | HSP-20: (PBI/sPPSU 80:20)/DMAc/LiCl 22/79.8/1.2 (wt.%). Before silicon rubber coating | ||||||||
| 1.54 | 0.25 | 6.2 | HSP-0 after silicon rubber coating | ||||||||
| 3.39 | 0.74 | 4.6 | HSP-5 after silicon rubber coating | ||||||||
| 6.14 | 1.42 | 4.3 | HSP-10 after silicon rubber coating | ||||||||
| 7.44 | 2.14 | 3.5 | HSP-20 after silicon rubber coating | ||||||||
| 7.6 | 1.4 | 5.5 | HSP-10-40 thermal treatment 40 °C | ||||||||
| 7.8 | 1.3 | 6.2 | HSP-10-80 thermal treatment 80 °C | ||||||||
| 7.6 | 1.1 | 6.8 | HSP-10-120 thermal treatment 120 °C | ||||||||
| 5.0 | 0.7 | 7.3 | HSP-10-120 chemical crosslinking 3% DBX | ||||||||
| 3.4 | 0.5 | 6.6 | HSP-10-120 chemical crosslinking 6% DBX | ||||||||
| 30 | 14.0 | 13.8 | 2.4 | 5.8 | Mixed gas. HSP-10-120-30 | ||||||
| 60 | 26.1 | 4.4 | 5.9 | Mixed gas. HSP-10-120-60 | |||||||
| 90 | 35.6 | 5.7 | 6.3 | Mixed gas. HSP-10-120-90 | |||||||
| 30 | 6.4 | 1.1 | 6.1 | Mixed gas. HSP-10-3%DBX-120-30 | |||||||
| 60 | 11.3 | 1.5 | 7.4 | Mixed gas. HSP-10-3%DBX-120-60 | |||||||
| 90 | 16.7 | 1.7 | 9.7 | Mixed gas. HSP-10-3%DBX-120-90 | |||||||
| 180 | 32.1 | 2.2 | 14.9 | Mixed gas. HSP-10-3%DBX-120-180 | |||||||
| Pan (2012) [109] | 22 | 1.0 | 4598 | 418 | 1194 | 358 | 11.0 | 3.9 | 12.8 | ytria-stabilized zirconia | ZIF-8 |
| Singh (2014) [110] | 250 | 540.0 | 9.3 | 28.4 | 58.0 | 19.0 | PBI | ||||
| 150.0 | 1.3 | 5.8 | 120.0 | 26.0 | |||||||
| Villalobos (2018) [111] | 35 | 0.05 | 0.01 | 4.8 | PBI | Pristine | |||||
| 45 | 0.07 | 0.01 | 5.0 | ||||||||
| 60 | 0.09 | 0.02 | 5.3 | ||||||||
| 22 | 29.0 | 4.14 | 7.0 | 0.05 M Pd NPs | |||||||
| 35 | 34.0 | 4.47 | 7.6 | ||||||||
| 45 | 40.0 | 4.71 | 8.5 | ||||||||
| 60 | 80.0 | 8.00 | 10.0 | ||||||||
| 22 | 0.55 | 0.06 | 9.0 | 0.1 M Pd NPs | |||||||
| 35 | 1.0 | 0.12 | 8.5 | ||||||||
| 45 | 1.0 | 0.12 | 8.3 | ||||||||
| 60 | 1.65 | 0.21 | 8.0 | ||||||||
| Wang (2016) [112] | 20 | 2.5 | 2493.3 | 886.8 | 343.4 | 2.8 | 7.3 | Silicon nitride ceramic | ZIF-8 | ||
| Yang (2012) [90] | 25 | 3.5 | 1.3 | 0.3 | 5.0 | Dual layer: inner Matrimid®; outer PBI/ZIF-8 | PZM00-MA 0% ZIF-8. Solvent-exchange: methanol. Single gas | ||||
| 0.8 | 0.1 | 6.2 | PZM00-MB 0% ZIF-8. Solvent-exchange: methanol. Single gas | ||||||||
| 0.8 | 0.1 | 7.0 | PZM00-MC 0% ZIF-8. Solvent-exchange: methanol. Single gas | ||||||||
| 1.7 | 0.2 | 7.7 | PZM00-IA: 0% ZIF-8. Solvent-exchange: isopropanol. Single gas | ||||||||
| 2.1 | 0.3 | 6.2 | PZM00-IB: 0% ZIF-8. Solvent-exchange: isopropanol. Single gas | ||||||||
| 1.8 | 0.2 | 8.2 | PZM00-IB: 0% ZIF-8. Solvent-exchange: isopropanol. Single gas | ||||||||
| 6.6 | 1.7 | 3.9 | PZM10-MA 10% ZIF-8. Solvent-exchange: methanol. Single gas | ||||||||
| 0.9 | 0.1 | 6.6 | PZM10-MB 10% ZIF-8. Solvent-exchange: methanol. Single gas | ||||||||
| 1.5 | 0.4 | 3.8 | PZM10-MC 10% ZIF-8. Solvent-exchange: methanol. Single gas | ||||||||
| 13.3 | 2.1 | 6.3 | PZM10-IA: 10% ZIF-8. Solvent-exchange: isopropanol. Single gas | ||||||||
| 8.9 | 0.9 | 9.5 | PZM10-IB: 10% ZIF-8. Solvent-exchange: isopropanol. Single gas | ||||||||
| 13.2 | 2.4 | 5.5 | PZM10-IB: 10% ZIF-8. Solvent-exchange: isopropanol. Single gas | ||||||||
| 8.9 | 3.7 | 2.4 | PZM20-MA 20% ZIF-8. Solvent-exchange: methanol. Single gas | ||||||||
| 21.0 | 4.6 | 4.6 | PZM20-MB 20% ZIF-8. Solvent-exchange: methanol. Single gas | ||||||||
| 57.4 | 12.4 | 4.6 | PZM20-MC 20% ZIF-8. Solvent-exchange: methanol. Single gas | ||||||||
| 28.3 | 8.2 | 3.5 | PZM20-IA: 20% ZIF-8. Solvent-exchange: isopropanol. Single gas | ||||||||
| 32.2 | 6.4 | 5.0 | PZM20-IB: 20% ZIF-8. Solvent-exchange: isopropanol. Single gas | ||||||||
| 66.8 | 14.5 | 4.6 | PZM20-IB: 20% ZIF-8. Solvent-exchange: isopropanol. Single gas | ||||||||
| 36.0 | 21.5 | 1.7 | PZM33-MA 33% ZIF-8. Solvent-exchange: methanol. Single gas | ||||||||
| 248.9 | 77.5 | 3.2 | PZM33-MB 33% ZIF-8. Solvent-exchange: methanol. Single gas | ||||||||
| 497.6 | 152.4 | 3.3 | PZM33-MC 33% ZIF-8. Solvent-exchange: methanol. Single gas | ||||||||
| 22.7 | 7.6 | 3.0 | PZM33-IA: 33% ZIF-8. Solvent-exchange: isopropanol. Single gas | ||||||||
| 34.9 | 8.7 | 4.0 | PZM33-IB: 33% ZIF-8. Solvent-exchange: isopropanol. Single gas | ||||||||
| 32.0 | 5.8 | 5.5 | PZM33-IB: 33% ZIF-8. Solvent-exchange: isopropanol. Single gas | ||||||||
| 25 | 6.0 | 3.0 | 0.6 | 4.8 | PZM10-IB, 10% ZIF-8. Mixed gas | ||||||
| 35 | 5.0 | 0.9 | 5.8 | ||||||||
| 50 | 8.0 | 1.0 | 8.0 | ||||||||
| 80 | 12.0 | 1.4 | 8.5 | ||||||||
| 120 | 22.0 | 2.1 | 10.7 | ||||||||
| 145 | 37.0 | 3.1 | 11.8 | ||||||||
| 180 | 45.0 | 3.7 | 12.2 | ||||||||
| 25 | 26.0 | 14.4 | 1.8 | PZM20-IB 20% ZIF-8. Mixed gas | |||||||
| 35 | 30.0 | 15.0 | 2.0 | ||||||||
| 50 | 40.0 | 16.0 | 2.5 | ||||||||
| 80 | 58.0 | 14.5 | 4.0 | ||||||||
| 120 | 76.0 | 13.6 | 5.6 | ||||||||
| 145 | 99.0 | 15.2 | 6.5 | ||||||||
| 180 | 123.0 | 14.8 | 8.3 | ||||||||
| 25 | 36.0 | 16.4 | 2.2 | PZM33-IB 33% ZIF-8. Mixed gas | |||||||
| 35 | 34.0 | 14.8 | 2.3 | ||||||||
| 50 | 40.0 | 13.3 | 3.0 | ||||||||
| 80 | 65.0 | 14.8 | 4.4 | ||||||||
| 120 | 100.0 | 17.5 | 5.7 | ||||||||
| 145 | 145.0 | 20.7 | 7.0 | ||||||||
| 180 | 201.0 | 25.8 | 7.8 | ||||||||
| Zhu (2018) [113] | 35.0 | 5.0 | 63.3 | 0.5 | 12.2 | 132.0 | 5.2 | Pure | |||
| 172.2 | 1.8 | 36.5 | 94.1 | 4.7 | Ultem® polyetherimide | 15% MIL-53 | |||||
| 127.1 | 0.9 | 31.4 | 144.5 | 4.1 | 15% S-MIL-53 |
3. Concluding Remarks
- Assurance of good dispersion of ZIF in the Matrimid® polymer and morphology in a wide range of filler contents.
- Guarantee of the highest hydrogen recovery yield providing an adequate sweep-gas flowrate, hindering the polarization concentration phenomenon and increasing the driving force across the membrane.
- Plasticization phenomenon avoided controlling feed pressures as a consequence of the swelling effect and the polymer chain packing disruption caused by highly condensable gases, such as carbon dioxide.
- Provision of Matrimid®/filler kinetic diameters that facilitate the molecular sieving effect. Due to the higher condensability of CO2, materials hindering its solubility in the membrane are required.
- Scarcity in gas mixture research has been detected, especially considering the demonstration of the competitive sorption between hydrogen and carbon dioxide, which prevents hydrogen molecule diffusion reduction; therefore, the permeability of both gases and H2/CO2 selectivity has been compared to single gas tests.
- Performance improvement by the membrane annealing procedure, although it may affect the mechanical stability, e.g., weakening the damage tolerance.
- Improved selectivity by using sealants, although permeance values could be compromised.
- Positive correlation operating temperature–H2 permeability and operating temperature–H2/CO2 selectivity owing to the Arrhenius behavior in gas transport and the change from a diffusion-limited to a sorption-limited regime, respectively.
- Positive influence of ZIF addition on permeability/permeance values and selectivity towards hydrogen as a consequence of the adsorption site availability and the polymeric chain packing modification.
- Negative influence of excess ZIF on the mechanical properties of the MM/MMHF membrane.
- Solution of agglomeration and aggregation phenomena by using nanosized fillers that provide higher surface areas susceptible to being coated by the polymer.
- Improvement of hydrogen recovery by crosslinking reactions but deterioration of permeance values.
- Enhancement of H2/CO2 selectivity in HFMs but poorer results in the separation of hydrogen from N2, CH4 and CO.
- Importance of operating parameters in HFM preparation in the final performance: draw rate, dopes solution, coagulation bath, solvent exchange and post-treatment.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFM | atomic force microscopy |
| APTES | 3-aminopropyltriethoxysilane |
| DCM | dichloromethane |
| DMA | dynamic mechanical analyzer |
| DMAc | N,N-dimethylacetamide |
| DMF | dimethyl formamide |
| DSC | differential scanning calorimetry |
| EDX | energy dispersive X-ray |
| 6FDA | 4,4′-(hexafluoroisopropylidene) diphthalic anhydride |
| FFV | fractional free volume |
| FTIR | Fourier transform infrared spectroscopy |
| GBL | gamma-butyrolactone |
| HFM | hollow fiber membrane |
| MMHFM | hollow fiber mixed matrix membrane |
| MMM | mixed matrix membrane |
| MOF | metal organic framework |
| NDA | 1,5-napthalenediamine |
| NP | nanoparticle |
| NMP | N-methylpyrrolidone |
| NMR | nuclear magnetic resonance |
| PALS | positron annihilation lifetime spectroscopy |
| PBI | polybenzimidazole |
| PDA | polydopamine |
| PEI | polyetherimide |
| PES | polyethersulfone |
| PMDA | pyromellitic dianhydride |
| PPSU | polyphenylsulfone |
| PSA | pressure swing adsorption |
| SEM | scanning electron microscopy |
| TCE | 1,1,2,2-tetrachloroethane |
| TEM | transmission electron microscopy |
| TGA | thermogravimetric analysis |
| THF | tetrahydrofuran |
| XPS | X-ray photoelectron spectroscopy |
| XRD | X-ray diffraction |
| ZIF | zeolitic imidazolate framework |
References
- Díaz, M.; Ortiz, A.; Ortiz, I. Progress in the use of ionic liquids as electrolyte membranes. J. Memb. Sci. 2014, 409, 379–396. [Google Scholar] [CrossRef]
- Gandía, L.M.; Arzamendi, G.; Diéguez, P.M. Renewable Hydrogen Technologies: Production, Purification, Storage, Applications and Safety; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 9780444563521. [Google Scholar]
- Fuel Cells and Hydrogen Joint Undertaking (FCH). Hydrogen Roadmap Europe; FCH: Bietlot, Belgium, 2019; ISBN 9789292463328. [Google Scholar]
- Ramsebner, J.; Haas, R.; Ajanovic, A.; Wietschel, M. The sector coupling concept: A critical review. Wiley Interdiscip. Rev. Energy Environ. 2021, 1–27. [Google Scholar] [CrossRef]
- Robinius, M.; Otto, A.; Heuser, P.; Welder, L.; Syranidis, K.; Ryberg, D.S.; Grube, T.; Markewitz, P.; Peters, R.; Stolten, D. Linking the power and transport sectors—Part 1: The principle of sector coupling. Energies 2017, 10, 956. [Google Scholar] [CrossRef]
- Buttler, A.; Spliethoff, H. Current status of water electrolysis for energy storage, grid balancing and sector coupling via power-to-gas and power-to-liquids: A review. Renew. Sustain. Energy Rev. 2018, 82, 2440–2454. [Google Scholar] [CrossRef]
- Emonts, B.; Reuß, M.; Stenzel, P.; Welder, L.; Knicker, F.; Grube, T.; Görner, K.; Robinius, M.; Stolten, D. Flexible sector coupling with hydrogen: A climate-friendly fuel supply for road transport. Int. J. Hydrogen Energy 2019, 44, 12918–12930. [Google Scholar] [CrossRef]
- Yáñez, M.; Ortiz, A.; Brunaud, B.; Grossmann, I.E.; Ortiz, I. Contribution of upcycling surplus hydrogen to design a sustainable supply chain: The case study of Northern Spain. Appl. Energy 2018, 231, 777–787. [Google Scholar] [CrossRef]
- Wokaun, A.; Wilhelm, E. Transition to Hydrogen: Pathways toward Clean Transportation; Cambridge University Press: Cambridge, UK, 2011; ISBN 9780521192880. [Google Scholar]
- Ball, M.; Martin, W. The Hydrogen Economy: Opportunities and Challenges; Cambridge University Press: Cambridge, UK, 2009; ISBN 9780521882163. [Google Scholar]
- Abejón, R.; Fernández-Ríos, A.; Domínguez-Ramos, A.; Laso, J.; Ruiz-Salmón, I.; Yáñez, M.; Ortiz, A.; Gorri, D.; Donzel, N.; Jones, D.; et al. Hydrogen recovery from waste gas streams to feed (High-temperature PEM) fuel cells: Environmental performance under a life-cycle thinking approach. Appl. Sci. 2020, 10, 7461. [Google Scholar] [CrossRef]
- Deng, X.; Wang, H.; Huang, H.; Ouyang, M. Hydrogen flow chart in China. Int. J. Hydrogen Energy 2010, 35, 6475–6481. [Google Scholar] [CrossRef]
- Yáñez, M. Hydrogen Recovery from Industrial Waste Gas Streams for Fuel Cell Application. Ph.D. Thesis, Universidad de Cantabria, Santander, Spain, 2019. [Google Scholar]
- Yáñez, M.; Relvas, F.; Ortiz, A.; Gorri, D.; Mendes, A.; Ortiz, I. PSA purification of waste hydrogen from ammonia plants to fuel cell grade. Sep. Purif. Technol. 2020, 240, 116334. [Google Scholar] [CrossRef]
- IPTS Reference Document on Best Available Techniques for the Manufacture of Large Volume Inorganic Chemicals—Ammonia, Acids and Fertilisers. Inst. Prospect. Technol. Stud. Edif. Expo. 2007, I, 513–527.
- Ockwig, N.W.; Nenoff, T.M. Membranes for hydrogen separation. Chem. Rev. 2007, 107, 4078–4110. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, G.; Araújo, T.; da Silva Lopes, T.; Sousa, J.; Mendes, A. Recent advances in membrane technologies for hydrogen purification. Int. J. Hydrogen Energy 2020, 45, 7313–7338. [Google Scholar] [CrossRef]
- Al-Mufachi, N.A.; Rees, N.V.; Steinberger-Wilkens, R. Hydrogen selective membranes: A review of palladium-based dense metal membranes. Renew. Sustain. Energy Rev. 2015, 47, 540–551. [Google Scholar] [CrossRef]
- Baker, R.W. Membrane Technologies and Applications, 2nd ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2004; ISBN 0470854456. [Google Scholar]
- Ismail, A.F.; Khulbe, K.C.; Matsuura, T. Gas Separation Membranes: Polymeric and Inorganic; Springer: Cham, Switzerland, 2015; ISBN 9783319010953. [Google Scholar]
- Li, P.; Wang, Z.; Qiao, Z.; Liu, Y.; Cao, X.; Li, W.; Wang, J.; Wang, S. Recent developments in membranes for efficient hydrogen purification. J. Memb. Sci. 2015, 495, 130–168. [Google Scholar] [CrossRef]
- Drioli, E.; Barbieri, G.; Brunetti, A. (Eds.) Membrane Engineering for the Treatment of Gases: Volume 1: Gas-Separation Issues with Membranes, 2nd ed.; Royal Society of Chemistry: London, UK, 2017; Volume 1, ISBN 9781782628743. [Google Scholar]
- Drioli, E.; Barbieri, G.; Brunetti, A. (Eds.) Membrane Engineering for the Treatment of Gases Volume 2: Gas-Separation Issues Combined with Membrane Reactors, 2nd ed.; Royal Society of Chemistry: London, UK, 2017; Volume 2, ISBN 9781782628743. [Google Scholar]
- Guo, X.; Qiao, Z.; Liu, D.; Zhong, C. Mixed-matrix membranes for CO2 separation: Role of the third component. J. Mater. Chem. A 2019, 7, 24738–24759. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Martin-Gil, V.; Ahmad, M.Z. Matrimid® 5218 in preparation of membranes for gas separation: Current state-of-the-art. Chem. Eng. Commun. 2018, 205, 161–196. [Google Scholar] [CrossRef]
- Dechnik, J.; Gascon, J.; Doonan, C.J.; Janiak, C.; Sumby, C.J. Mixed-Matrix Membranes. Angew. Chem. Int. Ed. 2017, 56, 9292–9310. [Google Scholar] [CrossRef]
- Hamid, M.R.A.; Jeong, H. Recent advances on mixed-matrix membranes for gas separation: Opportunities and engineering challenges. Korean J. Chem. Eng. 2018, 35, 1577–1600. [Google Scholar] [CrossRef]
- Ohya, H.; Kudryavtsev, V.V.; Semenova, S.I. Polyimide Membranes; Gordon and Breach Publishers: Amsterdam, The Netherlands, 1996; ISBN 9056990241. [Google Scholar]
- Clausi, D.T.; Koros, W.J. Formation of defect-free polyimide hollow fiber membranes for gas separations. J. Memb. Sci. 2000, 167, 79–89. [Google Scholar] [CrossRef]
- Sazali, N.; Mohamed, M.A.; Salleh, W.N.W. Membranes for hydrogen separation: A significant review. Int. J. Adv. Manuf. Technol. 2020, 107, 1859–1881. [Google Scholar] [CrossRef]
- David, O.C.; Gorri, D.; Ortiz, I.; Urtiaga, A.M. Dual-sorption model for H2/CO2 permeation in glassy polymeric Matrimid membrane. Desalin. Water Treat. 2011, 27, 31–36. [Google Scholar] [CrossRef]
- Huntsman Matrimid® 5218/Matrimid® 9725. Data Sheet 2007, 1–6. Available online: https://polymer-additives.specialchem.com/product/a-huntsman-matrimid-9725 (accessed on 16 March 2021).
- Lin, R.; Villacorta Hernandez, B.; Ge, L.; Zhu, Z. Metal organic framework based mixed matrix membranes: An overview on filler/polymer interfaces. J. Mater. Chem. A 2018, 6, 293–312. [Google Scholar] [CrossRef]
- Zhang, C.; Koros, W.J. Zeolitic Imidazolate Framework-Enabled Membranes: Challenges and Opportunities. J. Phys. Chem. Lett. 2015, 6, 3841–3849. [Google Scholar] [CrossRef]
- Yong, W.F.; Zhang, H. Recent advances in polymer blend membranes for gas separation and pervaporation. Prog. Mater. Sci. 2021, 116, 100713. [Google Scholar] [CrossRef]
- Friebe, S.; Diestel, L.; Knebel, A.; Wollbrink, A.; Caro, J. MOF-Based Mixed-Matrix Membranes in Gas Separation—Mystery and Reality. Chem. Ing. Tech. 2016, 88, 1788–1797. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Fíla, V. Progress on incorporating zeolites in Matrimid® 5218 mixed matrix membranes towards gas separation. Membranes 2018, 8, 30. [Google Scholar] [CrossRef]
- Chung, T.S.; Jiang, L.Y.; Li, Y.; Kulprathipanja, S. Mixed matrix membranes (MMMs) comprising organic polymers with dispersed inorganic fillers for gas separation. Prog. Polym. Sci. 2007, 32, 483–507. [Google Scholar] [CrossRef]
- Galizia, M.; Chi, W.S.; Smith, Z.P.; Merkel, T.C.; Baker, R.W.; Freeman, B.D. 50th Anniversary Perspective: Polymers and Mixed Matrix Membranes for Gas and Vapor Separation: A Review and Prospective Opportunities. Macromolecules 2017, 50, 7809–7843. [Google Scholar] [CrossRef]
- Seoane, B.; Coronas, J.; Gascon, I.; Benavides, M.E.; Karvan, O.; Caro, J.; Kapteijn, F.; Gascon, J. Metal-organic framework based mixed matrix membranes: A solution for highly efficient CO2 capture? Chem. Soc. Rev. 2015, 44, 2421–2454. [Google Scholar] [CrossRef]
- Iulianelli, A.; Drioli, E. Membrane engineering: Latest advancements in gas separation and pre-treatment processes, petrochemical industry and refinery, and future perspectives in emerging applications. Fuel Process. Technol. 2020, 206, 106464. [Google Scholar] [CrossRef]
- Song, Q.; Nataraj, S.K.; Roussenova, M.V.; Tan, J.C.; Hughes, D.J.; Li, W.; Bourgoin, P.; Alam, M.A.; Cheetham, A.K.; Al-Muhtaseb, S.A.; et al. Zeolitic imidazolate framework (ZIF-8) based polymer nanocomposite membranes for gas separation. Energy Environ. Sci. 2012, 5, 8359–8369. [Google Scholar] [CrossRef]
- Ordoñez, M.J.C.; Balkus, K.J.; Ferraris, J.P.; Musselman, I.H. Molecular sieving realized with ZIF-8/Matrimid® mixed-matrix membranes. J. Memb. Sci. 2010, 361, 28–37. [Google Scholar] [CrossRef]
- Mirzaei, A.; Navarchian, A.H.; Tangestaninejad, S. Mixed matrix membranes on the basis of Matrimid and palladium-zeolitic imidazolate framework for hydrogen separation. Iran. Polym. J. 2020, 29, 479–491. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Teoh, M.M.; Chung, T.S. Hydrogen separation and purification in membranes of miscible polymer blends with interpenetration networks. Polymer 2008, 49, 1594–1603. [Google Scholar] [CrossRef]
- Diestel, L.; Wang, N.; Schulz, A.; Steinbach, F.; Caro, J. Matrimid-based mixed matrix membranes: Interpretation and correlation of experimental findings for zeolitic imidazolate frameworks as fillers in H2/CO2 separation. Ind. Eng. Chem. Res. 2015, 54, 1103–1112. [Google Scholar] [CrossRef]
- David, O.C.; Gorri, D.; Urtiaga, A.; Ortiz, I. Mixed gas separation study for the hydrogen recovery from H2/CO/N2/CO2 post combustion mixtures using a Matrimid membrane. J. Memb. Sci. 2011, 378, 359–368. [Google Scholar] [CrossRef]
- Carter, D.; Tezel, F.H.; Kruczek, B.; Kalipcilar, H. Investigation and comparison of mixed matrix membranes composed of polyimide Matrimid with ZIF–8, silicalite, and SAPO–34. J. Memb. Sci. 2017, 544, 35–46. [Google Scholar] [CrossRef]
- Zhang, Y.; Balkus, K.J.; Musselman, I.H.; Ferraris, J.P. Mixed-matrix membranes composed of Matrimid® and mesoporous ZSM-5 nanoparticles. J. Memb. Sci. 2008, 325, 28–39. [Google Scholar] [CrossRef]
- Shishatskiy, S.; Nistor, C.; Popa, M.; Nunes, S.P.; Peinemann, K.V. Polyimide asymmetric membranes for hydrogen separation: Influence of formation conditions on gas transport properties. Adv. Eng. Mater. 2006, 8, 390–397. [Google Scholar] [CrossRef]
- Ullah Khan, I.; Othman, M.H.D.; Ismail, A.F.; Matsuura, T.; Hashim, H.; Nordin, N.A.H.M.; Rahman, M.A.; Jaafar, J.; Jilani, A. Status and improvement of dual-layer hollow fiber membranes via co-extrusion process for gas separation: A review. J. Nat. Gas Sci. Eng. 2018, 52, 215–234. [Google Scholar] [CrossRef]
- Huang, Y.; Xiao, C.; Huang, Q.; Liu, H.; Zhao, J. Progress on polymeric hollow fiber membrane preparation technique from the perspective of green and sustainable development. Chem. Eng. J. 2021, 403, 126295. [Google Scholar] [CrossRef]
- Krol, J.J.; Boerrigter, M.; Koops, G.H. Polyimide hollow fiber gas separation membranes: Preparation and the suppression of plasticization in propane/propylene environments. J. Memb. Sci. 2001, 184, 275–286. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Otitoju, T.A.; Ooi, B.S. Hollow fiber (HF) membrane fabrication: A review on the effects of solution spinning conditions on morphology and performance. J. Ind. Eng. Chem. 2019, 70, 35–50. [Google Scholar] [CrossRef]
- Dong, G.; Li, H.; Chen, V. Factors affect defect-free Matrimid® hollow fiber gas separation performance in natural gas purification. J. Memb. Sci. 2010, 353, 17–27. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Li, Y.; Chung, T.S.; Liu, Y. Enhanced gas separation performance of nanocomposite membranes using MgO nanoparticles. J. Memb. Sci. 2007, 302, 207–217. [Google Scholar] [CrossRef]
- Mahdi, E.M.; Tan, J.C. Mixed-matrix membranes of zeolitic imidazolate framework (ZIF-8)/Matrimid nanocomposite: Thermo-mechanical stability and viscoelasticity underpinning membrane separation performance. J. Memb. Sci. 2016, 498, 276–290. [Google Scholar] [CrossRef]
- Sánchez-Laínez, J.; Zornoza, B.; Mayoral, Á.; Berenguer-Murcia, Á.; Cazorla-Amorós, D.; Téllez, C.; Coronas, J. Beyond the H2/CO2 upper bound: One-step crystallization and separation of nano-sized ZIF-11 by centrifugation and its application in mixed matrix membranes. J. Mater. Chem. A 2015, 3, 6549–6556. [Google Scholar] [CrossRef]
- García, M.G.; Marchese, J.; Ochoa, N.A. Improved gas selectivity of polyetherimide membrane by the incorporation of PIM polyimide phase. J. Appl. Polym. Sci. 2017, 134, 1–11. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Memb. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Robeson, L.M. Correlation of separation factor versus permeability for polymeric membranes. J. Memb. Sci. 1991, 62, 165–185. [Google Scholar] [CrossRef]
- Yumru, A.B.; Safak Boroglu, M.; Boz, I. ZIF-11/Matrimid® mixed matrix membranes for efficient CO2, CH4, and H2 separations. Greenh. Gases Sci. Technol. 2018, 8, 529–541. [Google Scholar] [CrossRef]
- Esposito, E.; Mazzei, I.; Monteleone, M.; Fuoco, A.; Carta, M.; McKeown, N.B.; Malpass-Evans, R.; Jansen, J.C. Highly permeable Matrimid®/PIM-EA(H2)-TB blend membrane for gas separation. Polymers 2018, 11, 46. [Google Scholar] [CrossRef]
- Weigelt, F.; Georgopanos, P.; Shishatskiy, S.; Filiz, V.; Brinkmann, T.; Abetz, V. Development and characterization of defect-free Matrimid® mixed-matrix membranes containing activated carbon particles for gas separation. Polymers 2018, 10, 51. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Cao, Y.M.; Ding, X.L.; Zhou, M.Q.; Yuan, Q. Effects of cross-linkers with different molecular weights in cross-linked Matrimid 5218 and test temperature on gas transport properties. J. Memb. Sci. 2008, 323, 176–184. [Google Scholar] [CrossRef]
- Dean, J.A. Lange’s Handbook of Chemistry, 15th ed.; McGraw-Hill: New York, NY, USA, 1999; ISBN 0070163847. [Google Scholar]
- Breck, D.W. Zeolite Molecular Sieves: Structure, Chemistry and Use; John Wiley & Sons Inc.: New York, NY, USA, 1974; ISBN 0471099856. [Google Scholar]
- David, O.C. Membrane Technologies for Hydrogen and Carbon Monoxide Recovery from Residual Gas Streams. Ph.D. Thesis, Universidad de Cantabria, Santander, Spain, 2012. [Google Scholar]
- Yáñez, M.; Ortiz, A.; Gorri, D.; Ortiz, I. Comparative performance of commercial polymeric membranes in the recovery of industrial hydrogen waste gas streams. Int. J. Hydrogen Energy 2021. [Google Scholar] [CrossRef]
- Ansaloni, L.; Minelli, M.; Giacinti Baschetti, M.; Sarti, G.C. Effect of relative humidity and temperature on gas transport in Matrimid®: Experimental study and modeling. J. Memb. Sci. 2014, 471, 392–401. [Google Scholar] [CrossRef]
- Chen, X.Y.; Tien-Binh, N.; Kaliaguine, S.; Rodrigue, D. Polyimide Membranes for Gas Separation: Synthesis, Processing and Properties; Nova Science Publishers: New York, NY, USA, 2016; ISBN 9781536106237. [Google Scholar]
- Favvas, E.P.; Kapantaidakis, G.C.; Nolan, J.W.; Mitropoulos, A.C.; Kanellopoulos, N.K. Preparation, characterization and gas permeation properties of carbon hollow fiber membranes based on Matrimid® 5218 precursor. J. Mater. Process. Technol. 2007, 186, 102–110. [Google Scholar] [CrossRef]
- Peer, M.; Kamali, S.M.; Mahdeyarfar, M.; Mohammadi, T. Separation of hydrogen from carbon monoxide using a hollow fiber polyimide membrane: Experimental and simulation. Chem. Eng. Technol. 2007, 30, 1418–1425. [Google Scholar] [CrossRef]
- Bernardo, P.; Tasselli, F.; Chiappetta, G.; Clarizia, G. Effect of the post-spinning solvent exchange on the performance of asymmetric, polyimide hollow fibers prepared by using a triple-orifice spinneret. Materials 2019, 12, 3632. [Google Scholar] [CrossRef]
- Guan, W.; Dai, Y.; Dong, C.; Yang, X.; Xi, Y. Zeolite imidazolate framework (ZIF)-based mixed matrix membranes for CO2 separation: A review. J. Appl. Polym. Sci. 2020, 137, 1–13. [Google Scholar] [CrossRef]
- Assfour, B.; Leoni, S.; Seifert, G. Hydrogen adsorption sites in zeolite imidazolate frameworks ZIF-8 and ZIF-11. J. Phys. Chem. C 2010, 114, 13381–13384. [Google Scholar] [CrossRef]
- Boroglu, M.S.; Ugur, M.; Boz, I. Enhanced gas transport properties of mixed matrix membranes consisting of Matrimid and RHO type ZIF-12 particles. Chem. Eng. Res. Des. 2017, 123, 201–213. [Google Scholar] [CrossRef]
- Schneemann, A.; Bon, V.; Schwedler, I.; Senkovska, I.; Kaskel, S.; Fischer, R.A. Flexible metal-organic frameworks. Chem. Soc. Rev. 2014, 43, 6062–6096. [Google Scholar] [CrossRef] [PubMed]
- Abdul Hamid, M.R.; Park, S.; Kim, J.S.; Lee, Y.M.; Jeong, H.K. Synthesis of Ultrathin Zeolitic Imidazolate Framework ZIF-8 Membranes on Polymer Hollow Fibers Using a Polymer Modification Strategy for Propylene/Propane Separation. Ind. Eng. Chem. Res. 2019, 58, 14947–14953. [Google Scholar] [CrossRef]
- Lee, M.J.; Abdul Hamid, M.R.; Lee, J.; Kim, J.S.; Lee, Y.M.; Jeong, H.K. Ultrathin zeolitic-imidazolate framework ZIF-8 membranes on polymeric hollow fibers for propylene/propane separation. J. Memb. Sci. 2018, 559, 28–34. [Google Scholar] [CrossRef]
- Smith, Z.P.; Tiwari, R.R.; Murphy, T.M.; Sanders, D.F.; Gleason, K.L.; Paul, D.R.; Freeman, B.D. Hydrogen sorption in polymers for membrane applications. Polymer 2013, 54, 3026–3037. [Google Scholar] [CrossRef]
- Shao, L.; Low, B.T.; Chung, T.S.; Greenberg, A.R. Polymeric membranes for the hydrogen economy: Contemporary approaches and prospects for the future. J. Memb. Sci. 2009, 327, 18–31. [Google Scholar] [CrossRef]
- Knebel, A.; Wulfert-Holzmann, P.; Friebe, S.; Pavel, J.; Strauß, I.; Mundstock, A.; Steinbach, F.; Caro, J. Hierarchical Nanostructures of Metal-Organic Frameworks Applied in Gas Separating ZIF-8-on-ZIF-67 Membranes. Chem. A Eur. J. 2018, 24, 5728–5733. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, A.S.; Ba-Shammakh, M.; Usman, M.; Khan, M.F.; Dafallah, H.; Habib, M.A.M.; Al-Maythalony, B.A. High gas permselectivity in ZIF-302/polyimide self-consistent mixed-matrix membrane. J. Appl. Polym. Sci. 2020, 137, 1–11. [Google Scholar] [CrossRef]
- Mei, X.; Yang, S.; Lu, P.; Zhang, Y.; Zhang, J. Improving the Selectivity of ZIF-8/Polysulfone-Mixed Matrix Membranes by Polydopamine Modification for H2/CO2 Separation. Front. Chem. 2020, 8. [Google Scholar] [CrossRef]
- Mundstock, A.; Friebe, S.; Caro, J. On comparing permeation through Matrimid®-based mixed matrix and multilayer sandwich FAU membranes: H2/CO2 separation, support functionalization and ion exchange. Int. J. Hydrogen Energy 2017, 42, 279–288. [Google Scholar] [CrossRef]
- Perez, E.V.; Balkus, K.J.; Ferraris, J.P.; Musselman, I.H. Mixed-matrix membranes containing MOF-5 for gas separations. J. Memb. Sci. 2009, 328, 165–173. [Google Scholar] [CrossRef]
- Sánchez-Laínez, J.; Paseta, L.; Navarro, M.; Zornoza, B.; Téllez, C.; Coronas, J. Ultrapermeable Thin Film ZIF-8/Polyamide Membrane for H2/CO2 Separation at High Temperature without Using Sweep Gas. Adv. Mater. Interfaces 2018, 5, 1800647. [Google Scholar] [CrossRef]
- Yang, T.; Xiao, Y.; Chung, T.S. Poly-/metal-benzimidazole nano-composite membranes for hydrogen purification. Energy Environ. Sci. 2011, 4, 4171–4180. [Google Scholar] [CrossRef]
- Yang, T.; Shi, G.M.; Chung, T.S.C. Symmetric and asymmetric zeolitic imidazolate frameworks (ZIFs)/polybenzimidazole (PBI) nanocomposite membranes for hydrogen purification at high temperatures. Adv. Energy Mater. 2012, 2, 1358–1367. [Google Scholar] [CrossRef]
- Yang, T.; Chung, T.S. High performance ZIF-8/PBI nano-composite membranes for high temperature hydrogen separation consisting of carbon monoxide and water vapor. Int. J. Hydrogen Energy 2013, 38, 229–239. [Google Scholar] [CrossRef]
- Yang, T.; Chung, T.S. Room-temperature synthesis of ZIF-90 nanocrystals and the derived nano-composite membranes for hydrogen separation. J. Mater. Chem. A 2013, 1, 6081–6090. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Cao, Y.M.; Ding, X.L.; Zhou, M.Q.; Liu, J.H.; Yuan, Q. Poly(ethylene oxide) induced cross-linking modification of Matrimid membranes for selective separation of CO2. J. Memb. Sci. 2008, 320, 179–184. [Google Scholar] [CrossRef]
- Aceituno Melgar, V.M.; Kim, J.; Othman, M.R. Zeolitic imidazolate framework membranes for gas separation: A review of synthesis methods and gas separation performance. J. Ind. Eng. Chem. 2015, 28, 1–15. [Google Scholar] [CrossRef]
- Yun, S.; Ted Oyama, S. Correlations in palladium membranes for hydrogen separation: A review. J. Memb. Sci. 2011, 375, 28–45. [Google Scholar] [CrossRef]
- Conde, J.J.; Maroño, M.; Sánchez-Hervás, J.M. Pd-Based Membranes for Hydrogen Separation: Review of Alloying Elements and Their Influence on Membrane Properties. Sep. Purif. Rev. 2017, 46, 152–177. [Google Scholar] [CrossRef]
- Al-Maythalony, B.A.; Ghanem, A.S.; Ba-Shammakh, M.; Usman, M. Porous Membrane Containing Metal-Organic Frameworks. U.S. Patent App 16/285,724, 27 August 2020. [Google Scholar]
- García, M.G.; Marchese, J.; Ochoa, N.A. Aliphatic-aromatic polyimide blends for H2 separation. Int. J. Hydrogen Energy 2010, 35, 8983–8992. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Peng, N.; Chung, T.S. Gas separation membranes developed through integration of polymer blending and dual-layer hollow fiber spinning process for hydrogen and natural gas enrichments. J. Memb. Sci. 2010, 349, 156–166. [Google Scholar] [CrossRef]
- David, O.C.; Gorri, D.; Nijmeijer, K.; Ortiz, I.; Urtiaga, A. Hydrogen separation from multicomponent gas mixtures containing CO, N2 and CO2 using Matrimid® asymmetric hollow fiber membranes. J. Memb. Sci. 2012, 419–420, 49–56. [Google Scholar] [CrossRef]
- Lau, C.H.; Low, B.T.; Shao, L.; Chung, T.S. A vapor-phase surface modification method to enhance different types of hollow fiber membranes for industrial scale hydrogen separation. Int. J. Hydrogen Energy 2010, 35, 8970–8982. [Google Scholar] [CrossRef]
- Li, D.; Chung, T.S.; Wang, R. Morphological aspects and structure control of dual-layer asymmetric hollow fiber membranes formed by a simultaneous co-extrusion approach. J. Memb. Sci. 2004, 243, 155–175. [Google Scholar] [CrossRef]
- Clarizia, G.; Tasselli, F.; Bernardo, P. Effect of physical aging on gas transport in asymmetric polyimide hollow fibers prepared by triple-orifice spinneret. Polymers 2020, 12, 441. [Google Scholar] [CrossRef] [PubMed]
- Berchtold, K.A.; Dudeck, K.W.; Singh, R.P.; Dahe, G.J. Polybenzimidazole Hollow Fiber Membranes and Method for Making an Asymmetric Hollow Fiber Membrane. U.S. Patent 10071345B2, 11 September 2018. [Google Scholar]
- Dahe, G.J.; Singh, R.P.; Dudeck, K.W.; Yang, D.; Berchtold, K.A. Influence of non-solvent chemistry on polybenzimidazole hollow fiber membrane preparation. J. Memb. Sci. 2019, 577, 91–103. [Google Scholar] [CrossRef]
- Etxeberria-Benavides, M.; Johnson, T.; Cao, S.; Zornoza, B.; Coronas, J.; Sanchez-Lainez, J.; Sabetghadam, A.; Liu, X.; Andres-Garcia, E.; Kapteijn, F.; et al. PBI mixed matrix hollow fiber membrane: Influence of ZIF-8 filler over H2/CO2 separation performance at high temperature and pressure. Sep. Purif. Technol. 2020, 237, 116347. [Google Scholar] [CrossRef]
- Kumbharkar, S.C.; Liu, Y.; Li, K. High performance polybenzimidazole based asymmetric hollow fibre membranes for H2/CO2 separation. J. Memb. Sci. 2011, 375, 231–240. [Google Scholar] [CrossRef]
- Naderi, A.; Chung, T.S.; Weber, M.; Maletzko, C. High performance dual-layer hollow fiber membrane of sulfonated polyphenylsulfone/Polybenzimidazole for hydrogen purification. J. Memb. Sci. 2019, 591, 117292. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, B.; Lai, Z. Synthesis of ceramic hollow fiber supported zeolitic imidazolate framework-8 (ZIF-8) membranes with high hydrogen permeability. J. Memb. Sci. 2012, 421–422, 292–298. [Google Scholar] [CrossRef]
- Singh, R.P.; Dahe, G.J.; Dudeck, K.W.; Welch, C.F.; Berchtold, K.A. High temperature polybenzimidazole hollow fiber membranes for hydrogen separation and carbon dioxide capture from synthesis gas. Energy Procedia 2014, 63, 153–159. [Google Scholar] [CrossRef]
- Villalobos, L.F.; Hilke, R.; Akhtar, F.H.; Peinemann, K.V. Fabrication of Polybenzimidazole/Palladium Nanoparticles Hollow Fiber Membranes for Hydrogen Purification. Adv. Energy Mater. 2018, 8, 1701567. [Google Scholar] [CrossRef]
- Wang, J.W.; Li, N.X.; Li, Z.R.; Wang, J.R.; Xu, X.; Chen, C.S. Preparation and gas separation properties of Zeolitic imidazolate frameworks-8 (ZIF-8) membranes supported on silicon nitride ceramic hollow fibers. Ceram. Int. 2016, 42, 8949–8954. [Google Scholar] [CrossRef]
- Zhu, H.; Jie, X.; Wang, L.; Kang, G.; Liu, D.; Cao, Y. Enhanced gas separation performance of mixed matrix hollow fiber membranes containing post-functionalized S-MIL-53. J. Energy Chem. 2018, 27, 781–790. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, G.; Jiang, J. Adsorption and Diffusion of CO2 and CH4 in Zeolitic Imidazolate Framework-8: Effect of Structural Flexibility. J. Phys. Chem. C 2014, 118, 8788–8794. [Google Scholar] [CrossRef]
- Erucar, I.; Keskin, S. Computational Methods for MOF/Polymer Membranes. Chem. Rec. 2016, 16, 703–718. [Google Scholar] [CrossRef] [PubMed]





| Membrane Material | Strengths | Weaknesses |
|---|---|---|
| Metallic | Mechanical durability; resistance to H2 embrittlement; selectivity (dense) | H2 fluxes; chemical and thermal stability; cost and H2 embrittlement at low pressure and temperature in some materials, such as Pd |
| Silica | Tunable nature; high-temperature and high-pressure stability of microporous silica; high surface area; resistance to H2 embrittlement | Cost; manufacture reproducibility; stability at high temperature and embrittlement |
| Zeolite | Chemical, mechanical and thermal stability | Cost; manufacture reproducibility |
| Carbon-based | Versatility | Cost; selectivity; brittleness; chemical, mechanical and thermal stability |
| Polymer | Diffusivity; selectivity; H2 fluxes; permeabilities; cost and processability | Chemical, mechanical and thermal stability |
| Ref. | T (°C) | ΔP (Bar) | PeH2 (GPU) | PeN2 (GPU) | PeCO2 (GPU) | PeCH4 (GPU) | PeCO (GPU) | αH2/N2 | αH2/CO2 | αH2/CH4 | αH2/CO | Comments on Membrane Fabrication |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bernardo (2019) [74] | 25 | 1.0 | 40.0 | 0.53 | 13.3 | 0.44 | 75.5 | 3.0 | 90.9 | M1: Shell fluid none. Dope flow rate 5 g min−1 Protocol 1: no solvent-exchange | ||
| 47.0 | 0.63 | 21.9 | 0.54 | 74.6 | 2.1 | 87.0 | M2: Shell fluid NMP/water. Dope flow rate 5 g min−1 Protocol 1 | |||||
| 41.6 | 0.58 | 17.0 | 0.52 | 71.7 | 2.4 | 80.0 | M3: Shell fluid NMP/water. Dope flow rate 3.6 g min−1 Protocol 1 | |||||
| David (2012) [68] | 30 | 2.3 | 66.7 | 0.91 | 13.4 | 1.6 | 73.3 | 5.0 | 41.7 | air gap 12 cm | ||
| 4.1 | 65.8 | 0.94 | 14.4 | 1.6 | 70.0 | 4.6 | 41.1 | |||||
| 6.1 | 66.9 | 0.93 | 15.9 | 1.7 | 71.9 | 4.2 | 39.4 | |||||
| 8.0 | 64.1 | 0.92 | 16.8 | 1.6 | 69.7 | 3.8 | 40.1 | |||||
| 10.0 | 65.5 | 0.93 | 19.1 | 1.7 | 70.4 | 3.4 | 38.5 | |||||
| 2.2 | 159.0 | 9.1 | 40 | 9.1 | 17.5 | 4.0 | 17.5 | air gap 3 cm | ||||
| 4.1 | 164.2 | 10.0 | 41.0 | 9.5 | 16.4 | 4.0 | 17.3 | |||||
| 6.1 | 169.6 | 10.3 | 43.0 | 10.1 | 16.5 | 3.9 | 16.8 | |||||
| Favvas (2007) [72] | 40 | 342.2 | 23.1 | 84.9 | 30.6 | 26.0 | 14.8 | 4.0 | 11.2 | 13.2 | Without pyrolysis | |
| 40 | 2.9 | 0.11 | 0.08 | 0.09 | 0.07 | 26.1 | 37.8 | 31.9 | 43.5 | M1, N2 atmosphere | ||
| 20.5 | 0.27 | 6.3 | 0.30 | 0.59 | 75.8 | 3.2 | 68.2 | 34.7 | M2, H2O atmosphere | |||
| 17.6 | 0.12 | 2.6 | 0.16 | 0.33 | 146.5 | 6.7 | 109.9 | 53.3 | M3, CO2 atmosphere | |||
| 60 | 4.1 | 0.05 | 0.15 | 0.06 | 0.09 | 81.6 | 27.2 | 68.0 | 45.3 | M1 | ||
| 27.6 | 0.54 | 9.9 | 0.44 | 1.00 | 51.1 | 2.8 | 62.4 | 27.6 | M2 | |||
| 26.1 | 0.27 | 4.0 | 0.19 | 0.48 | 96.7 | 6.5 | 137.4 | 54.4 | M3 | |||
| 100 | 7.8 | 0.08 | 0.28 | 0.08 | 0.16 | 101.6 | 27.9 | 97.8 | 48.9 | M1 | ||
| 53.3 | 1.22 | 17.3 | 1.91 | 2.08 | 43.7 | 3.1 | 27.9 | 25.6 | M2 | |||
| 36.5 | 0.57 | 6.8 | 0.37 | 0.85 | 64.0 | 5.4 | 98.6 | 42.9 | M3 | |||
| Peer (2007) [73] | 20 | 9.0 | 70.2 | 4.1 | 17.0 | UBE polyimide | ||||||
| 40 | 9.0 | 74.0 | 4.3 | 17.3 | ||||||||
| 60 | 9.0 | 76.7 | 3.7 | 21.0 | ||||||||
| 80 | 9.0 | 80.7 | 2.2 | 37.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Castro, P.; Ortiz, A.; Gorri, D. Exploring the Potential Application of Matrimid® and ZIFs-Based Membranes for Hydrogen Recovery: A Review. Polymers 2021, 13, 1292. https://doi.org/10.3390/polym13081292
Fernández-Castro P, Ortiz A, Gorri D. Exploring the Potential Application of Matrimid® and ZIFs-Based Membranes for Hydrogen Recovery: A Review. Polymers. 2021; 13(8):1292. https://doi.org/10.3390/polym13081292
Chicago/Turabian StyleFernández-Castro, Pablo, Alfredo Ortiz, and Daniel Gorri. 2021. "Exploring the Potential Application of Matrimid® and ZIFs-Based Membranes for Hydrogen Recovery: A Review" Polymers 13, no. 8: 1292. https://doi.org/10.3390/polym13081292
APA StyleFernández-Castro, P., Ortiz, A., & Gorri, D. (2021). Exploring the Potential Application of Matrimid® and ZIFs-Based Membranes for Hydrogen Recovery: A Review. Polymers, 13(8), 1292. https://doi.org/10.3390/polym13081292






