Fabrication of Polycaprolactone/Nano Hydroxyapatite (PCL/nHA) 3D Scaffold with Enhanced In Vitro Cell Response via Design for Additive Manufacturing (DfAM)
Abstract
1. Introduction
2. Materials and Methods
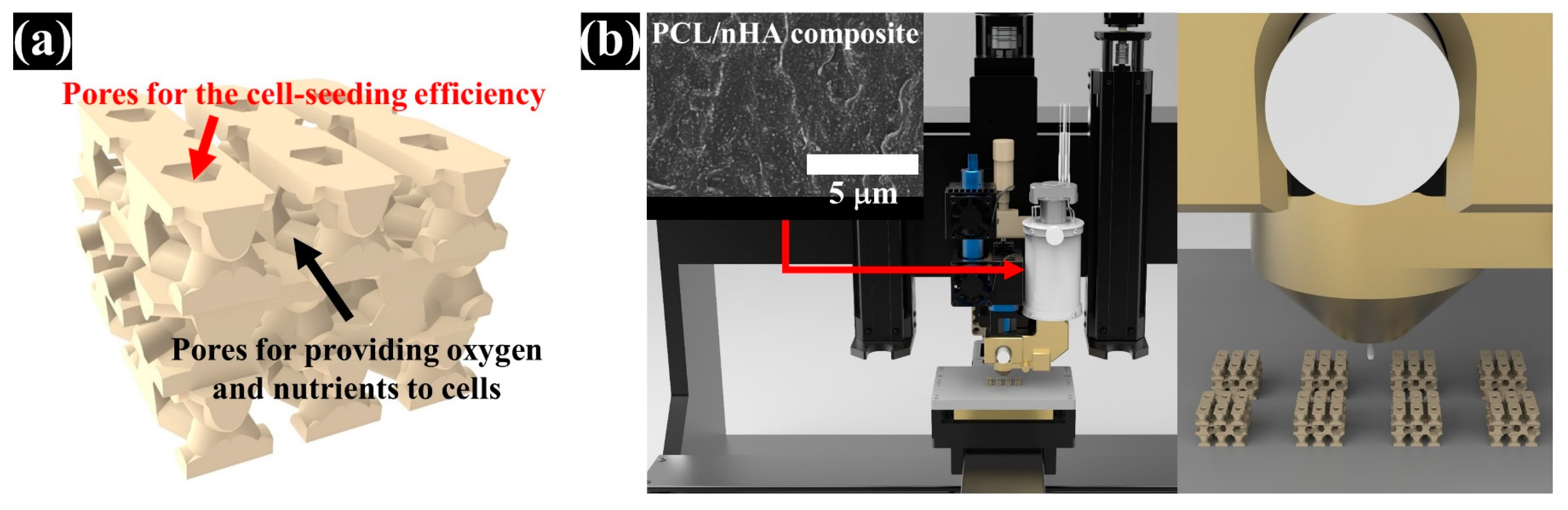
2.1. Design of PCL/nHA Scaffold with Dual-Pore Kagome Structure
2.2. Preparation and Fabrication of the PCL/nHA Scaffold with The Dual-Pore Kagome Structure
2.3. Characterization of the Fabricated Scaffolds
2.4. Osteoblast-Like Cell Culture and Cell-Growth Analysis of the Fabricated Scaffolds
2.5. Statistical Analysis
3. Results
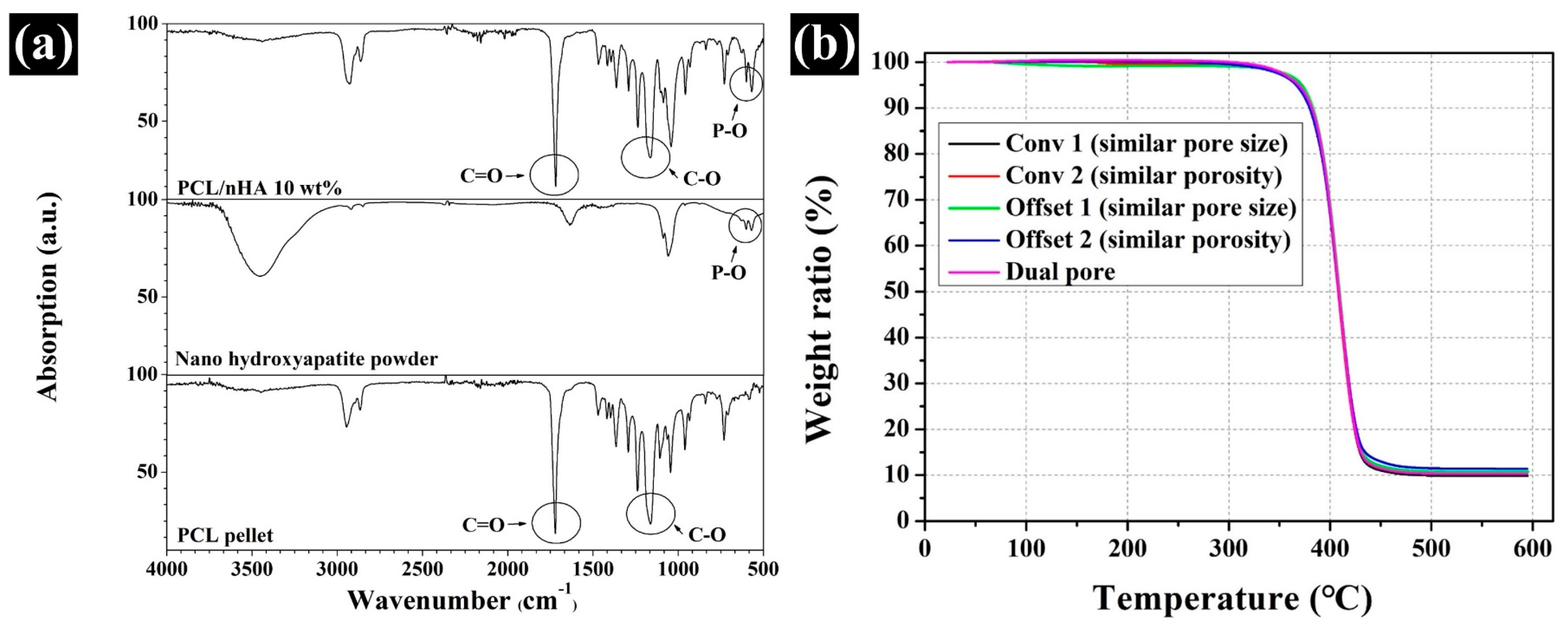
3.1. Comparison of the Chemical Component and nHA Content in the Fabricated Scaffold
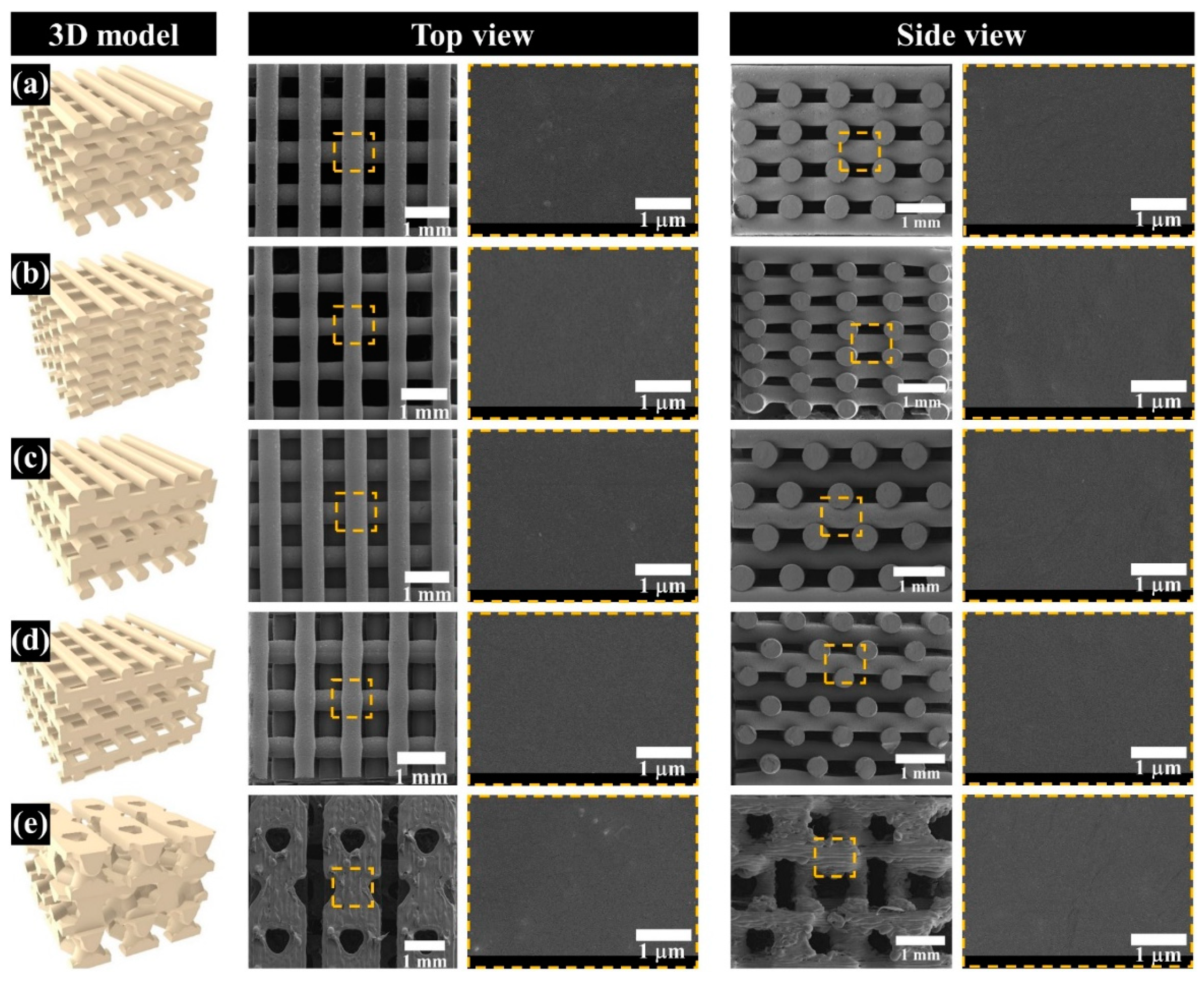
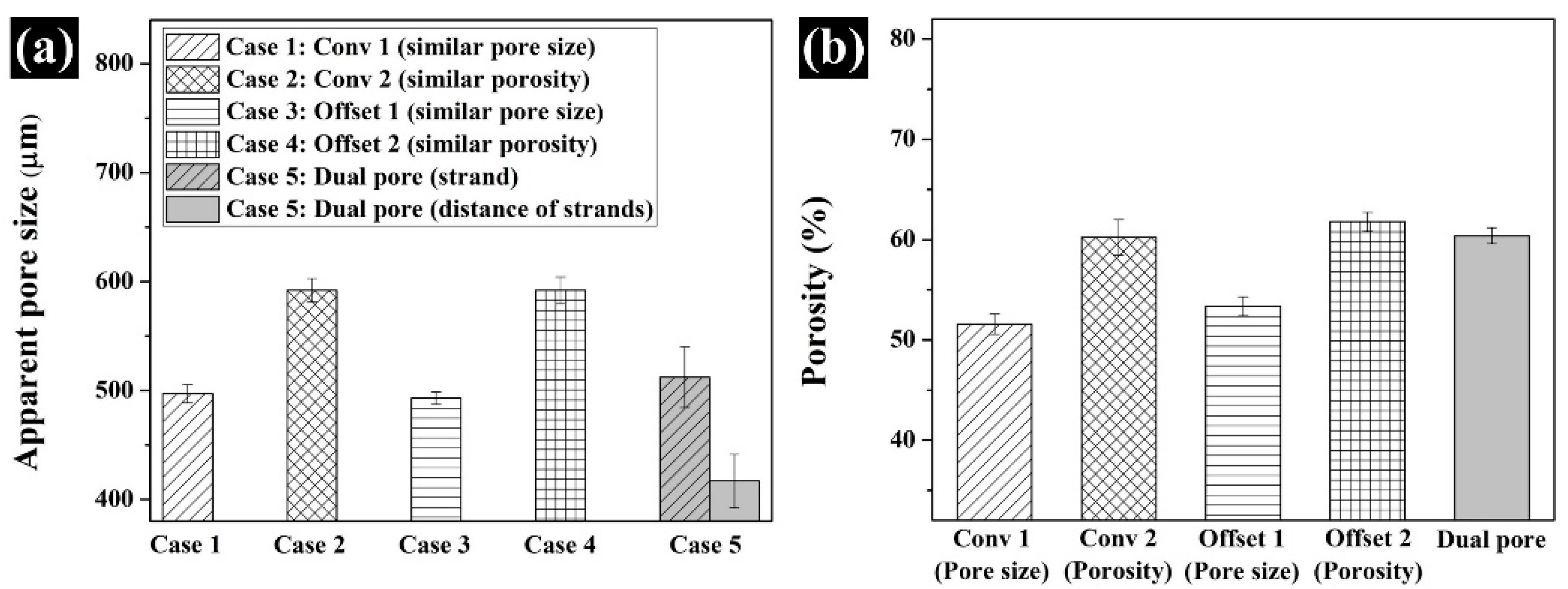
3.2. Comparison of the Morphological and Structural Characteristics of the Fabricated Scaffold
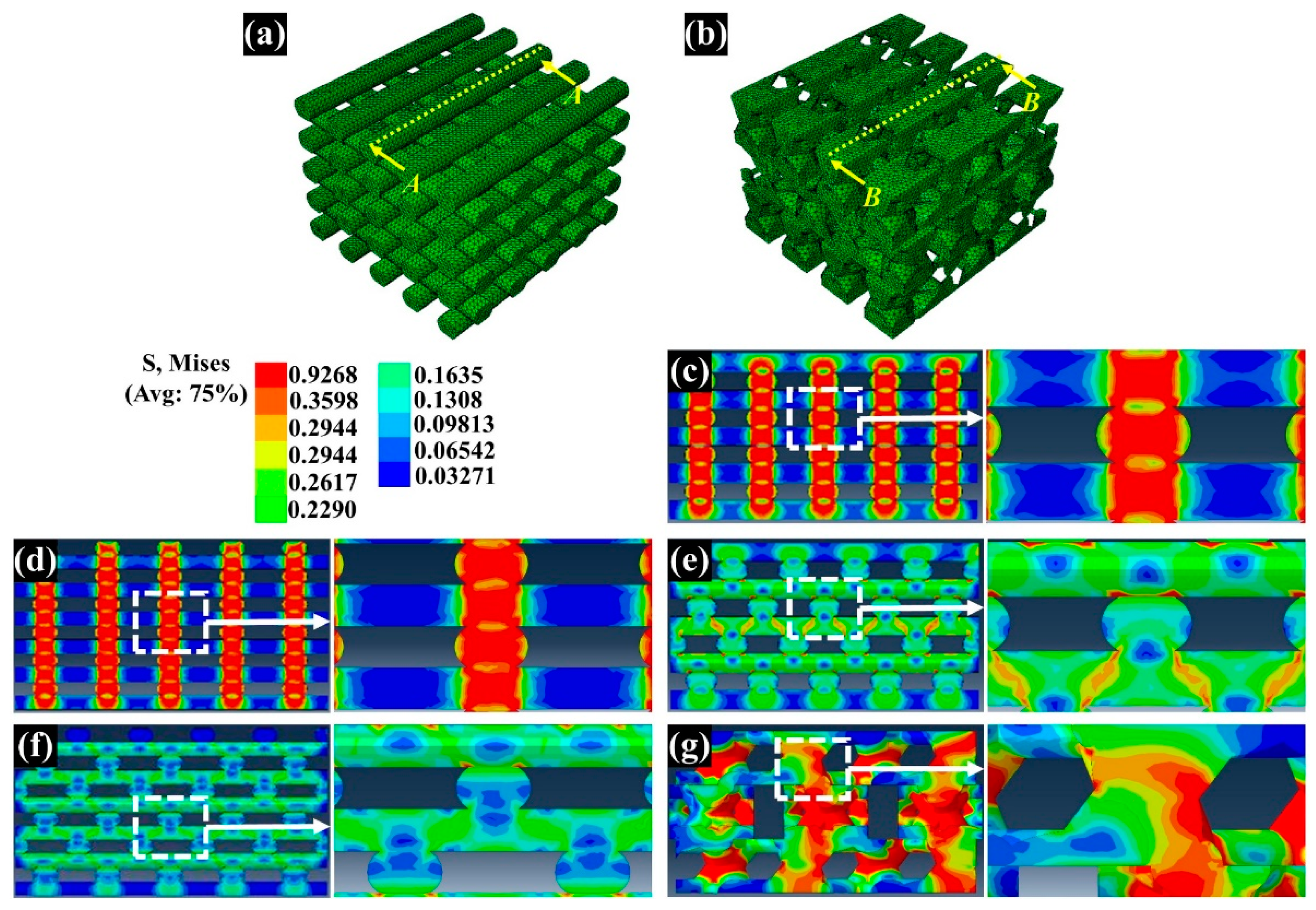
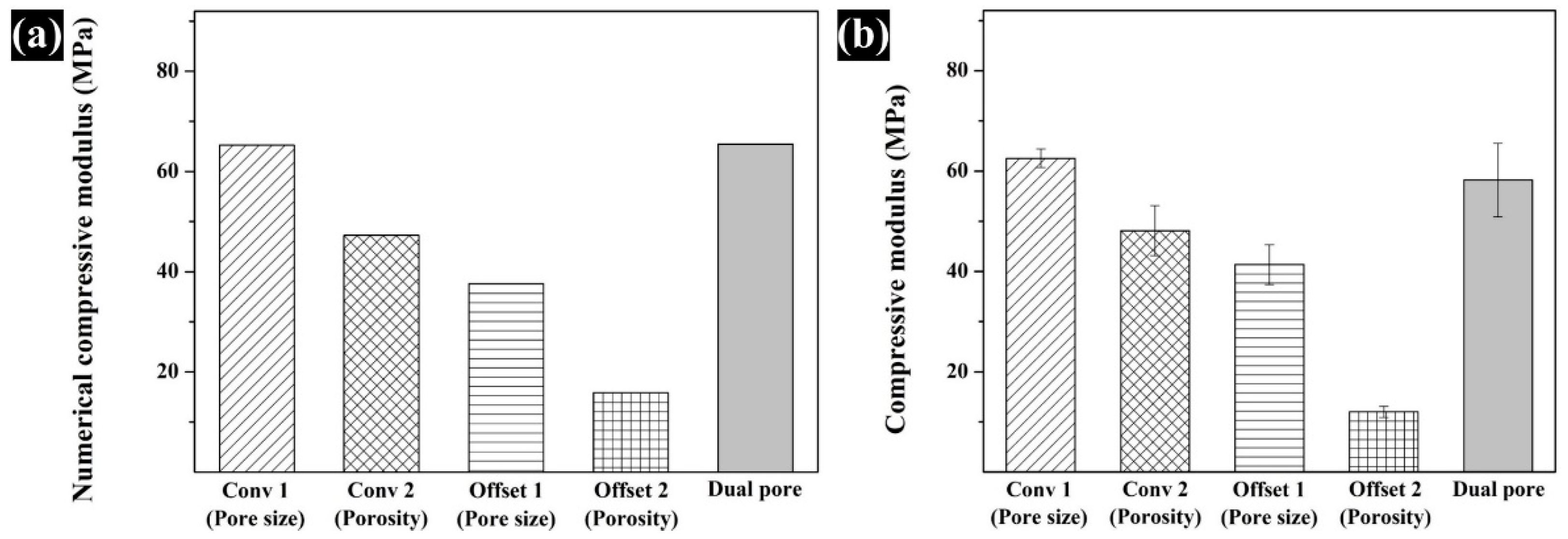
3.3. Assessment of the Compressive Modulus by Numerical and Experimental Analyses
3.4. Assessment of the In Vitro Cell Response on the Fabricated Scaffolds
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ma, H.; Feng, C.; Chang, J.; Wu, C. 3D-printed bioceramic scaffolds: From bone tissue engineering to tumor therapy. Acta Biomater. 2018, 79, 37–59. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xu, Q.; Teng, B.; Yu, C.; Li, J.; Song, L.; Lai, Y.-X.; Zhang, J.; Zheng, W.; Ren, P.-G. Investigation of angiogenesis in bioactive 3-dimensional poly (D,L-lactide-co-glycolide)/nano-hydroxyapatite scaffolds by in vivo multiphoton microscopy in murine calvarial critical bone defect. Acta Biomater. 2016, 42, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Dias, G.J.; Mahoney, P.; Hung, N.A.; Sharma, L.A.; Kalita, P.; Smith, R.A.; Kelly, R.J.; Ali, A. Osteoconduction in keratin–hydroxyapatite composite bone-graft substitutes. J. Biomed. Mater. Res. Part B 2017, 105, 2034–2044. [Google Scholar] [CrossRef]
- Cancedda, R.; Dozin, B.; Giannoni, P.; Quarto, R. Tissue engineering and cell therapy of cartilage and bone. Matrix Biol. 2003, 22, 81–91. [Google Scholar] [CrossRef]
- Hollister, S.J.; Maddox, R.D.; Taboas, J.M. Optimal design and fabrication of scaffolds to mimic tissue properties and satisfy biological constraints. Biomaterials 2002, 23, 4095–4103. [Google Scholar] [CrossRef]
- Holy, C.E.; Shoichet, M.S.; Davies, J.E. Engineering three-dimensional bone tissue in vitro using biodegradable scaffolds: Investigating initial cell-seeding density and culture period. J. Biomed. Mater. Res. Part A 2000, 51, 376–382. [Google Scholar] [CrossRef]
- Yoon, H.; Kim, G.H.; Koh, Y.H. A micro-scale surface-structured PCL scaffold fabricated by a 3D plotter and a chemical blowing agent. J. Biomat. Sci. Polym. E 2010, 21, 159–170. [Google Scholar] [CrossRef]
- Seyednejad, H.; Gawlitta, D.; Kuioer, R.; Bruin, A.; Nostrum, C.; Vermonden, T.; Dhert, W.; Hennink, W. In vivo biocompatibility and biodegradation of 3D-printed porous scaffolds based on a hydroxyl-functionalized poly(ε-caprolactone). Biomaterials 2012, 33, 4309–4318. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.J.; Lee, H.J.; Kim, G.H. A surface-modified poly(ε-caprolactone) scaffold comprising variable nanosized surface-roughness using a plasma treatment. Tissue Eng. Part C 2014, 20, 951–963. [Google Scholar] [CrossRef]
- Skoog, S.; Goering, P.; Narayan, R. Stereolithography in tissue engineering. J. Mater. Sci. Mater. Med. 2014, 25, 846–856. [Google Scholar] [CrossRef] [PubMed]
- Shuai, C.; Nie, Y.; Gao, C.; Feng, P.; Zhuang, J.; Zhou, Y.; Peng, S. The microstructure evolution of nanohydroxapatite powder sintered for bone tissue engineering. J. Exp. Nanosci. 2013, 8, 598–609. [Google Scholar] [CrossRef]
- Cho, Y.S.; Quan, M.; Lee, S.-H.; Hong, M.W.; Kim, Y.Y.; Cho, Y.-S. Assessment of ostegenesis for 3D-printed polycaprolactone/hydroxyapatite composite scaffold with enhanced exposure of hydroxyapatite using rat calvarial defect model. Compos. Sci. Technol. 2019, 184, 107844. [Google Scholar] [CrossRef]
- Prasadh, S.; Wong, R.C.W. Unraveling the mechanical strength of biomaterials used as a bone scaffold in oral and maxillofacial defects. Oral Sci. Int. 2018, 15, 48–55. [Google Scholar] [CrossRef]
- Liu, X.; Ma, P.X. Polymeric scaffolds for bone tissue engineering. Ann. Biomed. Eng. 2004, 32, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Dorj, B.; Park, J.-H.; Kim, H.-W. Robocasting chitosan/nanobioactive glass dual-pore structured scaffolds for bone engineering. Mater. Lett. 2012, 73, 119–122. [Google Scholar] [CrossRef]
- Kang, H.-W.; Rhie, J.-W.; Cho, D.-W. Development of a bi-pore scaffold using indirect solid freeform fabrication based on microstereolithography technology. Microelectron. Eng. 2009, 86, 941–944. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, T.G.; Kim, H.C.; Yang, D.-Y.; Park, T.G. Development of dual scale scaffolds via direct polymer melt deposition and electrospinning for applications in tissue regeneration. Acta Biomater. 2008, 4, 1198–1207. [Google Scholar] [CrossRef]
- Kang, N.-U.; Hong, M.W.; Kim, Y.Y.; Cho, Y.-S.; Lee, S.-J. Development of a powder extruder system for dual-pore tissue-engineering scaffold fabrication. J. Bionic Eng. 2019, 16, 686–695. [Google Scholar] [CrossRef]
- Cho, Y.S.; Hong, M.W.; Kim, S.-Y.; Lee, S.-J.; Lee, J.H.; Kim, Y.Y.; Cho, Y.-S. Fabrication of dual-pore scaffolds using SLUP (salt leaching using powder) and WNM (wire-network molding). Mat. Sci. Eng. C-Mater. 2014, 45, 546–555. [Google Scholar] [CrossRef]
- Lee, M.-Y.; Liu, S.-W.; Chen, J.-P.; Liao, H.-T.; Tsai, W.-W.; Wang, H.-C. In vitro experiments on laser sintered porous PCL scaffolds with polymer hydrogel for bone repair. J. Mech. Med. Biol. 2011, 11, 983–992. [Google Scholar] [CrossRef]
- Ferreira, J.; Gloria, A.; Cometa, S.; Coellho, J.F.J.; Domingos, M. Effect of in vitro enzymatic degradation on 3D printed poly(ε-caprolactone) scaffolds: Morphological, chemical and mechanical properties. J. Appl. Biomater. Funct. Mater. 2017, 15, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Domingos, M.; Intranuovo, F.; Russo, T.; De Santis, R.; Gloria, A.; Ambrosio, L.; Ciurana, J.; Bartolo, P. The first systematic analysis of 3D rapid prototyped poly(ε-caprolactone) scaffolds manufactured through BioCell printing: The effect of pore size and geometry on compressive mechanical behaviour and in vitro hMSC viability. Biofabrication 2013, 5, 045004. [Google Scholar] [CrossRef]
- Hoque, M.E.; Hutmacher, D.W.; Feng, W.; Li, S.; Huang, M.-H.; Vert, M.; Wong, Y.S. Fabrication using a rapid prototyping system and in vitro characterization of PEG-PCL-PLA scaffolds for tissue engineering. J. Biomater. Sci. Polym. Ed. 2005, 16, 1595–1610. [Google Scholar] [CrossRef] [PubMed]
- Robionet, M.; Polonio, E.; Guerra, A.J.; Martin, J.; Puig, T.; Ciurana, J. Design of a scaffold parameter selection system with additive manufacturing for a biomedical cell culture. Materials 2018, 11, 1427. [Google Scholar] [CrossRef] [PubMed]
- Park, S.A.; Lee, S.H.; Kim, W.D. Fabrication of porous polycaprolactone/hydroxyapatite (PCL/HA) blend scaffolds using a 3D plotting system for bone tissue engineering. Bioprocess. Biosyst. Eng. 2011, 34, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Yilgor, P.; Sousa, R.A.; Reis, R.L.; Hasirci, N.; Hasirci, V. 3D plotted PCL scaffolds for stem cell based bone tissue engineering. Macromol. Symp. 2008, 269, 92–99. [Google Scholar] [CrossRef]
- Cho, Y.S.; Quan, M.; Kang, N.-U.; Jeong, H.-J.; Hong, M.W.; Kim, Y.Y.; Cho, Y.-S. Strategy for enhancing mechanical properties and bone regeneration of 3D polycaprolactone kagome scaffold: Nano hydroxyapatite composite and its exposure. Eur. Polym. J. 2020, 134, 109814. [Google Scholar] [CrossRef]
- Lee, S.-H.; Cho, Y.S.; Hong, M.W.; Lee, B.-K.; Park, Y.; Park, S.-H.; Kim, Y.Y.; Cho, Y.-S. Mechanical properties and cell-culture characteristics of a polycaprolactone kagome-structure scaffold fabricated by a precision extruding deposition system. Biomed. Mater. 2017, 12, 055003. [Google Scholar] [CrossRef]
- Murphy, C.M.; Haugh, M.G.; O’Brien, F.J. The effect of mean pore size on cell attachment, proliferation and migration in collagen-glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 2010, 31, 461–466. [Google Scholar] [CrossRef]
- Zadpoor, A.A. Bone tissue regeneration: The role of scaffold geometry. Biomater. Sci. 2015, 3, 231–245. [Google Scholar] [CrossRef]
- Mantila Roosa, S.M.; Kemppainen, J.M.; Moffitt, E.N.; Krebsbach, P.H.; Hollister, S.J. The pore size of polycaprolactone scaffolds has limited influence on bone regeneration in an in vivo model. J. Biomed. Mater. Res. Part A 2010, 92, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Abd Razak, S.I.; Ahmad Sharif, N.F.; Abdul Rahman, W.A.W. Biodegradable polymers and their bone applications: A review. Int. J. Sci. Basic Appl. 2012, 12, 31–49. [Google Scholar]
- Henkel, J.; Woodruff, M.A.; Epari, D.R.; Steck, R.; Clatt, V.; Dickinson, I.C.; Choong, P.F.M.; Schuetz, M.A.; Hutmacher, D.W. Bone regeneration based on tissue engineering conceptions—A 21st century perspective. Bone Res. 2013, 3, 216–248. [Google Scholar] [CrossRef] [PubMed]
- Baino, F.; Fiorilli, S.; Vitale-Brovarone, C. Bioactive glass-based materials with hierarchical porosity for medical applications: Review of recent advances. Acta Biomater. 2016, 42, 18–32. [Google Scholar] [CrossRef] [PubMed]

| 2D Image | 3D Image | Target Pore Size | Target Porosity | |
|---|---|---|---|---|
| Conv 1 (similar pore size) |  |  | 500 μm (distance of strands) | 50% |
| Conv 2 (similar porosity) |  |  | 600 μm (distance of strands) | 60% |
| Offset 1 (similar pore size) |  |  | 500 μm (distance of strands) | 50% |
| Offset 2 (similar porosity) |  |  | 600 μm (distance of strands) | 60% |
| Dual pore |  |  | 500 μm (strand) | 60% |
| 500 μm (distance of strands) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, Y.S.; Gwak, S.-J.; Cho, Y.-S. Fabrication of Polycaprolactone/Nano Hydroxyapatite (PCL/nHA) 3D Scaffold with Enhanced In Vitro Cell Response via Design for Additive Manufacturing (DfAM). Polymers 2021, 13, 1394. https://doi.org/10.3390/polym13091394
Cho YS, Gwak S-J, Cho Y-S. Fabrication of Polycaprolactone/Nano Hydroxyapatite (PCL/nHA) 3D Scaffold with Enhanced In Vitro Cell Response via Design for Additive Manufacturing (DfAM). Polymers. 2021; 13(9):1394. https://doi.org/10.3390/polym13091394
Chicago/Turabian StyleCho, Yong Sang, So-Jung Gwak, and Young-Sam Cho. 2021. "Fabrication of Polycaprolactone/Nano Hydroxyapatite (PCL/nHA) 3D Scaffold with Enhanced In Vitro Cell Response via Design for Additive Manufacturing (DfAM)" Polymers 13, no. 9: 1394. https://doi.org/10.3390/polym13091394
APA StyleCho, Y. S., Gwak, S.-J., & Cho, Y.-S. (2021). Fabrication of Polycaprolactone/Nano Hydroxyapatite (PCL/nHA) 3D Scaffold with Enhanced In Vitro Cell Response via Design for Additive Manufacturing (DfAM). Polymers, 13(9), 1394. https://doi.org/10.3390/polym13091394






