Kinetic Analysis of the Curing Process of Biobased Epoxy Resin from Epoxidized Linseed Oil by Dynamic Differential Scanning Calorimetry
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Kinetic Study by Differential Scanning Calorimetry (DSC)
2.3. Theoretical Background
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Charlet, K.; Jernot, J.P.; Eve, S.; Gomina, M.; Breard, J. Multi-scale morphological characterisation of flax: From the stem to the fibrils. Carbohydr. Polym. 2010, 82, 54–61. [Google Scholar] [CrossRef]
- Cloutier, S. Linseed. Ref. Modul. Food Sci. 2016. [Google Scholar] [CrossRef]
- Gruia, A.; Dumbravă, D.-G.; Moldovan, C.; Bordean, D.-M. Fatty acids composition and oil characteristics of linseed (Linum usitatissimum L.) from Romania. J. Agroaliment. Process. Technol. 2012, 18, 136–140. [Google Scholar]
- Dixit, S.; Kanakraj, S.; Rehman, A. Linseed oil as a potential resource for bio-diesel: A review. Renew. Sustain. Energy Rev. 2012, 16, 4415–4421. [Google Scholar] [CrossRef]
- Concenço, F.I.G.d.R.; Gonzales, R.N.d.L.; Vizzotto, M.; Nora, L. Manufacturing and sensorial acceptance of cereal bars enriched with flaxseed (Linum usitatissimum) flour. J. Food Res. 2019, 8, 1–11. [Google Scholar] [CrossRef]
- Uyumaz, A. Experimental evaluation of linseed oil biodiesel/diesel fuel blends on combustion, performance and emission characteristics in a DI diesel engine. Fuel 2020, 267, 117150. [Google Scholar] [CrossRef]
- Wang, Y.; Li, D.; Wang, L.-J.; Chiu, Y.L.; Chen, X.D.; Mao, Z.-H.; Song, C.-F. Optimization of extrusion of flaxseeds for in vitro protein digestibility analysis using response surface methodology. J. Food Eng. 2008, 85, 59–64. [Google Scholar] [CrossRef]
- Sanyaolu, N.; Awosanya, A.; Sonde, O.; Kareem, F.; Yussuf, S.; Akinwunmi, F.; Ibikunle, A. Comparative Evaluation of the Alkyd Resins of the Composite Oils of Soybean (Ricinus Communis) and Castor (Glycine Max) Seed Oils With Castor Seed Oil for Alkyd Paint Formulation. J. Chem. Soc. Niger. 2019, 44, 914–921. [Google Scholar]
- Copaci, S.; Jurcoane, Ş. Researches Concerning the Use of Camelina Oil in the Composition of Cosmetic Products. Sci. Bull. Ser. F. Biotechnol. 2019, 23, 173–177. [Google Scholar]
- Guzman Barrera, N.I. Eco-Compatible Syntheses of Bio-Based Solvents for the Paint and Coating Industry; OATO: Toulouse, France, 2018. [Google Scholar]
- Pan, X.; Sengupta, P.; Webster, D.C. High biobased content epoxy-anhydride thermosets from epoxidized sucrose esters of Fatty acids. Biomacromolecules 2011, 12, 2416–2428. [Google Scholar] [CrossRef]
- Vereshchagin, A.; Novitskaya, G.V. The triglyceride composition of linseed oil. J. Am. Oil Chem. Soc. 1965, 42, 970–974. [Google Scholar] [CrossRef] [PubMed]
- Fombuena, V.; Petrucci, R.; Dominici, F.; Jorda-Vilaplana, A.; Montanes, N.; Torre, L. Maleinized Linseed Oil as Epoxy Resin Hardener for Composites with High Bio Content Obtained from Linen Byproducts. Polymers 2019, 11, 301. [Google Scholar] [CrossRef] [PubMed]
- Carbonell-Verdu, A.; Garcia-Garcia, D.; Dominici, F.; Torre, L.; Sanchez-Nacher, L.; Balart, R. PLA films with improved flexibility properties by using maleinized cottonseed oil. Eur. Polym. J. 2017, 91, 248–259. [Google Scholar] [CrossRef]
- Liminana, P.; Garcia-Sanoguera, D.; Quiles-Carrillo, L.; Balart, R.; Montanes, N. Optimization of Maleinized Linseed Oil Loading as a Biobased Compatibilizer in Poly(Butylene Succinate) Composites with Almond Shell Flour. Materials 2019, 12, 685. [Google Scholar] [CrossRef] [PubMed]
- Fombuena, V.; Sanchez-Nacher, L.; Samper, M.D.; Juarez, D.; Balart, R. Study of the Properties of Thermoset Materials Derived from Epoxidized Soybean Oil and Protein Fillers. J. Am. Oil Chem. Soc. 2013, 90, 449–457. [Google Scholar] [CrossRef]
- Quiles-Carrillo, L.; Duart, S.; Montanes, N.; Torres-Giner, S.; Balart, R. Enhancement of the mechanical and thermal properties of injection-molded polylactide parts by the addition of acrylated epoxidized soybean oil. Mater. Des. 2018, 140, 54–63. [Google Scholar] [CrossRef]
- Musa, C.; Kervoëlen, A.; Danjou, P.-E.; Bourmaud, A.; Delattre, F. Bio-based unidirectional composite made of flax fibre and isosorbide-based epoxy resin. Mater. Lett. 2020, 258, 126818. [Google Scholar] [CrossRef]
- Espinosa, J.P.; Hanazumi, V.; Stefani, P.M.; Ruseckaite, R.A. Curing behavior and properties of high biosourced epoxy resin blends based on a triepoxy monomer and a tricarboxylic acid hardener from 10-undecenoic acid. Polym. Test. 2020, 81, 106208. [Google Scholar] [CrossRef]
- Falcão, V.G.O.; de Carvalho Carneiro, D.; Pereira, S.A.; da Silva, M.R.D.; Candé, A.; da Cunha Lima, S. Analyzing the toxicity of bisphenol-A to microalgae for ecotoxicological applications. Environ. Monit. Assess. 2020, 192, 8. [Google Scholar] [CrossRef]
- Janković, M.R.; Govedarica, O.M.; Sinadinović-Fišer, S.V. The epoxidation of linseed oil with in situ formed peracetic acid: A model with included influence of the oil fatty acid composition. Ind. Crop. Prod. 2020, 143, 111881. [Google Scholar] [CrossRef]
- Miyagawa, H.; Mohanty, A.K.; Misra, M.; Drzal, L.T. Thermo-physical and impact properties of epoxy containing epoxidized linseed oil, 1. Macromol. Mater. Eng. 2004, 289, 629–635. [Google Scholar] [CrossRef]
- Boquillon, N.; Fringant, C. Polymer networks derived from curing of epoxidised linseed oil: Influence of different catalysts and anhydride hardeners. Polymer 2000, 41, 8603–8613. [Google Scholar] [CrossRef]
- Lascano, D.; Quiles-Carrillo, L.; Balart, R.; Boronat, T.; Montanes, N. Kinetic analysis of the curing of a partially biobased epoxy resin using dynamic differential scanning calorimetry. Polymers 2019, 11, 391. [Google Scholar] [CrossRef] [PubMed]
- Pin, J.M.; Sbirrazzuoli, N.; Mija, A. From epoxidized linseed oil to bioresin: An overall approach of epoxy/anhydride cross-linking. ChemSusChem 2015, 8, 1232–1243. [Google Scholar] [CrossRef]
- Roşu, D.; Caşcaval, C.; Mustątǎ, F.; Ciobanu, C. Cure kinetics of epoxy resins studied by non-isothermal DSC data. Thermochim. Acta 2002, 383, 119–127. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Chrissafis, K.; Di Lorenzo, M.L.; Koga, N.; Pijolat, M.; Roduit, B.; Sbirrazzuoli, N.; Suñol, J.J. ICTAC Kinetics Committee recommendations for collecting experimental thermal analysis data for kinetic computations. Thermochim. Acta 2014, 590, 1–23. [Google Scholar] [CrossRef]
- Yi, C.; Rostron, P.; Vahdati, N.; Gunister, E.; Alfantazi, A. Curing kinetics and mechanical properties of epoxy based coatings: The influence of added solvent. Prog. Org. Coat. 2018, 124, 165–174. [Google Scholar] [CrossRef]
- Wan, J.; Li, C.; Bu, Z.-Y.; Xu, C.-J.; Li, B.-G.; Fan, H. A comparative study of epoxy resin cured with a linear diamine and a branched polyamine. Chem. Eng. J. 2012, 188, 160–172. [Google Scholar] [CrossRef]
- Kissinger, H.E. Variation of Peak Temperature With Heating Rute in Differential Thermal Analysis. J. Res. Natl. Bur. Stand. 1956, 57, 217. [Google Scholar] [CrossRef]
- Budrugeac, P.; Segal, E. Applicability of the Kissinger equation in thermal analysis. J. Therm. Anal. Calorim. 2007, 88, 703–707. [Google Scholar] [CrossRef]
- Farjas, J.; Butchosa, N.; Roura, P. A simple kinetic method for the determination of the reaction model from non-isothermal experiments. J. Therm. Anal. Calorim. 2010, 102, 615–625. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Friedman, H.L. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic plastic. J. Polym. Sci. Part C Polym. Symp. 1964, 6, 183–195. [Google Scholar] [CrossRef]
- Sbirrazzuoli, N. Advanced Isoconversional Kinetic Analysis for the Elucidation of Complex Reaction Mechanisms: A New Method for the Identification of Rate-Limiting Steps. Molecules 2019, 24, 1683. [Google Scholar] [CrossRef] [PubMed]
- Doyle, C. Estimating isothermal life from thermogravimetric data. J. Appl. Polym. Sci. 1962, 6, 639–642. [Google Scholar] [CrossRef]
- Ozawa, T. A new method of analyzing thermogravimetric data. Bull. Chem. Soc. Jpn. 1965, 38, 1881–1886. [Google Scholar] [CrossRef]
- Akahira, T.; Sunose, T. Transactions of Joint Convention of Four Electrical Institutes; Res. Rep. Chiba Inst. Technol.: Chiba, Japan, 1969; p. 246. [Google Scholar]
- Starink, M. Analysis of hydrogen desorption from linear heating experiments: Accuracy of activation energy determinations. Int. J. Hydrog. Energy 2018, 43, 6632–6641. [Google Scholar] [CrossRef]
- Málek, J. The kinetic analysis of non-isothermal data. Thermochim. Acta 1992, 200, 257–269. [Google Scholar] [CrossRef]
- Senum, G.I.; Yang, R.T. Rational approximations of the integral of the Arrhenius function. J. Therm. Anal. 1977, 11, 445–447. [Google Scholar] [CrossRef]
- Flynn, J.H. The ‘temperature integral’—Its use and abuse. Thermochim. Acta 1997, 300, 83–92. [Google Scholar] [CrossRef]
- Rucigaj, A.; Alic, B.; Krajnc, M.; Sebenik, U. Investigation of cure kinetics in a system with reactant evaporation: Epoxidized soybean oil and maleic anhydride case study. Eur. Polym. J. 2014, 52, 105–116. [Google Scholar] [CrossRef]
- Santacesaria, E.; Turco, R.; Russo, V.; Di Serio, M.; Tesser, R. Kinetics of Soybean Oil Epoxidation in a Semibatch Reactor. Ind. Eng. Chem. Res. 2020, 59, 21700–21711. [Google Scholar] [CrossRef]
- Santacesaria, E.; Turco, R.; Russo, V.; Tesser, R.; Di Serio, M. Soybean Oil Epoxidation: Kinetics of the Epoxide Ring Opening Reactions. Processes 2020, 8, 1134. [Google Scholar] [CrossRef]
- Tan, S.G.; Ahmad, Z.; Chow, W.S. Relationships of cure kinetics and processing for epoxidized soybean oil bio-thermoset. Ind. Crop. Prod. 2013, 43, 378–385. [Google Scholar] [CrossRef]
- Wuzella, G.; Mahendran, A.R.; Mueller, U.; Kandelbauer, A.; Teischinger, A. Photocrosslinking of an Acrylated Epoxidized Linseed Oil: Kinetics and its Application for Optimized Wood Coatings. J. Polym. Environ. 2012, 20, 1063–1074. [Google Scholar] [CrossRef]
- Menager, C.; Guigo, N.; Vincent, L.; Sbirrazzuoli, N. Polymerization kinetic pathways of epoxidized linseed oil with aliphatic bio-based dicarboxylic acids. J. Polym. Sci. 2020, 58, 1717–1727. [Google Scholar] [CrossRef]
- Mahendran, A.R.; Wuzella, G.; Kandelbauer, A.; Aust, N. Thermal cure kinetics of epoxidized linseed oil with anhydride hardener. J. Therm. Anal. Calorim. 2012, 107, 989–998. [Google Scholar] [CrossRef]
- Carbonell-Verdu, A.; Bernardi, L.; Garcia-Garcia, D.; Sanchez-Nacher, L.; Balart, R. Development of environmentally friendly composite matrices from epoxidized cottonseed oil. Eur. Polym. J. 2015, 63, 1–10. [Google Scholar] [CrossRef]
- Rocks, J.; George, G.A.; Vohwinkel, F. Curing kinetics and thermomechanical behaviour of co-anhydride cured aminoglycidyl epoxy resins. Polym. Int. 2003, 52, 1758–1766. [Google Scholar] [CrossRef]
- Samper, M.D.; Ferri, J.M.; Carbonell-Verdu, A.; Balart, R.; Fenollar, O. Properties of biobased epoxy resins from epoxidized linseed oil (ELO) crosslinked with a mixture of cyclic anhydride and maleinized linseed oil. Express Polym. Lett. 2019, 13, 407–418. [Google Scholar] [CrossRef]
- Fischer, R. Polyesters from expoxides and anhydrides. J. Polym. Sci. 1960, 44, 155–172. [Google Scholar] [CrossRef]
- Tanaka, Y.; Kakiuchi, H. Study of epoxy compounds. Part I. curing reactions of epoxy resin and acid anhydride with amine and alcohol as catalyst. J. Appl. Polym. Sci. 1963, 7, 1063–1081. [Google Scholar] [CrossRef]
- Tanaka, Y.; Kakiuchi, H. Study of epoxy compounds. Part VI. Curing reactions of epoxy resin and acid anhydride with amine, acid, alcohol, and phenol as catalysts. J. Polym. Sci. Part A Gen. Pap. 1964, 2, 3405–3430. [Google Scholar] [CrossRef]
- Mark, H.F.; Kroschwitz, J.I. Encyclopedia of Polymer Science and Engineering; Wiley: New York, NY, USA, 1985. [Google Scholar]
- Samper, M.D.; Petrucci, R.; Sanchez-Nacher, L.; Balart, R.; Kenny, J.M. New environmentally friendly composite laminates with epoxidized linseed oil (ELO) and slate fiber fabrics. Compos. Part B Eng. 2015, 71, 203–209. [Google Scholar] [CrossRef]
- Sbirrazzuoli, N.; Brunel, D.; Elegant, L. Neural networks for kinetic parameters determination, signal filtering and deconvolution in thermal analysis. J. Therm. Anal. 1997, 49, 1553–1564. [Google Scholar] [CrossRef]
- Flynn, J.H.; Wall, L.A. General treatment of the thermogravimetry of polymers. J. Res. Natl. Bur. Stand. Part A 1966, 70, 487–523. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction kinetics in differential thermal analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Akahira, T.; Sunose, T. Research Report Chiba Institute of Technology. In Proceedings of the Joint Convention of Four Electrical Institutes; Chiba Institute of Technology: Chiba, Japan, 1971; pp. 22–31. [Google Scholar]
- Jouyandeh, M.; Paran, S.M.R.; Khadem, S.S.M.; Ganjali, M.R.; Akbari, V.; Vahabi, H.; Saeb, M.R. Nonisothermal cure kinetics of epoxy/MnxFe3-xO4 nanocomposites. Prog. Org. Coat. 2020, 140, 105505. [Google Scholar] [CrossRef]
- Lv, J.; Hong, J.; Liang, B.; Zhao, E.; Zeng, K.; Chen, M.; Hu, J.; Yang, G. Study of the curing kinetics of melamine/phthalonitrile resin system. Thermochim. Acta 2020, 683, 178442. [Google Scholar] [CrossRef]
- Morancho, J.M.; Fernández-Francos, X.; Acebo, C.; Ramis, X.; Salla, J.M.; Serra, À. Thermal curing of an epoxy-anhydride system modified with hyperbranched poly(ethylene imine)s with different terminal groups. J. Therm. Anal. Calorim. 2016, 127, 645–654. [Google Scholar] [CrossRef]
- Criado, J.; Malek, J.; Ortega, A. Applicability of the Master Plots in Kinetic Analysis. Thermochim. Acta 1989, 147, 377–385. [Google Scholar] [CrossRef]
- Criado, J.; Málek, J.; Gotor, F. The applicability of the Šesták-Berggren kinetic equation in constant rate thermal analysis (CRTA). Thermochim. Acta 1990, 158, 205–213. [Google Scholar] [CrossRef]
- Krishnaswamy, S.; Marchante, V.; Abhyankar, H.; Huang, Z.; Brighton, J. Non-isothermal cure kinetics of aerogel/epoxy composites using differential scanning calorimetry. Polym. Plast. Technol. Mater. 2019, 58, 1757–1765. [Google Scholar] [CrossRef]
- El-Thaher, N.; Mekonnen, T.; Mussone, P.; Bressler, D.; Choi, P. Nonisothermal DSC Study of Epoxy Resins Cured with Hydrolyzed Specified Risk Material. Ind. Eng. Chem. Res. 2013, 52, 8189–8199. [Google Scholar] [CrossRef]





| Reaction Model | Code | f(α) | |
|---|---|---|---|
| 2D-Reaction | R2 | (15) | |
| 2D-Diffusion | D2 | (16) | |
| Johnson-Mehl-Avrami | JMA(n) | (17) | |
| Jander | D3 | (18) |
| β (°C·min−1) | Curing Cycle | |
|---|---|---|
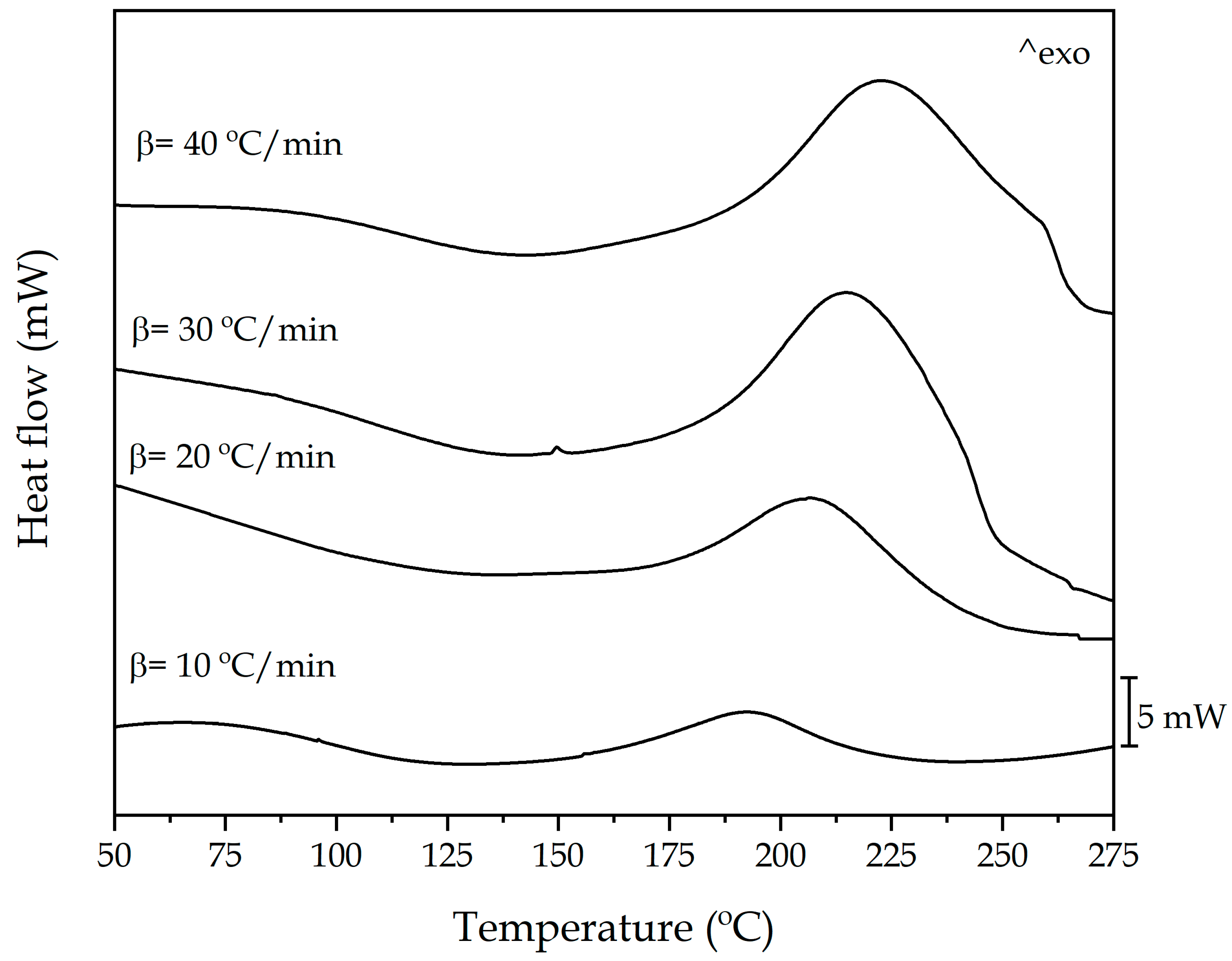
| Tp (°C) | ΔHT (J·g−1) | |
| 10 | 192.80 | 140.72 |
| 20 | 206.70 | 146.07 |
| 30 | 216.17 | 198.51 |
| 40 | 223.32 | 215.77 |
| Isoconversional Method | Apparent Activation Energy, Ea (kJ/mol) |
|---|---|
| Friedman | 66.83 ± 2.70 |
| Flynn–Wall–Ozawa (FWO) | 66.27 ± 3.56 |
| Kissinger–Akahira–Sunose (KAS) | 66.22 ± 2.62 |
| Starink | 68.57 ± 4.38 |
| β (°C/min) | |||
|---|---|---|---|
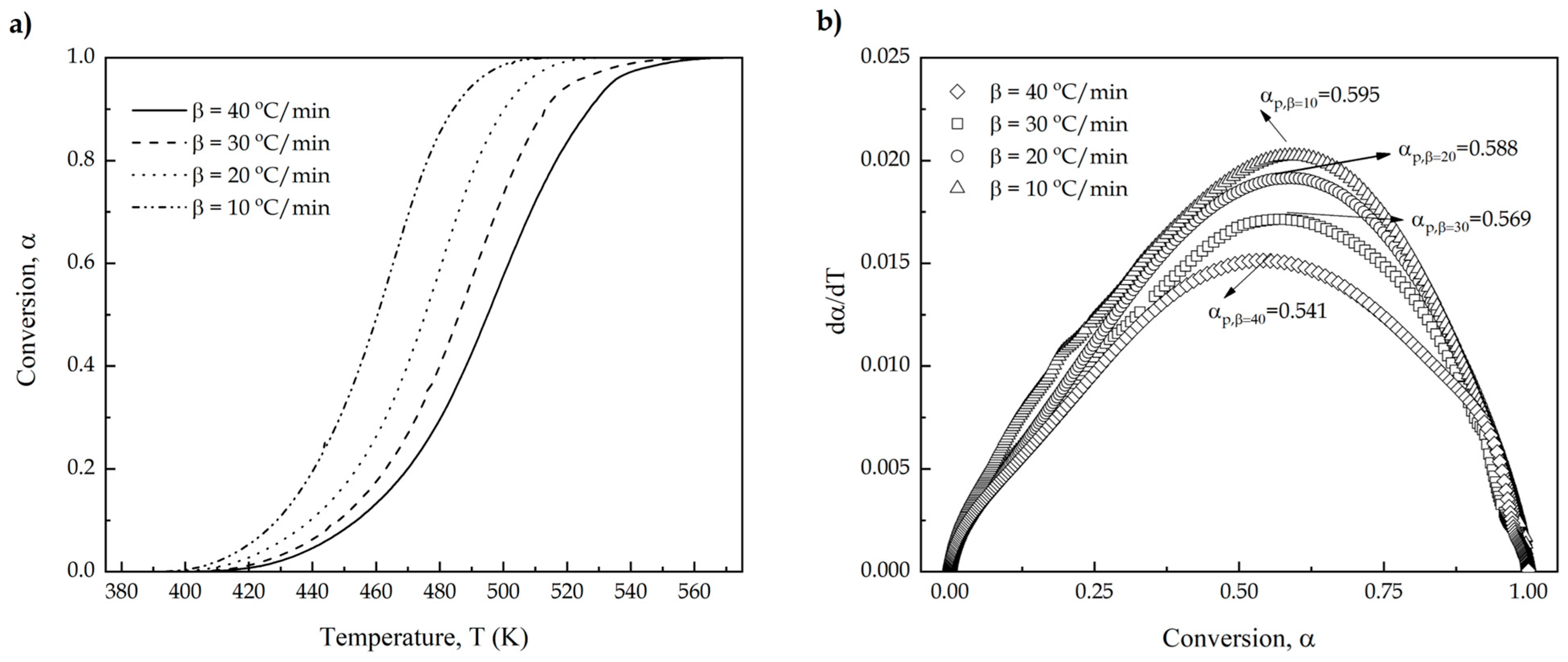
| 10 | 0.595 | 0.1038 | 0.6284 |
| 20 | 0.588 | 0.0284 | 0.6193 |
| 30 | 0.569 | 0.0151 | 0.5997 |
| 40 | 0.541 | 0.0401 | 0.5903 |
| β (°C/min) | ln(A) | m | n |
|---|---|---|---|
| 10 | 17.015 ± 0.011 | 0.185 ± 0.041 | 1.011 ± 0.007 |
| 20 | 16.939 ± 0.018 | 0.127 ± 0.006 | 0.916 ± 0.011 |
| 30 | 16.939 ± 0.019 | 0.0283 ± 0.006 | 0.975 ± 0.013 |
| 40 | 16.805 ± 0.019 | 0.089 ± 0.006 | 0.993 ± 0.013 |
| Average Value | 16.924 ± 0.087 | 0.107 ± 0.065 | 0.973 ± 0.041 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lascano, D.; Lerma-Canto, A.; Fombuena, V.; Balart, R.; Montanes, N.; Quiles-Carrillo, L. Kinetic Analysis of the Curing Process of Biobased Epoxy Resin from Epoxidized Linseed Oil by Dynamic Differential Scanning Calorimetry. Polymers 2021, 13, 1279. https://doi.org/10.3390/polym13081279
Lascano D, Lerma-Canto A, Fombuena V, Balart R, Montanes N, Quiles-Carrillo L. Kinetic Analysis of the Curing Process of Biobased Epoxy Resin from Epoxidized Linseed Oil by Dynamic Differential Scanning Calorimetry. Polymers. 2021; 13(8):1279. https://doi.org/10.3390/polym13081279
Chicago/Turabian StyleLascano, Diego, Alejandro Lerma-Canto, Vicent Fombuena, Rafael Balart, Nestor Montanes, and Luis Quiles-Carrillo. 2021. "Kinetic Analysis of the Curing Process of Biobased Epoxy Resin from Epoxidized Linseed Oil by Dynamic Differential Scanning Calorimetry" Polymers 13, no. 8: 1279. https://doi.org/10.3390/polym13081279
APA StyleLascano, D., Lerma-Canto, A., Fombuena, V., Balart, R., Montanes, N., & Quiles-Carrillo, L. (2021). Kinetic Analysis of the Curing Process of Biobased Epoxy Resin from Epoxidized Linseed Oil by Dynamic Differential Scanning Calorimetry. Polymers, 13(8), 1279. https://doi.org/10.3390/polym13081279










