Polymer Nanocomposites for Photocatalytic Degradation and Photoinduced Utilizations of Azo-Dyes
Abstract
1. Introduction
2. Polymer Nanocomposites for Photocatalytic Degradation of Azo-Dyes
2.1. Nanocomposite Photocatalysts from Natural Polymers
2.2. TiO2 Based Nanocomposites
2.3. Photocatalytic Mechanism of TiO2 Nanocomposites
H2O + h+ → ·OH
2.4. Other Nanocomposite Photocatalysts
3. Polymer Nanocomposites Utilizing Azo Dyes
3.1. Photo-Control Machinery
3.2. Nonlinear Optical Materials
3.3. Light Aligning Materials
4. Self-Assembly Method to Introduce Azo Dyes into Polymer Nanocomposites
5. Conclusions and Outlook
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Some Aromatic Amines, Organic Dyes, and Related Exposures. Lyon (FR): International Agency for Research on Cancer; 2010. (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, No. 99. Available online: https://www.ncbi.nlm.nih.gov/books/NBK385442/ (accessed on 24 March 2021).
- Weglarz-Tomczak, E.; Gorecki, L. Azo dyes—Biological activity and synthetic strategy. Chemik 2012, 66, 1298–1307. [Google Scholar]
- Price, R. Aspects of the chemistry of metal complex dyes. Chimia 1974, 28, 221–231. [Google Scholar]
- Rauf, M.A.; Hisaindee, S.; Saleh, N. Spectroscopic studies of keto-enol tautomeric equilibrium of azo dyes. RSC Adv. 2015, 5, 18097–18110. [Google Scholar] [CrossRef]
- Lesch, J.E. The First Miracle Drugs: How the Sulfa Drugs Transformed Medicine; Chapter 3: Prontosil; Oxford University Press: Oxford, UK, 2007; p. 51. [Google Scholar]
- Chung, K.-T. Azo dyes and human health: A review. J. Environ. Sci. Health Part C 2016, 34, 33–261. [Google Scholar] [CrossRef] [PubMed]
- Bafana, A.; Devi, S.S.; Chakrabarti, T. Azo dyes: Past, present and the future. Environ. Rev. 2011, 19, 350–370. [Google Scholar] [CrossRef]
- Fatima, M.; Farooq, R.; Lindstrom, R.W.; Saeed, M. A review on biocatalytic decomposition of azo dyes and electrons recovery. J. Liq. 2017, 246, 275–281. [Google Scholar] [CrossRef]
- Singh, R.L.; Singh, P.K.; Singh, R.P. Enzymatic decolourization and degradation of azo dyes—A review. Int. Biodeterior. Biodegrad. 2015, 104, 21–31. [Google Scholar] [CrossRef]
- Kishor, R.; Purchase, D.; Saratale, G.D.; Saratale, R.G.; Ferreira, L.F.R.; Bilal, M.; Chandra, R.; Bharagava, R.N. Ecotoxicological and health concerns of persistent coloring pollutants of textile industry wastewater and treatment approaches for environmental safety. J. Chem. Environ. Eng. 2021, 9, 105012. [Google Scholar] [CrossRef]
- Hartley, G.S. The cis-form of azobenzene. Nature 1937, 140, 281. [Google Scholar] [CrossRef]
- Fischer, E.; Frankel, M.; Wolovslcy, R. Wavelength Dependence of Photoisomerization Equilibria in Azocompounds. J. Chem. Phys. 1955, 23, 1367. [Google Scholar] [CrossRef]
- Bortolus, P.; Monti, S. Cis-Trans Photoisomerization of Azobenzene. Solvent and Triplet Donor Effects. J. Phys. Chem. 1979, 83, 648–652. [Google Scholar] [CrossRef]
- Loper, G.L.; Dorer, F.H. Primary Processes in the Photochemistry of 1 -Pyrazoline. J. Am. Amer. Chem. Soc. 1973, 95, 20–26. [Google Scholar] [CrossRef]
- Solomon, B.S.; Thomas, T.F.; Steel, C. Primary Processes in the Photochemistry of Bicyclic Azo Compounds. J. Am. Chem. Soc. 1968, 90, 2249–2258. [Google Scholar] [CrossRef]
- Bandara, H.M.D.; Friss, T.R.; Enriquez, M.M.; Isley, W.; Incarvito, C.; Frank, H.A.; Gascon, J.; Burdette, S.C. Proof for the Concerted Inversion Mechanism in the transfcis Isomerization of Azobenzene Using Hydrogen Bonding To Induce Isomer Locking. J. Org. Chem. 2010, 75, 4817–4827. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, J.; Kano, N.; Kawashima, T. Synthesis of the most intensely fluorescent azobenzene by utilizing the B–N interaction. Chem. Commun. 2007, 6, 559–561. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.-Y.; Xiao, L.; Jiang, R.; Zeng, G.-M.; Liu, L. Efficient decolorization of azo-dye solution by visible light-induced photocatalytic process using SnO2/ZnO heterojunction immobilized in Chitosan matrix. Chem. Eng. J. 2011, 172, 746–753. [Google Scholar] [CrossRef]
- Bhatt, R.; Ageetha, V.; Rathod, S.B.; Padmaja, P. Self-assembled chitosan-zirconium phosphate nanostructures for adsorption of chromium and degradation of dyes. Carbohydr. Polym. 2019, 208, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Sathiyavimal, S.; Vasantharaj, S.; Kaliannan, T.; Pugazhendhi, A. Eco-biocompatibility of chitosan coated biosynthesized copper oxide nanocompoistes for enhanced industrial (Azo) dye removal from aqueous solution and antibacterial properties. Carbohydr. Polym. 2020, 241, 116243. [Google Scholar] [CrossRef] [PubMed]
- Kafil, M.; Nasab, B.; Moazed, H.; Jokiniemi, J.; Laehde, A.; Bhatnagar, A. Efficient removal of azo dyes from water with chitosan/carbon nanoflower as a novel nanocomposite synthesized by pyrolysis technique. Desalin. Water Treat. 2019, 142, 308–320. [Google Scholar] [CrossRef]
- Banerjee, P.; Barman, S.R.; Mukhopadhayay, A.; Das, P. Ultrasound assisted mixed azo dye adsorption by chitosan-graphene oxide nanocomposite. Chem. Eng. Res. Des. 2017, 117, 43–56. [Google Scholar] [CrossRef]
- Mohagheghian, A.; Vahidi-Kolur, R.; Pourmohseni, M.; Yang, J.-K.; Shirzad-Siboni, M. Application of Scallop shell-Fe3O4 nano-composite for the removal of azo dye from aqueous solutions. Water Air Soil Pollut. 2015, 226, 1–16. [Google Scholar] [CrossRef]
- Dey, S.C.; Moztahida, M.; Sarker, M.; Ashaduzzaman, M.; Shamsuddin, S.M. pH-triggered interfacial interaction of kaolinite/chitosan nanocomposites with anionic azo dye. J. Compos. Sci. 2019, 3, 39. [Google Scholar] [CrossRef]
- Mohagheghian, A.; Pourmohseni, M.; Vahidi-Kolur, R.; Yang, J.-K.; Shirzad-Siboni, M. Application of kaolin-Fe3O4 nano-composite for the removal of azo dye from aqueous solutions. Desalin. Water Treat. 2017, 58, 308–319. [Google Scholar] [CrossRef]
- Stengl, V.; Henych, J.; Slusna, M. h-BN-TiO2 nanocomposite for photocatalytic applications. J. Nanomater. 2016, 2016, 4580516. [Google Scholar] [CrossRef]
- Sharma, A.K.; Lee, B.-K. Surfactant-aided sol-gel synthesis of TiO2-MgO nanocomposite and their photocatalytic azo dye degradation activity. J. Compos. Mater. 2020, 54, 1561–1570. [Google Scholar] [CrossRef]
- Karimzadeh, S.; Bahrami, K. Role of L-cysteine and CdS as promoted agents in photocatalytic activity of TiO2 nanocomposite. J. Environ. Chem. Eng. 2020. ahead of print. [Google Scholar]
- Katancic, Z.; Gavran, I.; Smolkovic, J.; Hrnjak-Murgic, Z. Fly ash supported photocatalytic nanocomposite poly(3,4-ethylenedioxythiophene)/TiO2 for azo dye removal under simulated solar irradiation. J. Appl. Polym. Sci. 2018, 135, 46316. [Google Scholar] [CrossRef]
- Zangeneh, H.; Farhadian, M.; Zinatizadeh, A.A. N(Urea) and C-N (L-Asparagine) doped TiO2-CuO nanocomposites: Fabrication, characterization and photodegradation of direct red 16. J. Environ. Chem. Eng. 2020, 8, 103639. [Google Scholar] [CrossRef]
- Ghanbari, S.; Givianrad, M.H.; Aberoomand, A.P. Synthesis and characterization of visible light driven N-Fe-codoped TiO2/SiO2 for simultaneous photoremoval of Cr(VI) and azo dyes in a novel fixed bed continuous flow photoreactor. Can. J. Chem. Eng. 2020, 98, 705–716. [Google Scholar] [CrossRef]
- Barahimi, V.; Moghimi, H.; Taheri, R.A. Cu doped TiO2-Bi2O3 nanocomposite for degradation of azo dye in aqueous solution: Process modeling and optimization using central composite design. J. Environ. Chem. Eng. 2019, 7, 103078. [Google Scholar] [CrossRef]
- Jorfi, S.; Mirali, S.; Mostoufi, A.; Ahmadi, M. Visible light photocatalytic degradation of azo dye and a real texile waste water using Mn, Mo, La/TiO2/AC nanocomposite. Chem. Biochem. Eng. Q. 2018, 32, 215–227. [Google Scholar] [CrossRef]
- Feng, J.; Wong, R.S.K.; Hu, X.; Yue, P.L. Discoloration and mineralization of orange II by using Fe3+-doped TiO2 and bentonite clay-based Fe nanocatalysts. Catal. Today 2004, 98, 441–446. [Google Scholar] [CrossRef]
- Janitarbar-Darzi, S.; Mahjoub, A.R. Investigation of phase transformations and phtocatalystic properties of sol-gel prepared nanostructured ZnO/TiO2 composite. J. Alloys Compd. 2009, 486, 805–808. [Google Scholar] [CrossRef]
- Aquino, S.F.; Lacerda, C.A.; Ribeiro, D.R. Use of ferrites encapsulated with titanium dioxide for photodegradation of azo dyes and color removal of textile effluents. Environ. Eng. Sci. 2010, 27, 1049–1059. [Google Scholar] [CrossRef]
- Alzahrani, E. Photodegradation of binary azo dyes using core-shell Fe3O4/SiO2/TiO2 nanospheres. Am. J. Anal. Chem. 2017, 8, 95–115. [Google Scholar] [CrossRef]
- Lucic, M.; Milosavljevic, N.; Radetic, M.; Saponjic, Z.; Radoicic, M.; Kalagasidis, M. The potential application of TiO2/hydrogel nanocomposite for removal of various textile azo dyes. Sep. Purif. Technol. 2014, 122, 206–216. [Google Scholar] [CrossRef]
- Bahram, M.; Salami, S.; Moghtader, M.; Moghadam, P.N.; Fareghi, A.R.; Rasouli, M.; Salimpour, S. Photocatalytic degradation of anionic azo dyes acid orange 7 and acid red 88 in aqueous solutions using TiO2-containing hydrogel. Anal. Bioanal. Chem. 2017, 4, 53–63. [Google Scholar]
- Guo, N.; Liang, Y.; Lan, S.; Liu, L.; Ji, G.; Gan, S.; Zou, H.; Xu, X. Uniform TiO2-SiO2 hollow nanoshperes: Synthesis, charaterization and enhanced adsorption-photodegradation of azo dyes and phenol. Appl. Surf. Sci. 2014, 305, 562–574. [Google Scholar] [CrossRef]
- Nilchi, A.; Janitabar-Darzi, S.; Rasouli-Garmarodi, S. Sol-gel preparation of nanoscale TiO2/SiO2 composite for eliminating con red azo dye. Mater. Sci. Appl. 2011, 2, 476–480. [Google Scholar]
- Ayati, A.; Ahmadpour, A.; Bamoharram, F.F.; Tanhaei, B.; Manttari, M.; Lahtinen, M.; Sillanpaa, M. Novel Au NPs/preyssler acid/TiO2 nanocomposite for the photocatalytic removal of azo dye. Sep. Purif. Technol. 2014, 133, 415–420. [Google Scholar] [CrossRef]
- An, L.; Wang, G.; Cheng, Y.; Zhao, L.; Gao, F.; Tian, Y. Ultrasonic-assisted synthesis of visible-light-driven TiO2/Bi2O3 nanocomposite photocatalysts: Characterization, properties and azo dye removal application. Res. Chem. Intermed. 2015, 41, 7449–7461. [Google Scholar] [CrossRef]
- Rosu, M.-C.; Socaci, C.; Floare-Avram, V.; Borodi, G.; Pogacean, F.; Coros, M.; Magerusan, L.; Pruneanu, S. Photocatalytic performance of graphene/TiO2-Ag composites on amaranth dye degradation. Mater. Chem. Phys. 2016, 179, 232–241. [Google Scholar] [CrossRef]
- Ghalebizade, M.; Ayati, B. Solar photoelectrocatalytic degradation of acid orange 7 with ZnO/TiO2 nanocomposites coated on stainless steel electrode. Process Saf. Environ. Prot. 2016, 103, 192–202. [Google Scholar] [CrossRef]
- Yu, A.; Lu, G.Q.M.; Drennan, J.; Gentle, I.R. Tubular titania nanostructures via layer-by-layer self-assembly. Adv. Funct. Mater. 2007, 17, 2600–2605. [Google Scholar] [CrossRef]
- Latifi, A.M.; Mirzaei, M.; Mousavi-Kamazani, M.; Zarghami, Z. Rice-like Ag/Al2O3 nanocomposites preparation from AlOOH nanostructures synthesized via a facile hydrothermal route for azo dyes photocatalytic degradation and Pd2+ adsorption. J. Mater. Sci. Mater. Electron. 2018, 29, 10234–10245. [Google Scholar] [CrossRef]
- Rohani, N.; Bamoharram, F.F.; Marijani, A.; Heravi, M.M. Gold nanoparticles Wells-Dawson heteropolyacid nanocomposite film as an effective nanocatalyst in photocatalytic removal of azo dyes from wastewaters. J. Nanostruct. Chem. 2017, 7, 171–178. [Google Scholar] [CrossRef]
- Najafian, H.; Manteghi, F.; Beshkar, F.; Salavati-Niasari, M. Fabrication of nanocomposite photocatalyst CuBi2O4/Bi3ClO4 for removal of acid brown 14 as water pollutant under visible light. J. Hazard. Mater. 2019, 361, 210–220. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, D.; Filice, S.; Scarangella, A.; Iannazzo, D.; Compagnini, G.; Scalese, S. Bi2O3/Nexar polymer nanocomposite memberanes for azo dyes removal by UV-vis or visible light irradiation. Catal. Today 2019, 321, 158–163. [Google Scholar] [CrossRef]
- Najafian, H.; Manteghi, F.; Beshkar, F.; Salavati-Niasari, M. Enhanced photocatalytic activity of a novel NiO/Bi2O3/Bi3ClO4 nanocomposite for the degradation of azo dye pollutants under visible light irradiation. Sep. Purif. Technol. 2019, 209, 6–17. [Google Scholar] [CrossRef]
- Khaghani, S.; Ghanbari, D.; Khaghani, S. Green synthesis of iron oxide-palladium nanocomposites by pepper extract and its application in removing of colored pollutants from water. J. Nanostruct. 2017, 7, 175–182. [Google Scholar] [CrossRef]
- Sahoo, A.; Patra, S. A magnetically separable and recyclable g-C3N4/Fe3O4/porous ruthenium nanocatalyst for photocatalytic degradation of water-soluable aromatic amines and azo dyes. RSC Adv. 2020, 10, 6043–6051. [Google Scholar] [CrossRef]
- Eskandari, N.; Nabiyouni, G.; Masoumi, S.; Ghanbari, D. Preparation of a new magnetic and photocatalyst CoFe2O4-SrTiO3 perovskite nanocomposite for photo-degradation of toxic dyes under short time visible irradiation. Compos. Part B: Eng. 2019, 176, 107343. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, F.; An, L.; Li, X.; Wang, G. Different combinations of Fe3O4 microshere, polypyrrole and silver as core-shell nanocomposites for adsorption and photocatalytic application. Adv. Powder Technol. 2014, 25, 1600–1607. [Google Scholar] [CrossRef]
- Ranjeh, M.; Beshkar, F.; Salavati-Niasari, M. Sol-gel synthesis of novel Li-based boron oxides nanocomposite for photodegradation of azo-dye pollutant under UV light irradiation. Compos. Part B: Eng. 2019, 172, 33–40. [Google Scholar] [CrossRef]
- Cai, X.; Cai, Y.; Liu, Y.; Deng, S.; Wang, Y.; Wang, Y.; Djerdj, I. Enhanced photocatalytic degradation properties of Ni(OH)2 nanosheets/ZnO nanorods composites for azo dyes under visible-light irradiation. Ceram. Int. 2014, 40, 57–65. [Google Scholar] [CrossRef]
- Krishnakumar, B.; Imae, T.; Miras, J.; Esquena, J. Synthesis and azo dye photodegradation activity of ZrS2-ZnO nano-composites. Sep. Purif. Technol. 2014, 132, 281–288. [Google Scholar] [CrossRef]
- Saratale, G.D.; Saratale, R.G.; Cho, S.-K.; Ghodake, G.; Bharagava, R.N.; Park, Y.; Mulla, S.I.; Kim, D.-S.; Kadam, A.; Nair, S.; et al. Investigation of photocatalytic degradation of reactive textile dyes by Portulaca Oleracea-Functionalized silver nanocomposites and exploration of their antibacterial and antidiabetic potentials. J. Alloys Compd. 2020, 833, 155083. [Google Scholar] [CrossRef]
- Sanderson, K. Lighting the way to more targeted treatments. C E News 2018, 2, 25–27. [Google Scholar]
- Borowiak, M.; Nahaboo, W.; Reynders, M.; Nekolla, K.; Jalinot, P.; Hasserodt, J.; Rehberg, M.; Delattre, M.; Zahler, S.; Vollmar, A.; et al. Photoswitchable Inhibitors of Microtubule Dynamics Optically Control Mitosis and Cell Death. Cell 2015, 162, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Kondo, M.; Mamiya, J.; Yu, Y.; Kinoshita, M.; Barrett, C.J.; Ikeda, T. Photomobile Polymer Materials: Towards Light- Driven Plastic Motors. Angew. Chem. Int. Ed. 2008, 47, 4986–4988. [Google Scholar] [CrossRef] [PubMed]
- Bushuyev, O.S.; Aizawa, M.; Shishido, A.; Barrett, C.J. Shape-Shifting Azo Dye Polymers: Towards Sunlight-Driven Molecular Devices. Macromol. Rapid Commun. 2018, 39, 1700253. [Google Scholar] [CrossRef] [PubMed]
- Crespi, S.; Simeth, N.A.; Konig, B. Heteroaryl azo dyes as molecular photoswitches. Nat. Rev. Chem. 2019, 3, 133–146. [Google Scholar] [CrossRef]
- Feringa, B.L.; van Delden, R.A.; Koumura, N.; Geertsema, E.M. Chiroptical Molecular Switches. Chem. Rev. 2000, 100, 1789–1816. [Google Scholar] [CrossRef] [PubMed]
- Zapata, F.; Fernández-González, M.Á.; Rivero, D.; Álvarez, Á.; Marazzi, M.; Frutos, L.M. Toward an Optomechanical Control of Photoswitches by Tuning Their Spectroscopical Properties: Structural and Dynamical Insights into Azobenzene. J. Chem. Theory Comput. 2014, 10, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Chang, V.Y.; Fedele, C.; Priimagi, A.; Shishido, A.; Barrett, C.J. Photoreversible soft azo dye materials: Toward optical control of bio-interfaces. Adv. Opt. Mater. 2019, 7, 1900091. [Google Scholar] [CrossRef]
- Diaz Costanzo, G.; Ribba, L.; Goyanes, S.; Ledesma, S. Enhancement of the optical response in a biodegradable polymer/azo-dye film by the addition of carbon nanotubes. J. Phys. D: Appl. Phys. 2014, 47, 135103/1–135103/8. [Google Scholar] [CrossRef]
- Georgiev, A.; Dimov, D.; Stoilova, A.; Markova, F.; Nazarova, D. Vapor deposited nanocomposite films of perylene bis azo-imides with improved photoresponsiveness by visible light. Opt. Mater. 2019, 89, 5–13. [Google Scholar] [CrossRef]
- Zhang, L.; Cole, M. TiO2-assisted phtoisomerization of azo dyes using self-assembled monolayers: Case study on para-methyl red towards solar-cell applications. ACS Appl. Mater. Interfaces 2014, 6, 3742–3749. [Google Scholar] [CrossRef] [PubMed]
- Raposo, M.; Manuela, M.; Sousa, A.M.R.C.; Fonseca, A.; Mauricio, C.; Kirsch, G. Donor-acceptor substituted thienylpyrrole azo dyes: Synthesis, solvatochromic and electrochemical properties. Mater. Sci. Forum 2006, 514, 103–107. [Google Scholar] [CrossRef]
- Churikov, V.M.; Hsu, C.-C. Photoinduced third-order nonlinear optical phenomena in azo-dye polymers. In Photoreactive Organic Thin Films; Sekkat, Z., Knoll, W., Eds.; Academic Press: Waltham, MA, USA, 2002; pp. 365–395. [Google Scholar]
- Chigrinov, V.; Kwok, H.S.; Takada, H.; Takatsu, H. Photoaligning by azo-dyes: Physics and applications. Liq. Cryst. Today 2005, 14, 1–15. [Google Scholar] [CrossRef]
- Simoni, F.; Francescangeli, O. Effects of light on molecular orientation of liquid crystals. J. Phys. Condens. Mater. 1999, 11, R439–R487. [Google Scholar] [CrossRef]
- Lucchetti, L.; Simoni, F. Light-induced adsorption and desorption in Methyl-Red-doped liquid crystals: A review. Liq. Cryst. Rev. 2015, 3, 79–98. [Google Scholar] [CrossRef]
- Kumar, V.; Ye, Z.; Jiang, H.; Shi, Y.; Li, K.; Gerard, D.; Luo, D.; Mu, Q.; Liu, Y.J. Highly Stable Pretilted Homeotropic Alignment of Liquid Crystals Enabled by In Situ Self-Assembled Dual-Wavelength Photoalignment. ACS Appl. Electr. Mater. 2020, 2, 2017–2025. [Google Scholar] [CrossRef]
- Kundu, S.; Lee, M.-H.; Lee, S.H.; Kang, S.-W. In Situ Homeotropic Alignment of Nematic Liquid Crystals Based on Photoisomerization of Azo-Dye, Physical Adsorption of Aggregates, and Consequent Topographical Modification. Adv. Mater. 2013, 25, 3365–3370. [Google Scholar] [CrossRef]
- Yeung, C.L.; Charlesworth, S.; Iqbal, P.; Bowen, J.; Preece, J.A.; Mendes, P.M. Different Formation Kinetics and Photoisomerization Behavior of Self-Assembled Monolayers of Thiols and Dithiolanes Bearing Azobenzene Moieties. Phys. Chem. Chem. Phys. 2013, 15, 11014–11024. [Google Scholar] [CrossRef] [PubMed]
- Kasha, M.; Rawls, H.R.; El-Bayoumi, M.A. The Excition Model in Molecular Spectroscopy. Pure Appl. Chem. 1965, 11, 371–392. [Google Scholar] [CrossRef]
- Tong, X.; Cui, L.; Zhao, Y. Confinement Effects on Photoalignment, Photochemical Phase Transition, and Thermochromic Behavior of Liquid Crystalline Azobenzene-Containing Diblock Copolymers. Macromolecules 2004, 37, 3101–3112. [Google Scholar] [CrossRef]
- Menzel, H.; Weichart, B.; Schmidt, A.; Paul, S.; Knolls, W.; Stumpe, J.; Fischer, T. Small -Angle X-ray Scattering and Ultraviolet-Visible Spectroscopy Studies on the Structure and Structural Changes in Langmuir-Blodgett Films of Polyglutamates with Azobenzene Moieties Tethered by Alkyl Spacers of Different Length. Langmuir 1994, 10, 1926–1933. [Google Scholar] [CrossRef]
- Stumpe, J.; Fischer, T.; Menzel, H. Langmuir-Blodgett Films of Photochromic Polyglutamates. 9. Relation between Photochemical Modification and Thermotropic Properties. Macromolecules 1996, 29, 2831–2842. [Google Scholar] [CrossRef]
- Shimomura, M.; Kunitake, T. Fluorescence and Photoisomerization of Azobenzene-Containing Bilayer Membranes. J. Am. Chem. Soc. 1987, 109, 5175–5183. [Google Scholar] [CrossRef]
- Tamai, N.; Miyasaka, H. Ultrafast Dynamics of Photochromic Systems. Chem. Rev. 2000, 100, 1875–1890. [Google Scholar] [CrossRef]
- He, Y.; Wang, R.; Sun, C.; Liu, S.; Zhou, J.; Zhang, L.; Jiao, T.; Peng, Q. Facile Synthesis of Self-Assembled NiFe Layered Double Hydroxide-Based Azobenzene Composite Films with Photoisomerization and Chemical Gas Sensor Performances. ACS Omega 2020, 5, 3689–3698. [Google Scholar] [CrossRef]
- Zhuang, X.-D.; Chen, Y.; Liu, G.; Zhang, B.; Neoh, K.-G.; Kang, E.-T.; Zhu, C.-X.; Li, Y.-X.; Niu, L.-J. Preparation and memory performance of a nanoaggregated Dispersed Red 1-functionalized poly (N-vinylcarbazole) film via solution-phase self-assembly. Adv. Funct. Mater. 2010, 20, 2916–2922. [Google Scholar] [CrossRef]
- Zakrevskyy, Y.; Stumpe, J.; Faul, C.F.J.A. Supramolecular Approach to Optically Anisotropic Materials: Photosensitive Ionic Self-assembly Complexes. Adv. Mater. 2006, 18, 2133–2136. [Google Scholar] [CrossRef]
- Vasantha, V.A.; Chen, J.; Zhao, W.; van Herk, A.M.; Parthiban, A. Reversible Photo- and Thermoresponsive, Self-Assembling Azobenzene Containing Zwitterionic Polymers. Langmuir 2019, 35, 1465–1474. [Google Scholar] [CrossRef]
- Kim, S.H.; Park, Y.M.; Lee, C.S.; Son, Y.A. Self-Assembly Fabrication Using Diazo Coupling Dye and Spiroxazine. Mol. Cryst. Liq. Cryst. 2008, 491, 94–102. [Google Scholar] [CrossRef]
- He, J.-A.; Bian, S.; Li, L.; Kumar, J.; Tripathy, S.K.; Samuelson, L.A. Photochemical Behavior and Formation of Surface Relief Grating on Self-Assembled Polyion/Dye Composite Film. J. Phys. Chem. B 2000, 104, 10513–10521. [Google Scholar] [CrossRef]
- Hansda, C.; Dutta, B.; Chakraborty, U.; Singha, T.; Hussain, S.A.; Bhattacharhee, D.; Paul, S.; Paul, P.K. Photophysical Behavior of Layer-by-layer Electrostatic Self-assembled Film of Azo Dye Chromotrope-2R and a Polycation. J. Lumin. 2016, 178, 347–355. [Google Scholar] [CrossRef]
- Cui, L.; Dahmane, S.; Tong, X.; Zhu, L.; Zhao, Y. Using Self-assembly to Prepare Multifunctional Diblock Copolymers Containing Azopyridine Moiety. Macromolecules 2005, 38, 2076–2084. [Google Scholar] [CrossRef]
- Hu, D.; Hu, Y.; Huang, W.; Zhang, Q. Hydrogen Bonded Supermolecular azopolymers: A Media for Multilayered and Polarization-mulitiplexed Data Storage Based Two-photon Process. Proc. SPIE 2012, 8559, 85590. [Google Scholar]
- Dalkiranis, G.G.; Therezio, E.M.; da Silva, M.A.T.; Pocas, L.C.; Duarte, J.L.; Laureto, E.; Dias, I.F.L.; Tozoni, J.R.; Silva, R.A.; Marletta, A. Annealing temperature effects on the azodye photoisomerization processes of layer-by-layer self-assembled poly(p-phenylenevinylene)/Congo red thin films. J. Lumin. 2013, 134, 842–846. [Google Scholar] [CrossRef]
- Moldenhauer, D.; Groehn, F. Nanoassemblies with Light-responsive Size and Density from Linear Flexible Polyelectrolytes. J. Polym. Sci. Part B: Polym. Phys. 2013, 51, 802–816. [Google Scholar] [CrossRef]
- Jin, C.; Zhang, T.; Liu, F.; Wang, L.; Yin, Q.; Xiao, D. Fabrication of size controllable polymeric hollow nanospheres containing azo functional groups via ionic self-assembly. RSC Adv. 2014, 4, 8216–8223. [Google Scholar] [CrossRef]
- Jin, C.; Zhao, Y.; Wang, H.; Lin, K.; Yin, Q. Synthesis and self-assembly of side-chain azocomplex for preparation of solid and hollow nanosphere. Colloid Polym. Sci. 2012, 290, 741–749. [Google Scholar] [CrossRef]
- Jin, C.; Zhang, T.; Liu, F.; Wang, L.; He, M.; Yuan, T.; Jiang, B.; Xiao, D.; Yin, Q. Photoinduced deformation of hollow nanospheres formed by the self-assembly of amphiphilic random copolymers and small azo molecules. RSC Adv. 2014, 4, 45890–45894. [Google Scholar] [CrossRef]
- Zhang, T.; Jin, C.; Wang, L.; He, M.; Yuan, T.; Yin, Q. One-step synthesis of hollow polymeric nanospheres: Self-assembly of amphiphilic azo polymers via hydrogen bond formation. RSC Adv. 2014, 4, 36882–36889. [Google Scholar] [CrossRef]
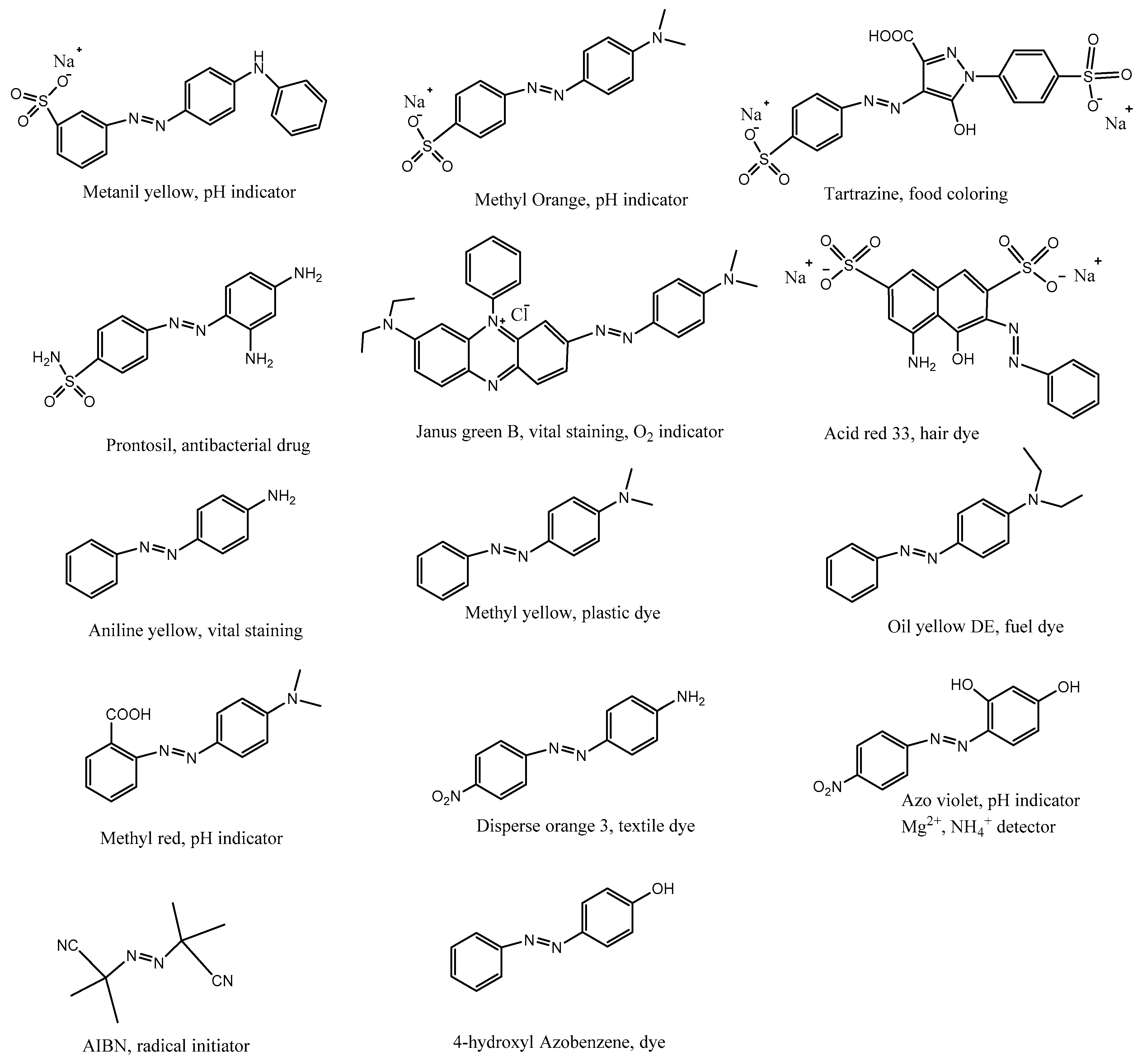



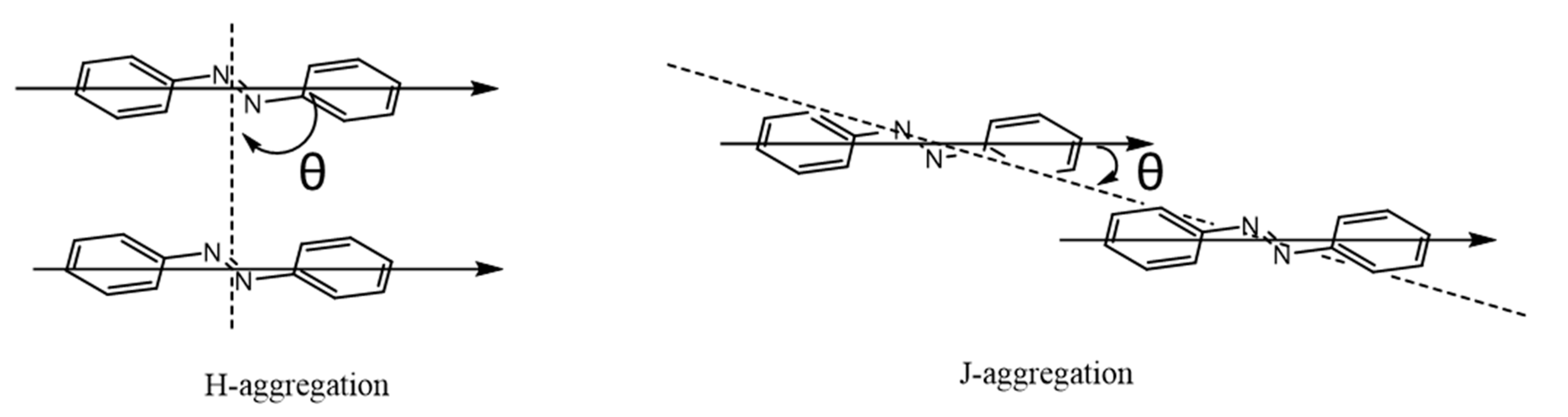
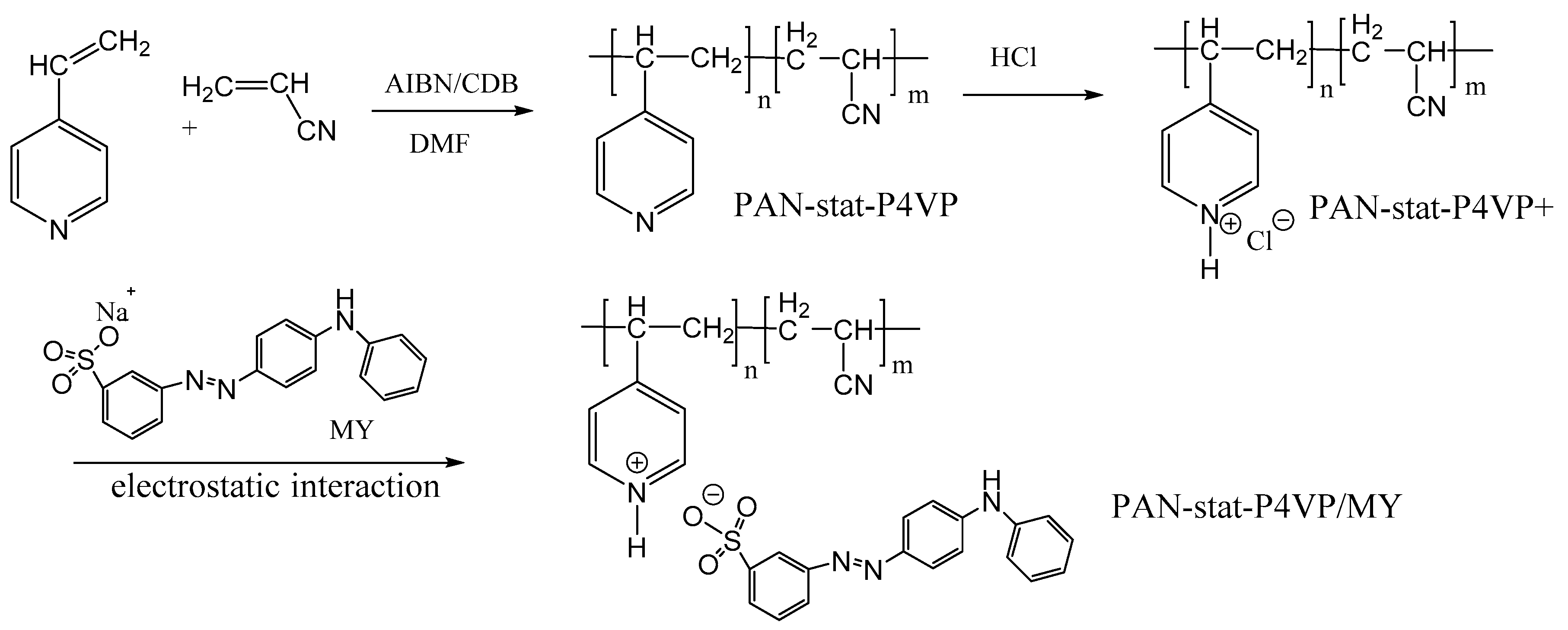

| Base | Morphology | Advantages | Mechanisms | Usage | Other Requirements | Stability + Reusability | Reference |
|---|---|---|---|---|---|---|---|
| Chitosan (CS) | Quantum dot SnO2/ZnO (3–5 nm) on chitosan film | Reduced heterojunctions are immobilized on an absorbent matrix; easy separation | ·OH and ·O2− radicals are active species | Methyl orange (MO) degradation | Visible light | Efficiency only drops after 3 runs | [18] |
| Chitosan | Chitosan-layered ZrP nanoparticles (NPs) | Synergetic effect (chitosan reduces Cr(VI) then ZrP absorbs Cr(III)) | The superoxide anions were stabilized by ZrP or CZrP | Cr(VI), Reactive blue 21 (RB-21), and Reactive red 141 (R-141) | H2O2 | Efficiency unchanged after 3 cycles | [19] |
| Chitosan | Chitosan-coated CuO NPs | Ecofriendly | Bacteria inhibitor; produces ·OH and superoxide radicals | Bacteria inhibition; Methyl blue (MB) and Congo red (CR) removal | Sunlight | [20] | |
| Chitosan | Chitosan/carbon nanoflowers (CNFs) | Chitosan is biodegradable; carbon nanoflowers act as an absorbent | CS/CNFs have a positively charged surface and can form hydrogen bonds | Acid black 1 (AB-1) and Congo red (CR) removal | 50–79% efficiency after 4 cycles (remains) | [21] | |
| Chitosan | Graphene oxide platelets are embedded in a chitosan matrix | Cost effective; sustainable | Absorbent | Acid yellow (AY) and Acid blue (AB) removal | Ultrasound | 87% efficiency after 8 cycles | [22] |
| Scallop shell | Fe3O4 NPs are coated on a shell surface | Efficient; low cost | Absorbent | Reactive black 5 (RB-5) removal | 100% after 6 consecutive runs | [23] | |
| Kaolinite/Chitosan | Composite beads with different ratios; self-assembly | Easy fabrication; eco-friendly | Chitosan has polycationic nature | Remazol red (RR) | pH | [24] | |
| Kaolin | Kaolin is coated with Fe3O4 NPs | Easy separation by magnets | Adsorbent | Acid red 14 (AR-14) | Efficiency 88.43% after 6 cycles | [25] |
| Morphology | Improvements Over TiO2 | Mechanisms | Usage | Light | Stability + Reusability | References |
|---|---|---|---|---|---|---|
| h-BN–TiO2 | Band gap energy Eg = ~2.3–3.3 eV | h-BN (like graphene) accepts electrons and inhibits hole/electron (h+/e−) recombination | Orange II (OII); Reactive black 5 (RB-5) | UV Visible | k = 0.0762 min−1 (OII); k = 0.0164 min−1 (RB-5) | [26] |
| MgO nanoparticles within a TiO2 framework via surfactant-aided sol-gel approaches | Surfactant ensures uniform dispersion of the magnesium dopant onto titanium | High MgO content provides a high energy level for photo-degradation | Methyl orange (MO); Methylene blue (MB) | Visible | 82.4% (MB); 77.8% (MO) | [27] |
| TiO2 was immobilized on the surface of fly ash, on which conductive polymer was grafted | Reduced band gap (Eg = 1.5–3.0 eV for conductive polymer) easy removal and reuse | Semiconductor produces ·OH and ·O2− radicals | Reactive red 45 (RR-45) | Visible + Solar | Catalytic efficiency dropped quickly after the first run | [29] |
| N-(urea) and C–N (L-asparagine)-doped TiO2–CuO nanocomposite | TiO2 band gap was narrowed to the visible light region | CuO is a p-type semiconductor (Eg = 1.2–1.5 eV), and N-cap reduces h+/e− recombination | Direct red 16 (DR-16) | Visible | k = 0.0403 min−1 | [30] |
| N–Fe co-doped-TiO2/SiO2 | Simultaneously remove Cr(VI) and azo dyes (using continuous flow photo reactor) | The non-metal anion dopants increase the percentage of the anatase phase of TiO2; SiO2 increases surface area | Cr(VI) Basic red 29 (BR-29); Basic blue 41 (BB-41); Basic yellow 51 (BY-51) | Visible or solar | 91.73% (Cr6+) 85.64% (BR-29) 87.23% (BB-41); 58.59% (BY-51); 70% efficiency after the 7th 12 h cycle | [31] |
| Cu-doped TiO2–Bi2O3 | Better efficiency using visible light | TiO2/Bi2O3 prevents recombination rates of photo-induced h+/e− pairs; Cu reduce band gap to visible region | Methyl orange (MO) | LED lamp (λ > 410 nm) | 79% efficiency after 5 cycles | [32] |
| Mn, Mo, and La/TiO2 activated carbon (AC) | High absorption ability in the visible light region; cost effective; easy removal | Metals reduce the recombination rates of hole/electron (h+/e−) pairs | Reactive red 198 (RR-198); textile wastewater | Visible | 91% (RB-198) 52.78% efficiency after the 4th run | [33] |
| Fe3+-doped TiO2–bentonite clay | Remove total organic carbon (TOC) | Photo-Fenton reaction; nanocomposite reduces hole/electron (h+/e−) recombination rate | Orange II (OII) | 254 nm UVC | 100% decoloring | [34] |
| ZnO/TiO2 Phase transformations | ZnO absorbs a larger fraction of UV light than TiO2 | Large surface area; hole can transfer from TiO2 valence band to ZnO valence band | Congo red (CR) | UVC lamp | discoloring after 10 min | [35] |
| Ferrites encapsulated with TiO2 | Easy catalyst recovery | Larger specific area (43.2 m2/g) than TiO2 (31.1 m2/g) Dyes degrades in bulk solution by radicals | Textile effluents | UV (253.7 nm) | 65% efficiency at pH = 7; 80% efficiency at pH = 12 | [36] |
| Fe3O4/SiO2/TiO2 | Fe3O4-core was coated with silica (SiO2), which was, in turn, coated with TiO2 | Core Fe3O4 NPs SiO2—inner layer TiO2—outer layer | Methyl Orange (MO); Methylene blue (MB) | UV (365 nm) | 90.2% (MO) 100% (MB); no efficiency decrease after 5 runs | [37] |
| TiO2–hydrogel | Good removal performance; pH sensitivity facilitates retrieval of catalyst at the end | TiO2 entrapped in the hydrogel | Acid orange 7 (AO-7); Acid red 88 (AR-88) | UV | 98% AR-88 71% AO-7 | [38] |
| TiO2 NPs/hydrogel | Hydrogel as supporting material for TiO2; easy removal | Hydrogel both supports TiO2 and absorbs pollutants | Acid red 18 (AR-18); Acid blue 113 (AB-113); Reactive black 5 (RB-5) Direct black-78 | UV–sun-like illumination | 85% efficiency after 3rd cycle | [39] |
| TiO2–SiO2 hollow nanospheres | Enhanced photocatalytic performance | Larger surface area (up to 1105 m2/g) | Methyl orange (MO); Phenol | UV light; sunlight | 95% MO | [40] |
| TiO2–SiO2 | Sol-gel methods; enhanced photocatalytic and thermal properties | Ti–O–Si bond and amorphous SiO2 increases the stability of anatase TiO2, increases surface area, and limits the growth of crystallites | Congo red (CR) | UVC lamp | 98% CR | [41] |
| Au NPs/Preyssler acid/TiO2 | Eco-friendly; no free Au contamination | Au-NPs increase charge separation between the excited electron (e−) and hole (h+); Preyssler is both a stabilizer and a reducing agent | Malachite green | UV | 95% efficiency after the 3rd cycle | [42] |
| Sonic-TiO2/Bi2O3 | More efficient than pure TiO2 and stirred TiO2/Bi2O3 | High energy of ultrasonic wave promotes the crystallization of the TiO2 NPs and Bi2O3 NPs that grow on the surface to benefit the charge transfer | Orange II (OII) | Visible (λ > 400 nm) | 94.7% OII | [43] |
| Graphene/TiO2–Ag | Extended light absorption range | Band gaps of nanocomposites are ~3.05–3.09 eV | Amaranth dye | UV; Sun | 85.3–98% graphene/TiO2–Ag; 99% reduced graphene/TiO2–Ag | [44] |
| ZnO/TiO2 coated on stainless-steel electrode | Combined photoelectrocatalytic and electrochemical methods | ZnO/TiO2 composite reduced the band gap; electrical field separate photo-induced electron–hole pairs | Acid orange 7 (AO-7); Chemical (C1); chemicaloxygen demand (COD) | Solar irradiation | 97% AO-7 99% COD | [45] |
| Morphology | Advantages | Mechanisms | Usage | Light | Stability + Reusability | References |
|---|---|---|---|---|---|---|
| Rice-like Ag/Al2O3 | Good surface:volume ratio; good absorption; Capacity for Pb2+ (250 mg/g) | Surface area 241.219 m2/g | Pd2+; Acid violet 7 (AV-7) | Visible | 86.7–100% AV-7; absorbs Pb2+ (215 mg/g) | [47] |
| Au NPs/(H6P2W18O62) | Au nanoparticles were incorporated in Wells–Dawson hetero-polyacid nanocomposite film | Heteropolyacids are powerful oxidants; reduced form is a strong reducing reagent and stabilizer | Methyl orange (MeO); Methylene read (MR) | UV 254 nm | 96% MeO; 87.2% MR | [48] |
| CuBi2O4/Bi3ClO4 | Strong visible light absorption; fine particle size; proper band gap; high charge separation efficiency | Hole (h+) and superoxide radical (·O2−) are prevailing active species | Acid brown 14 (AC-14) | Visible | 92% efficiency (AC-14); 75% TOC removal | [49] |
| Bi2O3/Nexar polymer | Efficient; environmentally friendly; easy regeneration and reuse | Bi2O3 Eg = 2.1–2.8 eV; Nexar—membrane | Methyl orange (MO); Methylene blue (MB) | UV-vis or Blue | 53% dyes membranes are processed and restored | [50] |
| NiO/Bi2O3/Bi3ClO4 | Effective separation and transfer of the photoexcited electron–hole pairs | Photoexcited superoxide radicals and holes are prevailing active sites | Acid red 88 (AR-88) | Visible | 89% AR-88; 95% efficiency after 4 cycles | [51] |
| Fe3O4-Pd/ pepper-extract | Magnetic separation; hydrothermal procedure (nanostructures have preferential and controlled shape and size) | Free electron gas of noble metal forms localized surface plasmon (LSP) | Acid black; Acid brown | UV | 97% (Acid black); 85% (Acid brown); magnetic removal of catalyst | [52] |
| g-C3N4/Fe3O4/p-RuNP | Simple and convenient (efficiency, energy, and reusability), and facile separation from the reaction mixture | Eg = 2.76 eV ·OH; radical is responsible for the reactions | Congo red (CR); Chlorazol black (CB); Evans blue (EB); Reactive read (RR) | Visible; LED; Sunlight | 85% (CR); 73% (CB); 65% (EB); 80%(RR); 65–85% efficiency; 95% efficiency after 5 cycles | [53] |
| CoFe2O4/SrTiO3 perovskite | Efficient, stable, and magnetic photocatalyst; easy separation from water | N:SrTiO3 Eg = 2.58 eV CoFe2O4 Eg = 1.76 eV | Acid brown; Acid black | Visible | Acid brown 44%; Acid black 99% | [54] |
| Fe3O4-microsphere/ polypyrrole-Ag | Core–shell morphology Fe3O4 as N-type semiconductor; Polypyrrole (PPY) as p-type semiconductor | The doped Ag improve transportation and separation of photo-induced charges on the surface interface; Band gaps are Eg = ~2.5 eV for nanocomposites of conducting polymer | Methyl orange (MO); Orange II (OII) | UV | 86.2% MeO; 80.4% OII | [55] |
| Li2B4O7/LiBO2/Li3BO3 (LBO) | Strong photo absorption; high-efficiency charge carrier separation; uniform particle size; Appropriate band gap | Superoxide radicals (·O2−) and holes (h+) are the prevailing active species | Acid violet 7 | UV | 91% AV7; 70% TOC | [56] |
| Ni(OH)2 nanosheets/ZnO nanorods | Well-crystallized ZnO; poorly crystallized Ni(OH)2·0.75H2O; absorbance edge shifts to red | Interface charge transfer (IFCT) from conducting band of ZnO to Ni(II) heterojunction | Rhodamine B; CR; MB; MO | Visible | 82.6–98.4% MB | [57] |
| ZrS2–ZnO | ZrS2 has Eg = 1.68 eV The coexistence of ZrS2 increases the absorbance of ZnO in the UV region | e− in the conducting bands of ZrS2 and ZnO reduce O2 to ·O2−, h+ in valence band of ZnO oxidize H2O to ·OH | Naphthol blue black (NBB) | UV | 98.5% Efficiency after 4th run | [58] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, E.Z.; Wang, Y.; Xiao, D. Polymer Nanocomposites for Photocatalytic Degradation and Photoinduced Utilizations of Azo-Dyes. Polymers 2021, 13, 1215. https://doi.org/10.3390/polym13081215
Wang EZ, Wang Y, Xiao D. Polymer Nanocomposites for Photocatalytic Degradation and Photoinduced Utilizations of Azo-Dyes. Polymers. 2021; 13(8):1215. https://doi.org/10.3390/polym13081215
Chicago/Turabian StyleWang, Emily Z., Yigui Wang, and Dequan Xiao. 2021. "Polymer Nanocomposites for Photocatalytic Degradation and Photoinduced Utilizations of Azo-Dyes" Polymers 13, no. 8: 1215. https://doi.org/10.3390/polym13081215
APA StyleWang, E. Z., Wang, Y., & Xiao, D. (2021). Polymer Nanocomposites for Photocatalytic Degradation and Photoinduced Utilizations of Azo-Dyes. Polymers, 13(8), 1215. https://doi.org/10.3390/polym13081215







