Green Design of Novel Starch-Based Packaging Materials Sustaining Human and Environmental Health
Abstract
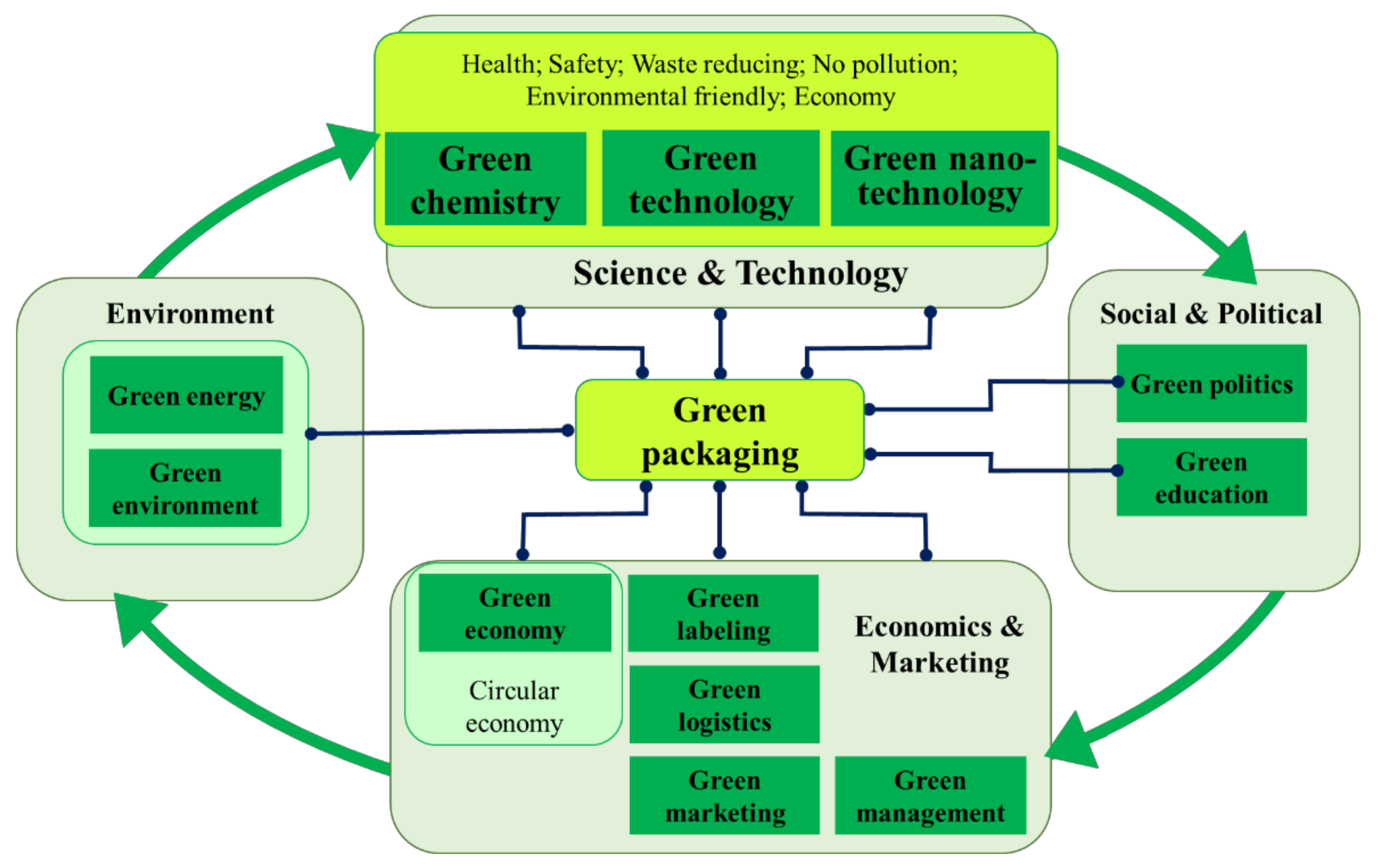
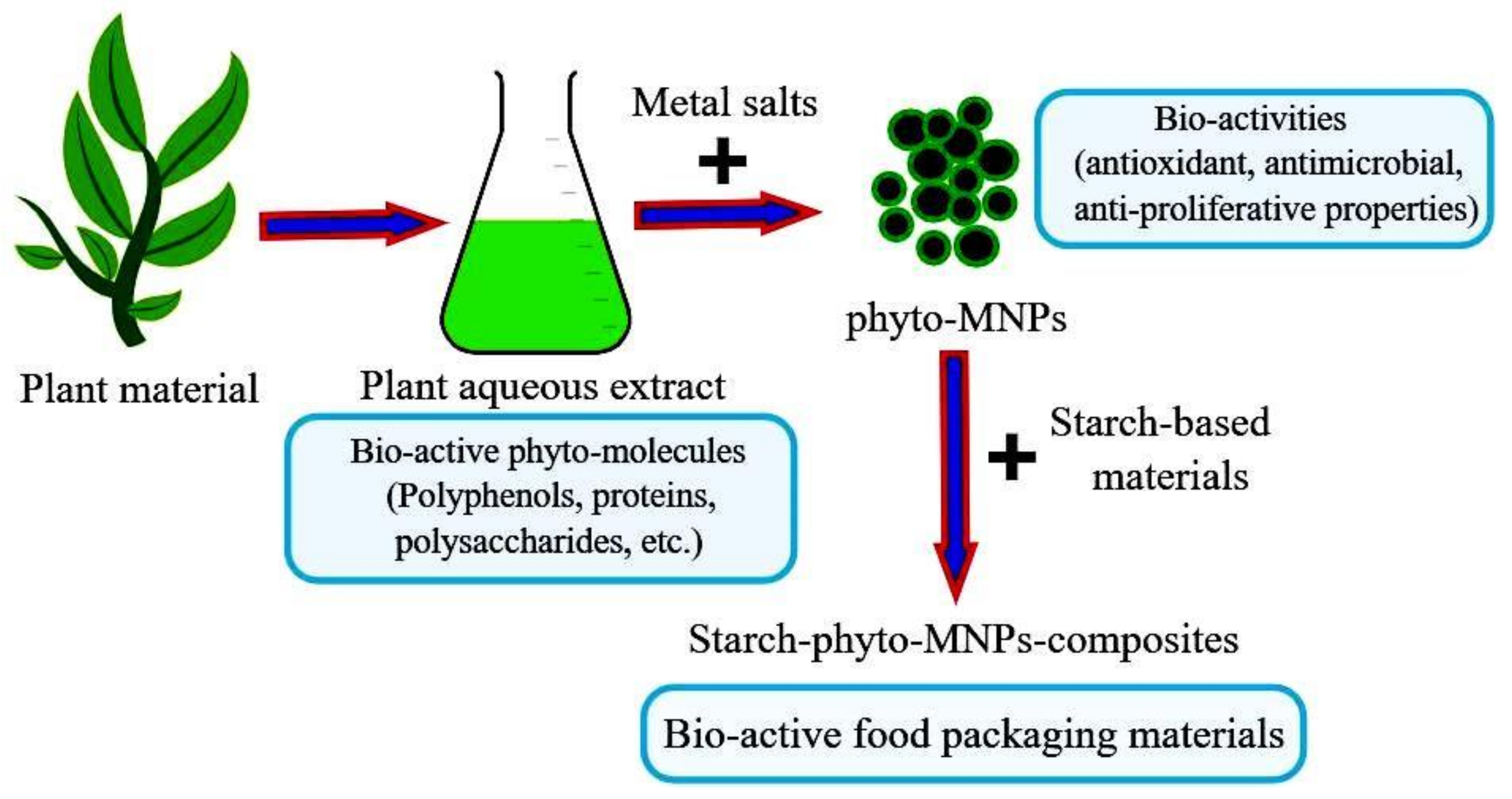
1. The “Green” Context
- -
- Bioactive compounds (vitamins, polyphenols, essential oils, plant extracts, amino acids, etc.) and their role for the immune system;
- -
- GC methods to incorporate bioactive compounds into the starch matrix and their role for the human health;
- -
- GT, with emphasis on physical treatments with low environmental impact (UV irradiation, plasma and others) on the starch-based packaging with or without incorporated bioactive ingredients, together with a Green Nanotechnology (GN) approach in developing nanomaterials combined with starch for food packaging.
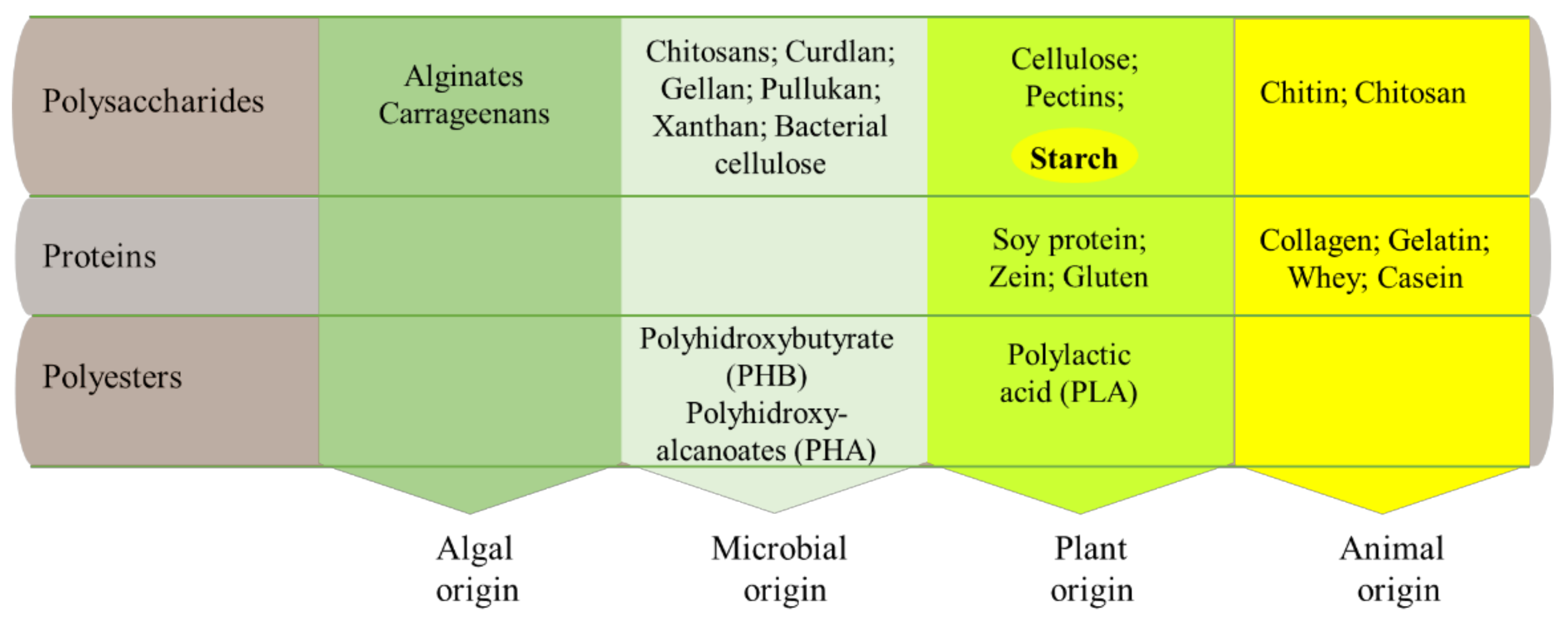
2. Starch-Based Films and Coatings
3. Plant-Derived Bioactive Compounds Promoting the Human Health
4. Green Chemical Treatments of the Starch-Based Films and Coatings by Incorporating Bioactive Compounds
4.1. Vitamins Incorporation into Starchy Films and Coatings
4.2. Polyphenols
4.3. Essential Oils
4.4. Minerals
4.5. Amino Acids, Peptides, Proteins and Enzymes in Starch-Based Food Packaging
4.6. Lipids and Lipid-Based Nanostructures in Starch Packaging Systems
4.7. Vegetal Extracts in Starch Food Packaging
- -
- Actions sustaining consumers’ health and safety: boosting the immune system, antimicrobial and antioxidant activity;
- -
- Actions on the packaged products: increasing their shelf life, improving of the mechanical and sensorial properties.
5. Application of Green Physical Treatments on Starch and Starch-Based Films
5.1. UV Radiation
5.2. Ionizing Radiation
5.2.1. Electron Beam (EB) Irradiation
5.2.2. Gamma Irradiation
5.3. Other Green Physical Treatments
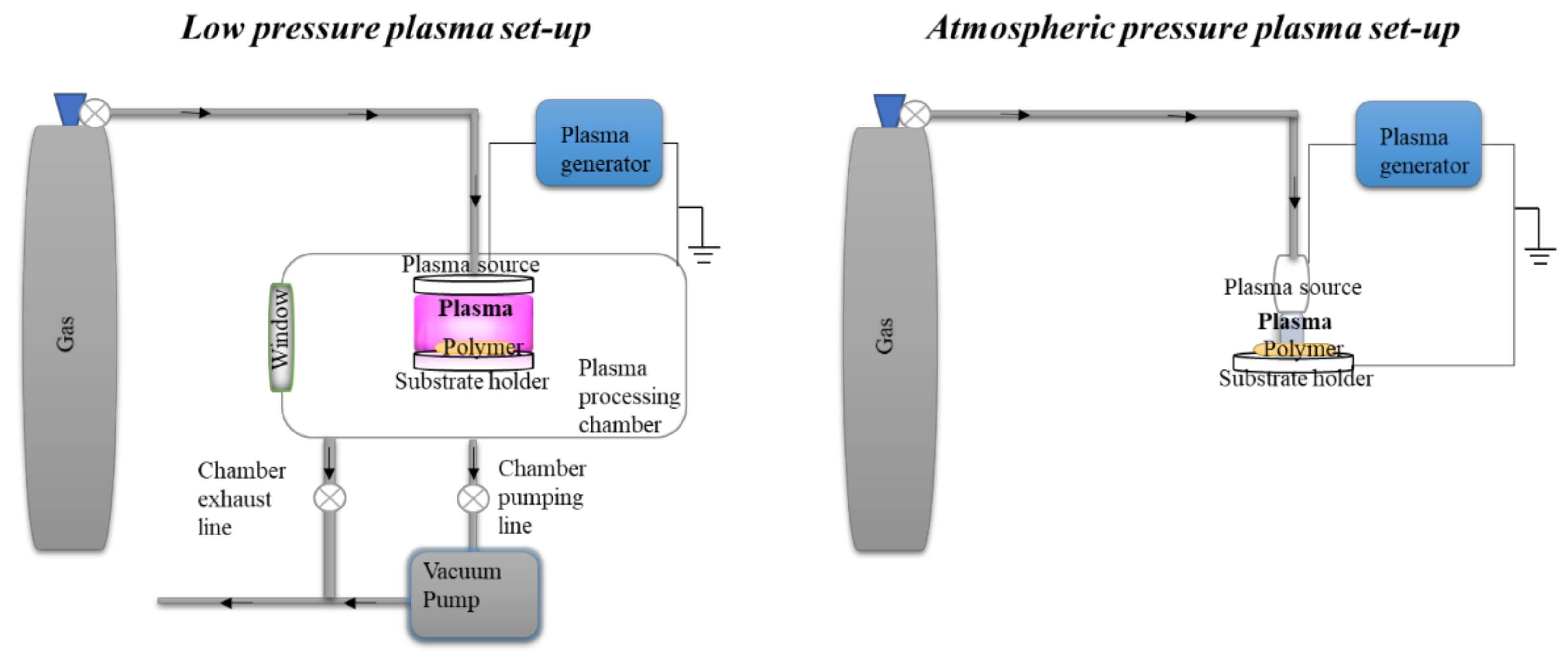
Plasma Treatment of Starch
6. Nanotechnology in Starch Food Packaging
- Particulated 3D nanoreinforcements (nanoparticles and nanocrystals);
- Fibrillated 2D nanoreinforcements (nanofibers, nanotubes);
- Laminated 1D nanoreinforcements (nanoclays).
7. Frontier Technologies in Green Starch-Based Films and Coatings Research
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boz, Z.; Korhonen, V.; Koelsch Sand, C. Consumer Considerations for the Implementation of Sustainable Packaging: A Review. Sustainability 2020, 12, 2192. [Google Scholar] [CrossRef]
- Pauer, E.; Wohner, B.; Heinrich, V.; Tacker, M. Assessing the Environmental Sustainability of Food Packaging: An Extended Life Cycle Assessment Including Packaging-Related Food Losses and Waste and Circularity Assessment. Sustainability 2019, 11, 925. [Google Scholar] [CrossRef]
- Guillard, V.; Gaucel, S.; Fornaciari, C.; Angellier-Coussy, H.; Buche, P.; Gontard, N. The Next Generation of Sustainable Food Packaging to Preserve Our Environment in a Circular Economy Context. Front. Nutr. 2018, 5, 121. [Google Scholar] [CrossRef] [PubMed]
- Molina-Besch, K.; Wikström, F.; Williams, H. The Environmental Impact of Packaging in Food Supply Chains—Does Life Cycle Assessment of Food Provide the Full Picture? Int. J. Life Cycle Assess 2019, 24, 37–50. [Google Scholar] [CrossRef]
- Pongrácz, E. The environmental impacts of packaging. In Environmentally Conscious Materials and Chemicals Processing; Kutz, M., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; pp. 237–278. [Google Scholar] [CrossRef]
- Motelica, L.; Ficai, D.; Ficai, A.; Oprea, O.C.; Kaya, D.A.; Andronescu, E. Biodegradable Antimicrobial Food Packaging: Trends and Perspectives. Foods 2020, 9, 1438. [Google Scholar] [CrossRef]
- Asgher, M.; Qamar, S.A.; Bilal, M.; Iqbal, H.M.N. Bio-Based Active Food Packaging Materials: Sustainable Alternative to Conventional Petrochemical-Based Packaging Materials. Food Res. Int. 2020, 137, 109625. [Google Scholar] [CrossRef]
- Sdrolia, E.; Zarotiadis, G. A comprehensive review for green product term: From definition to evaluation: A comprehensive review for green product term. J. Econ. Surv. 2019, 33, 150–178. [Google Scholar] [CrossRef]
- Knill, G. Towards the green paradigm. S. Afr. Geogr. J. 1991, 73, 52–59. [Google Scholar] [CrossRef]
- Zhang, G.; Zhao, Z. Green Packaging Management of Logistics Enterprises. Phys. Procedia 2012, 24, 900–905. [Google Scholar] [CrossRef]
- Singh, G.; Pandey, N. The Determinants of Green Packaging That Influence Buyers’ Willingness to Pay a Price Premium. Australas. Mark. J. 2018, 26, 221–230. [Google Scholar] [CrossRef]
- Robertson, G.L. Sustainable Food Packaging. In Handbook of Waste Management and Co-Product Recovery in Food Processing; Waldron, K., Ed.; Woodhead Publishing: Cambridge, UK, 2009; Volume 2, pp. 221–254. [Google Scholar] [CrossRef]
- Casarejos, F.; Bastos, C.R.; Rufin, C.; Frota, M.N. Rethinking Packaging Production and Consumption Vis-à-Vis Circular Economy: A Case Study of Compostable Cassava Starch-Based Material. J. Clean. Prod. 2018, 201, 1019–1028. [Google Scholar] [CrossRef]
- Dobrucka, R. Bioplastic Packaging Materials in Circular Economy. Logforum 2019, 15, 129–137. [Google Scholar] [CrossRef]
- Geueke, B.; Groh, K.; Muncke, J. Food Packaging in the Circular Economy: Overview of Chemical Safety Aspects for Commonly Used Materials. J. Clean. Prod. 2018, 193, 491–505. [Google Scholar] [CrossRef]
- Spierling, S.; Knüpffer, E.; Behnsen, H.; Mudersbach, M.; Krieg, H.; Springer, S.; Albrecht, S.; Herrmann, C.; Endres, H.-J. Bio-Based Plastics—A Review of Environmental, Social and Economic Impact Assessments. J. Clean. Prod. 2018, 185, 476–491. [Google Scholar] [CrossRef]
- Zhang, Q.; Mi, J.; Shen, H. Green Labeling and Sustainable Development. In Encyclopedia of Sustainability in Higher Education; Leal Filho, W., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–7. [Google Scholar] [CrossRef]
- Wei, L.T.; Yazdanifard, R. Edible Food Packaging as an Eco-Friendly Technology Using Green Marketing Strategy. Glob. J. Commer. Manag. Perspect. 2013, 2, 8–11. [Google Scholar]
- Mishra, P.; Jain, T.; Motiani, M. Have Green, Pay More: An Empirical Investigation of Consumer’s Attitude towards Green Packaging in an Emerging Economy. In Essays on Sustainability and Management; Sarkar, R., Shaw, A., Eds.; Springer: Singapore, 2017; pp. 125–150. [Google Scholar] [CrossRef]
- Wossen Kassaye, W. Green Dilemma. Mark. Intell. Plan 2001, 19, 444–455. [Google Scholar] [CrossRef]
- Selke, S.E.M. Green Packaging. In Green Technologies in Food Production and Processing; Boye, J.I., Arcand, Y., Eds.; Springer: Boston, MA, USA, 2012; pp. 443–468. [Google Scholar] [CrossRef]
- Yildirim, S.; Röcker, B.; Pettersen, M.K.; Nilsen-Nygaard, J.; Ayhan, Z.; Rutkaite, R.; Radusin, T.; Suminska, P.; Marcos, B.; Coma, V. Active Packaging Applications for Food. Compr. Rev. Food Sci. Food Saf. 2018, 17, 165–199. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Xiao, H.; Zhang, W.; Gong, G. Reactive Coating of Soybean Oil-Based Polymer on Nanofibrillated Cellulose Film for Water Vapor Barrier Packaging. Carbohydr. Polym. 2014, 111, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Wnek, P.H.; Lafferty, T.P.; Robison, R.G.; Cole, L.R.; O’Hagan, B.R.; Middleton, S.W. Insulating Microwave Interactive Packaging. US Patent US8563906B2, 22 October 2013. [Google Scholar]
- Müller, P.; Schmid, M. Intelligent Packaging in the Food Sector: A Brief Overview. Foods 2019, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.; Dhiman, R.; Rokana, N.; Panwar, H. Nanotechnology: An Untapped Resource for Food Packaging. Front. Microbiol. 2017, 8, 1735. [Google Scholar] [CrossRef] [PubMed]
- Shukla, P.; Bhise, S.; Thind, S. Role of Biodegradable Edible Films and Coatings in Food Industry. Acta Sci. Nutr. health 2019, 3, 138–147. [Google Scholar]
- Otoni, C.G.; Avena-Bustillos, R.J.; Azeredo, H.M.C.; Lorevice, M.V.; Moura, M.R.; Mattoso, L.H.C.; McHugh, T.H. Recent Advances on Edible Films Based on Fruits and Vegetables-A Review: Fruit and Vegetable Edible Films. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1151–1169. [Google Scholar] [CrossRef] [PubMed]
- Pavlath, A.E.; Orts, W. Edible Films and Coatings: Why, What, and How? In Edible Films and Coatings for Food Applications; Huber, K.C., Embuscado, M.E., Eds.; Springer: New York, NY, USA, 2009; pp. 1–23. [Google Scholar] [CrossRef]
- Versino, F.; Lopez, O.V.; Garcia, M.A.; Zaritzky, N.E. Starch-Based Films and Food Coatings: An Overview. Starch Stärke 2016, 68, 1026–1037. [Google Scholar] [CrossRef]
- Gabor, D.; Tita, O. Biopolymers Used in Food Packaging: A Review. Acta Univ. Cibiniensis Ser. E Food Technol. 2012, 16, 3–19. [Google Scholar]
- Jothimani, B.; Venkatachalapathy, B.; Karthikeyan, N.S.; Ravichandran, C. A Review on Versatile Applications of Degradable Polymers. In Green Biopolymers and Their Nanocomposites; Gnanasekaran, D., Ed.; Springer: Singapore, 2019; pp. 403–422. [Google Scholar] [CrossRef]
- Shah, U.; Naqash, F.; Gani, A.; Masoodi, F.A. Art and Science behind Modified Starch Edible Films and Coatings: A Review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 568–580. [Google Scholar] [CrossRef]
- Sapper, M.; Chiralt, A. Starch-Based Coatings for Preservation of Fruits and Vegetables. Coatings 2018, 8, 152. [Google Scholar] [CrossRef]
- Wróblewska-Krepsztul, J.; Rydzkowski, T.; Borowski, G.; Szczypiński, M.; Klepka, T.; Thakur, V.K. Recent Progress in Biodegradable Polymers and Nanocomposite-Based Packaging Materials for Sustainable Environment. Int. J. Polym. Anal. Charact. 2018, 23, 383–395. [Google Scholar] [CrossRef]
- Gadhave, R.V.; Das, A.; Mahanwar, P.A.; Gadekar, P.T. Starch Based Bio-Plastics: The Future of Sustainable Packaging. OJPChem 2018, 8, 21–33. [Google Scholar] [CrossRef]
- Pandiselvam, R.; Manikantan, M.R.; Divya, V.; Ashokkumar, C.; Kaavya, R.; Kothakota, A.; Ramesh, S.V. Ozone: An Advanced Oxidation Technology for Starch Modification. Ozone Sci. Eng. 2019, 41, 491–507. [Google Scholar] [CrossRef]
- Alcázar-Alay, S.C.; Meireles, M.A.A. Physicochemical Properties, Modifications and Applications of Starches from Different Botanical Sources. Food Sci. Technol. 2015, 35, 215–236. [Google Scholar] [CrossRef]
- Robyt, J.F. Starch: Structure, Properties, Chemistry, and Enzymology. In Glycoscience; Fraser-Reid, B.O., Tatsuta, K., Thiem, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 1437–1472. [Google Scholar] [CrossRef]
- Pokhrel, S. A review on introduction and applications of starch and its biodegradable polymers. Int. J. Environ. 2015, 4, 114–125. [Google Scholar] [CrossRef]
- Teck Kim, Y.; Min, B.; Won Kim, K. General Characteristics of Packaging Materials for Food System. In Innovations in Food Packaging; Han, J.H., Ed.; Academic Press: London, UK, 2014; pp. 13–15. [Google Scholar] [CrossRef]
- Masina, N.; Choonara, Y.E.; Kumar, P.; du Toit, L.C.; Govender, M.; Indermun, S.; Pillay, V. A Review of the Chemical Modification Techniques of Starch. Carbohydr. Polym. 2017, 157, 1226–1236. [Google Scholar] [CrossRef]
- Haroon, M.; Wang, L.; Yu, H.; Abbasi, N.M.; Zain-ul-Abdin, Z.-A.; Saleem, M.; Khan, R.U.; Ullah, R.S.; Chen, Q.; Wu, J. Chemical Modification of Starch and Its Application as an Adsorbent Material. RSC Adv. 2016, 6, 78264–78285. [Google Scholar] [CrossRef]
- Korma, S.A. Chemically Modified Starch and Utilization in Food Stuffs. IJNFS 2016, 5, 264. [Google Scholar] [CrossRef]
- Rapaille, A.; Vanhemelrijck, J. Modified Starches. In Thickening and Gelling Agents for Food; Imeson, A.P., Ed.; Springer: Boston, MA, USA, 1997; pp. 199–229. [Google Scholar] [CrossRef]
- López, O.V.; García, M.A.; Zaritzky, N.E. Film Forming Capacity of Chemically Modified Corn Starches. Carbohydr. Polym. 2008, 73, 573–581. [Google Scholar] [CrossRef]
- Chi, H.; Xu, K.; Wu, X.; Chen, Q.; Xue, D.; Song, C.; Zhang, W.; Wang, P. Effect of Acetylation on the Properties of Corn Starch. Food Chem. 2008, 106, 923–928. [Google Scholar] [CrossRef]
- Mohamad Yazid, N.S.; Abdullah, N.; Muhammad, N.; Matias-Peralta, H.M. Application of Starch and Starch-Based Products in Food Industry. JST 2018, 10, 144–174. [Google Scholar] [CrossRef]
- Rutenberg, M.W.; Solarek, D. Starch derivatives: Production and uses. In Starch: Chemistry and Technology; Academic Press Inc.: San Diego, CA, USA, 1984; pp. 311–388. [Google Scholar] [CrossRef]
- Perin, D.; Murano, E. Starch Polysaccharides in the Human Diet: Effect of the Different Source and Processing on Its Absorption. Nat. Prod. Commun. 2017, 12, 835–853. [Google Scholar] [CrossRef]
- Tian, S.; Chen, Y.; Chen, Z.; Yang, Y.; Wang, Y. Preparation and Characteristics of Starch Esters and Its Effects on Dough Physicochemical Properties. J. Food Qual. 2018, 2018, 1–7. [Google Scholar] [CrossRef]
- Zhang, Y.; Rempel, C. Retrogradation and Antiplasticization of Thermoplastic Starch. In Thermoplastic Elastomers; El-Sonbati, A., Ed.; IntechOpen: Rijeka, Croatia, 2012; pp. 117–134. [Google Scholar] [CrossRef]
- Niranjana Prabhu, T.; Prashantha, K. A Review on Present Status and Future Challenges of Starch Based Polymer Films and Their Composites in Food Packaging Applications. Polym. Compos. 2018, 39, 2499–2522. [Google Scholar] [CrossRef]
- Zuo, Y.; Gu, J.; Tan, H.; Zhang, Y. Thermoplastic Starch Prepared with Different Plasticizers: Relation between Degree of Plasticization and Properties. J. Wuhan Univ. Technol. Mat. Sci. Ed. 2015, 30, 423–428. [Google Scholar] [CrossRef]
- Das, D.K.; Dutta, H.; Mahanta, C.L. Development of a Rice Starch-Based Coating with Antioxidant and Microbe-Barrier Properties and Study of Its Effect on Tomatoes Stored at Room Temperature. LWT Food Sci. Technol. 2013, 50, 272–278. [Google Scholar] [CrossRef]
- Beilvert, A.; Chaubet, F.; Chaunier, L.; Guilois, S.; Pavon-Djavid, G.; Letourneur, D.; Meddahi-Pellé, A.; Lourdin, D. Shape-Memory Starch for Resorbable Biomedical Devices. Carbohydr. Polym. 2014, 99, 242–248. [Google Scholar] [CrossRef]
- Chaunier, L.; Lourdin, D. The Shape Memory of Starch. Starch Stärke 2009, 61, 116–118. [Google Scholar] [CrossRef]
- Véchambre, C.; Chaunier, L.; Lourdin, D. Novel Shape-Memory Materials Based on Potato Starch: Novel Shape-Memory Materials Based on Potato Starch. Macromol. Mater. Eng. 2010, 295, 115–122. [Google Scholar] [CrossRef]
- Brockgreitens, J.; Abbas, A. Responsive Food Packaging: Recent Progress and Technological Prospects: Responsive Food Packaging. Compr. Rev. Food Sci. Food Saf. 2016, 15, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Domene-López, D.; García-Quesada, J.C.; Martin-Gullon, I.; Montalbán, M.G. Influence of Starch Composition and Molecular Weight on Physicochemical Properties of Biodegradable Films. Polymers 2019, 11, 1084. [Google Scholar] [CrossRef] [PubMed]
- Shafik, S.S.; Majeed, K.J.; Kamil, M.I. Preparation of PVA/Corn Starch Blend Films and Studying the Influence of Gamma Irradiation on Mechanical Properties. Int. J. Mater. Sci. Appl. 2014, 3, 25–28. [Google Scholar] [CrossRef]
- Medina-Jaramillo, C.; Ochoa-Yepes, O.; Bernal, C.; Famá, L. Active and Smart Biodegradable Packaging Based on Starch and Natural Extracts. Carbohydr. Polym. 2017, 176, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Tomova, A.; Bukovsky, I.; Rembert, E.; Yonas, W.; Alwarith, J.; Barnard, N.D.; Kahleova, H. The Effects of Vegetarian and Vegan Diets on Gut Microbiota. Front. Nutr. 2019, 6, 47. [Google Scholar] [CrossRef]
- Calder, P.C. Feeding the Immune System. Proc. Nutr. Soc. 2013, 72, 299–309. [Google Scholar] [CrossRef]
- Farhan Aslam, M.; Majeed, S.; Aslam, S.; Irfan, J.A. Vitamins: Key Role Players in Boosting Up Immune Response-A Mini Review. Vitam. Min. 2017, 6, 1000153. [Google Scholar] [CrossRef]
- Alpert, P.T. The Role of Vitamins and Minerals on the Immune System. Home Health Care Manag. Pract. 2017, 29, 199–202. [Google Scholar] [CrossRef]
- Ross, A.C. Vitamin A and Retinoic Acid in T Cell–Related Immunity. Am. J. Clin. Nutr. 2012, 96, 1166S–1172S. [Google Scholar] [CrossRef]
- Chacón-Ordóñez, T.; Esquivel, P.; Quesada, S.; Jiménez, R.R.; Cordero, A.; Carle, R.; Schweiggert, R. Mamey Sapote Fruit and Carotenoid Formulations Derived Thereof Are Dietary Sources of Vitamin A—A Comparative Randomized Cross-over Study. Food Res. Int. 2019, 122, 340–347. [Google Scholar] [CrossRef]
- Beltrán-de-Miguel, B.; Estévez-Santiago, R.; Olmedilla-Alonso, B. Assessment of Dietary Vitamin A Intake (Retinol, α-Carotene, β-Carotene, β-Cryptoxanthin) and Its Sources in the National Survey of Dietary Intake in Spain (2009–2010). Int. J. Food Sci. Nutr. 2015, 66, 706–712. [Google Scholar] [CrossRef]
- Carr, A.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef] [PubMed]
- Devaki, S.J.; Raveendran, R.L. Vitamin C: Sources, Functions, Sensing and Analysis. In Vitamin C; Hamza, A.H., Ed.; IntechOpen: Rijeka, Croatia, 2017; pp. 3–20. [Google Scholar] [CrossRef]
- Aranow, C. Vitamin D and the Immune System. J. Investig. Med. 2011, 59, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Schmid, A.; Walther, B. Natural Vitamin D Content in Animal Products. Adv. Nutr. 2013, 4, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.; Raza, S.T.; Ahmed, F.; Ahmad, A.; Abbas, S.; Mahdi, F. The Role of Vitamin e in Human Health and Some Diseases. Sultan Qaboos Univ. Med. J. 2014, 14, e157–e165. [Google Scholar] [PubMed]
- Olszewska, M.A.; Gędas, A.; Simões, M. Antimicrobial Polyphenol-Rich Extracts: Applications and Limitations in the Food Industry. Food Res. Int. 2020, 134, 109214. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Jiang, H.; Fang, J. Regulation of Immune Function by Polyphenols. J. Immunol. Res. 2018, 2018, 1264074. [Google Scholar] [CrossRef] [PubMed]
- Diaconeasa, Z.; Știrbu, I.; Xiao, J.; Leopold, N.; Ayvaz, Z.; Danciu, C.; Ayvaz, H.; Stănilă, A.; Nistor, M.; Socaciu, C. Anthocyanins, Vibrant Color Pigments, and Their Role in Skin Cancer Prevention. Biomedicines 2020, 8, 336. [Google Scholar] [CrossRef]
- Biao, Y.; Yuxuan, C.; Qi, T.; Ziqi, Y.; Yourong, Z.; McClements, D.J.; Chongjiang, C. Enhanced Performance and Functionality of Active Edible Films by Incorporating Tea Polyphenols into Thin Calcium Alginate Hydrogels. Food Hydrocoll. 2019, 97, 105197. [Google Scholar] [CrossRef]
- Panja, P. Green Extraction Methods of Food Polyphenols from Vegetable Materials. Curr. Opin. Food Sci. 2018, 23, 173–182. [Google Scholar] [CrossRef]
- Aguirre-Joya, J.A.; Pastrana-Castro, L.; Nieto-Oropeza, D.; Ventura-Sobrevilla, J.; Rojas-Molina, R.; Aguilar, C.N. The Physicochemical, Antifungal and Antioxidant Properties of a Mixed Polyphenol Based Bioactive Film. Heliyon 2018, 4, e00942. [Google Scholar] [CrossRef]
- Denev, P.; Číž, M.; Kratchanova, M.; Blazheva, D. Black Chokeberry (Aronia Melanocarpa) Polyphenols Reveal Different Antioxidant, Antimicrobial and Neutrophil-Modulating Activities. Food Chem. 2019, 284, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Lei, Y.; Zhu, R.; Zhao, M.; Lu, J.; Xiao, D.; Jiao, C.; Zhang, Z.; Shen, G.; Li, S. Preparation and Characterization of Bioactive Edible Packaging Films Based on Pomelo Peel Flours Incorporating Tea Polyphenol. Food Hydrocoll. 2019, 90, 41–49. [Google Scholar] [CrossRef]
- Feng, M.; Yu, L.; Zhu, P.; Zhou, X.; Liu, H.; Yang, Y.; Zhou, J.; Gao, C.; Bao, X.; Chen, P. Development and Preparation of Active Starch Films Carrying Tea Polyphenol. Carbohydr. Polym. 2018, 196, 162–167. [Google Scholar] [CrossRef]
- Bouarab-Chibane, L.; Forquet, V.; Lantéri, P.; Clément, Y.; Léonard-Akkari, L.; Oulahal, N.; Degraeve, P.; Bordes, C. Antibacterial Properties of Polyphenols: Characterization and QSAR (Quantitative Structure–Activity Relationship) Models. Front. Microbiol. 2019, 10, 829. [Google Scholar] [CrossRef]
- Mishra, A.P.; Devkota, H.P.; Nigam, M.; Adetunji, C.O.; Srivastava, N.; Saklani, S.; Shukla, I.; Azmi, L.; Shariati, M.A.; Melo Coutinho, H.D.; et al. Combination of Essential Oils in Dairy Products: A Review of Their Functions and Potential Benefits. LWT 2020, 133, 110116. [Google Scholar] [CrossRef]
- Diao, W.-R.; Hu, Q.-P.; Zhang, H.; Xu, J.-G. Chemical Composition, Antibacterial Activity and Mechanism of Action of Essential Oil from Seeds of Fennel (Foeniculum Vulgare Mill.). Food Control 2014, 35, 109–116. [Google Scholar] [CrossRef]
- Carson, C.F.; Hammer, K.A. Chemistry and Bioactivity of Essential Oils. In Lipids and Essential Oils as Antimicrobial Agents; Thormar, H., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2011; pp. 203–238. [Google Scholar] [CrossRef]
- Khayyat, S.A.; Roselin, L.S. Recent Progress in Photochemical Reaction on Main Components of Some Essential Oils. J. Saudi Chem. Soc. 2018, 22, 855–875. [Google Scholar] [CrossRef]
- Asbahani, A.E.; Miladi, K.; Badri, W.; Sala, M.; Addi, E.H.A.; Casabianca, H.; Mousadik, A.E.; Hartmann, D.; Jilale, A.; Renaud, F.N.R.; et al. Essential Oils: From Extraction to Encapsulation. Int. J. Pharm. 2015, 483, 220–243. [Google Scholar] [CrossRef]
- Rassem, H.H.A.; Nour, A.H.; Yunus, R.M. Techniques for Extraction of Essential Oils from Plants: A Review. Aust. J. Basic Appl. Sci. 2016, 10, 117–127. [Google Scholar]
- Pavela, R.; Žabka, M.; Bednář, J.; Tříska, J.; Vrchotová, N. New Knowledge for Yield, Composition and Insecticidal Activity of Essential Oils Obtained from the Aerial Parts or Seeds of Fennel (Foeniculum Vulgare Mill.). Ind. Crop. Prod. 2016, 83, 275–282. [Google Scholar] [CrossRef]
- Ribeiro-Santos, R.; Andrade, M.; de Melo, N.R.; Sanches-Silva, A. Use of Essential Oils in Active Food Packaging: Recent Advances and Future Trends. Trends Food Sci. Technol. 2017, 61, 132–140. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S. Essential Oils: Extraction, Bioactivities, and Their Uses for Food Preservation. J. Food Sci. 2014, 79, R1231–R1249. [Google Scholar] [CrossRef] [PubMed]
- Sanches-Silva, A.; Costa, D.; Albuquerque, T.G.; Buonocore, G.G.; Ramos, F.; Castilho, M.C.; Machado, A.V.; Costa, H.S. Trends in the Use of Natural Antioxidants in Active Food Packaging: A Review. Food Addit. Contam. Part A 2014, 31, 374–395. [Google Scholar] [CrossRef] [PubMed]
- Niu, B.; Yan, Z.; Shao, P.; Kang, J.; Chen, H. Encapsulation of Cinnamon Essential Oil for Active Food Packaging Film with Synergistic Antimicrobial Activity. Nanomaterials 2018, 8, 598. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, M.; Khosravi-Daran, K. Essential Oils as Natural Food Preservatives: Antimicrobial and Antioxidant Applications. In Antimicrobials from Nature: Effective Control Agents for Drug Resistant Pathogens; Doughari, J.H., Ed.; Transworld Research Network: Kerala, India, 2013; pp. 15–37. [Google Scholar]
- Semeniuc, C.A.; Pop, C.R.; Rotar, A.M. Antibacterial Activity and Interactions of Plant Essential Oil Combinations against Gram-Positive and Gram-Negative Bacteria. J. Food Drug Anal. 2017, 25, 403–408. [Google Scholar] [CrossRef]
- Mendes, J.F.; Norcino, L.B.; Martins, H.H.A.; Manrich, A.; Otoni, C.G.; Carvalho, E.E.N.; Piccoli, R.H.; Oliveira, J.E.; Pinheiro, A.C.M.; Mattoso, L.H.C. Correlating Emulsion Characteristics with the Properties of Active Starch Films Loaded with Lemongrass Essential Oil. Food Hydrocoll. 2020, 100, 105428. [Google Scholar] [CrossRef]
- Cherrat, L.; Espina, L.; Bakkali, M.; García-Gonzalo, D.; Pagán, R.; Laglaoui, A. Chemical Composition and Antioxidant Properties of Laurus nobilis L. and Myrtus communis L. Essential Oils from Morocco and Evaluation of Their Antimicrobial Activity Acting Alone or in Combined Processes for Food Preservation: Antimicrobial and Antioxidant Activity of L. nobilis and M. communis EOs. J. Sci. Food Agric. 2014, 94, 1197–1204. [Google Scholar] [CrossRef]
- Abarca, R.L.; Rodríguez, F.J.; Guarda, A.; Galotto, M.J.; Bruna, J.E. Characterization of Beta-Cyclodextrin Inclusion Complexes Containing an Essential Oil Component. Food Chem. 2016, 196, 968–975. [Google Scholar] [CrossRef]
- Soetan, K.O.; Olaiya, C.O.; Oyewole, O.E. The Importance of Mineral Elements for Humans, Domestic Animals and Plants: A Review. Afr. J. Food Sci. 2010, 4, 200–222. [Google Scholar]
- Sigdel, A. Effect of Mineral Ions on the Functional Properties of Starch Films. Master’s Thesis, South Dakota State University, Vermillion, SD, USA, 2019. [Google Scholar]
- Tian, S.; Jones, S.M.; Solomon, E.I. Role of a Tyrosine Radical in Human Ceruloplasmin Catalysis. ACS Cent. Sci. 2020, 6, 1835–1843. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Raynor, J.; Nguyen, T.-L.M.; Chi, H. Nutrient and Metabolic Sensing in T Cell Responses. Front. Immunol. 2017, 8, 247. [Google Scholar] [CrossRef]
- Gray, H.B.; Winkler, J.R. Hole Hopping through Tyrosine/Tryptophan Chains Protects Proteins from Oxidative Damage. Proc. Natl. Acad. Sci. USA 2015, 112, 10920–10925. [Google Scholar] [CrossRef] [PubMed]
- Sikalidis, A.K. Amino Acids and Immune Response: A Role for Cysteine, Glutamine, Phenylalanine, Tryptophan and Arginine in T-Cell Function and Cancer? Pathol. Oncol. Res. 2015, 21, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Yin, Y.-L.; Li, D.; Woo Kim, S.; Wu, G. Amino Acids and Immune Function. Br. J. Nutr. 2007, 98, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Saljoughian, M. Whey Protein: Health Benefits at a Glance. US Pharm. 2009, 34, HS-14–HS-18. [Google Scholar]
- Bharti, S.K.; Pathak, V.; Alam, T.; Arya, A.; Basak, G.; Awasthi, M.G. Materiality of Edible Film Packaging in Muscle Foods: A Worthwhile Conception. J. Package Technol. Res. 2020, 4, 117–132. [Google Scholar] [CrossRef]
- Hoshi, T.; Wissuwa, B.; Tian, Y.; Tajima, N.; Xu, R.; Bauer, M.; Heinemann, S.H.; Hou, S. Omega-3 Fatty Acids Lower Blood Pressure by Directly Activating Large-Conductance Ca2+-Dependent K+ Channels. Proc. Natl. Acad. Sci. USA 2013, 110, 4816–4821. [Google Scholar] [CrossRef]
- Gil-de-Gómez, L.; Balgoma, D.; Montero, O. Lipidomic-Based Advances in Diagnosis and Modulation of Immune Response to Cancer. Metabolites 2020, 10, 332. [Google Scholar] [CrossRef]
- Da Silva Lannes, S.C.; Maria, R. Structuring Fat Foods. In Food Industry; Muzzalupo, I., Ed.; IntechOpen: Rijeka, Croatia, 2013; Available online: https://www.intechopen.com/books/food-industry/structuring-fat-foods (accessed on 26 November 2020). [CrossRef][Green Version]
- Radzikowska, U.; Rinaldi, A.O.; Çelebi Sözener, Z.; Karaguzel, D.; Wojcik, M.; Cypryk, K.; Akdis, M.; Akdis, C.A.; Sokolowska, M. The Influence of Dietary Fatty Acids on Immune Responses. Nutrients 2019, 11, 2990. [Google Scholar] [CrossRef]
- Kumar, A.; Mastana, S.S.; Lindley, M.R. N-3 Fatty Acids and Asthma. Nutr. Res. Rev. 2016, 29, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Storsve, A.B.; Johnsen, L.; Nyborg, C.; Melau, J.; Hisdal, J.; Burri, L. Effects of Krill Oil and Race Distance on Serum Choline and Choline Metabolites in Triathletes: A Field Study. Front. Nutr. 2020, 7, 133. [Google Scholar] [CrossRef]
- Liu, C.; Huang, J.; Zheng, X.; Liu, S.; Lu, K.; Tang, K.; Liu, J. Heat Sealable Soluble Soybean Polysaccharide/Gelatin Blend Edible Films for Food Packaging Applications. Food Packag. Shelf Life 2020, 24, 100485. [Google Scholar] [CrossRef]
- De Azeredo, H.M.C.; Rosa, M.F.; De Sá, M.; Souza Filho, M.; Waldron, K.W. The Use of Biomass for Packaging Films and Coatings. In Advances in Biorefineries; Woodhead Publishing House: Cambridge, UK, 2014; pp. 819–874. [Google Scholar] [CrossRef]
- Barbinta-Patrascu, M.E.; Ungureanu, C.; Badea, N.; Bacalum, M.; Lazea-Stoyanova, A.; Zgura, I.; Negrila, C.; Enculescu, M.; Burnei, C. Novel Ecogenic Plasmonic Biohybrids as Multifunctional Bioactive Coatings. Coatings 2020, 10, 659. [Google Scholar] [CrossRef]
- Barbinta-Patrascu, M.E.; Ungureanu, C.; Badea, N.; Constantin, M.; Purcar, V.; Ispas, A. Bioperformances of Honey-Phytonanosilver in Silica Materials. J. Optoelectron. Adv. Mater. 2020, 22, 310–315. [Google Scholar]
- Barbinta-Patrascu, M.E.; Badea, N.; Bacalum, M.; Ungureanu, C.; Suica-Bunghez, I.R.; Iordache, S.M.; Pirvu, C.; Zgura, I.; Maraloiu, V.A. 3D Hybrid Structures Based on Biomimetic Membranes and Caryophyllus Aromaticus—“Green” Synthesized Nano-Silver with Improved Bioperformances. Mater. Sci. Eng. C 2019, 101, 120–137. [Google Scholar] [CrossRef]
- Ultee, A.; Bennik, M.H.J.; Moezelaar, R. The Phenolic Hydroxyl Group of Carvacrol Is Essential for Action against the Food-Borne Pathogen Bacillus Cereus. AEM 2002, 68, 1561–1568. [Google Scholar] [CrossRef]
- Piñeros-Hernandez, D.; Medina-Jaramillo, C.; López-Córdoba, A.; Goyanes, S. Edible Cassava Starch Films Carrying Rosemary Antioxidant Extracts for Potential Use as Active Food Packaging. Food Hydrocoll. 2017, 63, 488–495. [Google Scholar] [CrossRef]
- Benbettaïeb, N.; Debeaufort, F.; Karbowiak, T. Bioactive Edible Films for Food Applications: Mechanisms of Antimicrobial and Antioxidant Activity. Crit. Rev. Food Sci. Nutr. 2019, 59, 3431–3455. [Google Scholar] [CrossRef]
- Basics of Green Chemistry, United States Environmental Protection Agency Web Site. Available online: https://www.epa.gov/greenchemistry/basics-green-chemistry (accessed on 6 March 2021).
- Manahan, S.E. Green Chemistry and the Ten Commandments of Sustainability, 3rd ed.; ChemChar Research: Columbia, MO, USA, 2011. [Google Scholar]
- Anastas, P.T.; Warner, J.C. Principles of Green Chemistry. In Green Chemistry: Theory and Practice; Oxford University Press: Oxford, UK, 1998; pp. 29–56. [Google Scholar]
- Han, J.H.; Aristippos, G. Edible Films and Coatings: A Review. In Innovations in Food Packaging; Academic Press: Amsterdam, The Netherlands, 2005; pp. 239–262. [Google Scholar] [CrossRef]
- Pushpadass, H.A.; Kumar, A.; Jackson, D.S.; Wehling, R.L.; Dumais, J.J.; Hanna, M.A. Macromolecular Changes in Extruded Starch-Films Plasticized with Glycerol, Water and Stearic Acid. Starch Stärke 2009, 61, 256–266. [Google Scholar] [CrossRef]
- Pervaiz, M.; Oakley, P.; Sain, M. Extrusion of Thermoplastic Starch: Effect of “Green” and Common Polyethylene on the Hydrophobicity Characteristics. MSA 2014, 5, 845–856. [Google Scholar] [CrossRef]
- Mironescu, M.; Fratila, L.; Hupert, A.; Mironescu, I.D. Obtaining and Characterisation of Starch-Based Edible Films Incorporating Honey, Propolis and Bee Bread. Acta Univ. Cibiniensis. Ser. E Food Technol. 2019, 23, 193–198. [Google Scholar] [CrossRef][Green Version]
- Matta Fakhouri, F.; Nogueira, G.F.; de Oliveira, R.A.; Velasco, J.I. Bioactive Edible Films Based on Arrowroot Starch Incorporated with Cranberry Powder: Microstructure, Thermal Properties, Ascorbic Acid Content and Sensory Analysis. Polymers 2019, 11, 1650. [Google Scholar] [CrossRef]
- Ashwar, B.A.; Shah, A.; Gani, A.; Shah, U.; Gani, A.; Wani, I.A.; Wani, S.M.; Masoodi, F.A. Rice Starch Active Packaging Films Loaded with Antioxidants-Development and Characterization. Starch Stärke 2015, 67, 294–302. [Google Scholar] [CrossRef]
- Cai, C.; Ma, R.; Duan, M.; Deng, Y.; Liu, T.; Lu, D. Effect of Starch Film Containing Thyme Essential Oil Microcapsules on Physicochemical Activity of Mango. LWT 2020, 131, 109700. [Google Scholar] [CrossRef]
- Li, L.; Chen, H.; Wang, M.; Lv, X.; Zhao, Y.; Xia, L. Development and Characterization of Irradiated-Corn-Starch Films. Carbohydr. Polym. 2018, 194, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Salaberria, A.M.; Diaz, R.H.; Labidi, J.; Fernandes, S.C.M. Preparing Valuable Renewable Nanocomposite Films Based Exclusively on Oceanic Biomass—Chitin Nanofillers and Chitosan. React. Funct. Polym. 2015, 89, 31–39. [Google Scholar] [CrossRef]
- González, A.; Alvarez Igarzabal, C.I. Nanocrystal-Reinforced Soy Protein Films and Their Application as Active Packaging. Food Hydrocoll. 2015, 43, 777–784. [Google Scholar] [CrossRef]
- Luchese, C.L.; Brum, L.F.W.; Piovesana, A.; Caetano, K.; Flôres, S.H. Bioactive Compounds Incorporation into the Production of Functional Biodegradable Films—A Review. Polym. Renew. Resour. 2017, 8, 151–176. [Google Scholar] [CrossRef]
- Riaz, A.; Lei, S.; Akhtar, H.M.S.; Wan, P.; Chen, D.; Jabbar, S.; Abid, M.; Hashim, M.M.; Zeng, X. Preparation and Characterization of Chitosan-Based Antimicrobial Active Food Packaging Film Incorporated with Apple Peel Polyphenols. Int. J. Biol. Macromol. 2018, 114, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Nisa, I.U.; Ashwar, B.A.; Shah, A.; Gani, A.; Gani, A.; Masoodi, F.A. Development of Potato Starch Based Active Packaging Films Loaded with Antioxidants and Its Effect on Shelf Life of Beef. J. Food Sci. Technol. 2015, 52, 7245–7253. [Google Scholar] [CrossRef]
- Sun, L.; Sun, J.; Chen, L.; Niu, P.; Yang, X.; Guo, Y. Preparation and Characterization of Chitosan Film Incorporated with Thinned Young Apple Polyphenols as an Active Packaging Material. Carbohydr. Polym. 2017, 163, 81–91. [Google Scholar] [CrossRef]
- Jang, S.-A.; Shin, Y.-J.; Song, K.B. Effect of Rapeseed Protein-Gelatin Film Containing Grapefruit Seed Extract on ‘Maehyang’ Strawberry Quality: Edible Film Packaging of Strawberry. Int. J. Food Sci. Technol. 2011, 46, 620–625. [Google Scholar] [CrossRef]
- Wang, L.-F.; Rhim, J.-W. Preparation and Application of Agar/Alginate/Collagen Ternary Blend Functional Food Packaging Films. Int. J. Biol. Macromol. 2015, 80, 460–468. [Google Scholar] [CrossRef]
- Reis, L.C.B.; de Souza, C.O.; da Silva, J.B.A.; Martins, A.C.; Nunes, I.L.; Druzian, J.I. Active Biocomposites of Cassava Starch: The Effect of Yerba Mate Extract and Mango Pulp as Antioxidant Additives on the Properties and the Stability of a Packaged Product. Food Bioprod. Process. 2015, 94, 382–391. [Google Scholar] [CrossRef]
- Akram, M.Z.; Yaman Fırıncıoğlu, S.; Jalal, H.; Canoğulları Doğan, S. The Use of Essential Oils in Active Food Packaging: A Review of Recent Studies. Turk. J. Agric. Food Sci. Technol. 2019, 7, 1799. [Google Scholar] [CrossRef]
- Nisar, T.; Wang, Z.-C.; Yang, X.; Tian, Y.; Iqbal, M.; Guo, Y. Characterization of Citrus Pectin Films Integrated with Clove Bud Essential Oil: Physical, Thermal, Barrier, Antioxidant and Antibacterial Properties. Int. J. Biol. Macromol. 2018, 106, 670–680. [Google Scholar] [CrossRef]
- Talón, E.; Vargas, M.; Chiralt, A.; González-Martínez, C. Antioxidant Starch-Based Films with Encapsulated Eugenol. Application to Sunflower Oil Preservation. LWT 2019, 113, 108290. [Google Scholar] [CrossRef]
- Brandelero, R.P.H.; Brandelero, E.M.; Almeida, F.M.d. Biodegradable Films of Starch/PVOH/Alginate in Packaging Systems for Minimally Processed Lettuce (Lactuca sativa L.). Ciênc. Agrotec. 2016, 40, 510–521. [Google Scholar] [CrossRef]
- Malik, T. Perspective Uses of Essential Oils in Functional Foods and Antimicrobial Packaging Material. In Examining the Development, Regulation, and Consumption of Functional Foods; Benjamin, S., Wang, Y., Eds.; IGI Global: Hersey, PA, USA, 2017; pp. 230–270. [Google Scholar] [CrossRef]
- Marra, A.; Silvestre, C.; Duraccio, D.; Cimmino, S. Polylactic Acid/Zinc Oxide Biocomposite Films for Food Packaging Application. Int. J. Biol. Macromol. 2016, 88, 254–262. [Google Scholar] [CrossRef]
- Kanmani, P.; Rhim, J.-W. Properties and Characterization of Bionanocomposite Films Prepared with Various Biopolymers and ZnO Nanoparticles. Carbohydr. Polym. 2014, 106, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Malagurski, I.; Levic, S.; Pantic, M.; Matijasevic, D.; Mitric, M.; Pavlovic, V.; Dimitrijevic-Brankovic, S. Synthesis and Antimicrobial Properties of Zn-Mineralized Alginate Nanocomposites. Carbohydr. Polym. 2017, 165, 313–321. [Google Scholar] [CrossRef]
- Visakh, P.M.; Yu, L. Starch-Based Blends, Composites and Nanocomposites; Royal Society of Chemistry: Cambridge, UK, 2015. [Google Scholar] [CrossRef]
- Chollakup, R.; Pongburoos, S.; Boonsong, W.; Khanoonkon, N.; Kongsin, K.; Sothornvit, R.; Sukyai, P.; Sukatta, U.; Harnkarnsujarit, N. Antioxidant and Antibacterial Activities of Cassava Starch and Whey Protein Blend Films Containing Rambutan Peel Extract and Cinnamon Oil for Active Packaging. LWT 2020, 130, 109573. [Google Scholar] [CrossRef]
- Cakmak, H.; Sogut, E. Functional Biobased Composite Polymers for Food Packaging Applications. In Reactive and Functional Polymers Volume One; Gutiérrez, T.J., Ed.; Springer International Publishing: Cham, Switzerland, 2020; Volume 1, pp. 95–136. [Google Scholar] [CrossRef]
- Quéré, D. Wetting and Roughness. Annu. Rev. Mater. Res. 2008, 38, 71–99. [Google Scholar] [CrossRef]
- Leroy, E.; Jacquet, P.; Coativy, G.; Reguerre, A.L.; Lourdin, D. Compatibilization of Starch–Zein Melt Processed Blends by an Ionic Liquid Used as Plasticizer. Carbohydr. Polym. 2012, 89, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yong, H.; Liu, Y.; Qin, Y.; Kan, J.; Liu, J. Preparation and Characterization of Active and Intelligent Films Based on Fish Gelatin and Haskap Berries (Lonicera caerulea L.) Extract. Food Packag. Shelf Life 2019, 22, 100417. [Google Scholar] [CrossRef]
- Duconseille, A.; Astruc, T.; Quintana, N.; Meersman, F.; Sante-Lhoutellier, V. Gelatin Structure and Composition Linked to Hard Capsule Dissolution: A Review. Food Hydrocoll. 2015, 43, 360–376. [Google Scholar] [CrossRef]
- Tosati, J.V.; Messias, V.C.; Carvalho, P.I.N.; Rodrigues Pollonio, M.A.; Meireles, M.A.A.; Monteiro, A.R. Antimicrobial Effect of Edible Coating Blend Based on Turmeric Starch Residue and Gelatin Applied onto Fresh Frankfurter Sausage. Food Bioprocess Technol. 2017, 10, 2165–2175. [Google Scholar] [CrossRef]
- Satpati, G.G. A Preliminary Report on Plant Based Immunity against SARS-CoV-2 (COVID-19) in Pandemic 2020. Res. J. Biotechnol. 2020, 15, 174–176. [Google Scholar]
- Dangaran, K.; Tomasula, P.M.; Qi, P. Structure and Function of Protein-Based Edible Films and Coatings. In Edible Films and Coatings for Food Applications; Huber, K.C., Embuscado, M.E., Eds.; Springer: New York, NY, USA, 2009; pp. 25–56. [Google Scholar] [CrossRef]
- Ollé Resa, C.P.; Gerschenson, L.N.; Jagus, R.J. Natamycin and Nisin Supported on Starch Edible Films for Controlling Mixed Culture Growth on Model Systems and Port Salut Cheese. Food Control 2014, 44, 146–151. [Google Scholar] [CrossRef]
- Meira, S.M.M.; Zehetmeyer, G.; Werner, J.O.; Brandelli, A. A Novel Active Packaging Material Based on Starch-Halloysite Nanocomposites Incorporating Antimicrobial Peptides. Food Hydrocoll. 2017, 63, 561–570. [Google Scholar] [CrossRef]
- Huang, T.; Qian, Y.; Wei, J.; Zhou, C. Polymeric Antimicrobial Food Packaging and Its Applications. Polymers 2019, 11, 560. [Google Scholar] [CrossRef]
- Bhatia, S.; Bharti, A. Evaluating the Antimicrobial Activity of Nisin, Lysozyme and Ethylenediaminetetraacetate Incorporated in Starch Based Active Food Packaging Film. J. Food Sci. Technol. 2015, 52, 354–3512. [Google Scholar] [CrossRef] [PubMed]
- Al-Tayyar, N.A.; Youssef, A.M.; Al-hindi, R. Antimicrobial Food Packaging Based on Sustainable Bio-Based Materials for Reducing Foodborne Pathogens: A Review. Food Chem. 2020, 310, 125915. [Google Scholar] [CrossRef] [PubMed]
- Shah, U.; Gani, A.; Ashwar, B.A.; Shah, A.; Ahmad, M.; Gani, A.; Wani, I.A.; Masoodi, F.A.; Yildiz, F. A Review of the Recent Advances in Starch as Active and Nanocomposite Packaging Films. Cogent Food Agric. 2015, 1, 1115640. [Google Scholar] [CrossRef]
- Mohammad Amini, A.; Razavi, S.M.A.; Zahedi, Y. The Influence of Different Plasticisers and Fatty Acids on Functional Properties of Basil Seed Gum Edible Film. Int. J. Food Sci. Technol. 2015, 50, 1137–1143. [Google Scholar] [CrossRef]
- Salleh, E.; Muhamad, I.I.; Khairuddin, N. Structural Characterization and Physical Properties of Antimicrobial (AM) Starch-Based Films. World Acad. Sci. Eng. Technol. 2009, 31, 428–436. [Google Scholar]
- Makwana, S.; Choudhary, R.; Haddock, J.; Kohli, P. In-Vitro Antibacterial Activity of Plant Based Phenolic Compounds for Food Safety and Preservation. LWT Food Sci. Technol. 2015, 62, 935–939. [Google Scholar] [CrossRef]
- Nobrega, M.M.; Olivato, J.B.; Müller, C.M.O.; Yamashita, F. Biodegradable Starch-Based Films Containing Saturated Fatty Acids: Thermal, Infrared and Raman Spectroscopic Characterization. Polímeros 2012, 22, 475–480. [Google Scholar] [CrossRef]
- Kapusniak, J.; Siemion, P. Thermal Reactions of Starch with Long-Chain Unsaturated Fatty Acids. Part 2. Linoleic Acid. J. Food Eng. 2007, 78, 323–332. [Google Scholar] [CrossRef]
- Slavutsky, A.M.; Bertuzzi, M.A. Formulation and Characterization of Nanolaminated Starch Based Film. LWT Food Sci. Technol. 2015, 61, 407–413. [Google Scholar] [CrossRef]
- Gonzalez-Calderon, J.A.; Vallejo-Montesinos, J.; Martínez-Martínez, H.N.; Cerecero-Enríquez, R.; López-Zamora, L. Effect of chemical modification of titanium dioxide particles via silanization under properties of chitosan/potato-starch films. Rev. Mex. Ing. Quim. 2019, 18, 913–927. [Google Scholar] [CrossRef]
- Subramani, T.; Ganapathyswamy, H. An Overview of Liposomal Nano-Encapsulation Techniques and Its Applications in Food and Nutraceutical. J. Food Sci. Technol. 2020, 57, 3545–3555. [Google Scholar] [CrossRef] [PubMed]
- Lacatusu, I.; Badea, N.; Niculae, G.; Bordei, N.; Stan, R.; Meghea, A. Lipid Nanocarriers Based on Natural Compounds: An Evolving Role in Plant Extract Delivery: Lipid Nanocarriers Based on Natural Compounds. Eur. J. Lipid Sci. Technol. 2014, 116, 1708–1717. [Google Scholar] [CrossRef]
- Hupfeld, S.; Holsæter, A.M.; Skar, M.; Frantzen, C.B.; Brandl, M. Liposome Size Analysis by Dynamic/Static Light Scattering upon Size Exclusion-/Field Flow-Fractionation. J. Nanosci. Nanotechnol. 2006, 6, 3025–3031. [Google Scholar] [CrossRef]
- Sebaaly, C.; Charcosset, C.; Fourmentin, S.; Greige-Gerges, H. Potential Applications of Cyclodextrin Inclusion Complexes, Liposomes, and Drug-in-Cyclodextrin-in-Liposome in Food Industry and Packaging. In Role of Materials Science in Food Bioengineering; Elsevier: Amsterdam, The Netherlands, 2018; pp. 187–234. [Google Scholar] [CrossRef]
- Cui, H.; Yuan, L.; Li, W.; Lin, L. Antioxidant Property of SiO2-Eugenol Liposome Loaded Nanofibrous Membranes on Beef. Food Packag. Shelf Life 2017, 11, 49–57. [Google Scholar] [CrossRef]
- Becerril, R.; Nerín, C.; Silva, F. Encapsulation Systems for Antimicrobial Food Packaging Components: An Update. Molecules 2020, 25, 1134. [Google Scholar] [CrossRef]
- Lopes, N.A.; Brandelli, A. Nanostructures for Delivery of Natural Antimicrobials in Food. Crit. Rev. Food Sci. Nutr. 2018, 58, 2202–2212. [Google Scholar] [CrossRef] [PubMed]
- Boelter, J.F.; Brandelli, A. Innovative Bionanocomposite Films of Edible Proteins Containing Liposome-Encapsulated Nisin and Halloysite Nanoclay. Colloids Surf. B Biointerfaces 2016, 145, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Revol-Junelles, A.-M.; René, N.; Jamshidian, M.; Akhtar, M.J.; Arab-Tehrany, E.; Jacquot, M.; Desobry, S. Microstructure and Physico-Chemical Evaluation of Nano-Emulsion-Based Antimicrobial Peptides Embedded in Bioactive Packaging Films. Food Hydrocoll. 2012, 29, 407–419. [Google Scholar] [CrossRef]
- Talón, E.; Trifkovic, K.T.; Nedovic, V.A.; Bugarski, B.M.; Vargas, M.; Chiralt, A.; González-Martínez, C. Antioxidant Edible Films Based on Chitosan and Starch Containing Polyphenols from Thyme Extracts. Carbohydr. Polym. 2017, 157, 1153–1161. [Google Scholar] [CrossRef]
- Luchese, C.L.; Uranga, J.; Spada, J.C.; Tessaro, I.C.; de la Caba, K. Valorisation of Blueberry Waste and Use of Compression to Manufacture Sustainable Starch Films with Enhanced Properties. Int. J. Biol. Macromol. 2018, 115, 955–960. [Google Scholar] [CrossRef]
- Go, E.; Song, K.B. Antioxidant Properties of Rye Starch Films Containing Rosehip Extract and Their Application in Packaging of Chicken Breast. Starch Stärke 2019, 71, 1900116. [Google Scholar] [CrossRef]
- Menzel, C.; González-Martínez, C.; Vilaplana, F.; Diretto, G.; Chiralt, A. Incorporation of Natural Antioxidants from Rice Straw into Renewable Starch Films. Int. J. Biol. Macromol. 2020, 146, 976–986. [Google Scholar] [CrossRef]
- Baek, S.-K.; Kim, S.; Song, K.B. Cowpea Starch Films Containing Maqui Berry Extract and Their Application in Salmon Packaging. Food Packag. Shelf Life 2019, 22, 100394. [Google Scholar] [CrossRef]
- Go, E.-J.; Song, K.B. Development and Characterization of Citrus Junos Pomace Pectin Films Incorporated With Rambutan (Nephelium lappaceum) Peel Extract. Coatings 2020, 10, 714. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, A.K.; Kumar, R.; Ganguly, R.; Rana, H.K.; Pandey, P.K.; Sethi, G.; Bishayee, A.; Pandey, A.K. Corilagin in Cancer: A Critical Evaluation of Anticancer Activities and Molecular Mechanisms. Molecules 2019, 24, 3399. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-M.; Zhao, L.; Li, H.; Xu, H.; Chen, W.-W.; Tao, L. Research Progress on the Anticarcinogenic Actions and Mechanisms of Ellagic Acid. Cancer Biol. Med. 2014, 11, 92–100. [Google Scholar] [CrossRef]
- Subramanian, A.P.; John, A.A.; Vellayappan, M.V.; Balaji, A.; Jaganathan, S.K.; Supriyanto, E.; Yusof, M. Gallic Acid: Prospects and Molecular Mechanisms of Its Anticancer Activity. RSC Adv. 2015, 5, 35608–35621. [Google Scholar] [CrossRef]
- Singh, A.P.; Kumar, S. Applications of Tannins in Industry. In Tannins—Structural Properties, Biological Properties and Current Knowledge; Aires, A., Ed.; IntechOpen: Rijeka, Croatia, 2019; pp. 1–19. [Google Scholar] [CrossRef]
- Chakravartula, S.S.N.; Lourenço, R.V.; Balestra, F.; Bittante, A.M.Q.B.; Sobral, P.J.d.A.; Dalla Rosa, M. Influence of Pitanga (Eugenia uniflora L.) Leaf Extract and/or Natamycin on Properties of Cassava Starch/Chitosan Active Films. Food Packag. Shelf Life 2020, 24, 100498. [Google Scholar] [CrossRef]
- Mir, S.A.; Dar, B.N.; Wani, A.A.; Shah, M.A. Effect of Plant Extracts on the Techno-Functional Properties of Biodegradable Packaging Films. Trends Food Sci. Technol. 2018, 80, 141–154. [Google Scholar] [CrossRef]
- BeMiller, J.N.; Huber, K.C. Physical Modification of Food Starch Functionalities. Annu. Rev. Food Sci. Technol. 2015, 6, 19–69. [Google Scholar] [CrossRef]
- Klanarong, N.; Hirunpraditkoon, S.; Saito, N.; Prasertsung, I. Characterization of Native and Oxidized Cassava Starch Prepared Using a Solution Plasma Process. Naresuan Univ. Eng. J. 2020, 15, 81–87. [Google Scholar]
- Ali, H.E.; Abdel Ghaffar, A.M. Preparation and Effect of Gamma Radiation on The Properties and Biodegradability of Poly(Styrene/Starch) Blends. Radiat. Phys. Chem. 2017, 130, 411–420. [Google Scholar] [CrossRef]
- Zhu, F. Plasma Modification of Starch. Food Chem. 2017, 232, 476–486. [Google Scholar] [CrossRef]
- Thirumdas, R.; Kadam, D.; Annapure, U.S. Cold Plasma: An Alternative Technology for the Starch Modification. Food Biophys. 2017, 12, 129–139. [Google Scholar] [CrossRef]
- Teixeira, B.S.; Garcia, R.H.L.; Takinami, P.Y.I.; del Mastro, N.L. Comparison of Gamma Radiation Effects on Natural Corn and Potato Starches and Modified Cassava Starch. Radiat. Phys. Chem. 2018, 142, 44–49. [Google Scholar] [CrossRef]
- Wani, I.A.; Jabeen, M.; Geelani, H.; Masoodi, F.A.; Saba, I.; Muzaffar, S. Effect of Gamma Irradiation on Physicochemical Properties of Indian Horse Chestnut (Aesculus Indica Colebr.) Starch. Food Hydrocoll. 2014, 35, 253–263. [Google Scholar] [CrossRef]
- Bhat, R.; Karim, A.A. Impact of Radiation Processing on Starch. Compr. Rev. Food Sci. Food Saf. 2009, 8, 44–58. [Google Scholar] [CrossRef]
- Brant, A.J.C.; Naime, N.; Lugão, A.B.; Ponce, P. Influence of Ionizing Radiation on Biodegradable Foam Trays for Food Packaging Obtained from Irradiated Cassava Starch. Braz. Arch. Biol. Technol. 2018, 61, e18160520. [Google Scholar] [CrossRef]
- Nemtan, M.R.; Brasoveanu, M. Aspects regarding the rheological behavior of the wheat starch treated with accelerated electron beam. Rom. J. Phys. 2010, 55, 111–117. [Google Scholar]
- Cleland, M.R.; Stichelbaut, F. Radiation processing with high-energy X-rays. Radiat. Phys. Chem. 2013, 84, 91–99. [Google Scholar] [CrossRef]
- Nasir, N.; Othman, S.A. Gamma Radiation Effects on Biodegradable Starch Based Blend with Different Polyester: A Review. J. Adv. Res. Fluid Mech. Therm. Sci. 2019, 62, 245–250. [Google Scholar]
- Vasile, C.; Butnaru, E. Radiation Chemistry of Organic Solids. In Applications of Ionizing Radiation in Materials Processing; Sun, Y., Chmielewsky, A.G., Eds.; Institute of Nuclear Chemistry and Technology: Warszawa, Poland, 2017; Volume I, pp. 117–142. [Google Scholar]
- Aveline, D.C.; Williams, J.R.; Elliott, E.R.; Dutenhoffer, C.; Kellogg, J.R.; Kohel, J.M.; Lay, N.E.; Oudrhiri, K.; Shotwell, R.F.; Yu, N.; et al. Observation of Bose–Einstein Condensates in an Earth-Orbiting Research Lab. Nature 2020, 582, 193–197. [Google Scholar] [CrossRef]
- Bogaerts, A.; Neyts, E.; Gijbels, R.; van der Mullen, J. Gas Discharge Plasmas and Their Applications. Spectrochim. Acta Part B At. Spectrosc. 2002, 57, 609–658. [Google Scholar] [CrossRef]
- Misra, N.N.; Pankaj, S.K.; Segat, A.; Ishikawa, K. Cold Plasma Interactions with Enzymes in Foods and Model Systems. Trends Food Sci. Technol. 2016, 55, 39–47. [Google Scholar] [CrossRef]
- Detduangchan, N.; Wittaya, T. Effect of Uv-Treatment on Properties of Biodegradable Film from Rice Starch. World Acad. Sci. Eng. Technol. Int. J. Mater. Metall. Eng. 2011, 5, 829–834. [Google Scholar] [CrossRef]
- Nasir, N.N.; Othman, S.A. Effect of radiation treatment on starch bioplastic—A review. IJALSR 2019, 2, 1–7. [Google Scholar] [CrossRef]
- Nawapat, D.; Thawien, W. Effect of UV-Treatment on the Properties of Biodegradable Rice Starch Films. IFRJ 2013, 20, 1313–2013. [Google Scholar]
- Khan, M.; Bhattacharia, S.; Kader, M.; Bahari, K. Preparation and Characterization of Ultra Violet (UV) Radiation Cured Bio-Degradable Films of Sago Starch/PVA Blend. Carbohydr. Polym. 2006, 63, 500–506. [Google Scholar] [CrossRef]
- Babaei-Ghazvini, A.; Shahabi-Ghahfarrokhi, I.; Goudarzi, V. Preparation of UV-Protective Starch/Kefiran/ZnO Nanocomposite as a Packaging Film: Characterization. Food Packag. Shelf Life 2018, 16, 103–111. [Google Scholar] [CrossRef]
- Villarruel, S.; Giannuzzi, L.; Rivero, S.; Pinotti, A. Changes Induced by UV Radiation in the Presence of Sodium Benzoate in Films Formulated with Polyvinyl Alcohol and Carboxymethyl Cellulose. Mater. Sci. Eng. C 2015, 56, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Martin, O.; Schwach, E.; Avérous, L.; Couturier, Y. Properties of Biodegradable Multilayer Films Based on Plasticized Wheat Starch. Starch Stärke 2001, 53, 372–380. [Google Scholar] [CrossRef]
- Mali, S.; Grossmann, M.V.E.; García, M.A.; Martino, M.N.; Zaritzky, N.E. Mechanical and Thermal Properties of Yam Starch Films. Food Hydrocoll. 2005, 19, 157–164. [Google Scholar] [CrossRef]
- Veiga-Santos, P.; Oliveira, L.M.; Cereda, M.P.; Alves, A.J.; Scamparini, A.R.P. Mechanical Properties, Hydrophilicity and Water Activity of Starch-Gum Films: Effect of Additives and Deacetylated Xanthan Gum. Food Hydrocoll. 2005, 19, 341–349. [Google Scholar] [CrossRef]
- Khan, B.; Khan Niazi, M.B.; Jahan, Z.; Farooq, W.; Naqvi, S.R.; Ali, M.; Ahmed, I.; Hussain, A. Effect of Ultra-Violet Cross-Linking on the Properties of Boric Acid and Glycerol Co-Plasticized Thermoplastic Starch Films. Food Packag. Shelf Life 2019, 19, 184–192. [Google Scholar] [CrossRef]
- Braşoveanu, M.; Nemţanu, M.R.; Duţă, D. Electron-Beam Processed Corn Starch: Evaluation of Physicochemical and Structural Properties and Technical-Economic Aspects of the Processing. Braz. J. Chem. Eng. 2013, 30, 847–856. [Google Scholar] [CrossRef]
- Kamal, H.; Sabry, G.M.; Lotfy, S.; Abdallah, N.M.; Ulanski, P.; Rosiak, J.; Hegazy, E.A. Controlling of Degradation Effects in Radiation Processing of Starch. J. Macromol. Sci. Part A 2007, 44, 865–875. [Google Scholar] [CrossRef]
- Nemţanu, M.R.; Braşoveanu, M.; Iovu, H. Degradation rate of some electron beam irradiated starches. Sci. Bull. Univ. Politeh. Buchar. Ser. B Chem. Mater. Sci. 2010, 72, 69–74. [Google Scholar]
- Uehara, V.B.; del Mastro, N.L. Characteristics of Biodegradable Films Based on Cassava Starch and Soy Isolate Protein Treated by Electron Beam Radiation. Acad. J. Agric. Res. 2017, 5, 68–74. [Google Scholar] [CrossRef]
- Nemtanu, M.R.; Brasoveanu, M. Radio-Sensitivity of Some Starches Treated with Accelerated Electron Beam. Starch Stärke 2012, 64, 435–440. [Google Scholar] [CrossRef]
- Ershov, B.G. Radiation-Chemical Degradation of Cellulose and Other Polysaccharides. Russ. Chem. Rev. 1998, 67, 315–334. [Google Scholar] [CrossRef]
- Pimpa, B.; Muhammad, S.K.S.; Hassan, M.A.; Ghazali, Z.; Kamaruddin, H.; Kanjanasopa, D. Effect of Electron Beam Irradiation on Physicochemical Properties of Sago Starch. Songklanakarin J. Sci. Technol. 2007, 29, 759–768. [Google Scholar]
- Zhou, X.; Ye, X.; He, J.; Wang, R.; Jin, Z. Effects of Electron Beam Irradiation on the Properties of Waxy Maize Starch and Its Films. Int. J. Biol. Macromol. 2020, 151, 239–246. [Google Scholar] [CrossRef]
- Zhai, M.; Yoshii, F.; Kume, T. Radiation Modification of Starch-Based Plastic Sheets. Carbohydr. Polym. 2003, 52, 311–317. [Google Scholar] [CrossRef]
- Naime, N.; Ponce, P.; Lugão, A.B. Gamma Irradiation Effect on Mechanical and Barrier Properties of Foamed Articles Based on Cassava Starch, International Nuclear Atlantic Conference—INAC 2009; Associacao Brasileira de Energia Nuclear ABEN: Rio de Janeiro, Brazil, 2009. [Google Scholar]
- Wittaya, T. Rice Starch-Based Biodegradable Films: Properties Enhancement. In Structure and Function of Food Engineering; Amer Eissa, A., Ed.; IntechOpen: Rijeka, Croatia, 2012; pp. 103–134. [Google Scholar] [CrossRef]
- Bhattacharya, A. Radiation and Industrial Polymers. Prog. Polym. Sci. 2000, 25, 371–401. [Google Scholar] [CrossRef]
- Conrads, H.; Schmidt, M. Plasma Generation and Plasma Sources. Plasma Sources Sci. Technol. 2000, 9, 441–454. [Google Scholar] [CrossRef]
- Lii, C.; Liao, C.; Stobinski, L.; Tomasik, P. Behaviour of Granular Starches in Low-Pressure Glow Plasma. Carbohydr. Polym. 2002, 49, 499–507. [Google Scholar] [CrossRef]
- Lii, C.; Liao, C.; Stobinski, L.; Tomasik, P. Effects of Hydrogen, Oxygen, and Ammonia Low-Pressure Glow Plasma on Granular Starches. Carbohydr. Polym. 2002, 49, 449–456. [Google Scholar] [CrossRef]
- Lii, C.; Liao, C.; Stobinski, L.; Tomasik, P. Exposure of Granular Starches to Low-Pressure Glow Ethylene Plasma. Eur. Polym. J. 2002, 38, 1601–1606. [Google Scholar] [CrossRef]
- Zou, J.-J.; Liu, C.-J.; Eliasson, B. Modification of Starch by Glow Discharge Plasma. Carbohydr. Polym. 2004, 55, 23–26. [Google Scholar] [CrossRef]
- Ma, Y.C.; Manolache, S.; Sarmadi, M.; Denes, F.S. Synthesis of Starch Copolymers by Silicon Tetrachloride Plasma-Induced Graft Polymerization. Starch Stärke 2004, 56, 47–57. [Google Scholar] [CrossRef]
- Szymanowski, H.; Kaczmarek, M.; Gazicki-Lipman, M.; Klimek, L.; Woźniak, B. New Biodegradable Material Based on RF Plasma Modified Starch. Surf. Coat. Technol. 2005, 200, 539–543. [Google Scholar] [CrossRef]
- Pankaj, S.K.; Bueno-Ferrer, C.; Misra, N.N.; Milosavljević, V.; O’Donnell, C.P.; Bourke, P.; Keener, K.M.; Cullen, P.J. Applications of Cold Plasma Technology in Food Packaging. Trends Food Sci. Technol. 2014, 35, 5–17. [Google Scholar] [CrossRef]
- Noman, A.; Yanshun, X.; Alfarga, A.; Abed, S.M.; Mahdi, A.A.; Al-ansi, W.A.; Wenshui, X. Study the Classification, Chemical, Physical, Methods Modification and Starch Applications in Some Industrial Processes. Review. Int. J. Res. Agric. Sci. 2016, 3, 2348–3997. [Google Scholar]
- Neelam, K.; Vijay, S.; Lalit, S. Various Techniques for the Modification of Starch and the Applications of Its Derivatives. Int. Res. J. Pharm. 2012, 3, 25–31. [Google Scholar]
- Beikzadeh, S.; Ghorbani, M.; Shahbazi, N.; Izadi, F.; Pilevar, Z.; Mortazavian, A.M. The Effects of Novel Thermal and Nonthermal Technologies on the Properties of Edible Food Packaging. Food Eng. Rev. 2020, 12, 333–345. [Google Scholar] [CrossRef]
- Kulkarni, S. Plasma Assisted Polymer Synthesis and Processing. In Non-Thermal Plasma Technology for Polymeric Materials; Elsevier: Amsterdam, The Netherlands, 2019; pp. 67–93. [Google Scholar] [CrossRef]
- Wongsagonsup, R.; Deeyai, P.; Chaiwat, W.; Horrungsiwat, S.; Leejariensuk, K.; Suphantharika, M.; Fuongfuchat, A.; Dangtip, S. Modification of Tapioca Starch by Non-Chemical Route Using Jet Atmospheric Argon Plasma. Carbohydr. Polym. 2014, 102, 790–798. [Google Scholar] [CrossRef]
- Zhou, Y.; Yan, Y.; Shi, M.; Liu, Y. Effect of an Atmospheric Pressure Plasma Jet on the Structure and Physicochemical Properties of Waxy and Normal Maize Starch. Polymers 2018, 11, 8. [Google Scholar] [CrossRef]
- Lazea, A.; Kravets, L.I.; Albu, B.; Ghica, C.; Dinescu, G. Modification of Polyester Track Membranes by Plasma Treatments. Surf. Coat. Technol. 2005, 200, 529–533. [Google Scholar] [CrossRef]
- Ionita, E.R.; Ionita, M.D.; Stancu, E.C.; Teodorescu, M.; Dinescu, G. Small Size Plasma Tools for Material Processing at Atmospheric Pressure. Appl. Surf. Sci. 2009, 255, 5448–5452. [Google Scholar] [CrossRef]
- Deeyai, P.; Jitsomboonmit, P.; Soonthonchaikul, W.; Suphantharik, M.; Dangtip, S. Effect of Atmospheric Argon-Plasma on Morphology of Cassava Starch Granule. J. Microsc. Soc. Thail. 2010, 24, 112–116. [Google Scholar]
- Deeyai, P.; Suphantharika, M.; Wongsagonsup, R.; Dangtip, S. Characterization of Modified Tapioca Starch in Atmospheric Argon Plasma under Diverse Humidity by FTIR Spectroscopy. Chin. Phys. Lett. 2013, 30, 018103. [Google Scholar] [CrossRef]
- Bie, P.; Pu, H.; Zhang, B.; Su, J.; Chen, L.; Li, X. Structural Characteristics and Rheological Properties of Plasma-Treated Starch. Innov. Food Sci. Emerg. Technol. 2016, 34, 196–204. [Google Scholar] [CrossRef]
- Pankaj, S.K.; Wan, Z.; De León, J.E.; Mosher, C.; Colonna, W.; Keener, K.M. High-Voltage Atmospheric Cold Plasma Treatment of Different Types of Starch Films: Cold Plasma Treatment of Starch Films. Starch Stärke 2017, 69, 1700009. [Google Scholar] [CrossRef]
- Trinh, K.S. Formation of Boiling-Stable Resistant Cassava Starch Using the Atmospheric Argon-Plasma Treatment. J. Bioen. Food Sci. 2018, 5, 97–105. [Google Scholar] [CrossRef]
- Trịnh, K.S.; Nguyễn, L.T. Structural, functional properties and in vitro digestibiligy of maize starch under heat-moisture and atmospheric-cold plasma treatments. JST 2018, 56, 751–760. [Google Scholar] [CrossRef]
- Wu, T.-Y.; Sun, N.-N.; Chau, C.-F. Application of Corona Electrical Discharge Plasma on Modifying the Physicochemical Properties of Banana Starch Indigenous to Taiwan. J. Food Drug Anal. 2018, 26, 244–251. [Google Scholar] [CrossRef]
- Yan, Y.; Zhou, Y.; Shi, M.; Liu, H.; Liu, Y. Influence of Atmospheric Pressure Plasma Jet on the Structure of Microcrystalline Starch with Different Relative Crystallinity. Int. J. Food Sci. Technol. 2019, 54, 567–575. [Google Scholar] [CrossRef]
- Trinh, K.S.; Nguyen, T.L. Atmospheric Argon-Plasma Treatment of Maltodextrin: Changes in Structure and Physico-Chemical Properties. Int. J. Adv. Appl. Sci. 2019, 6, 43–47. [Google Scholar] [CrossRef]
- Gao, S.; Liu, H.; Sun, L.; Liu, N.; Wang, J.; Huang, Y.; Wang, F.; Cao, J.; Fan, R.; Zhang, X.; et al. The Effects of Dielectric Barrier Discharge Plasma on Physicochemical and Digestion Properties of Starch. Int. J. Biol. Macromol. 2019, 138, 819–830. [Google Scholar] [CrossRef]
- Wu, T.-Y.; Chang, C.-R.; Chang, T.-J.; Chang, Y.-J.; Liew, Y.; Chau, C.-F. Changes in Physicochemical Properties of Corn Starch upon Modifications by Atmospheric Pressure Plasma Jet. Food Chem. 2019, 283, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Chen, G.; Hou, Y.; Chen, Y. Improved Solubility of Banana Starch by Dielectric Barrier Discharge Plasma Treatment. Int. J. Food Sci. Technol. 2020, 55, 641–648. [Google Scholar] [CrossRef]
- González, A.; Contreras, C.B.; Alvarez Igarzabal, C.I.; Strumia, M.C. Study of the Structure/Property Relationship of Nanomaterials for Development of Novel Food Packaging. In Food Packaging; Elsevier: Amsterdam, The Netherlands, 2017; pp. 265–294. [Google Scholar] [CrossRef]
- Baysal, G.; Çelik, B.Y. Synthesis and Characterization of Antibacterial Bio-Nano Films for Food Packaging. J. Environ. Sci. Health Part B 2019, 54, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Martelli-Tosi, M.; Esposto, B.S.; da Silva, N.C.; Tapia-Blácido, D.R.; Jafari, S.M. Reinforced Nanocomposites for Food Packaging. In Handbook of Food Nanotechnology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 533–574. [Google Scholar] [CrossRef]
- Azeredo, H.M.C. Nanocomposites for Food Packaging Applications. Food Res. Int. 2009, 42, 1240–1253. [Google Scholar] [CrossRef]
- Nafchi, A.M.; Nassiri, R.; Sheibani, S.; Ariffin, F.; Karim, A.A. Preparation and Characterization of Bionanocomposite Films Filled with Nanorod-Rich Zinc Oxide. Carbohydr. Polym. 2013, 96, 233–239. [Google Scholar] [CrossRef]
- Hietala, M.; Mathew, A.P.; Oksman, K. Bionanocomposites of Thermoplastic Starch and Cellulose Nanofibers Manufactured Using Twin-Screw Extrusion. Eur. Polym. J. 2013, 49, 950–956. [Google Scholar] [CrossRef]
- Polat, S.; Ağçam, E.; Dündar, B.; Akyildiz, A. Nanoparticles in Food Packaging: Opportunities and Challenges. In Health and Safety Aspects of Food Processing Technologies; Malik, A., Erginkaya, Z., Erten, H., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 577–611. [Google Scholar] [CrossRef]
- Cano, A.; Cháfer, M.; Chiralt, A.; González-Martínez, C. Development and Characterization of Active Films Based on Starch-PVA, Containing Silver Nanoparticles. Food Packag. Shelf Life 2016, 10, 16–24. [Google Scholar] [CrossRef]
- Ali, M.A.; Ahmed, T.; Wu, W.; Hossain, A.; Hafeez, R.; Islam Masum, M.d.M.; Wang, Y.; An, Q.; Sun, G.; Li, B. Advancements in Plant and Microbe-Based Synthesis of Metallic Nanoparticles and Their Antimicrobial Activity against Plant Pathogens. Nanomaterials 2020, 10, 1146. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, V.; Vasanthi, S.; Shalini, S.; Ali Shah, S.A.; Harish, R. Green Synthesis of Silver Nanoparticles Using Atrocarpus Altilis Leaf Extract and the Study of Their Antimicrobial and Antioxidant Activity. Mater. Lett. 2016, 180, 264–267. [Google Scholar] [CrossRef]
- Hoseinnejad, M.; Jafari, S.M.; Katouzian, I. Inorganic and Metal Nanoparticles and Their Antimicrobial Activity in Food Packaging Applications. Crit. Rev. Microbiol. 2018, 44, 161–181. [Google Scholar] [CrossRef]
- Zgura, I.; Enculescu, M.; Istrate, C.; Negrea, R.; Bacalum, M.; Nedelcu, L.; Barbinta-Patrascu, M.E. Performant Composite Materials Based on Oxide Semiconductors and Metallic Nanoparticles Generated from Cloves and Mandarin Peel Extracts. Nanomaterials 2020, 10, 2146. [Google Scholar] [CrossRef]
- Barbinta-Patrascu, M.E.; Ungureanu, C.; Besliu, D.; Lazea-Stoyanova, A.; Iosif, L. Bio-Active Nanomaterials Phyto-Generated from Weed Herb Cirsium Arvense. Optoelectron Adv. Mater. 2020, 14, 459–465. [Google Scholar]
- Barbinta-Patrascu, M.E.; Badea, N.; Ungureanu, C.; Besliu, D.; Antohe, S. Bioactive Phyto-Nanosilver Particles “Green” Synthesized from Clary Sage, Burdock, Southernwood and Asparagus. Rom. Rep. Phys. 2020, 72, 606. [Google Scholar]
- Ramachandraiah, K.; Han, S.G.; Chin, K.B. Nanotechnology in Meat Processing and Packaging: Potential Applications—A Review. Asian Australas. J. Anim. Sci. 2014, 28, 290–302. [Google Scholar] [CrossRef]
- Simon, P.; Chaudhry, Q.; Bakoš, D. Migration of Engineered Nanoparticles from Polymer Packaging to Food—A Physicochemical View. J. Food Nutr. Res. 2008, 47, 105–113. [Google Scholar]
- Abreu, A.S.; Oliveira, M.; de Sá, A.; Rodrigues, R.M.; Cerqueira, M.A.; Vicente, A.A.; Machado, A.V. Antimicrobial Nanostructured Starch Based Films for Packaging. Carbohydr. Polym. 2015, 129, 127–134. [Google Scholar] [CrossRef]
- Fahmy, H.M.; Eldin, R.E.S.; Serea, E.S.; Gomaa, N.M.; AboElmagd, G.M.; Salem, S.A.; Elsayed, Z.A.; Edrees, A.; Shams-Eldin, E.; Shalan, A.E. Advances in Nanotechnology and Antibacterial Properties of Biodegradable Food Packaging Materials. RSC Adv. 2020, 10, 20467–20484. [Google Scholar] [CrossRef]
- Takebe, H.; Kobayashi, S.; Aono, H.; Yamamuro, S. Fabrication and Characterization of Natural/Synthesized, Micro-, and Nanostructured Materials for Biomedical Applications. In Nanostructures for Novel Therapy; Elsevier: Amsterdam, The Netherlands, 2017; pp. 81–106. [Google Scholar] [CrossRef]
- Li, J.; Zhou, M.; Cheng, F.; Lin, Y.; Zhu, P. Bioinspired Approach to Enhance Mechanical Properties of Starch Based Nacre-Mimetic Nanocomposite. Carbohydr. Polym. 2019, 221, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Duan, J.; Zhang, Q.; Jiang, L. Learning from Nature: Constructing Integrated Graphene-Based Artificial Nacre. ACS Nano 2015, 9, 2231–2234. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Xu, D. Starch-MMT Composite Films: Effects of Bio-Inspired Modification on MMT. Starch Stärke 2015, 67, 470–477. [Google Scholar] [CrossRef]
- Zhou, M.; Liu, Q.; Wu, S.; Gou, Z.; Wu, X.; Xu, D. Starch/Chitosan Films Reinforced with Polydopamine Modified MMT: Effects of Dopamine Concentration. Food Hydrocoll. 2016, 61, 678–684. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, H.; Yu, L.; Duan, Q.; Ji, Z.; Chen, L. Superhydrophobic Modification on Starch Film Using PDMS and Ball-Milled MMT Coating. ACS Sustain. Chem. Eng. 2020, 8, 10423–10430. [Google Scholar] [CrossRef]
- Basu, S.; Dasgupta, P.S. Dopamine, a Neurotransmitter, Influences the Immune System. J. Neuroimmunol. 2000, 102, 113–124. [Google Scholar] [CrossRef]
- Barthlott, W.; Neinhuis, C. Purity of the Sacred Lotus, or Escape from Contamination in Biological Surfaces. Planta 1997, 202, 1–8. [Google Scholar] [CrossRef]
- De Gennes, P.G. Wetting: Statics and Dynamics. Rev. Mod. Phys. 1985, 57, 827–863. [Google Scholar] [CrossRef]

| Source | Film Matrix | Polyphenols Concentration | Application | References |
|---|---|---|---|---|
| Tea | Pomelo peel flours | 5–20% | Soybean oil preservation | [82] |
| Tea | Alginate | 1–5% | [78] | |
| Tea | Starch | 0.06–0.6% | [83] | |
| Green tea extract | Starch | 5% | Beef | [139] |
| Apple peel | Chitosan | 0.25–1% | [138] | |
| Young apple polyphenols extract | Chitosan | 0.25–1% | [140] | |
| Grapefruit seed extract | Rapeseed protein–gelatin | 1% | Strawberries | [141] |
| Grapefruit seed extract | Agar/alginate/collagen hydrogel | 0.2% | Potatoes | [142] |
| Yerba mate extract and mango pulp | Cassava starch | Mango pulp 20% Yerba mate extract 30% | Palm oil | [143] |
| Starch Type and Form | Plasma Experimental Conditions | Main Findings after Plasma Treatment | Ref. |
|---|---|---|---|
| Tapioca starch tablets | High voltage dielectric barrier discharge (DBD), 17 kHz frequency of power supply, 40 watts, treatment time 30 min | Higher degree of crystallinity in starch for high humidity conditions, increase in the degree of crosslinking, for all humidity conditions | [250,251] |
| Tapioca starch slurry | Atmospheric pressure argon plasma jet, 600 MHz high frequency, 50 or 100 W power, treatment time 5 min | Cross-linking or depolymerization of starch determined by the preparation of starch slurry and the plasma input power | [246] |
| Corn starch | Dielectric barrier discharge plasma, 50 V voltage, 1.5 A current, 75 W power, air gas, treatment time 1, 5 or 10 min | Larger channels of the starch granules, decrease in the degree of crystallinity, oxidation of partial hydroxyl groups to carboxyl groups, and molecular degradation, the viscosity decreased | [252] |
| Rice, potato, tapioca and corn starch films | High voltage dielectric barrier discharge (DBD) atmospheric cold plasma, frequency of 60 Hz, voltage 80 kV, treatment time 5 min | Increase in the glass transition temperature, surface roughness and surface oxygenation, the amylose content and the starch source play an important role in determining the polymer’s interaction with cold plasma | [253] |
| Granular cassava starch | Atmospheric dielectric barrier discharge (DBD) plasma, argon gas, electric current of 1.0 A, power supply 4–9 kV, treatment time 0–40 min | Increase in the crosslinking, effects in morphological properties, treated starch became highly resistant to enzymatic hydrolysis leading to the increasing of resistant starch content | [254] |
| Maize starch powder | Dielectric Barrier Discharge (DBD) cold argon-plasma treatment at atmospheric pressure, input parameters: 1.0 A, 176 V and 50 Hz, treatment time 10 min | Increase in crystallinity, reduction of rapidly digestible starch, water absorbance index and swelling factor, reduced molecular weight, more compact in structure than its raw starch | [255] |
| Waxy maize starchand normal maize starch as suspension | Atmospheric pressure plasma jet, 750 W input power, 25 kHz frequency of power supply, treatment time 1, 3, 5, or 7 min | Slight breakage of the surface of the starch granules, increases in waxy maize starch and swelling volume, and decreases in gelatinization temperature and enthalpy, decreases the relative crystallinity, reduces short-range molecular order | [247] |
| Banana starch suspension | Corona electrical discharge (CED), current intensity of 60 A at 30 kV/cm, 40 kV/cm, and 50 kV/cm, treatment time 3 min | Surface damage of the starch granules, reduction in the total area of diffraction peak, gelatinization enthalpy, and different pasting behaviors | [256] |
| Potato starch slurry | Atmospheric pressure plasma jet, power supply of 750 W, 25 kHz frequency of power supply, treatment time 1, 3, 5 or 7 min | Decreased relative crystallinity and short-range molecular order, slight damage in starch granule morphology | [257] |
| Maltodextrin (incomplete hydrolysate of starch) powder | Argon-plasma cold dielectric barrier discharge (DBD) at atmospheric pressure, 1 ampere, 120 voltages, 50 Hz, treatment time 0, 5, 10, 15 and 20 min | Reduce the level of polymerization and molecular weight, increase dextrose equivalents (higher sweetness) | [258] |
| Tatary buckwheat, quinoa and sorghum dry starches | Atmospheric plasma operated at 20 kV and at a frequency of 1 kHz, treatment time 30 s | Reduced amylose content and swelling power, and higher relative crystallinity, pasting temperature and syneresis, different surface modifications depending on the starch type, dramatic decrease in viscosities, higher degree of hydrolysis | [259] |
| Corn starch (S-41260) powder | Atmospheric pressure air cold plasma jet, high voltage input powers: 400 W, 600 W, and 800 W, treatment time 30 min | Production of small molecular fragments and hydrophilic functional groups, reduction in viscosity and an increase in solubility and starch paste clarity | [260] |
| Banana starch suspension | Atmospheric cold plasma dielectric barrier discharge, 30–50 V, treatment time 3 min | The amylose content increase as the treatment intensity increased, decomposition of the outer layers of banana starch granules depending on the treatment intensity, partial decomposition and etching of DBD treatment | [261] |
| Cassava starch colloid | Direct current (DC) pulsed plasma, 16 kV voltage, 3 µs pulse width, 20–30 kHz applied pulsed frequency, treatment time 0–300 min | Increased carbonyl and carboxyl groups of oxidized cassava starch, increase in hydroxyl radicals with increasing treatment time and pulsed frequency, reduction in amylose content | [197] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mironescu, M.; Lazea-Stoyanova, A.; Barbinta-Patrascu, M.E.; Virchea, L.-I.; Rexhepi, D.; Mathe, E.; Georgescu, C. Green Design of Novel Starch-Based Packaging Materials Sustaining Human and Environmental Health. Polymers 2021, 13, 1190. https://doi.org/10.3390/polym13081190
Mironescu M, Lazea-Stoyanova A, Barbinta-Patrascu ME, Virchea L-I, Rexhepi D, Mathe E, Georgescu C. Green Design of Novel Starch-Based Packaging Materials Sustaining Human and Environmental Health. Polymers. 2021; 13(8):1190. https://doi.org/10.3390/polym13081190
Chicago/Turabian StyleMironescu, Monica, Andrada Lazea-Stoyanova, Marcela Elisabeta Barbinta-Patrascu, Lidia-Ioana Virchea, Diana Rexhepi, Endre Mathe, and Cecilia Georgescu. 2021. "Green Design of Novel Starch-Based Packaging Materials Sustaining Human and Environmental Health" Polymers 13, no. 8: 1190. https://doi.org/10.3390/polym13081190
APA StyleMironescu, M., Lazea-Stoyanova, A., Barbinta-Patrascu, M. E., Virchea, L.-I., Rexhepi, D., Mathe, E., & Georgescu, C. (2021). Green Design of Novel Starch-Based Packaging Materials Sustaining Human and Environmental Health. Polymers, 13(8), 1190. https://doi.org/10.3390/polym13081190






