Motility Improvement of Biomimetic Trachea Scaffold via Hybrid 3D-Bioprinting Technology
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
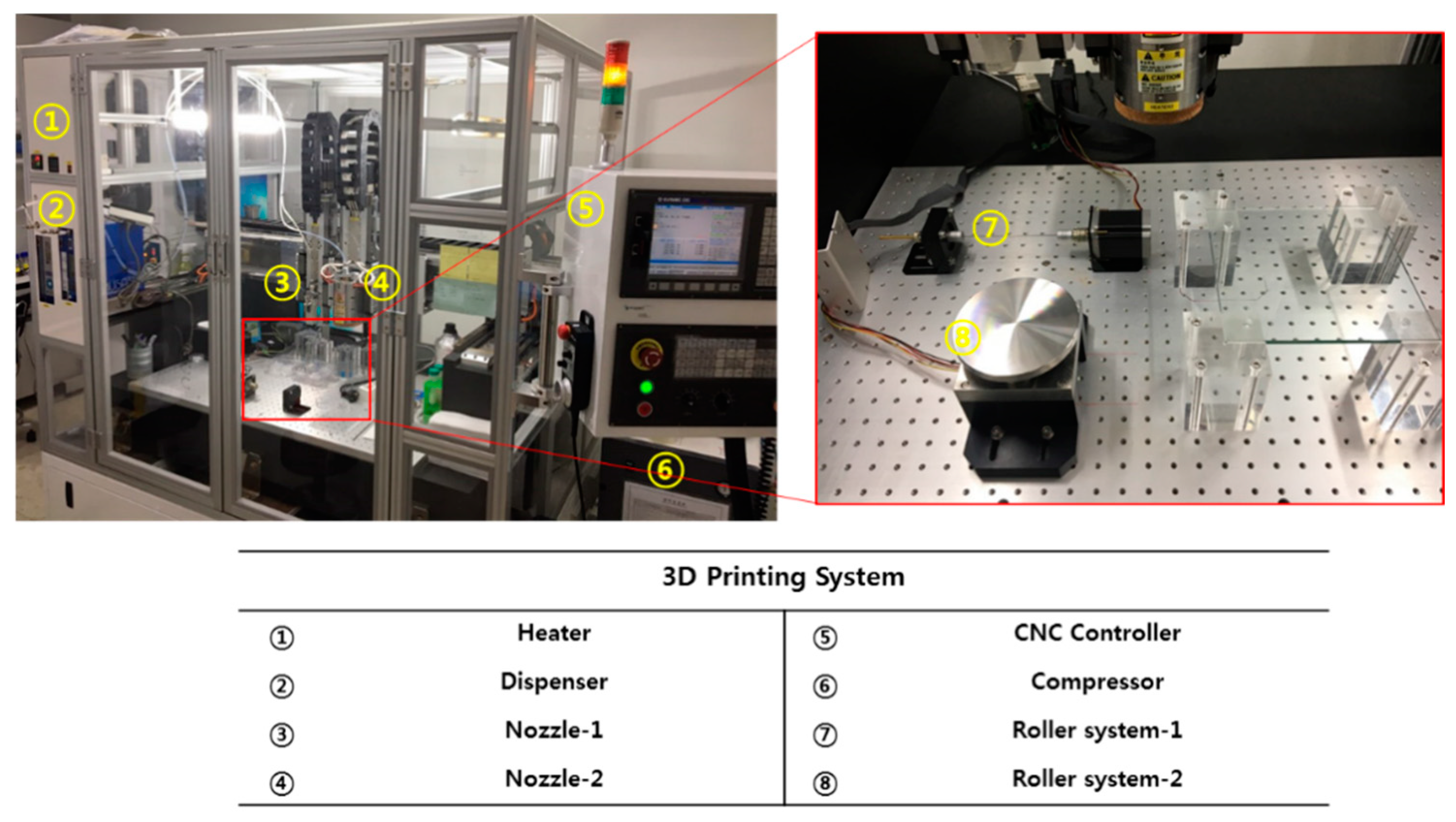
2.2. FDM (Fused Deposition Modeling)-Based 3D Printing System
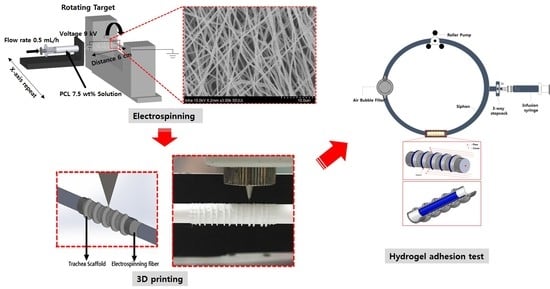
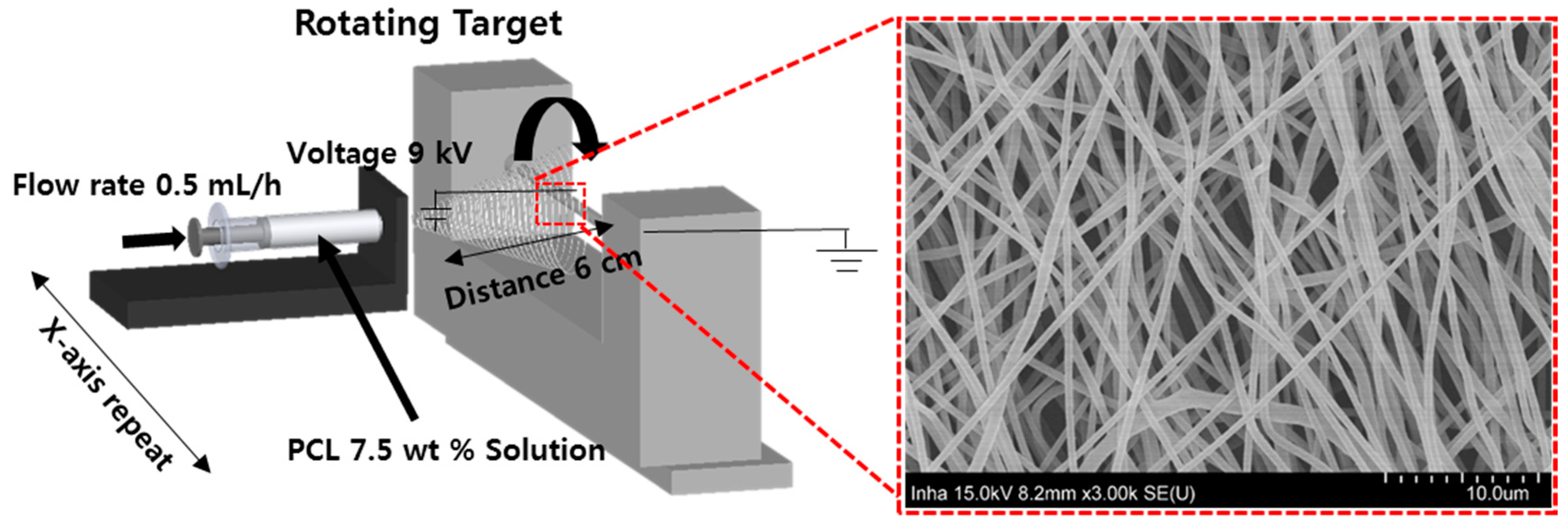
2.3. Electrospinning
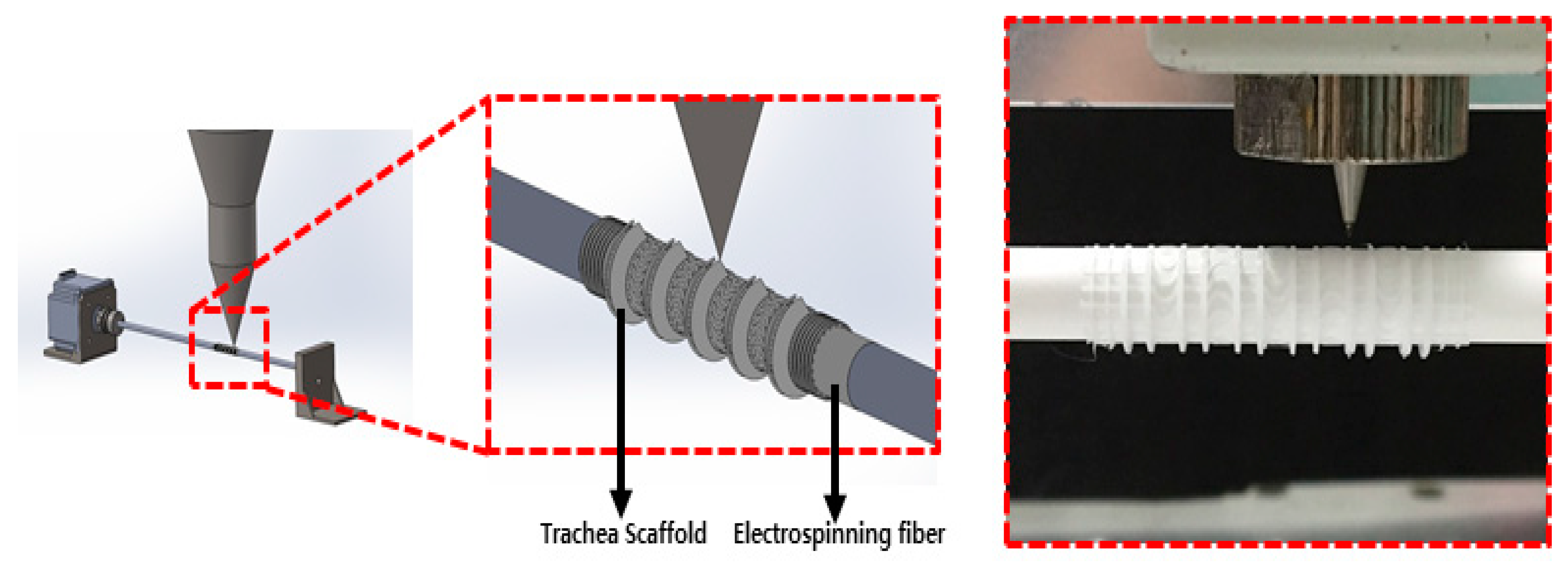
2.4. Design and Manufacturing Process Using CAD (Computer Aided Design) and CAM (Computer Aided Manufacturing)
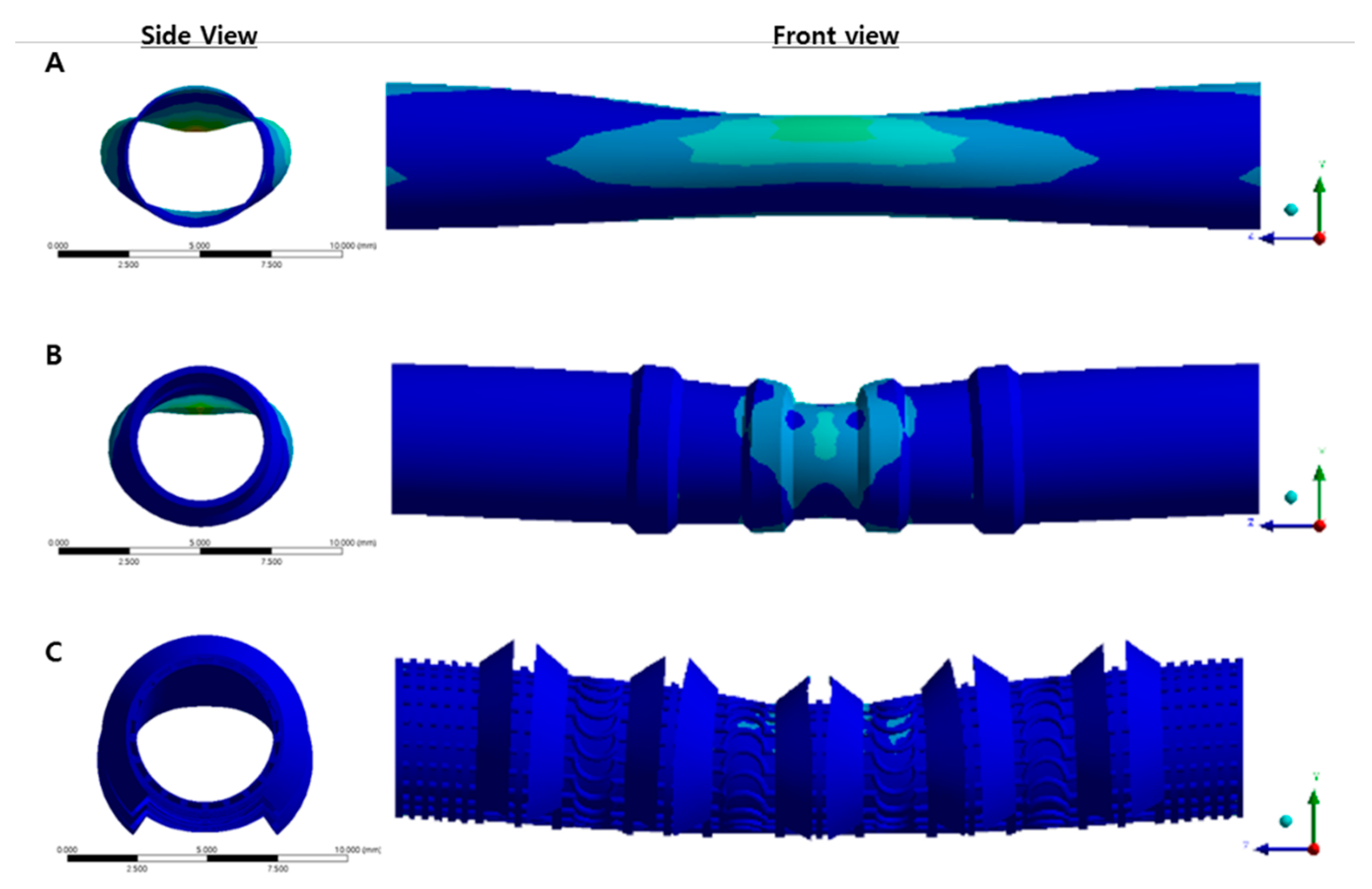
2.5. Simulation Test
2.6. Fabrication of Three Types of Trachea Scaffolds
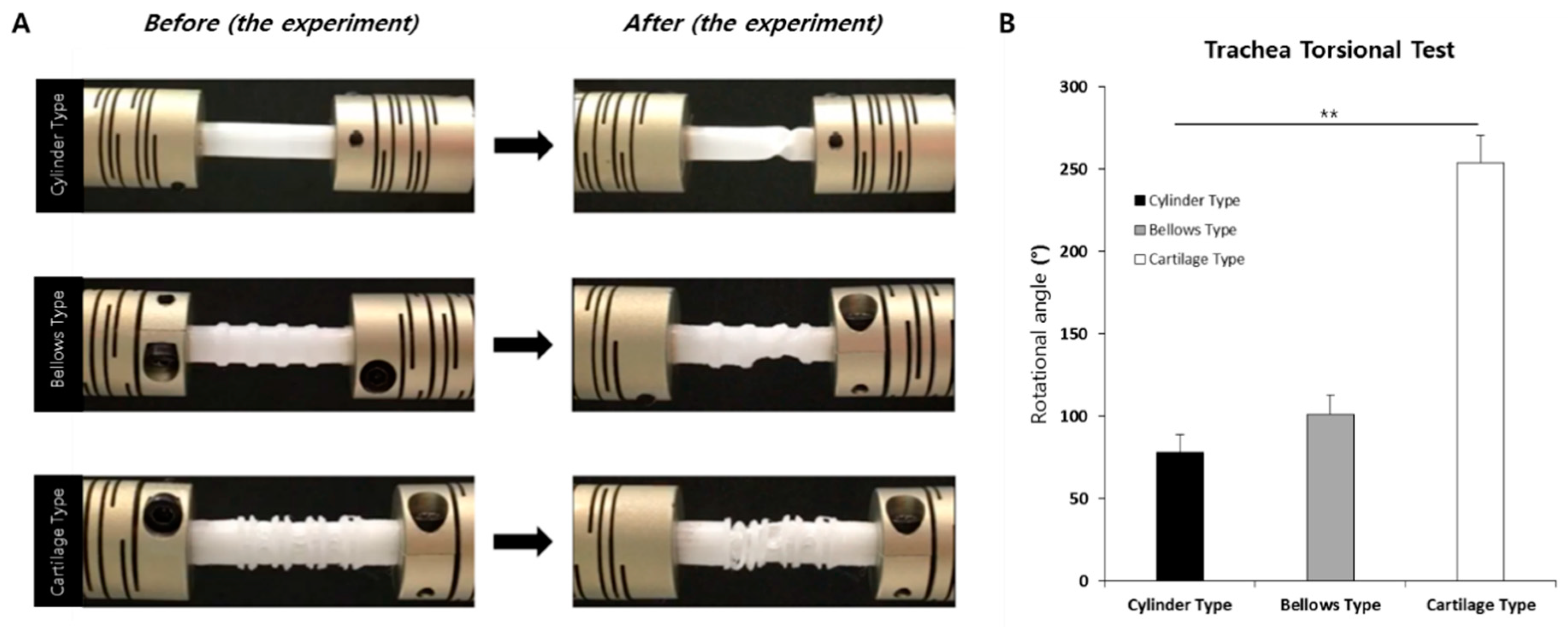
2.7. Measurement of Rotation Angle
2.8. Measurements of Mechanical Properties
2.9. Hydrogel Residual on the Scaffolds in a Circulation Mock-Up System
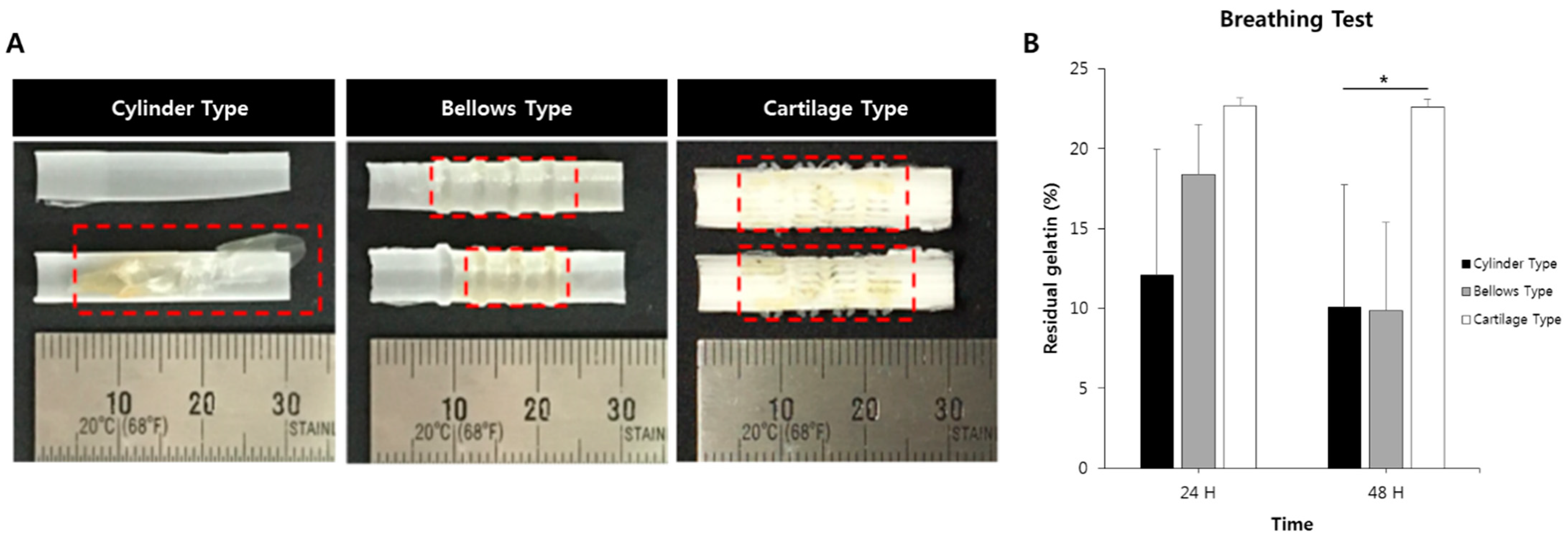
2.10. Hydrogel Residual Inside of the Scaffold Assessed Using a Ventilation System
2.11. Statistical Analysis
3. Results
3.1. Trachea Scaffold Fabrication
3.2. Simulation Result
3.3. Rotation Angles of Trachea Scaffolds
3.4. Comparison of Mechanical Properties among Various Trachea Scaffolds
3.5. Outside Flow Results for Hydrogel-Attached Scaffolds Using Circulation System
3.6. Breathing Results for Hydrogel-Attached Scaffolds Using Clinical Ventilator System
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Griffith, L.G.; Naughton, G. Tissue engineering e current challenges and expanding opportunities. Science 2002, 295, 1009–1014. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, C.E.; Marelli, B.; Donelli, I.; Alessandrino, A.; Freddi, G.; Nazhat, S.N. The role of physiological mechanical cues on mesenchymal stem cell differentiation in an airway tract-like dense collagen–silk fibroin construct. Biomaterials 2014, 35, 6236–6247. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-C.; Kim, M.-S.; Kim, D.-J.; Park, D.-H.; Lee, I.-W.; Roh, H.-J.; Lee, B.-J.; Kim, Y.-A.; Ko, S.; Sung, E.-S. Subglottic stenosis in children: Our experience at a pediatric tertiary center for 8 years in South Korea. Int. J. Pediatr. Otorhinolaryngol. 2019, 121, 64–67. [Google Scholar] [CrossRef]
- Ochando, J.; Charron, D.; Baptista, P.M.; Uygun, B.E. Immune responses to bioengineered organs. Curr. Opin. Organ Transp. 2017, 22, 79. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.Y.; Lee, S.J.; Kim, H.Y.; Park, H.S.; Wang, Z.; Kim, H.J.; Yoo, J.J.; Chung, S.M.; Kim, H.S. 3D printed polyurethane prosthesis for partial tracheal reconstruction: A pilot animal study. Biofabrication 2016, 8, 045015. [Google Scholar] [CrossRef]
- Fishman, J.M.; Wiles, K.; Lowdell, M.W.; De Coppi, P.; Elliott, M.J.; Atala, A.; Birchall, M.A. Airway tissue engineering: An update. Expert Opin. Biol. Ther. 2014, 14, 1477–1491. [Google Scholar] [CrossRef] [PubMed]
- Maughan, E.F.; Butler, C.R.; Crowley, C.; Teoh, G.Z.; Den Hondt, M.; Hamilton, N.J.; Hynds, R.E.; Lange, P.; Ansari, T.; Urbani, L. A comparison of tracheal scaffold strategies for pediatric transplantation in a rabbit model. Laryngoscope 2017, 127, E449–E457. [Google Scholar] [CrossRef] [PubMed]
- Bernhard, J.-C.; Isotani, S.; Matsugasumi, T.; Duddalwar, V.; Hung, A.J.; Suer, E.; Baco, E.; Satkunasivam, R.; Djaladat, H.; Metcalfe, C. Personalized 3D printed model of kidney and tumor anatomy: A useful tool for patient education. World J. Urol. 2016, 34, 337–345. [Google Scholar] [CrossRef]
- Lee, J.W.; Choi, Y.-J.; Yong, W.-J.; Pati, F.; Shim, J.-H.; Kang, K.S.; Kang, I.-H.; Park, J.; Cho, D.-W. Development of a 3D cell printed construct considering angiogenesis for liver tissue engineering. Biofabrication 2016, 8, 015007. [Google Scholar] [CrossRef]
- Vukicevic, M.; Mosadegh, B.; Min, J.K.; Little, S.H. Cardiac 3D printing and its future directions. JACC Cardiovasc. Imaging 2017, 10, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Askari, M.; Naniz, M.A.; Kouhi, M.; Saberi, A.; Zolfaghariane, A.; Bodaghi, M. Recent progress in extrusion 3D bioprinting of hydrogel biomaterials for tissue regeneration: A comprehensive review with focus on advanced fabrication techniques. Biomater. Sci. 2021, 9, 535. [Google Scholar] [CrossRef]
- Ghavidelnia, N.; Bodaghi, M.; Hedayat, R. Femur auxetic meta-implants with tuned micromotion distribution. Materials 2021, 14, 114. [Google Scholar] [CrossRef]
- Lee, J.W.; Soman, P.; Park, J.H.; Chen, S.; Cho, D.-W. A tubular biomaterial construct exhibiting a negative Poisson’s ratio. PLoS ONE 2016, 11, e0155681. [Google Scholar] [CrossRef]
- Ahn, G.; Min, K.-H.; Kim, C.; Lee, J.-S.; Kang, D.; Won, J.-Y.; Cho, D.-W.; Kim, J.-Y.; Jin, S.; Yun, W.-S. Precise stacking of decellularized extracellular matrix based 3D cell-laden constructs by a 3D cell printing system equipped with heating modules. Sci. Rep. 2017, 7, 8624. [Google Scholar] [CrossRef]
- Jang, J.; Park, H.-J.; Kim, S.-W.; Kim, H.; Park, J.Y.; Na, S.J.; Kim, H.J.; Park, M.N.; Choi, S.H.; Park, S.H. 3D printed complex tissue construct using stem cell-laden decellularized extracellular matrix bioinks for cardiac repair. Biomaterials 2017, 112, 264–274. [Google Scholar] [CrossRef]
- Zolfagharian, A.; Kaynak, A.; Bodaghi, M.; Kouzani, A.Z.; Gharaie, S.; Nahavandi, S. Control-based 4D printing: Adaptive 4D-printed systems. Appl. Sci. 2020, 10, 3020. [Google Scholar] [CrossRef]
- Bodaghi, M.; Damanpack, A.R.; Liao, W.H. Self-expanding/shrinking structures by 4D printing. Smart Mater. Struct. 2016, 25, 105034. [Google Scholar] [CrossRef]
- Kim, S.H.; Seo, Y.B.; Yeon, Y.K.; Lee, Y.J.; Park, H.S.; Sultan, M.T.; Lee, J.M.; Lee, J.S.; Lee, O.J.; Hong, H.; et al. 4D-bioprinted silk hydrogels for tissue engineering. Biomaterials 2020, 260, 120281. [Google Scholar] [CrossRef] [PubMed]
- Zopf, D.A.; Hollister, S.J.; Nelson, M.E.; Ohye, R.G.; Green, G.E. Bioresorbable airway splint created with a three-dimensional printer. N. Engl. J. Med. 2013, 368, 2043–2045. [Google Scholar] [CrossRef]
- Morrison, R.J.; Hollister, S.J.; Niedner, M.F.; Mahani, M.G.; Park, A.H.; Mehta, D.K.; Ohye, R.G.; Green, G.E. Mitigation of tracheobronchomalacia with 3D-printed personalized medical devices in pediatric patients. Sci. Transl. Med. 2015, 7, 285ra64. [Google Scholar] [CrossRef]
- Huang, L.; Wang, L.; He, J.; Zhao, J.; Zhong, D.; Yang, G.; Guo, T.; Yan, X.; Zhang, L.; Li, D. Tracheal suspension by using 3-dimensional printed personalized scaffold in a patient with tracheomalacia. J. Thorac. Dis. 2016, 8, 3323. [Google Scholar] [CrossRef]
- Morrison, R.J.; Sengupta, S.; Flanangan, C.L.; Ohye, R.G.; Hollister, S.J.; Green, G.E. Treatment of severe acquired tracheomalacia with a patient-specific, 3D-printed, permanent tracheal splint. JAMA Otolaryngol. 2017, 143, 523–525. [Google Scholar] [CrossRef] [PubMed]
- Les, A.S.; Ohye, R.G.; Filbrun, A.G.; Ghadimi Mahani, M.; Flanagan, C.L.; Daniels, R.C.; Kidwell, K.M.; Zopf, D.A.; Hollister, S.C.; Green, G.E. 3D-printed, externally-implanted, bioresorbable airway splints for severe tracheobronchomalacia. Laryngoscope 2019, 129, 1763–1771. [Google Scholar] [CrossRef] [PubMed]
- Zopf, D.A.; Flanagan, C.L.; Wheeler, M.; Hollister, S.J.; Green, G.E. Treatment of severe porcine tracheomalacia with a 3-dimensionally printed, bioresorbable, external airway splint. JAMA Otolaryngol. 2014, 140, 66–71. [Google Scholar] [CrossRef]
- Park, J.H.; Park, J.Y.; Nam, I.-C.; Hwang, S.-H.; Kim, C.-S.; Jung, J.W.; Jang, J.; Lee, H.; Choi, Y.; Park, S.H.; et al. Human turbinate mesenchymal stromal cell sheets with bellows graft for rapid tracheal epithelial regeneration. Acta Biomater. 2015, 25, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, T.A.; Smith, B.D.; Zeltsman, D.; Grande, D.; Smith, L.P. Introducing a 3-dimensionally printed, tissue-engineered graft for airway reconstruction: A pilot study. JAMA Otolaryngol. 2015, 153, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- Kaye, R.; Goldstein, T.; Aronowitz, D.; Grande, D.A.; Zeltsman, D.; Smith, L.P. Ex vivo tracheomalacia model with 3D-printed external tracheal splint. Laryngoscope 2017, 127, 950–955. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Park, S.A.; Lee, S.J.; Kim, T.H.; Oh, S.H.; Lee, J.H.; Kwon, S.K. Segmental tracheal reconstruction by 3 D-printed scaffold: Pivotal role of asymmetrically porous membrane. Laryngoscope 2016, 126, E304–E309. [Google Scholar] [CrossRef]
- Bhora, F.Y.; Lewis, E.E.; Rehmani, S.S.; Ayub, A.; Raad, W.; Al-Ayoubi, A.M.; Lebovics, R.S. Circumferential three-dimensional–printed tracheal grafts: Research model feasibility and early results. Ann. Thorac. Surg. 2017, 104, 958–963. [Google Scholar] [CrossRef]
- Rehmani, S.S.; Al-Ayoubi, A.M.; Ayub, A.; Barsky, M.; Lewis, E.; Flores, R.; Lebovics, R.; Bhora, F.Y. Three-dimensional-printed bioengineered tracheal grafts: Preclinical results and potential for human use. Ann. Thorac. Surg. 2017, 104, 998–1004. [Google Scholar] [CrossRef]
- Gao, M.; Zhang, H.; Dong, W.; Bai, J.; Gao, B.; Xia, D.; Feng, B.; Chen, M.; He, X.; Yin, M. Tissue-engineered trachea from a 3D-printed scaffold enhances whole-segment tracheal repair. Sci. Rep. 2017, 7, 5246. [Google Scholar] [CrossRef]
- Taniguchi, D.; Matsumoto, K.; Tsuchiya, T.; Machino, R.; Takeoka, Y.; Elgalad, A.; Gunge, K.; Takagi, K.; Taura, Y.; Hatachi, G. Scaffold-free trachea regeneration by tissue engineering with bio-3D printing. Interact. Cardiovasc. Thorac. Surg. 2018, 26, 745–752. [Google Scholar] [CrossRef]
- Park, J.H.; Park, J.Y.; Nam, I.-C.; Ahn, M.; Lee, J.Y.; Choi, S.H.; Kim, S.W.; Cho, D.-W. A rational tissue engineering strategy based on three-dimensional (3D) printing for extensive circumferential tracheal reconstruction. Biomaterials 2018, 185, 276–283. [Google Scholar] [CrossRef]
- Park, J.-H.; Yoon, J.-K.; Lee, J.B.; Shin, Y.M.; Lee, K.-W.; Bae, S.-W.; Lee, J.; Yu, J.; Jung, C.-R.; Youn, Y.-N. Experimental tracheal replacement using 3-dimensional bioprinted artificial trachea with autologous epithelial cells and chondrocytes. Sci. Rep. 2019, 9, 2103. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Jing, H.; Gao, M.; Wang, S.; Fu, W.; Zhang, X.; He, X.; Zheng, J. Long-segmental tracheal reconstruction in rabbits with pedicled Tissue-engineered trachea based on a 3D-printed scaffold. Acta Biomater. 2019, 97, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Kaye, R.; Goldstein, T.; Grande, D.A.; Zeltsman, D.; Smith, L.P. A 3-dimensional bioprinted tracheal segment implant pilot study: Rabbit tracheal resection with graft implantation. Int. J. Pediatr. Otorhinolaryngol. 2019, 117, 175–178. [Google Scholar] [CrossRef]
- Machino, R.; Matsumoto, K.; Taniguchi, D.; Tsuchiya, T.; Takeoka, Y.; Taura, Y.; Moriyama, M.; Tetsuo, T.; Oyama, S.; Takagi, K. Replacement of rat tracheas by layered, trachea-like, scaffold-free structures of human cells using a bio-3D printing system. Adv. Healthc. Mater. 2019, 8, 1800983. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.; Jin, D.; Wang, Q.; Gao, M.; Zhang, J.; Zhang, H.; Bai, J.; Feng, B.; Chen, M.; Huang, Y. Tissue-engineered trachea from a 3D-printed scaffold enhances whole-segment tracheal repair in a goat model. J. Tissue Eng. Regen. Med. 2019, 13, 694–703. [Google Scholar] [CrossRef]
- Best, C.A.; Pepper, V.K.; Ohst, D.; Bodnyk, K.; Heuer, E.; Onwuka, E.A.; King, N.; Strouse, R.; Grischkan, J.; Breuer, C.K. Designing a tissue-engineered tracheal scaffold for preclinical evaluation. Int. J. Pediatr. Otorhinolaryngol. 2018, 104, 155–160. [Google Scholar] [CrossRef]
- Kang, Y.; Wang, C.; Qiao, Y.; Gu, J.; Zhang, H.; Peijs, T.; Kong, J.; Zhang, G.; Shi, X. Tissue-engineered trachea consisting of electrospun patterned sc-PLA/GO-g-IL fibrous membranes with antibacterial property and 3D-printed skeletons with elasticity. Biomacromolecules 2019, 20, 1765–1776. [Google Scholar] [CrossRef]
- Pan, S.; Zhong, Y.; Shan, Y.; Liu, X.; Xiao, Y.; Shi, H. Selection of the optimum 3D-printed pore and the surface modification techniques for tissue engineering tracheal scaffold in vivo reconstruction. J. Biomed. Mater. Res. A 2019, 107, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Hong, J.M.; Ju, Y.M.; Jung, J.W.; Kang, H.-W.; Lee, S.J.; Yoo, J.J.; Kim, S.W.; Kim, S.H.; Cho, D.-W. A novel tissue-engineered trachea with a mechanical behavior similar to native trachea. Biomaterials 2015, 62, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Jung, J.W.; Kang, H.-W.; Joo, Y.H.; Lee, J.-S.; Cho, D.-W. Development of a 3D bellows tracheal graft: Mechanical behavior analysis, fabrication and an in vivo feasibility study. Biofabrication 2012, 4, 035004. [Google Scholar] [CrossRef] [PubMed]
- Minnich, D.J.; Mathisen, D.J. Anatomy of the trachea, carina, and bronchi. Thorac. Surg. Clin. 2007, 17, 571–585. [Google Scholar] [CrossRef] [PubMed]
- Swartz, E.E.; Floyd, R.; Cendoma, M. Cervical spine functional anatomy and the biomechanics of injury due to compressive loading. J. Athl. Train. 2005, 40, 155. [Google Scholar]






| Mechanical Property | Type of Trachea Scaffold | ||
|---|---|---|---|
| Cylinder Type | Bellows Type | Cartilage Type | |
| Tensile strength (kPa) | 4087 ± 76 | 3143 ± 40 | 470 ± 140 |
| Yield strength (kPa) | 3320 ± 350 | 2740 ± 270 | 237 ± 166 |
| Compressive yield strength (kPa) | 3672 ± 586 | 1731 ± 282 | 475 ± 193 |
| Flexural Stress (kPa) | 4117 ± 550 | 3191 ± 384 | 1079 ± 556 |
| Elongation ratio (%) | 60.83 ± 12.62 | 23.77 ± 3.94 | 194.71 ± 9.13 |
| Displacement (mm) | 15.20 ± 3.15 | 5.94 ± 0.99 | 48.68 ± 2.28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.S.; Ahn, C.B.; Son, K.H.; Lee, J.W. Motility Improvement of Biomimetic Trachea Scaffold via Hybrid 3D-Bioprinting Technology. Polymers 2021, 13, 971. https://doi.org/10.3390/polym13060971
Yu YS, Ahn CB, Son KH, Lee JW. Motility Improvement of Biomimetic Trachea Scaffold via Hybrid 3D-Bioprinting Technology. Polymers. 2021; 13(6):971. https://doi.org/10.3390/polym13060971
Chicago/Turabian StyleYu, Young Soo, Chi Bum Ahn, Kuk Hui Son, and Jin Woo Lee. 2021. "Motility Improvement of Biomimetic Trachea Scaffold via Hybrid 3D-Bioprinting Technology" Polymers 13, no. 6: 971. https://doi.org/10.3390/polym13060971
APA StyleYu, Y. S., Ahn, C. B., Son, K. H., & Lee, J. W. (2021). Motility Improvement of Biomimetic Trachea Scaffold via Hybrid 3D-Bioprinting Technology. Polymers, 13(6), 971. https://doi.org/10.3390/polym13060971