Hyaluronic Acid Treatment Improves Healing of the Tenorrhaphy Site by Suppressing Adhesions through Extracellular Matrix Remodeling in a Rat Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
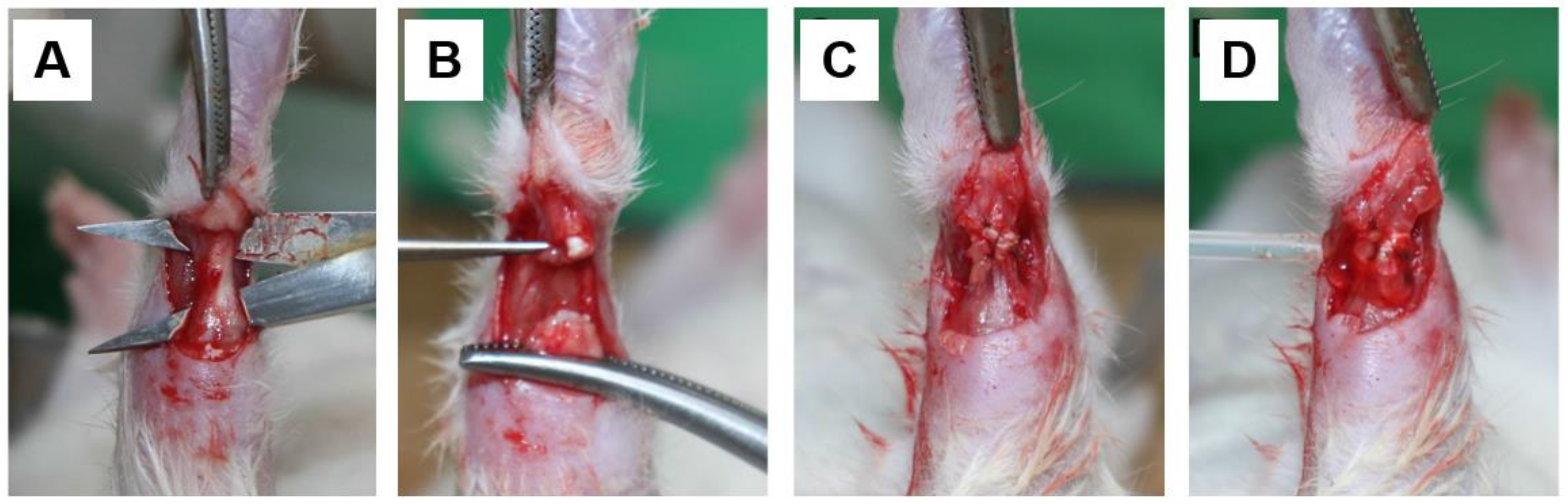
2.2. Surgical Procedures
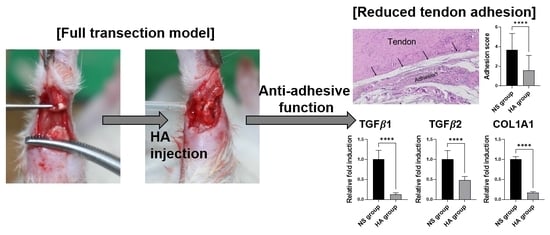
2.3. Macroscopic Examination
2.4. Histological Evaluation
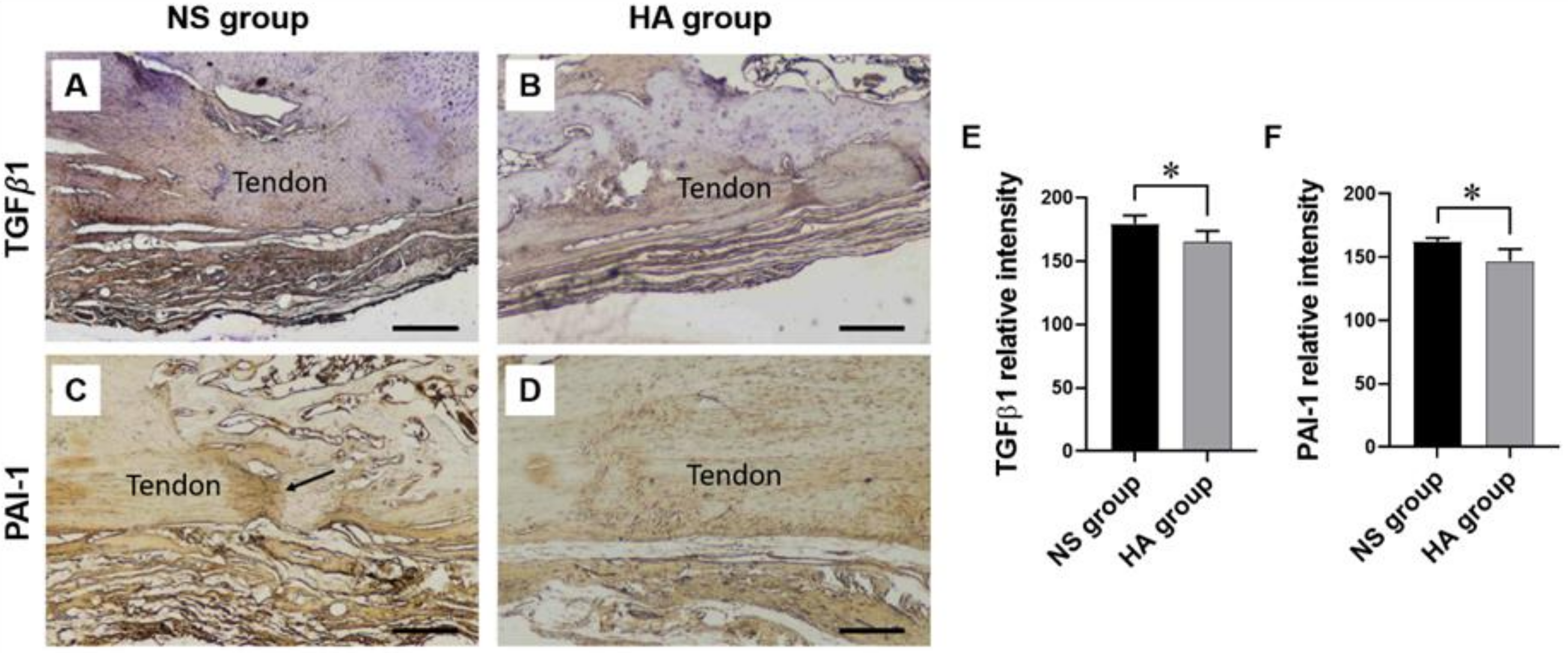
2.5. Immunohistochemistry
2.6. Immunofluorescence
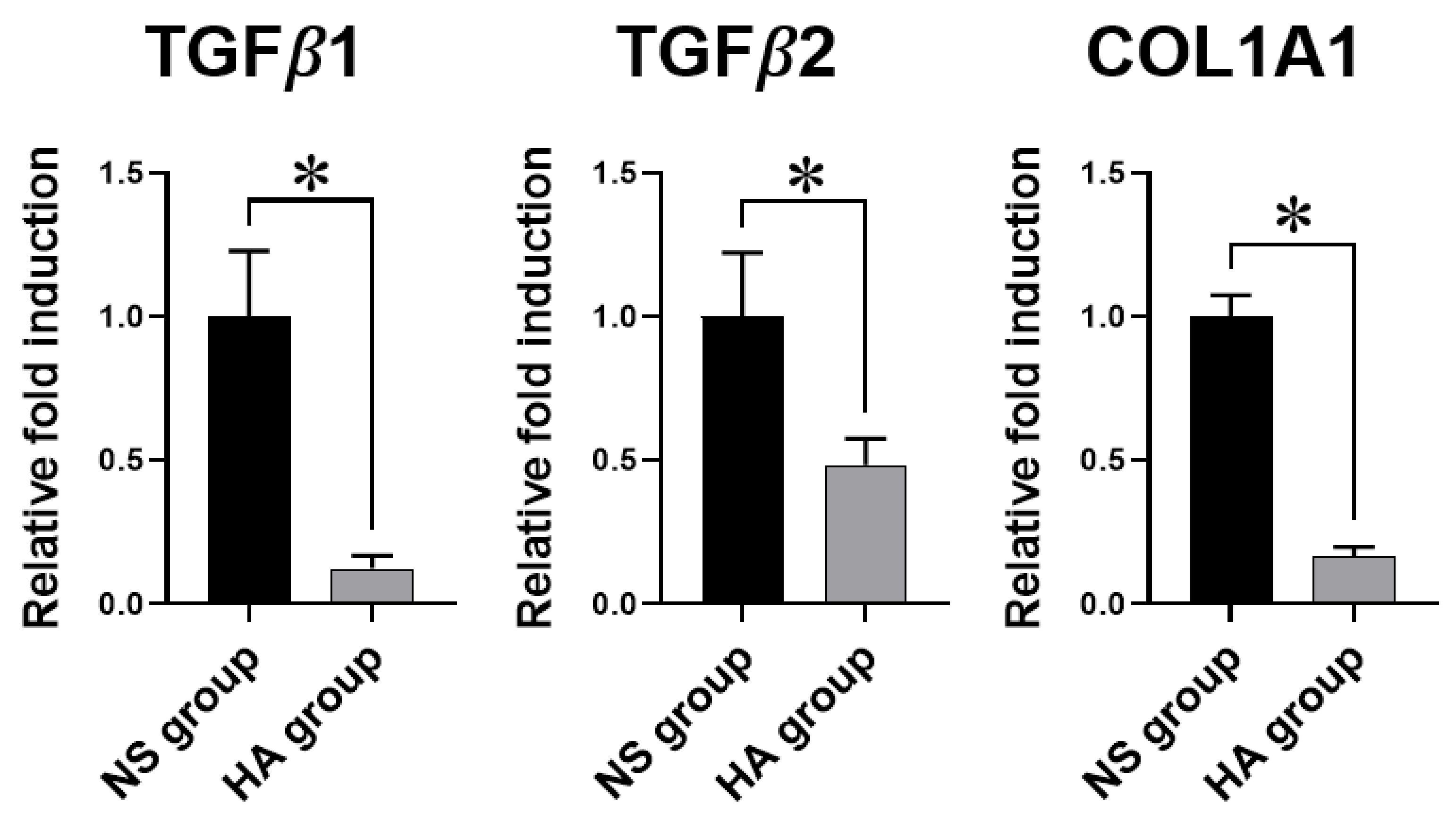
2.7. Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR) Analysis
2.8. Statistical Analysis
3. Results
3.1. Macroscopic and Histological Evaluation
3.2. Immunohistochemistry
3.3. Immunofluorescence
3.4. qRT-PCR Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TGF-β1 | transforming growth factor-beta 1 |
| TGF-β2 | transforming growth factor-beta 2 |
| COL1A1 | collagen type 1 alpha 1 chain |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase |
References
- Jo, C.H.; Lim, H.J.; Yoon, K.S. Characterization of Tendon-Specific Markers in Various Human Tissues, Tenocytes and Mesenchymal Stem Cells. Tissue Eng. Regen. Med. 2019, 16, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.L.; Liau, L.L.; Ng, M.H.; Chowdhury, S.R.; Law, J.X. Current Progress in Tendon and Ligament Tissue Engineering. Tissue Eng. Regen. Med. 2019, 16, 549–571. [Google Scholar] [CrossRef] [PubMed]
- Aydin, A.; Topalan, M.; Mezdeği, A.; Sezer, I.; Ozkan, T.; Erer, M.; Ozkan, S. Single-stage flexor tendoplasty in the treatment of flexor tendon injuries. Acta Orthop. Traumatol. Turc. 2004, 38, 54–59. [Google Scholar]
- Zhou, H.; Lu, H. Advances in the Development of Anti-Adhesive Biomaterials for Tendon Repair Treatment. Tissue Eng Regen Med 2021, 18, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Gelberman, R.H.; Manske, P.R. Factors influencing flexor tendon adhesions. Hand Clin. 1985, 1, 35–42. [Google Scholar]
- Azad-Tirgan, M.; Sarrafzadeh-Rezaei, F.; Malekinejad, H.; Hobbenaghi, R.; Heshmatian, B. Evaluation of tendon healing using fibroblast like synoviocytes in rabbits: A biomechanical study. Vet. Res. Forum 2016, 7, 21–26. [Google Scholar]
- Kuzumaki, T.; Yamazaki, K.; Suzuki, K.; Torigoe, K. Appropriate Tensile Mode and Timing of Applying Tension to Promote Tendon Gel Regeneration. Tissue Eng. Regen. Med. 2017, 14, 465–475. [Google Scholar] [CrossRef]
- Kim, S.E.; Kim, J.G.; Park, K. Biomaterials for the Treatment of Tendon Injury. Tissue Eng. Regen. Med. 2019, 16, 467–477. [Google Scholar] [CrossRef] [PubMed]
- James, R.; Kesturu, G.; Balian, G.; Chhabra, A.B. Tendon: Biology, biomechanics, repair, growth factors, and evolving treatment options. J. Hand Surg. Am. 2008, 33, 102–112. [Google Scholar] [CrossRef]
- Titan, A.L.; Foster, D.S.; Chang, J.; Longaker, M.T. Flexor Tendon: Development, Healing, Adhesion Formation, and Contributing Growth Factors. Plast. Reconstr. Surg. 2019, 144, 639e–647e. [Google Scholar] [CrossRef] [PubMed]
- Capella-Monsonis, H.; Kearns, S.; Kelly, J.; Zeugolis, D.I. Battling adhesions: From understanding to prevention. BMC Biomed. Eng. 2019, 1, 5. [Google Scholar] [CrossRef]
- Noble, P.W. Hyaluronan and its catabolic products in tissue injury and repair. Matrix Biol. 2002, 21, 25–29. [Google Scholar] [CrossRef]
- Kabra, H.; Hwang, Y.; Lim, H.L.; Kar, M.; Arya, G.; Varghese, S. Biomimetic Material-Assisted Delivery of Human Embryonic Stem Cell Derivatives for Enhanced In Vivo Survival and Engraftment. ACS Biomater. Sci. Eng. 2015, 1, 7–12. [Google Scholar] [CrossRef]
- Park, H.; Baek, S.; Kang, H.; Lee, D. Biomaterials to Prevent Post-Operative Adhesion. Materials 2020, 13. [Google Scholar] [CrossRef] [PubMed]
- Kaux, J.F.; Samson, A.; Crielaard, J.M. Hyaluronic acid and tendon lesions. Muscles Ligaments Tendons J. 2015, 5, 264–269. [Google Scholar] [CrossRef]
- Hagberg, L.; Heinegard, D.; Ohlsson, K. The contents of macromolecule solutes in flexor tendon sheath fluid and their relation to synovial fluid. A quantitative analysis. J. Hand Surg. Br. 1992, 17, 167–171. [Google Scholar] [CrossRef]
- Abate, M.; Schiavone, C.; Salini, V. The use of hyaluronic acid after tendon surgery and in tendinopathies. Biomed. Res. Int. 2014, 2014, 783632. [Google Scholar] [CrossRef] [PubMed]
- St Onge, R.; Weiss, C.; Denlinger, J.L.; Balazs, E.A. A preliminary assessment of Na-hyaluronate injection into “no man’s land” for primary flexor tendon repair. Clin. Orthop. Relat. Res. 1980, 146, 269–275. [Google Scholar]
- Rydell, N. Decreased granulation tissue reaction after installment of hyaluronic acid. Acta Orthop. Scand. 1970, 41, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Barmakian, J.T.; Lin, H.; Green, S.M.; Posner, M.A.; Casar, R.S. Comparison of a suture technique with the modified Kessler method: Resistance to gap formation. J. Hand Surg. Am. 1994, 19, 777–781. [Google Scholar] [CrossRef]
- Zhao, H.; Guan, H.G.; Gu, J.; Luo, Z.P.; Zhang, W.; Chen, B.; Gu, Q.L.; Yang, H.L.; Shi, Q. Collagen membrane alleviates peritendinous adhesion in the rat Achilles tendon injury model. Chin. Med. J. 2013, 126, 729–733. [Google Scholar]
- Rothkopf, D.M.; Webb, S.; Szabo, R.M.; Gelberman, R.H.; May, J.W., Jr. An experimental model for the study of canine flexor tendon adhesions. J. Hand Surg. Am. 1991, 16, 694–700. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Sandvall, B.K.; Kuhlman-Wood, K.; Recor, C.; Friedrich, J.B. Flexor tendon repair, rehabilitation, and reconstruction. Plast. Reconstr. Surg. 2013, 132, 1493–1503. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.B. New Developments Are Improving Flexor Tendon Repair. Plast. Reconstr. Surg. 2018, 141, 1427–1437. [Google Scholar] [CrossRef]
- Orner, C.A.; Geary, M.B.; Hammert, W.C.; O’Keefe, R.J.; Loiselle, A.E. Low-Dose and Short-Duration Matrix Metalloproteinase 9 Inhibition Does Not Affect Adhesion Formation during Murine Flexor Tendon Healing. Plast. Reconstr. Surg. 2016, 137, 545e–553e. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.I.; Lin, P.C.; Chen, M.Y.; Hsieh, T.H.; Chen, J.J.; Yeh, M.L. The effect of tenocyte/hyaluronic acid therapy on the early recovery of healing Achilles tendon in rats. J. Mater. Sci. Mater. Med. 2014, 25, 217–227. [Google Scholar] [CrossRef]
- Liu, S.H.; Yang, R.S.; al-Shaikh, R.; Lane, J.M. Collagen in tendon, ligament, and bone healing. A current review. Clin. Orthop. Relat. Res. 1995, 318, 265–278. [Google Scholar]
- Wong, J.K.; Lui, Y.H.; Kapacee, Z.; Kadler, K.E.; Ferguson, M.W.; McGrouther, D.A. The cellular biology of flexor tendon adhesion formation: An old problem in a new paradigm. Am. J. Pathol. 2009, 175, 1938–1951. [Google Scholar] [CrossRef]
- Matthews, P.; Richards, H. Factors in the adherence of flexor tendon after repair: An experimental study in the rabbit. J. Bone Jt. Surg. Br. 1976, 58, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Potenza, A.D. Critical Evaluation of Flexor-Tendon Healing and Adhesion Formation within Artificial Digital Sheaths. J. Bone Jt. Surg. Am. 1963, 45, 1217–1233. [Google Scholar] [CrossRef]
- Isik, S.; Ozturk, S.; Gurses, S.; Yetmez, M.; Guler, M.M.; Selmanpakoglu, N.; Gunhan, O. Prevention of restrictive adhesions in primary tendon repair by HA-membrane: Experimental research in chickens. Br. J. Plast. Surg. 1999, 52, 373–379. [Google Scholar] [CrossRef]
- Hagberg, L.; Gerdin, B. Sodium hyaluronate as an adjunct in adhesion prevention after flexor tendon surgery in rabbits. J. Hand Surg. Am. 1992, 17, 935–941. [Google Scholar] [CrossRef]
- Gaughan, E.M.; Nixon, A.J.; Krook, L.P.; Yeager, A.E.; Mann, K.A.; Mohammed, H.; Bartel, D.L. Effects of sodium hyaluronate on tendon healing and adhesion formation in horses. Am. J. Vet. Res. 1991, 52, 764–773. [Google Scholar] [PubMed]
- Farhat, Y.M.; Al-Maliki, A.A.; Chen, T.; Juneja, S.C.; Schwarz, E.M.; O’Keefe, R.J.; Awad, H.A. Gene expression analysis of the pleiotropic effects of TGF-β1 in an in vitro model of flexor tendon healing. PLoS ONE 2012, 7, e51411. [Google Scholar] [CrossRef]
- Freeberg, M.A.T.; Farhat, Y.M.; Easa, A.; Kallenbach, J.G.; Malcolm, D.W.; Buckley, M.R.; Benoit, D.S.W.; Awad, H.A. Serpine1 Knockdown Enhances MMP Activity after Flexor Tendon Injury in Mice: Implications for Adhesions Therapy. Sci. Rep. 2018, 8, 5810. [Google Scholar] [CrossRef]
- Hagberg, L. Exogenous hyaluronate as an adjunct in the prevention of adhesions after flexor tendon surgery: A controlled clinical trial. J. Hand Surg. Am. 1992, 17, 132–136. [Google Scholar] [CrossRef]
- Wight, T.N.; Potter-Perigo, S. The extracellular matrix: An active or passive player in fibrosis? Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G950–G955. [Google Scholar] [CrossRef]
- Brown, J.P.; Finley, V.G.; Kuo, C.K. Embryonic mechanical and soluble cues regulate tendon progenitor cell gene expression as a function of developmental stage and anatomical origin. J. Biomech. 2014, 47, 214–222. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, C.; Zhu, S.; Lu, P.; Zhu, T.; Gong, X.; Zhang, Z.; Hu, J.; Yin, Z.; Heng, B.C.; et al. Mohawk promotes the tenogenesis of mesenchymal stem cells through activation of the TGFbeta signaling pathway. Stem Cells 2015, 33, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.B.; Yalamanchi, N.; Pham, H.; Longaker, M.T.; Chang, J. Flexor tendon healing in vitro: Effects of TGF-beta on tendon cell collagen production. J. Hand Surg. Am. 2002, 27, 615–620. [Google Scholar] [CrossRef]
- Chan, K.M.; Fu, S.C.; Wong, Y.P.; Hui, W.C.; Cheuk, Y.C.; Wong, M.W. Expression of transforming growth factor beta isoforms and their roles in tendon healing. Wound Repair Regen. 2008, 16, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Nichols, A.E.C.; Settlage, R.E.; Werre, S.R.; Dahlgren, L.A. Novel roles for scleraxis in regulating adult tenocyte function. BMC Cell Biol. 2018, 19, 14. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lee, H.; Zhu, C. Molecular mechanisms of mechanotransduction in integrin-mediated cell-matrix adhesion. Exp. Cell Res. 2016, 349, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Geiger, B. A 130 K protein from chicken gizzard: Its localization at the termini of microfilament bundles in cultured chicken cells. Cell 1979, 18, 193–205. [Google Scholar] [CrossRef]
- McCrea, P.D.; Gottardi, C.J. Beyond β-catenin: Prospects for a larger catenin network in the nucleus. Nat. Rev. Mol. Cell Biol. 2016, 17, 55–64. [Google Scholar] [CrossRef] [PubMed]
- MacPherson, M.; Fagerholm, S.C. Filamin and filamin-binding proteins in integrin-regulation and adhesion. Focus on: “FilaminA is required for vimentin-mediated cell adhesion and spreading”. Am. J. Physiol. Cell Physiol. 2010, 298, C206–C208. [Google Scholar] [CrossRef] [PubMed]
- Coulombe, P.A.; Wong, P. Cytoplasmic intermediate filaments revealed as dynamic and multipurpose scaffolds. Nat. Cell Biol. 2004, 6, 699–706. [Google Scholar] [CrossRef]
- Lou, J.; Kubota, H.; Hotokezaka, S.; Ludwig, F.J.; Manske, P.R. In vivo gene transfer and overexpression of focal adhesion kinase (pp125 FAK) mediated by recombinant adenovirus-induced tendon adhesion formation and epitenon cell change. J. Orthop. Res. 1997, 15, 911–918. [Google Scholar] [CrossRef]
- Chen, C.T.; Chen, C.H.; Sheu, C.; Chen, J.P. Ibuprofen-Loaded Hyaluronic Acid Nanofibrous Membranes for Prevention of Postoperative Tendon Adhesion through Reduction of Inflammation. Int. J. Mol. Sci. 2019, 20, 5038. [Google Scholar] [CrossRef]


| Score | Features of Adhesion |
|---|---|
| Quantity | |
| 0 | No apparent adhesions |
| 1 | A number of scattered filaments |
| 2 | A large number of filaments |
| 3 | Countless filaments |
| Quality | |
| 0 | No apparent adhesions |
| 1 | Regular, elongated, fine filamentous |
| 2 | Irregular, mixed, shortened, filamentous |
| 3 | Dense, not filamentous |
| Grading of Adhesions | |
| 0 | None |
| 1–2 | Slight |
| 3–4 | Moderate |
| 5–6 | Severe |
| Accession Number | Gene | Forward Primer | Reverse Primer | Amplicon Size |
|---|---|---|---|---|
| NM_021578.2 | TGF-β1 | 5′-GCCTGAGTGGCTGTCTTTTGA-3′ | 5′-GGCTGATCCCGTTGATTTCCA-3′ | 146 bp |
| NM_031131.2 | TGF-β2 | 5′-CATCCCGCCCACTTTCTACAG-3′ | 5′-CACTCTGGCTTTGGGGTTTTG-3′ | 133 bp |
| NM_053304.1 | COL1A1 | 5′-CCCGAACCCCAAGGAAAAGAA-3′ | 5′-TAGGCTACGCTGTTCTTGCAG-3′ | 183 bp |
| NM_017008.4 | GAPDH | 5′-TCACCACCATGGAGAAGGC-3′ | 5′-GCTAAGCAGTTGGTGGTGCA-3′ | 169 bp |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, K.H.; Park, E.S.; Choi, C.Y.; Cha, H.G.; Hwang, Y.; Nam, S.M. Hyaluronic Acid Treatment Improves Healing of the Tenorrhaphy Site by Suppressing Adhesions through Extracellular Matrix Remodeling in a Rat Model. Polymers 2021, 13, 928. https://doi.org/10.3390/polym13060928
Ahn KH, Park ES, Choi CY, Cha HG, Hwang Y, Nam SM. Hyaluronic Acid Treatment Improves Healing of the Tenorrhaphy Site by Suppressing Adhesions through Extracellular Matrix Remodeling in a Rat Model. Polymers. 2021; 13(6):928. https://doi.org/10.3390/polym13060928
Chicago/Turabian StyleAhn, Kwang Hyeon, Eun Soo Park, Chang Yong Choi, Han Gyu Cha, Yongsung Hwang, and Seung Min Nam. 2021. "Hyaluronic Acid Treatment Improves Healing of the Tenorrhaphy Site by Suppressing Adhesions through Extracellular Matrix Remodeling in a Rat Model" Polymers 13, no. 6: 928. https://doi.org/10.3390/polym13060928
APA StyleAhn, K. H., Park, E. S., Choi, C. Y., Cha, H. G., Hwang, Y., & Nam, S. M. (2021). Hyaluronic Acid Treatment Improves Healing of the Tenorrhaphy Site by Suppressing Adhesions through Extracellular Matrix Remodeling in a Rat Model. Polymers, 13(6), 928. https://doi.org/10.3390/polym13060928