Synergistic Effects of Graphene and Ammonium Polyphosphate Modified with Vinyltrimethoxysilane on the Properties of High-Impact Polystyrene Composites
Abstract
1. Introduction
2. Experimental
2.1. Materials
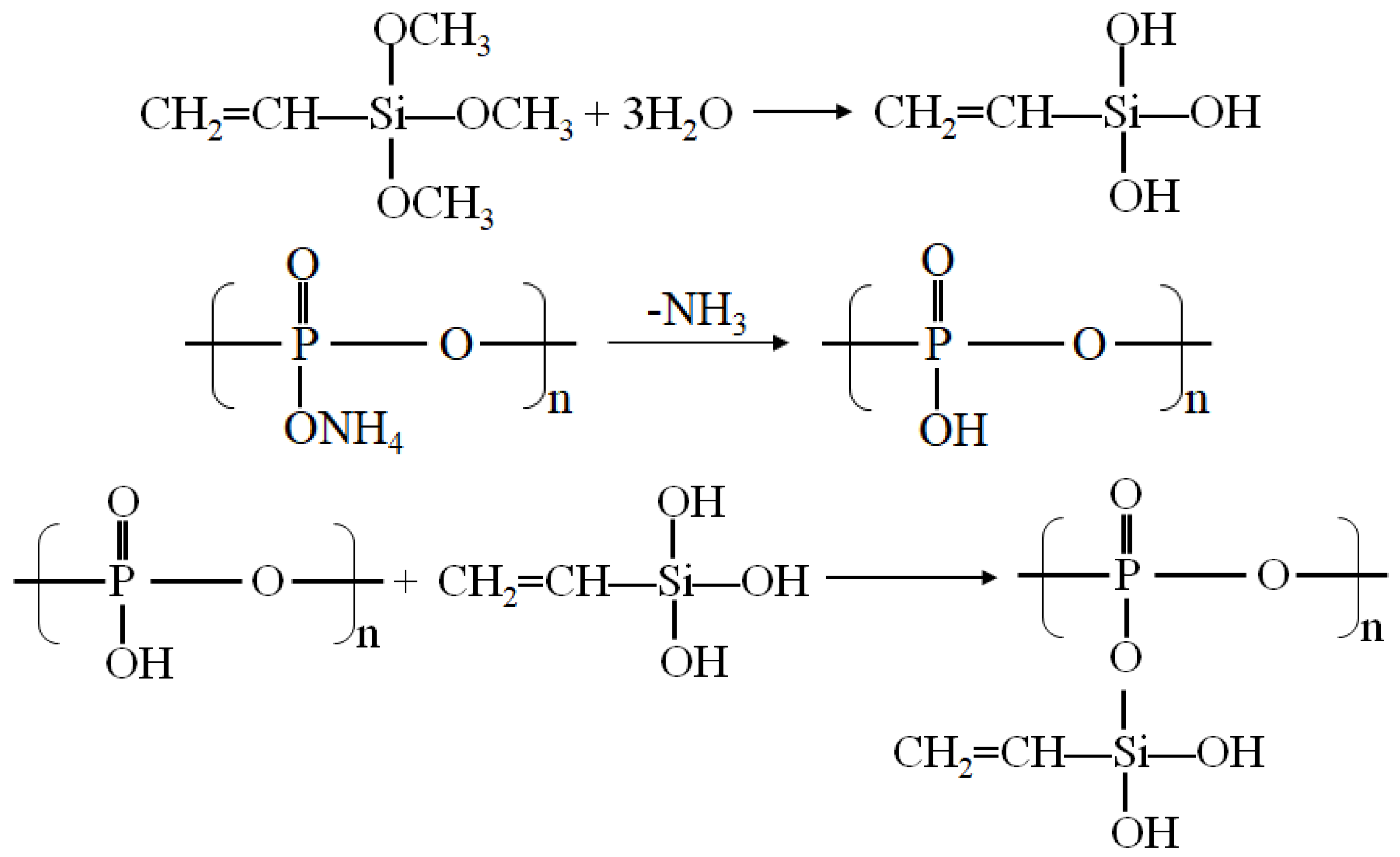
2.2. Surface Modification of Ammonium Polyphosphate
2.3. Preparation of Composites
2.4. Measurements and Characterization
2.5. Quantifying the Synergistic Effect in HIPS
3. Results and Discussion
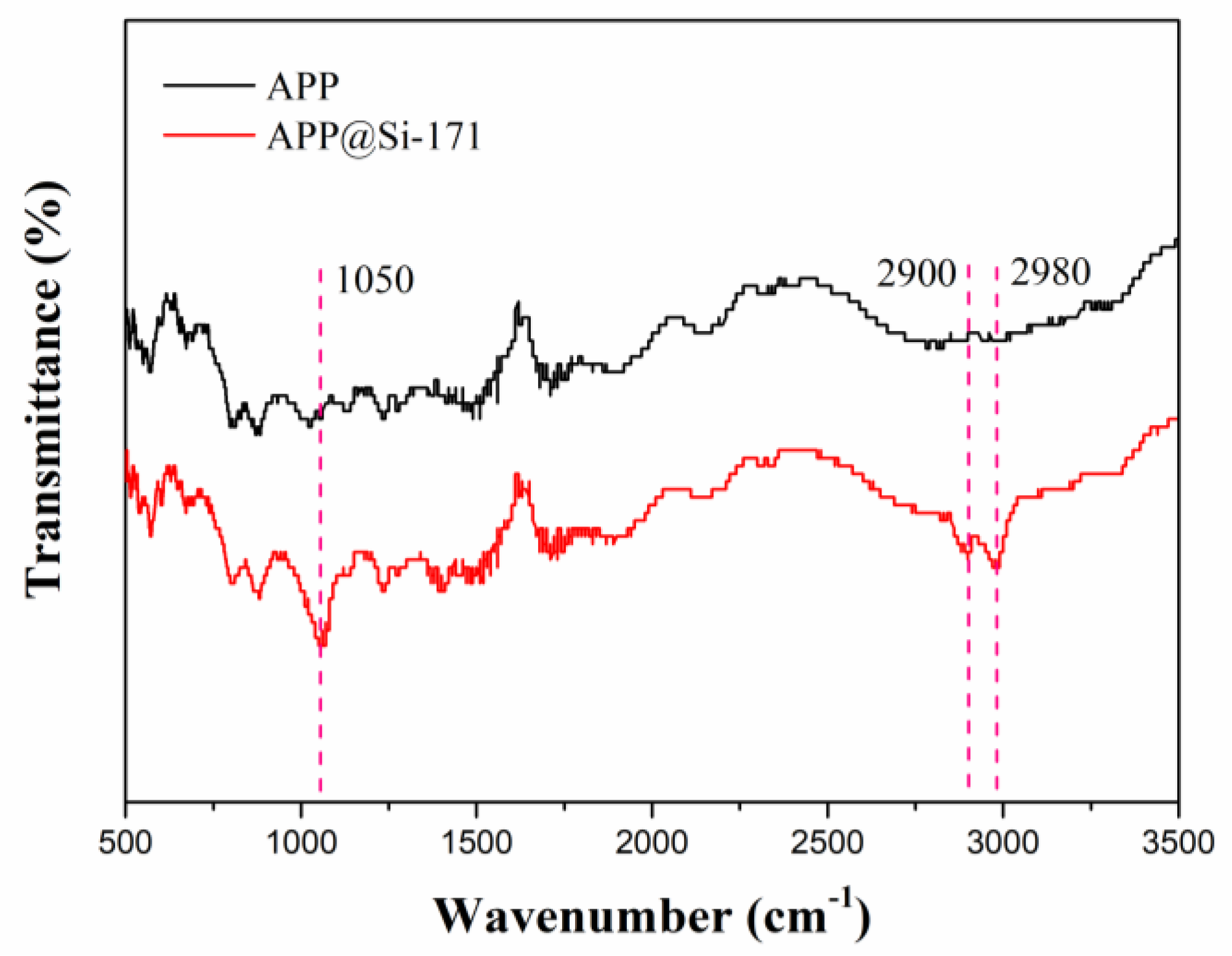
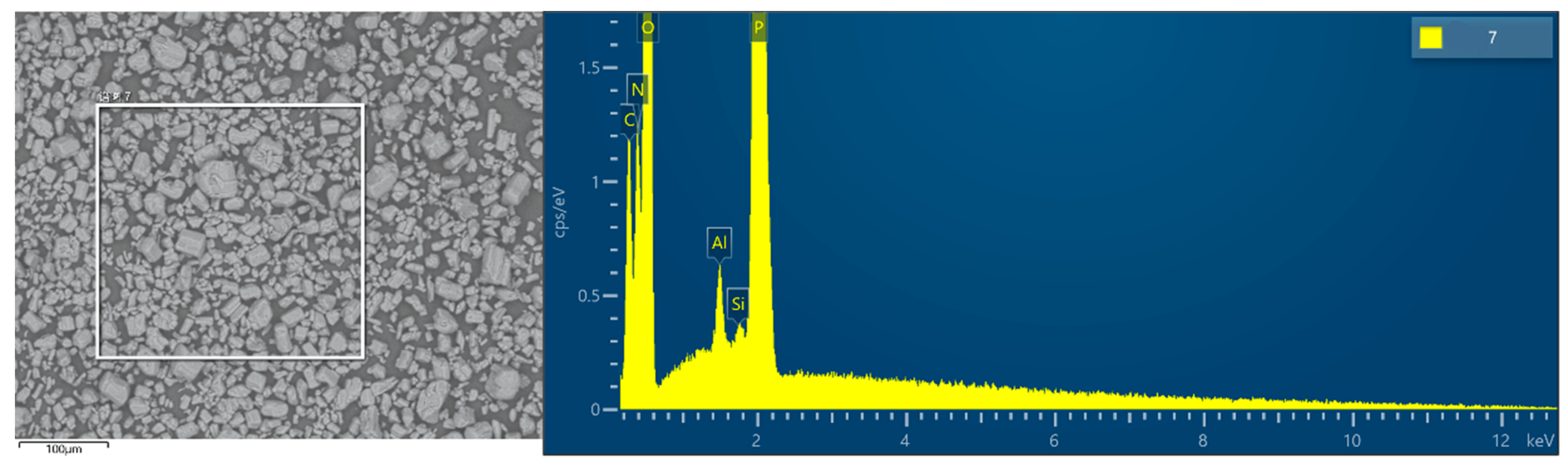
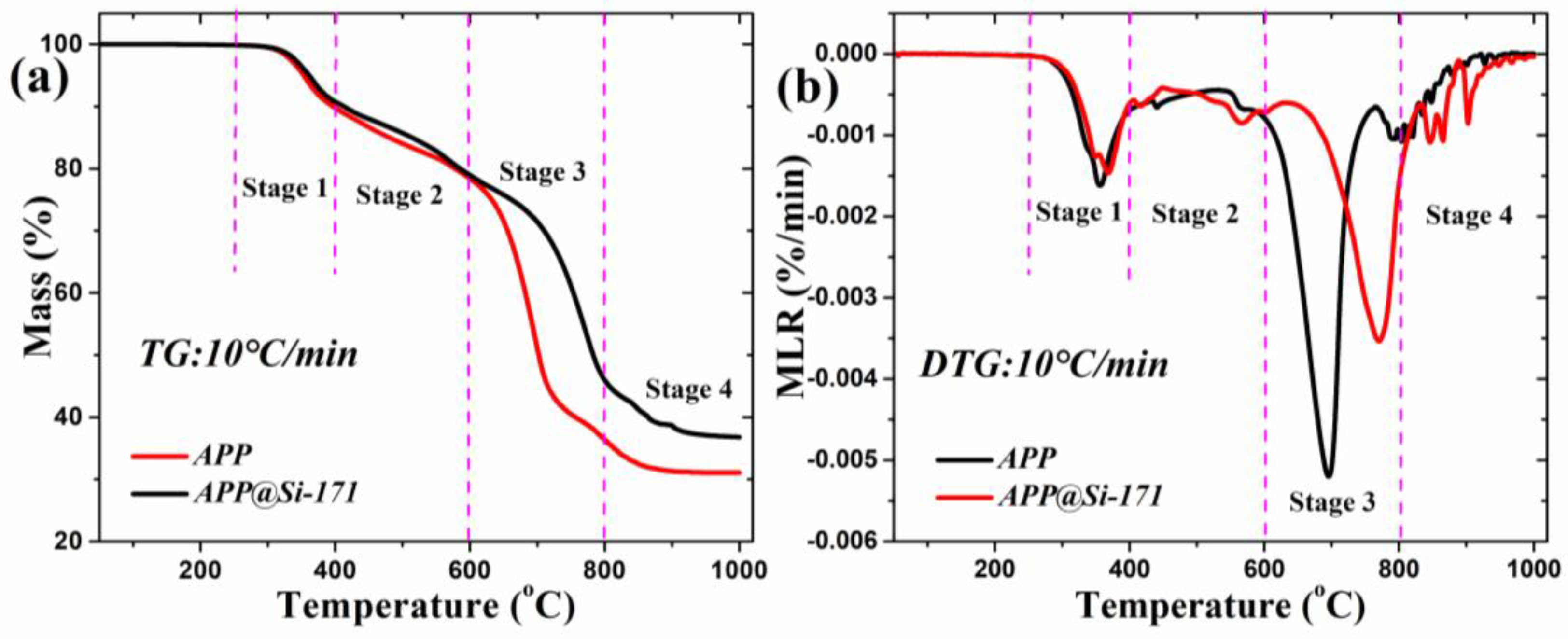
3.1. Surface Modification and Thermal Stability Characterization of APP
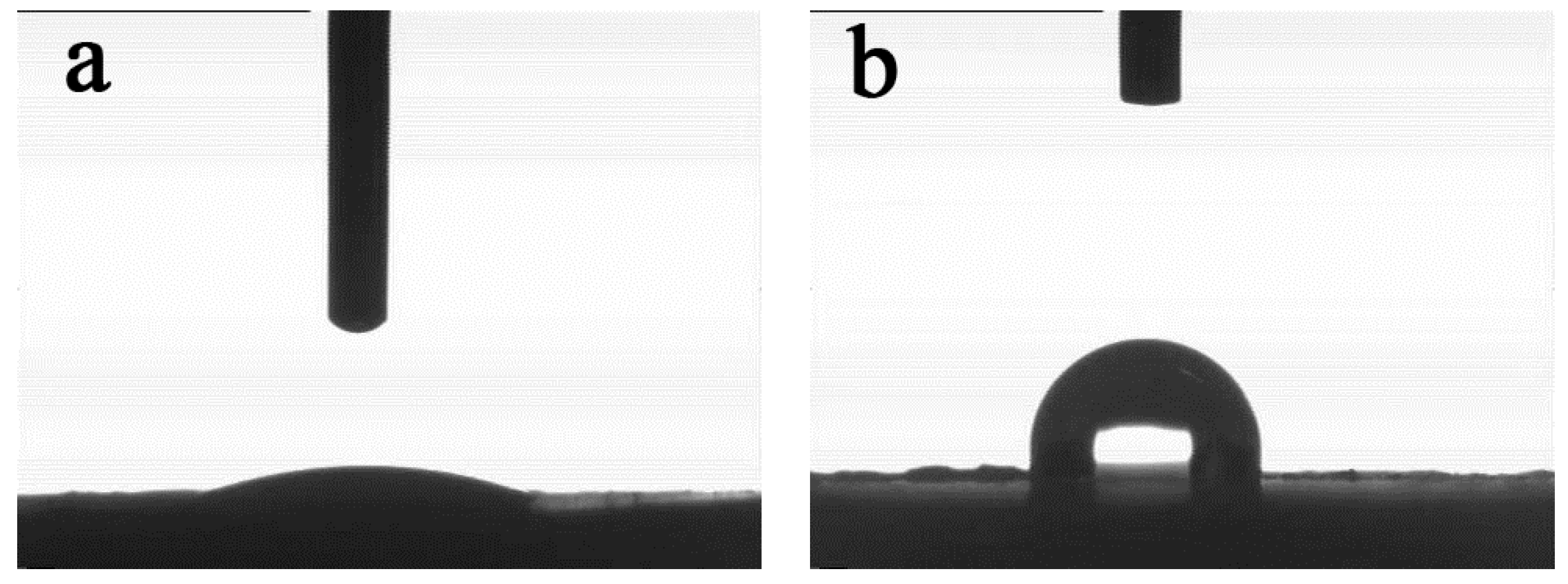
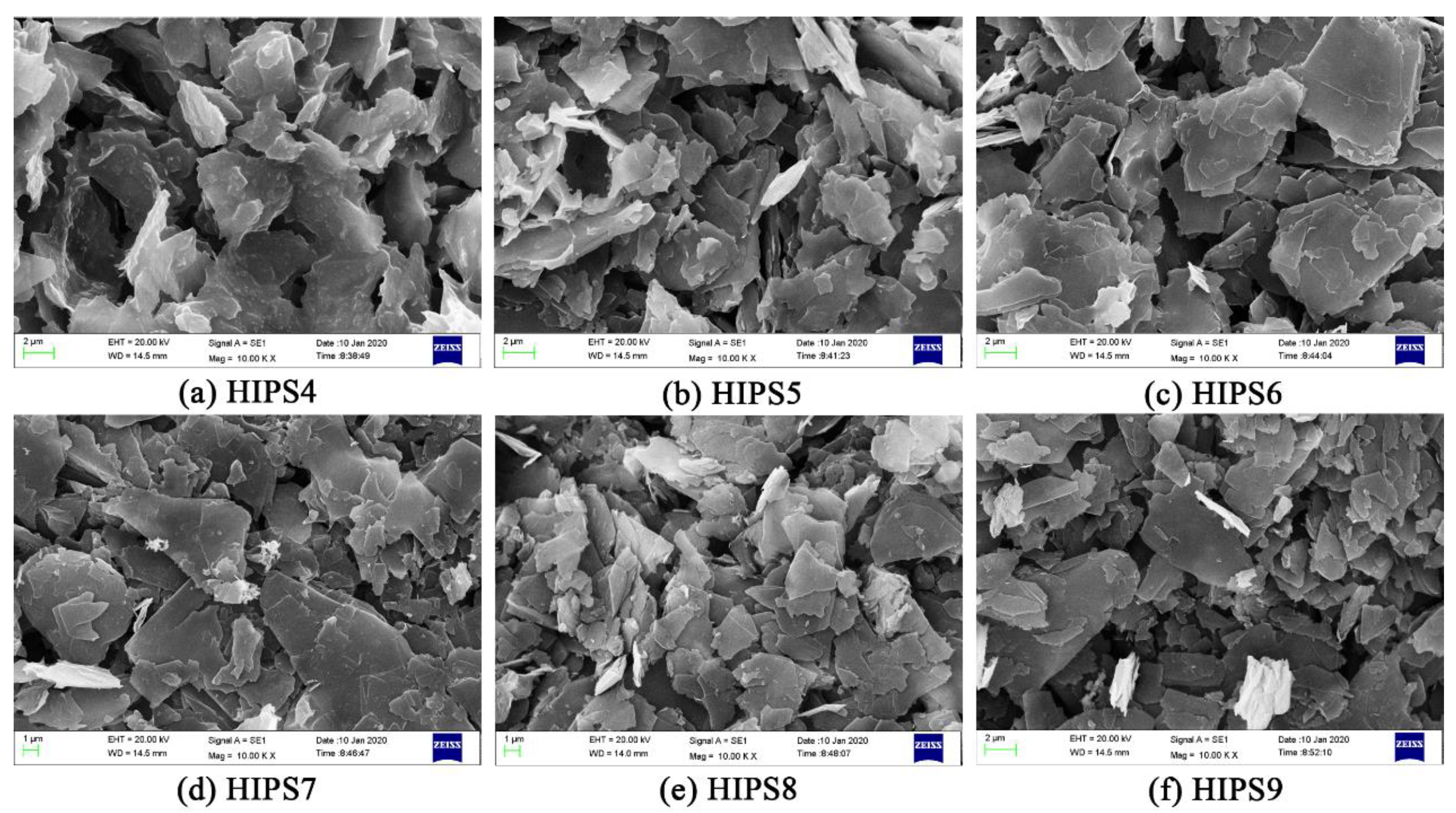
3.2. Dispersion of the Fillers
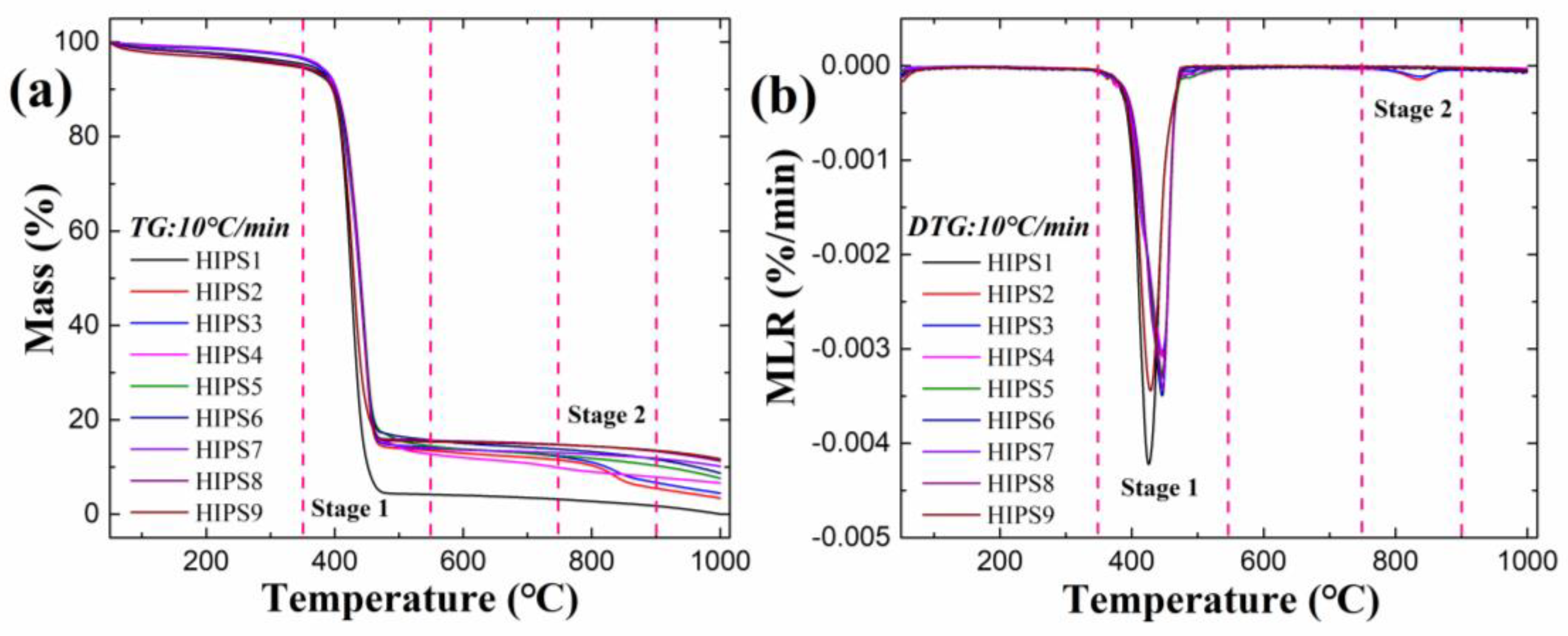
3.3. Thermogravimetric Analysis of Improved HIPS Composites
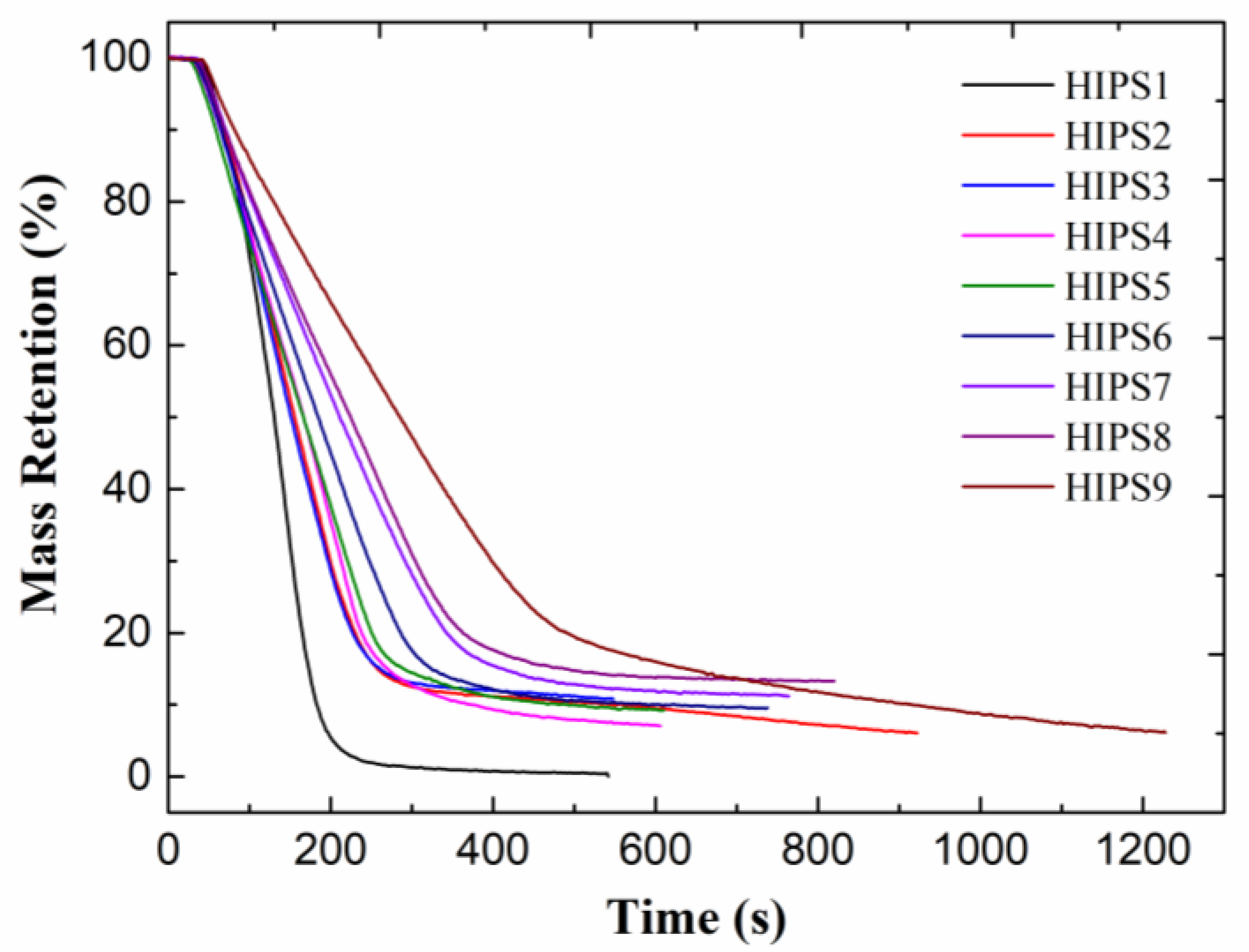
3.4. Cone Calorimeter Test Analysis of Improved HIPS Composites
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chang, S.Q.; Xie, T.X.; Yang, G.S. Effects of polystyrene-encapsulated magnesium hydroxide on rheological and flame-retarding properties of hips composites. Polym. Degrad. Stab. 2006, 91, 3266–3273. [Google Scholar] [CrossRef]
- Wang, B.B.; Zhang, Y.; Tao, Y.J.; Zhou, X.; Song, L.; Jie, G.X.; Hu, Y. Monitoring the degradation of physical properties and fire hazards of high-impact polystyrene composite with different ageing time in natural environments. J. Hazard. Mater. 2018, 352, 92–100. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, C.G.; Yang, H.; Cai, T.; Cai, J.; Li, H.B.; Zhou, D.; Chen, B.F.; Li, A.M.; Cheng, R.S. Improvement of the compatibilization of high-impact polystyrene/magnesium hydroxide composites with partially sulfonated polystyrene as macromolecular compatibilizers. Ind. Eng. Chem. Res. 2012, 51, 9204–9212. [Google Scholar] [CrossRef]
- Braun, U.; Schartel, B. Flame retardant mechanisms of red phosphorus and magnesium hydroxide in high impact polystyrene. Macromol. Chem. Phys. 2004, 205, 2185–2196. [Google Scholar] [CrossRef]
- Huang, C.Y.; Chen, X.F.; Yuan, B.H.; Zhang, H.M.; Dai, H.M.; He, S.; Zhang, Y.; Niu, Y.; Shen, S.F. Suppression of wood dust explosion by ultrafine magnesium hydroxide. J. Hazard. Mater. 2019, 378, 120723. [Google Scholar] [CrossRef]
- Lim, K.S.; Bee, S.T.; Sin, L.T.; Tee, T.T.; Ratnam, C.T.; Hui, D.; Rahmat, A.R. A review of application of ammonium polyphosphate as intumescent flame retardant in thermoplastic composites. Compos. Part B-Eng. 2016, 84, 155–174. [Google Scholar] [CrossRef]
- Yang, Z.; Cai, J.; Zhou, C.G.; Zhou, D.; Chen, B.F.; Yang, H.; Cheng, R.S. Effects of the content of silane coupling agent KH-560 on the properties of LLDPE/magnesium hydroxide composites. J. Appl. Polym. Sci. 2010, 118, 2634–2641. [Google Scholar] [CrossRef]
- Zhou, L.; Guo, C.G.; Li, L.P. Influence of ammonium polyphosphate modified with 3-(methylacryloxyl) propyltrimethoxy silane on mechanical and thermal properties of wood flour-polypropylene composites. J. Appl. Polym. Sci. 2011, 122, 849–855. [Google Scholar] [CrossRef]
- Jiao, C.M.; Wang, H.Z.; Zhang, Z.B.; Chen, X.L. Preparation and properties of an efficient smoke suppressant and flame-retardant agent for thermoplastic polyurethane. Polym. Adv. Technol. 2017, 28, 1690–1698. [Google Scholar] [CrossRef]
- Ge, L.L.; Duan, H.J.; Zhang, X.G.; Chen, C.; Tang, J.H.; Li, Z.M. Synergistic effect of ammonium polyphosphate and expandable graphite on flame-retardant properties of acrylonitrile-butadiene-styrene. J. Appl. Polym. Sci. 2012, 126, 1337–1343. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, B.B.; Yuan, B.H.; Yuan, Y.; Liew, K.M.; Song, L.; Hu, Y. Preparation of Large-Size Reduced Graphene Oxide-Wrapped Ammonium Polyphosphate and Its Enhancement of the Mechanical and Flame Retardant Properties of Thermoplastic Polyurethane. Ind. Eng. Chem. Res. 2017, 56, 7468–7477. [Google Scholar] [CrossRef]
- Seefeldt, H.; Braun, U.; Wagner, M.H. Residue Stabilization in the Fire Retardancy of Wood–Plastic Composites: Combination of Ammonium Polyphosphate, Expandable Graphite, and Red Phosphorus. Macromol. Chem. Phys. 2012, 213, 2370–2377. [Google Scholar] [CrossRef]
- Chen, X.S.; Ma, Y.J.; Cheng, Y.J.; Zhang, A.Q.; Liu, W. Enhanced mechanical and flame-resistant properties of polypropylene nanocomposites with reduced graphene oxide-functionalized ammonium polyphosphate and pentaerythritol. J. Appl. Polym. Sci. 2019, 136, 48036. [Google Scholar] [CrossRef]
- Chen, X.L.; Ma, C.Y.; Jiao, C.M. Synergistic effects between iron-graphene and ammonium polyphosphate in flame-retardant thermoplastic polyurethane. J. Therm. Anal. Calorim. 2016, 126, 633–642. [Google Scholar] [CrossRef]
- Lingamdinne, L.P.; Choi, Y.L.; Kim, I.S.; Yang, J.K.; Koduru, J.R.; Chang, Y.Y. Preparation and characterization of porous reduced graphene oxide based inverse spinel nickel ferrite nanocomposite for adsorption removal of radionuclides. J. Hazard. Mater. 2017, 326, 145–156. [Google Scholar] [CrossRef]
- Xiao, Y.L.; Jin, Z.Y.; He, L.X.; Ma, S.C.; Wang, C.Y.; Mu, X.W.; Song, L. Synthesis of a novel graphene conjugated covalent organic framework nanohybrid for enhancing the flame retardancy and mechanical properties of epoxy resins through synergistic effect. Compos. Part B-Eng. 2020, 182, 107616. [Google Scholar] [CrossRef]
- Amani, M.; Sharif, M.; Kashkooli, A.; Rahnama, N.; Fazli, A. Effect of mixing conditions on the selective localization of graphite oxide and the properties of polyethylene/high-impact polystyrene/graphite oxide nanocomposite blends. RSC Adv. 2015, 5, 77723–77733. [Google Scholar] [CrossRef]
- Rostampour, A.; Sharif, M.; Mouji, N. Synergetic effects of graphene oxide and clay on the microstructure and properties of HIPS/graphene oxide/clay nanocomposites. Polym.-Plast. Technol. Eng. 2017, 56, 171–183. [Google Scholar] [CrossRef]
- Song, P.A.; Liu, L.; Fu, S.; Yu, Y.; Jin, C.; Wu, Q.; Zhang, Y.; Li, Q. Striking multiple synergies created by combining reduced graphene oxides and carbon nanotubes for polymer nanocomposites. Nanotechnology 2013, 24, 125704. [Google Scholar] [CrossRef]
- Han, Y.Q.; Wu, Y.; Shen, M.X.; Huang, X.L.; Zhu, J.J.; Zhang, X.G. Preparation and properties of polystyrene nanocomposites with graphite oxide and graphene as flame retardants. J. Mater. Sci. 2013, 48, 4214–4222. [Google Scholar] [CrossRef]
- Gao, W.Y.; Qian, X.D.; Wang, S.J. Preparation of hybrid silicon materials microcapsulated ammonium polyphosphate and its application in thermoplastic polyurethane. J. Appl. Polym. Sci. 2018, 135, 45742. [Google Scholar] [CrossRef]
- Guo, C.G.; Zhou, L.; Lv, J.X. Effects of Expandable Graphite and Modified Ammonium Polyphosphate on the Flame-Retardant and Mechanical Properties of Wood Flour-Polypropylene Composites. Polym. Polym. Compos. 2013, 21, 449–456. [Google Scholar] [CrossRef]
- Meng, X.Y.; Ye, L.; Zhang, X.G.; Tang, P.M.; Tang, J.H.; Ji, X.; Li, Z.M. Effects of expandable graphite and ammonium polyphosphate on the flame-retardant and mechanical properties of rigid polyurethane foams. J. Appl. Polym. Sci. 2010, 114, 853–863. [Google Scholar] [CrossRef]
- Zhu, H.F.; Zhu, Q.L.; Li, J.; Tao, K.; Xue, L.X.; Yan, Q. Synergistic effect between expandable graphite and ammonium polyphosphate on flame retarded polylactide. Polym. Degrad. Stabil. 2011, 96, 183–189. [Google Scholar] [CrossRef]
- Tang, M.Q.; Qi, F.; Chen, M.; Sun, Z.D.; Xu, Y.; Chen, X.L.; Zhang, Z.B.; Shen, R. Synergistic effects of ammonium polyphosphate and red phosphorus with expandable graphite on flammability and thermal properties of HDPE/EVA blends. Polym. Adv. Technol. 2016, 27, 52–60. [Google Scholar] [CrossRef]
- Dittrich, B.; Wartig, K.A.; Mülhaupt, R.; Schartel, B. Flame-retardancy properties of intumescent ammonium poly (phosphate) and mineral filler magnesium hydroxide in combination with graphene. Polymers 2014, 6, 2875–2895. [Google Scholar] [CrossRef]
- Wu, G.M.; Schartel, B.; Yu, D.; Kleemeier, M.; Hartwig, A. Synergistic fire retardancy in layered-silicate nanocomposite combined with low-melting phenysiloxane glass. J. Fire Sci. 2012, 30, 69–87. [Google Scholar] [CrossRef]
- Zhu, C.S. Polymer Structure Analysis, 2nd ed.; Science Press: Beijing, China, 2016. [Google Scholar]
- Lin, H.J.; Yan, H.; Liu, B.; Wei, L.Q.; Xu, B.S. The influence of KH-550 on properties of ammonium polyphosphate and polypropylene flame retardant composites. Polym. Degrad. Stabil. 2011, 96, 1382–1388. [Google Scholar] [CrossRef]
- Bourbigot, S.; Le, B.M.; Gengembre, L.; Delobel, R. XPS study of an intumescent coating application to the ammonium polyphosphate/pentaerythritol fire-retardant system. Appl. Surf. Sci. 1994, 81, 299–307. [Google Scholar] [CrossRef]
- Li, J.; Stoliarov, S.I. Measurement of kinetics and thermodynamics of the thermal degradation for non-charring polymers. Combust. Flame 2013, 160, 1287–1297. [Google Scholar] [CrossRef]
- Li, J.; Stoliarov, S.I. Measurement of kinetics and thermodynamics of the thermal degradation for charring polymers. Polym. Degrad. Stabil. 2014, 106, 2–15. [Google Scholar] [CrossRef]
- Czégény, Z.; Blazsó, M. Effect of phosphorous flame retardants on the thermal decomposition of vinyl polymers and copolymers. J. Anal. Appl. Pyrolysis 2008, 81, 218–224. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J. Thermal stabilities of drops of burning thermoplastics under the UL 94 vertical test conditions. J. Hazard. Mater. 2013, 246, 103–109. [Google Scholar] [CrossRef] [PubMed]



| Samples | HIPS (wt%) | APP (wt%) | APP@Si-171 (wt%) | Graphene (wt%) |
|---|---|---|---|---|
| HIPS1 | 100 | 0 | 0 | 0 |
| HIPS2 | 85 | 15 | 0 | 0 |
| HIPS3 | 85 | 0 | 15 | 0 |
| HIPS4 | 85 | 0 | 12 | 3 |
| HIPS5 | 85 | 0 | 9 | 6 |
| HIPS6 | 85 | 0 | 7.5 | 7.5 |
| HIPS7 | 85 | 0 | 6 | 9 |
| HIPS8 | 85 | 0 | 3 | 12 |
| HIPS9 | 85 | 0 | 0 | 15 |
| Element Line | Weight (%) | Atom (%) |
|---|---|---|
| O K | 45.95 | 47.52 |
| Si K | 0.15 | 0.09 |
| C K | 15.59 | 21.47 |
| Al K | 0.46 | 0.28 |
| P K | 21.75 | 11.62 |
| N K | 16.10 | 19.01 |
| Td, onset (°C) | Tmax (°C) | Tend (°C) | MLRmax (‱/min) | Mass Residues (%) | A (s−1) | E (kJ/mol) | |
|---|---|---|---|---|---|---|---|
| APP | 317.2 | 694.3 | 915.2 | 0.52 | 31.1 | 11.2 | 205.9 |
| APP@Si-171 | 330.3 | 768.7 | 952.3 | 0.35 | 36.8 | 9.1 | 214.9 |
| Discrepancy | 13.1 | 74.4 | 37.1 | 0.17 | 5.7 | 2.1 | 9.0 |
| Samples | Td, onset (°C) | Tmax (°C) | MLRmax (‱/min) | Residue Yield (%) | A (s−1) | E (kJ/mol) |
|---|---|---|---|---|---|---|
| HIPS1 | 404.8 | 424.7 | 0.42 | 0.0 | 10.2 | 71.66 |
| HIPS2 | 410.3 | 447.3 | 0.31 | 3.4 | 9.6 | 74.0 |
| HIPS3 | 409.7 | 446.8 | 0.30 | 4.4 | 9.6 | 75.27 |
| HIPS4 | 415.2 | 448.3 | 0.31 | 6.6 | 9.2 | 78.2 |
| HIPS5 | 415.8 | 447.2 | 0.33 | 7.6 | 9.3 | 81.4 |
| HIPS6 | 421.8 | 446.3 | 0.35 | 8.7 | 9.6 | 86.3 |
| HIPS7 | 416.2 | 447.3 | 0.34 | 10.1 | 9.6 | 82.2 |
| HIPS8 | 417.5 | 444.8 | 0.33 | 11.2 | 9.4 | 81.2 |
| HIPS9 | 407.0 | 427.8 | 0.34 | 11.7 | 9.1 | 75.3 |
| Samples | SE of Td, onset | SE of Tmax | SE of Residue Yield | SE of E |
|---|---|---|---|---|
| HIPS4 | 2.40 | 1.28 | 1.12 | 1.78 |
| HIPS5 | 2.92 | 1.55 | 1.03 | 2.65 |
| HIPS6 | 4.85 | 1.71 | 1.08 | 3.97 |
| HIPS7 | 3.50 | 2.10 | 1.16 | 2.86 |
| HIPS8 | 4.69 | 2.89 | 1.10 | 2.58 |
| Samples | THR (MJ/m2) | PHRR (kW/m2) | CO2 Yield (kg/kg) | Residue Yield (%) |
|---|---|---|---|---|
| HIPS1 | 158.3 | 1450.1 | 5.5 | 0 |
| HIPS2 | 147.6 | 830.0 | 7.1 | 6.1 |
| HIPS3 | 146.5 | 840.7 | 4.3 | 10.8 |
| HIPS4 | 138.8 | 739.3 | 3.7 | 7.1 |
| HIPS5 | 131.2 | 635.7 | 3.5 | 9.2 |
| HIPS6 | 141.8 | 625.0 | 4.2 | 9.6 |
| HIPS7 | 144.5 | 588.3 | 3.6 | 11.2 |
| HIPS8 | 140.0 | 516.2 | 3.8 | 13.3 |
| HIPS9 | 177.2 | 461.6 | 3.7 | 6.1 |
| Samples | SE of PHRR | SE of CO2 Yield | SE of Residue Yield |
|---|---|---|---|
| HIPS4 | 1.04 | 1.01 | 0.72 |
| HIPS5 | 1.07 | 1.12 | 0.93 |
| HIPS6 | 1.03 | 0.75 | 0.97 |
| HIPS7 | 1.03 | 1.16 | 1.13 |
| HIPS8 | 1.02 | 0.94 | 1.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, X.; Pan, Y.; Wang, Y.; Jia, Z.; Chen, T.; Gong, J.; Jiang, J. Synergistic Effects of Graphene and Ammonium Polyphosphate Modified with Vinyltrimethoxysilane on the Properties of High-Impact Polystyrene Composites. Polymers 2021, 13, 881. https://doi.org/10.3390/polym13060881
Shi X, Pan Y, Wang Y, Jia Z, Chen T, Gong J, Jiang J. Synergistic Effects of Graphene and Ammonium Polyphosphate Modified with Vinyltrimethoxysilane on the Properties of High-Impact Polystyrene Composites. Polymers. 2021; 13(6):881. https://doi.org/10.3390/polym13060881
Chicago/Turabian StyleShi, Xianghui, Yong Pan, Yuguo Wang, Zhimeng Jia, Tingting Chen, Junhui Gong, and Juncheng Jiang. 2021. "Synergistic Effects of Graphene and Ammonium Polyphosphate Modified with Vinyltrimethoxysilane on the Properties of High-Impact Polystyrene Composites" Polymers 13, no. 6: 881. https://doi.org/10.3390/polym13060881
APA StyleShi, X., Pan, Y., Wang, Y., Jia, Z., Chen, T., Gong, J., & Jiang, J. (2021). Synergistic Effects of Graphene and Ammonium Polyphosphate Modified with Vinyltrimethoxysilane on the Properties of High-Impact Polystyrene Composites. Polymers, 13(6), 881. https://doi.org/10.3390/polym13060881







