Synthetic, Natural, and Semisynthetic Polymer Carriers for Controlled Nitric Oxide Release in Dermal Applications: A Review
Abstract
1. Introduction
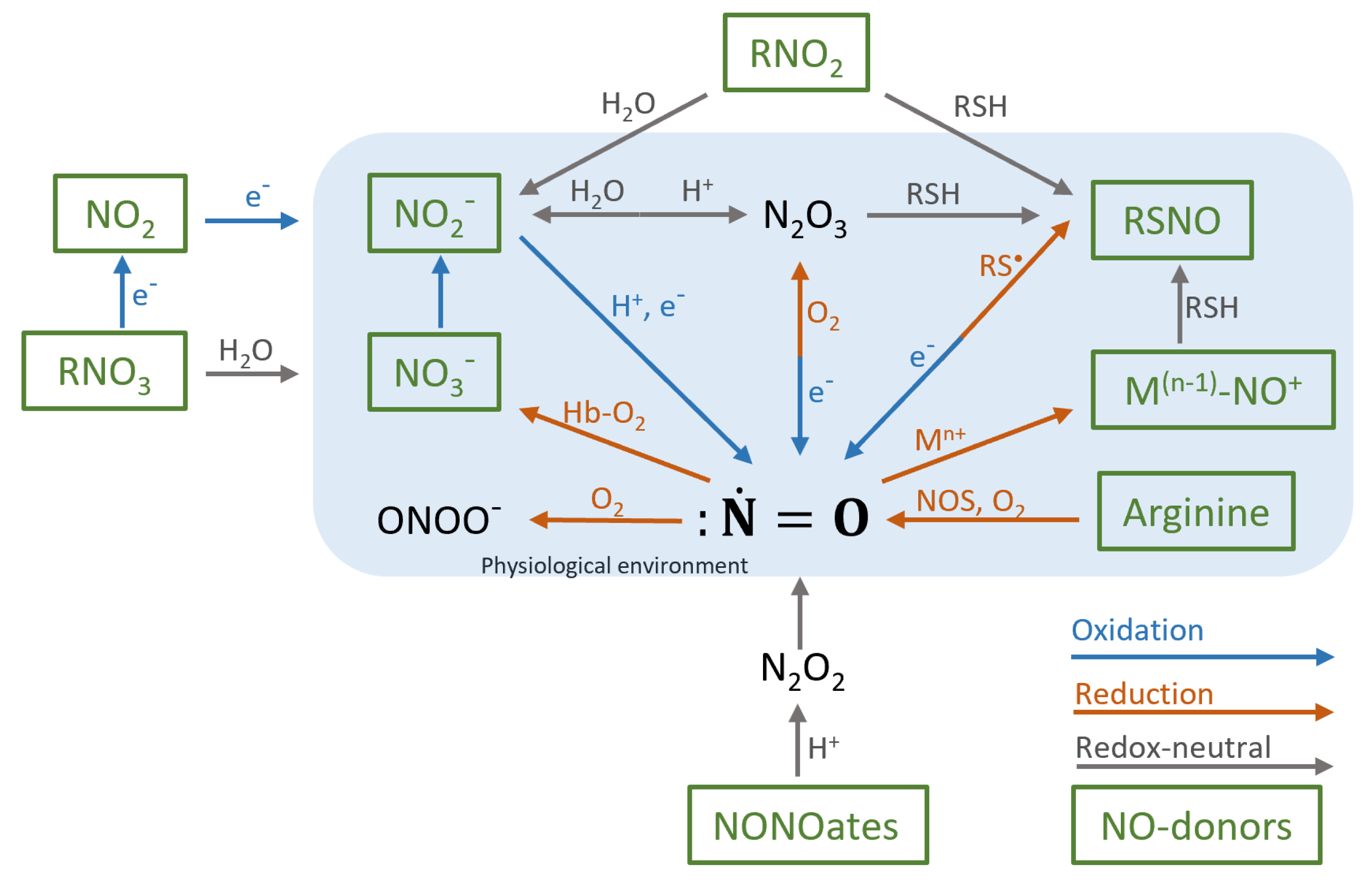
2. Biochemistry of Nitric Oxide
3. Nitric Oxide in Human Physiology
Dermatological Nitric Oxide
4. Nitric Oxide Skin Disorders
5. Nitric Oxide Donors
5.1. Controlled Release
5.2. Main NO-Donors
6. Carriers of Nitric Oxide Donors
6.1. Liposomal Carriers
6.2. Metallic Carriers
6.3. Polymeric Carriers
Polymer Carrier Preparation
7. Pharmaceutical Applications
7.1. Antimicrobial Applications
7.1.1. Synthetic Polymer Carriers
7.1.2. Natural Polymer Carriers
7.1.3. Semisynthetic Polymer Carriers
7.1.4. Further Enhancement of Polymer Carriers
7.2. Wound Healing Applications
7.2.1. Synthetic Polymer Carriers
7.2.2. Natural Polymer Carriers
7.2.3. Semisynthetic Polymer Carriers
7.2.4. Further Enhancement of Polymer Carriers
7.3. Circulatory Applications
8. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, B.; Ming, Y.; Liu, Y.; Xing, H.; Fu, R.; Li, Z.; Ni, R.; Li, L.; Duan, D.; Xu, J.; et al. Recent Developments in Pharmacological Effect, Mechanism and Application Prospect of Diazeniumdiolates. Front. Pharmacol. 2020, 11, 923. Available online: https://www.frontiersin.org/article/10.3389/fphar.2020.00923/full (accessed on 27 February 2021). [CrossRef]
- Pelegrino, M.T.; de Araújo, D.R.; Seabra, A.B. S-nitrosoglutathione-containing chitosan nanoparticles dispersed in Pluronic F-127 hydrogel: Potential uses in topical applications. J. Drug Deliv. Sci. Technol. 2018, 43, 211–220. [Google Scholar] [CrossRef]
- Seabra, A.B.; Justo, G.Z.; Haddad, P.S. State of the art, challenges and perspectives in the design of nitric oxide-releasing polymeric nanomaterials for biomedical applications. Biotechnol. Adv. 2015, 33, 1370–1379. [Google Scholar] [CrossRef] [PubMed]
- Opländer, C.; Römer, A.; Paunel-Görgülü, A.; Fritsch, T.; van Faassen, E.E.; Mürtz, M.; Bozkurt, A.; Grieb, G.; Fuchs, P.; Pallua, N.; et al. Dermal Application of Nitric Oxide In Vivo: Kinetics, Biological Responses, and Therapeutic Potential in Humans. Clin. Pharmacol. Ther. 2012, 91, 1074–1082. Available online: http://doi.wiley.com/10.1038/clpt.2011.366 (accessed on 27 February 2021). [CrossRef]
- Liechty, W.B.; Kryscio, D.R.; Slaughter, B.V.; Peppas, N.A. Polymers for Drug Delivery Systems. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 149–173. Available online: https://pmc/articles/PMC3438887/?report=abstract (accessed on 27 February 2021). [CrossRef]
- Marcato, P.D.; Adami, L.F.; de Melo, B.R.; Melo, P.S.; Ferreira, I.R.; de Paula, L.; Nelson, D.; Seabra, A.B. Development of a Sustained-release System for Nitric Oxide Delivery using Alginate/Chitosan Nanoparticles. Curr. Nanosci. 2013, 9, 1–7. [Google Scholar]
- Kesharwani, P.; Jain, K.; Jain, N.K. Dendrimer as nanocarrier for drug delivery. Prog. Polym. Sci. 2014, 39, 268–307. [Google Scholar] [CrossRef]
- Adler, B.L.; Friedman, A.J. Nitric oxide therapy for dermatologic disease. Future Sci. OA 2015, 1. Available online: https://pmc/articles/PMC5137922/?report=abstract (accessed on 27 February 2021). [CrossRef] [PubMed]
- Franke, A.; Oszajca, M.; Brindell, M.; Stochel, G.; van Eldik, R. Metal-assisted activation of nitric oxide-mechanistic aspects of complex nitrosylation processes. In Advances in Inorganic Chemistry; van Eldik, R., Ford, P., Eds.; Elsevier Academic Press: San Diego, CA, USA, 2015; pp. 171–241. [Google Scholar]
- Toledo, J.C.; Augusto, O. Connecting the Chemical and Biological Properties of Nitric Oxide. Chem. Res. Toxicol. 2012, 25, 975–989. Available online: https://pubs.acs.org/doi/full/10.1021/tx300042g (accessed on 27 February 2021). [CrossRef]
- Vercelino, R.; Cunha, T.M.; Ferreira, E.S.; Cunha, F.Q.; Ferreira, S.H.; de Oliveira, M.G. Skin vasodilation and analgesic effect of a topical nitric oxide-releasing hydrogel. J. Mater. Sci. Mater. Med. 2013, 24, 2157–2169. Available online: https://link.springer.com/article/10.1007/s10856-013-4973-7 (accessed on 27 February 2021). [CrossRef] [PubMed]
- Pub-Chem. Center for Biotechnology Information. Compound Summary for CID 145068, Nitric Oxide. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Nitric-oxide (accessed on 26 November 2020).
- Lancaster, J.R. Nitric oxide: A brief overview of chemical and physical properties relevant to therapeutic applications. Futur. Sci. OA 2015, 1, FSO59. Available online: http://www.future-science.com/doi/10.4155/fso.15.59 (accessed on 27 February 2021). [CrossRef]
- Hughes, M.N. Chemistry of Nitric Oxide and Related Species. In Cellulases; Elsevier BV: Amsterdam, The Netherlands, 2008; Volume 436, pp. 3–19. [Google Scholar]
- Coleman, J.W. Nitric oxide in immunity and inflammation. Int. Immunopharmacol. 2001, 1, 1397–1406. [Google Scholar] [CrossRef]
- Snyder, S.H.; Bredt, D.S. Biological Roles of Nitric Oxide. Sci. Am. 1992, 266, 68–77. Available online: https://www.jstor.org/stable/24939060 (accessed on 27 February 2021). [CrossRef] [PubMed]
- Ignarro, L.J. Nitric oxide is not just blowing in the wind. Br. J. Pharmacol. 2019, 176, 131–134. Available online: http://onlinelibrary.wiley.com/doi/10.1111/bph.v176.2/issuetoc (accessed on 27 February 2021). [CrossRef]
- PubChem. National Center for Biotechnology Information. PubChem Compound Summary for CID 6322, Arginine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Arginine (accessed on 18 December 2020).
- Ghavari, A.; Miller, C.C.; Mcmullin, B.; Ghahary, A. Potential application of gaseous nitric oxide as a topical antimicrobial agent. Nitric Oxide 2006, 14, 21–29. Available online: www.elsevier.com/locate/yniox (accessed on 27 February 2021). [CrossRef]
- Ma, L.; Hu, L.; Feng, X.; Wang, S. Nitrate and nitrite in health and disease. Aging Dis. 2018, 9, 938–945. Available online: https://pmc/articles/PMC6147587/?report=abstract (accessed on 27 February 2021). [CrossRef] [PubMed]
- Cracowski, J.; Roustit, M. Human Skin Microcirculation. In Comprehensive Physiology; Prakash, Y.S., Ed.; Wiley: Hoboken, NJ, USA, 2020; Volume 10, pp. 1105–1154. Available online: https://onlinelibrary.wiley.com/doi/10.1002/cphy.c190008 (accessed on 27 February 2021).
- Bruch-Gerharz, D.; Ruzicka, T.; Kolb-Bachofen, V. Nitric oxide and its implications in skin homeostasis and disease—A review. Arch. Dermatol. Res. 1998, 290, 643–651. Available online: https://link.springer.com/article/10.1007/s004030050367 (accessed on 27 February 2021). [CrossRef] [PubMed]
- Pieretti, J.C.; Seabra, A.B. Nitric Oxide-Releasing Nanomaterials and Skin Infections. In Nanotechnology in Skin, Soft Tissue, and Bone Infections; Mahendra, R., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 3–23. Available online: https://link.springer.com/chapter/10.1007/978-3-030-35147-2_1 (accessed on 27 February 2021).
- Cobbold, C. The role of nitric oxide in the formation of keloid and hypertrophic lesions. Med. Hypotheses 2001, 57, 497–502. [Google Scholar] [CrossRef]
- Limandjaja, G.C.; Niessen, F.B.; Scheper, R.J.; Gibbs, S. The Keloid Disorder: Heterogeneity, Histopathology, Mechanisms and Models. Front. Cell Dev. Biol. 2020, 8, 360. Available online: www.frontiersin.org (accessed on 27 February 2021). [CrossRef] [PubMed]
- Luo, J.D.; Chen, A.F. Nitric oxide: A newly discovered function on wound healing. Acta Pharmacol. Sin. 2005, 26, 259–264. Available online: https://www.nature.com/articles/aps200541 (accessed on 27 February 2021). [CrossRef] [PubMed]
- Holliman, G.; Lowe, D.; Cohen, H.; Felton, S.; Raj, K. Ultraviolet Radiation-Induced Production of Nitric Oxide: A multi-cell and multi-donor analysis. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.; Reske-Kunz, A.B. The role of NO in contact hypersensitivity. Int. Immunopharmacol. 2001, 1, 1469–1478. [Google Scholar] [CrossRef]
- Furchgott, R.F.; Zawadzki, J.V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 1980, 288, 373–376. Available online: https://www.nature.com/articles/288373a0 (accessed on 27 February 2021). [CrossRef]
- Luiking, Y.C.; Engelen, M.P.K.J.; Deutz, N.E.P. Regulation of nitric oxide production in health and disease. Curr. Opin. Clin. Nutr. Metab. Care. 2010, 13, 97–104. Available online: https://pmc/articles/PMC2953417/?report=abstract (accessed on 27 February 2021). [CrossRef] [PubMed]
- Hadi, H.A.R.; Carr, C.S.; al Suwaidi, J. Endothelial dysfunction: Cardiovascular risk factors, therapy, and outcome. Vasc. Health Risk Manag. 2005, 1, 183–198. Available online: https://pmc/articles/PMC1993955/?report=abstract (accessed on 27 February 2021).
- Hsu, Y.-C.; Hsiao, M.; Wang, L.-F.; Chien, Y.W.; Lee, W.-R. Nitric oxide produced by iNOS is associated with collagen synthesis in keloid scar formation. Nitric Oxide 2006, 14, 327–334. [Google Scholar] [CrossRef]
- Zhang, Z.; Luo, X.; Yang, Y. From Spontaneous to Photo-triggered and Photo-calibrated Nitric Oxide Donors. Isr. J. Chem. 2020, 202000084. Available online: https://onlinelibrary.wiley.com/doi/10.1002/ijch.202000084 (accessed on 27 February 2021). [CrossRef]
- Yang, T.; Zelikin, A.N.; Chandrawati, R. Progress and Promise of Nitric Oxide-Releasing Platforms. Adv. Sci. 2018, 5, 1701043. Available online: http://doi.wiley.com/10.1002/advs.201701043 (accessed on 27 February 2021). [CrossRef]
- Zhou, X.; Wang, H.; Zhang, J.; Li, X.; Wu, Y.; Wei, Y.; Ji, S.; Kong, D.; Zhao, Q. Functional poly(ε-caprolactone)/chitosan dressings with nitric oxide-releasing property improve wound healing. Acta Biomater. 2017, 54, 128–137. [Google Scholar] [CrossRef]
- Kutner, A.; Friedman, A. Nitric oxide nanoparticles for wound healing: Future directions to overcome challenges. Expert Rev. Dermatol. 2013, 8, 451–461. Available online: https://www.tandfonline.com/doi/abs/10.1586/17469872.2013.837670 (accessed on 27 February 2021). [CrossRef]
- Wang, P.G.; Xian, M.; Tang, X.; Wu, X.; Wen, Z.; Cai, T.; Janczuk, A.J. Nitric Oxide Donors: Chemical Activities and Biological Applications. Chem. Rev. 2002, 102, 1091–1134. Available online: https://pubs.acs.org/doi/full/10.1021/cr000040l (accessed on 27 February 2021). [CrossRef]
- Hayton, T.W.; Legzdins, A.P.; Sharp, W.B. Coordination and Organometallic Chemistry of Metal−NO Complexes. Chem. Rev. 2002, 102, 935–992. Available online: https://pubs.acs.org/doi/pdf/10.1021/cr000074t (accessed on 27 February 2021). [CrossRef] [PubMed]
- Simplicio, F.I.; de Oliveira, M.G.; de Souza, G.F.P. In vitro inhibition of linoleic acid peroxidation by primary S-nitrosothiols. J. Braz. Chem. Soc. 2010, 21, 1885–1895. Available online: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0103-50532010001000013&lng=en&nrm=iso&tlng=en (accessed on 27 February 2021). [CrossRef]
- Liang, H.; Nacharaju, P.; Friedman, A.; Friedman, J.M. Nitric oxide generating/releasing materials. Future Sci. OA 2015, 1. Available online: https://pmc/articles/PMC4739797/?report=abstract (accessed on 27 February 2021). [CrossRef] [PubMed]
- Baldim, V.; de Oliveira, M.G. Poly-ε-caprolactone/polysulfhydrylated polyester blend: A platform for topical and degradable nitric oxide-releasing materials. Eur. Polym. J. 2018, 109, 143–152. [Google Scholar] [CrossRef]
- De Oliveira, M.G. S-Nitrosothiols as Platforms for Topical Nitric Oxide Delivery. Basic Clin. Pharmacol. Toxicol. 2016, 119, 49–56. Available online: http://doi.wiley.com/10.1111/bcpt.12588 (accessed on 27 February 2021). [CrossRef]
- Miller, M.R.; Megson, I.L. Recent developments in nitric oxide donor drugs. Br. J. Pharmacol. 2007, 151, 305–321. Available online: https://pmc/articles/PMC2013979/?report=abstract (accessed on 27 February 2021). [CrossRef]
- Nakanishi, K.; Koshiyama, T.; Iba, S.; Ohba, M. Lipophilic ruthenium salen complexes: Incorporation into lipo-some bilayers and photoinduced release of nitric oxide. Dalt. Trans. 2015, 44, 14200–14203. Available online: https://pubmed.ncbi.nlm.nih.gov/26200295/ (accessed on 27 February 2021). [CrossRef]
- Connelly, J.T.; Kondapalli, S.; Skoupi, M.; Parker, J.S.L.; Kirby, B.J.; Baeumner, A.J. Micro-total analysis system for virus detection: Microfluidic pre-concentration coupled to liposome-based detection. Anal. Bioanal. Chem. 2011, 402, 315–323. Available online: https://link.springer.com/article/10.1007/s00216-011-5381-9 (accessed on 27 February 2021). [CrossRef] [PubMed]
- Chandrawati, R.; Städler, B.; Postma, A.; Connal, L.A.; Chong, S.F.; Zelikin, A.N.; Caruso, F. Cholesterol-mediated anchoring of enzyme-loaded liposomes within disulfide-stabilized polymer carrier capsules. Biomaterials 2009, 30, 5988–5998. [Google Scholar] [CrossRef] [PubMed]
- Duong, H.T.T.; Adnan, N.N.M.; Barraud, N.; Basuki, J.S.; Kutty, S.K.; Jung, K.; Kumar, N.; Davis, T.P.; Boyer, C. Functional gold nanoparticles for the storage and controlled release of nitric oxide: Applications in biofilm dispersal and intracellular delivery. J. Mater. Chem. B 2014, 2, 5003–5011. Available online: https://pubs.rsc.org/en/content/articlehtml/2014/tb/c4tb00632a (accessed on 27 February 2021). [CrossRef] [PubMed]
- Diring, S.; Wang, D.O.; Kim, C.; Kondo, M.; Chen, Y.; Kitagawa, S.; Kamei, K.-I.; Furukawa, S. Localized cell stimulation by nitric oxide using a photoactive porous coordination polymer platform. Nat. Commun. 2013, 4, 2684. Available online: https://www.nature.com/articles/ncomms3684 (accessed on 27 February 2021). [CrossRef] [PubMed]
- Lu, B.; Lu, F.; Zou, Y.; Liu, J.; Rong, B.; Li, Z.; Dai, F.; Wu, D.; Lan, G. In situ reduction of silver nanoparticles by chitosan-l-glutamic acid/hyaluronic acid: Enhancing antimicrobial and wound-healing activity. Carbohydr. Polym. 2017, 173, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Sithole, M.N.; Choonara, Y.E.; du Toit, L.C.; Kumar, P.; Pillay, V. A review of semi-synthetic biopolymer complexes: Modified polysaccharide nano-carriers for enhancement of oral drug bioavailability. Pharm. Dev. Technol. 2016, 22, 283–295. Available online: https://www.tandfonline.com/doi/abs/10.1080/10837450.2016.1212882 (accessed on 27 February 2021). [CrossRef]
- Dmour, I.; Taha, M.O. Natural and semisynthetic polymers in pharmaceutical nanotechnology. In Organic Materials as Smart Nanocarriers for Drug Delivery; Grumezescu, A.M., Ed.; Elsevier BV: Amsterdam, The Netherlands, 2018; pp. 35–100. [Google Scholar]
- Worley, B.V.; Soto, R.J.; Kinsley, P.C.; Schoenfisch, M.H. Active Release of Nitric Oxide-Releasing Dendrimers from Electrospun Polyurethane Fibers. ACS Biomater. Sci. Eng. 2016, 2, 426–437. Available online: https://pubs.acs.org/doi/full/10.1021/acsbiomaterials.6b00032 (accessed on 27 February 2021). [CrossRef]
- Dan Mogoşanu, G.; Grumezescu, A.M.; Bejenaru, L.E.; Bejenaru, C. Natural and synthetic polymers for drug delivery and targeting. In Nanobiomaterials in Drug Delivery: Applications of Nanobiomaterials; Grumezescu, A.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 229–284. [Google Scholar]
- Sikka, M.P.; Midha, V.K. The role of biopolymers and biodegradable polymeric dressings in managing chronic wounds. In Advanced Textiles for Wound Care; Rajendran, S., Ed.; Elsevier BV: Amsterdam, The Netherlands, 2019; pp. 463–488. [Google Scholar]
- Seabra, A.B.; de Oliveira, M.G. Poly (vinyl alcohol) and poly (vinyl pyrrolidone) blended films for local nitric oxide release. Biomaterials 2004, 25, 3773–3782. [Google Scholar] [CrossRef]
- Seabra, A.; Fitzpatrick, A.; Paul, J.; de Oliveira, M.; Weller, R. Topically applied S-nitrosothiol-containing hydrogels as experimental and pharmacological nitric oxide donors in human skin. Br. J. Dermatol. 2004, 151, 977–983. Available online: https://onlinelibrary.wiley.com/doi/full/10.1111/j.1365-2133.2004.06213.x (accessed on 27 February 2021). [CrossRef]
- Shishido, S.M.; Seabra, A.B.; Loh, W.; de Oliveira, M.G. Thermal and photochemical nitric oxide release from S-nitrosothiols incorporated in Pluronic F127 gel: Potential uses for local and controlled nitric oxide release. Biomaterials 2003, 24, 3543–3553. [Google Scholar] [CrossRef]
- Halpenny, G.M.; Steinhardt, R.C.; Okialda, K.A.; Mascharak, P.K. Characterization of pHEMA-based hydrogels that exhibit light-induced bactericidal effect via release of NO. J. Mater. Sci. Mater. Med. 2009, 20, 2353–2360. Available online: https://link.springer.com/article/10.1007/s10856-009-3795-0 (accessed on 27 February 2021). [CrossRef]
- Marcato, P.D.; Adami, L.F.; Melo, P.S.; de Paula, L.B.; Durán, N.; Seabra, A.B. Glutathione and S-nitrosoglutathione in alginate/chitosan nanoparticles: Cytotoxicity. J. Phys. Conf. Ser. 2011, 304, 012045. Available online: https://iopscience.iop.org/article/10.1088/1742-6596/304/1/012045 (accessed on 27 February 2021). [CrossRef]
- Cardozo, V.F.; Lancheros, C.A.C.; Narciso, A.M.; Valereto, E.C.S.; Kobayashi, R.K.T.; Seabra, A.B.; Nakazato, G. Evaluation of antibacterial activity of nitric oxide-releasing polymeric particles against Staphylococcus aureus and Escherichia coli from bovine mastitis. Int. J. Pharm. 2014, 473, 20–29. [Google Scholar] [CrossRef]
- Nguyen, T.K.; Selvanayagam, R.; Ho, K.K.K.; Chen, R.; Kutty, S.K.; Rice, S.A.; Kumar, N.; Barraud, N.; Duong, H.T.T.; Boyer, C. Co-delivery of nitric oxide and antibiotic using polymeric nanoparticles. Chem. Sci. 2016, 7, 1016–1027. Available online: https://pubs.rsc.org/en/content/articlehtml/2016/sc/c5sc02769a (accessed on 27 February 2021). [CrossRef] [PubMed]
- Sadrearhami, Z.; Yeow, J.; Nguyen, T.-K.; Ho, K.K.K.; Kumar, N.; Boyer, C. Biofilm dispersal using nitric oxide loaded nanoparticles fabricated by photo-PISA: Influence of morphology. Chem. Commun. 2017, 53, 12894–12897. Available online: https://pubs.rsc.org/en/content/articlehtml/2017/cc/c7cc07293g (accessed on 27 February 2021). [CrossRef]
- Shen, Z.; He, K.; Ding, Z.; Zhang, M.; Yu, Y.; Hu, J. Visible-Light-Triggered Self-Reporting Release of Nitric Oxide (NO) for Bacterial Biofilm Dispersal. Macromolecules 2019, 52, 7668–7677. Available online: https://pubs.acs.org/doi/full/10.1021/acs.macromol.9b01252 (accessed on 27 February 2021). [CrossRef]
- Namivandi-Zangeneh, R.; Sadrearhami, Z.; Bagheri, A.; Sauvage-Nguyen, M.; Ho, K.K.K.; Kumar, N.; Wong, E.H.H.; Boyer, C. Nitric Oxide-Loaded Antimicrobial Polymer for the Synergistic Eradication of Bacterial Biofilm. ACS Macro Lett. 2018, 7, 592–597. Available online: https://pubs.acs.org/doi/full/10.1021/acsmacrolett.8b00190 (accessed on 27 February 2021). [CrossRef]
- Weller, R.; Dykhuizen, R.; Leifert, C.; Ormerod, A.; Christophers, E.; Henseler, T. Nitric oxide release accounts for the reduced incidence of cutaneous infections in psoriasis. J. Am. Acad. Dermatol. 1997, 36, 281–282. Available online: http://www.jaad.org/article/S0190962297703074/fulltext (accessed on 27 February 2021). [CrossRef]
- Weller, R.; Price, R.; Ormerod, A.; Benjamin, N.; Leifert, C. Antimicrobial effect of acidified nitrite on dermatophyte fungi, Candida and bacterial skin pathogens. J. Appl. Microbiol. 2001, 90, 648–652. Available online: http://doi.wiley.com/10.1046/j.1365-2672.2001.01291.x (accessed on 27 February 2021). [CrossRef]
- Li, J.; Hunter, C.A.; Farrell, J.P. Anti-TGF-β Treatment Promotes Rapid Healing of Leishmania major Infection in Mice by Enhancing In Vivo Nitric Oxide Production. J. Immunol. 1999, 162, 974–979. [Google Scholar] [PubMed]
- López-Jaramillo, P.; Ruano, C.; Rivera, J.; Terán, E.; Salazar-Irigoyen, R.; Esplugues, J.V.; Moncada, S. Treatment of cutaneous leishmaniasis with nitric-oxide donor. Lancet 1998, 351, 1176–1177. [Google Scholar]
- Seabra, A.B.; Duran, N. Nanotechnology Allied to Nitric Oxide Release Materials for Dermatological Applications. Curr. Nanosci. 2012, 8, 520–525. Available online: http://www.eurekaselect.com/openurl/content.php?genre=article&issn=1573-4137&volume=8&issue=4&spage=520 (accessed on 27 February 2021). [CrossRef]
- Urzedo, A.L.; Gonçalves, M.C.; Nascimento, M.H.M.; Lombello, C.B.; Nakazato, G.; Seabra, A.B. Cytotoxicity and Antibacterial Activity of Alginate Hydrogel Containing Nitric Oxide Donor and Silver Nanoparticles for Topical Applications. ACS Biomater. Sci. Eng. 2020, 6, 2117–2134. Available online: https://dx.doi.org/10.1021/acsbiomaterials.9b01685 (accessed on 27 February 2021). [CrossRef]
- Urzedo, A.L.; Gonçalves, M.C.; Nascimento, M.H.M.; Lombello, C.B.; Nakazato, G.; Seabra, A.B. Multifunctional alginate nanoparticles containing nitric oxide donor and silver nanoparticles for biomedical applications. Mater. Sci. Eng. C 2020, 112, 110933. [Google Scholar] [CrossRef]
- Hebert, A.A.; Siegfried, E.C.; Durham, T.; de León, E.N.; Reams, T.; Messersmith, E.; Maeda-Chubachi, T. Efficacy and tolerability of an investigational nitric oxide–releasing topical gel in patients with molluscum contagiosum: A randomized clinical trial. J. Am. Acad. Dermatol. 2020, 82, 887–894. Available online: https://doi.org/10.1016/j.jaad.2019.09.064 (accessed on 27 February 2021). [CrossRef]
- Costa-Orlandi, C.B.; Mordorski, B.; Baltazar, L.M.; Mendes-Giannini, M.J.S.; Friedman, J.M.; Nosanchuk, J.D.; Friedman, A.J. Nitric Oxide Releasing Nanoparticles as a Strategy to Improve Cur-rent Onychomycosis Treatments. J. Drugs Dermatol. 2018, 7, 717–720. Available online: https://pubmed.ncbi.nlm.nih.gov/30005092/ (accessed on 27 February 2021).
- Stasko, N.; McHale, K.; Hollenbach, S.J.; Martin, M.; Doxey, R. Nitric Oxide-Releasing Macromolecule Exhibits Broad-Spectrum Antifungal Activity and Utility as a Topical Treatment for Superficial Fungal Infections. Antimicrob. Agents Chemother. 2018, 62. Available online: https://doi.org/10.1128/AAC.01026-17 (accessed on 27 February 2021). [CrossRef]
- Pelegrino, M.T.; Lima, B.D.A.; Nascimento, M.H.M.D.; Lombello, C.B.; Brocchi, M.; Seabra, A.B. Biocompatible and Antibacterial Nitric Oxide-Releasing Pluronic F-127/Chitosan Hydrogel for Topical Applications. Polymers 2018, 10, 452. Available online: www.mdpi.com/journal/polymers (accessed on 27 February 2021). [CrossRef] [PubMed]
- Mordorski, B.; Costa-Orlandi, C.B.; Baltazar, L.M.; Carreño, L.J.; Landriscina, A.; Rosen, J.; Navati, M.; Mendes-Giannini, M.J.S.; Friedman, J.M.; Nosanchuk, J.D.; et al. Topical nitric oxide releasing nanoparticles are effective in a murine model of dermal Trichophyton rubrum dermatophytosis. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 2267–2270. [Google Scholar] [CrossRef] [PubMed]
- Sulemankhil, I.; Ganopolsky, J.G.; Dieni, C.A.; Dan, A.F.; Jones, M.L.; Prakash, S. Prevention and Treatment of Virulent Bacterial Biofilms with an Enzymatic Nitric Oxide-Releasing Dressing. Antimicrob. Agents Chemother. 2012, 56, 6095–6103. Available online: https://pmc/articles/PMC3497171/?report=abstract (accessed on 27 February 2021). [CrossRef]
- López-Jaramillo, P.; Rincón, M.Y.; García, R.G.; Silva, S.Y.; Smith, E.; Kampeerapappun, P.; García, C.; Smith, D.J.; López, M.; Vélez, I.D. A controlled, randomized-blinded clinical trial to assess the efficacy of a nitric oxide releasing patch in the treatment of cutaneous leishmaniasis by Leishmania (V.) panamensis. Am. J. Trop. Med. Hyg. 2010, 83, 97–101. Available online: https://www.ajtmh.org/view/journals/tpmd/83/1/article-p97.xml (accessed on 27 February 2021). [CrossRef] [PubMed]
- Jones, M.L.; Ganopolsky, J.G.; Labbé, A.; Prakash, S. A novel nitric oxide producing probiotic patch and its antimicrobial efficacy: Preparation and in vitro analysis. Appl. Microbiol. Biotechnol. 2010, 87, 509–516. Available online: https://link.springer.com/article/10.1007/s00253-010-2490-x (accessed on 27 February 2021). [CrossRef] [PubMed]
- Han, G.; Martinez, L.R.; Mihu, M.R.; Friedman, A.J.; Friedman, J.M.; Nosanchuk, J.D. Nitric Oxide Releasing Nanoparticles Are Therapeutic for Staphylococcus aureus Abscesses in a Murine Model of Infection. PLoS ONE 2009, 4, e7804. Available online: https://dx.plos.org/10.1371/journal.pone.0007804 (accessed on 27 February 2021). [CrossRef] [PubMed]
- Friedman, A.J.; Han, G.; Navati, M.S.; Chacko, M.; Gunther, L.; Alfieri, A.; Friedman, J.M. Sustained release nitric oxide releas-ing nanoparticles: Characterization of a novel delivery platform based on nitrite containing hydrogel/glass composites. Nitric Oxide 2008, 19, 12–20. [Google Scholar] [CrossRef]
- Mir, M.; Ali, M.N.; Barakullah, A.; Gulzar, A.; Arshad, M.; Fatima, S.; Asad, M. Synthetic polymeric biomaterials for wound healing: A review. Prog. Biomater. 2018, 7, 1–21. Available online: https://doi.org/10.1007/s40204-018-0083-4 (accessed on 27 February 2021). [CrossRef] [PubMed]
- Coelho, J.F.; Ferreira, P.C.; Alves, P.; Cordeiro, R.; Fonseca, A.C.; Góis, J.R.; Gil, M.H. Drug delivery systems: Advanced technologies potentially applicable in personalized treatments. EPMA J. 2010, 1, 164–209. Available online: https://pmc/articles/PMC3405312/?report=abstract (accessed on 27 February 2021). [CrossRef]
- Bhatia, S. Natural Polymers vs Synthetic Polymer. In Natural Polymer Drug Delivery Systems; Springer Science and Business Media LLC: Cham, Switzerland, 2016; pp. 95–118. Available online: https://link.springer.com/chapter/10.1007/978-3-319-41129-3_3 (accessed on 27 February 2021).
- Banerjee, N.S.; Moore, D.W.; Wang, H.-K.; Broker, T.R.; Chow, L.T. NVN1000, a novel nitric oxide-releasing compound, inhibits HPV-18 virus production by interfering with E6 and E7 oncoprotein functions. Antivir. Res. 2019, 170, 104559. [Google Scholar] [CrossRef] [PubMed]
- Maeda-Chubachi, T.; Messersmith, E.; Hebert, D.; de Leon, E.; Reams, T. LB1096 Results of phase 2 study evaluating the efficacy and safety of SB206, topical berdazimer sodium gel, in subjects with Molluscum Contagiosum. J. Investig. Dermatol. 2019, 139, B14. [Google Scholar] [CrossRef]
- Abedi-Gaballu, F.; Dehghan, G.; Ghaffari, M.; Yekta, R.; Abbaspour-Ravasjani, S.; Baradaran, B.; Dolatabadie, J.E.N.; Hamblinfgh, M.R. PAMAM dendrimers as efficient drug and gene delivery nanosystems for cancer therapy. Appl. Mater. Today 2018, 12, 177–190. Available online: https://pmc/articles/PMC6269116/?report=abstract (accessed on 27 February 2021). [CrossRef] [PubMed]
- Jenkins, A.D.; Loening, K.L. Nomenclature. In Comprehensive Polymer Science and Supplements; Allen, G., Bevington, J.C., Eds.; Elsevier: Amsterdam, The Netherlands, 1989; pp. 13–54. Available online: https://linkinghub.elsevier.com/retrieve/pii/B9780080967011000021 (accessed on 27 February 2021).
- Witte, M.B.; Barbul, A. Role of nitric oxide in wound repair. Am. J. Surg. 2002, 183, 406–412. [Google Scholar] [CrossRef]
- Lee, J.; Kwak, D.; Kim, H.; Kim, J.; Hlaing, S.P.; Hasan, N.; Cao, J.; Yoo, J.-W. Nitric Oxide-Releasing S-Nitrosoglutathione-Conjugated Poly (Lactic-Co-Glycolic Acid) Nanoparticles for the Treatment of MRSA-Infected Cutaneous Wounds. Pharmaceutics 2020, 12, 618. Available online: https://www.mdpi.com/1999-4923/12/7/618 (accessed on 27 February 2021). [CrossRef]
- Nie, X.; Zhang, H.; Shi, X.; Zhao, J.; Chen, Y.; Wu, F.; Yang, J.; Li, X. Asiaticoside nitric oxide gel accelerates diabetic cutaneous ulcers healing by activating Wnt/β-catenin signaling pathway. Int. Immunopharmacol. 2020, 79, 106109. [Google Scholar] [CrossRef]
- Lee, J.; Hlaing, S.P.; Cao, J.; Hasan, N.; Yoo, J.-W. In vitro and in vivo evaluation of a novel nitric oxide-releasing ointment for the treatment of methicillin-resistant Staphylococcus aureus-infected wounds. J. Pharm. Investig. 2020, 50, 505–512. Available online: https://doi.org/10.1007/s40005-020-00472-1 (accessed on 27 February 2021). [CrossRef]
- Wan, X.; Liu, S.; Xin, X.; Li, P.; Dou, J.; Han, X.; Kang, I.-K.; Yuan, J.; Chi, B.; Shen, J. S-nitrosated keratin composite mats with NO release capacity for wound healing. Chem. Eng. J. 2020, 400, 125964. [Google Scholar] [CrossRef]
- Huang, S.; Liu, H.; Liao, K.; Hu, Q.; Guo, R.; Deng, K. Functionalized GO Nanovehicles with Nitric Oxide Release and Photothermal Activity-Based Hydrogels for Bacteria-Infected Wound Healing. ACS Appl. Mater. Interfaces 2020. Available online: https://pubs.acs.org/doi/full/10.1021/acsami.0c04080 (accessed on 27 February 2021). [CrossRef]
- Póvoa, V.C.; dos Santos, G.J.; Picheth, G.F.; Jara, C.P.; da Silva, L.C.; de Araújo, E.P.; de Oliveira, M.G. Wound healing action of nitric oxide-releasing self-expandable collagen sponge. J. Tissue Eng. Regen. Med. 2020, 14, 807–818. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/term.3046 (accessed on 27 February 2021). [CrossRef]
- Zhang, Y.; Tang, K.; Chen, B.; Zhou, S.; Li, N.; Liu, C.; Yang, J.; Lin, R.; Zhang, T.; He, W. A polyethylenimine-based diazeniumdiolate nitric oxide donor accelerates wound healing. Biomater. Sci. 2019, 7, 1607–1616. Available online: https://pubmed.ncbi.nlm.nih.gov/30702089/ (accessed on 27 February 2021). [CrossRef] [PubMed]
- Kulshrestha, S.; Chawla, R.; Alam, T.; Adhikari, J.; Basu, M. Efficacy and dermal toxicity analysis of Sildenafil citrate based topical hydrogel formulation against traumatic wounds. Biomed. Pharmacother. 2019, 112, 108571. [Google Scholar] [CrossRef] [PubMed]
- Hasan, N.; Cao, J.; Lee, J.; Naeem, M.; Hlaing, S.P.; Kim, J.; Jung, Y.; Lee, B.-L.; Yoo, J.-W. PEI/NONOates-doped PLGA nanoparticles for eradicating methicillin-resistant Staphylococcus aureus biofilm in diabetic wounds via binding to the biofilm matrix. Mater. Sci. Eng. C 2019, 103, 109741. [Google Scholar] [CrossRef] [PubMed]
- Hlaing, S.P.; Kim, J.; Lee, J.; Hasan, N.; Cao, J.; Naeem, M.; Lee, E.H.; Shin, J.H.; Jung, Y.; Lee, B.-L.; et al. S-Nitrosoglutathione loaded poly(lactic-co-glycolic acid) microparticles for prolonged nitric oxide release and enhanced healing of methicillin-resistant Staphy-lococcus aureus-infected wounds. Eur. J. Pharm. Biopharm. 2018, 132, 94–102. [Google Scholar] [CrossRef]
- Baldwin, H.; Blanco, D.; McKeever, C.; Paz, N.; Vasquez, Y.N.; Quiring, J.; Enloe, C.; de León, E.; Stasko, N. Results of a phase 2 efficacy and safety study with SB204, an investigational topical nitric oxide-releasing drug for the treatment of Acne vulgaris. J. Clin. Aesthetic Dermatol. 2016, 9, 12. Available online: https://pmc/articles/PMC5022991/?report=abstract (accessed on 27 February 2021).
- Schanuel, F.S.; Santos, K.S.R.; Monte-Alto-Costa, A.; de Oliveira, M.G. Combined nitric oxide-releasing poly (vinyl alcohol) film/F127 hydrogel for accelerating wound healing. Colloids Surf. B Biointerfaces 2015, 130, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Lowe, A.; Bills, J.; Verma, R.; Lavery, L.; Davis, K.; Balkus, K.J. Electrospun nitric oxide releasing bandage with enhanced wound healing. Acta Biomater. 2015, 13, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.O.; Noh, J.K.; Thapa, R.K.; Hasan, N.; Choi, M.; Kim, J.H.; Lee, J.-H.; Ku, S.K.; Yoo, J.-W. Nitric oxide-releasing chitosan film for enhanced antibacterial and in vivo wound-healing efficacy. Int. J. Biol. Macromol. 2015, 79, 217–225. [Google Scholar] [CrossRef]
- Gao, J.; Zheng, W.; Zhang, J.; Guan, D.; Yang, Z.; Kong, D.; Zhao, Q. Enzyme-controllable delivery of nitric oxide from a molecular hydrogel. Chem. Commun. 2013, 49, 9173–9175. Available online: https://pubmed.ncbi.nlm.nih.gov/23989671/ (accessed on 27 February 2021). [CrossRef] [PubMed]
- Jones, M.; Ganopolsky, J.G.; Labbé, A.; Gilardino, M.; Wahl, C.; Martoni, C.; Prakash, S. Novel nitric oxide producing probiotic wound healing patch: Preparation and in vivo analysis in a New Zealand white rabbit model of ischaemic and infected wounds. Int. Wound, J. 2012, 9, 330–343. Available online: http://doi.wiley.com/10.1111/j.1742-481X.2011.00889.x (accessed on 27 February 2021). [CrossRef]
- Georgii, J.L.; Amadeu, T.P.; Seabra, A.B.; de Oliveira, M.G.; Monte-Alto-Costa, A. Topical S-nitrosoglutathione-releasing hydrogel improves healing of rat ischaemic wounds. J. Tissue Eng. Regen. Med. 2010, 5, 612–619. Available online: http://doi.wiley.com/10.1002/term.353 (accessed on 27 February 2021). [CrossRef]
- Martinez, L.R.; Han, G.; Chacko, M.; Mihu, M.R.; Jacobson, M.; Gialanella, P.; Friedman, A.J.; Nosanchuk, J.D.; Friedman, J.M. Antimicrobial and healing efficacy of sustained release nitric oxide nanoparticles against Staphylococcus aureus skin infection. J. Investig. Dermatol. 2009, 129, 2463–2469. Available online: www.jidonline.org (accessed on 27 February 2021). [CrossRef]
- Amadeu, T.P.; Seabra, A.B.; de Oliveira, M.G.; Monte-Alto-Costa, A. Nitric Oxide Donor Improves Healing if Applied on Inflammatory and Proliferative Phase. J. Surg. Res. 2008, 149, 84–93. Available online: http://www.journalofsurgicalresearch.com/article/S0022480407006373/fulltext (accessed on 27 February 2021). [CrossRef]
- Amadeu, T.P.; Seabra, A.B.; de Oliveira, M.G.; Monte-Alto-Costa, A. S-nitrosoglutathione-containing hydrogel accelerates rat cutaneous wound repair. J. Eur. Acad. Dermatol. Venereol. 2007, 21, 629–637. Available online: http://doi.wiley.com/10.1111/j.1468-3083.2006.02032.x (accessed on 27 February 2021). [CrossRef] [PubMed]
- Masters, K.S.B.; Leibovich, S.J.; Belem, P.; West, J.L.; Poole-Warren, L.A. Effects of nitric oxide releasing poly (vinyl alcohol) hydrogel dressings on dermal wound healing in diabetic mice. Wound Repair Regen. 2002, 10, 286–294. Available online: https://onlinelibrary.wiley.com/doi/full/10.1046/j.1524-475X.2002.10503.x (accessed on 27 February 2021). [CrossRef]
- Arora, N.; Maurya, P.K.; Kacker, P. Translational Research in Drug Discovery and Development. Transl. Med. Res. 2017, 55–87. Available online: https://link.springer.com/chapter/10.1007/978-94-024-1045-7_3 (accessed on 27 February 2021).
- Souto, S.; Palma, P.; Riccetto, C.; Seabra, A.; Oliveira, M.; Palma, T.; Capmartin, R. Impact of topic administration of nitric oxide donor gel in the clitoridian blood flow, assessed by Doppler ultra-sound. Actas Urológicas Españolas 2010, 34, 708–712. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Soares, R.N.; Proctor, D.N.; de Oliveira, G.V.; Alvares, T.S.; Murias, J.M. Acute application of a transdermal nitroglycerin patch protects against prolonged forearm ischemia-induced microvascular dysfunction. Microcirculation 2019, 27, e12599. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1111/micc.12599 (accessed on 27 February 2021). [CrossRef]
- Pastore, M.N.; Kalia, Y.N.; Horstmann, M.; Roberts, M.S. Transdermal patches: History, development and pharmacology. Br. J. Pharmacol. 2015, 172, 2179–2209. Available online: https://pmc/articles/PMC4403087/?report=abstract (accessed on 27 February 2021). [CrossRef] [PubMed]
- Kirkland, Q.C. Product Monograph Including Patient Medication Information; Mylan Pharmaceuticals ULC: Etobicoke, ON, USA, 2016. [Google Scholar]
- Appleton, J.P.; Krishnan, K.; Bath, P.M. Transdermal delivery of glyceryl trinitrate: Clinical applications in acute stroke. Expert Opin. Drug Deliv. 2020, 17, 297–303. Available online: https://www.tandfonline.com/doi/abs/10.1080/17425247.2020.1716727 (accessed on 27 February 2021). [CrossRef]
- Giglio, L.P.; Picheth, G.F.; Løvschall, K.B.; Zelikin, A.N.; de Oliveira, M.G. S-nitrosothiol-terminated poly (vinyl alcohol): Nitric oxide release and skin blood flow response. Nitric Oxide 2020, 98, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Dawson, J.I.; Oreffo, R.O.C.; Kim, Y.-H.; Hong, J. Nanoclay–Polyamine Composite Hydrogel for Topical Delivery of Nitric Oxide Gas via Innate Gelation Characteristics of Laponite. Biomacromolecules 2020, 21, 2096–2103. Available online: https://dx.doi.org/10.1021/acs.biomac.0c00086 (accessed on 27 February 2021). [CrossRef] [PubMed]
- Gori, T. Exogenous no therapy for the treatment and prevention of atherosclerosis. Int. J. Mol. Sci. 2020, 21, 2703. Available online: www.mdpi.com/journal/ijms (accessed on 27 February 2021). [CrossRef] [PubMed]
| NO-donor. | Representative Examples | Trigger for NO• Generation | ||
|---|---|---|---|---|
| Structure | Name | Non-Enzymatic | Enzymatic | |
| Organic nitrate |  | GTN [33] | Thiols | Cyt-P450, GST, etc. |
| Organic nitrite |  | Tert-pentyl nitrite [33] | Hydrolysis, trans-nitrosation, thiols, light, heat | Xanthine oxidase |
| Metal–NO complex | 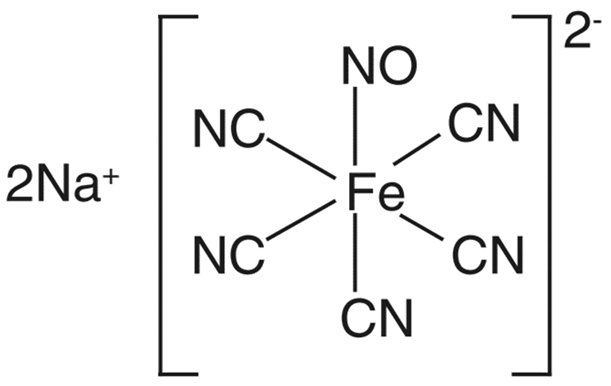 | Sodium nitroprusside [38] | Light, thiols, reductants, nucleophiles | Membrane-bound enzyme |
| Nitrosothiol | 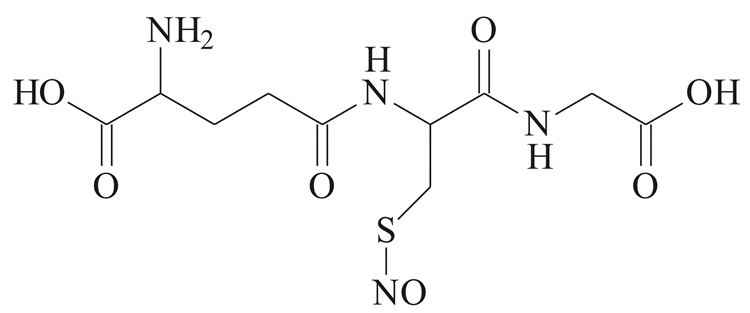 | GSNO [39] | Spontaneous, enhanced by thiols, light, metal ions | Unknown enzymes |
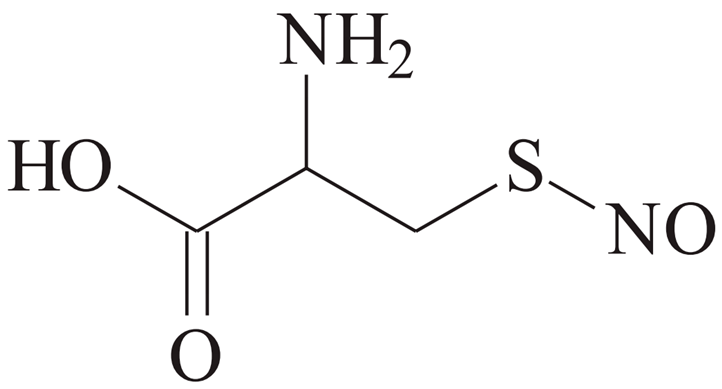 | CySNO [39] | |||
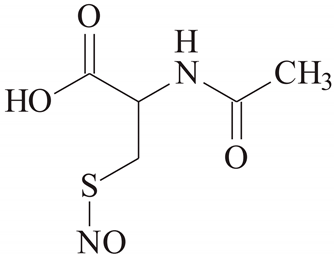 | SNAC [39] | |||
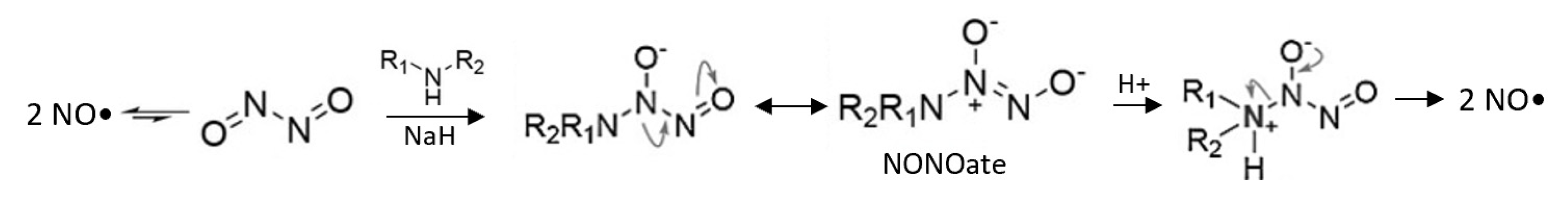
| NONOate | 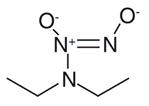 | DEA/NO [40] | Spontaneous, engineered to be light or pH-responsive | Caged for control by β-galactosidase |
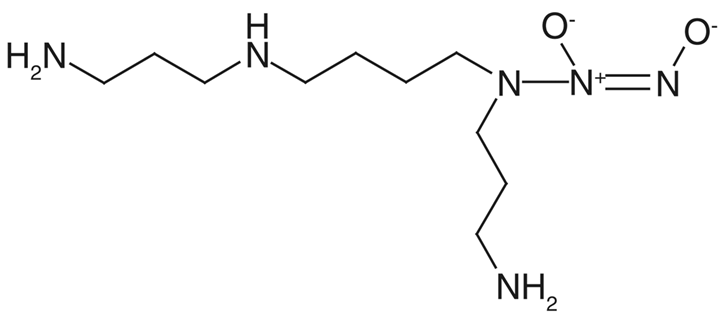 | SPER/NO [40] | |||
| Year | Origin | Carrier | Matrix | NO-Donor | Donor Coupling | Synergistic Additive | Release Trigger | Antimicrobial Test | Clinical Trial | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| 2020 | N | Alg | np | Nitrosothiol | - | Silver np | - | Bacterial | - | [70] |
| 2020 | N | Alginate | np | Nitrosothiol | - | Silver np | - | Bacterial | - | [71] |
| 2020 | N | Cellulose | Hydrogel | NONOate | - | - | - | Fungal | Yes | [72] |
| 2019 | S | PEO | Micelles | N-nitrosamine | Yes | - | Light | Bacterial | - | [63] |
| 2018 | S | PEO | Hydrogel | NO• gas | Yes | - | - | Bacterial | - | [64] |
| 2018 | S | PEG/Disaccharide | np | Inorganic nitrite | - | Antifungal | - | Fungal | - | [73] |
| 2018 | SS | PEO/Cellulose | np | NONOate | - | - | - | Fungal | Yes | [74] |
| 2018 | SS | Chitosan/PL | np | Nitrosothiol | - | - | - | - | [2] | |
| 2018 | SS | Chitosan/PL | Hydrogel | Nitrosothiol | - | - | Bacterial | - | [75] | |
| 2017 | S | PEG/Disaccharide | Hydrogel | Inorganic nitrite | - | - | - | Fungal | - | [76] |
| 2017 | S | POEGMA | np | NONOate | Yes | - | Bacterial | - | [62] | |
| 2016 | S | PU/PAMAM | Dendrimers | NONOate | Yes | - | - | Bacterial | - | [52] |
| 2016 | S | POEGMA | np | NONOate | - | Antibiotic | - | Bacterial | - | [61] |
| 2014 | N | Alginate/Chitosan | Capsules | Nitrosothiol | - | - | - | Bacterial | - | [60] |
| 2013 | N | Alginate/Chitosan | np | Nitrosothiol | - | - | - | - | - | [6] |
| 2012 | S | PEO | Chamber | NO• gas | - | - | Enzyme | Bacterial | - | [77] |
| 2011 | N | Alginate/Chitosan | np | Nitrosothiol | - | - | - | - | - | [59] |
| 2010 | S | PU | Hydrogel | Organic nitrite | - | - | - | Parasitic | Yes | [78] |
| 2010 | S | Acrylic polymer | Chamber | Inorganic nitrite | - | - | Bacteria | Bacterial, fungal | - | [79] |
| 2009 | S | PEG/Disaccharide | Hydrogel | Inorganic nitrite | - | - | - | Bacterial | - | [80] |
| 2009 | S | PU | Hydrogel | Metal-NO complex | - | H2O2, methyl-ene blue | Light | Bacterial | - | [58] |
| 2008 | S | PEG/Disaccharide | Hydrogel | Inorganic nitrite | - | - | - | - | - | [81] |
| 2004 | S | PEO | Hydrogel | Nitrosothiol | - | - | - | - | Yes | [56] |
| 2004 | S | PVA/PVP | Hydrogel | Nitrosothiol | - | - | - | - | - | [55] |
| 2003 | S | PL | Hydrogel | Nitrosothiol | - | - | Light | - | - | [57] |
| Year | Origin | Carrier | Matrix | NO-Donor | Coupled Donor | Synergistic Additive | Trigger for Release | Antibacterial Test | Trial | Extra Target Condition | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2020 | S | PLGA | np | Nitrosothiol | Yes | - | - | Yes | in vivo | Infection | [90] |
| 2020 | N | Cellulose | Hydrogel | NO• gas | - | Plant extract | - | Yes | in vivo | Diabetic skin ulcer | [91] |
| 2020 | S | PEG | Hydrogel | Nitrosothiol | Yes | - | - | Yes | in vivo, in vitro | Infection | [92] |
| 2020 | SS | PU, gelatin, keratin | Hydrogel | Nitrosothiol | Yes | - | - | Yes | in vivo, in vitro | - | [93] |
| 2020 | N | GelMA | Hydrogel | N-nitrosamine | Yes | Hyaluronic acid | Photons | Yes | in vivo, in vitro | Infection | [94] |
| 2020 | N | Collagen | Sponge | Nitrosothiol | - | - | - | - | in vivo | - | [95] |
| 2019 | S | PEI | Hydrogel | NONOate | Yes | - | - | - | in vivo | - | [96] |
| 2019 | S | PAA | Hydrogel | Sildenafil citrate | - | - | - | - | in vivo, in vitro | - | [97] |
| 2019 | S | PEI, PLGA | np | NONOate | Yes | - | - | Yes | in vivo, ex vivo, in vitro | Diabetic infection | [98] |
| 2018 | S | PLGA | Emulsion | Nitrosothiol | - | - | - | Yes | in vivo, in vitro | Infection | [99] |
| 2017 | SS | PCL/chitosan | Hydrogel | NONOate | Yes | - | Enzyme | - | in vivo | Ischemia | [35] |
| 2016 | S | Poly-siloxane | Hydrogel | NONOate NONOate | Yes | - | - | Yes | Clinical | Acne lesion | [100] |
| 2015 | S | PVA/PL | Hydrogel | Nitrosothiol | Yes | - | - | - | in vivo | - | [101] |
| 2015 | S | PAN | Hydrogel | Acrylonitrile | Yes | - | - | - | in vivo | - | [102] |
| 2015 | N | Chitosan | Hydrogel | Nitrosothiol | - | - | - | Yes | in vivo, in vitro | - | [103] |
| 2013 | N | Nap-FFGGG | Hydrogel | NONOate | Yes | - | Enzyme | - | in vivo | - | [104] |
| 2012 | SS | Alginate/Acrylic polymer | Chamber | Inorganic nitrite | - | Probiotics | - | Yes | in vivo, in vitro | Ischemic, infection | [105] |
| 2010 | S | PL | Hydrogel | Nitrosothiol | - | - | - | - | in vivo | Ischemia | [106] |
| 2009 | S | PEG/Disaccharide | Hydrogel | Inorganic nitrite | - | - | - | Yes | in vivo, in vitro | Infection | [107] |
| 2008 | S | PL | Hydrogel | Nitrosothiol | - | - | - | - | in vivo | - | [108] |
| 2007 | S | PL | Hydrogel | Nitrosothiol | - | - | - | - | in vivo | - | [109] |
| 2002 | S | PVA | Hydrogel | NO• gas | Yes | - | - | - | in vivo | Diabetes | [110] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutierrez Cisneros, C.; Bloemen, V.; Mignon, A. Synthetic, Natural, and Semisynthetic Polymer Carriers for Controlled Nitric Oxide Release in Dermal Applications: A Review. Polymers 2021, 13, 760. https://doi.org/10.3390/polym13050760
Gutierrez Cisneros C, Bloemen V, Mignon A. Synthetic, Natural, and Semisynthetic Polymer Carriers for Controlled Nitric Oxide Release in Dermal Applications: A Review. Polymers. 2021; 13(5):760. https://doi.org/10.3390/polym13050760
Chicago/Turabian StyleGutierrez Cisneros, Carolina, Veerle Bloemen, and Arn Mignon. 2021. "Synthetic, Natural, and Semisynthetic Polymer Carriers for Controlled Nitric Oxide Release in Dermal Applications: A Review" Polymers 13, no. 5: 760. https://doi.org/10.3390/polym13050760
APA StyleGutierrez Cisneros, C., Bloemen, V., & Mignon, A. (2021). Synthetic, Natural, and Semisynthetic Polymer Carriers for Controlled Nitric Oxide Release in Dermal Applications: A Review. Polymers, 13(5), 760. https://doi.org/10.3390/polym13050760








