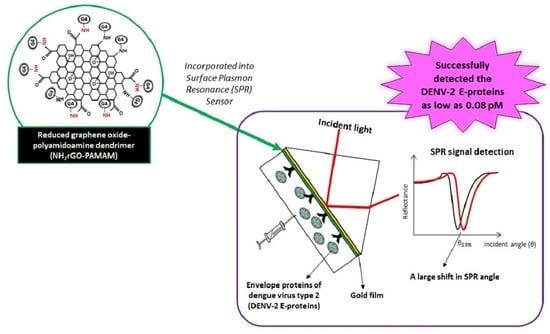
An Optical Sensor for Dengue Envelope Proteins Using Polyamidoamine Dendrimer Biopolymer-Based Nanocomposite Thin Film: Enhanced Sensitivity, Selectivity, and Recovery Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
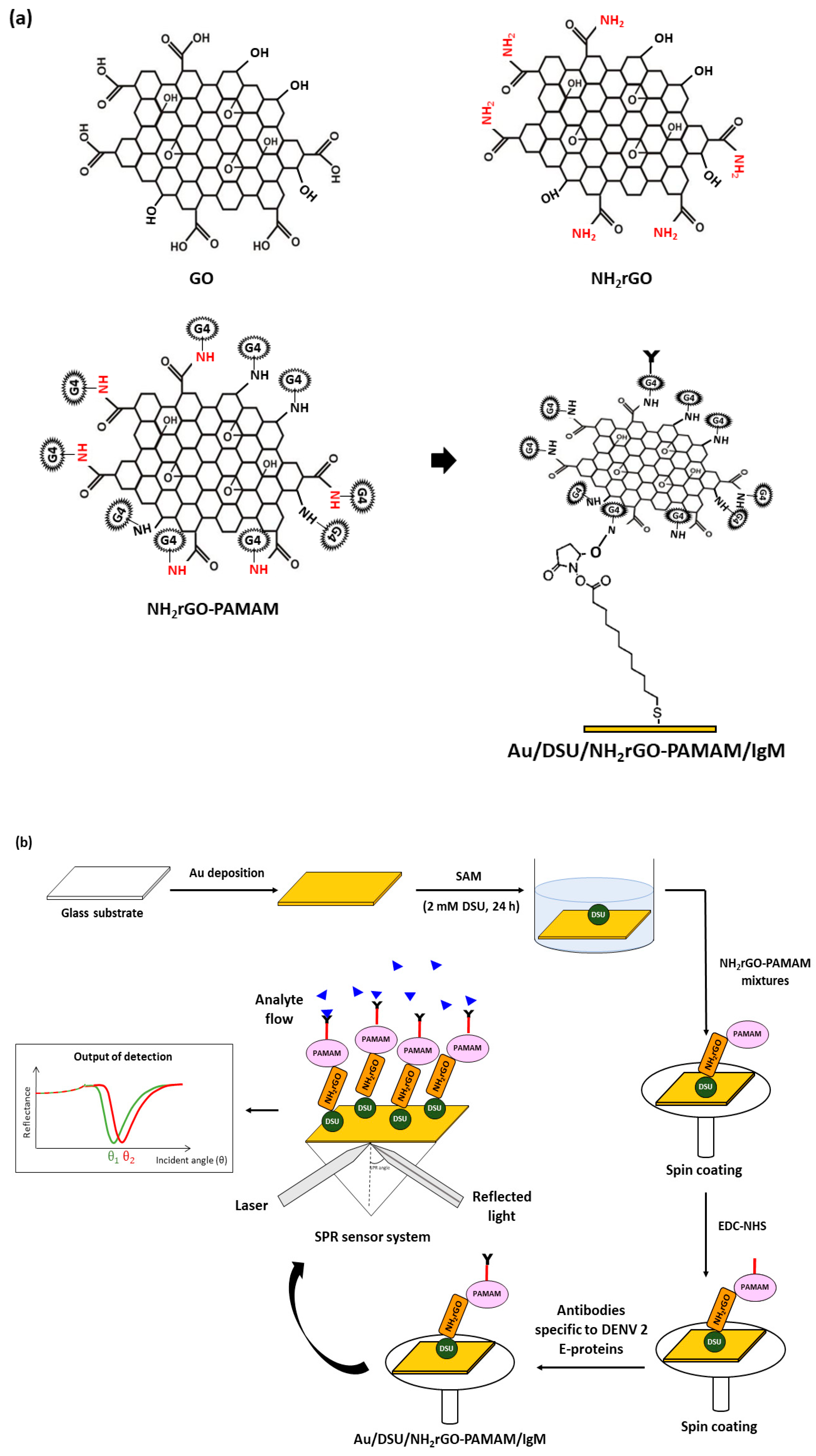
2.2. Preparation of NH2rGO–PAMAM-Based Nanocomposite Thin Film
2.3. Incorporation of Au/DSU/NH2rGO–PAMAM/IgM Sensor Film into SPR System
3. Results
3.1. FTIR Analysis of Au/DSU/NH2rGO–PAMAM/IgM Sensor Film
3.2. SPR Analysis of Au/DSU/NH2rGO–PAMAM/IgM Sensor Film towards DENV-2 E-Proteins
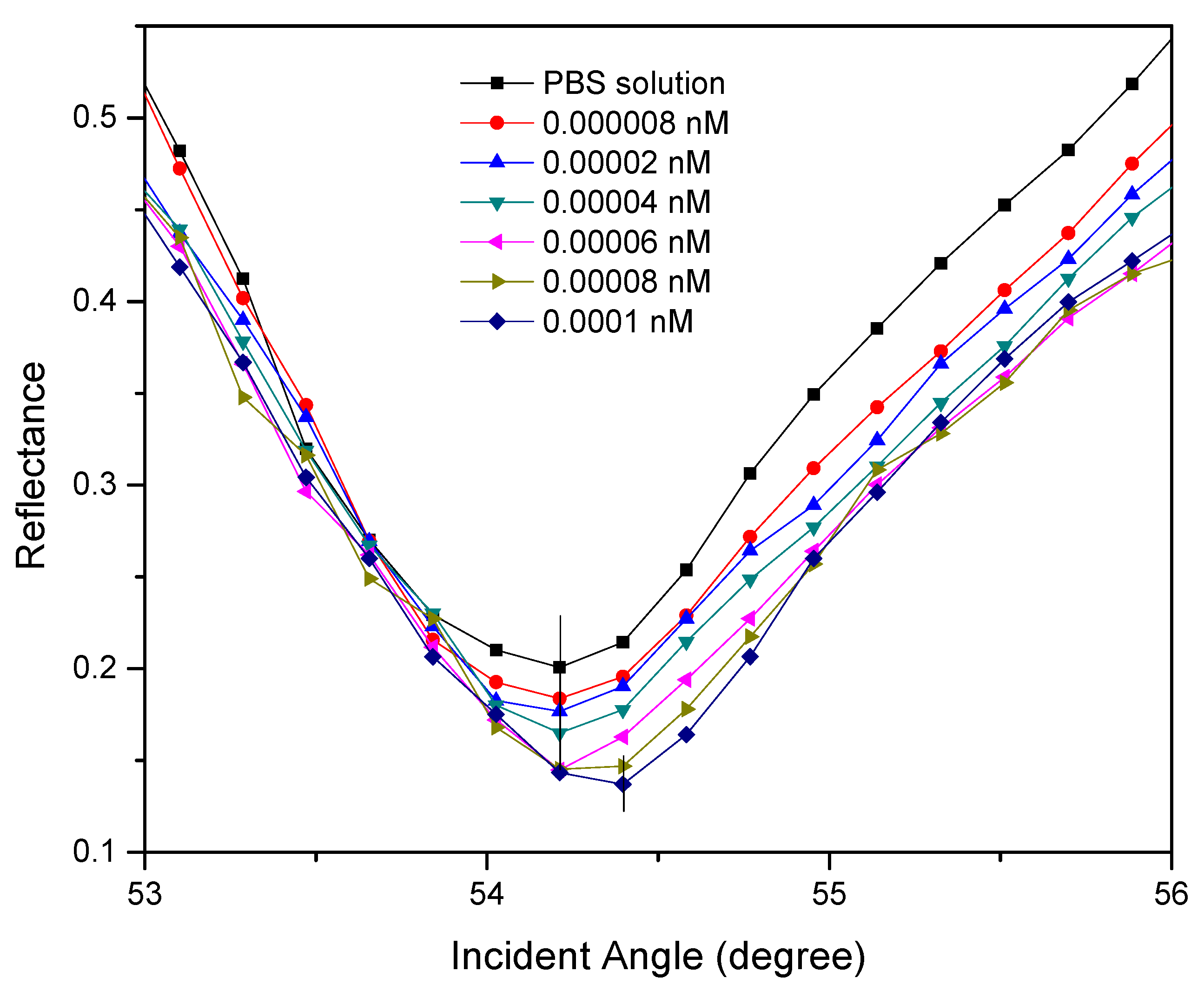
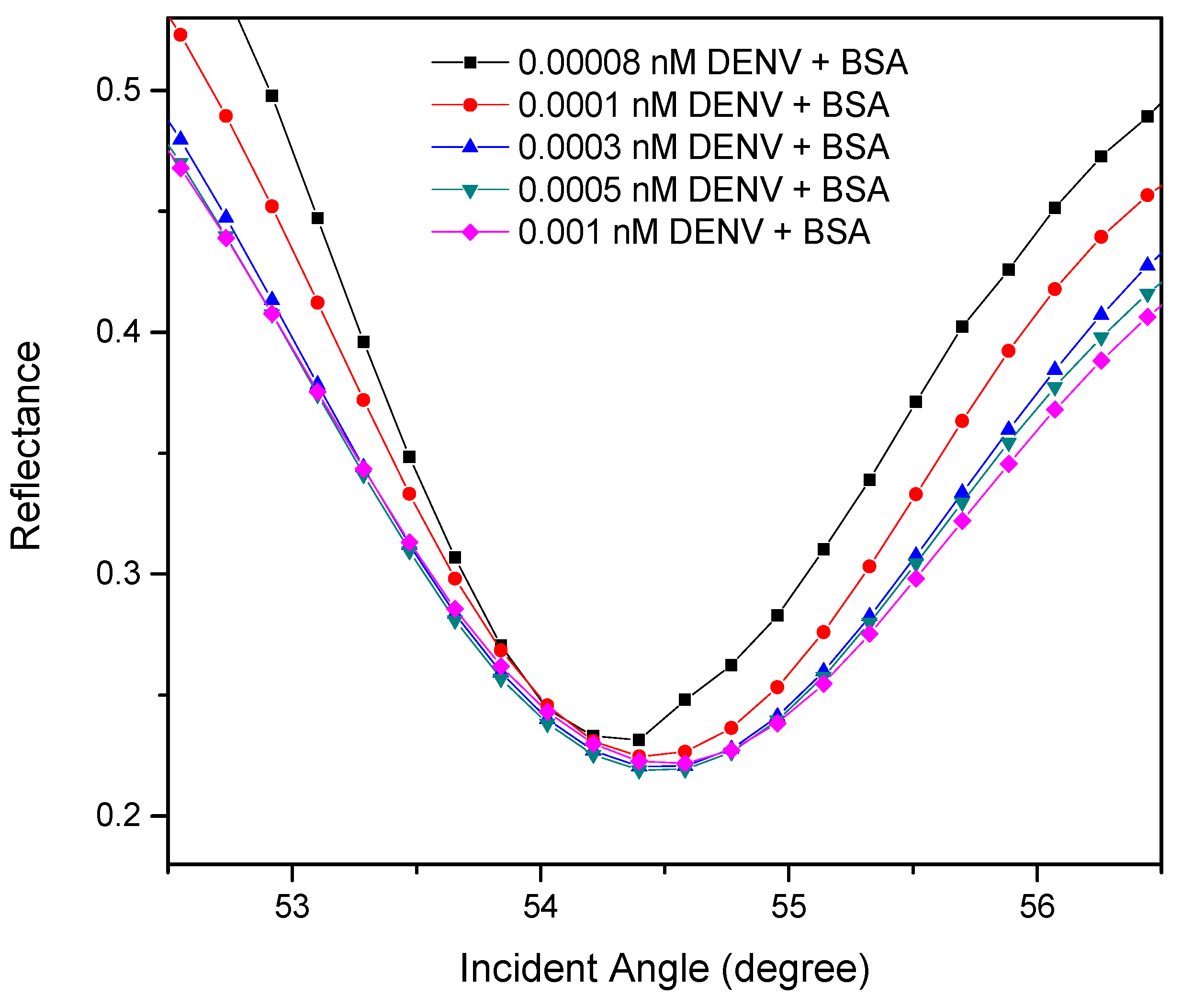
3.2.1. SPR Reflectivity
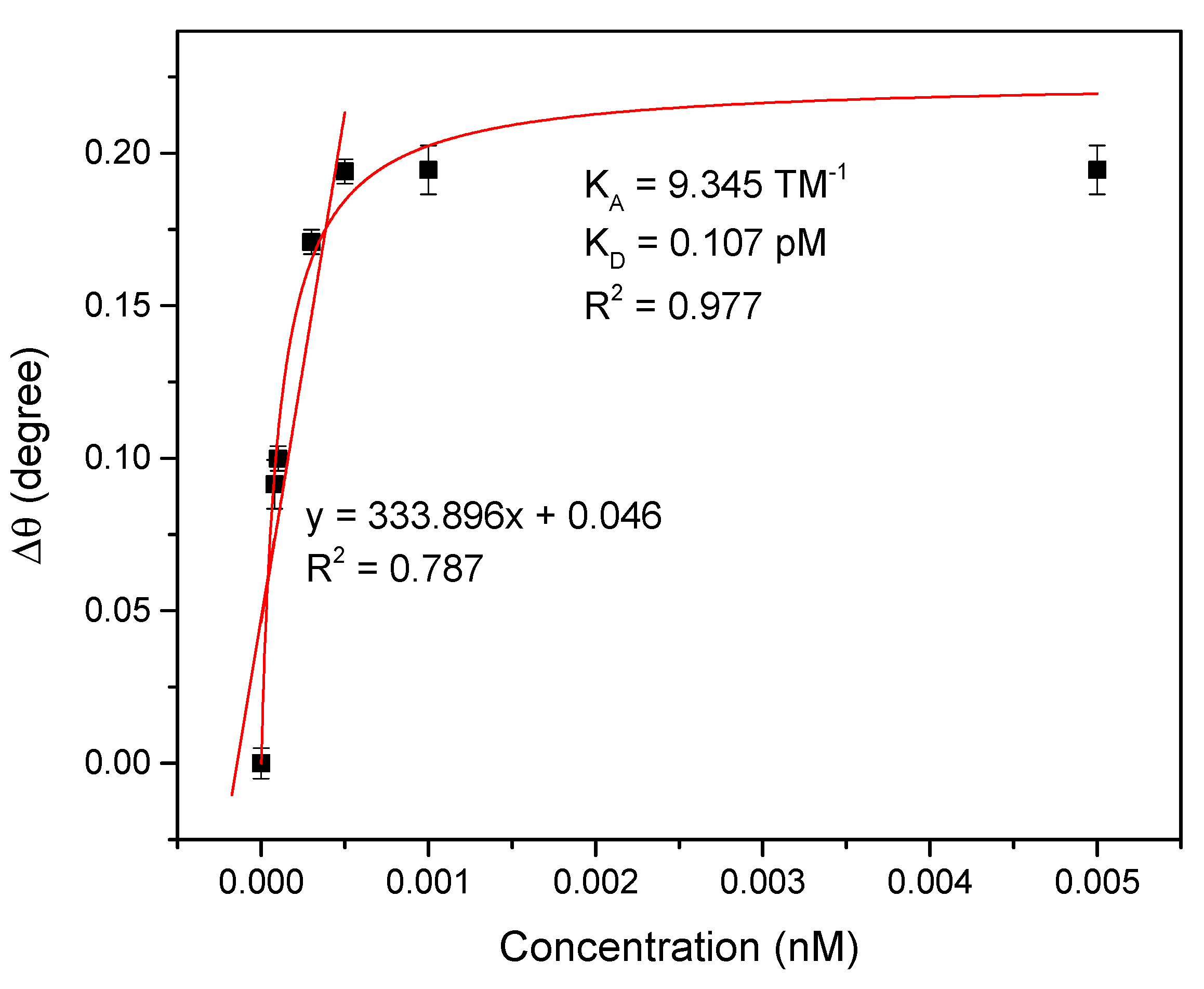
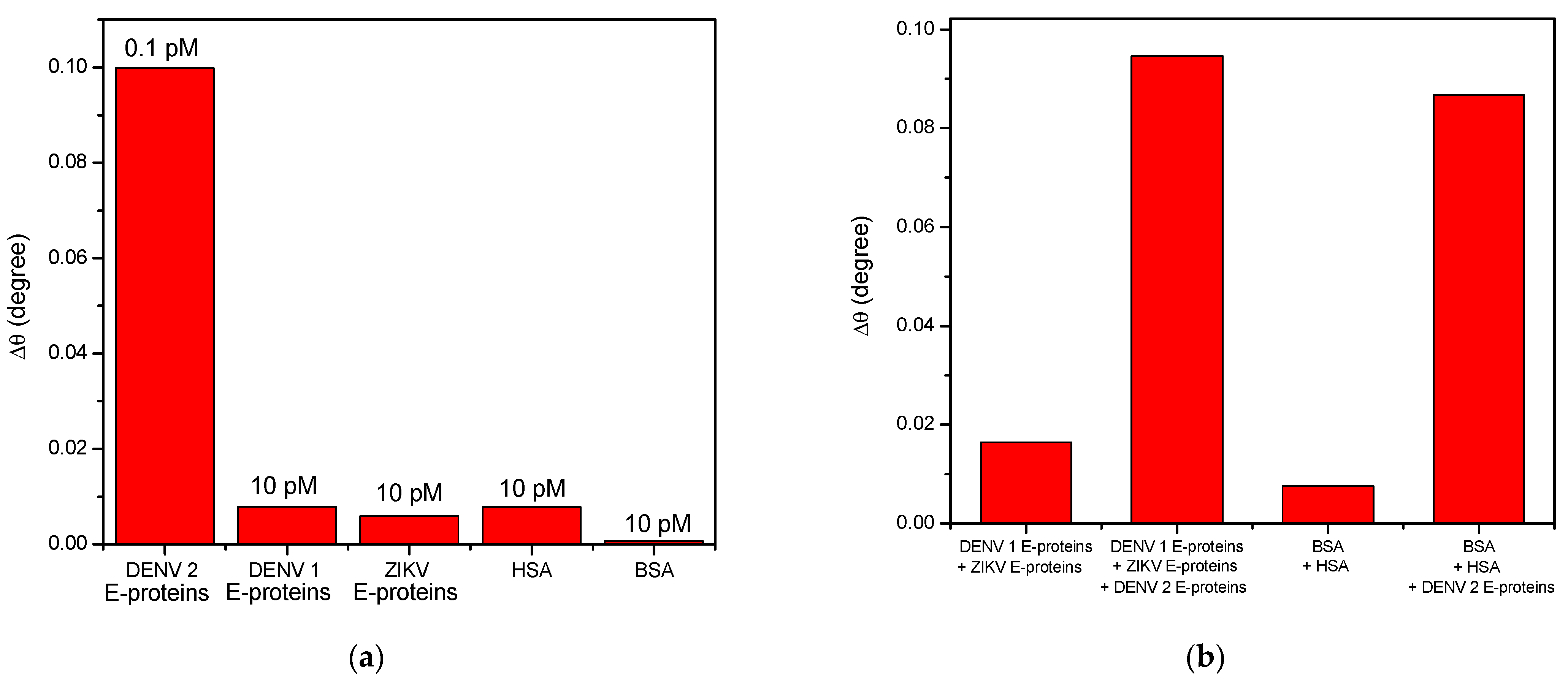
3.2.2. SPR Performances
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saleviter, S.; Fen, Y.W.; Daniyal, W.M.E.M.M.; Abdullah, J.; Sadrolhosseini, A.R.; Omar, N.A.S. Design and analysis of surface plasmon resonance optical sensor for determining cobalt ion based on chitosan-graphene oxide decorated quantum dots-modified gold active layer. Opt. Express 2019, 27, 32294–32307. [Google Scholar] [CrossRef] [PubMed]
- Ramdzan, N.S.M.; Fen, Y.W.; Anas, N.A.A.; Omar, N.A.S.; Saleviter, S. Development of biopolymer and conducting polymer-based optical sensors for heavy metal ion detection. Molecules 2020, 25, 2548. [Google Scholar] [CrossRef]
- Hashim, H.S.; Fen, Y.W.; Omar, N.A.S.; Daniyal, W.M.E.M.M.; Saleviter, S.; Abdullah, J. Structural, optical and potential sensing properties of tyrosinase immobilized graphene oxide thin film on gold surface. Optik 2020, 212, 1–11. [Google Scholar] [CrossRef]
- Daniyal, W.M.E.M.M.; Fen, Y.W.; Abdullah, J.; Sadrolhosseini, A.R.; Saleviter, S.; Omar, N.A.S. Label-free optical spectroscopy for characterizing binding properties of highly sensitive nanocrystalline cellulose-graphene oxide-based nanocomposite towards nickel ion. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2019, 212, 25–31. [Google Scholar] [CrossRef]
- Omar, N.A.S.; Fen, Y.W.; Saleviter, S.; Daniyal, W.M.E.M.M.; Anas, N.A.A.; Ramdzan, N.S.M.; Roshidi, M.D.A. Development of a graphene-based surface plasmon resonance optical sensor chip for potential biomedical application. Materials 2019, 12, 1928. [Google Scholar] [CrossRef]
- Teng, C.; Zheng, J.; Liang, Q.; Deng, S.; Deng, H.; Liu, H.; Yuan, L. The influence of structural parameters on the surface plasmon resonance sensor based on a side-polished macrobending plastic optical fiber. IEEE Sens. J. 2020, 20, 4245–4250. [Google Scholar] [CrossRef]
- Solis-Tinoco, V.; Acevedo-Barrera, A.; Vazquez-Estrada, O.; Munguia-Cervantes, J.; Hernandez-Como, N.; Olguin, L.F.; Garcia-Valenzuela, A. Fast and accurate optical determination of gold-nanofilms thickness. Opt. Laser Technol. 2021, 134, 1–11. [Google Scholar] [CrossRef]
- Omar, N.A.S.; Fen, Y.W.; Abdullah, J.; Kamil, Y.M.; Daniyal, W.M.E.M.M.; Sadrolhosseini, A.R.; Mahdi, M.A. Sensitive detection of dengue virus type 2 E-proteins signals using self-assembled monolayers/reduced graphene oxide-PAMAM dendrimer thin film-SPR optical sensor. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Daniyal, W.M.E.M.M.; Fen, Y.W.; Fauzi, N.I.M.; Hashim, H.S.; Ramdzan, N.S.M.; Omar, N.A.S. Recent advances in surface plasmon resonance optical sensors for potential application in environmental monitoring. Sens. Mater. 2020, 32, 4191–4200. [Google Scholar]
- Anas, N.A.A.; Fen, Y.W.; Yusof, N.A.; Omar, N.A.S.; Daniyal, W.M.E.M.M.; Ramdzan, N.S.M. Highly sensitive surface plasmon resonance optical detection of ferric ion using CTAB/hydroxylated graphene quantum dots thin film. J. Appl. Phys. 2020, 128, 1–11. [Google Scholar] [CrossRef]
- Fauzi, N.I.M.; Fen, Y.W.; Omar, N.A.S.; Saleviter, S.; Daniyal, W.M.E.M.M.; Hashim, H.S.; Nasrullah, M. Nanostructured chitosan/maghemite composites thin film for potential optical detection of mercury ion by surface plasmon resonance investigation. Polymers 2020, 12, 1497. [Google Scholar] [CrossRef] [PubMed]
- Ramdzan, N.S.M.; Fen, Y.W.; Omar, N.A.S.; Anas, N.A.A.; Daniyal, W.M.E.M.M.; Saleviter, S.; Zainudin, A.A. Optical and surface plasmon resonance sensing properties for chitosan/carboxyl-functionalized graphene quantum dots thin film. Optik 2019, 178, 802–812. [Google Scholar] [CrossRef]
- Fen, Y.W.; Yunus, W.M.M. Utilization of chitosan-based sensor thin films for the detection of lead ion by surface plasmon resonance optical sensor. IEEE Sens. J. 2012, 13, 1413–1418. [Google Scholar] [CrossRef]
- Fen, Y.W.; Yunus, W.M.M.; Yusof, N.A. Optical properties of crosslinked chitosan thin film as copper ion detection using surface plasmon resonance technique. Opt. Appl. 2011, 41, 999–1013. [Google Scholar]
- Fen, Y.W.; Yunus, W.M.M.; Moksin, M.M.; Talib, Z.A.; Yusof, N.A. Surface plasmon resonance optical sensor for mercury ion detection by crosslinked chitosan thin film. J. Optoelectron. Adv. Mater. 2011, 13, 279–285. [Google Scholar]
- Fen, Y.W.; Yunus, W.M.M.; Talib, Z.A. Analysis of Pb(II) ion sensing by crosslinked chitosan thin film using surface plasmon resonance spectroscopy. Optik 2013, 124, 126–133. [Google Scholar] [CrossRef]
- Zainudin, A.A.; Fen, Y.W.; Yusof, N.A.; Omar, N.A.S. Structural, optical and sensing properties of ionophore doped graphene based bionanocomposite thin film. Optik 2017, 144, 308–315. [Google Scholar] [CrossRef]
- Daniyal, W.M.E.M.M.; Saleviter, S.; Fen, Y.W. Development of surface plasmon resonance spectroscopy for metal ion detection. Sens. Mater. 2018, 30, 2023–2038. [Google Scholar] [CrossRef]
- Daniyal, W.M.E.M.M.; Fen, Y.W.; Abdullah, J.; Sadrolhosseini, A.R.; Saleviter, S.; Omar, N.A.S. Exploration of surface plasmon resonance for sensing copper ion based on nanocrystalline cellulose-modified thin film. Opt. Express 2018, 26, 34880–34893. [Google Scholar] [CrossRef]
- Daniyal, W.M.E.M.M.; Fen, Y.W.; Anas, N.A.A.; Omar, N.A.S.; Ramdzan, N.S.M.; Nakajima, H.; Mahdi, M.A. Enhancing the sensitivity of a surface plasmon resonance-based optical sensor for zinc ion detection by the modification of a gold thin film. RSC Adv. 2019, 9, 41729–41736. [Google Scholar] [CrossRef]
- Fen, Y.W.; Yunus, W.M.M.; Talib, Z.A.; Yusof, N.A. Development of surface plasmon resonance sensor for determining zinc ion using novel active nanolayers as probe. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 134, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Anas, N.A.A.; Fen, Y.W.; Omar, N.A.S.; Daniyal, W.M.E.M.M.; Ramdzan, N.S.M.; Saleviter, S. Development of graphene quantum dots-based optical sensor for toxic metal ion detection. Sensors 2019, 19, 3850. [Google Scholar] [CrossRef] [PubMed]
- Fen, Y.W.; Yunus, W.M.M. Surface plasmon resonance spectroscopy as an alternative for sensing heavy metal ions: A review. Sens. Rev. 2013, 33, 305–314. [Google Scholar]
- Fen, Y.W.; Yunus, W.M.M.; Yusof, N.A. Surface plasmon resonance optical sensor for detection of Pb2+ based on immobilized p-tert-butylcalix[4]arene-tetrakis in chitosan thin film as an active layer. Sens. Actuators B Chem. 2012, 171–172, 287–293. [Google Scholar] [CrossRef]
- Anas, N.A.A.; Fen, Y.W.; Omar, N.A.S.; Ramdzan, N.S.M.; Daniyal, W.M.E.M.M.; Saleviter, S.; Zainudin, A.A. Optical properties of chitosan/hydroxyl-functionalized graphene quantum dots thin film for potential optical detection of ferric(III) ion. Opt. Laser Technol. 2019, 120, 105724. [Google Scholar] [CrossRef]
- Kumar, H.; Kuča, K.; Bhatia, S.K.; Saini, K.; Kaushal, A.; Verma, R.; Bhalla, T.C.; Kumar, D. Applications of nanotechnology in biosensor-based detection of foodborne pathogens. Sensors 2020, 20, 1966. [Google Scholar] [CrossRef] [PubMed]
- Elancheziyan, M.; Theyagarajan, K.; Saravanakumar, D.; Thenmozhi, K.; Senthilkumar, S. Viologen-terminated polyamidoamine (PAMAM) dendrimer encapsulated with gold nanoparticles for nonenzymatic determination of hydrogen peroxide. Mater. Today Chem. 2020, 16, 1–9. [Google Scholar] [CrossRef]
- Ren, Y.; Wei, J.; He, Y.; Wang, Y.; Bai, M.; Zhang, C.; Luo, L.; Wang, J.; Wang, Y. Ultrasensitive label-free immunochromatographic strip sensor for Salmonella determination based on salt-induced aggregated gold nanoparticles. Food Chem. 2021, 343, 1–8. [Google Scholar] [CrossRef]
- Zhu, J.; Ye, Z.; Fan, X.; Wang, H.; Wang, Z.; Chen, B. A highly sensitive biosensor based on au NPs/rGo-PaMaM-Fc nanomaterials for detection of cholesterol. Int. J. Nanomed. 2019, 14, 835–849. [Google Scholar] [CrossRef]
- Salvi, L.; Dubey, C.K.; Sharma, K.; Nagar, D.; Meghani, M.; Goyal, S.; Nagar, J.C.; Sharma, A. A synthesis, properties and application as a possible drug delivery systems dendrimers–A review. Asian J. Pharm. Res. Dev. 2020, 8, 107–113. [Google Scholar] [CrossRef]
- Hajizadeh, F.; Maleki, B.; Zonoz, F.M.; Amiri, A. Application of structurally enhanced magnetite cored polyamidoamine dendrimer for knoevenagel condensation. J. Iran. Chem. Soc. 2020, 1–12. [Google Scholar] [CrossRef]
- Li, Z.; Hu, J.; Yang, L.; Zhang, X.; Liu, X.; Wang, Z.; Li, Y. Integrated POSS-dendrimer nanohybrid materials: Current status and future perspective. Nanoscale 2020, 12, 11395–11415. [Google Scholar] [CrossRef]
- Van Tuan, P.; Phuong, T.T.; Tan, V.T.; Nguyen, S.X.; Khiem, T.N. In-situ hydrothermal fabrication and photocatalytic behavior of ZnO/reduced graphene oxide nanocomposites with varying graphene oxide concentrations. Mater. Sci. Semicond. Process. 2020, 115, 105114. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, L.; Zhang, L.; Li, X.; Gu, J.; Si, H.; Wu, L.; Shi, Y.; Sun, C.; Zhang, Y. Highly porous oxygen-doped NiCoP immobilized in reduced graphene oxide for supercapacitive energy storage. Compos. Part. B Eng. 2020, 182, 1–43. [Google Scholar] [CrossRef]
- Syama, S.; Mohanan, P.V. Comprehensive application of graphene: Emphasis on biomedical concerns. Nano-Micro Lett. 2019, 11, 1–31. [Google Scholar] [CrossRef]
- Wang, X.; Li, K.; He, J.; Yang, J.; Dong, F.; Mai, W.; Zhu, M. Defect in reduced graphene oxide tailored selectivity of photocatalytic CO2 reduction on Cs4PbBr6 pervoskite hole-in-microdisk structure. Nano Energy 2020, 78, 1–8. [Google Scholar] [CrossRef]
- Sharma, N.; Sharma, V.; Sharma, S.K.; Sachdev, K. Gas sensing behaviour of green synthesized reduced graphene oxide (rGO) for H2 and NO. Mater. Lett. 2019, 236, 444–447. [Google Scholar] [CrossRef]
- Hu, S.; Tan, Y.; Feng, C.; Wu, H.; Zhang, J.; Mei, H. Synthesis of N doped NiZnCu-layered double hydroxides with reduced graphene oxide on nickel foam as versatile electrocatalysts for hydrogen production in hybrid-water electrolysis. J. Power Sources 2020, 453, 1–10. [Google Scholar] [CrossRef]
- Zeng, Y.; Li, T.; Yao, Y.; Li, T.; Hu, L.; Marconnet, A. Thermally conductive reduced graphene oxide thin films for extreme temperature sensors. Adv. Funct. Mater. 2019, 29, 1–7. [Google Scholar] [CrossRef]
- Shu, R.; Wu, Y.; Li, W.; Zhang, J.; Liu, Y.; Shi, J.; Zheng, M. Fabrication of ferroferric oxide–carbon/reduced graphene oxide nanocomposites derived from Fe-based metal–organic frameworks for microwave absorption. Compos. Sci. Technol. 2020, 196, 1–12. [Google Scholar] [CrossRef]
- Smith, A.T.; LaChance, A.M.; Zeng, S.; Liu, B.; Sun, L. Synthesis, properties, and applications of graphene oxide/reduced graphene oxide and their nanocomposites. Nano Mater. Sci. 2019, 1, 31–47. [Google Scholar] [CrossRef]
- Li, X.; Xie, H.; Mao, J. Ag nanoparticles-reduced graphene oxide hybrid: An efficient electrocatalyst for artificial N2 fixation to NH3 at ambient conditions. J. Mater. Sci. 2020, 55, 5203–5210. [Google Scholar] [CrossRef]
- Kubiszeski, J.R.; Vieira, C.J.D.S.P.; Thies, S.F.; Silva, D.J.F.D.; Barreto, E.S.; Mondini, A.; Bronzoni, R.V.D.M. Detection of the Asian II genotype of dengue virus serotype 2 in humans and mosquitoes in Brazil. Rev. Soc. Bras. Med. Trop. 2020, 53, e20190439. [Google Scholar] [CrossRef] [PubMed]
- Eldigail, M.H.; Abubaker, H.A.; Khalid, F.A.; Abdallah, T.M.; Musa, H.H.; Ahmed, M.E.; Adam, G.K.; Elbashir, M.I.; Aradaib, I.E. Association of genotype III of dengue virus serotype 3 with disease outbreak in Eastern Sudan, 2019. Virol. J. 2020, 17, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Omar, N.A.S.; Fen, Y.W.; Abdullah, J.; Zaid, M.H.M.; Mahdi, M.A. Structural, optical and sensing properties of CdS-NH2GO thin film as a dengue virus E-protein sensing material. Optik 2018, 171, 934–940. [Google Scholar] [CrossRef]
- Rana, V.S.; Popli, S.; Saurav, G.K.; Yadav, K.; Kumar, A.; Sunil, S.; Kumar, N.; Singh, O.P.; Natarajan, K.; Rajagopal, R. Aedes aegypti lachesin protein binds to the domain III of envelop protein of Dengue virus-2 and inhibits viral replication. Cell. Microbiol. 2020, 22, 1–15. [Google Scholar] [CrossRef]
- Andrade, P.; Narvekar, P.; Montoya, M.; Michlmayr, D.; Balmaseda, A.; Coloma, J.; Harris, E. Primary and secondary dengue virus infections elicit similar memory B-Cell responses, but breadth to other serotypes and cross-reactivity to Zika virus is higher in secondary dengue. J. Infect. Dis. 2020, 222, 590–600. [Google Scholar] [CrossRef]
- Tan, K.W.; Tan, B.; Thein, T.L.; Leo, Y.S.; Lye, D.C.; Dickens, B.L.; Wong, J.G.X.; Cook, A.R. Dynamic dengue haemorrhagic fever calculators as clinical decision support tools in adult dengue. Trans. R. Soc. Trop. Med. Hyg. 2020, 114, 7–15. [Google Scholar] [CrossRef]
- Sundaram, A.K.; Ewing, D.; Blevins, M.; Liang, Z.; Sink, S.; Lassan, J.; Raviprakash, K.; Defang, G.; Williams, M.; Porter, K.R.; et al. Comparison of purified psoralen-inactivated and formalin-inactivated dengue vaccines in mice and nonhuman primates. Vaccine 2020, 38, 3313–3320. [Google Scholar] [CrossRef]
- Xisto, M.F.; Prates, J.W.O.; Dias, I.M.; Dias, R.S.; Silva, C.C.D.; Paula, S.O.D. NS1 recombinant proteins are efficiently produced in pichia pastoris and have great potential for use in diagnostic kits for dengue virus infections. Diagnostics 2020, 10, 379. [Google Scholar] [CrossRef]
- Firdous, S. Laser scanning optical investigations of dengue virus infection from antibody fluorescence of human blood. Laser Phys. Lett. 2019, 16, 1–8. [Google Scholar] [CrossRef]
- Andryukov, B.G.; Besednova, N.N.; Romashko, R.V.; Zaporozhets, T.S.; Efimov, T.A. Label-free biosensors for laboratory-based diagnostics of infections: Current achievements and new trends. Biosensors 2020, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Mukherjee, N. Current Developments in diagnostic biosensor technology: Relevance to therapeutic intervention of infectious and inflammatory diseases of human. In Modern Techniques in Biosensors; Springer: Singapore, 2021; pp. 1–36. [Google Scholar]
- Rajamanonmani, R.; Nkenfou, C.; Clancy, P.; Yau, Y.H.; Shochat, S.G.; Sukupolvi-Petty, S.; Schul, W.; Diamond, M.S.; Vasudevan, S.G.; Lescar, J. On a mouse monoclonal antibody that neutralizes all four dengue virus serotypes Lescar. J. Gen. Virol. 2009, 90, 799–809. [Google Scholar] [CrossRef]
- Jahanshahi, P.; Zalnezhad, E.; Sekaran, S.D.; Adikan, F.R.M. Rapid immunoglobulin M-based dengue diagnostic test using surface plasmon resonance biosensor. Sci. Rep. 2014, 4, 1–7. [Google Scholar] [CrossRef]
- Wong, W.R.; Krupin, O.; Sekaran, S.D.; Adikan, F.R.M.; Berini, P. Serological diagnosis of dengue infection in blood plasma using long-range surface plasmon waveguides. Anal. Chem. 2014, 86, 1735–1743. [Google Scholar] [CrossRef]
- Jahanshahi, P.; Sekaran, S.D.; Adikan, F.R.M. Optical and analytical investigations on dengue virus rapid diagnostic test for IgM antibody detection. Med. Biol. Eng. Comput. 2015, 53, 679–687. [Google Scholar] [CrossRef]
- Kumbhat, S.; Sharma, K.; Gehlot, R.; Solanki, A.; Joshi, V. Surface plasmon resonance based immunosensor for serological diagnosis of dengue virus infection. J. Pharm. Biomed. Anal. 2010, 52, 255–259. [Google Scholar] [CrossRef]
- Fry, S.R.; Meyer, M.; Semple, M.G.; Simmons, C.P.; Sekaran, S.D.; Huang, J.X.; McElnea, C.; Huang, C.; Valks, A.; Young, P.R.; et al. The diagnostic sensitivity of dengue rapid test assays is significantly enhanced by using a combined antigen and antibody testing approach. PLoS Negl. Trop. Dis. 2011, 5, e1199. [Google Scholar] [CrossRef]
- Hu, D.; Fry, S.; Huang, J.; Ding, X.; Qiu, L.; Pan, Y.; Chen, Y.; Jin, J.; McElnea, C.; Buechler, J.; et al. Comparison of surface plasmon resonance, resonant waveguide grating biosensing and enzyme linked immunosorbent assay (ELISA) in the evaluation of a dengue virus immunoassay. Biosensors 2013, 3, 297–311. [Google Scholar] [CrossRef]
- Wong, W.R.; Sekaran, S.D.; Adikan, F.R.M.; Berini, P. Detection of dengue NS1 antigen using long-range surface plasmon waveguides. Biosens. Bioelectrons. 2016, 78, 132–139. [Google Scholar] [CrossRef]
- Jahanshahi, P.; Wei, Q.; Jie, Z.; Ghomeishi, M.; Sekaran, S.D.; Adikan, F.R.M. Kinetic analysis of IgM monoclonal antibodies for determination of dengue sample concentration using SPR technique. Bioengineered 2017, 8, 239–247. [Google Scholar] [CrossRef]
- Sjahrurachman, A.; Dewi, B.E.; Lischer, K.; Pratami, D.K.; Flamandita, D.; Sahlan, M. Surface plasmon resonance analysis for detecting non-structural protein 1 of dengue virus in Indonesia. Saudi J. Biol. Sci. 2020, 27, 1931–1937. [Google Scholar]
- Liu, L.T.; Chen, C.H.; Tsai, C.Y.; Lin, P.C.; Hsu, M.C.; Huang, B.Y.; Wang, Y.H.; Tsai, J.J. Evaluation of rapid diagnostic tests to detect dengue virus infections in Taiwan. PLoS ONE 2020, 15, 1–14. [Google Scholar] [CrossRef] [PubMed]
- da Cruz Santos, C.; Santos, P.C.M.; Rocha, K.L.S.; Thomasini, R.L.; de Oliveira, D.B.; Franco, D.L.; Ferreira, L.F. A new tool for dengue virus diagnosis: Optimization and detection of anti-NS1 antibodies in serum samples by impedimetric transducers. Microchem. J. 2020, 154, 1–10. [Google Scholar]
- Omar, N.A.S.; Fen, Y.W.; Abdullah, J.; Sadrolhosseini, A.R.; Mustapha Kamil, Y.; Fauzi, N.I.M.; Hashim, H.S.; Mahdi, M.A. Quantitative and selective surface plasmon resonance response based on a reduced graphene oxide–polyamidoamine nanocomposite for detection of dengue virus e-proteins. Nanomaterials 2020, 10, 569. [Google Scholar] [CrossRef] [PubMed]
- Omar, N.A.S.; Fen, Y.W.; Abdullah, J.; Zaid, M.H.M.; Daniyal, W.M.E.M.M.; Mahdi, M.A. Sensitive surface plasmon resonance performance of cadmium sulfide quantum dots-amine functionalized graphene oxide based thin film towards dengue virus E-protein. Opt. Laser Technol. 2019, 114, 204–208. [Google Scholar] [CrossRef]
- Wu, S.; Shi, T.; Zhang, L. Preparation and properties of amine-functionalized reduced graphene oxide/waterborne polyurethane nanocomposites. High. Perform. Polym. 2016, 28, 453–465. [Google Scholar] [CrossRef]
- Khosroshahi, M.E. Applications of Biophotonics and Nanobiomaterials in Biomedical Engineering; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Navaee, A.; Salimi, A. Efficient amine functionalization of graphene oxide through the Bucherer reaction: An extraordinary metal-free electrocatalyst for the oxygen reduction reaction. RSC Adv. 2015, 5, 59874–59880. [Google Scholar] [CrossRef]
- Sankari, G.; Krishnamoorthy, E.; Jayakumaran, S.; Gunasekaran, S.; Priya, V.V.; Subramaniam, S.; Subramaniam, S.; Mohan, S.K. Analysis of serum immunoglobulins using Fourier transform infrared spectral measurements. Biol. Med. 2010, 2, 42–48. [Google Scholar]
- Mossuto, M.F.; Ami, D.; Anelli, T.; Fagioli, C.; Doglia, S.M.; Sitia, R. Biochemical nature of Russell bodies. Sci. Rep. 2015, 5, 1–12. [Google Scholar]
- Kamil, Y.M.; Al-Rekabi, S.H.; Yaacob, M.H.; Syahir, A.; Chee, H.Y.; Mahdi, M.A.; Bakar, M.H.A. Detection of dengue using PAMAM dendrimer integrated tapered optical fiber sensor. Sci. Rep. 2019, 9, 1–10. [Google Scholar]
- Yoon, S.J.; Kim, D. Thin-film-based field penetration engineering for surface plasmon resonance biosensing. J. Opt. Soc. Am. A 2007, 24, 2543–2549. [Google Scholar] [CrossRef]
- Maharana, P.K.; Jha, R. Chalcogenide prism and graphene multilayer based surface plasmon resonance affinity biosensor for high performance. Sens. Actuators B Chem. 2012, 169, 161–166. [Google Scholar] [CrossRef]
- Benazize, S.; Dibi, Z.; Benaziez, N. Optimization of the graphene-silver based surface plasmon resonance (SPR) sensor. Univ. Politeh. Buchar. Sci. Bull. Ser. B 2018, 80, 1454–2331. [Google Scholar]
- Bhavsar, K.; Prabhu, R.; Pollard, P. Ultrasensitive graphene coated SPR sensor for biosensing applications. Proc. SPIE 2015, 9506. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, Y.; Zhang, H.; Xu, B.; Zhang, H.; Song, D. Preparation and application of triangular silver nanoplates/chitosan composite in surface plasmon resonance biosensing. Anal. Chim. Acta 2013, 769, 114–120. [Google Scholar] [CrossRef]
- Lee, Y.K.; Jang, D.H.; Lee, K.S.; Kim, W.M.; Sohn, Y.S. Enhancing performance of a miniaturized surface plasmon resonance sensor in the reflectance detection mode using a waveguide-coupled bimetallic chip. Nanoscale Res. Lett. 2013, 8, 1–8. [Google Scholar] [CrossRef]
- Hasan, M.R.; Akter, S.; Rifat, A.A.; Rana, S.; Ahmed, K.; Ahmed, R.; Subbaraman, H.; Abbott, D. Spiral photonic crystal fiber-based dual-polarized surface plasmon resonance biosensor. IEEE Sens. J. 2017, 18, 133–140. [Google Scholar] [CrossRef]
- Mustapa, M.A.; Bakar, M.H.A.; Kamil, Y.M.; Syahir, A.; Mahdi, M.A. Bio-functionalized tapered multimode fiber coated with dengue virus NS1 glycoprotein for label free detection of anti-Dengue virus NS1 IgG antibody. IEEE Sens. J. 2018, 18, 4066–4072. [Google Scholar] [CrossRef]
- Ida, J.; Kuzuya, A.; Choong, Y.S.; Lim, T.S. An intermolecular-split G-quadruplex DNAzyme sensor for dengue virus detection. RSC Adv. 2020, 10, 33040–33051. [Google Scholar] [CrossRef]
- Zakaria, R.; Fahri, M.A.S.A.; Thirunavakkarasu, P.M.; Patel, S.K.; Harun, S.W. High sensitivity refractive index sensor in long-range surface plasmon resonance based on side polished optical fiber. Opt. Fiber Technol. 2021, 61, 1–7. [Google Scholar] [CrossRef]
- Ekgasit, S.; Thammacharoen, C.; Knoll, W. Surface plasmon resonance spectroscopy based on evanescent field treatment. Anal. Chem. 2004, 76, 561–568. [Google Scholar] [CrossRef]
- Maharana, P.K.; Padhy, P.; Jha, R. On the field enhancement and performance of an ultra-stable SPR biosensor based on graphene. IEEE Photon. Technol. Lett. 2013, 25, 2156–2159. [Google Scholar] [CrossRef]
- Hoet, M.; Cohen, E.H.; Kent, R.B.; Rookey, K.; Schoonbroodt, S.; Hogan, S.; Rem, L.; Frans, N.; Daukandt, M.; Pieters, H.; et al. Generation of high-affinity human antibodies by combining donor-derived and synthetic complementarity-determining-region diversity. Nat. Biotechnol. 2005, 23, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Salahudeen, M.S.; Nishtala, P.S. An overview of pharmacodynamic modelling, ligand-binding approach and its application in clinical practice. Saudi Pharm. J. 2017, 25, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Peters Jr, T. Serum albumin. Adv. Protein Chem. 1985, 37, 161–245. [Google Scholar]
- Chew, M.; Poh, K.; Poh, C. Peptides as therapeutic agents for dengue virus. Int. J. Med. Sci. 2017, 14, 1342–1359. [Google Scholar] [CrossRef]
- Alen, M.M.F.; Schols, D. Dengue virus entry as target for antiviral therapy. J. Trop. Med. 2012, 2012, 1–13. [Google Scholar] [CrossRef]
- Salles, T.S.; Sá-Gulmarães, T.E.; Souza, D.F.D.; López, S.B.G.; Alvarenga, E.S.L.; Franco, T.A.; Melo, A.C.A.; Soares, M.R.; Ferreira, D.F.; Moreira, M.F. Quantitative dengue serotyping: The development of a higher performance method using SYBR Green Assay. Arch. Clin. Microbiol. 2017, 8, 1–12. [Google Scholar]
- Hu, Z.; Li, M.; Liu, J.; Yu, L.; Xue, Y.; Chen, Y. Detection of hepatitis b virus large surface protein using a time-resolved immunofluorometric assay. J. Clin. Lab. Anal. 2015, 29, 498–504. [Google Scholar] [CrossRef]
- Yañez, V.A.C.; Göpfert, J.C.; Otto, M.; Tumani, H.; Peter, A.; Joos, T.O. Development and validation of an ultrasensitive procalcitonin sandwich immunoassay. High. Throughput 2017, 6, 1–12. [Google Scholar]
- Oftedal, L.; Maple-Grødem, J.; Førland, M.G.G.; Alves, G.; Lange, J. Validation and assessment of preanalytical factors of a fluorometric in vitro assay for glucocerebrosidase activity in human cerebrospinal fluid. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bouquet, M.; Passmore, M.R.; Hoe, L.E.S.; Tung, J.P.; Simonova, G.; Boon, A.C.; Fraser, J.F. Development and validation of ELISAs for the quantitation of interleukin (IL)-1β, IL-6, IL-8 and IL-10 in ovine plasma. J. Immunol. Methods 2020, 486, 1–7. [Google Scholar] [CrossRef] [PubMed]



| Targeted Determinant | Detection Limit | References |
|---|---|---|
| IgM | - | [54,55,56,57] |
| DENV | - | [58] |
| DENV-2 NS1 | 0.25 ng/mL | [59] |
| NS1 proteins | 1 nM | [60] |
| NS1 | 5.73 pg/mm2 | [61] |
| IgM | 2.125 pM | [62] |
| NS1 proteins | 0.3 nM | [63] |
| Optical Sensor | Active Layer | Determinant | Detection Limit | References |
|---|---|---|---|---|
| Optical fiber | PAMAM 1 | DNEV-2 E-proteins | 1 pM | [73] |
| Optical fiber | - | DENV NS1 IgG antibody | 200 pM | [81] |
| SPR | CM5/EDC-NHS 2 | IgM antibody | 2.125 pM | [62] |
| SPR | CMD/EDC-NHS 3 | NS1 proteins | 0.3 nM | [63] |
| Colorimetric | G4-hemin DNAzyme | DENV-1 DNA; DENV-2 DNA; DENV-3 DNA, DENV-4 DNA | 8.8 nM; 4.9 nM; 9.3 nM; 5.1 nM | [82] |
| SPR | Au/DSU/NH2rGO–PAMAM | DENV-2 E-proteins | 0.08 pM | This work |
| Sample Concentration (pM) | Spike (%) | PBS + DENV-2 E-Protein Resonance Angle (Degree) | BSA + DENV-2 E-Protein Resonance Angle (Degree) | Recovery (%) |
|---|---|---|---|---|
| 0.08 | 4.5 | 54.305 | 54.400 | 100.174 |
| 0.1 | 4.5 | 54.313 | 54.480 | 100.307 |
| 0.3 | 4.5 | 54.392 | 54.496 | 100.191 |
| 0.5 | 4.5 | 54.400 | 54.496 | 100.176 |
| 1 | 4.5 | 54.408 | 54.500 | 100.169 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omar, N.A.S.; Fen, Y.W.; Ramli, I.; Sadrolhosseini, A.R.; Abdullah, J.; Yusof, N.A.; Kamil, Y.M.; Mahdi, M.A. An Optical Sensor for Dengue Envelope Proteins Using Polyamidoamine Dendrimer Biopolymer-Based Nanocomposite Thin Film: Enhanced Sensitivity, Selectivity, and Recovery Studies. Polymers 2021, 13, 762. https://doi.org/10.3390/polym13050762
Omar NAS, Fen YW, Ramli I, Sadrolhosseini AR, Abdullah J, Yusof NA, Kamil YM, Mahdi MA. An Optical Sensor for Dengue Envelope Proteins Using Polyamidoamine Dendrimer Biopolymer-Based Nanocomposite Thin Film: Enhanced Sensitivity, Selectivity, and Recovery Studies. Polymers. 2021; 13(5):762. https://doi.org/10.3390/polym13050762
Chicago/Turabian StyleOmar, Nur Alia Sheh, Yap Wing Fen, Irmawati Ramli, Amir Reza Sadrolhosseini, Jaafar Abdullah, Nor Azah Yusof, Yasmin Mustapha Kamil, and Mohd Adzir Mahdi. 2021. "An Optical Sensor for Dengue Envelope Proteins Using Polyamidoamine Dendrimer Biopolymer-Based Nanocomposite Thin Film: Enhanced Sensitivity, Selectivity, and Recovery Studies" Polymers 13, no. 5: 762. https://doi.org/10.3390/polym13050762
APA StyleOmar, N. A. S., Fen, Y. W., Ramli, I., Sadrolhosseini, A. R., Abdullah, J., Yusof, N. A., Kamil, Y. M., & Mahdi, M. A. (2021). An Optical Sensor for Dengue Envelope Proteins Using Polyamidoamine Dendrimer Biopolymer-Based Nanocomposite Thin Film: Enhanced Sensitivity, Selectivity, and Recovery Studies. Polymers, 13(5), 762. https://doi.org/10.3390/polym13050762