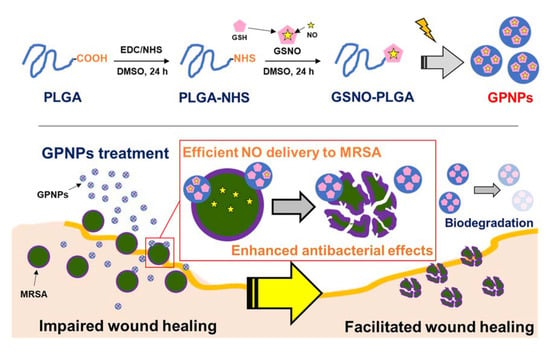
Nitric Oxide-Releasing S-Nitrosoglutathione-Conjugated Poly(Lactic-Co-Glycolic Acid) Nanoparticles for the Treatment of MRSA-Infected Cutaneous Wounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of GSNO
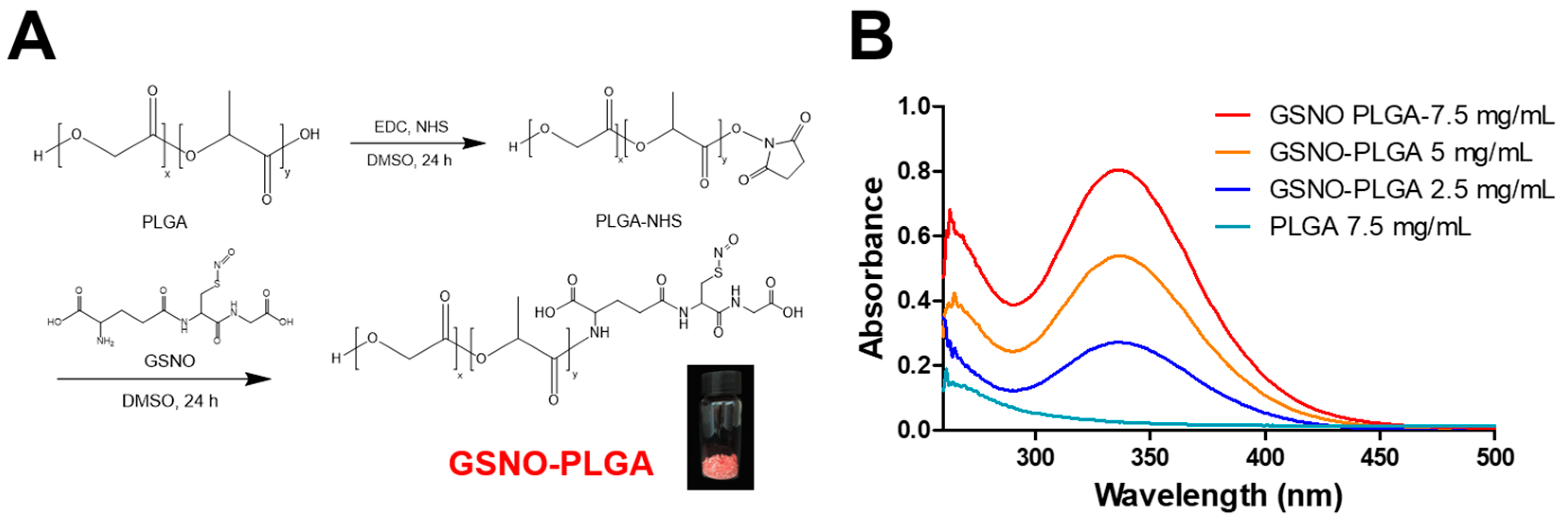
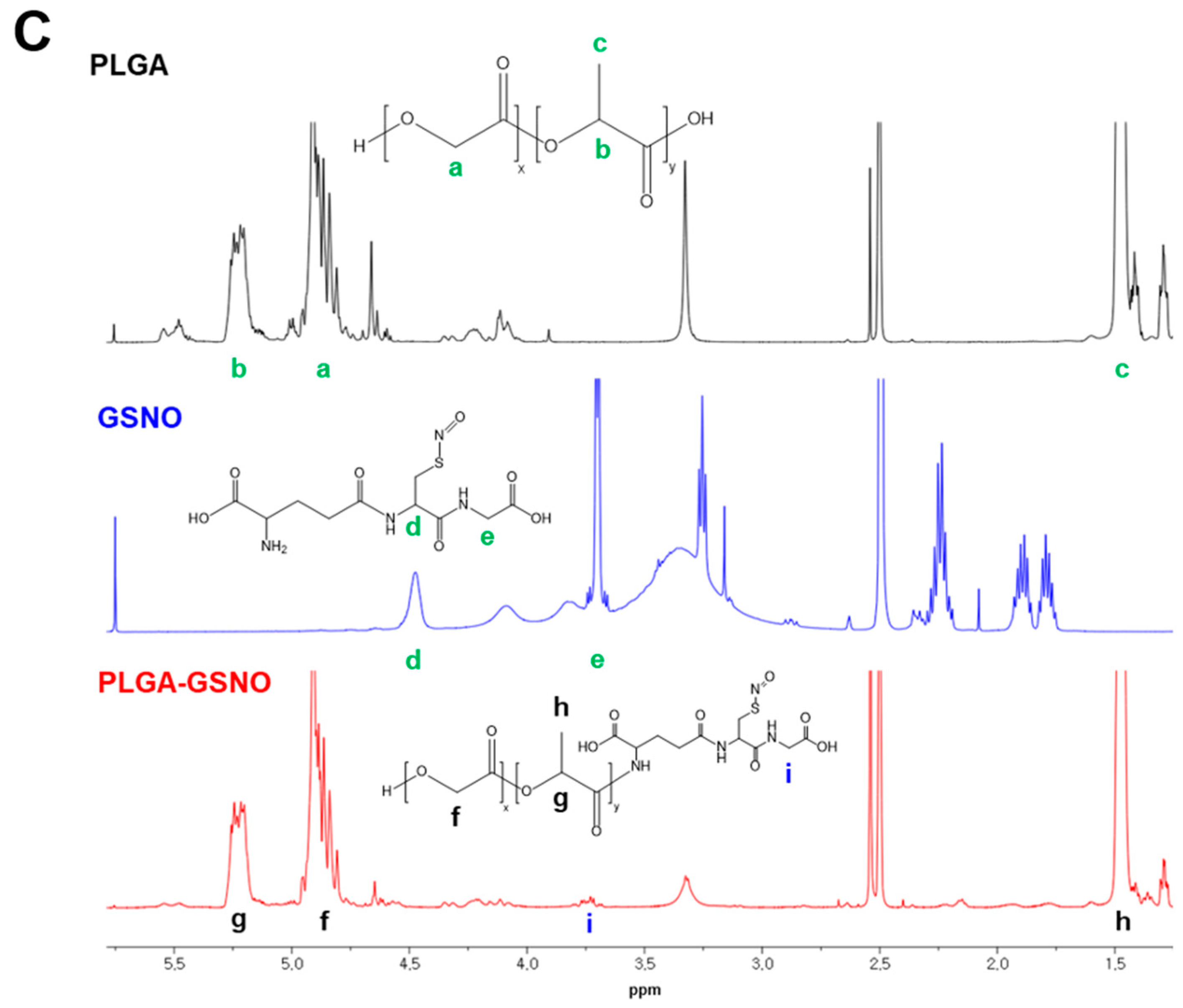
2.3. Synthesis of GSNO-Conjugated PLGA
2.4. Fabrication of GPNPs
2.5. NO Release from GPNPs
2.6. Antibacterial Assay
2.7. In vivo Wound Healing Study in an MRSA-Challenged Full-Thickness Wound Mouse Model
2.7.1. Macroscopic Assessment: Wound Size Reduction
2.7.2. Histological Assessment
2.8. Statistics
3. Results
3.1. Synthesis of GSNO-Conjugated PLGA
3.2. Fabrication of GPNPs
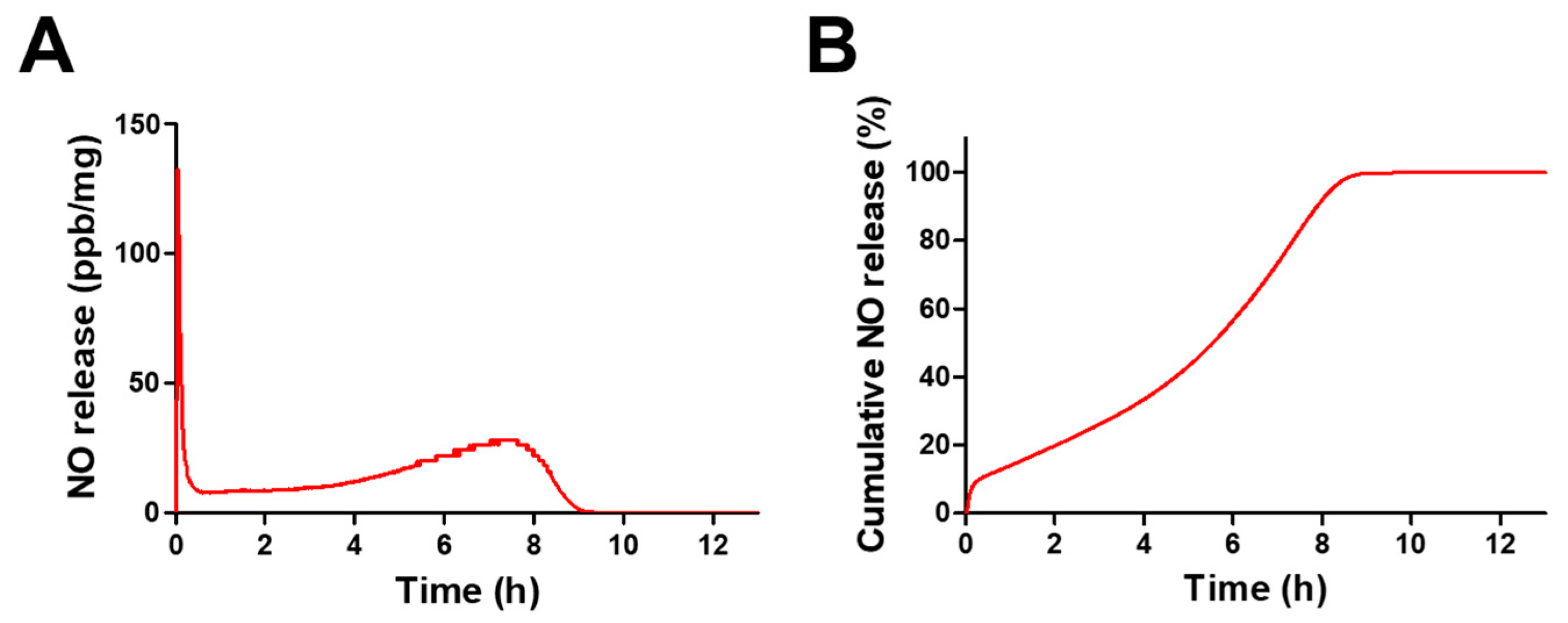
3.3. NO Release from GPNPs
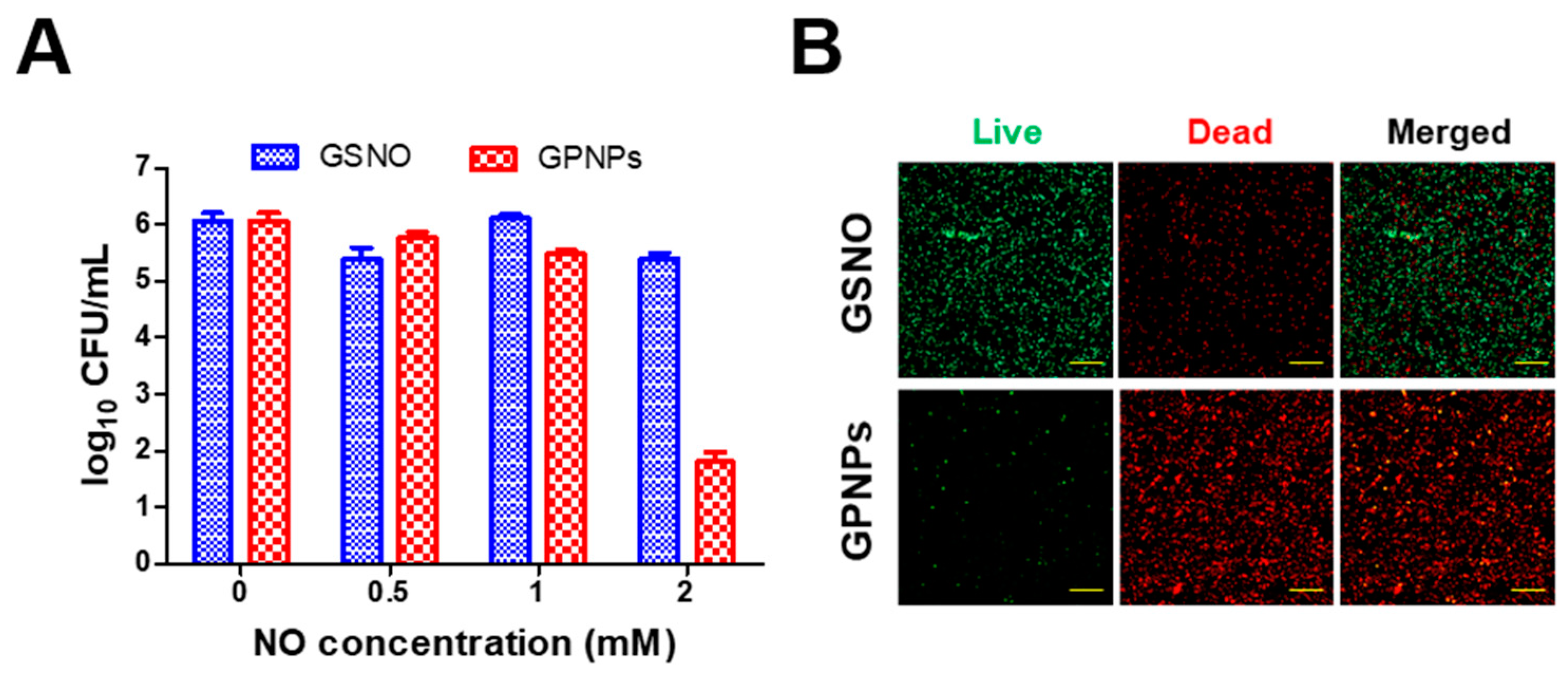
3.4. Antibacterial Assay
3.5. In vivo Wound Healing Study
3.5.1. Macroscopic Assessment: Wound Size Reduction
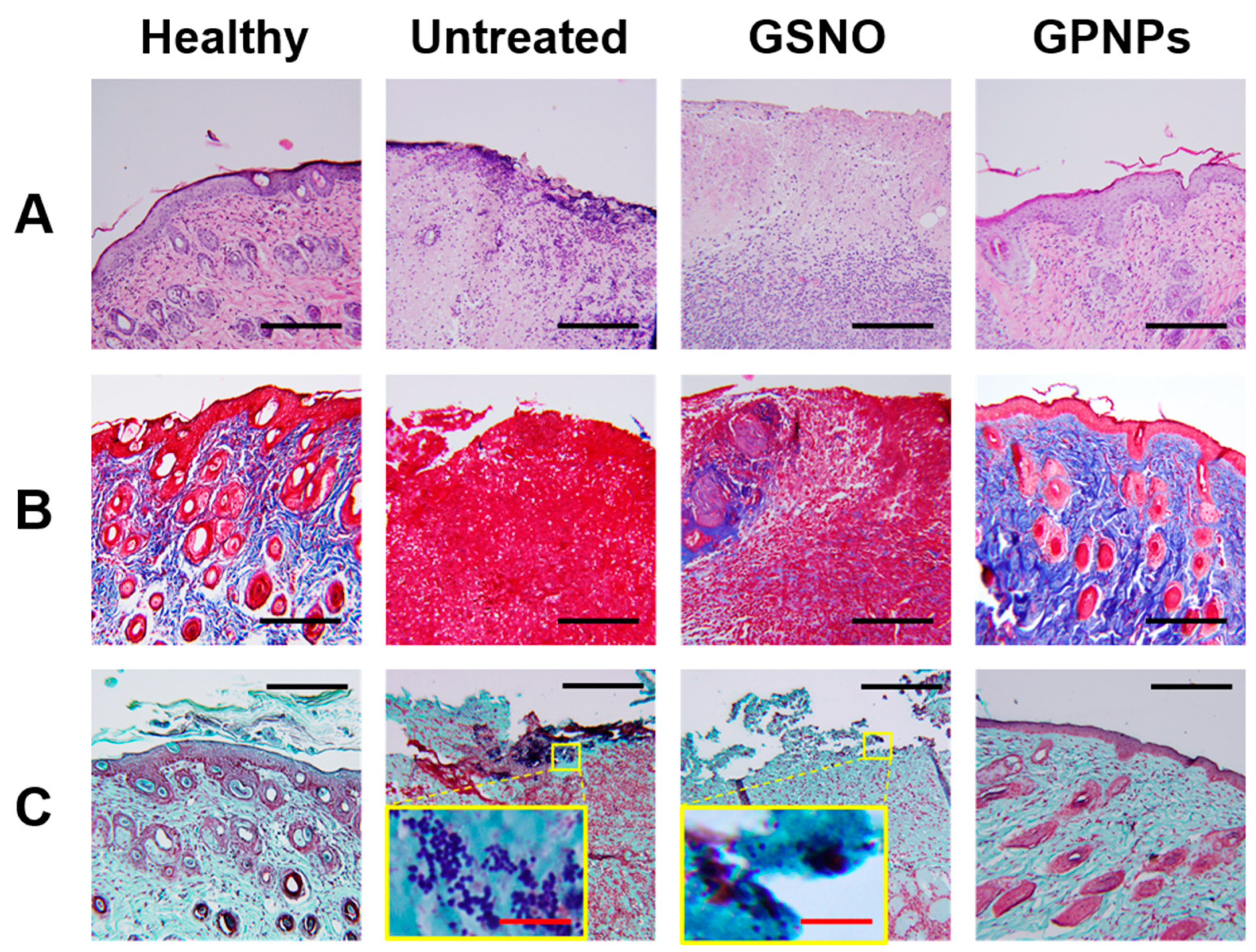
3.5.2. Histological Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Daeschlein, G. Antimicrobial and antiseptic strategies in wound management. Int. Wound J. 2013, 10, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Hlaing, S.P.; Cao, J.; Hasan, N.; Yoo, J.-W. In vitro and in vivo evaluation of a novel nitric oxide-releasing ointment for the treatment of methicillin-resistant Staphylococcus aureus-infected wounds. J Pharm. Investig. 2020, 1–8. [Google Scholar] [CrossRef]
- Fleischmann, C.; Scherag, A.; Adhikari, N.K.; Hartog, C.S.; Tsaganos, T.; Schlattmann, P.; Angus, D.C.; Reinhart, K. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am. J. Respir. Crit. Care Med. 2016, 193, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Bello, Y.M.; Phillips, T.J. Recent advances in wound healing. JAMA 2000, 283, 716–718. [Google Scholar] [CrossRef] [PubMed]
- George Broughton, I.; Janis, J.E.; Attinger, C.E. The basic science of wound healing. Plast. Reconstr. Surg. 2006, 117, 12S–34S. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.G.; Edwards, R.; Thomas, D.W. Inflammation and wound healing: The role of bacteria in the immuno-regulation of wound healing. Int. J. Low. Extrem. Wounds 2004, 3, 201–208. [Google Scholar] [CrossRef]
- Yurt, R.W.; McManus, A.T.; Mason, A.D.; Pruitt, B.A. Increased susceptibility to infection related to extent of burn injury. Arch. Surg. 1984, 119, 183–188. [Google Scholar] [CrossRef]
- Choi, M.; Hasan, N.; Cao, J.; Lee, J.; Hlaing, S.P.; Yoo, J.-W. Chitosan-based nitric oxide-releasing dressing for anti-biofilm and in vivo healing activities in MRSA biofilm-infected wounds. Int. J. Biol. Macromol. 2019, 142, 680–692. [Google Scholar] [CrossRef]
- Dabiri, G.; Damstetter, E.; Phillips, T. Choosing a wound dressing based on common wound characteristics. Adv. Wound Care 2016, 5, 32–41. [Google Scholar] [CrossRef]
- Fair, R.J.; Tor, Y. Antibiotics and bacterial resistance in the 21st century. Perspect. Med. Chem. 2014, 6, S14459. [Google Scholar] [CrossRef]
- Liu, S.; Cai, X.; Xue, W.; Ma, D.; Zhang, W. Chitosan derivatives co-delivering nitric oxide and methicillin for the effective therapy to the methicillin-resistant S. aureus infection. Carbohydr. Polym. 2020, 234, 115928. [Google Scholar] [CrossRef] [PubMed]
- Behzadi, P. DNA microarrays and multidrug resistant bacteria. Eur. Pharm. Rev. 2018, 1, 30–32. [Google Scholar]
- Heinze, K.; Kabeto, M.; Martin, E.T.; Cassone, M.; Hicks, L.; Mody, L. Predictors of methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci co-colonization among nursing facility patients. Am. J. Infect. Control 2019, 47, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Hasan, N.; Cao, J.; Lee, J.; Hlaing, S.P.; Oshi, M.A.; Naeem, M.; Ki, M.-H.; Lee, B.L.; Jung, Y.; Yoo, J.-W. Bacteria-Targeted Clindamycin Loaded Polymeric Nanoparticles: Effect of Surface Charge on Nanoparticle Adhesion to MRSA, Antibacterial Activity, and Wound Healing. Pharmaceutics 2019, 11, 236. [Google Scholar] [CrossRef]
- Bogdan, C. Nitric oxide and the immune response. Nat. Immunol. 2001, 2, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Schäffer, M.R.; Tantry, U.; Gross, S.S.; Wasserkrug, H.L.; Barbul, A. Nitric oxide regulates wound healing. J. Surg. Res. 1996, 63, 237–240. [Google Scholar] [CrossRef]
- Wallace, J.L. Nitric oxide as a regulator of inflammatory processes. Mem. Inst. Oswaldo Cruz 2005, 100, 5–9. [Google Scholar] [CrossRef]
- Smith, A.W. Biofilms and antibiotic therapy: Is there a role for combating bacterial resistance by the use of novel drug delivery systems? Adv. Drug Deliv. Rev. 2005, 57, 1539–1550. [Google Scholar] [CrossRef]
- Malone-Povolny, M.J.; Maloney, S.E.; Schoenfisch, M.H. Nitric Oxide Therapy for Diabetic Wound Healing. Adv. Healthc. Mater. 2019, 8, 1801210. [Google Scholar] [CrossRef]
- Hasan, N.; Cao, J.; Lee, J.; Naeem, M.; Hlaing, S.P.; Kim, J.; Jung, Y.; Lee, B.-L.; Yoo, J.-W. PEI/NONOates-doped PLGA nanoparticles for eradicating methicillin-resistant Staphylococcus aureus biofilm in diabetic wounds via binding to the biofilm matrix. Mater. Sci. Eng. C 2019, 103, 109741. [Google Scholar] [CrossRef]
- Jones, M.L.; Ganopolsky, J.G.; Labbé, A.; Wahl, C.; Prakash, S. Antimicrobial properties of nitric oxide and its application in antimicrobial formulations and medical devices. Appl. Microbiol. Biotechnol. 2010, 88, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, A.W.; Schoenfisch, M.H. Nitric oxide release: Part II. Therapeutic applications. Chem. Soc. Rev. 2012, 41, 3742–3752. [Google Scholar] [CrossRef] [PubMed]
- Schairer, D.O.; Chouake, J.S.; Nosanchuk, J.D.; Friedman, A.J. The potential of nitric oxide releasing therapies as antimicrobial agents. Virulence 2012, 3, 271–279. [Google Scholar] [CrossRef]
- Privett, B.J.; Broadnax, A.D.; Bauman, S.J.; Riccio, D.A.; Schoenfisch, M.H. Examination of bacterial resistance to exogenous nitric oxide. Nitric Oxide 2012, 26, 169–173. [Google Scholar] [CrossRef]
- Le, Q.-V.; Choi, J.; Oh, Y.-K. Nano delivery systems and cancer immunotherapy. J. Pharm. Investig. 2018, 48, 527–539. [Google Scholar] [CrossRef]
- Zeb, A.; Arif, S.T.; Malik, M.; Shah, F.A.; Din, F.U.; Qureshi, O.S.; Lee, E.-S.; Lee, G.-Y.; Kim, J.-K. Potential of nanoparticulate carriers for improved drug delivery via skin. J. Pharm. Investig. 2018, 49, 485–517. [Google Scholar] [CrossRef]
- Nurhasni, H.; Cao, J.; Choi, M.; Kim, I.; Lee, B.L.; Jung, Y.; Yoo, J.-W. Nitric oxide-releasing poly (lactic-co-glycolic acid)-polyethylenimine nanoparticles for prolonged nitric oxide release, antibacterial efficacy, and in vivo wound healing activity. Int. J. Nanomed. 2015, 10, 3065. [Google Scholar]
- Hetrick, E.M.; Shin, J.H.; Paul, H.S.; Schoenfisch, M.H. Anti-biofilm efficacy of nitric oxide-releasing silica nanoparticles. Biomaterials 2009, 30, 2782–2789. [Google Scholar] [CrossRef]
- Kafshgari, M.H.; Cavallaro, A.; Delalat, B.; Harding, F.J.; McInnes, S.J.; Mäkilä, E.; Salonen, J.; Vasilev, K.; Voelcker, N.H. Nitric oxide-releasing porous silicon nanoparticles. Nanoscale Res. Lett. 2014, 9, 1–9. [Google Scholar] [CrossRef]
- Ma, X.; Cheng, Y.; Jian, H.; Feng, Y.; Chang, Y.; Zheng, R.; Wu, X.; Wang, L.; Li, X.; Zhang, H. Hollow, Rough, and Nitric Oxide-Releasing Cerium Oxide Nanoparticles for Promoting Multiple Stages of Wound Healing. Adv. Healthc. Mater. 2019, 8, 1900256. [Google Scholar] [CrossRef]
- Pieretti, J.C.; Seabra, A.B. Nitric Oxide-Releasing Nanomaterials and Skin Infections. In Nanotechnology in Skin, Soft Tissue, and Bone Infections; Springer: New York, NY, USA, 2020; pp. 3–23. [Google Scholar]
- Niska, K.; Zielinska, E.; Radomski, M.W.; Inkielewicz-Stepniak, I. Metal nanoparticles in dermatology and cosmetology: Interactions with human skin cells. Chem. Biol. Interact. 2018, 295, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Szmyd, R.; Goralczyk, A.G.; Skalniak, L.; Cierniak, A.; Lipert, B.; Filon, F.L.; Crosera, M.; Borowczyk, J.; Laczna, E.; Drukala, J. Effect of silver nanoparticles on human primary keratinocytes. Biol. Chem. 2013, 394, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Lewinski, N.; Colvin, V.; Drezek, R. Cytotoxicity of nanoparticles. Small 2008, 4, 26–49. [Google Scholar] [CrossRef] [PubMed]
- Broniowska, K.A.; Diers, A.R.; Hogg, N. S-nitrosoglutathione. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 3173–3181. [Google Scholar] [CrossRef]
- Forman, H.J. Glutathione–From antioxidant to post-translational modifier. Arch. Biochem. Biophys. 2016, 595, 64–67. [Google Scholar] [CrossRef]
- Champeau, M.; Póvoa, V.; Militão, L.; Cabrini, F.M.; Picheth, G.F.; Meneau, F.; Jara, C.P.; de Araujo, E.P.; de Oliveira, M.G. Supramolecular poly (acrylic acid)/F127 hydrogel with hydration-controlled nitric oxide release for enhancing wound healing. Acta Biomater. 2018, 74, 312–325. [Google Scholar] [CrossRef]
- Amadeu, T.P.; Seabra, A.B.; De Oliveira, M.G.; Costa, A.M. Venereology. S-nitrosoglutathione-containing hydrogel accelerates rat cutaneous wound repair. J. Eur. Acad. Dermatol. Venereol. 2007, 21, 629–637. [Google Scholar]
- Lee, J.; Hlaing, S.P.; Cao, J.; Hasan, N.; Ahn, H.-J.; Song, K.-W.; Yoo, J.-W. In Situ Hydrogel-Forming/Nitric Oxide-Releasing Wound Dressing for Enhanced Antibacterial Activity and Healing in Mice with Infected Wounds. Pharmaceutics 2019, 11, 496. [Google Scholar] [CrossRef]
- Kim, J.O.; Noh, J.-K.; Thapa, R.K.; Hasan, N.; Choi, M.; Kim, J.H.; Lee, J.-H.; Ku, S.K.; Yoo, J.-W. Nitric oxide-releasing chitosan film for enhanced antibacterial and in vivo wound-healing efficacy. Int. J. Biol. Macromol. 2015, 79, 217–225. [Google Scholar] [CrossRef]
- Duong, H.T.; Kamarudin, Z.M.; Erlich, R.B.; Li, Y.; Jones, M.W.; Kavallaris, M.; Boyer, C.; Davis, T.P. Intracellular nitric oxide delivery from stable NO-polymeric nanoparticle carriers. Chem. Commun. 2013, 49, 4190–4192. [Google Scholar] [CrossRef]
- Marcato, P.D.; Adami, L.F.; de Melo Barbosa, R.; Melo, P.S.; Ferreira, I.R.; de Paula, L.; Duran, N.; Seabra, A.B. Development of a sustained-release system for nitric oxide delivery using alginate/chitosan nanoparticles. Curr. Nanosci. 2013, 9, 1–7. [Google Scholar]
- Wu, W.; Gaucher, C.; Diab, R.; Fries, I.; Xiao, Y.-L.; Hu, X.-M.; Maincent, P.; Sapin-Minet, A. Biopharmaceutics. Time lasting S-nitrosoglutathione polymeric nanoparticles delay cellular protein S-nitrosation. Eur J. Pharm. Biopharm. 2015, 89, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Park, D.J.; Choi, G.H.; Yang, D.-N.; Heo, J.S.; Lee, S.C. pH-Responsive mineralized nanoparticles as stable nanocarriers for intracellular nitric oxide delivery. Colloids Surf. B 2016, 146, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.-W.; Acharya, G.; Lee, C.H. In vivo evaluation of vaginal films for mucosal delivery of nitric oxide. Biomaterials 2009, 30, 3978–3985. [Google Scholar] [CrossRef] [PubMed]
- Valencia, P.M.; Hanewich-Hollatz, M.H.; Gao, W.; Karim, F.; Langer, R.; Karnik, R.; Farokhzad, O.C. Effects of ligands with different water solubilities on self-assembly and properties of targeted nanoparticles. Biomaterials 2011, 32, 6226–6233. [Google Scholar] [CrossRef]
- Malvern Instruments. Zetasizer Nano Series User Manual; Malvern: Worcestershire, UK, 2004. [Google Scholar]
- Danafar, H.; Rostamizadeh, K.; Hamidi, M. Polylactide/poly (ethylene glycol)/polylactide triblock copolymer micelles as carrier for delivery of hydrophilic and hydrophobic drugs: A comparison study. J. Pharm. Investig. 2018, 48, 381–391. [Google Scholar] [CrossRef]
- Heggers, J.P.; Robson, M.C.; Doran, E.T. Quantitative assessment of bacterial contamination of open wounds by a slide technique. Trans. R. Soc. Trop. Med. Hyg. 1969, 63, 532–534. [Google Scholar] [CrossRef]
- Siepmann, J.; Faisant, N.; Benoit, J.-P. A New Mathematical Model Quantifying Drug Release from Bioerodible Microparticles Using Monte Carlo Simulations. Pharm. Res. 2002, 19, 1885–1893. [Google Scholar] [CrossRef]
- Zolnik, B.S.; Leary, P.E.; Burgess, D.J. Elevated temperature accelerated release testing of PLGA microspheres. J. Control. Release 2006, 112, 293–300. [Google Scholar] [CrossRef]
- Aragón, D.; Rosas, J.; Martínez, F. Effect of the ibuprofen solubility in acetone and dichloromethane on the drug release profiles from PLGA microspheres. Latin Am. Appl. Res. 2014, 44, 87–92. [Google Scholar]
- Carpenter, A.W.; Worley, B.V.; Slomberg, D.L.; Schoenfisch, M.H. Dual action antimicrobials: Nitric oxide release from quaternary ammonium-functionalized silica nanoparticles. Biomacromolecules 2012, 13, 3334–3342. [Google Scholar] [CrossRef]
- Raghupathi, K.R.; Koodali, R.T.; Manna, A.C. Size-dependent bacterial growth inhibition and mechanism of antibacterial activity of zinc oxide nanoparticles. Langmuir 2011, 27, 4020–4028. [Google Scholar] [CrossRef] [PubMed]
- Macherla, C.; Sanchez, D.A.; Ahmadi, M.; Vellozzi, E.M.; Friedman, A.J.; Nosanchuk, J.D.; Martinez, L.R. Nitric oxide releasing nanoparticles for treatment of Candida albicans burn infections. Front. Microbiol. 2012, 3, 193. [Google Scholar] [CrossRef] [PubMed]
- Barone, M.; Sciortino, M.T.; Zaccaria, D.; Mazzaglia, A.; Sortino, S. Nitric oxide photocaging platinum nanoparticles with anticancer potential. J. Mater. Chem. 2008, 18, 5531–5536. [Google Scholar] [CrossRef]
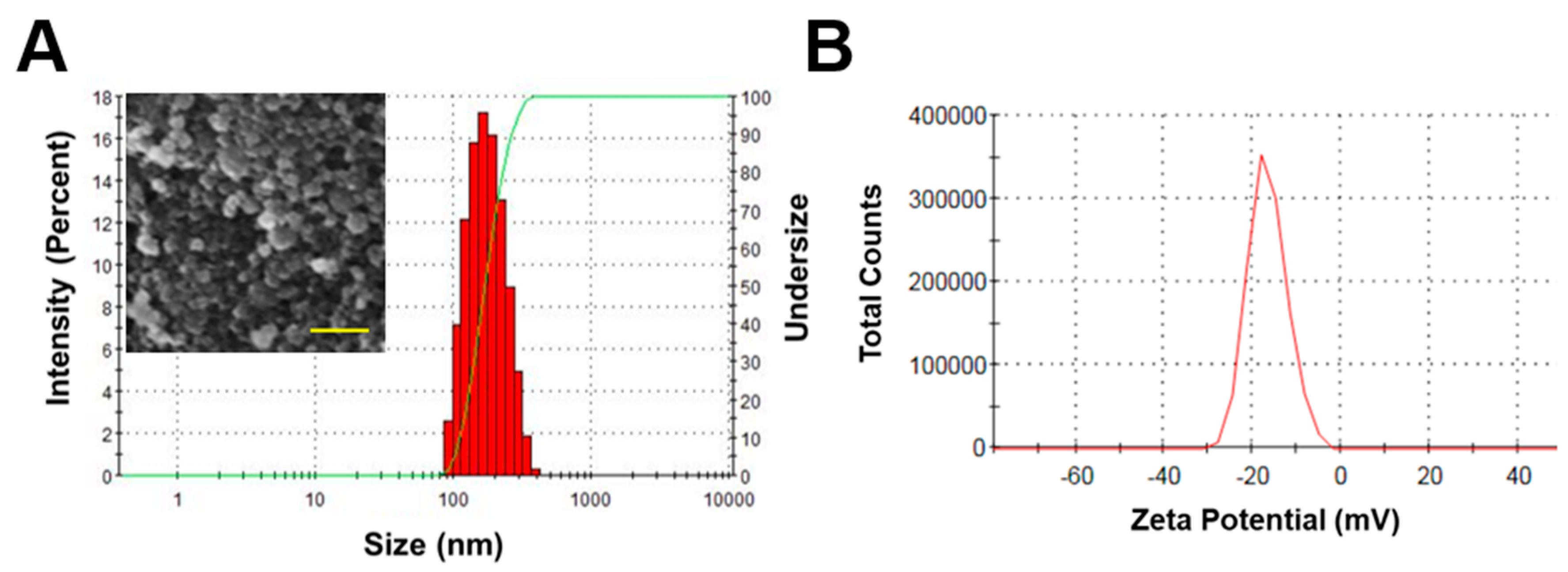

| Particle Size (Hydrodynamic Diameter) (nm) | Poly-Disperse Index | Zeta-Potential (mV) | Loading (GSNO, %) | Loading (NO, μmol/mg) |
|---|---|---|---|---|
| 164.5 ± 2.2 | 0.12 ± 0.03 | -17 ± 0.6 | 2.32 ± 0.27 | 0.07 ± 0.01 |
| Formulation | [NO]T (μmol/mg) | [NO]max (ppb/mg) | t[NO]max (h) | t1/2 (h) | td (h) |
|---|---|---|---|---|---|
| GPNPs | 0.07 | 132 | 0.05 | 5.55 | 11.27 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Kwak, D.; Kim, H.; Kim, J.; Hlaing, S.P.; Hasan, N.; Cao, J.; Yoo, J.-W. Nitric Oxide-Releasing S-Nitrosoglutathione-Conjugated Poly(Lactic-Co-Glycolic Acid) Nanoparticles for the Treatment of MRSA-Infected Cutaneous Wounds. Pharmaceutics 2020, 12, 618. https://doi.org/10.3390/pharmaceutics12070618
Lee J, Kwak D, Kim H, Kim J, Hlaing SP, Hasan N, Cao J, Yoo J-W. Nitric Oxide-Releasing S-Nitrosoglutathione-Conjugated Poly(Lactic-Co-Glycolic Acid) Nanoparticles for the Treatment of MRSA-Infected Cutaneous Wounds. Pharmaceutics. 2020; 12(7):618. https://doi.org/10.3390/pharmaceutics12070618
Chicago/Turabian StyleLee, Juho, Dongmin Kwak, Hyunwoo Kim, Jihyun Kim, Shwe Phyu Hlaing, Nurhasni Hasan, Jiafu Cao, and Jin-Wook Yoo. 2020. "Nitric Oxide-Releasing S-Nitrosoglutathione-Conjugated Poly(Lactic-Co-Glycolic Acid) Nanoparticles for the Treatment of MRSA-Infected Cutaneous Wounds" Pharmaceutics 12, no. 7: 618. https://doi.org/10.3390/pharmaceutics12070618
APA StyleLee, J., Kwak, D., Kim, H., Kim, J., Hlaing, S. P., Hasan, N., Cao, J., & Yoo, J.-W. (2020). Nitric Oxide-Releasing S-Nitrosoglutathione-Conjugated Poly(Lactic-Co-Glycolic Acid) Nanoparticles for the Treatment of MRSA-Infected Cutaneous Wounds. Pharmaceutics, 12(7), 618. https://doi.org/10.3390/pharmaceutics12070618