Terahertz-Based Method for Accurate Characterization of Early Water Absorption Properties of Epoxy Resins and Rapid Detection of Water Absorption
Abstract
:1. Introduction
2. Materials and Methods
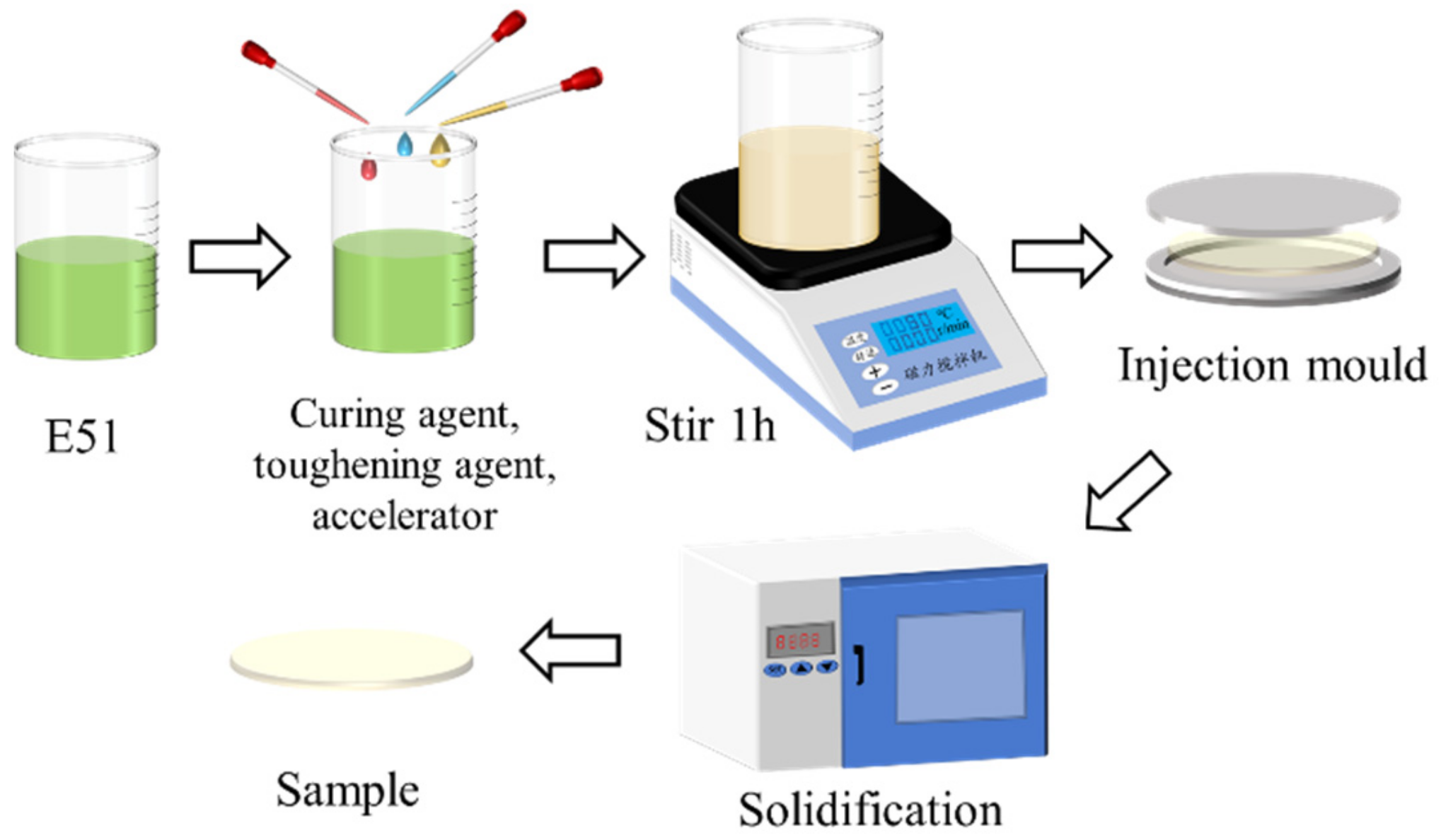
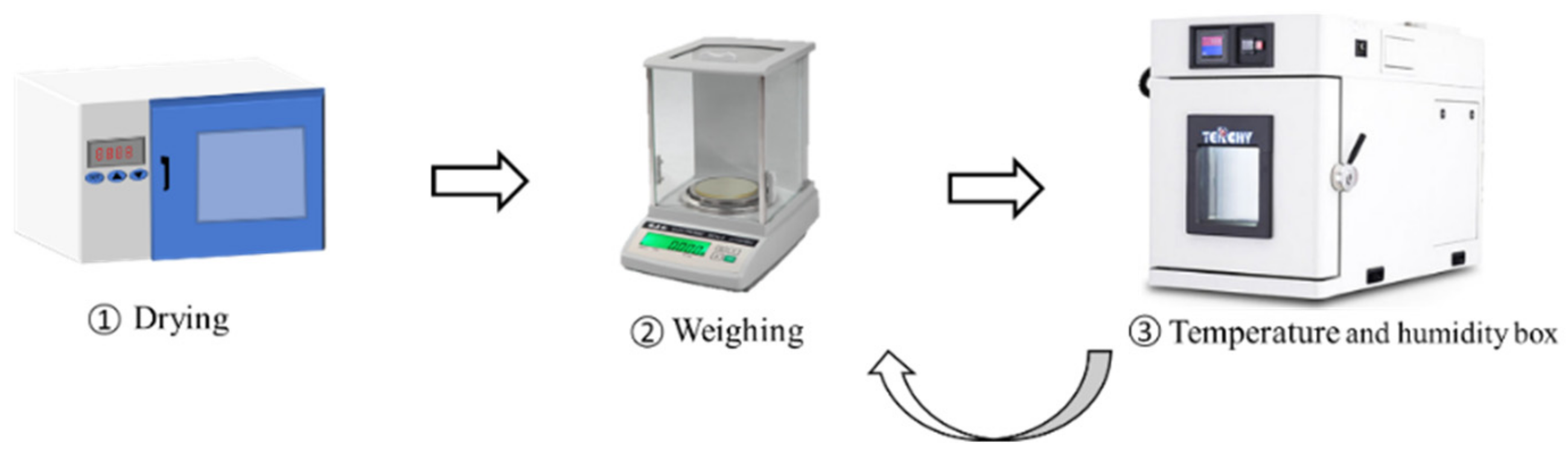
2.1. Materials and Experiments
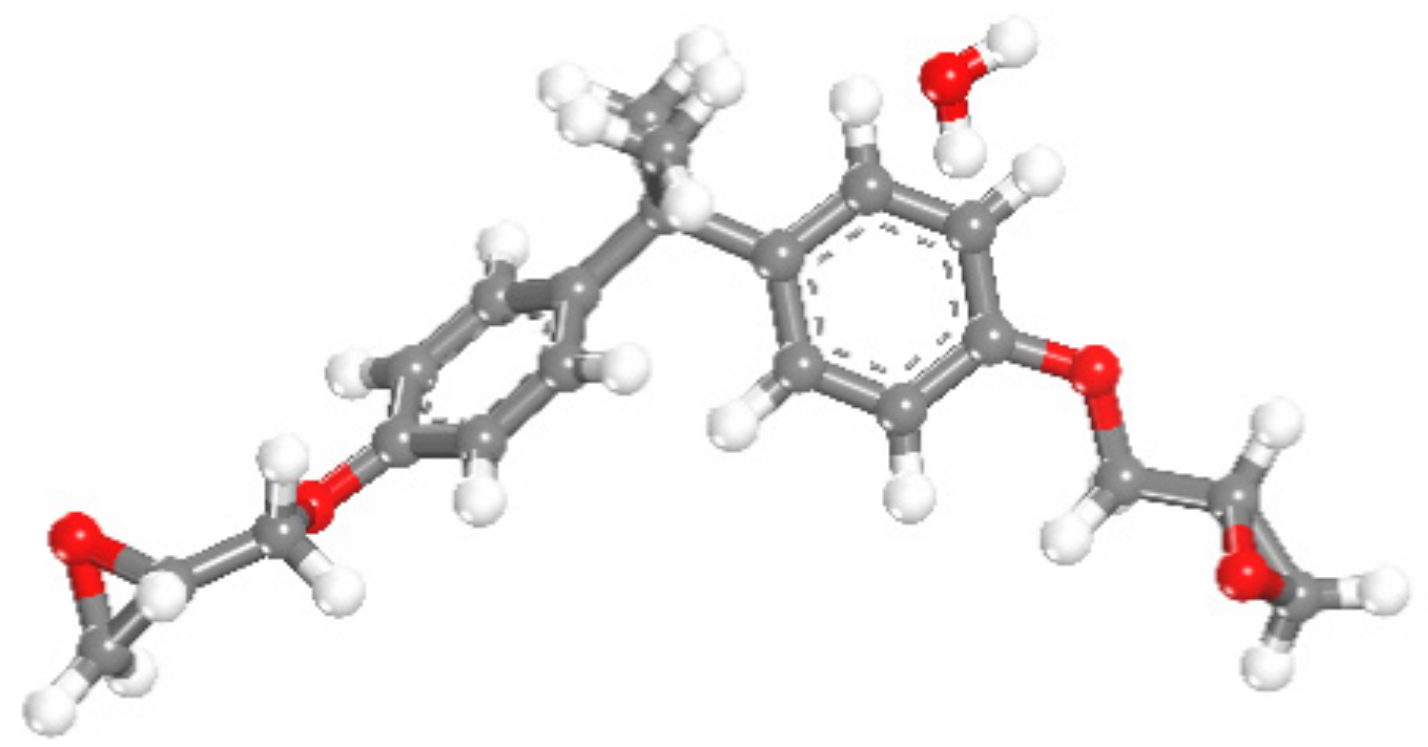
2.2. Molecular Dynamics Simulation
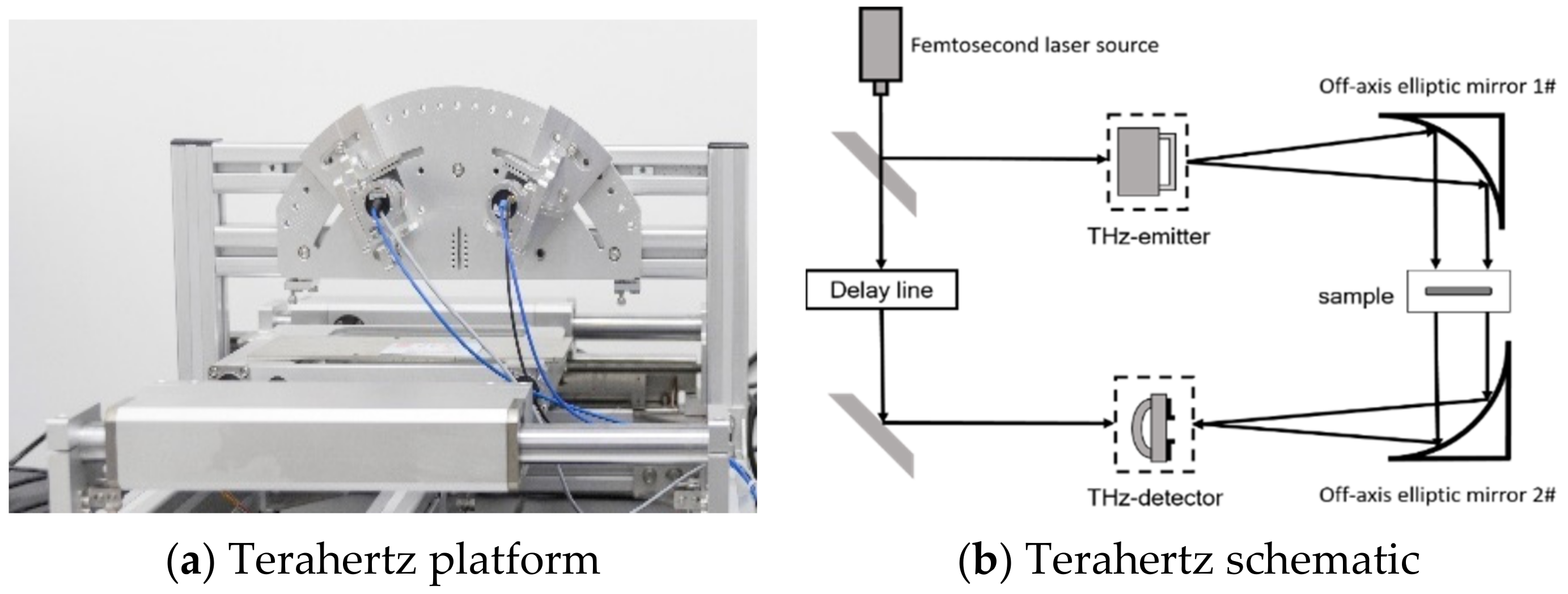
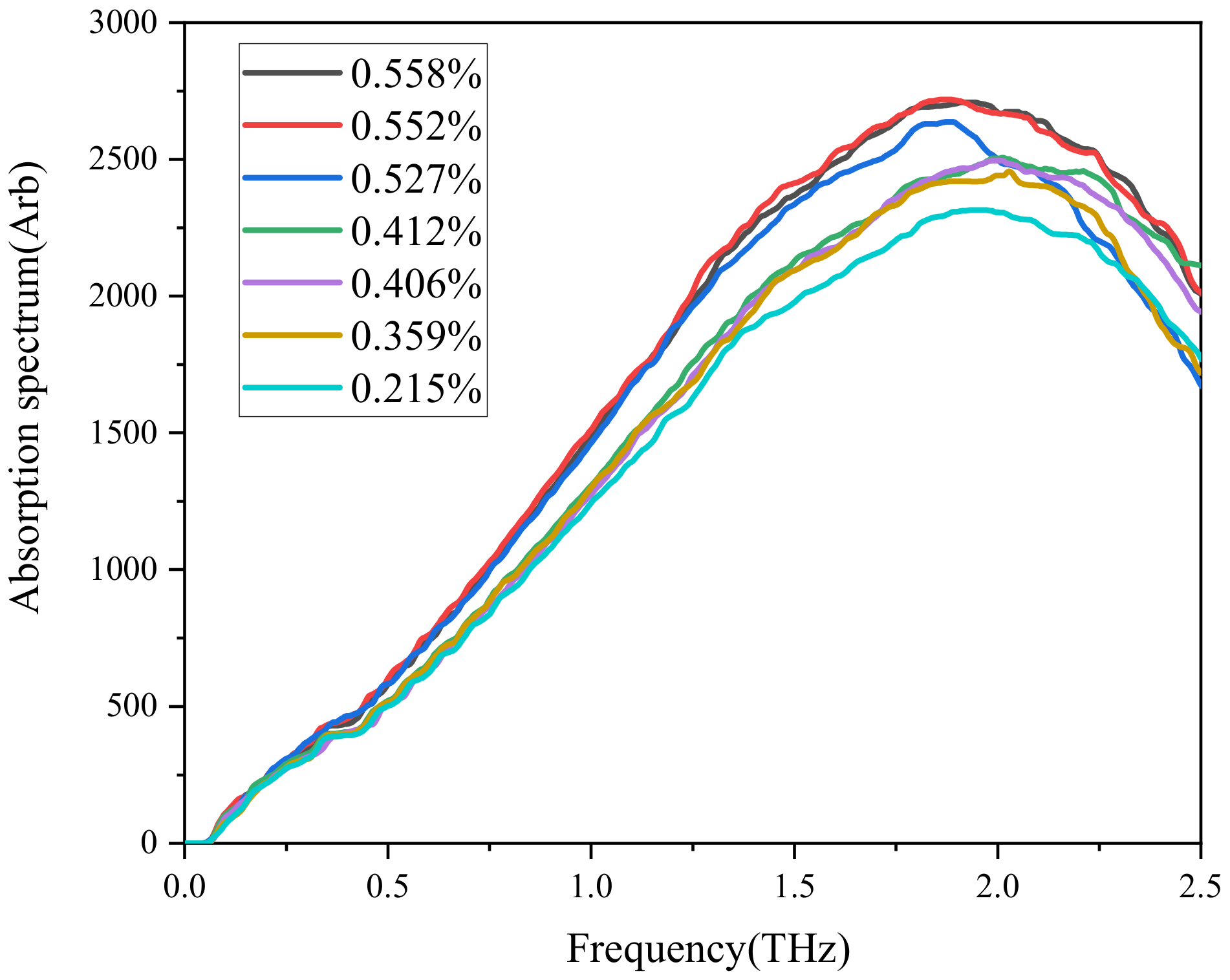
2.3. Terahertz Time-Domain Spectroscopy System
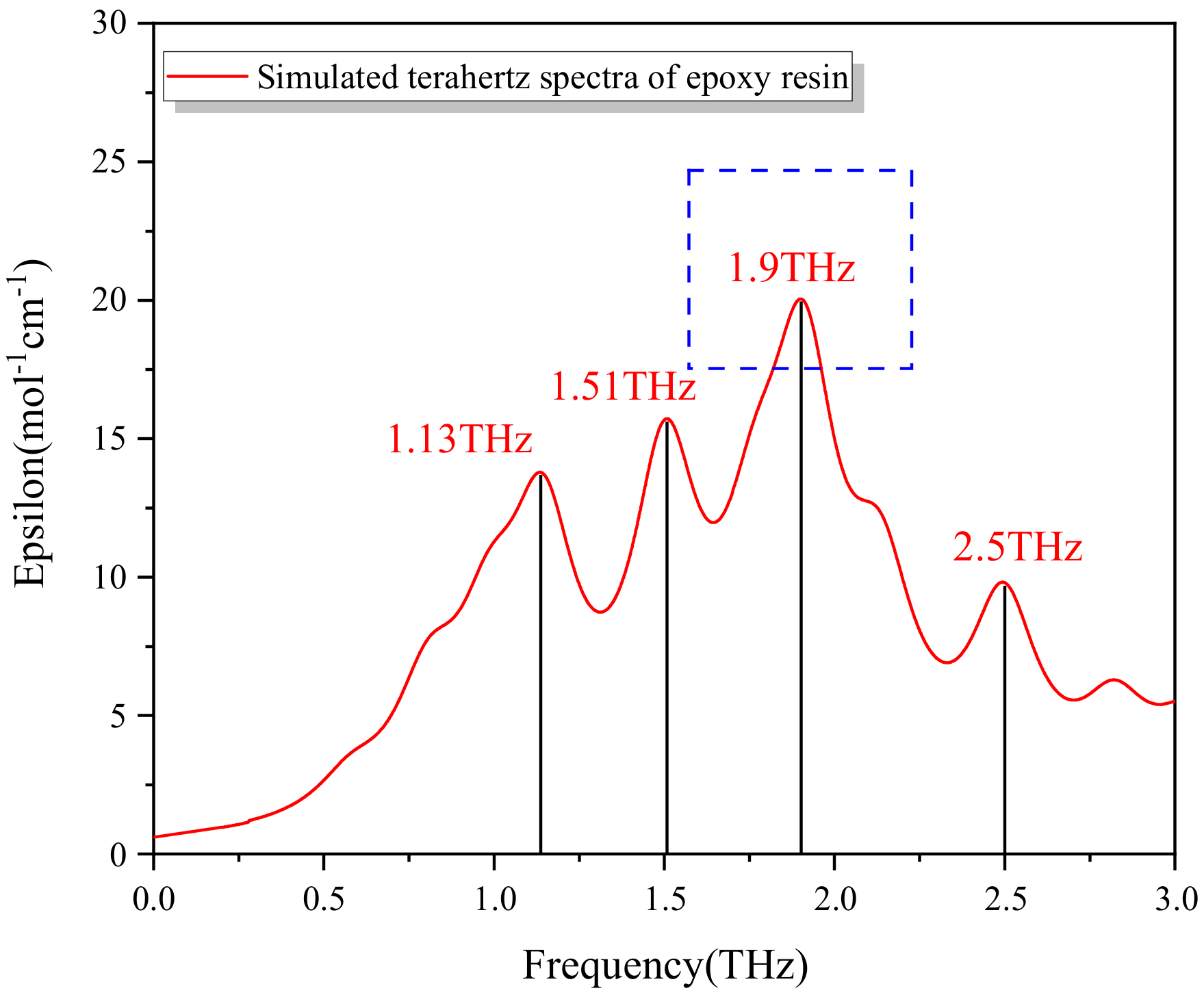
2.4. DFT Methods
3. Study Methods
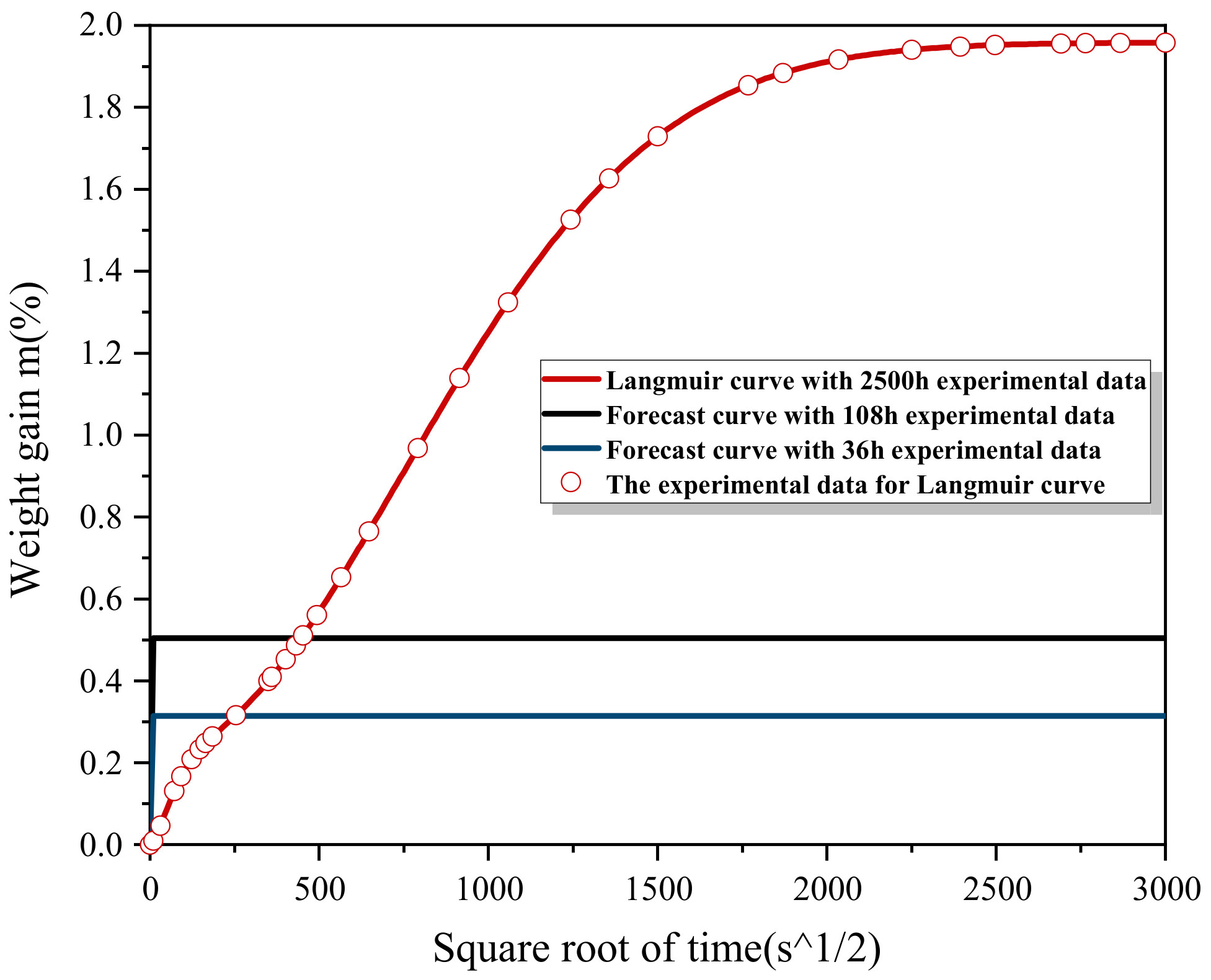
3.1. Physical Model of Water Absorption
3.2. Error Thershold Determination
3.3. ISO 62:2008 Prediction Method
3.4. Fitting Method Based on Langmuir Formula
4. Determination of the Initial Values of the Parameters
4.1. Search Range of Diffusion Coefficient D
4.2. Search Range of α and β
5. Prediction Results for Water Absorption of Epoxy Resin
6. Precise Representation of Early Water Absorbability
7. Conclusions
- (1)
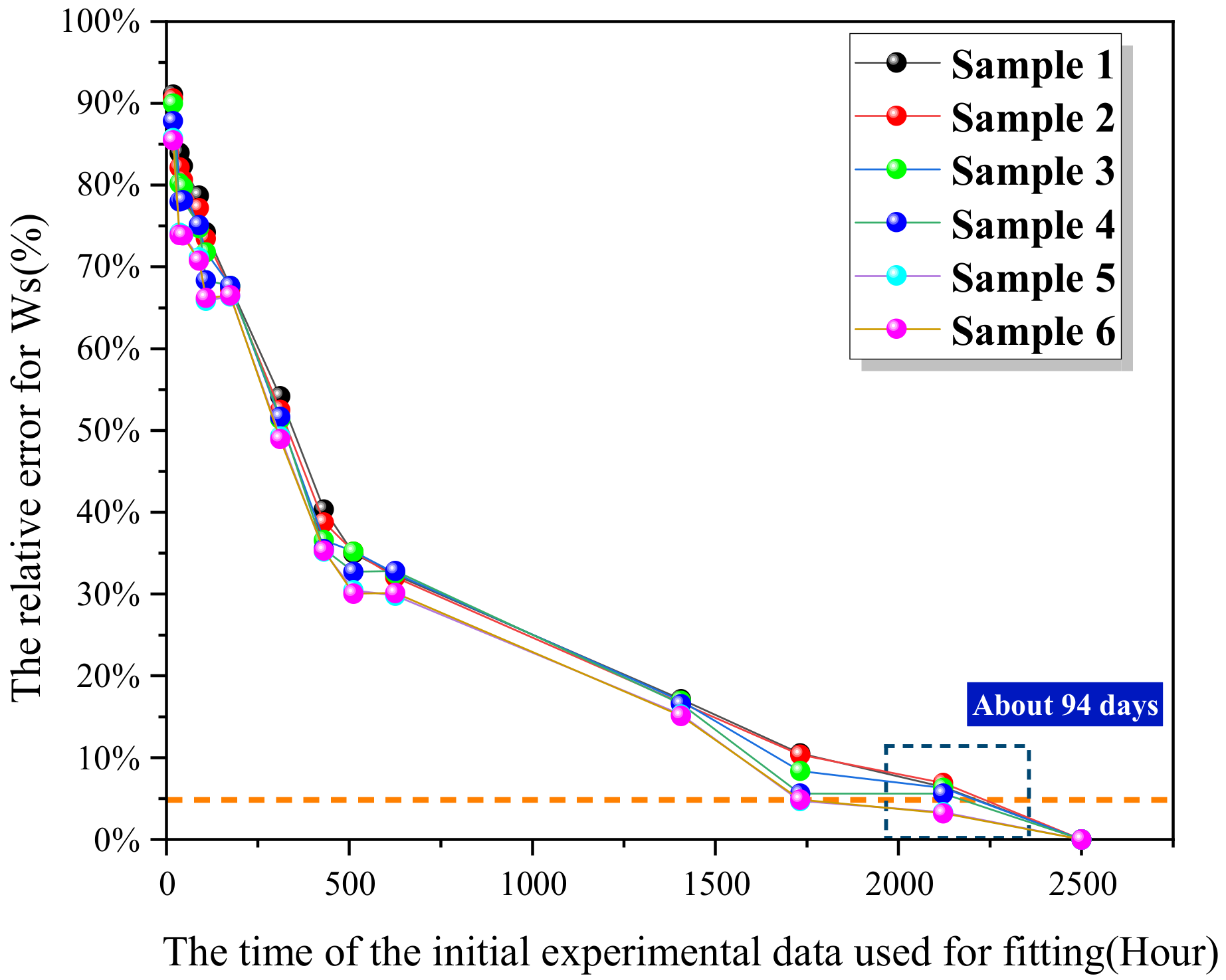
- Based on the nonlinearity and high latitude of the Langmuir diffusion equation, this study has proposed a shrinkage-expansion algorithm-based fitting method, which may realize the fitting of the water absorption process, but the algorithm is still to be improved, for the experimental time is merely reduced from 104 days to 94 days.
- (2)
- The diffusion coefficient of epoxy resin at different temperatures has been simulated and analyzed with a molecular simulation method, and the diffusion coefficient and the temperature satisfy the Arrhenius formula.
- (3)
- Based on the results of molecular simulation, experiment, and the relevant literature, the initial values of diffusion coefficient, bonding coefficient, and de-bonding coefficient have been determined, and the prediction model has been optimized. Thus, the experimental time is further reduced from 108 days to 1.5 days, and the prediction error is no greater than the experimental error of moisture absorption test (5%), and so the engineering requirements may be set generally.
- (4)
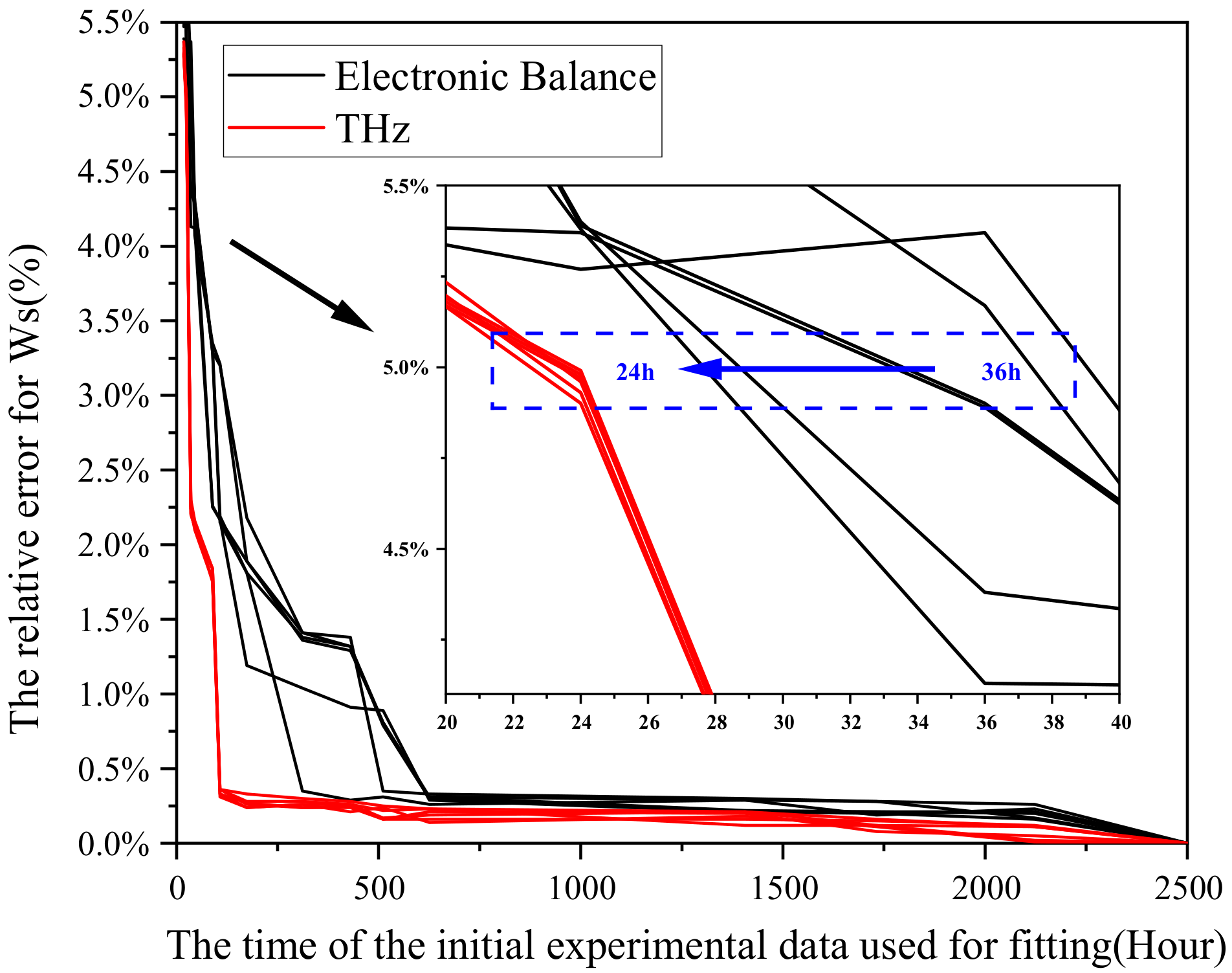
- In order to reduce the influence for fluctuation of balance and further shorten the experimental time required by the model, this study has proposed a method to improve the precision of the model based on terahertz. Under an error threshold of 5%, the terahertz method may shorten the experimental time from 36 h to 24 h.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Awais, M.; Chen, X.; Dai, C.; Meng, F.B.; Paramane, A.; Tanaka, Y. Tuning epoxy for medium frequency transformer application: Resin optimization and characterization of nanocomposites at high temperature. IEEE Trans. Dielectr. Electr. Insul. 2021, 28, 1751–1758. [Google Scholar] [CrossRef]
- Yin, Z.; Sun, P.; Sima, W.; Li, L.; Du, W.; Sui, H. Thermal response properties and surface insulation failure mechanism of epoxy resin under arc ablation. IEEE Trans. Dielectr. Electr. Insul. 2019, 26, 1503–1511. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, Y.; Kong, F.; Wang, R.; Ren, C.; Shao, T. Surface charge decay of epoxy resin treated by AP-DBD deposition and direct fluorination. IEEE Trans. Dielectr. Electr. Insul. 2019, 26, 768–775. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Y.; Chen, X.; Wen, H.; Xiao, S. Theoretical study on decomposition mechanism of insulating epoxy resin cured by anhydride. Polymers 2017, 9, 341. [Google Scholar] [CrossRef]
- He, S.J.; Luo, C.M.; Zheng, Y.Z.; Xue, Y.; Song, X.P.; Lin, J. Improvement in the charge dissipation performance of epoxy resin composites by incorporating amino-modified boron nitride nanosheets. Mater. Lett. 2021, 298, 130009. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, Z.; Gao, M.; Huang, Z. Hygrothermal aging characteristics of silicone-modified aging-resistant epoxy resin insulating material. Polymers 2021, 13, 2145. [Google Scholar] [CrossRef]
- Liao, Y.F.; Weng, Y.X.; Wang, J.Q.; Zhou, H.F.; Lin, J.; He, S.J. Silicone rubber composites with high breakdown strength and low dielectric loss based on polydopamine coated mica. Polymers 2019, 11, 2030. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Meng, Z.; Zhu, W. Hygrothermal aging behavior and aging mechanism of carbon nanofibers/epoxy composites. Constr. Build. Mater. 2021, 294, 123538. [Google Scholar] [CrossRef]
- Wang, B.; Li, D.; Xian, G.; Li, C. Effect of Immersion in water or alkali solution on the structures and properties of epoxy resin. Polymers 2021, 13, 1902. [Google Scholar] [CrossRef]
- Zou, C.; Fothergill, J.C.; Rowe, S.W. The effect of water absorption on the dielectric properties of epoxy nanocomposites. IEEE Trans. Dielectr. Electr. Insul. 2008, 15, 106–117. [Google Scholar] [CrossRef]
- Nakamura, T.; Tabuchi, H.; Hirai, T. Effects of silane coupling agent hydrophobicity and loading method on water absorption and mechanical strength of silica particle-filled epoxy resin. J. Appl. Polym. Sci. 2020, 137, 48615. [Google Scholar] [CrossRef]
- Cabanelas, J.C.; Prolongo, S.G.; Serrano, B.; Bravo, J.; Baselga, J. Water absorption in polyamino siloxane-epoxy thermosetting polymers. J. Mater. Process. Tech. 2003, 143–144, 311–315. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.; Bao, W.; Gao, Y. Decay-like fracture mechanism of silicone rubber composite insulator. IEEE Trans. Dielectr. Electr. Insul. 2018, 25, 110–119. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, J.; Liang, X.; Yan, Z.; Liu, Y.; Cai, Y. Investigation on permeation properties of liquids into HTV silicone rubber materials. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 2428–2437. [Google Scholar] [CrossRef]
- Chen, X.; Wang, J.Q.; Zhang, C.; Yang, W.; Lin, J.; Bian, X.M. Performance of silicone rubber composites using boron nitride to replace alumina tri-hydrate. High Volt. 2021, 6, 480–486. [Google Scholar] [CrossRef]
- Lutz, B. Water absorption and water vapor permeation characteristics of HTV silicone rubber material. In Proceedings of the 2012 IEEE International Symposium on Electrical Insulation, San Juan, PR, USA, 10–13 June 2012; pp. 478–482. [Google Scholar]
- Wang, Z.; Zhao, L.H.; Jia, Z.D.; Guan, Z.C. Water and moisture permeability of high-temperature vulcanized silicone rubber. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 2440–2448. [Google Scholar] [CrossRef]
- Wang, Z.; Jia, Z.D.; Fang, M.H.; Guan, Z.C. Absorption and permeation of water and aqueous solutions of high-temperature vulcanized silicone rubber. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 3357–3365. [Google Scholar] [CrossRef]
- He, S.J.; Wang, J.Q.; Hu, J.B.; Zhou, H.F.; Nguyen, H.; Luo, C.M. Silicone rubber composites incorporating graphitic carbon nitride and modified by vinyl tri-methoxysilane. Polym. Test 2019, 79, 106005. [Google Scholar] [CrossRef]
- Plastics—Determination of Water Absorption (ISO 62:2008). UNE-EN ISO 62-2008. Available online: https://www.iso.org/standard/41672.html (accessed on 1 February 2008).
- Simon, H.; Claus, H.; Michal, K. Temperature dependence of moisture diffusion in woven epoxy-glass composites: A theoretical and experimental study. Mater.Today Commun. 2021, 29, 102844. [Google Scholar]
- Fu, Y.Q.; Yao, L.; Jiang, C.Q. Prediction of water absorption of modification polyvinyl alcohol using BP Neural network. J. Anhui univ. Tech. Sci. (Nat. Sci.) 2007, 22, 44–47. [Google Scholar]
- Hui, L.; Wang, Y.G.; Xu, L.; Ma, S.H.; Zhang, X.; Fei, B.Q. Moisture absorption model of composites considering water temperature effect. J. Mater. Eng. 2016, 44, 83–87. [Google Scholar]
- Wang, L.; Nie, Z.; Zhao, C.; Zhou, J.; Geng, W. Water permeation characteristic in cycloaliphatic epoxy resin composite insulator sheath. High Volt. Eng. 2019, 45, 173–180. [Google Scholar]
- Gao, Y.; Wang, J.; Yan, Z.; Liang, X.; Liu, Y. Investigation on water diffusion property into HTV silicone rubber. Proc. CSEE 2015, 35, 231–239. [Google Scholar]
- Hassan, A.M.; Hufnagle, D.C.; El-Shenawee, M. Terahertz imaging for margin assessment of breast cancer tumors. In IEEE MTT-S international Microwave Symposium Digest; IEEE: Montreal, QC, Canada, 2012; pp. 1–3. [Google Scholar]
- Singh, A.K.; Pérez-López, A.V.; Simpson, J. Three-dimensional water mapping of succulent Agave victoriae-reginae leaves by terahertz imaging. Sci. Rep. 2020, 10, 1404. [Google Scholar] [CrossRef]
- Li, B.; Zhang, X.; Wang, R. Leaf water status monitoring by scattering effects at terahertz frequencies. Spectrochim. Acta. A 2020, 245, 118932. [Google Scholar] [CrossRef]
- Pagano, M.; Baldacci, L.; Ottomaniello, A. THz water transmittance and leaf surface area: An effective nondestructive method for determining leaf water content. Sensors 2019, 19, 4838. [Google Scholar] [CrossRef] [Green Version]
- Carter, H.G.; Kibler, K.G. Langmuir-type model for anomalous moisture diffusion in composite resins. J. Compos. Mater. 1978, 12, 118–131. [Google Scholar] [CrossRef]
- Gu, L.S.; Wan, L.S.; Huang, L.J.; Wang, W.P. Improved contraction-expansion algorithm for curve and surface fitting. Acta Agron. Sin. 2007, 4, 583–589. [Google Scholar]
- Yin, L. Research on water absorption characteristic of cycloaliphatic epoxy resin insulator. In Proceedings of the 2020 IEEE 1st China International Youth Conference on Electrical Engineering (CIYCEE), Wuhan, China, 1–4 November 2020; pp. 1–5. [Google Scholar]
- Popineau, S.; Rondeau-Mouro, C.; Sulpice-Gaillet, C. Free/bound water absorption in an epoxy adhesive. Polymer 2005, 46, 10733–10740. [Google Scholar]






| Temperature | a | R2 | D/cm2/s |
|---|---|---|---|
| 298 K | 0.024 | 0.98 | 3.96 × 10−7 |
| 313 K | 0.025 | 0.98 | 4.19 × 10−7 |
| 328 K | 0.037 | 0.98 | 6.11 × 10−7 |
| 343 K | 0.090 | 0.99 | 1.50 × 10−6 |
| 358 K | 0.222 | 0.99 | 3.71 × 10−6 |
| Water Absorption Test Value of Epoxy Resin/s−1 | Silicone Rubber [16] /s−1 | Epoxy Resin Adhesive (Type EC 2216 from 3M) [33]/s−1 | |
|---|---|---|---|
| α | 7.432 × 10−6 | 1.5 × 10−6 | 4 × 10−8 |
| β | 9.071 × 10−7 | 2.5 × 10−6 | 8.1 × 10−8 |
| The Experimental Time | D | The Relative Error | α/β | The Relative Error | The Relative Error | |
|---|---|---|---|---|---|---|
| The real value | 1.098 × 10−7 | 0 | 8.248 | 0 | 1.958 | 0 |
| 108 h | 1.082 × 10−7 | 1.457% | 8.003 | 2.970% | 1.95 | 0.409% |
| 36 h | 1.052 × 10−7 | 4.189% | 9.113 | 10.487% | 1.912 | 2.349% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, H.; Liu, Y.; Cao, Y.; Wu, J.; Zhang, S.; Zhang, X.; Cheng, L. Terahertz-Based Method for Accurate Characterization of Early Water Absorption Properties of Epoxy Resins and Rapid Detection of Water Absorption. Polymers 2021, 13, 4250. https://doi.org/10.3390/polym13234250
Dong H, Liu Y, Cao Y, Wu J, Zhang S, Zhang X, Cheng L. Terahertz-Based Method for Accurate Characterization of Early Water Absorption Properties of Epoxy Resins and Rapid Detection of Water Absorption. Polymers. 2021; 13(23):4250. https://doi.org/10.3390/polym13234250
Chicago/Turabian StyleDong, Hongchuan, Yunfan Liu, Yanming Cao, Juzhen Wu, Sida Zhang, Xinlong Zhang, and Li Cheng. 2021. "Terahertz-Based Method for Accurate Characterization of Early Water Absorption Properties of Epoxy Resins and Rapid Detection of Water Absorption" Polymers 13, no. 23: 4250. https://doi.org/10.3390/polym13234250
APA StyleDong, H., Liu, Y., Cao, Y., Wu, J., Zhang, S., Zhang, X., & Cheng, L. (2021). Terahertz-Based Method for Accurate Characterization of Early Water Absorption Properties of Epoxy Resins and Rapid Detection of Water Absorption. Polymers, 13(23), 4250. https://doi.org/10.3390/polym13234250







