Abstract
Waste materials are receiving more attention as concerns about the future of our planet increase. Cellulose is the most common substance in agricultural waste. Agricultural wastes containing cellulose are misplaced resources that could be reused in various fields for both environmental and economic benefits. In this work, 32 different kinds of waste are investigated for chemical modification in order to obtain carboxymethyl cellulose for the production of a superabsorbent hydrogel that can be applied in agriculture. A brief literature review is provided to help researchers wishing to obtain carboxymethyl cellulose by carboxymethylation starting with waste materials. We also provide details about methods to obtain as well as verify carboxymethylation. Carboxymethyl cellulose (CMC), as a constituent of cellulosic water and superabsorbent hydrogels with applications in agriculture, is described. Superabsorbent hydrogels with CMC are able to absorb huge amounts of water and are biodegradable.
1. Introduction
Agricultural waste material is receiving more attention as concerns about the future of our planet increase, and it is a resource whose utilization could make an important contribution to establishing a more sustainable living environment [1]. Global industrialization implies an increase in the populations living in small areas, with densely populated centers that present a threat to environmental safety and sustainability, as well food production. The results of the mentioned socio-agriculture management have resulted in global warming and the buildup of chemical and biological contaminants [2]. On the one side is waste material and sustainable agriculture, and on the other is the socio-economic circumstances of living [3]. From this perspective, agricultural wastes containing cellulose are misplaced resources that could be reused in various fields for both environmental and economic benefits. The mediating factor that could enable the transition from harmful waste material into a useful and environmental friendly product is superabsorbent polymers with applications in agriculture. Such polymers present a very desirable class of materials that can be used for different purposes. They have attracted the interest of researchers in many different laboratories. Products made with them fulfil not only their task requirements but also degrade easily in soil [4].
Cellulose is the most common substance in agricultural waste [5]. Moreover, the most abundant renewable carbon source from plant material on Earth is cellulose [6]. It can be extracted from waste materials through chemical processes and be used as a base component for carboxymethyl cellulose synthesis afterwards. In recent years, technological engineers and other scientists have developed new processes in order to produce superabsorbent hydrogels from different waste materials [7]. It was found that chemical methods are more cost-effective in comparison to physical methods [8]. Compared to other natural sources, such as starch and chitosan, cellulose is more readily available [9]. Along with its high absorption properties [10] and biodegradability [11,12], it is desirable and interesting for researchers.
Since not all countries experience sufficient precipitation for agricultural purposes during different times of the year, having a product that allows the slow release of water when it is needed would be very useful. As they can influence the soil permeability and the evaporation and infiltration rates of water through the soils, hydrogels composed of polymers are gaining popularity in agricultural science [13].
There are two major classes of hydrogels used in agriculture: (1) those that improve the water supply in soil for plants and (2) those that enable the controlled release of agrochemicals [14]. The affinity for water is adjustable via control of the crosslinking density of matrices [15]. A molecular crosslinked organization stops the burst release of water [14]. The continuous release of water is a property that has received increased interest in recent years. The superb hydrophilic properties and high swelling ratio of hydrogels have led to their recommendation for application in agriculture. CMC is a biocompatible and biodegradable polymer [16].
The aim of this review paper is to change the view of agricultural wastes as a material to be discard to that of a valuable resource that can be chemically modified and find secondary usage in agriculture. CMC is a substance that can be produced by the chemical modification of cellulose from agricultural wastes and afterwards find implementation in the preparation of a biodegradable superabsorbent hydrogel; for this reason, it is highlighted in this paper.
2. Materials and Methods
A detailed review of the available published scientific papers was conducted. Using the Google Scholar database, Scopus, Web of Science and PubMed, electronic research of published papers was performed for the period up to July 2021. There was no exclusion of any language when searching the literature. The used search terms were: cellulose, carboxymethyl cellulose, waste material, superabsorbent hydrogel carboxymethyl cellulose, superabsorbent polymer. We considered whether studies pertained to agriculture, polymer synthesis, sustainable development or biodegradation.
3. Useful Waste Material
Millions of tons of agricultural wastes (~731 rice straw, ~354 wheat straw, ~203 corn straw, ~180 bagasse) generated each year present a serious challenge for sustainability [8]. In reality, this waste material is burned [17], generating huge amounts of harmful gases, such as CO2, N2O and CH4; air pollutants, such as CO, NH3, NOx, SO2 and other volatile organic chemicals; and contaminating particulate matter [18]. As the concerns about CO2 emissions grow, interest in the utilization of lignocellulosic materials from wastes as abundant sources of substances is growing. This is where the sustainable management of agricultural waste is urgently needed. A move towards green material solutions is a step forward according to a sustainable development policy and environmental neutrality [19,20]. The most important advantage in the use of these materials is that they are affordable worldwide [21].
3.1. Characteristics of Cellulose in Agricultural Wastes
Plant biomass is a useful industrial material for researchers focusing on environmental concerns. It consists of cellulose, hemicelluloses, lignin, pectins, gums, mucilages, starch and proteins. Approximately 33% of all plant biomass contains cellulose as the major component because it is the constituent of the rigid cell walls. In fact, cellulose is the most common organic compound on Earth. For example, cotton consists of 90% cellulose and wood contains 40–50%. For industrial use, wood pulp and cotton are the primary sources of cellulose.
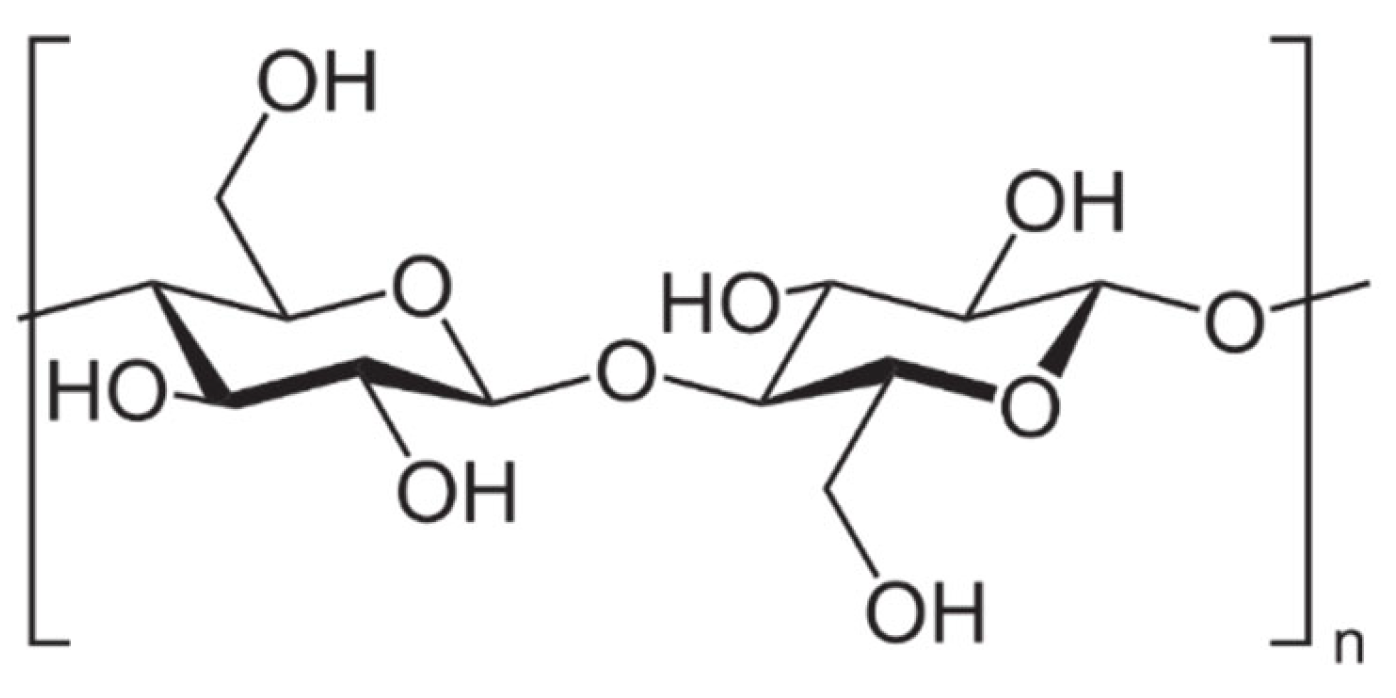
The cell walls of lignocellulosic agricultural wastes consist of cellulose (35–65%), hemicellulose (20–45%) and lignin (10–25%). Cellulose (Figure 1) is always the main component, regardless of the content of these three types of biopolymers, which can vary among different plant species [22,23]. In any case, cellulose is chemically described as a linear homopolysaccharide of β-D-glucopyranose units, i.e., a repeating unit known as cellubiose (a dimer of glucose) lined up by β-1,4-linkages [24]. The glucose units in the same chain and between different chains are linked by strong hydrogen bonds due to the hydroxyl groups of the anhydroglucose unit (the monomer of cellubiose) [25]. Because of the strong hydrogen bonding, cellulose is durable and insoluble in water and most organic solvents. The process of removing the non-cellulosic components by different mechanical operations, chemical reactions or biological processes constitutes the extraction process of cellulose from agricultural wastes [8].
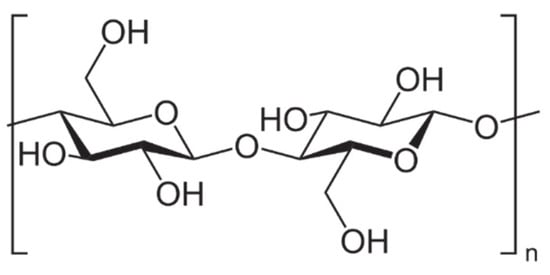
Figure 1.
Cellulose chain repeating unit.
3.2. Waste Material Treatment
A combination of acid–chlorite and alkaline processing is the most commonly used pretreatment method. With the chemical reaction using acid–chlorite, known as delignification or bleaching, most of the lignin and other components is eliminated with a mixture of water, acetic acid and sodium chlorite. The mixture solution with waste material needs to be stirred at 70–80 °C for 4–12 h, and after this process, the solid products of the chemical reaction (holocellulose, consisting dominantly of cellulose and hemicellulose) are washed wish distilled water until pH = 7 and then dried in oven at 50 °C. Then, the solid products need to be treated with an alkaline solution (4–20% (v/v) sodium hydroxide) for 1–5 h in order to remove the amorphous polymer of hemicellulose and residual lignin. The final solid products obtained after washing with distilled water until pH = 7 and dried in an oven at 50 °C consist mostly of cellulose [24].
4. Chemical Modification of Cellulose
4.1. Carboxymethylation by the Classic Method
Cellulose is an easy target for chemical derivatization and, for this reason, it is used as a raw material in the chemical industry [26]. The carboxymethylation reaction is a multivariable-dependent transformation whose outcome is a waterswellable or water-soluble polymer with highly desirable features [27,28,29,30]. Revised conditions and reagents for the chemical reaction are given in Table 1.

Table 1.
Reaction conditions for carboxymethylation.
By this method, used by Moussa et al. for the synthesis of CMC from agricultural waste consisting of almond shells, synthesis is carried out in two steps: alkalization of cellulose and its etherification (further details of this chemical reaction will be discussed below). In the mentioned study, during the alkalization of cellulose, 10 g of cellulose was suspended in a solvent solution under magnetic stirring at 80 °C. A volume of 60 mL of NaOH from a 40% aqueous NaOH solution was added to the reaction mixture after 1 h. In order to convert hydroxyl to alcoholate groups, stirring was continued for a further 14 h at the same temperature. Then, the reaction mixture was ready for the etherification reaction, which was conducted by adding 17.4 g of monochloroacetate (MCA). It was stirred for 8 h at a temperature of 80 °C. The etherification process took place as in Equation (2) to obtain CMC and a side reaction that results in the formation of sodium glycolate, as expressed in Equation (3). The surplus alkali was neutralized with 90% (v/v) acetic acid, and 500 mL of ethanol was added to induce precipitation. The obtained CMC was recovered by filtration and washed with a mixture of ethanol/water 80/20 (v/v). The final product was washed with pure ethanol and dried in oven at 50 °C for 24 h [31].
4.2. Microwave-Assisted Synthesis of CMC
By this method, used by Moussa et al. for the synthesis of CMC from agricultural waste consisting of almond shells, almond stems and fig stems, cellulose (3 g) was mixed with 30 mL of water in the reaction vessel. Magnetic stirring at room temperature was performed for 20 min, 20 mL of 40% (v/v) sodium hydroxide was added and the reaction was performed in a microwave reactor. After this, 5.8 g of MCA was added to the reaction mixture, in order to perform the etherification reaction. This was followed by the neutralization of surplus alkali with 90% (v/v) acetic acid. For precipitation, 200 mL of ethanol was added. The CMC was collected as a filtrate and washed with a mixture of ethanol/water 80/20 (v/v) in order to remove salt impurities. The final product was washed with pure ethanol and dried in an oven at 50 °C for 24 h [31].
4.3. Proof of Carboxymethylation
4.3.1. Degree of Substitution
After the carboxymethylation of cellulose is achieved, the success and efficiency of the process should be measured. The degree of substitution (DS) of CMC is defined as the average number of hydroxyl groups in the cellulose structure, which was substituted by carboxymethyl and sodium carboxymethyl groups at C2, 3 and 6.
The degree of substitution is dependent on the reaction conditions for the water-soluble products obtained by carboxymethylation [37]. The variables affecting the DS of CMC are: the concentration of monochloroacetic acid, the temperature and reaction time, the reaction medium and the concentration of NaOH, where a higher number for DS indicates higher solubility of the CMC [52,53,54].
The first chemical reaction is between the hydroxyl groups of cellulose and NaOH, where a more reactive alkaline form of cellulose is generated (1).
CLL–OH + NaOH → CLL–ONa + H2O
(Cellulose)(Reactive alkaline form)
This reaction is followed by the reaction of etherification, where CMC is obtained (2), and a reaction in which sodium glycolate is produced as a byproduct (3).
CLL–ONa + Cl–CH2–COONa → CLL–O–CH2–COONa + NaCl + H2O
(CMC)NaOH + Cl–CH2–COONa → HO–CH2–COONa + NaCl
(Sodium glycolate)
Since the transformation of cellulose to the desired carboxymethyl cellulose takes place during this step, it is of importance to know how each parameter contributes to the product of the chemical reaction. Pushpamalar et al. optimized the reaction conditions for preparing carboxymethyl cellulose from sago waste [49]. They explained the effect of varying parameters in this reaction, including different solvent media, time, temperature, the amount of sodium monochloroacetate and the concentration of sodium hydroxide.
The effects of different solvent media, such as water, dimethylformamide, methanol, dimethyl sulfoxide, isopropyl acohol, ethanol and butanol, were assessed by Pushpamalar et al. A maximum DS was obtained with isopropyl alcohol as a solvent medium. It was explained that solvent polarities and stereochemistry play a crucial role in this result, and as the polarity of the solvent decreases, the reaction efficiency increases. Regarding the reaction time, there was an increase in DS up to 180 min. By that time, the better reaction environment and prolonged time contributed to the higher availability of molecules for carboxymethylation, favoring the diffusion and absorption of reactants. After 180 min, a decrease was noticed, which may be attributed to the degradation of the polymer. Regarding the obtained results for DS with temperature as a changing parameter, there was an increase up to 45 °C, followed by a decrease. The explanation for this decrease is the degradation of cellulose, or, more precisely, the elimination of molecules of water with the formation of unsaturation on C2, C3 or a ketone functional group on C2. In parallel with this reaction, intermolecular elimination between nearby hydroxyl groups raises the crosslinking by ether linking, decreasing the number of hydroxyl groups available for carboxymethylation. The amount of chemical reactant NaMCA directly affects the formation of NaCMC. An increase in DS occurs until one point, after which it decreases. This is because of the higher concentration of acetate ions. Above the concentration that provides the maximum DS, it is to be expected that sodium glycolate formation is favored. The concentration of sodium hydroxide as a parameter that affects the formation of NaCMC also has one point until DS increases and reaches its maximum. The explanation for this can be found in the carboxymethylation process, where two competitive reactions take place. From the first one, CMC is obtained as a product (Equation (2)), and sodium glycolate is obtained from the second (Equation (3)). For concentrations until the maximum of DS is reached, the first reaction overcomes the second, but above this point, the formation of sodium glycolate prevails due to the inactivation of MCA and its depletion in the side reaction [49].
For the determination of DS, two methods are mostly applied: the USPXXXII method described for croscarmellose sodium, which is practically potentiometric back-titration [41,55], and the “ashing method” [56].
It should be noted that only for Mimosa pigra peel, DS is <0.4 (0.23; Table 1) and thus it could not be used for further investigation in this work.
4.3.2. Infrared Spectroscopy
The instrumental method of choice for confirmation of the carboxymethylation process is infrared spectroscopy. An infrared spectrophotometer allows observation of the carboxymethyl groups on molecules of cellulose. It is used in the process of the chemical transformation of cellulose from Mimosa pigra [41]. The instrument registered the same functional groups from cellulose and CMC, such as –OH, –CH2, C=O and –O–. However, the difference was noticeable in the high increase in –CH2, C=O and –O–, while the band of –OH decreased from the CMC sample when compared with molecules of cellulose itself.
5. CMC
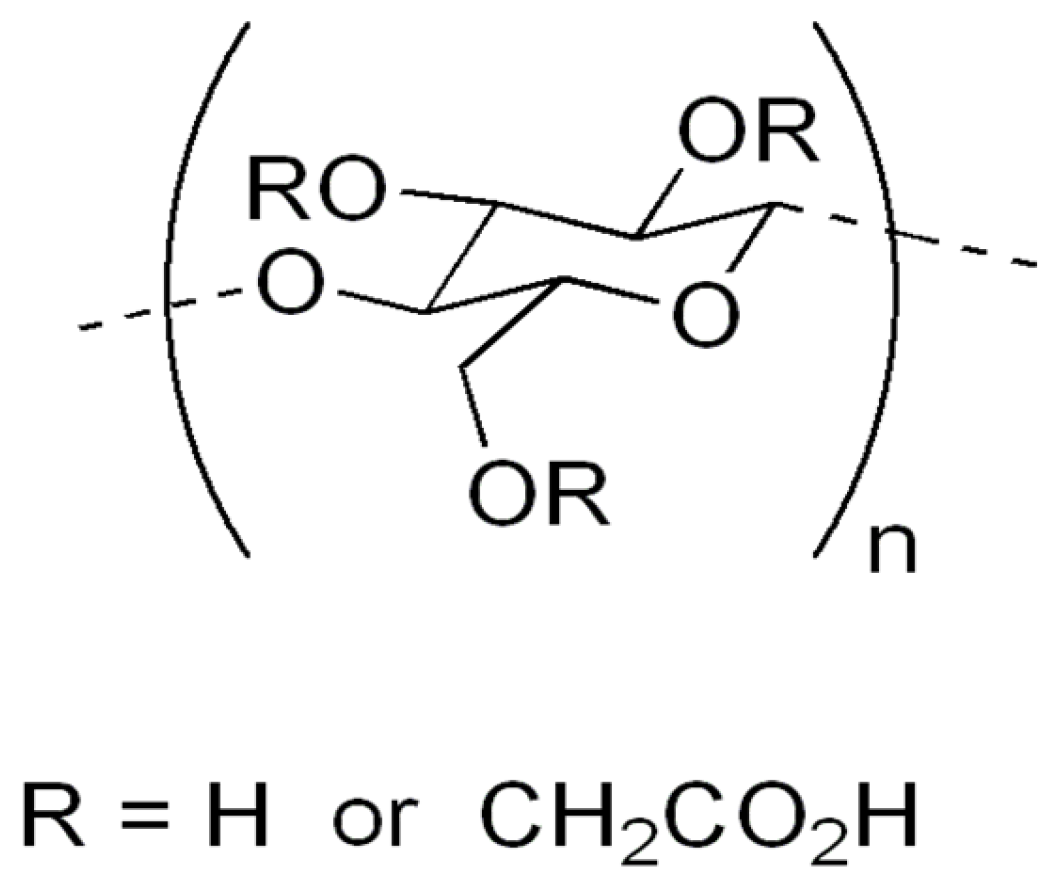
Carboxymethyl cellulose (CMC) (Figure 2) is probably the best-known cellulose derivative, which is water-soluble cellulose ether, obtained by reacting sodium monochloroacetate with cellulose in an alkaline medium [9]. This cellulose derivative is an anionic, linear, highly viscous polysaccharide, confirmed as a nontoxic, non-allergenic and biodegradable substance [57,58]. Because all reactions for synthesis are operated at atmospheric pressure, by using commercially available reagents, the production of CMC is simpler than that of most other cellulose ethers [59]. It has a huge range of different applications in different products and processes, such as oil drilling [60], detergents, food exploration, paper, textiles, pharmaceuticals [61] and the agricultural industry [13]. Recently, CMC has found new interest in advanced fields such as tissue engineering [62], dye and lithium ion batteries [63] and the adsorption of heavy metals [64,65,66].
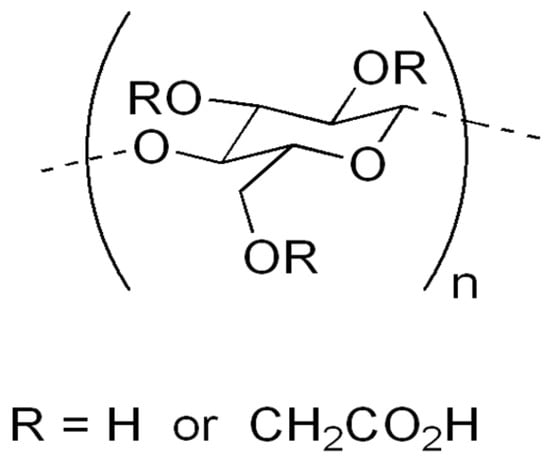
Figure 2.
Carboxymethyl cellulose.
This very important and frequently used biopolymer in industry (industrial production of CMC is approximately 360,000 metric tons per year) has partial substitution of the 2, 3 and 6 hydroxyl groups of cellulose by carboxymethyl groups [67,68]. Because of the countless hydroxyl and carboxylic groups provided, it is able to absorb and hold water. The cheapness and simplicity of the production process of this anionic and water-soluble natural polymer has already been shown [49,69,70,71]. The hydrophilicity of CMC increases with an increase in the DS number. Those with DS < 0.4 can swell but remain insoluble, while those with DS > 0.4 are water-soluble and have high viscosity [72]. Therefore, DS is a parameter that directs the final application [29] and thus makes it possible for the material to be applied in agriculture.
5.1. Cellulosic Water
A combination of carboxymethyl cellulose, as a cellulosic substrate, and hydrated metallic salts results in the formation of a soft gel named cellulosic water (CW) [73]. In direct physical contact with the growing medium, water is released due to the activity of cellulolytic enzymes of microorganisms living in the growing medium because the gel structure breaks down [57]. This is the mechanism by which soil obtains extra water moisture. CW does not have the ability to become rehydrated and it is applied fully hydrated, with limited contact with the growing medium. Stamps and Savage tested the possibility of delayed wilt of containerized poinsettias held under simulated conditions. It was effective in delaying wilt for 4 weeks [13]. In addition, another study considered the usage of CW for a containerized plant of Rhododendron indicum under greenhouse conditions. The growing medium was supplemented with CW mass 0.086 and 0.172 g/cm3 of growing medium. In this way, plants were kept from wilting for 4 weeks [74].
5.2. Superabsorbent Hydrogels
Superabsorbent hydrogels consist of three-dimensional networks structured by hydrophilic polymers formed by crosslinking, and they can absorb and hold significant amounts of water [75,76]. When applied on terrain, a superabsorbent hydrogel acts as a water reservoir. As a result, water-depleted regions can overcome hot periods with insufficient rainfall.
The interest in the application of superabsorbent hydrogels in agriculture is growing [77]. Carboxymethyl cellulose is an essential substance for preparing biodegradable superabsorbent hydrogels for agricultural purposes. This is supported by results that have shown that environmental sustainability and the circular economy in agriculture are promoted by the development of agricultural-waste-derived superabsorbent hydrogels [8]. In Table 2, the chemical composition of superabsorbent hydrogels containing CMC is given.

Table 2.
Superabsorbent hydrogels containing carboxymethyl cellulose in their composition.
Due to the simplicity of the composition, the most interesting superabsorbent may be the one consisting only of CMC. The water uptake for 1 g of 10% CMC gel was reported to be ~450 g of water. Swelling behavior is described by second-order kinetics. This material showed better swelling ability compared to hydroxylethyl cellulose and methyl cellulose [79]. The same authors produced a superabsorbent hydrogel consisting of CMC and starch in different ratios. The highest water uptake, ~350 g of water per 1 g of gel, was reported to be achieved with a hydrogel containing 70% CMC and 30% starch [80].
However, the results that are the most thematically significant because they are in accordance with the concept of sustainable agriculture are those obtained by Dai and Huang, who prepared a superabsorbent hydrogel consisting of CMC obtained from pineapple peel, acrylic acid and acrylamide [1], with water uptake of 420.17 g of water per 1 g of superabsorbent; meanwhile, the formulation prepared by Salleh et al. consisted of CMC obtained from oil palm, empty fruit bunches, cellulose and ammonium persulfate and N,N′-methylenebisacrylamide as crosslinkers, which was able to uptake 80 g of water per 1 g of superabsorbent [86].
5.3. Enzymatic Degradation of CMC Hydrogel
The term biodegradation has attracted increased attention as plastic materials used in everyday life contaminate the environment. The application of biodegradable polymers addresses this concern because of their conversion into carbon dioxide, water and energy. This desirable feature is limited to aliphatic polyesters, polyethers and some polysaccharides, including cellulose derivatives. Glycosidic linkages bonding polysaccharide rings are targets for microorganisms and hydrolytic enzymes in order to be biodegraded. Cellulose ethers, such as carboxymethyl cellulose, display this feature [90,91,92]. Compared to acrylate- and acrylamide-based hydrogels, these are biodegradable and biocompatible [93].
6. Conclusions
Plant waste material is a valuable source of cellulose. It is possible to produce superabsorbent hydrogels from plant waste material via chemical modification, which improves agriculture production. Carboxymethyl cellulose is a substance with a huge range of application, and there are more to be discovered. The discussion presented in this work is relevant to people and industries within different professional fields and has the goal of connecting them in order to achieved sustainable food production and environmental protection. With the ease of implementation, all parties will benefit. It is in everyone’s interest to apply this concept further within society.
Author Contributions
Conceptualization, V.M. and I.G.; methodology, V.M.; software, I.G.; validation, L.N.; formal analysis, I.G.; investigation, V.M. and I.G.; writing of the manuscript with input from all authors, V.M., I.G. and L.N.; visualization, V.M.; project supervision, L.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Republic of Serbia’s Ministry of Education, Science and Technological Development Program for financing scientific research work, number 451-03-9/2021-14/200133.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
References
- Dai, H.; Huang, Y.; Huang, H. Eco-friendly polyvinyl alcohol/carboxymethyl cellulose hydrogels reinforced with graphene oxide and bentonite for enhanced adsorption of methylene blue. Carbohydr. Polym. 2018, 185, 1–11. [Google Scholar] [CrossRef]
- Yazdani, F.; Allahdadi, I.; Abas Akbari, G. Impact of superabsorbent polymer on yield and growth analysis of soybean (Glycine max L.) under drought stress condition. Pak. J. Bio. Sci. 2007, 10, 4190–4196. [Google Scholar] [CrossRef] [Green Version]
- Xiquan, L.; Tingzhu, Q.; Shaoqui, Q. Kinetics of the carboxymethyl cellulose in the isopropyl alcohol system. Acta Polym. 1990, 41, 220. [Google Scholar] [CrossRef]
- Darwis, D.; Mitomo, H.; Yoshii, F. Degradability of radiation crosslinked PCL in the supercooled state under various environments. Polym. Degrad. Stab. 1999, 65, 279–285. [Google Scholar] [CrossRef]
- Li, S.; Chen, G.; Anandhi, A. Applications of emerging bioelectrochemical technologies in agricultural systems: A current review. Energies 2018, 11, 2951. [Google Scholar] [CrossRef] [Green Version]
- Van de Vyver, S.; Geboers, J.; Jacobs, P.A.; Sels, B.F. Recent advances in the catalytic conversion of cellulose. ChemCatChem 2011, 3, 82–94. [Google Scholar] [CrossRef]
- Montesano, F.; Parente, A.; Santamaria, P.; Sannino, A.; Serio, F. Biodegradable superabsorbent hydrogel increases water retention properties of growing media and plant growth. Agric. Agric. Sci. Procedia 2015, 4, 451–458. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Chen, G. Agricultural waste-derived superabsorbent hydrogels: Preparation, performance, and socioeconomic impacts. J. Clean. Prod. 2019, 251, 119669. [Google Scholar] [CrossRef]
- Bao, Y.; Ma, J.; Li, N. Synthesis and swelling behaviors of sodium carboxymethyl cellulose-g-poly (AA-co-AM-co-AMPS)/MMT superabsorbent hydrogel. Carbohydr. Polym. 2010, 84, 76–82. [Google Scholar] [CrossRef]
- Lionetto, F.; Sannino, A.; Maffezzoli, A. Ultrasonic monitoring of the network formation in superabsorbent cellulose based hydrogels. Polymer 2005, 46, 1796–1803. [Google Scholar] [CrossRef]
- Lim, K.Y.; Yoon, K.J.; Kim, B.C. Highly absorbable lyocell fiber spun from cellulose/hydrolyzed starch-g-PAN solution in NMMO monohydrate. Eur. Polym. J. 2003, 39, 2115–2120. [Google Scholar] [CrossRef]
- Sannino, A.; Maffezzoli, A.; Nicolais, L. Introduction of molecular spacers between the crosslinks of a cellulose-based superabsorbent hydrogel: Effects on the equilibrium sorption properties. J. Appl. Polym. Sci. 2003, 90, 168–174. [Google Scholar] [CrossRef]
- Stamps, R.H.; Savage, H.M. Cellulosic water and polyacrylamide gels can delay wilting and extend watering intervals for potted poinsettias displayed in interiorscapes. Horttechnology 2012, 22, 766–770. [Google Scholar] [CrossRef]
- Ganguly, S.; Das, N.C. Water uptake kinetics and control release of agrochemical fertilizers from nanoclay-assisted semi-interpenetrating sodium acrylate-based hydrogel. Polym. Plast. Technol. Eng. 2017, 56, 744–761. [Google Scholar] [CrossRef]
- Gajić, I.; Ilić-Stojanović, S.; Dinić, A.; Zdravković, A.; Stanojević, L.; Nikolić, V.; Nikolić, L. Modified biochanin a release from dual pH- and thermo-responsive copolymer hydrogels. Polymers 2021, 13, 426. [Google Scholar] [CrossRef]
- Hebeish, A.; Hashem, M.; El-Hady, M.M.A.; Sharaf, S. Development of CMC hydrogels loaded with silver nano-particles for medical applications. Carbohydr. Polym. 2013, 92, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Candido, R.G.; Goncalves, A.R. Synthesis of cellulose acetate and carboxymethylcellulose from sugarcane straw. Carbohydr. Polym. 2016, 152, 679–686. [Google Scholar] [CrossRef]
- Cao, G.; Zhang, X.; Gong, S.; Zheng, F. Investigation on emission factors of particulate matter and gaseous pollutants from crop residue burning. J. Environ. Sci. 2008, 20, 50–55. [Google Scholar] [CrossRef]
- Bendaoud, A.; Chalamet, Y. Plasticizing effect of ionic liquid on cellulose acetate obtained by melt processing. Carbohydr. Polym. 2014, 108, 75–82. [Google Scholar] [CrossRef]
- Heguaburu, V.; Franco, J.; Reina, L.; Tabarez, C.; Moyna, G.; Moyna, P. Dehydration of carbohydrates to 2-furaldehydes in ionic liquids by catalysis with ion exchange resins. Catal. Commun. 2012, 27, 88–91. [Google Scholar] [CrossRef]
- Daud, W.R.W.; Djuned, F.M. Cellulose acetate from oil palm empty fruit bunch via a one step heterogeneous acetylation. Carbohydr. Polym. 2015, 132, 252–260. [Google Scholar] [CrossRef]
- Sarkar, N.; Ghosh, S.K.; Bannerjee, S.; Aikat, K. Bioethanol production from agricultural wastes: An overview. Renew. Energy 2012, 37, 19–27. [Google Scholar] [CrossRef]
- Van Wyk, J.P.N. Biotechnology and the utilization of biowaste as a resource for bioproduct development. Trends Biotechnol. 2001, 19, 172–177. [Google Scholar] [CrossRef]
- Phanthong, P.; Reubroycharoen, P.; Hao, X.; Xu, G.; Abudula, A.; Guan, G. Nanocellulose: Extraction and application. Carbon Resour. Convers. 2018, 1, 32–43. [Google Scholar] [CrossRef]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef] [PubMed]
- Barkalow, D.G.; Young, R.A. Cellulose derivatives derived from pulp and paper mill sludge. J. Wood Chem. Technol. 1985, 5, 293–312. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.-P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Heinze, T.; Koschella, A. Carboxymethyl ethers of cellulose and starch-a review. Macromol. Symp. 2005, 223, 130–139. [Google Scholar] [CrossRef]
- Feddersen, R.L.; Thorp, S.N. Industrial Gums and Their Derivatives; Academic Press: New York, NY, USA, 1993; pp. 537–578. [Google Scholar]
- Sandford, P.A.; Baird, J. The Polysaccharides; Academic Press: Reading, MA, USA, 1983; pp. 411–490. [Google Scholar]
- Moussa, I.; Khiari, R.; Moussa, A.; Belgacem, M.H.; Mhenni, M.F. Preparation and characterization of carboxymethyl cellulose with a high degree of substitution from agricultural wastes. Fibers Polym. 2019, 20, 933–943. [Google Scholar] [CrossRef]
- Klunklin, W.; Jantanasakulwong, K.; Phimolsiripol, Y.; Leksawasdi, N.; Seesuriyachan, P.; Chaiyaso, T.; Insomphun, C.; Phongthai, S.; Jantrawut, P.; Sommano, S.R.; et al. Synthesis, Characterization, and Application of carboxymethyl cellulose from asparagus stalk end. Polymers 2021, 13, 81. [Google Scholar] [CrossRef]
- Huang, C.M.Y.; Chia, P.X.; Lim, C.S.S.; Nai, J.Q.; Ding, D.Y.; Seow, P.B.; Wong, C.W.; Chan, E.W.C. Synthesis and characterisation of carboxymethyl cellulose from various agricultural wastes. Cellul. Chem. Technol. 2017, 51, 665–672. [Google Scholar]
- Biswas, A.; Kim, S.; Selling, G.W.; Cheng, H.N. Conversion of agricultural residues to carboxymethylcellulose and carboxymethylcellulose acetate. Ind. Crops. Prod. 2014, 60, 259–265. [Google Scholar] [CrossRef]
- Adinugraha, M.P.; Marseno, D.W.; Haryadi. Synthesis and characterization of sodium carboxymethilcellulose from cavendish banana pseudo stem (Musa cavendishii LAMBERT). Carbohydr. Polym. 2005, 62, 164–169. [Google Scholar] [CrossRef]
- Silva, D.A.; de Paula, R.C.M.; Feitosa, J.P.A.; de Brito, A.C.F.; Maciel, J.S.; Paula, H.C.B. Carboxymethylation of cashew tree exudate polysaccharide. Carbohydr. Polym. 2004, 58, 163–171. [Google Scholar] [CrossRef]
- Singh, R.K.; Singh, A.K. Optimization of reaction conditions for preparing carboxymethyl cellulose from corn cobic agricultural waste. Waste Biomass Valorization 2013, 4, 129–137. [Google Scholar] [CrossRef]
- Haleem, N.; Arshad, M.; Shahid, M.; Tahir, M.A. Synthesis of carboxymethyl cellulose from waste of cotton ginning industry. Carbohydr. Polym. 2014, 113, 249–255. [Google Scholar] [CrossRef]
- Jahan, I.A.; Sultana, F.; Islam, M.N.; Hossain, M.A.; Abedin, J. Studies on indigenous cotton linters for preparation of carboxymethyl cellulose. Bangladesh J. Sci. Ind. Res. 2007, 42, 29–36. [Google Scholar] [CrossRef]
- Rachtanapun, P.; Luangkamin, S.; Tanprasert, K.; Suriyatem, R. Carboxymethyl cellulose film from durian rind. LWT Food Sci. Technol. 2012, 48, 52–58. [Google Scholar] [CrossRef]
- Rachtanapun, P.; Rattanapanone, N. Synthesis and characterization of carboxymethyl cellulose powder and films from Mimosa pigra. J. Appl. Polym. Sci. 2011, 122, 3218–3226. [Google Scholar] [CrossRef]
- Rachtanapun, P.; Kumthai, S.; Mulkarat, N.; Pintajam, N.; Suriyatem, R. Value added of mulberry paper waste by carboxymethylation for preparation a packaging film. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2015; Volume 87, p. 012081. [Google Scholar] [CrossRef] [Green Version]
- Yasar, F.; Togrul, H.; Arslan, N. Flow properties of cellulose and carboxymethyl cellulose from orange peel. J. Food Eng. 2007, 81, 187–199. [Google Scholar] [CrossRef]
- Suriyatem, R.; Noikang, N.; Kankam, T.; Jantanasakulwong, K.; Leksawasdi, N.; Phimolsiripol, Y.; Insomphun, C.; Seesuriyachan, P.; Chaiyaso, T.; Jantrawut, P.; et al. Physical properties of carboxymethyl cellulose from palm bunch and bagasse agricultural wastes: Effect of delignification with hydrogen peroxide. Polymers 2020, 12, 1505. [Google Scholar] [CrossRef]
- Rachtanapun, P. Blended films of carbixymethyl cellulose from papaya peel (CMCp) and corn strach. Kasetsart J. (Nat. Sci.) 2009, 43, 259–266. [Google Scholar]
- Chen, J.; Li, H.; Fang, C.; Cheng, Y.; Tan, T.; Han, H. Synthesis and structure of carboxymethylcellulose with a high degree of substitution derived from waste disposable paper cups. Carbohydr. Polym. 2020, 237, 116040. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Huang, H. Enhanced swelling and responsive properties of pineapple peel carboxymethyl cellulose-g-poly(acrylic acid-co-acrylamide) superabsorbent hydrogel by the introduction of carclazyte. J. Agric. Food Chem. 2017, 65, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Rodsamran, P.; Sothornvit, R. Rice stubble as a new biopolymer source to produce carboxymethyl cellulose-blended films. Carbohydr. Polym. 2017, 171, 94–101. [Google Scholar] [CrossRef]
- Pushpamalar, V.; Langford, S.J.; Ahmad, M.; Lim, Y.Y. Optimization of reaction conditions for preparing carboxymethyl cellulose from sago waste. Carbohydr. Polym. 2006, 64, 312–318. [Google Scholar] [CrossRef]
- Baiya, C.; Nannuan, L.; Tassanapukdee, Y.; Chailapakul, O.; Songsrirote, K. The synthesis of carboxymethyl cellulose-based hydrogel from sugarcane bagasse using microwave-assisted irradiation for selective adsorption of coper(II) ions. Environ. Prog. Sustain. Energy 2018, 38, S157–S165. [Google Scholar] [CrossRef]
- Togrul, H.; Arslan, N. Production of carboxymethyl cellulose from sugar beet pulp cellulose and rheological behaviour of carboxymethyl cellulose. Carbohydr. Polym. 2003, 54, 73–82. [Google Scholar] [CrossRef]
- Girard, M.; Turgeon, S.L.; Paquin, P. Emulsifying properties of whey protein-carboxymethylcellulose. J. Food Sci. 2002, 67, 113–119. [Google Scholar] [CrossRef]
- Rachtanapun, P.; Mulkarat, N.; Pintajam, N. Effect of chitosan coating combined with calcium gluconate on strawberry (Fragaria x ananassa) quality during refrigerated storage. In Proceedings of the 5th International Packaging Congress and Exhibition, Bayrakli, Izmir, Turkey, 22–24 November 2007; Chamber of Chemical Engineers Branch: Ankara, Turkey, 2007. [Google Scholar]
- Waring, M.J.; Parsons, D. Physico-chemical characterisation of carboxymethylated spun cellulose fibers. Biomaterials 2001, 22, 903–912. [Google Scholar] [CrossRef]
- Chia, P.X.; Tan, L.J.; Huang, C.M.J.; Chan, E.W.C.; Wong, S.Y.W. Hydrogel beads from sugar cane bagasse and palm kernel cake, and the viability of encapsulated Lactobacillus acidophilus. E Polym. 2015, 15, 411–418. [Google Scholar] [CrossRef]
- Wang, X.; Zhai, Y.; Zhan, W. Determination of the degree of substitution of carboxymethylcellulose sodium. J. Food Saf. 2015, 6, 3145–3148. [Google Scholar]
- Nie, H.R.; Liu, M.Z.; Chen, Z.B. Kinetic study on bio-degradation of carboxymethylcellulose hydrogel. Acta Phys. Sin. 2004, 20, 386–390. [Google Scholar] [CrossRef]
- Tongdeesoontorn, W.; Mauer, L.; Wongruong, S.; Sriburi, P.; Rachtanapun, P. Effect of carboxymethyl cellulose concentration on physical properties of biodegradable cassava starch-based films. Chem. Centr. J. 2011, 5, 6. [Google Scholar] [CrossRef] [Green Version]
- Dahlman, O.; Jacobs, A.; Sjoberg, J. Molecular properties of hemicelluloses located in the surface and inner layers of hard wood and softwood pulps. Cellulose 2003, 10, 325–334. [Google Scholar] [CrossRef]
- Oun, A.A.; Rhim, J.-W. Preparation and characterization of sodium carboxymethyl cellulose/cotton linter cellulose nanofibril composite films. Carbohydr. Polym. 2011, 18, 152–165. [Google Scholar] [CrossRef]
- Methacanon, P.; Chaikumpollert, O.; Thavorniti, P.; Suchiva, K. Hemicellulosic polymer from Vetiver grass and its physicochemical properties. Carbohydr. Polym. 2003, 54, 335–342. [Google Scholar] [CrossRef]
- Khan, F.; Ahmad, S.R. Polysaccharides and their derivatives for versatile tissue engineering application. Macromol. Biosci. 2013, 13, 395–421. [Google Scholar] [CrossRef]
- Zhu, Y.; Xiao, S.; Li, M.; Chang, Z.; Wang, F.; Gao, J.; Wu, Y.P. Natural macromolecule based carboxymethyl cellulose as a gel polymer electrolyte with adjustable porosity for lithium ion batteries. J. Power Sources 2015, 288, 368–375. [Google Scholar] [CrossRef]
- Miljković, V.; Momčilović, M.; Stanković, M.; Ćirković, B.; Laketić, D.; Nikolić, G.; Vujović, M. Remediation of arsenic contaminated water by a novel carboxymethyl cellulose bentonite adsorbent. Appl. Ecol. Environ. Res. 2019, 17, 733–744. [Google Scholar] [CrossRef]
- Miljković, V.; Jokanović, M.; Jokanović, M.; Đorđević, S.; Stanković, M.; Stojiljković, S.; Vujović, M.; Bojanić, N. The removal of lead (II) ions from aqueous solutions by acid-activated clay modified with sodium carboxymethil cellulose. Appl. Ecol. Environ. Res. 2017, 15, 1461–1472. [Google Scholar] [CrossRef]
- Wei, W.; Kim, S.; Song, M.-H.; Bediako, J.K.; Yun, Y.-S. Carboxymethylcellulose fiber as a fast binding and biodegradable adsorbent of heavy metals. J. Taiwan Inst. Chem. Eng. 2015, 54, 104–110. [Google Scholar] [CrossRef]
- Dapia, S.; Tovar, C.A.; Santos, V.; Parajo, J.C. Rheological behavior of carboxymethylcellulose manufactured from TCF-bleached milox pulps. Food Hydrocoll. 2005, 19, 313–320. [Google Scholar] [CrossRef]
- Son, Y.R.; Rhee, K.Y.; Park, S.J. Influence of reduced graphene oxide on mechanical behaviors of sodium carboxymethyl cellulose. Compos. B Eng. 2015, 83, 36–42. [Google Scholar] [CrossRef]
- Guo, J.H.; Skinner, G.W.; Harcum, W.W.; Barnum, P.E. Pharmaceutical applications of naturally occurring water-soluble polymers. Pharm. Sci. Technol. Today 1998, 1, 254–261. [Google Scholar] [CrossRef]
- Benchabane, A. Rheological Properties of Carboxymethyl Cellulose (CMC) Solutions. Ph.D. Thesis, University Louis Pasteur, Strasbourg, France, 2006. [Google Scholar]
- Thenapakiam, S.; Kumar, D.; Pushpamalar, J.; Saravanan, M. Aluminium and radiation cross-linked carboxymethyl sago pulp beads for colon targeted delivery. Carbohydr. Polym. 2013, 94, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Bono, A.; Ying, P.H.; Yan, F.Y.; Muei, C.L.; Sarbatly, R.; Krishnaiah, D. Synthesis and characterization of carboxymethyl cellulose from palm kernel cake. Adv. Nat. Appl. Sci. 2009, 3, 5–11. [Google Scholar]
- Avera, F.L. Moisturizing Agent. U.S. Patent 4,865,640, 12 September 1989. [Google Scholar]
- Dellavalle, N.B. Efficacy test of driwater—A slow water release substrate. Commun. Soil Sci. Plant Anal. 1992, 23, 2547–2553. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Ghasemzadeh, H.; Soleyman, R. Synthesis, characterization, and swelling and behavior of alginate-g-poly(sodium acrylate)/kaolin superabsorbent hydrogel composites. J. Appl. Polym. Sci. 2007, 105, 2631–2639. [Google Scholar] [CrossRef]
- Wang, W.; Wang, A. Nanocomposite of carboxymethyl cellulose and attapulgite as a novel pH-sensitive superabsorbent: Synthesis, characterization and properties. Carbohydr. Polym. 2010, 461, 83–91. [Google Scholar] [CrossRef]
- Ibrahim, S.M.; El Salmawi, K.M.; Zahran, A.H. Synthesis of crosslinked superabsorbent carboxymethyl cellulose/acrylamide hydrogels through electron-beam irradiation. J. Appl. Polym. Sci. 2007, 104, 2003–2008. [Google Scholar] [CrossRef]
- Chang, C.; Duan, B.; Cai, J.; Zhang, L. Superabsorbent hydrogels based on cellulose for smart swelling and controllable delivery. Eur. Polym. J. 2010, 46, 92–100. [Google Scholar] [CrossRef]
- Fekete, T.; Borsa, J.; Takács, E.; Wojnárovits, L. Synthesis of cellulose derivative based superabsorbent hydrogels by radiation induced crosslinking. Cellulose 2014, 21, 4157–4165. [Google Scholar] [CrossRef]
- Fekete, T.; Borsa, J.; Takács, E.; Wojnárovits, L. Synthesis of carboxymethylcellulose/ starch superabsorbent hydrogels by gamma-irradiation. Chem. Cent. J. 2017, 11, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klinpituksa, P.; Kosaiyakanon, P. Superabsorbent polymer based on sodium carboxymethyl cellulose grafted polyacrylic acid by inverse suspension polymerization. Int. J. Polym. Sci. 2017, 9, 3476921. [Google Scholar] [CrossRef]
- Lee, J.; Park, S.; Roh, H.-g.; Oh, S.; Sunghoon Kim, S.; Kim, M.; Kim, D.; Park, J. Preparation and characterization of superabsorbent polymers based on starch aldehydes and carboxymethyl cellulose. Polymers 2018, 10, 605. [Google Scholar] [CrossRef] [Green Version]
- Nnadi, F.; Brave, C. Environmentally friendly superabsorbent polymers for water conservation in agricultural lands. J. Soil Sci. Environ. Manag. 2011, 2, 206–211. [Google Scholar]
- Petroudy, S.R.D.; Ranjbar, J.; Garmaroody, E.R. Eco-friendly superabsorbent polymers based on carboxymethyl cellulose strengthened by TEMPO-mediated oxidation wheat straw cellulose nanofiber. Carbohydr. Polym. 2018, 197, 565–575. [Google Scholar] [CrossRef]
- Raafat, A.I.; Eid, M.; El-Arnaouty, M.B. Radiation synthesis of superabsorbent CMC based hydrogels for agriculture applications. Nucl. Instrum. Methods Phys. Res. B 2012, 283, 71–76. [Google Scholar] [CrossRef]
- Salleh, K.M.; Zakaria, S.; Sajab, M.S.; Gan, S.; Kaco, H. Superabsorbent hydrogel from oil palm empty fruit bunch cellulose and sodium carboxymethylcellulose. Int. J. Biol. Macromol. 2019, 131, 51–59. [Google Scholar] [CrossRef]
- Suo, A.; Qian, J.; Yao, Y.; Zhang, W. Synthesis and properties of carboxymethyl cellulosegraft-poly(acrylic acid-co-acrylamide) as a novel cellulose-based superabsorbent. J. Appl. Polym. Sci. 2007, 103, 1382–1388. [Google Scholar] [CrossRef]
- Sutradhar, S.C.; Khan, M.M.R.; Rahman, M.M.; Dafadar, N.C. The synthesis of superabsorbent polymers from a carboxymethylcellulose/acrylic acid blend using gamma radiation and its application in agriculture. J. Phys. Sci. 2015, 26, 23–39. [Google Scholar]
- Tang, H.; Chen, H.; Duan, B.; Lu, A.; Zhang, L. Swelling behaviors of superabsorbent chitin/carboxymethylcellulose hydrogels. J. Mater. Sci. 2014, 49, 2235–2242. [Google Scholar] [CrossRef]
- Wach, A.R.; Mitomo, H.; Yoshii, F.; Kume, T. Hydrogel of biodegradable cellulose derivatives. II. Effect of some factors on radiation-induced crosslinking of CMC. J. Appl. Polym. Sci. 2001, 81, 3030–3037. [Google Scholar] [CrossRef]
- Fei, B.; Wach, R.A.; Mitomo, H.; Yoshii, F.; Kume, T. Hydrogel of biodegradable cellulose derivatives. I. Radiation-Induced crosslinking of CMC. J. Appl. Polym. Sci. 2000, 78, 278–283. [Google Scholar] [CrossRef]
- Stahl, J.D.; Cameron, M.D.; Haselbach, J.; Aust, S.D. Biodegradation of superabsorbent polymers in soil. Environ. Sci. Pollut. Res. 2000, 7, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Kabiri, K.; Omidian, H.; Zohuriaan-Mehr, M.J.; Doroudiani, S. Superabsorbent hydrogel composites and nanocomposites: A review. Polym. Compos. 2010, 32, 277–289. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).