Abstract
An in-situ chemical oxidative method was used to effectively synthesize a promising supercapacitor material based on PPy/ZrO2 composites. The synthesized materials were characterized by different analytical techniques, such as UV/visible (UV/Vis) spectroscopy, Fourier-transform infra-red spectroscopy (FTIR), X-ray diffraction (XRD), thermogravimetric analysis (TGA), and scanning electron microscopy (SEM). The inclusion of ZrO2 into the PPy matrix was verified by vibrational spectra and structural analyses. The (TGA) results showed that incorporating ZrO2 into the polymeric matrix improved its thermal stability. In addition, the electrochemical properties of the synthesizedmaterials were investigated byusing cyclic voltammetry (CV) and galvanostatic charge/discharge (GCD). The PPy/ZrO2 composite demonstrated excellent super capacitive performance, and high specific capacity of 337.83 F/g, with an exceedingly high energy density of 187.68 Wh/kg at a power density of 1000 W/kg. The composite materials maintain good stability after 1000 charge and discharge cycles, with 85% capacitance retention. The PPy/ZrO2 possesses a high capacitance, an attractive micro-morphology, and a simple synthesis method. The findings indicate that the PPy/ZrO2 composite could be a promising electrode material for high-performance supercapacitor applications.
1. Introduction
Electrochemical capacitors, also known as supercapacitors, are known for their ideal power storage capacity, which is achieved through rapid/reversible redox reactions and/or phase-change processes on the surface or subsurface areas of modified electrodes in various types of portable electronic equipment [1,2,3]. Supercapacitors show a number of interesting characteristics, including fast charge/discharge rate, safe operational features, ideal cyclic stability, and highpower density [4,5]. Solid-state supercapacitors demonstrate advantages such as ideal safety, light weight, and high flexibility, as compared to that of liquid electrolyte-based supercapacitors, and they are essential for the development of state-of-the-artportable electronic devices [6,7,8]. Supercapacitors occupied a very large significant portion of the market and research area among many electrochemical energy storage devices, due to their high power density, which can store significant amount of energy, long life cyclic stability (100 to 1000 of charge/discharge cycles before any significant deterioration of the charge capacity), andalmost unlimited charge/discharge rate [9].
The conducting polymer Polypyrrole (PPy) is broadly considered as a perfect candidate for the development of both electric double-layer capacitors (EDLC) and pseudocapacitors due to its fast and reversible redox process caused by the pi-conjugated polymeric chain [10,11]. Due to its advantages over its equivalents among the numerous conducting polymers (CPs), PPy performs admirably as an electrode material for the creation of supercapacitors. These noteworthy advantages of PPy include promising mechanical strength, ideal specific capacity, high electrical conductivity, and biocompatibility [12,13,14]. Still, like other CPs, PPy has a number of drawbacks, such as volumetric shrinking during discharge and a decrease in cyclic stability, which could be mitigated by adding potential modifiers [15,16]. Because of their rapid redox kinetics and perfect capacitance, transition metal oxides were widely employed to improve the PPy’s cyclic stability [17,18]. Different researchers synthesized nanocomposites of PPy with numerous MOs, including TiO2 [19], Fe2O3 [20], SiO2 [21], ZnO [22] and CeO2 [23], due to the large potential of CPs and polymers doped with inorganic metal oxides (MOs), which increase the performance of materials in many applications. High electron affinity, better mechanical characteristics, and high electrical conductivity may all be achieved by doping MOs in CPs [24]. These nanomaterial-doped CPs are employed for a wide range of applications and purposes. For supercapacitors, high-performance ordered porous Polypyrrole/ZnO sheets with enhanced specific capacitance were developed [25]. PPy and its composites with titanium oxide demonstrate increased supercapacitative qualities [26]. Polypyrrole@silica composites with good performance as electrode materials for Lithium-ion batteries were prepared using a solution polymerization process [27]. The development of new materials with encouraging electrochemical properties is an important goal for energy storage applications. Furthermore, low working potential, low energy density, and large self-charge/discharge currents are all issues that must be addressed in the development of next-generation supercapacitors. Supercapacitors can use MOs electrodes, although they have limited current capability and poor cycle stability. As a result, there is a compelling need to enhance the electrochemical characteristics of MOs and CPs [28].
In the current study, PPy/ZrO2 composites were synthesized by an in-situ chemical oxidative polymerization method with improved electrochemical performance and specific capacitance at a scan rate of 100 mV s−1. The solid-state supercapacitor device, which is made up of as-prepared PPy/ZrO2 composite electrodes, has good specific capacitance of 337.83 F/g and a maximum energy density of 187.68 Wh/kg. These findings indicate that the PPy/ZrO2 composites could be promising electrode materials for high-performance supercapacitor applications, which was not previously reported.
2. Materials and Methods
2.1. Materials
The reagent grade pyrrole monomer (Fluka) was distilled twice before use. The following chemicals were used as received: dodecyl-benzenesulphonic acid (DBSA), chloroform, ammonium persulphate (APS), acetone and N-methyl-2-pyrrolidone (NMP) were purchased from Sigma–Aldrich (St. Louis, MO, USA). However, methanol and sulfuric acid were provided by Scharlau (Sentmenat, Spain). Double-distilled water was used for solution preparation and glassware washing/rinsing. The compounds that were utilized are listed in Table 1 below.

Table 1.
List of chemicals used and their molecular weight, density, and purity.
2.2. Synthesis of Polypyrrole (PPy)
In a round bottom flask placed in an ice bath, 0.2 mL pyrrole monomer and 50 mL deionized water were added and stirred to make PPy. After 10 min, 3.5 mL dodecyl-benzenesulfonic acid (DBSA) was added and stirred for another 10 min, then 10 mL ammonium per sulphate (APS) solution (0.1 M) was added dropwise as an initiator. The reaction mixture was stirred for 24 h at 5 to 10 °C. The precipitate was separated by centrifugation (7000 rpm) after 24 h and washed several times with water followed by acetone. The resulting black powder was vacuum-dried at 60 °C for 12 h and labelled as PPy.
2.3. Synthesis of Polypyrrole/Zirconium Dioxide (PPy/ ZrO2) Composites
The same procedure was followed for the synthesis of PPy/ZrO2 composites by adding different amounts (0.1 g, 0.15 g, 0.2 g, 0.25 g and 0.3 g) of ZrO2 into the reaction mixture just after the addition of DBSA. The samples thus obtained were named as PPy/ZrO2 1, PPy/ZrO2 2, PPy/ZrO2 3, PPy/ZrO2 4, and PPy/ZrO2 5, respectively.
2.4. Apparatus
For electrochemical measurements Autolab, and three electrode cell having different electrodes such as glassy carbon as working electrode, gold as counter electrode, and silver-silver chloride as reference electrode, were used.
3. Results and Discussion
3.1. UV-Visible Characterization
The UV-visible spectra of pure PPy and its ZrO2 composites were measured in the 200–900 nm region (Figure 1a–f). Pure PPy spectra reveal two distinct wide absorption bands, located at 359 nm and 589 nm, respectively (Figure 1a). The π-π* transition is responsible for the initial absorption band. At 584 nm, the second wide absorption band suggests that the PPy is in oxidized and non-conducting state [29]. Figure 1b–f shows the absorption spectra of PPy/ZrO2 composites. The composites also show two absorption peaks, with a peak in the region of 401–410 nm indicating the PPy ring’s π-π* transition. The second absorption peak above 700 nm in the composites belongs to the sum of polarons and bipolarons [30]. The broad peaks for PPy/ZrO2 composites curves (b), (c), (d), (e) and (f) show that when the amount of ZrO2 in the composites rises, the peak for the sum of polaron and bipolaron shifts towards the higher wavelength [30]. This results in a narrowing of the band gap and an increase in the composites’ conductivity.
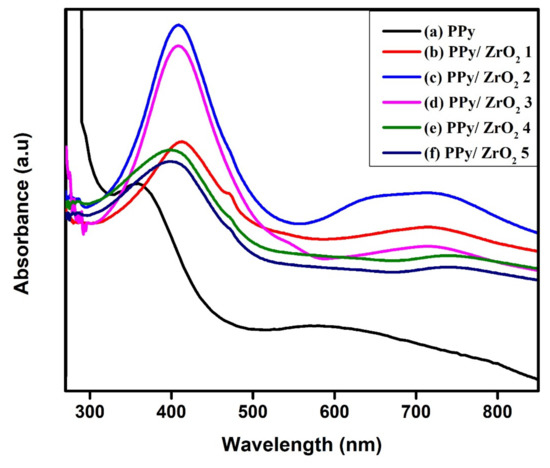
Figure 1.
UV-Visible spectra of (a) PPy, (b) PPy/ZrO2 1, (c) PPy/ZrO2 2, (d) PPy/ZrO2 3, (e) PPy/ZrO2 4, (f) PPy/ZrO2 5.
3.2. UV Band Gap Calculation
The optical band gap was computed using the Tauc Equation (1). The UV-visible spectra of PPy and its composites aid in the identification of their optical band gaps, where αand h indicate the absorption coefficients and Planck’s constant, respectively. The symbols ν, A, and Eg stand for light frequency, a constant, and band gap, respectively.
PPy and PPy/ZrO2 1, PPy/ZrO2 2, PPy/ZrO2 3, PPy/ZrO2 4, and PPy/ZrO2 5 had band gaps of 2.8, 2.36, 2.28, 2.29, 2.4, and 2.39, respectively, as shown in Figure 2. These findings reveal that increasing the quantity of ZrO2 in the polymer matrix reduces the band gap, resulting in an increase in conductivity. As demonstrated in Figure 2, the band gap of PPy/ZrO2 2 is less than that of other composites and pure PPy.
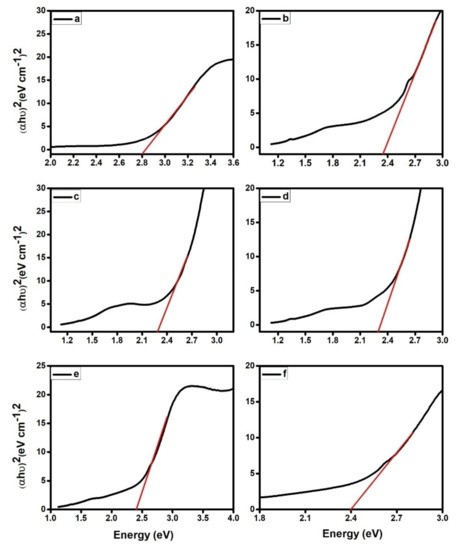
Figure 2.
Band gap calculation for (a) PPy, (b) PPy/ZrO2 1, (c) PPy/ZrO2 2, (d) PPy/ZrO2 3, (e) PPy/ZrO2 4, (f) PPy/ZrO2 5.
3.3. Vibrational Spectroscopy/FTIR Analysis
FTIR spectra of PPy and PPy/ZrO2 composites are shown in Figure 3. The C–C stretching vibration in the pyrrole ring shows up as an absorption band at 1540 cm−1 in PPy. The ring’s C–N stretching vibration is represented by the band at 1452 cm−1 in the PPy spectrum [21]. The C–N in-plane band is at 1290 cm−1, and the C–H bending modes are at 1164 cm−1 and 1031 cm−1 [21,22]. The C–C and C–H out-of-plane deformation vibration bands are at 963 and 891 cm−1, respectively [21,22]. Except for the C–C, C–N and C–H bending modes of the pyrrole ring, the PPy/ZrO2 composite spectra generated with various ZrO2 weights displayed superimposed absorption bands of both components [21]. For pure PPy, the bands at 1540 cm−1correspond to C–C stretching vibration, and 1452 cm−1 to C–N stretching vibration, and 1031 cm−1 to C–H bending modes are red-shifted to 1516, 1431, and 1000cm−1 for the PPy/ZrO2 5 composite, respectively. The small absorption band at 963 cm−1 was slightly red shifted down to 956 cm−1. The peak intensity of pure PPy declines as the concentration of ZrO2 grows and for the PPy/ZrO2 5 composite, it virtually vanishes (Figure 3). The formation of PPy/ZrO2 composites is indicated by these findings.
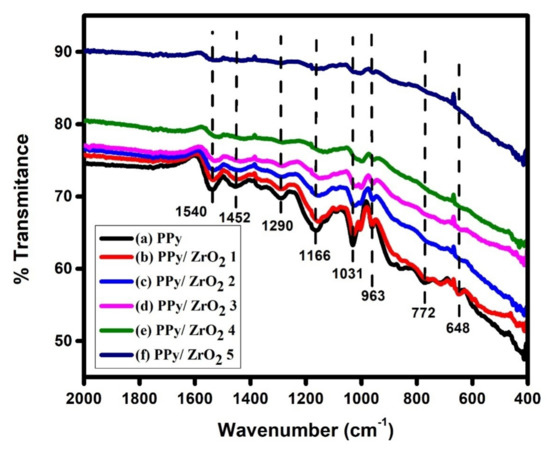
Figure 3.
FTIR spectra (a) PPy, (b) PPy/ZrO2 1, (c) PPy/ZrO2 2, (d) PPy/ZrO2 3, (e) PPy/ZrO2 4, (f) PPy/ZrO2 5.
3.4. X-ray Diffraction Studies
The X-ray diffraction patterns of PPy and PPy/ZrO2 composites are shown in Figure 4. Only PPy has a broad peak at 2θ = 12° to 30°, with tiny shoulders at 17°, 19°, and 21° in the sample. In the PPy sample, the shoulder at 2θ = 17° and 2θ = 19° reflects the distance between the benzene ring planes in adjacent PPy chains. The PPy, like other CPs, has a broad peak, which is thought to imply a semicrystalline nature [31,32].
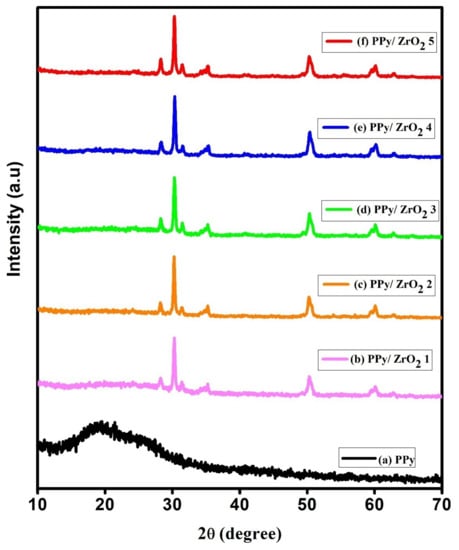
Figure 4.
XRD spectra (a) PPy, (b) PPy/ZrO2 1, (c) PPy/ZrO2 2, (d) PPy/ZrO2 3, (e) PPy/ZrO2 4, (f) PPy/ZrO2 5.
The composites’ XRD diffractograms exhibit a prominent and intense peak at 2θ = 30°, as well as less intense peaks at 2θ = 35°, 50°, and 60°, all of which may be attributed to ZrO2 inside the polymer matrix [33]. These peaks for ZrO2 in the PPy matrix imply an increase in crystallinity, which is consistent with the band gap calculation and related increase in conductivity. The broad PPy peak is flattened by ZrO2 inclusion, and the ZrO2 peaks dominate the diffractograms. It may be deduced from this that the addition of ZrO2 had a significant impact on the crystallinity of PPy [34]. Scherrer’s Equation (2) was used to compute the average crystallite size.
where β is the full width at half maximum (FWHM), λ is the X-ray wavelength, θ is the diffraction angle, and k is the Scherrer’s constant of order one. The average crystallite size in PPy/ZrO2 composites is 19.56 nm.
3.5. Thermogravimetric Analysis (TGA)
Figure 5 shows the thermograms of PPy and PPy/ZrO2 composites, which demonstrate three step weight losses. For pure PPy, the absorbed water molecules are evaporated in the first stage, resulting in a weight loss of 5% at temperatures ranging from 25 °C to 150 °C. The DBSA molecules are removed from the polymer matrix in the second stage. The decomposition of the main polymer chain occurs in the third stage at high temperatures ranging from 425 °C to 800 °C, with 21.29% weight retention. In composites, ZrO2 plays a crucial function in modifying the composites’ thermal behavior. The addition of ZrO2 to PPy/ZrO2 composites improves thermal stability significantly, as evidenced by curves (b–e) in Figure 5 [32]. The strong association/interaction between PPy and ZrO2 particles-particles is responsible for the improved thermal stability [35]. In comparison to pure PPy, the major decomposition in composites begins at high temperatures, resulting in a 63.27% increase in weight retention. Table 2 summarizes weight loss and weight retention at different temperatures.
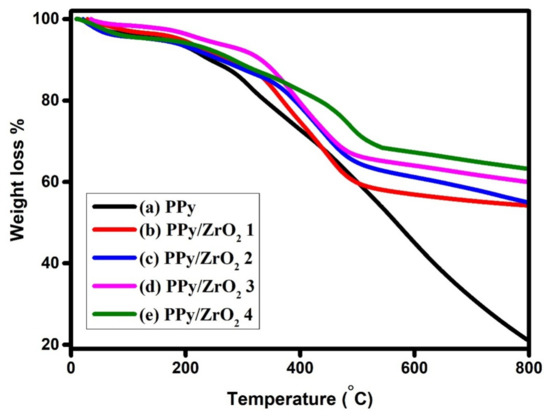
Figure 5.
TGA curves of (a) PPy (b) PPy/ZrO2 1 (c) PPy/ZrO2 2 (d) PPy/ZrO2 3 (e) PPy/ZrO2 4.

Table 2.
Weight loss at different temperature and weight retention.
3.6. Morphological Study
Figure 6 shows SEM images of pure PPy and a PPy/ZrO24 composite. Pure PPy has a porous morphology with bigger aggregated particles and a large particle size. In the segregated form, the PPy/ZrO24 composite has a granular morphology with smaller particles and reduced particle size. The ZrO2 particles appear to be evenly distributed over the porous surface of the PPy matrix. This causes an increase in the conductivity of the composite because of the ease of an electron hoping mechanism [36].
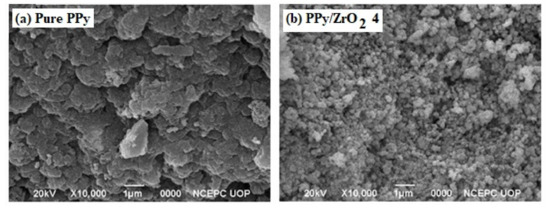
Figure 6.
SEM images of (a) pure PPy (b) PPy/ZrO2 4.
3.7. Cyclic Voltammetric (CV) Analysis
The electrochemical properties were studied by cyclic voltammetry (CV). Figure 7a,b shows the CV curves of neat PPy and tPPy/ZrO24 composite, respectively. CV curves were recorded using a three-electrode setup in acidic medium containing a 1 molar H2SO4 solution as the electrolyte, with glassy carbon, gold and silver-silver chloride electrodes serving as working, counter and reference electrodes, respectively. A potential window ranging from −1 to 1 V was selected at a scan rate of 100 mV/s. PPy’s CV curves have well-defined redox peaks. At a scan rate of 100 mV/s, the PPy voltammogram reveals an anodic peak potential (Epa) of 0.419 V with current density 0.214 mA and a cathodic peak potential (Epc) of 0.280 V with current density −0.125 mA. The cyclic voltammogram of the PPy/ZrO24 composite in Figure 7b shows an anodic peak potential (Epa) of 0.462 V with current density 0.241 mA and a cathodic peak potential (Epc) of 0.151 V with current density −0.196 mA. According to the CV curves, the current density has risen from 0.214 to 0.241 mA at the same scan rate (100 mV/s) and potential window, demonstrating that the composite material has a better response than the neat PPy modified electrode.
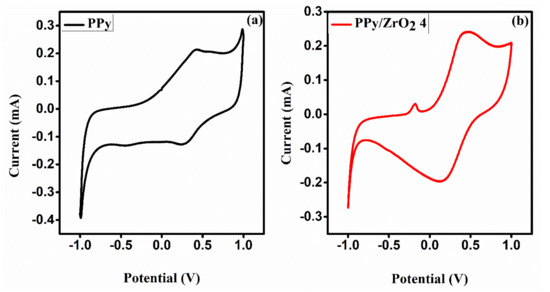
Figure 7.
Cyclic voltammograms of (a) PPy and (b) PPy/ZrO2 4 composite.
3.8. Effect of Scan Rate
Figure 8a demonstrates the influence of scan rate on the PPy/ZrO2 4 modified electrode, which displays an increase in redox peaks as the scan rate increases from 25 to 200 mV/s. Figure 8b shows that the square root of scan rate and anodic peak current (Ipa) have an excellent linearity (R2 = 0.97792), indicating that the electron transfer reaction of the PPy/ZrO2 4 modified electrode with the electrolyte solution is a diffusion controlled process.
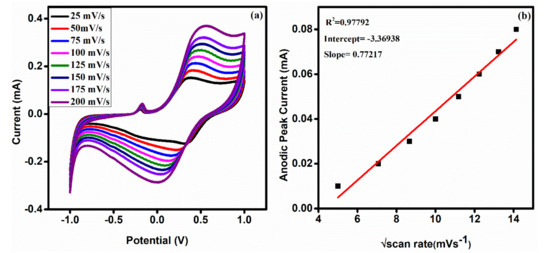
Figure 8.
(a) CV of PPy/ZrO2 4 at different scan rates (25–200 mV/s), and (b) plot of Ipa versus square root of scan rate.
3.9. Galvanostatic Charge Discharge Studies
Figure 9a shows the galvanostatic charge discharge (GCD) of PPy and PPy/ZrO2 4 composite in a potential window of −1 to 1 V in 1.0 M H2SO4 solution. The applicability of the fabricated supercapacitor electrodes was studied using various parameters such as specific capacitance (Cs), energy density (E), and power density (P). The specific capacitance of the fabricated supercapacitor electrodes was calculated using Equation (3).
where I represents the current charge/discharge (A), Δt is the time of discharge, ΔV and m represent the potential window and mass deposited on the GSC electrode, respectively. Equations (4) and (5) help to deduce the energy and power densities, respectively.
where Cs represent specific capacitance, E and P symbolized energy and power densities.
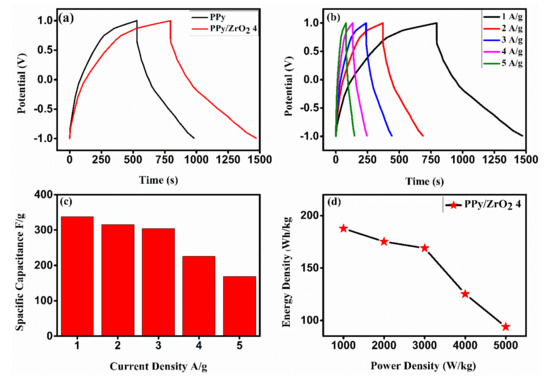
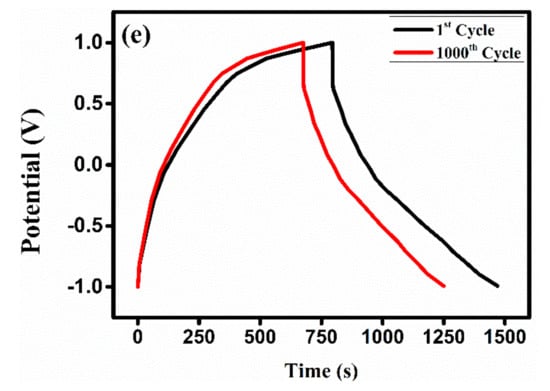
Figure 9.
GCD curve of (a) PPy and PPy/ZrO2 4, and (b) PPy/ZrO2 4 at different current density. (c) Specific capacitance versus current density. (d) Ragone plot. (e) Comparison of GCD curves of PPy/ZrO2 4, i.e., 1st cycle and 1000th cycle.
As can be seen in Figure 9b, the PPy/ZrO2 4 composite shows a long charge discharge time at a current density of 1 A/g. The charge/discharge time versus potential is linear in all of the curves, which is characteristic of capacitors. The specific capacitance of the PPy/ZrO2 composite was 337.83 F/g at a current density of 1 A/g, whereas PPy had a specific capacitance of 225.225 F/g. The high specific capacitance of the PPy/ZrO2 4 electrode can be attributed to its smaller particle size and well-defined structure, which reduce ion diffusion length and increase ion and electron transport kinetics in electrodes and at the electrode/electrolyte interface [37]. Furthermore, at current densities ranging from 01 to 05 A/g, the specific capacitance of PPy/ZrO2 4 was 337.83 F/g to 168.92 F/g respectively. As a result, the electrode material in a supercapacitor, PPy/ZrO24, has good capacitance performance. Simultaneously, comparing GCD curves at various current densities reveals that the capacitance of sample PPy/ZrO2 4 increases as the current density decreases [38]. At a higher value of current density, the ions or electrons are mainly adsorbed on the upper surface of the working electrode and hindered because of a short time at a high current density to reach the whole surface. Table 3 compares the specific capacitance and current density of the PPy/ZrO2 electrode used in this work to those of other well-known electrode materials, demonstrating that PPy/ZrO2 outperforms them.

Table 3.
Comparative analysis of PPy/ZrO2 with other electrode materials.
4. Conclusions
We found that in-situ chemical oxidative polymerization is a simple way to successfully synthesized PPy and PPy/ZrO2 composites with outstanding electrochemical characteristics. The aromatic ring of the polymer exhibits π-π* transition in the UV-visible region. The presence of ZrO2 in the PPy matrix was verified by vibrational spectroscopy. Sharp and fine XRD spectra were detected after the inclusion of ZrO2 particles in PPy. The surface morphology showed a granular structure and agglomeration of particles with a higher concentration of ZrO2. The thermograms show an excellent thermal stability of PPy by the addition of ZrO2. Correspondingly, the modified electrode with the PPy/ZrO2 4 composite through three electrode system showed the specific capacitance of 337.83 F/g at a current density of 1 A/g and excellent energy density of 187.67 Wh/kg and an outstanding power density of 1000 W/kg. Moreover, the modified electrode PPy/ZrO2 4 demonstrated excellent specific capacitance performance and retained 85% of its specific capacitance after 1000 charge-discharge cycles. The modified PPy/ZrO2 4 electrode is a promising material for making CPs supercapacitor electrodes due to its excellent electrochemical performance and cycle stability.
Author Contributions
Conceptualization, R.U., M.K. and N.K.; methodology, R.U., M.K. and N.K.; software, R.U., M.K., N.K. and M.S.K.; validation, R.U., M.K., N.K. and M.S.K.; formal analysis, R.U., M.K. and R.K.; investigation, R.U., M.K. and R.K.; resources, R.U., R.K., M.S.K. and Y.A.E.-B.; writing—original draft preparation, R.U. and M.K.; writing—review and editing, R.K. and Y.A.E.-B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors gratefully acknowledge the financial support provided from the Taif University Researchers Supporting Project number TURSP-2020/106, Taif University, Taif, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Fang, S.; Li, J.; Xiang, C.; Zou, Y.; Xu, F.; Sun, L.; Zhang, J. Anchoring sea urchin-like cobalt-nickel carbonate hydroxide on 3D carbon sponge for electrochemical energy storage. J. Alloy. Compd. 2020, 845, 156024. [Google Scholar] [CrossRef]
- Cai, C.; Zou, Y.; Xiang, C.; Chu, H.; Qiu, S.; Sui, Q.; Xu, F.; Sun, L.; Shah, A. Broccoli-like porous carbon nitride from ZIF-8 and melamine for high performance supercapacitors. Appl. Surf. Sci. 2018, 440, 47–54. [Google Scholar] [CrossRef]
- Liu, Y.; Xiang, C.; Chu, H.; Qiu, S.; McLeod, J.; She, Z.; Xu, F.; Sun, L.; Zou, Y. Binary Co–Ni oxide nanoparticle-loaded hierarchical graphitic porous carbon for high-performance supercapacitors. J. Mater. Sci. Technol. 2020, 37, 135–142. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef] [PubMed]
- Arico, A.S.; Bruce, P.; Scrosati, B.; Tarascon, J.; van Schalkwijk, W. Nanostructured materials for advanced energy conversion and storage devices. In Materials for Sustainable Energy: A Collection of Peer-Reviewed Research and Review Articles from Nature Publishing Group; World Scientific: Singapore, 2011; pp. 148–159. [Google Scholar]
- Meng, C.; Liu, C.; Chen, L.; Hu, C.; Fan, S. Highly Flexible and All-Solid-State Paperlike Polymer Supercapacitors. Nano Lett. 2010, 10, 4025–4031. [Google Scholar] [CrossRef] [PubMed]
- Kaempgen, M.; Chan, C.K.; Ma, J.; Cui, Y.; Gruner, G. Printable Thin Film Supercapacitors Using Single-Walled Carbon Nanotubes. Nano Lett. 2009, 9, 1872–1876. [Google Scholar] [CrossRef]
- Weng, Z.; Su, Y.; Wang, D.-W.; Li, F.; Du, J.; Cheng, H.-M. Graphene-Cellulose Paper Flexible Supercapacitors. Adv. Energy Mater. 2011, 1, 917–922. [Google Scholar] [CrossRef]
- Noori, A.; El-Kady, M.F.; Rahmanifar, M.S.; Kaner, R.B.; Mousavi, M.F. Towards establishing standard performance metrics for batteries, supercapacitors and beyond. Chem. Soc. Rev. 2019, 48, 1272–1341. [Google Scholar] [CrossRef]
- Woo, S.-W.; Dokko, K.; Kanamura, K. Composite electrode composed of bimodal porous carbon and polypyrrole for electrochemical capacitors. J. Power Sources 2008, 185, 1589–1593. [Google Scholar] [CrossRef]
- Kim, B.; Ko, J.; Wallace, G. A novel capacitor material based on Nafion-doped polypyrrole. J. Power Sources 2008, 177, 665–668. [Google Scholar] [CrossRef]
- Ghenaatian, H.; Mousavi, M.; Rahmanifar, M. High performance hybrid supercapacitor based on two nanostructured conducting polymers: Self-doped polyaniline and polypyrrole nanofibers. Electrochim. Acta 2012, 78, 212–222. [Google Scholar] [CrossRef]
- Yuan, L.; Yao, B.; Hu, B.; Huo, K.; Chen, W.; Zhou, J. Polypyrrole-coated paper for flexible solid-state energy storage. Energy Environ. Sci. 2013, 6, 470–476. [Google Scholar] [CrossRef]
- Wang, Z.-L.; Guo, R.; Ding, L.-X.; Tong, Y.-X.; Li, G.-R. Controllable Template-Assisted Electrodeposition of Single- and Multi-Walled Nanotube Arrays for Electrochemical Energy Storage. Sci. Rep. 2013, 3, srep01204. [Google Scholar] [CrossRef] [PubMed]
- Snook, G.A.; Kao, P.; Best, A.S. Conducting-polymer-based supercapacitor devices and electrodes. J. Power Sources 2011, 196, 1–12. [Google Scholar] [CrossRef]
- Chen, S.S.; Zhang, H.R.; Todd, I. Phase-separation-enhanced plasticity in a Cu47.2Zr46.5Al5.5Nb0.8 bulk metallic glass. Scr. Mater. 2014, 72–73, 47–50. [Google Scholar] [CrossRef]
- Yu, M.; Zeng, Y.; Zhang, C.; Lu, X.; Zeng, C.; Yao, C.; Yang, Y.; Tong, Y. Titanium dioxide@polypyrrole core–shell nanowires for all solid-state flexible supercapacitors. Nanoscale 2013, 5, 10806–10810. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zeng, X.; Yang, M.; Qi, Y. Investigation of a Branchlike MoO3/Polypyrrole Hybrid with Enhanced Electrochemical Performance Used as an Electrode in Supercapacitors. ACS Appl. Mater. Interfaces 2014, 6, 1125–1130. [Google Scholar] [CrossRef]
- Gao, F.; Hou, X.; Wang, A.; Chu, G.; Wu, W.; Chen, J.; Zou, H. Preparation of polypyrrole/TiO2 nanocomposites with enhanced photocatalytic performance. Particuology 2016, 26, 73–78. [Google Scholar] [CrossRef]
- Jadhav, N.; Kasisomayajula, S.; Gelling, V.J. Polypyrrole/Metal Oxides-Based Composites/Nanocomposites for Corrosion Protection. Front. Mater. 2020, 7, 95. [Google Scholar] [CrossRef]
- Grari, O.; Taouil, A.E.; Dhouibi, L.; Buron, C.; Lallemand, F. Multilayered polypyrrole–SiO2 composite coatings for functionalization of stainless steel: Characterization and corrosion protection behavior. Prog. Org. Coat. 2015, 88, 48–53. [Google Scholar] [CrossRef]
- Dey, S.; Kar, A.K. Effect of Forster resonance energy transfer on the photoluminescence of PPy-ZnO composite. J. Sol.-Gel. Sci. Technol. 2021. [Google Scholar] [CrossRef]
- Chigondo, M.; Chigondo, F.; Nyamunda, B. Synthesis of hydrous CeO2 polypyrrole nanocomposite as a rapid and efficient adsorbent for defluoridation of drinking water. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100462. [Google Scholar] [CrossRef]
- Dakshayini, B.; Reddy, K.R.; Mishra, A.; Shetti, N.P.; Malode, S.J.; Basu, S.; Naveen, S.; Raghu, A.V. Role of conducting polymer and metal oxide-based hybrids for applications in ampereometric sensors and biosensors. Microchem. J. 2019, 147, 7–24. [Google Scholar] [CrossRef]
- Xue, J.; Yang, Q.; Guan, R.; Shen, Q.; Liu, X.; Jia, H.; Li, Q. High-performance ordered porous Polypyrrole/ZnO films with improved specific capacitance for supercapacitors. Mater. Chem. Phys. 2020, 256, 123591. [Google Scholar] [CrossRef]
- Kim, M.S.; Park, J.H. Polypyrrole/titanium oxide nanotube arrays composites as an active material for supercapacitors. J. Nanosci. Nanotechnol. 2011, 11, 4522–4526. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, K.; Pan, D.; Liu, X.; Yang, M.; Zhu, X.J. Polypyrrole@silica composites as high performance electrode materials for Lithium-ion batteries. Mater. Sci. Mater. Electron. 2018, 29, 6098–6104. [Google Scholar]
- Bekhoukh, A.; Moulefera, I.; Sabantina, L.; Benyoucef, A. Development, Investigation, and Comparative Study of the Effects of Various Metal Oxides on Optical Electrochemical Properties Using a Doped PANI Matrix. Polymers 2021, 13, 3344. [Google Scholar] [CrossRef]
- Habelhames, F.; Nessark, B.; Bouhafs, D.; Cheriet, A.; Derbal, H. Synthesis and characterisation of polypyrrole–indium phosphide composite film. Ionics 2010, 16, 177–184. [Google Scholar] [CrossRef]
- Vasilyeva, S.V.; Vorotyntsev, M.A.; Bezverkhyy, I.; Lesniewska, E.; Heintz, O.; Chassagnon, R. Synthesis and Characterization of Palladium Nanoparticle/Polypyrrole Composites. J. Phys. Chem. C 2008, 112, 19878–19885. [Google Scholar] [CrossRef]
- Bilal, S.; Perveen, F.; Shah, A. Chemical synthesis of polypyrrole doped with dodecyl benzene sulfonic acid. J. SciInnov Res. 2015, 4, 33–42. [Google Scholar]
- Irfan, M.; Shakoor, A.; Majid, A.; Hassam, N.; Ahmed, N. Study of Structural, Thermal and Dielectric Modulus of PPy–DBSA–Zirconium Oxide Composites. Russ. J. Phys. Chem. B 2019, 13, 1057–1063. [Google Scholar] [CrossRef]
- Wang, J.; Yin, W.; He, X.; Wang, Q.; Guo, M.; Chen, S. Good Biocompatibility and Sintering Properties of Zirconia Nanoparticles Synthesized via Vapor-phase Hydrolysis. Sci. Rep. 2016, 6, 35020. [Google Scholar] [CrossRef] [Green Version]
- Sultan, A.; Ahmad, S.; Mohammad, F. Synthesis, Characterization and Electrical Properties of Polypyrrole/ Zirconia Nanocomposite and its Application as Ethene Gas Sensor. Polym. Polym. Compos. 2017, 25, 695–704. [Google Scholar] [CrossRef]
- Yamani, K.; Berenguer, R.; Benyoucef, A.; Morallón, E. Preparation of polypyrrole (PPy)-derived polymer/ZrO2 nanocomposites. J. Therm. Anal. Calorim. 2019, 135, 2089–2100. [Google Scholar] [CrossRef]
- Jayamurgan, P.; Ponnuswamy, V.; Ashokan, S.; Mahalingam, T. The effect of dopant on structural, thermal and morphological properties of DBSA-doped polypyrrole. Iran. Polym. J. 2013, 22, 219–225. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; Yang, L.; Chen, S.; Shao, Y.; Jing, L.; Zhao, G.; Wei, H. Core–shell nanospherical polypyrrole/graphene oxide composites for high performance supercapacitors. RSC Adv. 2015, 5, 91645–91653. [Google Scholar] [CrossRef]
- Liu, X.; Yang, J.; Li, X.; Li, Q.; Xia, Y. Fabrication of polypyrrole (PPy) nanotube electrode for supercapacitors with enhanced electrochemical performance. J. Mater. Sci. Mater. Electron. 2020, 31, 581–586. [Google Scholar] [CrossRef]
- Wang, P.; Zheng, Y.; Li, B. Preparation and electrochemical properties of polypyrrole/graphite oxide composites with various feed ratios of pyrrole to graphite oxide. Synth. Met. 2013, 166, 33–39. [Google Scholar] [CrossRef]
- Yuan, L.; Wan, C.; Zhao, L. Facial in-situ synthesis of MnO2/PPy composite for supercapacitor. Int. J. Electrochem. Sci. 2015, 10, 9456–9465. [Google Scholar]
- Wang, X.; Wang, T.; Liu, D.; Guo, J.; Liu, P. Synthesis and Electrochemical Performance of CeO2/PPy Nanocomposites: Interfacial Effect. Ind. Eng. Chem. Res. 2016, 55, 866–874. [Google Scholar] [CrossRef]
- Zang, J.; Li, X. In situ synthesis of ultrafine β-MnO2/polypyrrole nanorod composites for high-performance supercapacitors. J. Mater. Chem. 2011, 21, 10965–10969. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).